Abstract
BACKGROUND AND PURPOSE
Despite the abundant expression of the UDP-sensitive P2Y6 receptor in urothelial cells and sub-urothelial myofibroblasts its role in the control of bladder function is not well understood.
EXPERIMENTAL APPROACH
We compared the effects of UDP and of the selective P2Y6 receptor agonist, PSB0474, on bladder urodynamics in anaesthetized rats; the voided fluid was tested for ATP bioluminescence. The isolated urinary bladder was used for in vitro myographic recordings and [3H]-ACh overflow experiments.
KEY RESULTS
Instillation of UDP or PSB0474 into the bladder increased the voiding frequency (VF) without affecting the amplitude (A) and the duration (Δt) of bladder contractions; an effect blocked by the P2Y6 receptor antagonist, MRS2578. Effects mediated by urothelial P2Y6 receptors required extrinsic neuronal circuitry as they were not detected in the isolated bladder. UDP-induced bladder hyperactvity was also prevented by blocking P2X3 and P2Y1 receptors, respectively, with A317491 and MRS2179 applied i.v.. UDP decreased [3H]-ACh release from stimulated bladder strips with urothelium, but not in its absence. Inhibitory effects of UDP were converted into facilitation by the P2Y1 receptor antagonist, MRS2179. The P2Y6 receptor agonist increased threefold ATP levels in the voided fluid.
CONCLUSIONS AND IMPLICATIONS
Activation of P2Y6 receptors increased the voiding frequency indirectly by releasing ATP from the urothelium and activation of P2X3 receptors on sub-urothelial nerve afferents. Bladder hyperactivity may be partly reversed following ATP hydrolysis to ADP by E-NTPDases, thereby decreasing ACh release from cholinergic nerves expressing P2Y1 receptors.
Keywords: urinary bladder, urothelium, micturition reflex, P2Y6 receptor, ATP release, UDP, acetylcholine release, anaesthetized rat
Introduction
Purinergic transmission has increasingly been accepted as having an important modulatory role in many aspects of bladder function. There is good evidence that the primary source of ATP in the bladder is the urothelium (Ferguson et al., 1997; Kumar et al., 2004). This nucleotide exerts its effects predominantly through the activation of P2 purinoceptors located in the detrusor smooth muscle (P2X1) and in sub-urothelial pelvic nerve afferents (P2X3) that are involved in the micturition reflex triggered by bladder filling (Burnstock, 1999; Cockayne et al., 2000; Ito et al., 2008; receptor nomenclature follows Alexander et al., 2013a). Thus, intravesical ATP decreases bladder capacity and micturition volume in a concentration-dependent manner, with limited effects on detrusor tension (Pandita and Andersson, 2002). ATP stimulation of micturition may be partly counteracted by its catabolism, by ecto-nucleoside triphosphate diphosphohydrolases (E-NTPDases) of the urinary bladder, into ADP, which could then act through inhibitory P2Y1 receptors on cholinergic nerve endings in the human bladder (Silva et al., 2011; nomenclature see Alexander et al., 2013b). Recently, evidence has emerged supporting a role for sub-urothelial myofibroblasts in detrusor contraction, which also express P2X and P2Y purinoceptors (Fry and Rossen, 2007; Fry et al., 2007).
Deregulation of purinergic signalling through the abnormal production and release of ATP or altered expression of various P2 purinoceptors is a common feature of many urological diseases (Ruggieri, 2006; Fry and Rossen, 2007). Substantial amounts of ATP are released from urothelial cells in response to mechanical stretch, inflammatory mediators and chemical irritation (see Ferguson et al., 1997; Vlaskovska et al., 2001; Wang et al., 2005). Increase in the purinergic tone of the bladder has been associated with interstitial cystitis, detrusor overactivity, outlet obstruction, inflammation, neurogenic bladder, spinal cord lesions and ageing. Our group has provided evidence that urinary ATP may be a dynamic biomarker of detrusor activity in women with overactive bladder syndrome (Silva-Ramos et al., 2013). There is, however, very limited understanding of the molecular mechanisms involved in purinergic signalling changes underlying these pathologies, limiting the efficacy of treatment options.
In contrast to the compelling evidence for the extracellular signalling role of ATP, the hypothesis that uracil nucleotides may also fulfil an autocrine/paracrine role has only recently gained experimental support. Previous studies showed that rat urothelial cells express P2Y2 (and to a lesser extent P2Y4) receptors recognizing UTP as the most potent agonist, which upon activation lead to the release of ATP (Chopra et al., 2014). On the other hand, UDP-sensitive P2Y6 receptors have been involved in the generation of large spontaneous contractions and propagating waves of intracellular Ca2+ and membrane depolarization originating in sub-urothelial myofibroblasts and spreading to the detrusor smooth muscle, in rats submitted to spinal cord transection (Fry et al., 2012). Furthermore, Yu et al. (2013,2013) using myographic recordings demonstrated that UDP, acting on P2Y6 receptors, interplays with P2X1 receptors in a synergistic manner to increase bladder smooth muscle tone. Despite the signalling effects of P2Y6 receptors in sub-urothelial myofibroblasts and detrusor smooth muscle fibres, we were intrigued by the observation that P2Y6 receptors are most abundantly expressed in urothelial cells with discrete labelling in the sub-urothelial layer (see immunostaining in Sui et al., 2006), but no data have been produced to date regarding its role in the urothelium, to control bladder function.
This study was designed to investigate the ability of urinary UDP and its stable analogue PSB0474, which exhibits selectivity for P2Y6 receptors (El-Tayeb et al., 2006), to modulate bladder urodynamics in the anaesthetized rat in vivo. The rat has been widely used as a model to study the purinergic mechanisms underlying bladder function in health and disease (see Burnstock, 2014), which in most instances compared well with that observed in humans. Given our findings, showing that both UDP and PSB0474 increased the voiding frequency in the anaesthetized rat only if the bladder nervous circuitry was left intact, we hypothesized that ATP released from urothelium could be the mediator involved in P2Y6 receptor-operated bladder hyperactivity. Therefore, we measured the ATP levels in the voiding fluid before and after P2Y6 receptor activation. The participation of excitatory P2X3 and inhibitory P2Y1 purinoceptors located, respectively, on sub-urothelial nerve afferents and cholinergic nerve efferents was investigated in the anaesthetized rat in vivo and in stimulated bladder strips in vitro.
Methods
Animals
Animal care and experimental procedures were in accordance with the guidelines prepared by Committee on Care and Use of Laboratory Animal Resources (National Research Council, USA) and followed the European Communities Council Directive (86/609/EEC). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 1974; McGrath et al., 2004). A total of 105 animals were used in the experiments described here. Male rats (Wistar, 300–450 g; Charles River, Barcelona, Spain) were kept at a constant temperature (21°C) and a regular light (06:30–19:30 h)–dark (19:30–06:30 h) cycle, with food and water provided ad libitum.
In vivo cystometric recordings
The experiments were carried out in spontaneously breathing rats, anaesthetized with urethane (1.0−1.2 g·kg−1). Core body temperature was kept between 36 and 38°C with the help of a heating pad controlled by a thermosensor connected to a rectal probe. A catheter connected to an injection pump was inserted into the left jugular vein to permit saline infusion (4 mL·h−1·kg−1) and i.v. drugs application. After exposing the urinary bladder through a medial abdominal incision, a three-barrel catheter was inserted through its dome. One barrel was connected to an automated perfusion pump for saline and/or drugs infusion; a second barrel was attached to a pressure transducer for continuous monitoring of intravesical pressure; the third barrel was used either to drain or to close the bladder circuit in order to initiate the micturition reflex. The bladder pressure was continuously monitored on a computer screen with a PowerLab data acquisition system (Chart 5, version 4.2 software; AD Instruments, Colorado Springs, CO, USA), which was also used to record haemodynamic and respiratory parameters in the anaesthetized rat.
After surgical preparation, a 60 min equilibration period was undertaken during which saline was infused into the urinary bladder at 0.04 mL·min−1 and allowed to freely drain out of the bladder (open circuit). The micturition reflex was initiated by closing the draining barrel while keeping intravesical infusion of saline at a constant flow rate (0.04 mL·min−1), which is within the range used by other authors to obtained stable micturition cycles during continuous cystometrograms in anaesthetized rats (see Honda et al., 2012). The flow rate was two- to fourfold higher than the normal urinary debit in experimental rats (15–30 mL·d−1) and compared with the conditions used in standard filling cystometry (urodynamic test) in humans. Voiding contractions were assumed as large-amplitude rhythmic bladder contractions accompanied by urine draining through the urethra when bladder pressure reached a certain threshold (see Figure 1A). The intercontraction interval (ICI, min) and the pressure threshold (PTh, cm of H2O) that is required to initiate the voiding reflex are normally associated with the sensitive component of the micturition reflex (filling phase); conversely, the amplitude (A, cm of H2O) and the duration (Δt, s) of the voiding contractions are mostly associated with the motor component of the micturition reflex (emptying phase). For the sake of clarity, the results presented in this study will consider, in most instances, the percentage change in of ICI and Δt values, compared with the control situation achieved after six consecutive voiding contractions of similar amplitude. Test drugs were applied either into the bladder lumen (by changing the syringe connected to the automated perfusion pump, 0.04 mL·min−1) or i.v. through the catheter inserted into the left jugular vein.
Figure 1.
