Abstract
BACKGROUND AND PURPOSE
Suramin is a clinically prescribed drug for treatment of human African trypanosomiasis, cancer and infection. It is also a well-known pharmacological antagonist of P2 purinoceptors. Despite its clinical use and use in research, the biological actions of this molecule are still incompletely understood. Here, we investigated the effects of suramin on membrane channels, as exemplified by its actions on non-junctional connexin43 (Cx43) hemichannels, pore-forming α-haemolysin and channels involved in ATP release under hypotonic conditions.
EXPERIMENTAL APPROACH
Hemichannels were activated by removing extracellular Ca2+. The influences of suramin on hemichannel activities were evaluated by its effects on influx of fluorescent dyes and efflux of ATP. The membrane permeability and integrity were assessed through cellular retention of preloaded calcein and LDH release.
KEY RESULTS
Suramin blocked Cx43 hemichannel permeability induced by removal of extracellular Ca2+ without much effect on Cx43 expression and gap junctional intercellular communication. This action of suramin was mimicked by its analogue NF023 and NF449 but not by another P2 purinoceptor antagonist PPADS. Besides hemichannels, suramin also significantly blocked intracellular and extracellular exchanges of small molecules caused by α-haemolysin from Staphylococcus aureus and by exposure of cells to hypotonic solution. Furthermore, it prevented α-haemolysin- and hypotonic stress-elicited cell injury.
CONCLUSION AND IMPLICATIONS
Suramin blocked membrane channels and protected cells against toxin- and hypotonic stress-elicited injury. Our finding provides novel mechanistic insights into the pharmacological actions of suramin. Suramin might be therapeutically exploited to protect membrane integrity under certain pathological situations.
Keywords: suramin, connexin 43, hemichannels, hypotonic stress, α-haemolysin, channel permeability
Introduction
Suramin is an anti-protozoal drug developed more than 90 years ago and now is known to exhibit remarkably diverse pharmacological actions. It has been used for treatment of infection, inflammation and cancer (McGeary et al., 2008) and recently, suramin was reported to protect cells from injuries caused by various insults such as ischaemia, inflammation and toxic chemicals (Kharlamov et al., 2002; Doebler, 2003; Zhuang et al., 2009; Liu et al., 2011a; 2012). Many of suramin's actions have clinical relevance. For example, suramin inhibits the binding of several growth factors (such as PDGF, TGF-β, fibroblast growth factor, insulin-like growth factor and EGF) to their cell surface receptors and this property of suramin is related to its anti-tumour actions (Sullivan et al., 1997; Davol et al., 1999; Kathir et al., 2006; McGeary et al., 2008). Suramin also has antiviral actions and has been used for human immunodeficiency virus infection (Voogd et al., 1993; Kreimeyer et al., 1998). The multifaceted actions of suramin make it an attractive agent for treatment of a growing repertoire of diseases (Voogd et al., 1993; McGeary et al., 2008; Liu and Zhuang, 2011b).
Suramin is also well known in basic scientific research. It is a widely used P2 purinoceptor antagonist (Charlton et al., 1996; Brown et al., 1997; receptor nomenclature follows Alexander et al., 2013). Furthermore, it directly modulates many signalling molecules, including kinases and phosphatases (McCain et al., 2004; Nakata, 2004). Suramin also blocks several types of membrane channels, including cystic fibrosis transmembrane conductance regulator (CFTR), GABA and glutamate receptor channels, Ca2+ release channels, NMDA-gated ion channels, as well as the P2Z ATP receptor-operated channels (now P2X7 receptors) (Wiley et al., 1993; Nakazawa et al., 1995; Peoples and Li, 1998; Bachmann et al., 1999; O'Neill et al., 2003). During a recent study of connexin 43 (Cx43) hemichannel-mediated activation of the purinergic signalling pathway (Chi et al., 2014), we noticed that suramin potently blocked Cx43 hemichannels. We therefore conducted a more detailed investigation into these channel-blocking actions of suramin and explored the therapeutic potential of suramin in channel-related cell injury.
Here, we present our data that suramin potently blocked membrane channels, as exemplified by its action on non-junctional Cx43 hemichannels, and protected cells against α-haemolysin and hypotonic stress-elicited extracellular loss of ATP, as well as cell damage.
Methods
Cells
Normal rat kidney (NRK) epithelial cell line NRK and porcine kidney epithelial cell line LLC-PK1 cells were purchased from American Type Culture Collection (Rockville, MD, USA). Cells were maintained in DMEM (Gibco-BRL, Gaithersburg, MD, USA) supplemented with 5% FBS. For comparison of cell responses between normal Ca2+ and Ca2+-free situations, cells were exposed to Ca2+-free DMEM (Gibco-BRL, catalogue number: #21068) with or without supplementation of 1.8 mM Ca2+.
Dye uptake assay
The presence of functional hemichannels was evaluated by the cellular uptake of ethidium bromide (EtBr) as described previously (Garre et al., 2010; Fang et al., 2011). NRK monolayers cultured in DMEM containing 1.8 mM Ca2+ were pretreated with or without suramin, as described in the Figure legends, and exposed to Ca2+-free medium or α-haemolysin in the presence or absence of 0.1% Lucifer yellow (LY) or 10 μM EtBr for 15 min. The cells were then rinsed twice with DMEM containing 1.8 mM Ca2+ and fixed with 3% paraformaldehyde in PBS. Immunofluorescent images were photographed using a CCD camera attached to an Olympus IX71 microscope (Tokyo, Japan). The microscope incorporates a 1.6× magnification changer that offers increased magnification to eyepieces, when it is switched on. The fluorescence intensity was measured either using NIH ImageJ software (http://rsb.info.nih.gov/ij/).
ATP measurement
ATP was measured using a luciferin/luciferase bioluminescence assay kit (Roche, Mannheim, Germany). The intensity of chemiluminescent signal was determined by a luminometer (Gene Light 55; Microtech Nition, Chiba, Japan) as described previously (Fang et al., 2011; Li et al., 2013).
Western blot analysis
Total cellular protein was extracted by suspending the prewashed cells in SDS lysis buffer (62.5 mM Tris-HCl, 2% SDS, 10% glycerol) together with freshly added proteinase inhibitor cocktail (Nacalai Tesque, Kyoto, Japan). Lysates were incubated on ice for 30 min with intermittent mixing and then centrifuged at 15,350× g for 10 min at 4°C. Supernatant was recovered, and protein concentration was determined using the Micro BCA Protein Assay Kit (Pierce, Rockford, IL, USA).
Western blot was performed by the enhanced chemiluminescence system (Chi et al., 2011; Fang et al., 2011; Li et al., 2013). Briefly, extracted cellular proteins were separated by 10% SDS-polyacrylamide gels and electrotransferred onto polyvinylidine difluoride membranes. After blocking with 3% BSA in PBS, the membranes were incubated with the primary antibody for 1.5 h at room temperature or at 4°C overnight. After washing, the membranes were probed with HRP-conjugated anti-rabbit IgG (Cell Signaling; Beverly, MA, USA), and the bands were visualized by the enhanced chemiluminescence system (Nacalai Tesque). The chemiluminescent signal is captured with a Fujifilm luminescent image LAS-1000 analyser (Fujifilm, Tokyo, Japan) and quantified with the NIH ImageJ software (http://rsb.info.nih.gov/ij). The results of quantification were expressed as OD. To confirm equal loading of proteins, the membranes were stripped with 62.5 mM Tris-HCl (pH 6.8) containing 2% SDS and 100 mM 2-mercaptoethanol for 30 min at 60°C and probed for β-actin.
Scrape-loading dye transfer assay
Gap junctional intercellular communication (GJIC) was assessed by transfer of the membrane-impermeant fluorescent dye LY from scrape-loaded cell to neighbouring cells (Yao et al., 2010; Chi et al., 2011). Briefly, confluently cultured cells in 12-well plate were exposed to 0.05% LY. A scrape line on the cell monolayer was made with a surgical blade. After a period of 2 min to allow diffusion of LY, cells were washed with the same culture medium to remove the background fluorescence and fixed with 3% paraformaldehyde in PBS. Immunofluorescent signals were captured by using a CCD camera attached to the Olympus fluorescent microscope. The distance of LY diffusion was assessed by counting the cell layer from the cells proximal to the scrape line to the most distant LY-positive cells.
