Abstract
Objectives
This study was conducted to assess the management of incidental gallbladder cancer and indeterminate gallbladder lesions. Its secondary aim referred to the devising of a management pathway for these patients.
Methods
Patients referred with incidental gallbladder cancer and indeterminate gallbladder lesions during 2002–2011 were identified from a prospectively maintained database. Collated data included operative findings, histopathological data and survival outcomes.
Results
The study included a total of 104 patients, 40 of whom had incidental gallbladder cancer following cholecystectomy. In this group, the index cholecystectomy was considered curative (T-is/T1a stage) in three patients; 11 patients underwent further resection, and 26 patients were inoperable. One-, 3- and 5-year overall survival rates were 91.1%, 91.0% and 60.7%, respectively, in patients who underwent re-resection. Of the 64 patients with indeterminate gallbladder lesions, 54 patients underwent modified radical cholecystectomy. Seven patients were found to have gallbladder cancer. One-, 3- and 5-year overall survival rates were 85.9%, 43.1% and 42.8%, respectively. Five-year overall survival in patients treated with surgery for gallbladder cancer was 59.9%.
Conclusions
The majority of patients with incidental gallbladder cancer were not amenable to further potentially curative resection. The radiological suspicion of gallbladder cancer should lead to prompt referral to a tertiary hepatobiliary unit for further management.
Introduction
Gallbladder cancer is a rare malignancy in Western populations and is associated with a poor prognosis,1 mainly as a result of rapid tumour progression. Patients usually present with metastatic disease. In patients who are suitable for surgery, extensive liver resection, with or without portal lymphadenectomy and bile duct resection, is frequently required for disease eradication, but is associated with high morbidity and occasional mortality.2 However, the definitive surgical treatment for gallbladder cancer remains controversial, especially with respect to the extent of resection in different stages and modes of presentation. Some centres would consider radical surgery for advanced T3 gallbladder cancer.2,3
In the majority of cases, gallbladder cancers are discovered incidentally following cholecystectomy.4,5 Using the American Joint Committee on Cancer (AJCC) staging system, simple cholecystectomy alone is considered definitive treatment if the histological T-stage is T in situ or T1a, and provided there is no biliary spillage during surgery.6 Some centres have reported reduced disease recurrence rates and better survival outcomes with resection of the liver bed following the incidental diagnosis of gallbladder cancer post-cholecystectomy.2,7,8 The recommended treatment for suspected but resectable gallbladder cancer on imaging is hepatic resection with or without lymphadenectomy and bile duct resection.2,9 However, the management strategy is unclear when cross-sectional imaging is indeterminate. There is a risk that either a patient with gallbladder cancer will be undertreated with simple cholecystectomy, which may potentially affect his or her longterm survival, or that a patient with a benign inflammatory process may be overtreated by liver resection with portal lymphadenectomy, in which there is significant increase in risk for postoperative complications.
The aim of this study was to assess the management of incidental gallbladder cancer found following simple cholecystectomy and of gallbladder lesions that are indeterminate on imaging. The secondary aim referred to the devising of a management pathway for patients in whom possible gallbladder cancer is suspected on initial diagnostic imaging.
Materials and methods
Patients in whom an initial diagnostic scan raised radiological suspicion and patients with histologically proven gallbladder cancer following cholecystectomy were identified from a prospectively maintained hepatobiliary database. All patients had been referred to the study centre during the 9.5-year period from January 2002 to August 2011. Ethics approval for this study was obtained from the University Hospital Aintree.
There were two patterns of referral to this tertiary centre: (i) patients with incidental gallbladder cancer after cholecystectomy carried out at a non-tertiary centre were referred for further management, and (ii) patients with radiologically indeterminate gallbladder lesions were referred for modified radical cholecystectomy. A radiologically indeterminate gallbladder lesion was defined as focal or diffuse thickening of the gallbladder wall, a mass in the gallbladder fossa or an intraluminal mass, with or without associated findings of cholelithiasis, biliary duct dilatation, invasion of the adjacent structures, distant metastases other than those of the liver, and porcelain gallbladder.10,11 Patients were excluded if preoperative imaging clearly suggested gallbladder cancer, N2 stage or M1 stage disease, and if their follow-up after surgery amounted to <12 months.