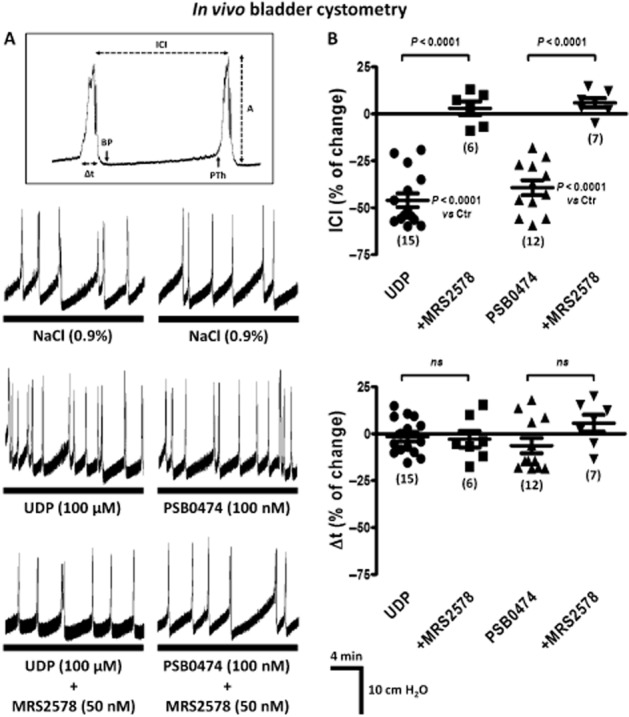
(A) Bladder cystometry recordings during normal saline (0.9%·w/v of NaCl) infusion into the urinary bladder of urethane-anaesthetized rats: comparison of the effects of UDP (100 μM) and PSB0474 (100 nM) in the absence and in the presence of the selective P2Y6 receptor antagonist, MRS2578 (50 nM). Large-amplitude rhythmic bladder contractions correspond to voiding contractions when they were accompanied by urine draining through the urethra. The inset shows the urodynamic parameters evaluated: ICI (min), PTh (cm of H2O), amplitude (A, cm of H2O) and duration (Δt, s) of the voiding contractions. Stable urodynamic responses to UDP and PSB0474 were reached in 10–15 min. Co-application of MRS2578 with the agonists preceded urodynamic measurements by at least 20 min. (B) Scatter plots representing the percentage change of the ICI and of the duration (Δt) of voiding contractions as compared with control values (Ctr, 0%). The vertical bars represent SEM of a n number of animals (shown in parenthesis). P values as shown; significantly different from control samples (saline infusion) or from the effects of UDP or PSB0474, applied alone; unpaired Student's t-test with Welch's correction.
In vitro myographic recordings
Myographic recordings were performed in vitro in whole-mounts of the rat urinary bladder. After removal of the urinary bladder from the animal, a three-barrel catheter was inserted through its dome as described for the in vivo cystometric assays. The preparation was then mounted along its longitudinal axis in a 12 mL capacity perfusion chamber and connected to an isometric force transducer via a thread tied to the proximal urethra. Tension responses were recorded isometrically at a resting tension of 10 mN with a force transducer and displayed on a Hugo-Sachs (March-Hugstetten, Germany) thermo-sensitive paper recorder. Preparations were allowed to equilibrate for 60 min under continuous superfusion of both the outside and the inside of the bladder with gassed (95% O2 and 5% CO2) Tyrode's solution containing (mM): NaCl 137, KCl 2.7, CaCl2 1.8, MgCl2 1, NaH2PO4 0.4, NaHCO3 11.9, glucose 11.2, at 37°C. After closing the draining barrel of the catheter inserted into the lumen, bladders were then filled with Tyrode's solution to a maximum of 0.15 mL at increments of 10 μL to simulate the conditions used in the in vivo cystometric assays (0.04 mL·min−1). UDP (300 μM) was superfused either through the catheter inserted into the bladder dome or directly into the bathing solution outside the bladder wall. The effect of UDP was compared with that of the muscarinic receptor agonist, oxotremorine (30 μM), and the ATP analogue, α,β-methylene ATP (30 μM).
Measurement of urinary ATP
For measuring urinary ATP content, samples were collected from the draining barrel of the catheter inserted in the bladder during in vivo fluid cystometry experiments. Sterile samples were immediately freeze-dried in liquid nitrogen and preserved at −80°C until ATP determination (see Silva-Ramos et al., 2013). Undiluted samples were unfrozen to 25°C and afterwards centrifuged at 3000× g at room temperature for 20 s. A mixture of luciferine-luciferase was added to the supernatant according to the manufacturer's instructions (ATP Bioluminescence Assay Kit HS II, Roche Applied Science Indianapolis, IN, USA). Luminescence was detected using a microplate reader (Synergy HT, BioTek Instruments Inc., Winooski, VT, USA). Sample bioluminescence was compared with external ATP standards prepared daily within the same concentration range. All samples were run in duplicate. Each sample remaining was used to quantify the LDH (EC 1.1.1.27) activity (Keiding et al., 1974). LDH is an intracellular enzyme that is commonly used as an indicator of cell integrity.
[3H]-ACh release experiments
The experiments were performed at 37°C in urinary bladder strips with or without urothelium mounted in 365 μL capacity chambers of a Brandel SF-12 automated superfusion system (Valley International Corp., Austin, TX, USA). Preparations were continuously superfused with gassed (95% O2 and 5% CO2) Tyrode's solution. Urinary bladders were cut in half; one half was left intact and the other half had its urothelium gently removed with a cottonwool swab.
After a 30 min equilibration period, the preparations were loaded for 40 min with 1 μM [3H]-choline (specific activity 5 μCi·nmol−1) under electrical field stimulation at 1 Hz frequency (EFS, 0.2 ms pulse width). Washout (1 mL·min−1) of the preparations was performed over 120 min with Tyrode's solution supplemented with the choline uptake inhibitor, hemicholinium-3 (10 μM). Tritium outflow was evaluated by liquid scintillation spectrometry (TriCarb2900TR, Perkin Elmer, Boston, MA, USA) (percentage of counting efficiency: 55 ± 2%) after appropriate background subtraction, using 1 mL bath samples automatically collected every 1 min.
[3H]-ACh release was evoked by two periods of EFS (S1 and S2) each consisting of 200 square wave pulses of 0.2 ms duration delivered at 10 Hz frequency. EFS of the rat urinary bladder increased the release of [3H]-ACh in a Ca2+- and tetrodotoxin (TTX)-sensitive manner, and hence, originates from vesicle exocytosis from cholinergic nerves. Test drugs were added 8 min before S2 and were present up to the end of the experiments. In control conditions, S2/S1 ratios were 0.91 ± 0.03 (n = 5) and 0.91 ± 0.07 (n = 4) when bladder strips were used with and without the urothelium respectively. None of the drugs significantly (P > 0.05) changed the basal tritium outflow.
At the end of the experiments, the preparations were fixed in PLP solution (paraformaldehyde 2%, lysine 0.075 M, sodium phosphate 0.037 M, sodium periodate 0.01 M) for 16 h at 4°C and stained with haematoxylin-eosin for histological observation, to confirm the presence or the absence of the urothelium.
Immunofluorescence staining and confocal microscopy observation
Excised bladder fragments were stretched in all directions, pinned onto Petri dishes coated with Sylgard and fixed in PLP solution for 16 h at 4°C. Fixed tissue was cryoprotected with a solution containing 20% anhydrous glycerol dissolved in 0.1 M phosphate buffer, frozen, sectioned (16 μm) and incubated with a blocking buffer solution consisting of FBS 10%, BSA 1%, Triton X-100 0.3% in PBS, for 2 h with constant stirring. After blocking and permeabilization, samples were incubated with selected primary antibodies (see Table 1) diluted in the incubation buffer (FBS 5%, serum albumin 1%, Triton X-100 0.3% in PBS), at 4°C, for 16 h. For double immunostaining, antibodies were combined before application to tissue samples. After washing away unbound primary antibody, the sections were incubated with secondary antibodies (Table 1) in the dark for 2 h, at room temperature. Finally, tissue samples were mounted on optical-quality glass slides using VectaShield with DAPI as mounting media (VectorLabs, Peterborough, UK) and stored in the dark at 4°C. Observations were performed and analysed with a laser-scanning confocal microscope (Olympus FluoView, FV1000, Tokyo, Japan).
Table 1.
Primary and secondary antibodies used in immunohistochemistry experiments
| Antigen | Code | Host | Dilution | Supplier |
|---|---|---|---|---|
| Primary antibodies | ||||
| P2Y1 | APR-009 | Rabbit | 1:75 | Alomone |
| P2Y6 | APR-011 | Rabbit | 1:100 | Alomone |
| P2Y6 | ABIN1386282 | Rabbit | 1:150 | Antibodies Online |
| P2X1 | APR-001 | Rabbit | 1:50 | Alomone |
| P2X3 | APR-016 | Rabbit | 1:100 | Alomone |
| NTPDase2 | BZ1-5F | Rabbit | 1:400 | J. Sevigny |
| Cytokeratin 20 (CK20) | M7019 | Mouse | 1:100 | Dako |
| Vimentin | M0725 | Mouse | 1:150 | Dako |
| NF160 | Ab7794 | Mouse | 1:600 | Abcam |
| VAChT | AB1578 | Goat | 1:750 | Chemicon |
| Secondary antibodies | ||||
| Alexa Fluor 488 anti-rb | A-21206 | Donkey | 1:1500 | Mol. Probes |
| Alexa Fluor 568 anti-ms | A-10037 | Donkey | 1:1500 | Mol. Probes |
| Alexa Fluor 633 anti-gt | A-21082 | Donkey | 1:1500 | Mol. Probes |
To test the specificity of the antibody for P2Y6 receptors (APR-011), some sections were processed with the primary antibody pre-adsorbed with a control antigen corresponding to the amino acid sequence 311–328 of the rat P2Y6 receptor (Q63371, Alomone Labs, Jerusalem, Israel). Pre-adsorption was performed by incubating the P2Y6 primary antibody overnight at 4°C with 10-fold molar excess of the antigen peptide sequence. Sections were then processed as described earlier with the pre-absorbed antiserum and with the normal antiserum, in parallel. During documentation of P2Y6 receptor pre-absorption controls, settings on the confocal microscope were adjusted appropriately to show P2Y6-immunoreactivity for sections that were processed normally (no pre-absorption) and these settings were maintained when documenting pre-absorption controls to minimize bias, during capture and printing of digital images.