Treatment of cells with siRNA
NRK cells were transiently transfected with siRNA specifically targeting Cx43 (Mm_Gja1_2 HP siRNA; Qiagen, Tokyo, Japan) or a negative control siRNA (AllStars Negative Control siRNA) at a final concentration of 20 nM using Hyperfect transfection reagent for 48 h (Chi et al., 2011; Fang et al., 2011).
Cell transfection
Wild-type Cx43-pEGFP1 vectors were kindly provided by Dr. Oyamada (Department of Pathology, Kyoto Prefectural University of Medicine, Kyoto, Japan). These vectors were transfected into LLC-PK1 cells by using Lipofectamin Plus reagent (Invitrogen, Carlsbad, CA, USA), following the manufacturer's instruction (Fang et al., 2011; Li et al., 2013). Clones with high levels of GFP were selected under the fluorescence microscope and used for this study.
Evaluation of membrane permeability and integrity using calcein AM
Calcein AM was used to evaluate the permeability and integrity of cytoplasmic membrane. Briefly, cells were preloaded with 10 μM calcein AM for 1 h at 37°C in culture medium. After washing once with the culture medium, cells were exposed to hypotonic solution (distilled water) in the presence or absence of the related agents for the indicated time intervals. The fluorescent image was visualized and captured using an Olympus IX71 inverted fluorescence microscope equipped with a standard green fluorescence cube. The intensity of the remaining fluorescence in the adherent cells was measured at 480-nm excitation and 535-nm emission by using a fluorescence multi-well plate reader (Molecular Devices, Osaka, Japan).
LDH release assay
Cytotoxicity was evaluated by the release of LDH using an LDH cytotoxicity detection kit (Takara Bio, Inc., Otsu, Shiga, Japan), as described previously (Fang et al., 2011; Li et al., 2013). Briefly, cells in 96-well culture plate were exposed to various stimulants for the described time interval. Culture medium was collected and measured for LDH activity. Culture medium was used as background control, while cells treated with 2% Triton X-100 were considered as 100% release. LDH release was calculated and expressed as percentage of total release.
Data analysis
Values are expressed as mean ± SE. Comparison of two populations was made by Student's t-test. For multiple comparisons, one-way anova followed by Dunnett's test was employed. Both analyses were done by using SigmaStat statistical software (Systat Software Inc., San Jose, CA, USA). P < 0.05 was considered to be a statistically significant difference.
Materials
FBS, trypsin/EDTA, antibiotics, cadmium chloride, heptanol, lindane, LY, EtBr, lanthanum chloride, gadolinium chloride, suramin, pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid (PPADS), α-haemolysin from Staphylococcus aureus were obtained from Sigma (Tokyo, Japan). Antibody against Cx43 (product number C6219) was also bought from Sigma. The antibody was developed in rabbit using a synthetic peptide corresponding to a C-terminal segment of the cytoplasmic domain of human and rat Cx43. NF023 and NF449 were purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA), calcein AM was obtained from Invitrogene (Tokyo, Japan). Antibodies against β-actin and Akt were purchased from Cell Signaling, Inc. (Beverly, MA, USA).
Results
Suramin inhibits hemichannel opening induced by removal of extracellular Ca2+
To determine the potential role of suramin on non-junctional hemichannels, we examined the influence of suramin on hemichannel activity triggered by removing extracellular Ca2+ (Quist et al., 2000; Stout et al., 2002; Li et al., 2013). As shown in Figure 1A, exposure of NRK cells to Ca2+-free culture medium triggered an influx of small MW fluorescent dye EtBr, as indicated by the increased fluorescent intensity. In the presence of suramin, however, the influx of EtBr was suppressed. This effect of suramin was concentration-dependent, being observable at the concentration as low as 10–50 μM (Figure 1B). Higher concentration of suramin almost completely blocked the entry of EtBr, which was comparable with hemichannel inhibitor lindane (Figure 1C).
Figure 1.
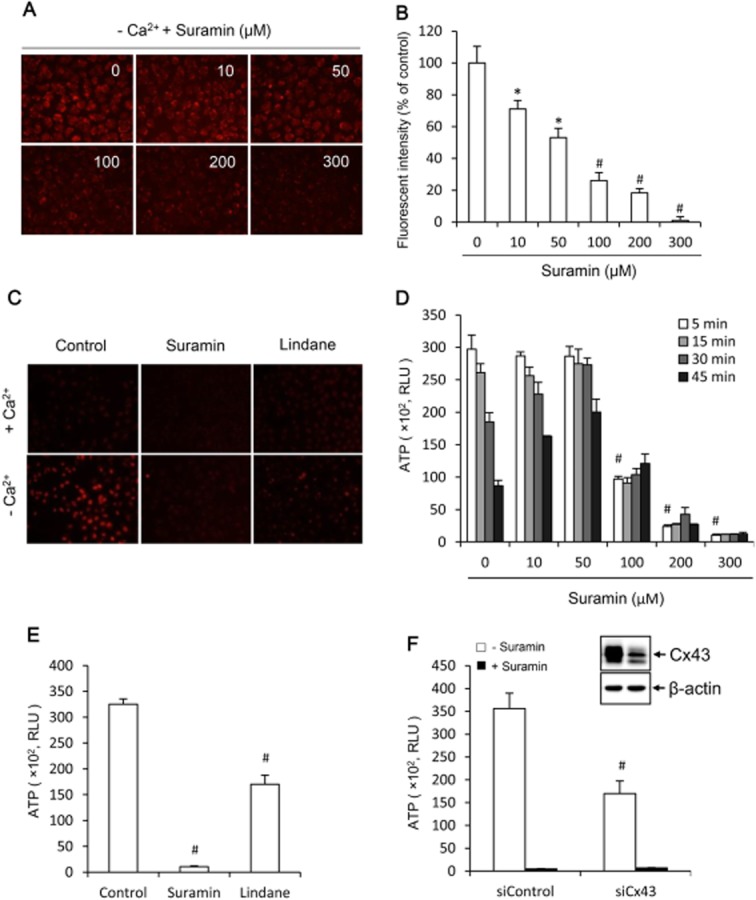
Effects of suramin on hemichannel permeability. (A) Effects of suramin on cellular uptake of EtBr following removal of extracellular Ca2+. NRK cells were pretreated with the indicated concentrations of suramin for 20 min. After that, they were exposed to either normal or Ca2+-free culture medium that contained 10 μM EtBr in the presence of the same concentrations of suramin for an additional 15 min. The cellular uptake of EtBr was photographed (magnification, ×320). (B) Quantitation of the cellular fluorescent intensity in (A). Results are expressed as means ± SEM (n = 10, *P < 0.05, #P < 0.01 vs. zero control). (C) Blockade of EtBr uptake by suramin and hemichannel inhibitor. Cells were treated the same as earlier in the presence of 300 μM suramin or 100 μM lindane (magnification, ×160). (D) Effects of suramin on Ca2+ depletion triggers ATP release. NRK cells were exposed to Ca2+-free culture medium in the presence of the indicated concentrations of suramin for the indicated time intervals. Cell supernatants were collected and quantitated for ATP concentration. Results are expressed as relative light unit (RLU; mean ± SE, n = 3). #P < 0.01 versus zero point control. (E) Effects of suramin and hemichannel inhibitors on ATP release. Cells were exposed to Ca2+-free medium in the presence or absence of 300 μM suramin or 100 μM lindane. (F) Effects of suramin and down-regulation of Cx43 with specific siRNA on ATP release. NRK cells were treated with either control siRNA or siRNA against Cx43 for 48 h. Thereafter, cells were exposed to Ca2+-free medium in the presence or absence of 300 μM suramin for 5 min. Cell supernatants were collected and quantitated for ATP concentration. Results are expressed as RLU (mean ± SE, n = 3). #P < 0.01 versus control. To verify the effectiveness of Cx43 siRNA in down-regulation of Cx43, the cellular lysates extracted from siControl and siCx43 were subjected to Western blot analysis of Cx43 (Figure 1F, insert). Note the obvious reduced level of Cx43 in Cx43 siRNA-treated cells (right lane).