Collated data included patient demographics, laboratory analyses, type of surgical resection, histopathology analysis and clinical outcome. Preoperative radiological assessment included abdominal ultrasonography (US), computed tomography (CT) scans of the thorax, abdomen and pelvis, and magnetic resonance imaging (MRI) of the liver (from 2008).
All patients were discussed in a specialist multidisciplinary team (MDT) meeting that included hepatobiliary surgeons, and a hepatologist, medical oncologist, radiologist and pathologist prior to surgery or systemic or palliative management.
Revision radical cholecystectomy was offered to patients with incidental gallbladder cancer after cholecystectomy, and without distant metastases on staging CT. T-stages of T-is or T1a disease were considered as curative following the initial cholecystectomy and no further radical intervention was provided.6 In patients with indeterminate gallbladder lesions on imaging, modified radical cholecystectomy was offered. No preoperative histological diagnosis is obtained in this group of patients.
Surgical data
The operative data from the initial cholecystectomy in the incidental gallbladder cancer group were obtained from referral centres and surgical procedures were graded as simple or difficult. A procedure was defined as ‘difficult’ either according to clear documentation in the operation notes and/or because a laparoscopic procedure had required to be converted to an open approach. Other data collated included: bile spillage during cholecystectomy; surgical incision (laparoscopic versus open); type of surgery (simple versus resection of the gallbladder bed); other organ involvement, and intraoperative tumour status.
Modified radical cholecystectomy was performed using either a laparoscopic or an open approach. The laparoscopic approach involved the placement of four ports as per standard laparoscopic cholecystectomy. Following initial assessment, a decision was made to proceed either laparoscopically or to convert to an open procedure based on whether the critical view of safety could be dissected.12 Following dissection, the proximal cystic duct was sent for frozen section examination. The gallbladder was removed en bloc with a 1–2-cm cuff of segment IVb/V of the liver using a Harmonic scalpel (Ethicon Endo-Surgery, Inc, Cincinnati, OH, USA). If the frozen sections were suspicious or positive for gallbladder cancer, the operation was converted to open in order to facilitate a more extensive lymphadenectomy and bile duct excision with biliary reconstruction using Roux-en-Y hepaticojejunostomy.13 All gallbladders harvested using the laparoscopic approach were routinely retrieved using a specimen retrieval bag. Trocar sites were not routinely excised in this cohort. Open procedures were performed in exactly the same manner.
The length of hospital stay, postoperative complications and 30-day mortality were recorded.
Histopathology analysis
Histological tumour–node–metastasis (TNM) staging, tumour status at the cystic duct and resection margins were recorded. Microvascular involvement was also noted. As the majority of patients in the incidental gallbladder cancer group were referred from other hospitals, highly accurate histological T-staging was not always available for all patients for data analysis. However, provided that there was an indication for re-resection after the confirmation of incidental gallbladder cancer, all histological sections of the gallbladder were reviewed at the study centre.
Follow-up protocol
Patients were followed up in a specialist hepatobiliary clinic. Following initial postoperative review at 1 month, all patients were examined in the outpatient clinic at 3, 6, 12, 18 and 24 months and annually thereafter. All patients in this study had a minimum follow-up of 1 year following surgery. Surveillance imaging included CT scans of the thorax, abdomen and pelvis. Patients underwent 6-monthly CT during the first 2 years postoperatively and annual CT scans thereafter.
Overall and disease-free survival data were recorded. Disease-free survival was defined as the time from primary resection to the first documented recurrence of disease on imaging. Overall survival was defined as the time interval between the date of primary resection and the date of death or most recent date of follow-up if the patient was still alive.
Statistical analysis
Categorical data were presented as frequencies and proportions (%) and were analysed using Pearson's chi-squared test or Fisher's exact test. Medians (range) were used to describe continuous data. The Kaplan–Meier method was used to assess actuarial survival and disease-free survival. Univariate analysis was performed to assess for significant differences in clinicopathological characteristics that influenced disease recurrence and survival following resection. A multivariate analysis was performed using Cox regression (stepwise forward model) for variables significant on univariate analysis. All statistical analyses were performed using SigmaPlot for Windows Version 12 (Systat Software, Inc., Chicago, IL, USA) and statistical significance was set at the 5% level.