In situ E-NTPDase activity experiments
For the histochemical localization of E-NTPDase activity, formation of phosphate anion was evaluated by the Wachstein/Meisel lead phosphate method. Briefly, excised bladder fragments were frozen in a bath of isopentane pre-cooled in liquid N2, sectioned (8 μm) and preincubated for 45 min at room temperature in 50 mM Tris-maleate-sucrose (TMS) buffer, pH 7.4 containing 2 mM CaCl2, 0.25 M sucrose and 2.5 mM β-glycerophosphate, an inhibitor of the alkaline phosphatase (AP) activity. Enzymic reactions were performed for 1 h at 37°C in TMS buffer supplemented with 5 mM MnCl2, 2 mM Pb(NO3)2, 3% dextran T250, and in the presence of 30 μM of each substrate (ATP and ADP). The substrate was omitted in control experiments. After the enzymatic reaction, sections were washed with TMS buffer and revealed by incubation with (NH4)2S (1%v·v−1) for exactly 1 min. Samples were then mounted using Faramount Aqueous Mounting Medium (Dako, Lisboa, Portugal), stored at 4°C and observed under a FV1000 microscope running the Cell F software (Olympus, Tokyo, Japan).
Data analysis
Results are expressed as mean ± SEM, with n indicating the number of animals used for a particular set of experiments. Only one experimental procedure (e.g. agonist in the absence and in the presence of the antagonist) was performed per rat in vivo. Because of limited inter-individual variation, randomly chosen groups of five to seven animals of the same strain, gender and weight (male Wistar rats of 300–450 g) were considered sufficient to replicate each experimental protocol. Statistical analysis of data was carried out using GraphPad Prism 5.04 software (La Jolla, CA, USA). Unpaired Student's t-test with Welch's correction was used for statistical analysis when parametric data was considered. For multiple comparisons, one-way anova followed by Dunnett's modified t-test was used. P < 0.05 (two-tailed) values were considered to show significant differences between means.
Materials
A317491 (5-[[[(3-phenoxyphenyl) methyl] [(1S)-1,2,3,4-tetrahydro-1-naphthalenyl] amino] carbonyl]-1,2,4-benzenetricarboxylic acid sodium salt hydrate), ADP, ARL67156 (6-N,N-diethyl-D-β,γ-dibromomethylene ATP trisodium salt), ATP, α,β-methylene ATP, MRS2578 (N,N″-1,4-butanediylbis[N′-(3-isothiocyanatophenyl) thiourea), oxotremorine sesquifumarate, PSB0474 (3-(2-oxo-2-phenylethyl)-uridine-5′-diphosphate disodium salt), TTX citrate were obtained from Tocris Bioscience (Bristol, UK); Choline chloride, FBS, lysine, pyridoxal phosphate-6-azo(benzene-2,4-disulfonic acid) tetrasodium salt hydrate (PPADS), paraformaldehyde, sodium periodate, UDP and urethane were obtained from Sigma (St. Louis, MO, USA); MRS2179 (2′-deoxy-N6-methyladenosine 3′,5′-bisphosphate tetrasodium salt) was obtained from ABCAM (Cambridge, UK); [methyl-3H]choline chloride (in ethanol, 85.5 Ci·mmol−1) was from Perkin Elmer; serum albumin and Triton X-100 were obtained from Merck (Darmstadt, Germany). The antibody against E-NTPDase2 was a kind gift from J. Sévigny (Univ. Laval, Québec, QC, Canada). Luciferin-luciferase ATP bioluminescence assay kit HS II was purchased from Roche Applied Science. Stock solutions of MRS2578 and A317491 were prepared in DMSO. Other drugs were prepared in saline (in vivo cystometric recordings) or in Tyrode's solution (in vitro experiments). All stock solutions were stored as frozen aliquots at −20°C. Dilutions of these stock solutions were made daily and appropriate solvent controls were done. No statistical differences between control experiments, made in the absence or in the presence of the solvents at the maximal concentrations used (0.5% v/v), were observed.
Results
Activation of urothelial P2Y6 receptors increases the voiding frequency in the anaesthetized rat in vivo
Figure 1 shows the effect of UDP (100 μM) and of its synthetic analogue that is highly selective for P2Y6 receptors, PSB0474 (100 nM) (El-Tayeb et al., 2006), on bladder urodynamics in the anaesthetized rat. Infusion of UDP (100 μM) and PSB0474 (100 nM) into the lumen of the urinary bladder decreased the ICI, to about the same extent, without significantly affecting both the amplitude (A) and the duration (Δt) of voiding bladder contractions. The selective non-competitive P2Y6 receptor antagonist, MRS2578 (50 nM) (Mamedova et al., 2004), prevented the increases in the voiding frequency caused by UDP (100 μM) and PSB0474 (100 nM) (see Figure 1B). Blockade of the increased bladder activity caused by UDP (100 μM) was also achieved with the non-selective P2 receptor antagonist, PPADS (10 μM, n = 3) (data not shown).
When applied alone into the bladder lumen, the P2Y6 receptor antagonist MRS2578 (50 nM, n = 6) did not significantly change any of the urodynamic parameters (ICI, PTh, A and Δt) analysed (Figure 2). Interestingly, higher concentrations (100 and 300 nM) of the P2Y6 receptor antagonist increased both the ICI and the PTh required to initiate the voiding reflex without significantly affecting the motor components (A and Δt) of the micturition reflex (Figure 2). The decrease in the voiding frequency (prolongation of the ICI) observed with MRS2578 infused into the bladder lumen at 100 and 300 nM concentrations was respectively 35 ± 9% (n = 5) and 53 ± 6% (n = 5). MRS2578 was even more effective at increasing the PTh to trigger the voiding reflex, by 59 ± 14% (n = 5) with 100 nM and 63 ± 8% (n = 5) with 300 nM. These results suggest that the afferent component of the micturition reflex in the rat in vivo is under the tonic control of UDP-sensitive P2Y6 receptors activation because the affected urodynamic parameters, ICI and PTh, are normally associated with the filling sensitive phase of the reflex.
Figure 2.
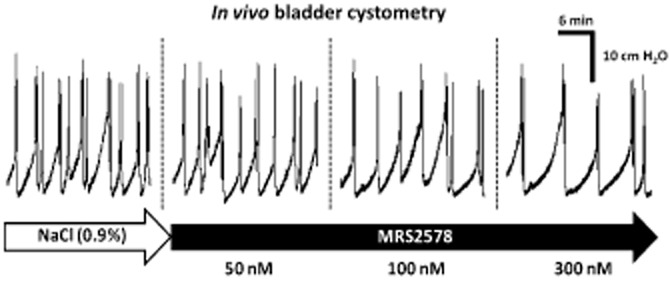
Concentration-dependent inhibition of the voiding frequency by the P2Y6 receptor antagonist, MRS2578, during normal saline (0.9%·w/v of NaCl) infusion into the urinary bladder of urethane-anaesthetized rats. MRS2578 (50–300 nM) was applied in a cumulative manner into the bladder lumen by changing the content of the syringe connected to the automated perfusion system. Stable urodynamic responses to MRS2578 were reached in 10–15 min.
Participation of excitatory P2X3 and inhibitory P2Y1 receptors on UDP-induced increase in the voiding frequency in anaesthetized rats
The effects of UDP and its stable analogue, PSB0474, resembled the increase in the voiding frequency observed with ATP applied into the bladder lumen of anaesthetized animals (Pandita and Andersson, 2002), which is known to be predominantly mediated by P2X3 receptors on sub-urothelial nerve afferents (Burnstock, 1999; Cockayne et al., 2000; Ito et al., 2008). Besides the relevance of sub-urothelial P2X3 receptors to initiate the micturition reflex, blockade of P2Y1 receptors may remove an accommodatory, inhibitory drive to the detrusor muscle in urethane-anaesthetized female rats (King et al., 2004). In the human urinary bladder, ATP stimulation of bladder activity may be partly reversed by its catabolism by E-NTPDases to ADP, leading to the activation of inhibitory P2Y1 receptors on cholinergic nerve endings (Silva et al., 2011). In this context, we investigated the contribution of P2X3 and P2Y1 receptors to bladder hyperactivity caused by UDP (100 μM). I.v. application of the selective P2X3 receptor antagonist, A317491 (100 nM), completely prevented the increase in the voiding frequency produced by UDP (100 μM) (Figure 3); A317491 (100 nM) was devoid of effects in the amplitude (A) and the duration (Δt) of voiding bladder contractions when applied together with UDP (100 μM) (see Figure 3A,B). When used alone, i.v. A317491 (100 nM) had no effects on the parameters evaluated during saline distension cystometry recordings in the anaesthetized rat. In the presence of the P2Y1 receptor antagonist, MRS2179 (300 nM, applied i.v.), UDP (100 μM) increased, rather than decreased, the ICI (Figure 3A,B). Under these conditions, UDP-induced decrease in the voiding frequency may be, at least partly, due to a significant increase in the duration (Δt) of voiding contractions (Figure 3A,B).
Figure 3.