Deprivation of extracellular Ca2+ also caused a rapid efflux of ATP, which peaked at 5 min and gradually returned to near basal level. In the presence of suramin, the release of ATP was significantly suppressed (Figure 1D). Interestingly, the lower concentration of suramin (10–50 μM) shifted the peak release to a relatively later time point, suggesting a partial blockade of hemichannels. Higher concentration of suramin completely abolished ATP release. As a positive control, treatment of cells with hemichannel inhibitor lindane or down-regulation of Cx43 with specific siRNA significantly prevented the efflux of ATP (Figure 1E,F). siRNA treatment also reduced the influx of EtBr (Supporting Information Fig. S1). The effectiveness of Cx43 siRNA was confirmed by the markedly reduced level of Cx43 in Western blot analysis (Figure 1F, insert). Intriguingly, suramin exerted a much potent suppression on ATP release than hemichannel inhibitor lindane and Cx43 siRNA. These results indicate that suramin may not only suppress Cx43 hemichannels but also other ATP release channels as well.
Of note, the counting of basal and control level of ATP was very low (usually less than 100), and it was not greatly altered by suramin. For the purpose of clarity, the values are not indicated in figures.
The suppression of hemichannels by suramin is not related to its action on P2 purinoceptors
Given that suramin is a well-used pharmacological antagonist of P2 purinoceptor (Charlton et al., 1996) and that hemichannel opening is associated with an increased extracellular release of ATP as well as activation of purinergic signalling pathway (Baroja-Mazo et al., 2013), one might speculate that inhibition of hemichannels could be a consequence of its action on P2 purinoceptors. To examine this possibility, we have compared the effects of suramin with PPADS, another broad-spectrum antagonist of P2 purinoceptors (Charlton et al., 1996), and KN62, an antagonist of P2X7 (Humphreys et al., 1998; Baraldi et al., 2003). As shown in Figure 2A,B, the suppressive effect of suramin on the influx of EtBr and efflux of ATP was not reproduced by PPADS and KN62. PPADS used at the concentration up to 100 μM did not affect ATP release (Figure 2C). However, it was mimicked by NF023 and NF449 (Figure 2D,E,F), structural analogues of suramin that selectively antagonizes P2X1 receptors (Soto et al., 1999; El-Ajouz et al., 2012). These analogues suppressed low Ca2+-induced influx of EtBr and efflux of ATP in a way very much similar to suramin (Figure 2D,E,F). These results indicate that the action of suramin on hemichannels is independent of its action on P2 purinoceptors.
Figure 2.
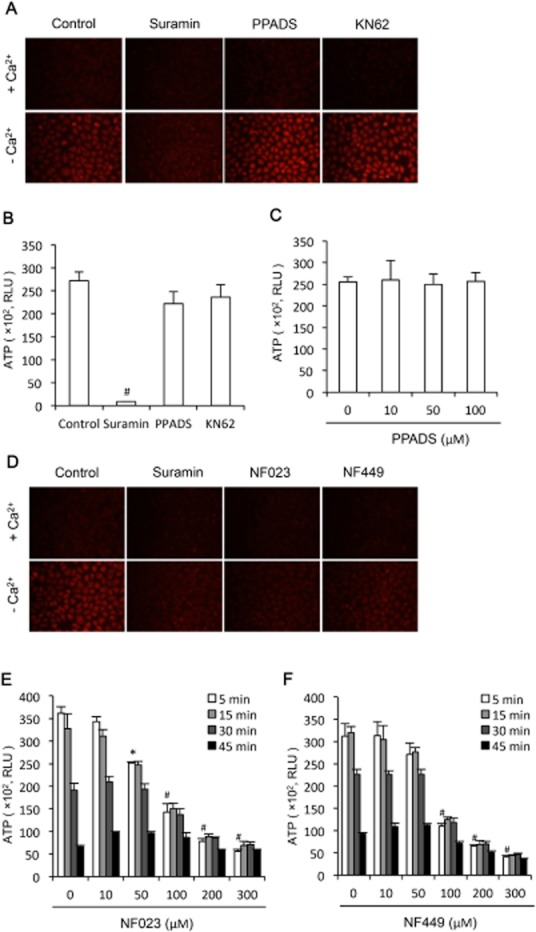
Effects of other P2 purinoceptor antagonists on hemichannel permeability. (A) Effects of P2 purinoceptor antagonist PPADS on cellular uptake of EtBr following removal of extracellular Ca2+. NRK cells were pretreated with 300 μM suramin, 3 mM heptanol, 30 μM PPADS and 100 μM NK-62 for 20 min. After that, they were exposed to either normal or Ca2+-free culture medium that contained 10 μM EtBr in the presence of the same concentrations of the earlier agents for an additional 15 min. The cellular uptake of EtBr was photographed (magnification, ×200). (B) Effects of interception of P2 purinoceptor signalling pathway on Ca2+ depletion-triggers ATP release. NRK cells were exposed to Ca2+-free culture medium in the presence of 300 μM suramin, 30 μM PPADS or 100 μM KN-62 for 5 min. Cell supernatants were collected and quantitated for ATP concentration. Results are expressed as relative light unit (RLU; mean ± SE, n = 3). #P < 0.01 compared with control. (C) Effects of various concentrations of PPADS on ATP release. Cells were treated the same as earlier in the presence or absence of the indicated concentrations of PPADS. (D) Effect of suramin, NF-023 and NF-449 on cellular uptake of EtBr. NRK cells were pretreated with 300 μM suramin, NF-023 or NF-449 for 20 min. Thereafter, cells were exposed to either normal or Ca2+-free culture medium that contained 10 μM EtBr in the presence of the same concentrations of the previous agents for an additional 15 min. The cellular uptake of EtBr was photographed (magnification, ×200). (E,F) Effect of suramin analogue NF-023 and NF-449 on ATP release. NRK cells were exposed to Ca2+-free culture medium in the presence of the indicated concentrations of NF023 or NF449 for the indicated time intervals. Cell supernatants were collected and quantitated for ATP concentration. Results are expressed as RLU (mean ± SE, n = 3). #P < 0.01, *P < 0.05 as compared with zero point control.
Suramin does not affect total Cx43 expression level
The rapid and potent inhibitory effect of suramin on non-junctional hemichannels promoted us to examine the possible influence of suramin on expression and function of Cx43, the only functional connexin expressed in NRK cells (Yao et al., 2010). As shown in Figure 3A,B, in SDS-PAGE, Cx43 was detected as two bands, representing phosphorylated and non-phosphorylated Cx43, respectively. Incubation of NRK-E52 cells with suramin up to 300 μM for 12 h did not affect total Cx43 expression level. It also did not affect Cx43 phosphorylation, as indicated by the intensity of slowly migrating bands. Consistently, suramin did not affect basal GJIC (Figure 3C,D). Scrape-loading dye-transfer assay revealed that NRK-E52 cells were well coupled by gap junctions. LY was diffused from the scrape-loaded cells to many surrounding cells, which was not altered by suramin and PPADS. As a positive control, heptanol, an inhibitor of both gap junctions and hemichannels, effectively blocked dye transfer. These observations thus indicate that suramin neither affects Cx43 expression nor its function as a gap junction channel.
Figure 3.