Results
A total of 104 patients were identified for the study period, 40 of whom had incidental gallbladder cancer following cholecystectomy.
Incidental gallbladder cancer
Of the 40 patients with incidental gallbladder cancer following cholecystectomy, 21 (52.5%) patients were not offered further surgery (Fig. 1, Table 1). In three patients, the index cholecystectomy was considered curative (T1a stage); in the remaining 18 patients, subsequent cross-sectional imaging performed for staging revealed locally unresectable or metastatic disease. Of these 18 patients, nine (50.0%) had biliary spillage secondary to gallbladder perforation during initial cholecystectomy. In addition, the completeness of cholecystectomy was not recorded in six patients.
Figure 1.
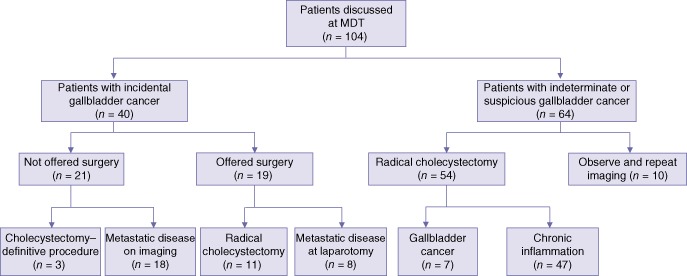
Outcomes in patients with incidental gallbladder cancer and patients with indeterminate or suspicious gallbladder cancer. MDT, multidisciplinary team
Table 1.
Demographic, operative and histopathological data for patients with incidental gallbladder cancer (n = 40)
| Demographic, clinical and pathological factors | Operable patients (n = 14)a n (%) | Inoperable patients (n = 26)b n (%) | P-value |
|---|---|---|---|
| Demographic factors | |||
| Age ≥ 65 years | 5 (35.7%) | 15 (57.7%) | 0.320 |
| Male gender | 3 (21.4%) | 8 (30.8%) | 0.715 |
| Surgical factors | |||
| Gallbladder perforationc | 1 (9.1%) | 8 (57.1%) | 0.033 |
| Histopathological factor | |||
| Tumour–node–metastasis staging | 0.037 | ||
| pT1a | 3 (21.4%) | 0 | |
| pT1b | 2 (14.3%) | 4 (15.4%) | |
| pT2 | 8 (57.1%) | 5 (19.2%) | |
| pT3 | 0 | 4 (15.4%) | |
| pT4 | 1 (7.1%) | 7 (26.9%) | |
| Resection margin (R0 ≥ 1 mm) | 14 (100%) | NA | – |
The operable group consists of patients who were sufficiently treated by cholecystectomy (n = 3) and subsequent hepatic resection (n = 11).
Histology details of six patients in the inoperable group were not available.
Information was unavailable for one patient in the operable group and 12 patients in the inoperable group.
P-values in bold indicate statistically significant differences (P < 0.05).
NA, not applicable.
Of the 19 patients with incidental gallbladder cancer who were offered surgery, eight (42.1%) were found to have metastatic peritoneal disease at laparotomy, which was confirmed by frozen section examination. Six of the eight (75.0%) patients had biliary spillage during the index cholecystectomy. The remaining 11 patients underwent open resection of the gallbladder bed (segments IVb/V). The overall morbidity rate was 15.8% (n = 3). Two patients suffered Grade II complications (nosocomial pneumonia and supraventricular tachycardia), and one suffered a Grade III complication of biliary leak without further intervention.14 One of these 11 patients, who had biliary spillage during initial cholecystectomy, was subsequently found to have recurrent disease within 1 year after re-resection. Records of initial cholecystectomy status were not available in 10 patients.
Rates of 1-, 3- and 5-year overall survival in patients submitted to gallbladder bed re-resection were 91.1%, 91.0% and 60.7%, respectively. By contrast, patients found to be unresectable on cross-sectional imaging or at laparotomy had a median survival of 8 months following referral. One patient had disease recurrence at 33 months after hepatic resection following the finding of an incidental T1a gallbladder cancer at the index cholecystectomy.