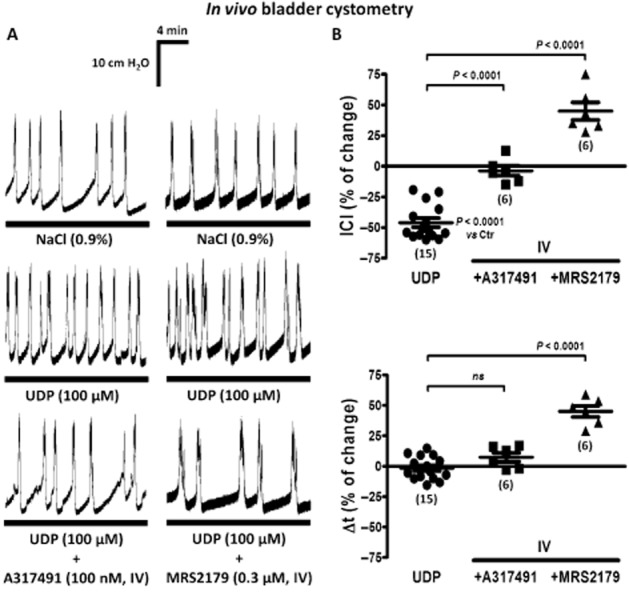
Modification of UDP (100 μM)-induced increase in the voiding frequency by i.v. application of A317491 (100 nM, a selective P2X3 receptor antagonist) and MRS2179 (0.3 μM, a P2Y1 receptor antagonist) in the anaesthetized rat. (A) Bladder cystometry recordings during normal saline (0.9%·w/v of NaCl) infusion into the urinary bladder in the absence and in the presence of UDP (100 μM). A317491 (100 nM) and MRS2179 (0.3 μM) were continuously perfused through the catheter inserted into the left jugular vein starting at least 20 min before UDP application. (B) Scatter plots representing the percent change in the ICI and of the duration (Δt) of voiding contractions, compared with control values (Ctr, 0%). The vertical bars represent SEM of a n number of animals (shown in parenthesis). P values as shown; significantly different from UDP alone; unpaired Student's t-test with Welch's correction.
Immunolocalization of P2Y6, P2Y1 and P2X3 purinoceptors in urothelium and sub-urothelial layers of the rat urinary bladder
The presence of P2Y6 receptors in the rat urinary bladder was demonstrated by immunofluorescence confocal microscopy using two distinct commercially available antibodies (Figure 4; for details, see Table 1). The antibody from Alomone Labs (APR-011) is directed against the intracellular C-terminus domain of the rat P2Y6 receptor; this antibody is considered highly specific for this species although in our hands it was also able to recognize P2Y6 receptors in human bone marrow stromal cells (Noronha-Matos et al., 2012). This antibody may lack specificity towards the mouse receptor as staining was not totally lacking in the P2Y6 receptor knockout mouse (Yu and Hill, 2013). For this reason, we used another antibody from Antibodies Online (ABIN1386282), which was designed to recognize the human P2Y6 receptor, while cross-reacting significantly with the rat receptor. Our data showed that the P2Y6 receptor was most abundantly expressed in the urothelium (Figure 4A), although labelling was also observed in sub-urothelial cells staining positively for the intermediate filament protein vimentin (presumably myofibroblasts) (cf. Fry et al., 2012; Sui et al., 2006). The P2Y6 receptor immunostaining was equally srong with either antibody, APR-011 or ABIN1386282. Pre-adsorption with the peptide corresponding to the amino acid sequence 311–328 of the rat P2Y6 receptor abolished staining with the APR-011 antibody, while keeping the same acquisition settings on the confocal microscope (Figure 4A, bottom panels). Notably, we observed P2Y6 receptor immunoreactivity in all layers of the urothelium including the terminally differentiated superficial umbrella cells which also stained positively with cytokeratin 20 (Figure 4A, upper panels).
Figure 4.
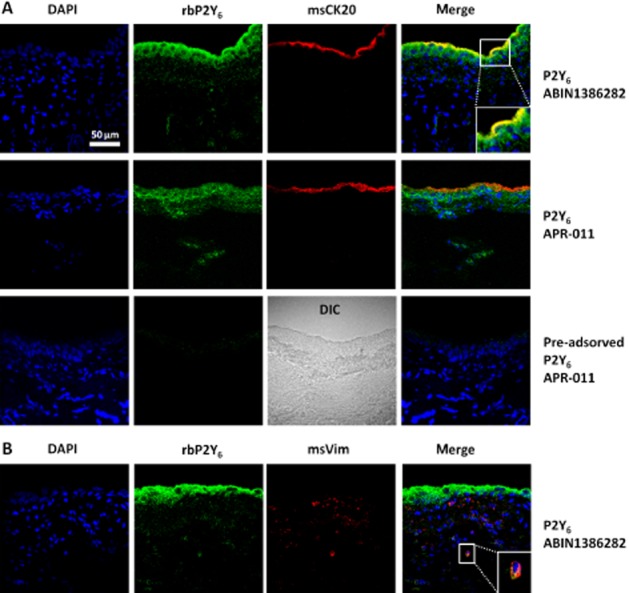
Immunolocalization of P2Y6 receptors in the uroepithelium and sub-urothelial layers of transverse sections of the rat urinary bladder by confocal microscopy. Two distinct P2Y6 receptor antibodies, APR-011 and ABIN1386282, were used as indicated. Terminally differentiated urothelial cells (umbrella cells) and sub-urothelial myofibroblasts are labelled with cytokeratin 20 (CK20, red, in panel A) and vimentin (Vim, red, in panel B) respectively. Nuclei are stained with DAPI (blue). Yellow staining denotes co-localization of P2Y6 receptor (green) with CK20 (red) or Vim (red). Pre-adsorption with the peptide corresponding to the amino acid sequence 311–328 of the rat P2Y6 receptor abolished staining with the APR-011 antibody. Differential interference contrast (DIC) image is shown for comparison in the latter condition. Scale bar = 50 μm.
We confirmed data from previous reports showing that P2X3 and P2Y1 receptor subtypes are expressed throughout the bladder epithelium of rats, rabbits, cats and humans (Elneil et al., 2001; Birder et al., 2004; Wang et al., 2005; see Burnstock, 2014) (Figure 5). Localization of P2X3 and P2Y1 immunoreactivity within the urothelium suggests an additional sensory role for these receptors in regulating uroepithelial function. According to the model of Wang et al. (2005) ATP released from the urothelium upon bladder filling acts through a diversity of P2 purinoceptors (including P2X3 and P2Y1, but also P2Y6) expressed in the bladder epithelium to promote signal amplification to release the nucleotide and to stimulate apical membrane insertion in umbrella cells.
Figure 5.
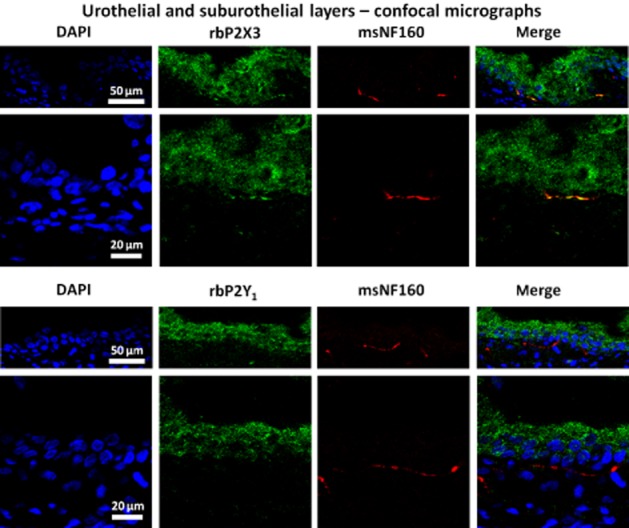
Immunolocalization of P2X3 and P2Y1 purinoceptors in the uroepithelium and sub-urothelial layer of transverse sections of the rat urinary bladder by confocal microscopy. Both, P2X3 and P2Y1, receptor subtypes are expressed throughout the bladder epithelium. P2X3, but not P2Y1, receptors (green) co-localize (yellow staining) with NF160 (red) labelling in small sub-urothelial nerve fibres. Nuclei are stained with DAPI (blue). Scale bars = 20 or 50 μm (as indicated).
Interestingly, in light of our results on bladder urodynamics in the anaesthetized rat, we demonstrated the presence of ionotropic P2X3 receptors on small sub-urothelial nerve fibres staining positively against the neurofilament 160 (NF160) in the rat urinary bladder, although we were unable to show any P2Y1 receptor immunoreactivity in NF160-labelled sub-urothelial nerve fibres using the same acquisition settings (Figure 5). These findings agree with the results suggesting that P2X3 receptors on sub-urothelial sensory nerves have a role in the purinergic mechanosensory transduction underlying initiation of voiding reflexes in rabbits and mice (Ferguson et al., 1997; Cockayne et al., 2000; Vlaskovska et al., 2001), and sustain the hypothesis that P2Y1 receptors play a predominant inhibitory role in the rat detrusor tone (King et al., 2004).
UDP-induced hyperactivity requires intact bladder nervous circuitry
To evaluate the contribution of the extrinsic nervous circuitry to UDP-induced bladder hyperactivity, we recorded the myographic activity of the isolated rat urinary bladder in vitro. Although small-amplitude spontaneous detrusor twitching was detected, no voiding contractions were elicited upon bladder filling in conditions similar to those used in the in vivo cystometric assays (see Methods). When UDP (300 μM) was applied through the catheter inserted inside the bladder, we observed no changes in the myographic recordings compared with the control situation where only Tyrode's solution was infused (Figure 6B,C). The frequency and the amplitude of spontaneous detrusor twitching increased significantly (P < 0.05) when UDP (300 μM) was added directly into the bathing solution outside the bladder wall (Figure 6C). Although the concentration of UDP used in myographic recordings in vitro was threefold higher (300 μM) than that required to increase bladder overactivity in the anaesthetized rat in vivo, the excitatory effect of UDP did not exceed 10% of the contractions produced by oxotremorine (30 μM) or α,β-methylene ATP (30 μM), which selectively activate muscarinic and P2X1 receptors in the detrusor smooth muscle respectively (data not shown).