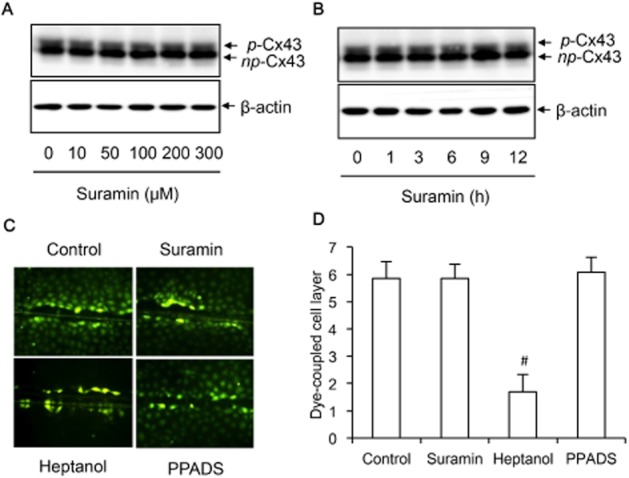
Effects of suramin on Cx43 expression and function. (A,B) NRK cells were treated with the indicated concentrations of suramin for 30 min (A) or 300 μM suramin for the indicated time intervals (B). The cellular lysates were extracted and subjected to Western blot analysis of Cx43. The upper band represents phosphorylated Cx43 (p-Cx43), and the lower band indicates non-phosphorylated Cx43 (np-Cx43). (C,D) Effects of suramin on GJIC. NRK-E52 cells were treated with 300 μM suramin, 3 mM heptanol or 30 μM PPADS for 30 min. The micrographs of LY diffusion into cellular monolayer after scrape-loading were shown (magnification, ×200). (D) The distance of LY diffusion as shown in C. Results were expressed as cell layer of dye-coupled cells (mean ± SE, n = 13). #P < 0.01 versus control.
Suramin suppresses the extracellular release of ATP induced by several different hemichannel activators
To determine whether the suppressive effect of suramin on hemichannels was activator-specific or not, we determined the influence of suramin on ATP release under several different hemichannel-activating situations (Quist et al., 2000; Thompson et al., 2006; Fang et al., 2011). Figure 4A,C shows that suramin similarly suppressed ATP release initiated by exposure of cells to HBSS (lack of both Ca2+ and glucose) and glucose-deficient medium. The participation of Cx43-forming channels in ATP release under these situations was verified by treatment of cells with hemichannel inhibitors, heptanol and lindane, or down-regulation of Cx43 with specific siRNA (Figure 4A–D). The effectiveness of Cx43 siRNA was confirmed by the markedly reduced level of Cx43 in Western blot analysis (Figure 4B, inset).
Figure 4.
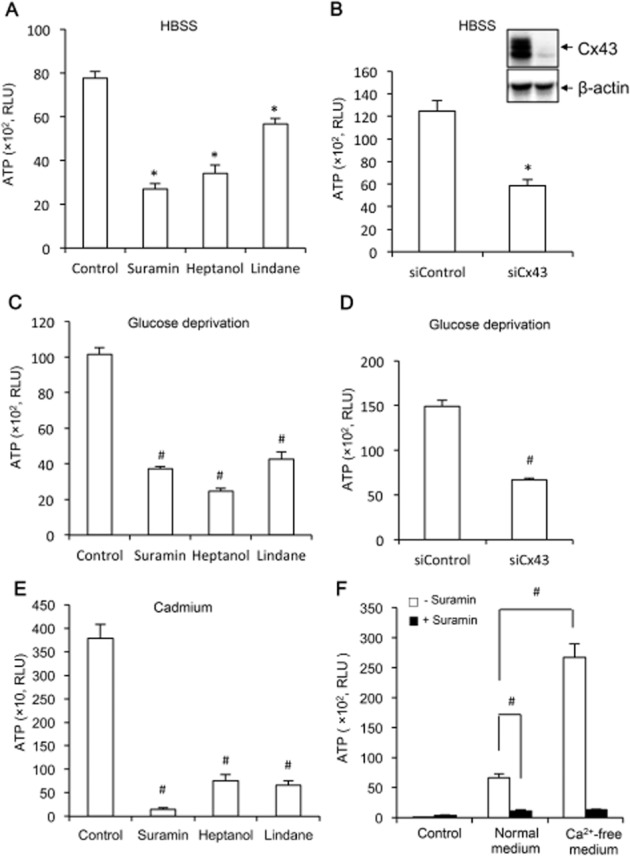
Effects of suramin on ATP release under several different conditions. (A,C,E) NRK (A,C) or EGFP-Cx43 LLC-PK1 cells (E) were pretreated with 300 μM suramin, 3 mM heptanol or 100 μM lindane for 20 min, and exposed to HBSS (A), glucose-deprived medium (C), or 50 μM Cd2+ (E) for an additional 30 min (A,B) or 3 h (E). Cell supernatants were collected and quantitated for ATP concentration. Results are expressed as relative light unit (RLU; mean ± SE, n = 3). *P < 0.05, #P < 0.01 compared with respective control. (B,D) Down-regulation of Cx43 with specific siRNA on HBSS and glucose deprivation-triggered ATP release. NRK cells were treated with either control siRNA or siRNA against Cx43 for 48 h. After that, they were exposed to HBSS or glucose-free medium for 30 min. The cellular lysates were also subjected to Western blot analysis of Cx43 to verify the effectiveness of Cx43 siRNA in down-regulation of Cx43 (Figure 4B, insert). Note the obvious reduced level of Cx43 in Cx43 siRNA-treated cells. Results are expressed as RLU (mean ± SE, n = 3). (F) Effect of suramin on basal ATP release triggered by medium exchange. NRK cells cultured in normal Ca2+ medium were incubated with or without 300 μM suramin for 20 min. Thereafter, culture medium were either left untouched (control) or changed to the same normal Ca2+ medium or Ca2+-free medium for additional 5 min. Cell supernatants were collected and quantitated for ATP concentration. Results are expressed as RLU (mean ± SE, n = 6). #P < 0.01.
Our previous studies proved that Cd2+ activates hemichannels in Cx43 overexpressing LLC-PK1 cells (Fang et al., 2011; Li et al., 2013). We therefore examined the effect of suramin on Cd2+-induced release of ATP in EGFP-Cx43 LLC-PK1 cells (Fang et al., 2011). Figure 4E shows that suramin similarly suppressed ATP release induced by Cd2+.
Several reports described that mechanical stretch induces ATP release through activation of hemichannels (Zhao et al., 2005; Garcia and Knight, 2010; Richter et al., 2014). We therefore examined the effect of suramin on ATP release induced by medium exchange that causes shear stress. As shown in Figure 4F, exchange of medium alone was enough to trigger ATP release, which accounted for about 1/5 of that induced by Ca2+-free medium. In the presence of suramin, however, the elevation was completely blocked. It appears that suramin also blocks shear stress-induced ATP release. Collectively, these observations indicate that the blocking effect of suramin on hemichannels is not cell type- and activator-dependent.
Suramin inhibits pore-forming toxin-elicited membrane hyperpermeability and cell injury
The potent suppression of Cx43 hemichannels promoted us to test whether suramin also interferes with membrane pores formed by bacterial toxin, which shares common properties with cell surface channels (Bhakdi et al., 1981; Menestrina et al., 1990; Bhakdi and Tranum-Jensen, 1991; Furini et al., 2008). Incubation of NRK cells with α-haemolysin, a pore-forming toxin from Staphylococcus aureus, led to a concentration-dependent release of ATP (Figure 5A). In the presence of suramin, this action of haemolysin was almost completely blocked. Interestingly, this action of suramin was also not mimicked by PPADS (Figure 5B). Consistent with its blocking action on extracellular release of ATP, suramin also potently blocked the pore-mediated influx of EtBr (Figure 5C).
Figure 5.