Indeterminate/suspicious gallbladder cancer
Of the 64 patients with indeterminate gallbladder lesions, 54 patients underwent modified radical cholecystectomy on an intention-to-treat basis (open surgery, n = 38; laparoscopic surgery, n = 7; laparoscopic converted to open surgery, n = 9). Seven of these 54 patients were found to have gallbladder cancer and 47 patients had severe chronic inflammation on histopathological analysis (Table 2). In addition to radical cholecystectomy, en bloc resections of the colon or duodenum (n = 4) and common bile duct (n = 2) were performed. Among those requiring multiple visceral resections, three of four patients had a histological diagnosis of gallbladder cancer.
Table 2.
Demographic, operative and histopathological data for patients with suspicious gallbladder lesions (n = 64)a
| Demographic, clinical and pathological factors | Patients with malignant disease (n = 7) n (%) | Patients with benign disease (n = 47)a n (%) | P-value |
|---|---|---|---|
| Demographic factors | |||
| Age ≥ 65 years | 4 (57.1%) | 17 (38.6%) | 0.411 |
| Male gender | 5 (71.4%) | 17 (38.6%) | 0.107 |
| Surgery | |||
| Additional procedure | 3 (42.9%) | 4 (9.1%) | 0.039 |
| Histopathological factor | |||
| Tumour–node–metastasis staging | – | ||
| pT1a | 0 | 0 | |
| pT1b | 0 | 0 | |
| pT2 | 3 (42.9%) | 0 | |
| pT3 | 3 (42.9%) | 0 | |
| pT4 | 1 (14.3%) | 0 | |
| Dysplasia | NA | 2 (4.5%) | |
| Porcelain gallbladder | NA | 5 (11.4%) | |
| Resection margin (R0 ≥ 1 mm) | 7 (100) | NA | – |
The benign group consists of patients with a histological diagnosis of benign inflammation (n = 47). Patients who underwent cross-sectional imaging on follow-up were excluded from this analysis (n = 10).
P-values in bold indicate statistically significant differences (P < 0.05).
NA, not applicable.
The overall morbidity rate was 14.8% (n = 8). Grade I complications (post-epidural headache and wound infection) were observed in two patients, Grade II complications (biliary leak and acute renal failure) were reported in two patients, Grade IIIa complications (duodenal fistula formation, pulmonary embolism and biliary fistula) were seen in three patients, and Grade IIIb complications (wound dehiscence and persistent biliary fistula) occurred in one patient.14 Median survival in patients with gallbladder malignancy was 18.9 months (range: 13–77 months). Rates of 1-, 3- and 5-year overall survival were 85.9%, 43.1% and 42.8%, respectively. Two of these seven patients had local recurrence and one had distant metastases. Rates of 1-, 3- and 5-year disease-free survival in these patients were 57.2%, 43.0% and 42.8%, respectively.
The remaining 10 patients underwent a period of observation and repeat cross-sectional imaging; none developed recurrence during the follow-up period.
Overall, four of 21 (19.0%) patients with curative resection for gallbladder cancer had disease recurrence. Five-year overall survival in these patients was 59.9%. Although 1-, 3- and 5-year survival appeared to be better in the group undergoing re-resection for incidental gallbladder cancer than in patients undergoing modified radical cholecystectomy, the difference between the groups in survival was not statistically significant (P = 0.23).