Figure 6.
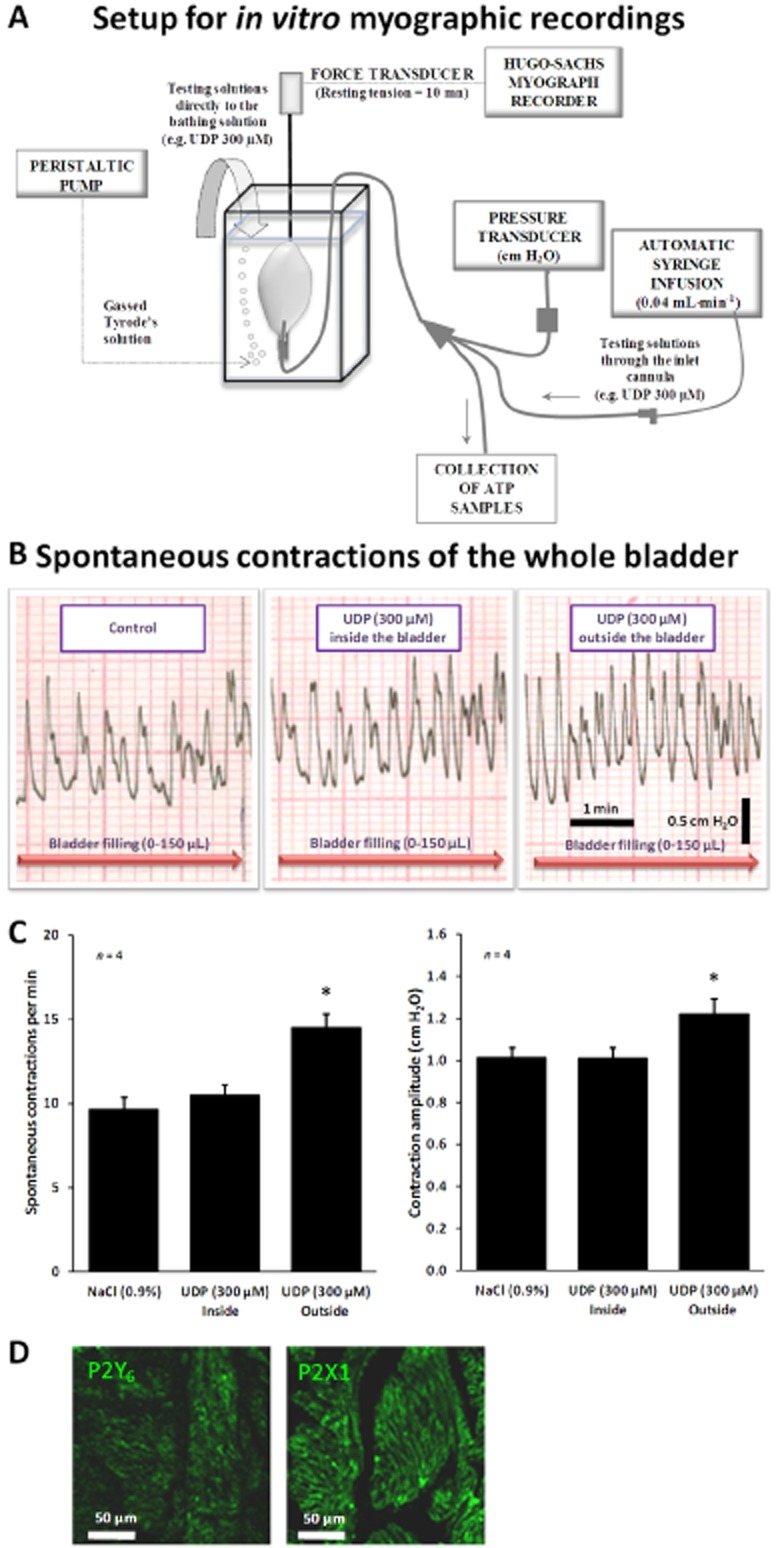
(A) Setup for myographic recordings of the whole urinary bladder of the rat in vitro. (B) Spontaneous contractile activity of the rat urinary bladder in response to bladder filling with Tyrode's solution, up to 150 μL, infused at a constant flow rate (40 μL·min−1) to mimic in vivo cystometry experiments. UDP (300 μM) was superfused either into the bladder lumen (by changing the syringe connected to the automated perfusion system) or directly to the bathing solution outside the bladder. (C) Quantification of the frequency and magnitude of spontaneous contractions of the whole bladder in vitro in the absence and in the presence of UDP (300 μM) applied inside and outside the bladder. The vertical bars represent SEM of four isolated bladders. *P < 0.05; significantly different from control (saline superfusion); one-way anova followed by Dunnett's modified t-test. (D) Confocal micrographs of transverse sections of rat urinary bladder detrusor muscle immunostained for P2Y6 (APR-011) and P2X1 (APR-001) receptors. Scale bars = 50 μm.
Figure 6D shows confocal micrographs of transverse sections of rat urinary bladder detrusor muscle immunostained for P2Y6 and P2X1 receptors. The greater contractile efficacy of the P2X1 receptor agonist, α,β-methylene ATP (30 μM), compared with UDP (300 μM), agrees with immunohistochemical studies showing that the P2X1 receptor is the dominant subtype in smooth muscle cell membranes of the rat detrusor (Lee et al., 2000; see Figure 6D). Despite the small effect of direct application of UDP (300 μM) in to the bath on spontaneous contractions of the whole bladder in vitro, our data do not exclude the possibility that co-localized P2Y6 receptors may enhance P2X1-mediated contractions in the abnormal urinary bladder as previously suggested (Yu and Hill, 2013; Yu et al., 2013). The point that we would emphasize is that effectiveness of urothelial P2Y6 receptors requires extrinsic neuronal circuitry, as it was not detected in the isolated bladder in vitro, even though sub-urothelial myofibroblasts and intrinsic neuronal networks were preserved.
P2Y6 receptor agonist, PSB0474, increases urinary ATP content
The increase in the voiding frequency caused by the P2Y6 receptor agonist, PSB0474 (100 nM), was mimicked by the E-NTPDase inhibitor, ARL 67156 (100 μM), which increased urinary ATP levels when applied inside the bladder (Figure 7A). Infusion of PSB0474 (100 nM) through the catheter inserted into the bladder increased, by more than threefold, the ATP content of the voided cystometry fluid evaluated by the luciferin-luciferase bioluminescence method (Figure 7B). No significant changes were observed in the LDH activity of samples collected before (7.3 ± 1.4 mU·mL−1, n = 5) and after PSB0474 (100 nM, 8.0 ± 3.7 mU·mL−1, n = 5) application.
Figure 7.
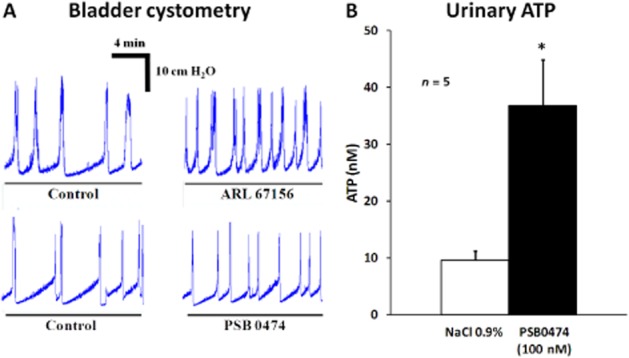
(A) Comparison of bladder cystometry recordings obtained during infusion of ARL 67156 (100 μM, an E-NTPDase inhibitor) and PSB0474 (100 nM, a selective P2Y6 receptor agonist) into the urinary bladder lumen of anaesthetized rats. (B) Intravesical infusion of PSB0474 (100 nM) increases ATP levels in the urinary fluid collected during cystometry recordings. The ATP content of the samples was quantified by the luciferin-luciferase bioluminescence assay. The vertical bars represent SEM of five animals. *P < 0.05; significantly different from control (saline infusion); one-way anova followed by Dunnett's modified t-test.
UDP indirectly inhibits [3H]-ACh release from the stimulated urinary bladder: involvement of P2Y6 and P2Y1 receptors on urothelial cells and cholinergic nerves respectively
UDP (100 μM) significantly decreased [3H]-ACh release from rat urinary bladder preparations stimulated electrically (10 Hz, 200 pulses) (Figure 8A). The inhibitory effect of UDP (100 μM) was evident in intact bladder preparations, but not in those where the urothelium has been gently removed with a cotton swab (Figure 8B). Pretreatment of the preparations with the P2Y6 receptor selective antagonist, MRS2578 (50 nM), blocked the inhibitory effects of UDP (100 μM) on transmitter release (Figure 8A). The inhibitory effect of UDP (100 μM) was transformed into facilitation upon blockade of P2Y1 receptors with MRS2179 (300 nM), but only when the urothelium was left intact (Figure 8A).
Figure 8.