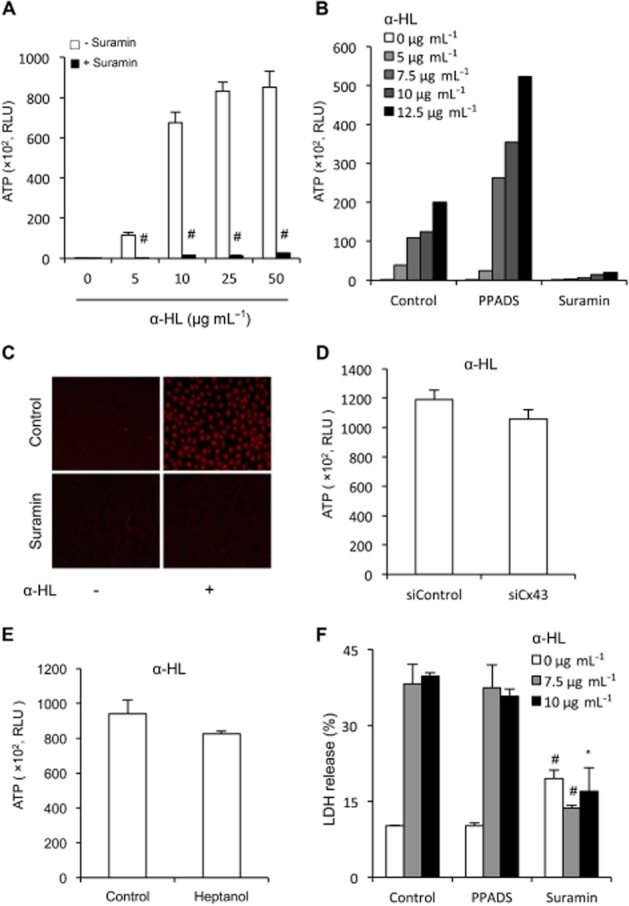
Effects of suramin on α-HL-mediated permeability and cell injury. (A,B) Effect of suramin and PPADS on pore-mediated release of ATP. NRK cells were exposed to the indicated concentration of α-HL in the presence or absence of 300 μM suramin or 30 μM PPADS for 30 min. Cell supernatant were collected and quantitated for ATP activities. Results are expressed as relative light unit (RLU; mean ± SE, n = 4 in A and n = 1 in B). #P < 0.01 compared with control. (C) Effect of suramin on pore-mediated uptake of EtBr. NRK cells were treated with 10 μg·mL−1 α-HL in the presence or absence of 300 μM suramin for 30 min. The cells were exposed to 10 μM EtBr for 15 min. Cellular uptake of EtBr were photographed (magnification, ×160). (D,E) Effects of knockdown of Cx43 or blockade of hemichannels with heptanol on haemolysin-induced release of ATP. (D) NRK cells were treated with either control siRNA or siRNA against Cx43 for 48 h. Thereafter, cells were exposed to 10 μg·mL−1 alpha-haemolysin for 90 min. (E) NRK cells were exposed to 10 μg·mL−1 alpha-haemolysin in the presence or absence of 3 mM heptanol for 90 min. Cell supernatants were collected and quantitated for ATP activities. Results are expressed as RLU (mean ± SE, n = 6 for D and n = 4 for E). (F) Effect of suramin on α-HL-induced cell injury. NRK cells were exposed to the indicated concentration of α-HL in the presence of 300 μM suramin or 30 μM PPADS for 24 h. Cell supernatants were collected and assayed for LDH release. The results are expressed as % of total release (RLU; mean ± SE, n = 3). #P < 0.01, *P < 0.05 compared with respective control.
As a previous report described an involvement of pannexin1 hemichannel-derived ATP in haemolysin-induced lysis of red blood cells (Skals et al., 2009), we therefore examined the possible participation of Cx43 hemichannels under our experimental setting. Figure 5D,E shows that knockdown of Cx43 with specific siRNA or blockade of hemichannels with heptanol did not greatly affect haemolysin-induced ATP release, thus excluding a potential contribution of Cx43 hemichannels in haemolysin-induced ATP release.
Prolonged incubation of cells with haemolysin led to cell death, as evidenced by the increased extracellular release of LDH. Consistent with the blocking effect on the pore permeability, suramin also significantly prevented haemolysin-induced cell injury (Figure 5F). Interestingly, this cytoprotective action of suramin was observed under the situation that suramin itself induced a modest but statistically significant elevation in LDH release, suggestive of cytotoxicity. These observations thus indicate that suramin blocks the channel activities of pore-forming toxin. They also indicate that suramin could have cytotoxicity in long-term culture.
Suramin protect cells from hypotonic stress-elicited eruption of cell membrane
The potent antagonistic actions of suramin on hemichannels and pores promoted us to test whether suramin also affected other channels. For this purpose, we have examined the effect of suramin on hypotonicity-induced ATP release, an event that involves many different types of membrane channels (Menestrina et al., 1990; Taouil and Hannaert, 1999; Hazama et al., 2000; Braunstein et al., 2001; Sabirov et al., 2001; Boudreault and Grygorczyk, 2002; Dutta et al., 2002; Okada et al., 2004; Calloe et al., 2007; Liu et al., 2008; Shi et al., 2009; Lu et al., 2012). Exposure of NRK cells to distilled water led to a rapid release of ATP, which was significantly blocked by suramin, and hemichannel inhibitor lindane and heptanol as well (Figure 6A). Down-regulation of Cx43 with siRNA also significantly blocked ATP release (Figure 6B). These results indicate an involvement of hemichannels in hypotonicity-induced release of ATP.
Figure 6.
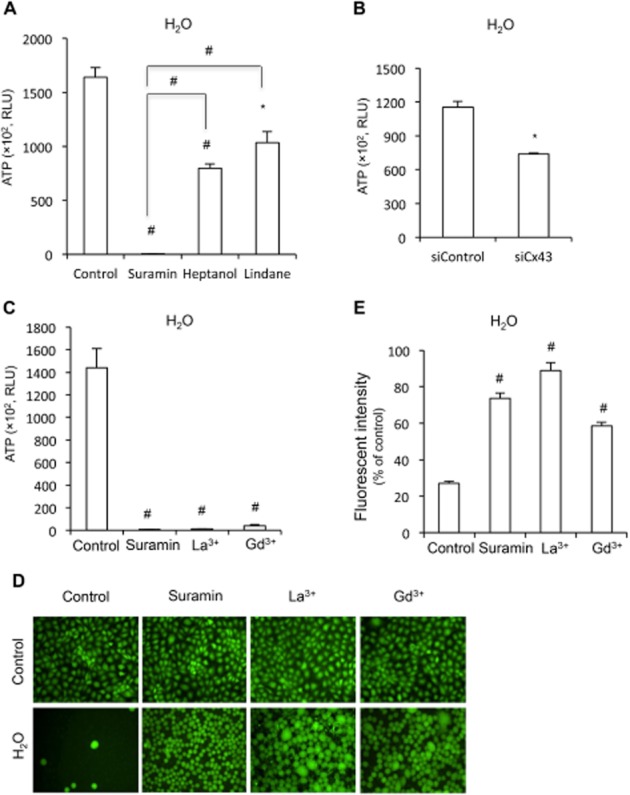
Effect of suramin on membrane permeability and integrity under hypotonic condition. (A,B) Effect of suramin, hemichannel inhibitor or Cx43 siRNA on ATP release under hypotonic condition. (A,B) NRK cells were pretreated with 300 μM suramin, 3 mM heptanol or 100 μM lindane for 20 min, or Cx43 siRNA for 48 h. After that, cells were exposed to distilled water in the presence or absence of the same amount of agents for 5 min. Cell supernatants were collected and quantitated for ATP concentration. Results are expressed as relative light unit (RLU; mean ± SE, n = 3). *P < 0.05, #P < 0.01 compared with untreated control. (C) Effect of suramin and non-specific channel blockers on hypotonicity-induced release of ATP. NRK cells were pretreated with 300 μM suramin, 500 μM La3+ or 500 μM Gd3+ for 20 min. Cells were exposed to distilled water in the presence or absence of the same amount of agents for 5 min. Cell supernatants were collected and quantitated for ATP concentration. Results are expressed as RLU (mean ± SE, n = 4). #P < 0.01 compared with untreated control. (D,E) Effect of suramin and non-specific channel blockers on membrane integrity under hypotonic stress. NRK cells were preincubated with 10 μM calcein for 1 h. After that, they were treated with 300 μM suramin, 500 μM La3+ or 500 μM Gd3+ and then exposed to distilled water in the presence or absence of the same amount of the earlier agents for an additional 45 min. The cell membrane integrity was evaluated by the retention of the preloaded calcein inside the cells under fluorescent microscopy (D; magnification, ×200) or the fluorescent intensity of the remaining cells under a fluorescent reader (E). Results in E are expressed as % of fluorescence relative to normal control (mean ± SE, n = 4). #P < 0.01 compared with H2O alone.