Discussion
Gallbladder cancer remains a difficult malignancy to manage, particularly in those patients referred to tertiary centres with an incidental histopathological diagnosis of underlying malignancy following apparently straightforward cholecystectomy. In the present cohort, the further management of 104 patients was discussed in MDT meetings following either incidental histology-proven gallbladder malignancy or suspicious gallbladder cancer on cross-sectional imaging. Over the last decade, regional policy at the study centre has required the tertiary referral of all suspicious or confirmed gallbladder malignancies to the unit's MDT meeting. Hence, this study reports a true denominator for the incidence of incidental gallbladder cancers following cholecystectomy in a general European population. This is a key difference between the present study and other studies published in the literature.15–18
With respect to patients in whom incidental gallbladder cancer was found following cholecystectomy, the proportions of patients subsequently found to be eligible for further hepatic resection varied among centres.2,7,19 This difference may reflect referral bias dependent on the policies of the referring hospital and the respective tertiary centre. Fong et al.2 and Paolucci et al.20 reported rates of hepatic resection with curative intent for incidental gallbladder cancer of 32% and 29%, respectively. By contrast, the present series observed a resection rate of 25.0% in such patients. The exact reason for this small difference is unknown, but it may be explained by the delay in referral from the district general hospital following cholecystectomy and subsequent histology analysis. In addition, the majority of published studies were retrospective in nature and hence did not report the proportion of patients undergoing subsequent futile laparotomy.7
The present series also demonstrated that further potentially curative surgery could not be offered to more than half of 50.0% of the patients found to have incidental gallbladder cancer following cholecystectomy, and hence indicated the loss of any survival benefit of resection.21 In patients who proceeded to surgery with curative intent in this incidental group, curative resection was not feasible in 42.1% as a result of either locally advanced or peritoneal disease following the initial cholecystectomy. A possible contributing factor was biliary spillage secondary to gallbladder perforation.
Fong and co-workers2 compared findings in patients with gallbladder cancer diagnosed after cholecystectomy with findings in those without histological diagnoses and discovered that patients without previous cholecystectomy had higher T-stage disease and were therefore less likely to be suitable for curative resection. These results suggested that initial cholecystectomy was not associated with a lower likelihood of definitive curative resection. In the present cohort, a higher T-stage in the primary resection group than in the incidental group was similarly observed.
Current 5-year survival data for patients with gallbladder cancer who undergo radical cholecystectomy or subsequent hepatic resection range from 21% to 69%.6,22–27 The survival outcome in the present series is similar to those in these published reports. In addition, there was no survival difference between patients who underwent subsequent segment IVb/V re-resection and those submitted to modified radical cholecystectomy in the current patient cohort. These results are consistent with the findings of other published studies.17,28,29 Other authors have also reported a survival benefit in patients with incidental gallbladder cancer who undergo subsequent hepatic resection compared with patients who are considered to be unresectable as a result of disease progression,2,8,17 as was observed in the present study.
Based on the findings above, Fig. 2 summarizes a suggested management algorithm for both incidental gallbladder cancers and indeterminate gallbladder lesions. It is recommended that all patients with suspicious or histologically proven gallbladder cancer should be referred to a tertiary hepatobiliary centre for further management within 2 weeks.
Figure 2.
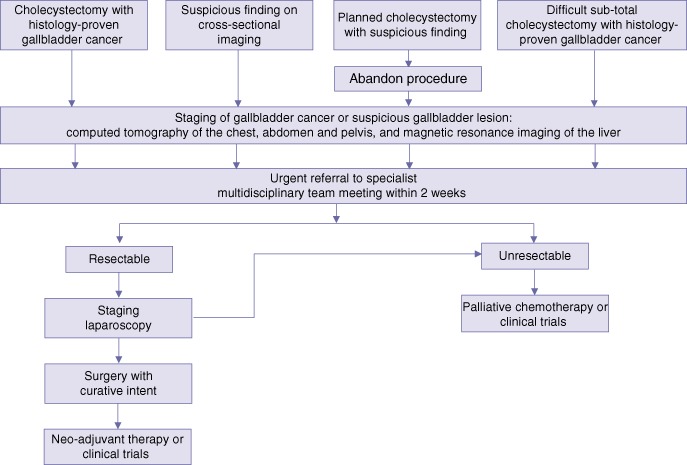
Suggested management algorithm for patients with suspected gallbladder cancer
Given the present finding that more than 50.0% of patients would not be offered re-resection following cholecystectomy for incidental gallbladder cancer, and hence would lose any survival benefit to be derived from re-resection, it would be reasonable to recommend that when a non-hepatobiliary surgeon is confronted by a suspicious gallbladder lesion during laparoscopic cholecystectomy, the procedure should be abandoned and the patient referred to the respective tertiary hepatobiliary centre for further management. It may be that the management approach outlined in Fig. 2 would minimize the number of incidental gallbladder cancers and optimize potential curative resection rates in this group of patients. In line with current national guidelines, which require a referral to be made within 2 weeks for any suspected malignancy,30 the 2-week rule was applied in the management algorithm presented here.