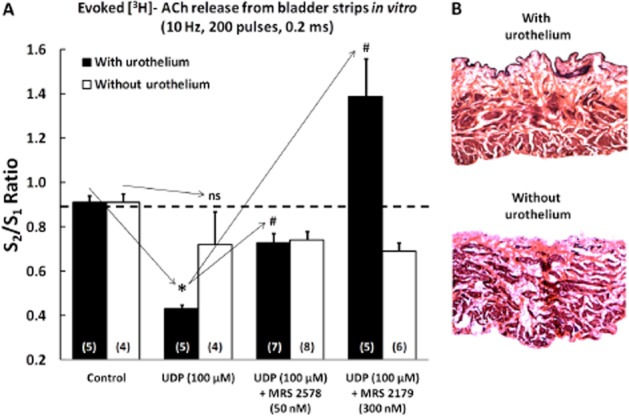
(A) Effect of UDP (100 μM) on electrically-evoked [3H]-ACh release from intact urinary bladder strips and in preparations without the urothelium UDP (100 μM) was applied 8 min before S2. MRS2578 (50 nM) and MRS2179 (300 nM) were added to the incubation media at the beginning of the release period (time zero) and were present throughout the assay, including S1 and S2. The ordinates represent evoked tritium outflow expressed by S2/S1 ratios, i.e. the ratio between the evoked [3H]-ACh release during the second period of stimulation (in the presence of UDP) and the evoked [3H]-ACh release during the first stimulation period (without UDP). The vertical bars represent SEM. *P < 0.05; significantly different from control; #P < 0.05: significantly different from UDP alone; unpaired Student's t-test with Welch's correction. (B) Representative microscopic images of rat urinary bladder strips stained with haematoxylin-eosin to confirm the presence or the absence of the urothelium. Magnification, 40×.
Confocal microscopy studies demonstrated that the P2Y6 receptor immunoreactivity did not co-localize with the vesicular ACh transporter (VAChT) in the detrusor muscle layer of the rat urinary bladder (Figure 9). This finding excludes a direct inhibitory action of UDP on ACh release from stimulated cholinergic nerve terminals. Immunohistochemical data support the hypothesis that UDP might exert an indirect effect operated by inhibitory P2Y1 receptors localized on VAChT-positive cholinergic nerve terminals (Figure 9) via ADP resulting from the extracellular catabolism of ATP predominantly released from the urothelium.
Figure 9.
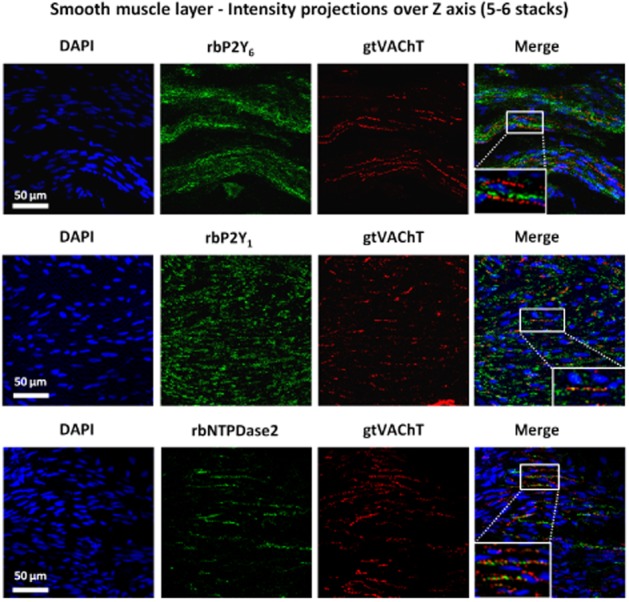
Confocal micrographs showing P2Y6, P2Y1 and NTPDase2 immunoreactivity in transverse sections of the detrusor smooth layer of rat urinary bladder. To facilitate visualization of small cholinergic nerve terminals staining for VAChT (red) images correspond to the intensity projections over Z axis of five to six confocal microscopy stacks taken at the smooth muscle layer. No co-localization was found between P2Y6 receptor (green) and VAChT (red) immunoreactivity. Conversely, VAChT-positive cholinergic nerve terminals (red) stained positively with antibodies against the P2Y1 receptor and E-NTPDase2 (green); yellow staining denotes co-localization. Nucleic DNA is stained with DAPI (blue). Scale bars = 50 μm.
Histochemical studies of E-NTPDases in the rat bladder demonstrated that phosphate deposition resulting from the extracellular catabolism of ATP (30 μM) occurred predominantly in the urothelium, but was also detectable in the sub-urothelial and smooth muscle layers (Figure 10A). Interestingly, the extracellular catabolism of ADP (30 μM) was observed predominantly in the apical urothelium and in smooth muscle, but was minimal in the sub-urothelial layer (Figure 10B). Moreover, we found that VAChT-positive cholinergic nerve terminals also stained positively with the antibody against E-NTPDase2 (Figure 9). Because E-NTPDase2 (CD39L1, ATPase, EC 3.6.1.3) is a nucleoside triphosphatase hydrolysing ATP 10–15 times more efficiently than ADP (Matsuoka and Ohkubo, 2004), the bladder neuromuscular synapse may accumulate ADP to the levels required to activate the P2Y1 receptors on cholinergic nerve terminals which inhibit Ach release. Thus, blockade of these inhibitory P2Y1 receptors with MRS2179 resulted in the amplification of evoked [3H]-ACh release from cholinergic nerve efferents (Figure 8A) and, consequently, may increase the duration (Δt) and/or the amplitude (A) of voiding contractions (Figure 3B) produced by UDP.
Figure 10.
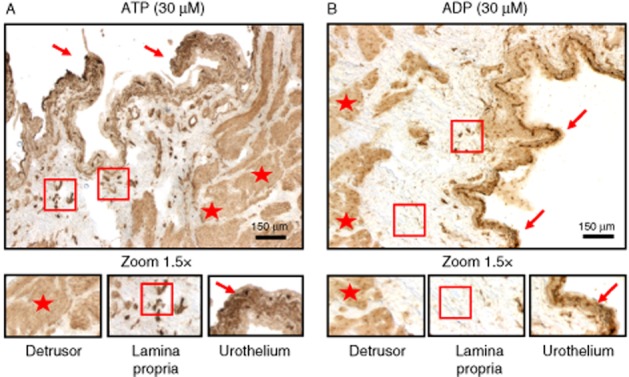
Histochemical E-NTPDase activity in the rat urinary bladder. Phosphate deposition resulting from extracellular catabolism of ATP (30 μM, A) was found predominantly in the urothelium (arrows), but was also present in the sub-urothelial (square) and smooth muscle (star) layers: Extracellular ADP (30 μM, B) was dephosphorylated predominantly in apical urothelial cells (arrows) and in the smooth muscle (stars), but only at very low levels in the sub-urothelial layer (square). These details are better appreciated in the higher magnification (1.5×) images in the lower panels. Scale bars = 150 μm.
Discussion and conclusions
Urothelial cells are highly deformable and can increase several fold in size during bladder filling (Truschel et al., 2002; Yu et al., 2009). Mechanical distension of urothelial cells release significant amounts of ATP (and probably UTP) to initiate the micturition reflex and to stimulate membrane insertion at the apical pole of umbrella cells (Wang et al., 2005). Upon binding to a diversity of P2 purinoceptors (including P2X3 and P2Y1) expressed in uroepithelial cells, released ATP and/or its metabolites formed by membrane-bound ecto-NTPDases may trigger a self-regenerating purinergic wave propagating to sub-urothelial myofibroblasts and sensory nerve fibres to initiate the voiding reflex. Immunofluorescent confocal microscopy data from this study demonstrated that P2Y6 receptors were abundantly expressed in the urothelium of the rat urinary bladder, including the highly differentiated superficial umbrella cells. These receptors have also been reported in sub-urothelial myofibroblasts and detrusor smooth muscle fibres (see Sui et al., 2006; Yu and Hill, 2013; Yu et al., 2013). To our knowledge, this is the first report showing that the UDP-sensitive P2Y6 receptor is an important player in the mechanosensory purinergic pathway initiated by distension of epithelial cells, leading to increases in the voiding frequency in rats by promoting the release of ATP from the urothelium (Figure 11). A similar effect was found in the human urinary bladder (Silva-Ramos et al., 2012).
Figure 11.
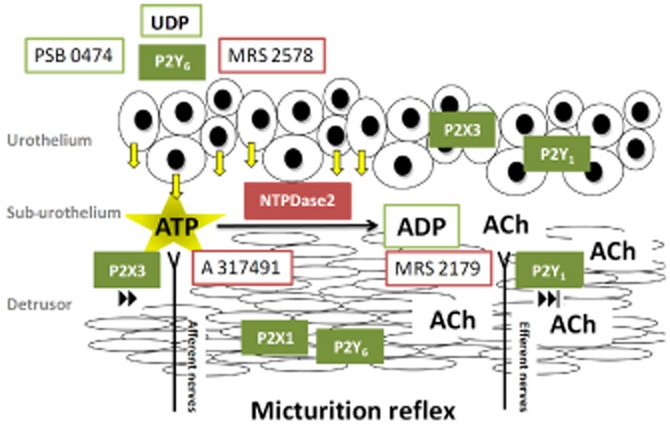
Schematic representation of the putative mechanisms underlying the control of the voiding frequency by urothelial UDP-sensitive P2Y6 receptors in the anaesthetized rat. Activation of P2Y6 receptors on distended umbrella cells during bladder filling increased, by threefold, the release of ATP from the urothelium. Released ATP, acting via multiple urothelial P2 purinoceptors, triggers a self-regenerating purinergic wave propagating to sensory nerve afferents endowed with P2X3 receptors to initiate the voiding reflex. Bladder activity may be partly reversed by the hydrolysis of ATP into ADP by E-NTPDases, namely E-NTPDase2 located in the lamina propria (probably on interstitial cells) and on cholinergic nerve efferents. ADP accumulation at the neuromuscular synapse decreases ACh release and smooth muscle contraction through the activation of prejunctional inhibitory P2Y1 receptors. The diagram also shows the locus of action of the main drugs used in this study.