Interestingly, as compared with hemichannel inhibitors, suramin exhibited a significantly more potent suppression on ATP release (Figure 6A), implying an involvement of other channels that were similarly suppressed by suramin. To confirm this speculation, we have compared the effect of suramin with two non-specific channel blockers, La3+ and Gd3+, which have previously been documented to be able to inhibit hypotonic release of ATP and to protect cells from hypotonicity-induced cell injury (Berrier et al., 1992; De Smet et al., 1998; Braunstein et al., 2001; Boudreault and Grygorczyk, 2002; Liu et al., 2008). As shown in Figure 6C, suramin suppressed hypotonicity-induced ATP release to the magnitude similar to these non-specific channel blockers.
Exposure of NRK cells to H2O led to a concentration-dependent disruption of cell membrane integrity, as evaluated by the retention of the preloaded calcein inside the cells, as well as detection of fluorescent intensity of the cells under a fluorescent reader (Supporting Information Fig. S2). Consistent with the channel-blocking actions, suramin significantly protected cells from hypotonic stress-elicited membrane eruption. This action of suramin was also comparable with that of non-specific channel blockers, although La3+ and Gd3+-treated cells appeared to have a much larger cell body than those of suramin-treated cells under hypotonic condition (Figure 6D,E). These results thus indicate that suramin protects cells against hypotonicity-induced cell injury.
Discussion
Suramin has been used for treatment of many diseases in clinic and has been reported to suppress the functions of a wide range of cell membrane proteins, including receptors, channels and adhesive molecules (Supporting Information Table S1). In this study, we demonstrated, for the first time, that suramin interfered with non-junctional Cx43 hemichannels and protected cells from toxin- and hypotonic stress-elicited membrane hyperpermeability and cell injury. Given the importance of suramin in both clinic and basic research, our findings could have great implications for a better understanding of the roles and mechanisms of suramin.
In this study, the action of suramin on cell surface channels was exemplified by its role on non-junctional Cx43 hemichannels. Hemichannels is activated under many pathophysiological situations, including Cx mutations, depolarization of the membrane potential, hypoxia, change of intracellular and extracellular Ca2+, as well as cellular redox status (Anselmi et al., 2008; Saez et al., 2010), and has been shown to be critically involved in a variety of pathophysiological processes (Fang et al., 2011; Scheckenbach et al., 2011; Baroja-Mazo et al., 2013; Li et al., 2013). In this investigation, we have employed a simple method to induce hemichannel opening, i.e. through removing extracellular Ca2+. Activation of hemichannels by lowering Ca2+ has been previously demonstrated at both structural and functional levels. Using an atomic force microscope, Muller et al. observed that removal of extracellular Ca2+ enhanced outer hemichannel pore diameter (Muller et al., 2002). Consistently, many investigators observed increased hemichannel permeability following elimination of extracellular Ca2+ (Stout et al., 2002; Li et al., 2013). In line with these observations, we detected an increased exchange of small molecules between the inside and the outside of cell membrane following Ca2+ deprivation, which was blocked by suramin in a way similar to hemichannel inhibitors. These results thus indicate that suramin inhibits hemichannel activities.
Suramin have many pharmacological functions. The question naturally occurs as to whether the effect of suramin on hemichannels was a result of its actions on other signalling molecules. In this study, we excluded a participation of P2 purinoceptors. This is shown by the observation that the effect of suramin was not mimicked by PPADS, a structurally different, broad-spectrum P2 purinoceptor antagonist (Charlton et al., 1996) and KN62, a potent P2X7 antagonist (Humphreys et al., 1998; Baraldi et al., 2003), but reproduced by NF023 and NF449, structural analogues of suramin that selectively block P2X1 receptors (Humphreys et al., 1998; Soto et al., 1999; El-Ajouz et al., 2012). It is worth mentioning that our undisclosed data indicated that suramin and PPADS similarly suppressed P2-receptor-mediated activation of AKT and mTOR under Ca2+-free condition (Supporting Information Fig. S3; manuscript in submission). It appears that the action of suramin on hemichannels was not due to its action on P2 purinoceptors.
Our study also suggests that the effect of suramin was not channel activator-dependent. Suramin similarly affected ATP release initiated by removing extracellular Ca2+, glucose deprivation, mechanical strain as well as Cd2+ stimulation. Activation of hemichannels under these conditions has been previously reported with little information available regarding the activation mechanisms (Quist et al., 2000; Zhao et al., 2005; Thompson et al., 2006; Garcia and Knight, 2010; Fang et al., 2011; Richter et al., 2014). The similar inhibition of hemichannels triggered by different stimuli implies that the effect of suramin might be through direct interaction with channels rather than through interference of stimuli or stimuli-elicited signalling pathways.
It is intriguing to mention that suramin also blocked the membrane permeability caused by α-haemolysin, a pore-forming toxin from Staphylococcus aureus (Bhakdi and Tranum-Jensen, 1991; Walev et al., 1993). In fact, hemichannels and the pores share many properties in common. They are hexamer transmembrane channels that mediate intracellular and extracellular exchange of small molecules, like ATP, amino acids and nucleotides (Bhakdi et al., 1981; Bhakdi and Tranum-Jensen, 1991; Saez et al., 2010; Baroja-Mazo et al., 2013). The reported MW cut-off of material passing through the hemichannels and pores are approximately 1000 and 2000 Daltons respectively (Bhakdi and Tranum-Jensen, 1991; Saez et al., 2010; Baroja-Mazo et al., 2013). The similar inhibition of Cx43 hemichannels and pores suggests that suramin might affect channels with common structure through a common regulating mechanism.
Of note, a previous study by Skals et al. (2009) demonstrated that haemolytic lysis caused by the bacterial toxin HlyA was suppressed by non-selective ATP-receptor antagonist PPADS and suramin. Furthermore, they demonstrated an implication of P2X receptors and pannexin1 in augmentation of haemolysis. Different from their observations, we did not detect a protective effect of PPADS on haemolysin-triggered ATP release and cell injury. The reason for the discrepancy is presently unclear. It could be due to different experimental settings. Different from red blood cells, NRK cells used in the current study are pannexin-deficient (Penuela et al., 2007). Thus, an implication of pannexin in our system was less likely. Furthermore, our study also excluded an implication of hemichannel in augmentation of the effect of haemolysin. Knockdown of Cx43 with specific siRNA or blockade of hemichannels with heptanol did not greatly affect haemolysin-induced ATP release. Our observations thus support a direct blocking effect of suramin on haemolysin-forming pore rather than an indirect action through blocking hemichannels or purinergic signalling.
The suppression of multiple membrane channels by suramin was partially verified by the observation that suramin potently blocked hypotonicity-induced ATP release. Previous studies have demonstrated that hypotonic release of ATP was derived from multiple membrane channels, including K/ATP channel, KCNQ channel, Cx hemichannel, voltage-dependent anion channel, ATP-conductive large-conductance anion channel, maxi-anion channel and CFTR (Taouil and Hannaert, 1999; Hazama et al., 2000; Braunstein et al., 2001; Sabirov et al., 2001; Boudreault and Grygorczyk, 2002; Dutta et al., 2002; Okada et al., 2004; Calloe et al., 2007; Liu et al., 2008; Shi et al., 2009; Lu et al., 2012). Using hemichannel inhibitors and Cx43 siRNA, we clearly demonstrated an involvement of hemichannels. However, the much more potent suppression of ATP release by suramin pointed to a participation of other channels. Indeed, suramin is reported to inhibit several membrane channels (Wiley et al., 1993; Nakazawa et al., 1995; Bachmann et al., 1999), including CFTR known to be involved in ATP release (Hazama et al., 2000; Braunstein et al., 2001). In further support of a role of suramin on other channels, suramin mimicked the suppressive effects of non-specific channel blockers, La3+ and Gd3+, on hypotonicity-induced ATP release and cell injury (Berrier et al., 1992; De Smet et al., 1998; Braunstein et al., 2001; Boudreault and Grygorczyk, 2002; Liu et al., 2008).