It is challenging to fully differentiate chronic cholecystitis from a malignant process based on CT imaging.31 This is one of the reasons why 13% of gallbladder cancer is identified in patients with indeterminate gallbladder lesions. In addition, most series do not report the denominators for indeterminate gallbladder lesions on cross-sectional imaging,2,7,32 but simply use histologically proven gallbladder cancers as the denominator in the series. It is therefore impossible to compare actual management strategies for gallbladder lesions that are indeterminate on imaging among hepatobiliary centres.
Furthermore, in patients in this cohort with benign chronic inflammatory disease, surgical intervention is frequently complex and additional procedures are required. These cases are difficult to complete laparoscopically and frequently require to be converted to open procedures, even in the hands of skilled laparoscopic hepatobiliary surgeons. The degree of inflammation and fibrosis also increases the risk for bile duct injury in these patients.33 Hence, it is more appropriate to perform such complex biliary surgery in a tertiary hepatobiliary centre.34
In conclusion, the majority of patients found to have incidental gallbladder cancer following cholecystectomy are not amenable to further potentially curative hepatic resection and present with metastatic disease on cross-sectional imaging post-cholecystectomy. This significantly lowers their overall survival. The presence of clinical, radiological and/or intraoperative suspicions of gallbladder cancer should lead to a prompt referral to the tertiary hepatobiliary unit for further management.
Acknowledgments
The authors would like to thank Dr N. Grimes, Mr A. Al-Sariah and Mr S. Staettner, Department of Hepatobiliary Surgery, University Hospital Aintree, Liverpool, for their help in contributing parts of this manuscript.
Conflicts of interest
None declared.
References
- 1.White K, Kraybill WG, Lopez MJ. Primary carcinoma of the gallbladder: TNM staging and prognosis. J Surg Oncol. 1988;39:251–255. doi: 10.1002/jso.2930390407. [DOI] [PubMed] [Google Scholar]
- 2.Fong Y, Jarnagin W, Blumgart LH. Gallbladder cancer: comparison of patients presenting initially for definitive operation with those presenting after prior non-curative intervention. Ann Surg. 2000;232:557–569. doi: 10.1097/00000658-200010000-00011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kai M, Chijiiwa K, Ohuchida J, Nagano M, Hiyoshi M, Kondo K. A curative resection improves the postoperative survival rate even in patients with advanced gallbladder carcinoma. J Gastrointest Surg. 2007;11:1025–1032. doi: 10.1007/s11605-007-0181-4. [DOI] [PubMed] [Google Scholar]
- 4.Misra MC, Guleria S. Management of cancer gallbladder found as a surprise on a resected gallbladder specimen. J Surg Oncol. 2006;93:690–698. doi: 10.1002/jso.20537. [DOI] [PubMed] [Google Scholar]
- 5.Steinert R, Nestler G, Sagynaliev E, Muller J, Lippert H, Reymond MA. Laparoscopic cholecystectomy and gallbladder cancer. J Surg Oncol. 2006;93:682–689. doi: 10.1002/jso.20536. [DOI] [PubMed] [Google Scholar]
- 6.Taner CB, Nagorney DM, Donohue JH. Surgical treatment of gallbladder cancer. J Gastrointest Surg. 2004;8:83–89. doi: 10.1016/j.gassur.2003.09.022. discussion 89. [DOI] [PubMed] [Google Scholar]