Large amounts of extracellular ATP may leak from injured cells, but the mechanism of ATP release from urothelial cells under physiological conditions is still unresolved. Nucleotides-releasing pathways in intact cells include (1) electrodiffusional translocation via connexin- and pannexin-containing hemichannels and voltage-dependent anion channels; (2) facilitated diffusion by nucleotide-specific ATP-binding cassette transporters; and (3) vesicle exocytosis (Knight et al., 2002; reviewed in Burnstock, 2006). In this study, ATP release from the urothelium was not due to cell damage because we detected no significant changes in the activity of the intracellular enzyme, LDH, in the voiding fluid. Recent data from our group point to a significant role of pannexin-1 containing hemichannels on P2Y6 receptor-mediated ATP release to control bladder urodynamics in the anaesthetized rat (Timóteo et al., 2014). Further studies are required to investigate whether this is the only mechanism involved and whether changes in this pathway occur under the conditions of the overactive bladder, along with altered expression of P2 purinoceptors (Birder et al., 2004).
Our data provided functional and biochemical evidence that activation of P2Y6 receptors increased the voiding frequency in the anaesthetized rat, indirectly, by releasing ATP from the urothelium. Fry et al. (2012) showed that sub-urothelial myofibroblasts were supersensitive to stretch-released ATP from the urothelium giving rise to augmented intrinsic spontaneous contractions of the urinary bladder in spinal cord transected rats. Interestingly, they also showed that stretch-induced bladder hyperactivity in these animals may be decreased by the P2Y6 receptor antagonist, MRS2578, but they needed a 100-fold higher (10 μM) concentration of the antagonist. Although the expression of UTP-sensitive P2Y2 (and P2Y4) receptors has been shown by real-time PCR, Western blotting and immunocytochemistry at the rat urothelium (see Chopra et al., 2014), activation of these receptors might play a minor role under the present conditions because MRS2578 displays no activity at P2Y1, P2Y2, P2Y4 and P2Y11 receptors at concentrations below 10 μM (Mamedova et al., 2004).
It is known that stimulation of high-threshold sub-urothelial nerve afferents containing ionotropic P2X3 receptors by ATP released from the urothelium is involved in the micturition reflex following urine bladder distention (Burnstock, 1999; Cockayne et al., 2000; Ito et al., 2008). Non-neuronal (urothelial) localization of P2X3 receptors supports the idea that in addition to stimulating sensory afferent nerves, ATP may play additional roles to regulate urothelial function. Here we showed that selective blockade of P2X3 receptors with i.v. A317491 prevented the increase in the voiding frequency produced by UDP-sensitive P2Y6 receptor activation in the rat in vivo. As UDP does not bind to ionotropic P2X purinoceptors, these results indicate that increases in the voiding frequency triggered by P2Y6 receptors may be indirectly mediated via P2X3 receptors on neighbouring uroepithelial cells and/or sub-urothelial sensory nerve fibres by ATP released from the urothelium. Proximity of sub-urothelial nerve fibres to blood vessels of the lamina propria makes it more likely any interference with the micturition reflex caused by i.v. application of the P2X3 receptor antagonist, but one cannot exclude an effect of this drug on the urothelial self-propagating purinergic wave.
In spite of our findings demonstrating that activation of P2Y6 receptors by UDP or its stable analogue, PSB0474, applied into the bladder lumen, increased the voiding frequency, we failed to detect changes in the motor components of the micturition reflex, both the amplitude (A) and the duration (Δt) of the voiding contractions. This contrasts with the findings of Yu et al. (2013,2013) who demonstrated that P2Y6 receptors synergise with P2X1 receptors to increase bladder smooth muscle tone. Using myographic recordings in the isolated bladder in vitro, we confirmed that UDP increased the frequency and the amplitude of spontaneous detrusor twitching, a situation that required application of a threefold higher concentration (300 μM) of UDP directly outside the bladder wall, but was not detected when the nucleotide was infused into the bladder lumen. Even so, increments on spontaneous detrusor twitching caused by UDP (300 μM) were minimal (less than 10%) when compared with the effects of oxotremorine (30 μM) and α,β-methylene ATP (30 μM), which selectively activate muscarinic and P2X1 receptors in the detrusor smooth muscle respectively. Thus, compared with UDP-induced increases in the voiding frequency that implicate the sequential activation of urothelial P2Y6 receptors and ATP release leading to stimulation of urothelial/sub-urothelial P2X3 receptors, direct activation of detrusor smooth muscle via co-expressed P2Y6 and P2X1 receptors might represent a minor component of UDP urodynamic response at least under the present experimental conditions.
Bladder overactivity may be partly reversed through the hydrolysis of ATP into ADP by E-NTPDases. Previous reports from our group demonstrated that ADP acts through inhibitory P2Y1 receptors to decrease nerve-evoked ACh release in the human bladder (Silva et al., 2011). Here, we showed that VAChT-positive cholinergic nerve efferents to the detrusor, but not NF160-labelled sub-urothelial sensory nerves, exhibit immunoreactive P2Y1 receptors. Upon blocking P2Y1 receptors with i.v. MRS2179, UDP increased rather than decreased the ICI and enhanced the duration (Δt) of the voiding contractions. Thus, blockade of P2Y1 receptors may unravel an effect of the nucleotide on the motor component of the micturition reflex. In fact, UDP acting via P2Y6 receptors decreases [3H]-ACh release from rat urinary bladder strips stimulated electrically, but this effect was observed only if the urothelium was left intact. Blockade of ADP-sensitive P2Y1 receptors with MRS2179 converted the inhibitory effects of UDP on ACh release into a facilitatory action, but only in those preparations with urothelium. These findings convincingly indicated that the increase in the voiding frequency produced by UDP was partly reversed by the activation of inhibitory P2Y1 receptors on cholinergic nerve efferents.
ADP resulting from the extracellular catabolism of ATP released from the urothelium may be the endogenous activator of P2Y1 receptors in the urinary bladder. By histochemical analysis of the E-NTPDase activity in the rat urinary bladder in situ, we found that the dephosphorylation of ATP occurred predominantly in the urothelium, but was also detectable in the sub-urothelial and smooth muscle layers, whereas the further hydrolysis of ADP was observed predominantly in the superficial layer of the urothelium and in the smooth muscle. These findings are consistent with the results obtained by Yu et al. (2011) in the mouse urinary bladder. They demonstrated that, in contrast to the urothelial layer, which is rich in E-NTPDase3 (and 8)-positive cells, only the E-NTPDase2 subtype is significantly expressed in the lamina propria (probably in interstitial cells of Cajal, see Yu et al., 2012) as well as in cells in close proximity to smooth muscle bundles. As E-NTPDase2 is a nucleoside triphosphatase and hydrolyses ATP 10 to 15 times more efficiently than ADP (Matsuoka and Ohkubo, 2004), this might explain why ADP hydrolysis was only minimal in the sub-urothelium. Therefore, it is reasonable to suppose that ADP generated from ATP released from the urothelium may reach its target, the P2Y1 receptor, on cholinergic nerve efferents. In addition, we found that VAChT-positive cholinergic nerve terminals innervating the detrusor also express E-NTPDase2 immunoreactivity. As ATP is co-released with ACh in most cholinergic synapses, the bladder neuromuscular synapse may be able to accumulate ADP to the levels required to activate inhibitory P2Y1 receptors on cholinergic nerve terminals. From the histoenzymic analysis, it is also likely that the ATP levels measured in the voiding fluid might have been underestimates of the amounts released because of the hydrolysis of this nucleotide by E-NTPDases bound to urothelial cells, during bladder filling.
Unlike the well-recognized extracellular signalling role of ATP, little is known about the UTP and UDP release within the bladder wall. The observation that blockade of UDP-sensitive P2Y6 receptors with MRS2578 (100 and 300 nM, IC50 value for the rat P2Y6 receptor is 98 nM) increased the ICI and the PTh that is required to initiate the voiding reflex during bladder filling in the anaesthetized rat demonstrated that either UTP or UDP was endogenously released and modulated the micturition reflex. Further studies are required to investigate which of these nucleotides is actually being released, because the latter could be generated from the former through E-NTPDases. Given that blockade of the P2Y6 receptor tonus was more effective in the filling phase, rather than the contractile phase, of the micturition cycle, it is possible that urothelium and/or the sub-urothelial layer, including afferent nerves and/or myofibroblasts, would be the most likely production sites of UTP and UDP.
In conclusion, this study provides strong evidence that activation of P2Y6 receptors increased the voiding frequency in the anaesthetized rat, indirectly, by releasing ATP from the urothelium and subsequent activation of P2X3 receptors on sub-urothelial sensory nerve fibres. Under these circumstances, bladder hyperactivity may be partly reversed through the hydrolysis of ATP into ADP by E-NTPDases, which through the activation of inhibitory P2Y1 receptors might then inhibit ACh release from stimulated nerve efferents (Figure 11). Although we do not know at this stage whether UDP-sensitive P2Y6 receptors play any prominent role in overactive bladder or painful bladder syndromes in humans, we have recently confirmed that activation of P2Y6 receptors favours ATP release from strips of the human urothelium (unpublished work; Silva-Ramos et al., 2012).
Acknowledgments
The authors wish to thank Drs Nuno Silva, Diogo Monteiro and José Marinhas for their collaboration in some in vivo experiments. We also thank Mrs. Maria Helena Costa e Silva and Belmira Silva for their valuable technical assistance. This research was partially supported by Fundação para a Ciência e a Tecnologia (FCT projects PTDC/SAU-OSM/104369/2008 and Pest/OE/UI0215/2011), Associação Portuguesa de Urologia and the University of Porto/Caixa Geral de Depósitos (Investigação Científica na Pré-Graduação).