The molecular mechanisms underlying the inhibitory action of suramin on multiple membrane channels are presently unclear. Suramin bears fixed-negative charges by virtue of their polysulfonates. It has been reported that suramin interacts with positive ectodomain of receptors and prevents their binding of multiple cytokines and growth factors (Stratmann et al., 2000; North, 2002; McGeary et al., 2008). A similar mechanism might be behind the channel-blocking action of suramin. Indeed, the suppressive action of suramin on CFTR is thought to be through electrostatic interactions with the positive charge of a lysine side chain within the channel pore (Bachmann et al., 1999; St Aubin et al., 2007). Similarly, an ectodomain lysine residue in P2X1 receptors has been reported to be responsible for the species difference in receptor binding to suramin (Sim et al., 2008). Intriguingly, the positively charged lysine residues also exist in α-toxin, which determines the electrical properties of the pore (Cescatti et al., 1991). Suramin might interfere with channel activity through interaction with some common structure of channels, such as positively charged resides. Identification and characterization of the residues and structures would provide more information about the structure and function relationship of these channels and help us to designate more effective channel blockers.
The suppressive effect of suramin on hemichannels promoted us to speculate that suramin may also affect pannexin activity, a channel that shares many common properties with hemichannels in structure and function (Penuela et al., 2007; Wang et al., 2013). Indeed, suramin and a suramin analogue, food dye FD&C blue no. 1, have been reported to inhibit pannexin 1 channels (Qiu and Dahl, 2009; Wang et al., 2013). Of note, NRK cells are previously reported to be pannexin-deficient (Penuela et al., 2007), the possible implication of pannexin in this study was less likely.
Our findings have multifold implications for both clinic and basic application of suramin. First, our results indicate that suppression of hemichannels could be a presently unrecognized mechanism behind the pharmacological actions of suramin. For example, suramin has been shown to be able to suppress inflammatory response and ischaemic cell injuries (Kharlamov et al., 2002; Liu et al., 2012). Interestingly, the similar effects have also been reported by blockade of hemichannels (Scheckenbach et al., 2011; Davidson et al., 2013). It is conceivable that the effect of suramin could be through interference of hemichannels. Second, because the concentrations of suramin used for suppression of hemichannels (100∼300 μM) overlap with those reported for antagonizing P2 purinoceptors, cautions should be taken in interpreting the results obtained from studies in which suramin is used as a purinoceptor antagonist. Third, suramin might have advantages over the other hemichannel inhibitors or P2 purinoceptor antagonists in suppression of channel-mediated activation of purinergic signalling pathway because of its dual-blocking effect on channels and receptors. Fourth, our data indicate that suppression of non-junctional hemichannels by suramin was not associated with altered GJIC. This feature of suramin might be used to distinguish the effects of gap junctions and hemichannels that usually coexist under various pathophysiological situations. The available chemical hemichannel inhibitors usually also suppress gap junctions. Fifth, as a multiple-channel blocker, suramin might be exploited to protect cells against the injury caused by membrane channel hyperpermeability and instability under the situation of hypotonic stress and pathogen invasion. It should be mentioned that suramin has also cytotoxicity (Dhar et al., 2000). This property has been exploited for tumour therapy. In this investigation, we noticed that long-term exposure of cells to suramin caused LDH release, indicative of cytotoxicity. Therefore, cautions should be taken when suramin is used for chronic experiments or treatment of chronic illnesses.
In conclusion, our results revealed that suramin inhibits cell membrane permeability, as exemplified by its suppressive actions on Cx43 hemichannels and pore formed by α-haemolysin. Our findings thus provide novel mechanistic insights into the pharmacological actions of suramin. Suramin might be used as a pharmacological tool for investigation of the role and mechanisms of membrane channels, as well as channel-derived mediators. It also has the potential to be developed as a novel therapeutic agent for prevention and treatment of certain diseases, which are caused by or associated with the elevated channel activities and membrane permeability.
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research from the Ministry of Education, Culture, Sports, Science and Technology, Japan (17659255 and 20590953 to JY). We thank Drs. Masanori Kitamura (University of Yamanashi) and Hiroyuki Matsue (Chiba University, Japan) for helpful discussion and valuable advices.
Glossary
- Cx43
connexin 43
- EtBr
ethidium bromide
- GJIC
gap junctional intercellular communication
- LY
Lucifer yellow
- NRK
Normal rat kidney
- PPADS
pyridoxalphosphate-6-azophenyl-2′,4′-disulfonic acid
Conflict of interest
None.
Supporting information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
http://dx.doi.org/10.1111/bph.12693
Figure S1 Effects of knockdown of Cx43 on cellular uptake of EtBr following removal of extracellular Ca2+. NRK cells were treated with either control siRNA or siRNA against Cx43 for 48 h. Thereafter, cells were exposed to normal or Ca2+-free culture medium that contained 10 μM EtBr 15 min. The cellular uptake of EtBr was photographed (magnification, ×320).
Figure S2 Hypotonic stress-induced disruption of membrane integrity. NRK cells were pre-incubated with 10 μM calcein for 1 h. After that, they were exposed to distilled water for the indicated time intervals. Cell membrane integrity was evaluated by the retention of fluorescent dye calcein inside the cells under microscope (A; magnification, ×160) or the fluorescent intensity of the remaining cells under a fluorescent reader (B). Results in B are expressed as % of fluorescence relative to normal control (mean ± SE, n = 4). *P < 0.05 compared with untreated control.
Figure 3 Suppression of low Ca2+-induced activation of Akt by suramin and PPADS. NRK cells were pretreated with or without 300 μM suramin or 10 μM PPADS for 30 min before exposing to Ca2+-free culture medium for an additional 5 min. Cellular proteins were extracted and subjected to Western analysis for phosphorylation of Akt and β-actin. Note the similar potency of PPADS and suramin on Ca2+ deprivation-induced activation of Akt.