- 7.Clemente G, Nuzzo G, De Rose AM, Giovannini I, La Torre G, Ardito F, et al. Unexpected gallbladder cancer after laparoscopic cholecystectomy for acute cholecystitis: a worrisome picture. J Gastrointest Surg. 2012;16:1462–1468. doi: 10.1007/s11605-012-1915-5. [DOI] [PubMed] [Google Scholar]
- 8.Goetze TO, Paolucci V. Adequate extent in radical re-resection of incidental gallbladder carcinoma: analysis of the German Registry. Surg Endosc. 2010;24:2156–2164. doi: 10.1007/s00464-010-0914-4. [DOI] [PubMed] [Google Scholar]
- 9.Pitt HA, Nakeeb A. Operative approach to gallbladder cancer. Curr Gastroenterol Rep. 2006;8:161–167. doi: 10.1007/s11894-006-0013-9. [DOI] [PubMed] [Google Scholar]
- 10.Soyer P, Gouhiri M, Boudiaf M, Brocheriou-Spelle I, Kardache M, Fishman EK, et al. Carcinoma of the gallbladder: imaging features with surgical correlation. AJR Am J Roentgenol. 1997;169:781–785. doi: 10.2214/ajr.169.3.9275896. [DOI] [PubMed] [Google Scholar]
- 11.Rooholamini SA, Tehrani NS, Razavi MK, Au AH, Hansen GC, Ostrzega N, et al. Imaging of gallbladder carcinoma. Radiographics. 1994;14:291–306. doi: 10.1148/radiographics.14.2.8190955. [DOI] [PubMed] [Google Scholar]
- 12.Strasberg SM, Brunt LM. Rationale and use of the critical view of safety in laparoscopic cholecystectomy. J Am Coll Surg. 2010;211:132–138. doi: 10.1016/j.jamcollsurg.2010.02.053. [DOI] [PubMed] [Google Scholar]
- 13.Jensen EH, Abraham A, Jarosek S, Habermann EB, Al-Refaie WB, Vickers SA, et al. Lymph node evaluation is associated with improved survival after surgery for early stage gallbladder cancer. Surgery. 2009;146:706–711. doi: 10.1016/j.surg.2009.06.056. discussion 711–713. [DOI] [PubMed] [Google Scholar]
- 14.Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg. 2004;240:205–213. doi: 10.1097/01.sla.0000133083.54934.ae. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lendoire JC, Gil L, Duek F, Quarin C, Garay V, Raffin G, et al. Relevance of residual disease after liver resection for incidental gallbladder cancer. HPB. 2012;14:548–553. doi: 10.1111/j.1477-2574.2012.00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Glauser PM, Strub D, Kaser SA, Mattiello D, Rieben F, Maurer CA. Incidence, management, and outcome of incidental gallbladder carcinoma: analysis of the database of the Swiss Association of Laparoscopic and Thoracoscopic Surgery. Surg Endosc. 2010;24:2281–2286. doi: 10.1007/s00464-010-0952-y. [DOI] [PubMed] [Google Scholar]
- 17.Cho SY, Park SJ, Kim SH, Han SS, Kim YK, Lee KW. Comparative analysis between clinical outcomes of primary radical resection and second completion radical resection for T2 gallbladder cancer: single-centre experience. World J Surg. 2010;34:1572–1578. doi: 10.1007/s00268-010-0522-4. [DOI] [PubMed] [Google Scholar]
- 18.Butte JM, Waugh E, Meneses M, Parada H, De La Fuente HA. Incidental gallbladder cancer: analysis of surgical findings and survival. J Surg Oncol. 2010;102:620–625. doi: 10.1002/jso.21681. [DOI] [PubMed] [Google Scholar]
- 19.Goetze TO, Paolucci V. [Immediate radical re-resection of incidental T1b gallbladder cancer and the problem of an adequate extent of resection (results of the German Registry ‘Incidental Gallbladder Cancer’).] Zentralbl Chir. 2011 doi: 10.1055/s-0030-1262698. [Epub ahead of print] German. PMID: 21365537. [DOI] [PubMed] [Google Scholar]
- 20.Paolucci V, Neckell M, Gotze T. [Unsuspected gallbladder carcinoma – the CAE-S/CAMIC registry.] Zentralbl Chir. 2003;128:309–312. doi: 10.1055/s-2003-38795. [DOI] [PubMed] [Google Scholar]