Glossary
- EFS
electrical field stimulation
- E-NTPDases
ecto-nucleoside triphosphate diphosphohydrolases
- NF160
neurofilament 160
- TTX
tetrodotoxin
- VAChT
vesicular ACh transporter
Conflict of interest
None.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-Gated Ion Channels. Br J Pharmacol. 2013a;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013b;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birder LA, Ruan HZ, Chopra B, Xiang Z, Barrick S, Buffington CA, et al. Alterations in P2X and P2Y purinergic receptor expression in urinary bladder from normal cats and cats with interstitial cystitis. Am J Physiol Renal Physiol. 2004;287:F1084–F1091. doi: 10.1152/ajprenal.00118.2004. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Release of vasoactive substances from endothelial cells by shear stress and purinergic mechanosensory transduction. J Anat. 1999;194:335–342. doi: 10.1046/j.1469-7580.1999.19430335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. Pathophysiology and therapeutic potential of purinergic signaling. Pharmacol Rev. 2006;58:58–86. doi: 10.1124/pr.58.1.5. [DOI] [PubMed] [Google Scholar]
- Burnstock G. Purinergic signalling in the urinary tract in health and disease. Purinergic Signal. 2014;10:103–155. doi: 10.1007/s11302-013-9395-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra B, Gever J, Barrick SR, Hanna-Mitchell AT, Beckel JM, Ford AP, et al. Expression and function of rat urothelial P2Y receptors. Am J Physiol Renal Physiol. 2008;294:F821–F829. doi: 10.1152/ajprenal.00321.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, et al. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- El-Tayeb A, Qi A, Müller CE. Synthesis and structure-activity relationships of uracil nucleotide derivatives and analogues as agonists at human P2Y2, P2Y4, and P2Y6 receptors. J Med Chem. 2006;49:7076–7087. doi: 10.1021/jm060848j. [DOI] [PubMed] [Google Scholar]
- Elneil S, Skepper JN, Kidd EJ, Williamson JG, Ferguson DR. Distribution of P2X1 and P2X3 receptors in the rat and human urinary bladder. Pharmacology. 2001;63:120–128. doi: 10.1159/000056122. [DOI] [PubMed] [Google Scholar]
- Ferguson DR, Kennedy I, Burton TJ. ATP is released from rabbit urinary bladder epithelial cells by hydrostatic pressure changes – a possible sensory mechanism? J Physiol (London) 1997;505:503–511. doi: 10.1111/j.1469-7793.1997.503bb.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fry CH, Rossen A. Urothelial targets in the overactivity bladder. J Urol Urogynäkol. 2007;1:20–23. [Google Scholar]
- Fry CH, Sui GP, Kanai AJ, Wu C. The function of suburothelial myofibroblasts in the bladder. Neurourol Urodyn. 2007;26:914–919. doi: 10.1002/nau.20483. [DOI] [PubMed] [Google Scholar]
- Fry CH, Young HS, Jabr RI, McCarthy C, Ikeda Y, Kanai AJ. Modulation of spontaneous activity in the overactive bladder: the role of P2Y agonists. Am J Physiol Renal Physiol. 2012;302:F1447–1454. doi: 10.1152/ajprenal.00436.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honda M, Takenaka A, Inoue S, Chancellor MB, Yoshimura N. Sensory neurone-specific receptor-mediated regulation of micturition reflex in urethane-anaesthetized rats. BJU Int. 2012;109:628–633. doi: 10.1111/j.1464-410X.2011.10400.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito K, Iwami A, Katsura H, Ikeda M. Therapeutic effects of the putative P2X3/P2X2/3 antagonist A-317491 on cyclophosphamide-induced cystitis in rats. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:483–490. doi: 10.1007/s00210-007-0197-z. [DOI] [PubMed] [Google Scholar]
- Keiding R, Hörde M, Gerhard W, Denmark E. Recommended methods for the determination of four enzymes in blood. Scand J Clin Lab Invest. 1974;1:271–274. doi: 10.1080/00365517409082499. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King BF, Knowles ID, Burnstock G, Ramage AG. Investigation of the effects of P2 purinoceptor ligands on the micturition reflex in female urethane-anaesthetized rats. Br J Pharmacol. 2004;142:519–530. doi: 10.1038/sj.bjp.0705790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight GE, Bodin P, de Groat WC, Burnstock G. ATP is released from guinea pig ureter epithelium on distension. Am J Physiol Renal Physiol. 2002;282:F281–F288. doi: 10.1152/ajprenal.00293.2000. [DOI] [PubMed] [Google Scholar]
- Kumar V, Chapple CC, Chess-Williams R. Characteristics of adenosine triphosphate release from porcine and human normal bladder. J Urol. 2004;172:744–747. doi: 10.1097/01.ju.0000131244.67160.f4abstract. [DOI] [PubMed] [Google Scholar]
- Lee H-Y, Bardini M, Burnstock G. Distribution of P2X receptors in the urinary bladder and ureter of the rat. J Urol. 2000;163:2002–2007. [PubMed] [Google Scholar]
- Mamedova LK, Joshi BV, Gao ZG, von Kügelgen I, Jacobson KA. Diisothiocyanate derivatives as potent, insurmountable antagonists of P2Y6 nucleotide receptors. Biochem Pharmacol. 2004;67:1763–1770. doi: 10.1016/j.bcp.2004.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka I, Ohkubo S. ATP- and adenosine-mediated signaling in the central nervous system: adenosine receptor activation by ATP through rapid and localized generation of adenosine by ecto-nucleotidases. J Pharmacol Sci. 2004;94:95–99. doi: 10.1254/jphs.94.95. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha-Matos JB, Costa MA, Magalhães-Cardoso MT, Ferreirinha F, Pelletier J, Freitas R, et al. Role of ecto-NTPDases on UDP-sensitive P2Y(6) receptor activation during osteogenic differentiation of primary bone marrow stromal cells from postmenopausal women. J Cell Physiol. 2012;227:2694–2709. doi: 10.1002/jcp.23014. [DOI] [PubMed] [Google Scholar]
- Pandita RK, Andersson KE. Intravesiacal adenosine triphosphate stimulates the micturition reflex in awake, freely moving rats. J Urol. 2002;168:1230–1234. doi: 10.1016/S0022-5347(05)64631-9. [DOI] [PubMed] [Google Scholar]
- Ruggieri MR., Sr Mechanisms of disease: role of purinergic signaling in the pathophysiology of bladder dysfunction. Nat Clin Pract Urol. 2006;3:206–215. doi: 10.1038/ncpuro0456. [DOI] [PubMed] [Google Scholar]
- Silva I, Correia J, Ferreirinha F, Magalhães-Cardoso MT, Silva-Ramos M, Sévigny J, et al. Bladder overactivity due to global impairment of ecto-NTPDases in humans with lower urinary tract disorders exhibiting unbalanced ATP / adenosine formation. Auton Neurosci Basic Clinical. 2011;163:49–50. [Google Scholar]
- Silva-Ramos M, Silva I, Timoteo MA, Carneiro I, Vieira C, Silva N, et al. UDP-sensitive P2Y6 receptors play a dual role in the human urinary bladder indirectly via the release of ATP from urothelium. Neurourol Urodyn. 2012;31:1023. [Google Scholar]
- Silva-Ramos M, Silva I, Oliveira O, Ferreira S, Reis MJ, Oliveira JC, et al. Urinary ATP may be a dynamic biomarker of detrusor overactivity in women with overactive bladder syndrome. PLoS ONE. 2013;8:e64696. doi: 10.1371/journal.pone.0064696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sui GP, Wu C, Fry CH. Characterization on the purinergic receptor subtype on guinea-pig suburothelial myofibroblasts. BJU Int. 2006;97:1327–1331. doi: 10.1111/j.1464-410X.2006.06200.x. [DOI] [PubMed] [Google Scholar]
- Timóteo MA, Carneiro I, Silva I, Noronha-Matos JB, Ferreirinha F, Silva-Ramos M, et al. ATP released via pannexin-1 hemichannels mediates bladder overactivity triggered by urothelial P2Y6 receptors. Biochem Pharmacol. 2014;87:371–379. doi: 10.1016/j.bcp.2013.11.007. [DOI] [PubMed] [Google Scholar]
- Truschel ST, Wang E, Ruiz WG, Leung SM, Rojas R, Lavelle J, et al. Stretch-regulated exocytosis/endocytosis in bladder umbrella cells. Mol Biol Cell. 2002;13:830–846. doi: 10.1091/mbc.01-09-0435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlaskovska M, Kasakov L, Rong W, Bodin P, Bardini M, Cockayne DA, et al. P2X3 knock-out mice reveal a major sensory role for urothelially released ATP. J Neurosci. 2001;21:5670–5677. doi: 10.1523/JNEUROSCI.21-15-05670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang EC, Lee JM, Ruiz WG, Balestreire EM, von Bodungen M, Barrick S, et al. ATP and purinergic receptor-dependent membrane traffic in bladder umbrella cells. J Clin Invest. 2005;115:2412–2422. doi: 10.1172/JCI24086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Hill WG. Lack of specificity shown by P2Y6 receptor antibodies. Naunyn Schmiedebergs Arch Pharmacol. 2013;386:885–891. doi: 10.1007/s00210-013-0894-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Khanderwal P, Apodaca G. Distinct apical and basolateral membrane requirements for stretch-induced membrane traffic at the apical of bladder umbrella cells. Mol Biol Cell. 2009;20:282–295. doi: 10.1091/mbc.E08-04-0439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Robson SC, Hill WG. Expression and distribution of ectonucleotidases in mouse urinary bladder. PLoS ONE. 2011;6:e18704. doi: 10.1371/journal.pone.0018704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Zeidel ML, Hill WG. Cellular expression profile for interstitial cells of Cajal in bladder – a cell often misidentified as myocyte or myofibroblast. PLoS ONE. 2012;7:e48897. doi: 10.1371/journal.pone.0048897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu W, Sun X, Robson SC, Hill WG. Extracellular UDP enhances P2X-mediated bladder smooth muscle contractility via P2Y6 activation of the phospholipase C/inositol trisphosphate pathway. FASEB J. 2013;27:1895–1903. doi: 10.1096/fj.12-219006. [DOI] [PMC free article] [PubMed] [Google Scholar]