- 21.Jensen EH, Abraham A, Habermann EB, Al-Refaie WB, Vickers SM, Virnig BA, et al. A critical analysis of the surgical management of early-stage gallbladder cancer in the United States. J Gastrointest Surg. 2009;13:722–727. doi: 10.1007/s11605-008-0772-8. [DOI] [PubMed] [Google Scholar]
- 22.Duffy A, Capanu M, Abou-Alfa GK, Huitzil D, Jarnagin W, Fong Y, et al. Gallbladder cancer (GBC): 10-year experience at Memorial Sloan–Kettering Cancer Center (MSKCC) J Surg Oncol. 2008;98:485–489. doi: 10.1002/jso.21141. [DOI] [PubMed] [Google Scholar]
- 23.Dixon E, Vollmer CM, Jr, Sahajpal A, Cattral M, Grant D, Doig C, et al. An aggressive surgical approach leads to improved survival in patients with gallbladder cancer: a 12-year study at a North American centre. Ann Surg. 2005;241:385–394. doi: 10.1097/01.sla.0000154118.07704.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito H, Matros E, Brooks DC, Osteen RT, Zinner MJ, Swanson RS, et al. Treatment outcomes associated with surgery for gallbladder cancer: a 20-year experience. J Gastrointest Surg. 2004;8:183–190. doi: 10.1016/j.gassur.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Lai EC, Lau WY. Aggressive surgical resection for carcinoma of gallbladder. ANZ J Surg. 2005;75:441–444. doi: 10.1111/j.1445-2197.2005.03401.x. [DOI] [PubMed] [Google Scholar]
- 26.Sakamoto Y, Kosuge T, Shimada K, Sano T, Hibi T, Yamamoto J, et al. Clinical significance of extrahepatic bile duct resection for advanced gallbladder cancer. J Surg Oncol. 2006;94:298–306. doi: 10.1002/jso.20585. [DOI] [PubMed] [Google Scholar]
- 27.Tsukada K, Hatakeyama K, Kurosaki I, Uchida K, Shirai Y, Muto T, et al. Outcome of radical surgery for carcinoma of the gallbladder according to the TNM stage. Surgery. 1996;120:816–821. doi: 10.1016/s0039-6060(96)80089-4. [DOI] [PubMed] [Google Scholar]
- 28.Foster JM, Hoshi H, Gibbs JF, Iyer R, Javle M, Chu Q, et al. Gallbladder cancer: defining the indications for primary radical resection and radical re-resection. Ann Surg Oncol. 2007;14:833–840. doi: 10.1245/s10434-006-9097-6. [DOI] [PubMed] [Google Scholar]
- 29.Shih SP, Schulick RD, Cameron JL, Lillemoe KD, Pitt HA, Choti MA, et al. Gallbladder cancer: the role of laparoscopy and radical resection. Ann Surg. 2007;245:893–901. doi: 10.1097/SLA.0b013e31806beec2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.National Institute for Health and Care Excellence (NICE) London: NICE; 2005. Referral for suspected cancer (CG27) [Google Scholar]
- 31.Yun EJ, Cho SG, Park S, Park SW, Kim WH, Kim HJ, et al. Gallbladder carcinoma and chronic cholecystitis: differentiation with two-phase spiral CT. Abdom Imaging. 2004;29:102–108. doi: 10.1007/s00261-003-0080-4. [DOI] [PubMed] [Google Scholar]
- 32.Goetze TO, Paolucci V. Immediate re-resection of T1 incidental gallbladder carcinomas: a survival analysis of the German Registry. Surg Endosc. 2008;22:2462–2465. doi: 10.1007/s00464-008-9747-9. [DOI] [PubMed] [Google Scholar]
- 33.Wolf AS, Nijsse BA, Sokal SM, Chang Y, Berger DL. Surgical outcomes of open cholecystectomy in the laparoscopic era. Am J Surg. 2009;197:781–784. doi: 10.1016/j.amjsurg.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 34.Harrison EM, O'Neill S, Meurs TS, Wong PL, Duxbury M, Paterson-Brown S, et al. Hospital volume and patient outcomes after cholecystectomy in Scotland: retrospective, national population-based study. BMJ. 2012;344:e3330. doi: 10.1136/bmj.e3330. [DOI] [PubMed] [Google Scholar]