Table S1 Effects of suramin on cell membrane receptors, channels and adhesive molecules.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ligand-Gated Ion Channels. Br J Pharmacol. 2013;170:1582–1606. doi: 10.1111/bph.12446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anselmi F, Hernandez VH, Crispino G, Seydel A, Ortolano S, Roper SD, et al. ATP release through connexin hemichannels and gap junction transfer of second messengers propagate Ca2+ signals across the inner ear. Proc Natl Acad Sci U S A. 2008;105:18770–18775. doi: 10.1073/pnas.0800793105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachmann A, Russ U, Quast U. Potent inhibition of the CFTR chloride channel by suramin. Naunyn Schmiedebergs Arch Pharmacol. 1999;360:473–476. doi: 10.1007/s002109900096. [DOI] [PubMed] [Google Scholar]
- Baraldi PG, del Carmen Nuñez M, Morelli A, Falzoni S, Di Virgilio F, Romagnoli R. Synthesis and biological activity of N-arylpiperazine-modified analogues of KN-62, a potent antagonist of the purinergic P2X7 receptor. J Med Chem. 2003;46:1318–1329. doi: 10.1021/jm021049d. [DOI] [PubMed] [Google Scholar]
- Baroja-Mazo A, Barbera-Cremades M, Pelegrin P. The participation of plasma membrane hemichannels to purinergic signaling. Biochim Biophys Acta. 2013;1828:79–93. doi: 10.1016/j.bbamem.2012.01.002. [DOI] [PubMed] [Google Scholar]
- Berrier C, Coulombe A, Szabo I, Zoratti M, Ghazi A. Gadolinium ion inhibits loss of metabolites induced by osmotic shock and large stretch-activated channels in bacteria. Eur J Biochem. 1992;206:559–565. doi: 10.1111/j.1432-1033.1992.tb16960.x. [DOI] [PubMed] [Google Scholar]
- Bhakdi S, Tranum-Jensen J. Alpha-toxin of Staphylococcus aureus. Microbiol Rev. 1991;55:733–751. doi: 10.1128/mr.55.4.733-751.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhakdi S, Fussle R, Tranum-Jensen J. Staphylococcal alpha-toxin: oligomerization of hydrophilic monomers to form amphiphilic hexamers induced through contact with deoxycholate detergent micelles. Proc Natl Acad Sci U S A. 1981;78:5475–5479. doi: 10.1073/pnas.78.9.5475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreault F, Grygorczyk R. Cell swelling-induced ATP release and gadolinium-sensitive channels. Am J Physiol Cell Physiol. 2002;282:C219–C226. doi: 10.1152/ajpcell.00317.2001. [DOI] [PubMed] [Google Scholar]
- Braunstein GM, Roman RM, Clancy JP, Kudlow BA, Taylor AL, Shylonsky VG, et al. Cystic fibrosis transmembrane conductance regulator facilitates ATP release by stimulating a separate ATP release channel for autocrine control of cell volume regulation. J Biol Chem. 2001;276:6621–6630. doi: 10.1074/jbc.M005893200. [DOI] [PubMed] [Google Scholar]
- Brown CA, Charlton SJ, Boarder MR. Enhancement of the response to purinergic agonists in P2Y1 transfected 1321N1 cells by antagonists suramin and PPADS. Br J Pharmacol. 1997;120:1049–1052. doi: 10.1038/sj.bjp.0701010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calloe K, Nielsen MS, Grunnet M, Schmitt N, Jorgensen NK. KCNQ channels are involved in the regulatory volume decrease response in primary neonatal rat cardiomyocytes. Biochim Biophys Acta. 2007;1773:764–773. doi: 10.1016/j.bbamcr.2007.02.008. [DOI] [PubMed] [Google Scholar]
- Cescatti L, Pederzolli C, Menestrina G. Modification of lysine residues of Staphylococcus aureus alpha-toxin: effects on its channel-forming properties. J Membr Biol. 1991;119:53–64. doi: 10.1007/BF01868540. [DOI] [PubMed] [Google Scholar]
- Charlton SJ, Brown CA, Weisman GA, Turner JT, Erb L, Boarder MR. PPADS and suramin as antagonists at cloned P-2Y- and P-2U-purinoceptors. Br J Pharmacol. 1996;118:704–710. doi: 10.1111/j.1476-5381.1996.tb15457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chi Y, Li K, Yan Q, Koizumi S, Shi L, Takahashi S, et al. Nonsteroidal anti-inflammatory drug flufenamic acid is a potent activator of AMP-activated protein kinase. J Pharmacol Exp Ther. 2011;339:257–266. doi: 10.1124/jpet.111.183020. [DOI] [PubMed] [Google Scholar]
- Chi Y, Gao K, Li K, Nakajima S, Kira S, Takeda M, et al. Purinergic control of AMPK activation by ATP released through connexin 43 hemichannels: pivotal roles in hemichannel-mediated cell injury. J Cell Sci. 2014;127:1487–1499. doi: 10.1242/jcs.139089. [DOI] [PubMed] [Google Scholar]
- Davidson JO, Green CR, Bennet L, Nicholson LF, Danesh-Meyer H, O'Carroll SJ, et al. A key role for connexin hemichannels in spreading ischemic brain injury. Curr Drug Targets. 2013;14:36–46. doi: 10.2174/138945013804806479. [DOI] [PubMed] [Google Scholar]
- Davol PA, Garza S, Frackelton AR., Jr Combining suramin and a chimeric toxin directed to basic fibroblast growth factor receptors increases therapeutic efficacy against human melanoma in an animal model. Cancer. 1999;86:1733–1741. doi: 10.1002/(sici)1097-0142(19991101)86:9<1733::aid-cncr15>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- De Smet P, Li J, Van Driessche W. Hypotonicity activates a lanthanide-sensitive pathway for K+ release in A6 epithelia. Am J Physiol. 1998;275((1 Pt 1)):C189–C199. doi: 10.1152/ajpcell.1998.275.1.C189. [DOI] [PubMed] [Google Scholar]
- Dhar S, Gullbo J, Csoka K, Eriksson E, Nilsson K, Nickel P, et al. Antitumour activity of suramin analogues in human tumour cell lines and primary cultures of tumour cells from patients. Eur J Cancer. 2000;36:803–809. doi: 10.1016/s0959-8049(00)00024-1. [DOI] [PubMed] [Google Scholar]
- Doebler JA. Blockade of sulfur mustard cytotoxicity in human epidermal keratinocytes with the purinergic receptor antagonist suramin. Vet Hum Toxicol. 2003;45:14–17. [PubMed] [Google Scholar]
- Dutta AK, Okada Y, Sabirov RZ. Regulation of an ATP-conductive large-conductance anion channel and swelling-induced ATP release by arachidonic acid. J Physiol. 2002;542((Pt 3)):803–816. doi: 10.1113/jphysiol.2002.019802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Ajouz S, Ray D, Allsopp RC, Evans RJ. Molecular basis of selective antagonism of the P2X1 receptor for ATP by NF449 and suramin: contribution of basic amino acids in the cysteine-rich loop. Br J Pharmacol. 2012;165:390–400. doi: 10.1111/j.1476-5381.2011.01534.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang X, Huang T, Zhu Y, Yan Q, Chi Y, Jiang JX, et al. Connexin43 hemichannels contribute to cadmium-induced oxidative stress and cell injury. Antioxid Redox Signal. 2011;14:2427–2439. doi: 10.1089/ars.2010.3150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furini S, Domene C, Rossi M, Tartagni M, Cavalcanti S. Model-based prediction of the alpha-hemolysin structure in the hexameric state. Biophys J. 2008;95:2265–2274. doi: 10.1529/biophysj.107.127019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia M, Knight MM. Cyclic loading opens hemichannels to release ATP as part of a chondrocyte mechanotransduction pathway. J Orthop Res. 2010;28:510–515. doi: 10.1002/jor.21025. [DOI] [PubMed] [Google Scholar]
- Garre JM, Retamal MA, Cassina P, Barbeito L, Bukauskas FF, Saez JC, et al. FGF-1 induces ATP release from spinal astrocytes in culture and opens pannexin and connexin hemichannels. Proc Natl Acad Sci U S A. 2010;107:22659–22664. doi: 10.1073/pnas.1013793107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazama A, Fan HT, Abdullaev I, Maeno E, Tanaka S, Ando-Akatsuka Y, et al. Swelling-activated, cystic fibrosis transmembrane conductance regulator-augmented ATP release and Cl(-) conductances in murine C127 cells. J Physiol-London. 2000;523:1–11. doi: 10.1111/j.1469-7793.2000.t01-6-00001.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphreys BD, Virginio C, Surprenant A, Rice J, Dubyak GR. Isoquinolines as antagonists of the P2X7 nucleotide receptor: high selectivity for the human versus rat receptor homologues. Mol Pharmacol. 1998;54:22–32. doi: 10.1124/mol.54.1.22. [DOI] [PubMed] [Google Scholar]
- Kathir KM, Kumar TK, Yu C. Understanding the mechanism of the antimitogenic activity of suramin. Biochemistry. 2006;45:899–906. doi: 10.1021/bi051389b. [DOI] [PubMed] [Google Scholar]
- Kharlamov A, Jones SC, Kim DK. Suramin reduces infarct volume in a model of focal brain ischemia in rats. Exp Brain Res. 2002;147:353–359. doi: 10.1007/s00221-002-1251-1. [DOI] [PubMed] [Google Scholar]
- Kreimeyer A, Muller G, Kassack M, Nickel P, Gagliardi AR. Suramin analogues with a 2-phenylbenzimidazole moiety as partial structure; potential anti HIV- and angiostatic drugs, 2: sulfanilic acid-, benzenedisulfonic acid-, and naphthalenetrisulfonic acid analogues. Arch Pharm. 1998;331:97–103. doi: 10.1002/(sici)1521-4184(199803)331:3<97::aid-ardp97>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Li K, Chi Y, Gao K, Yan Q, Matsue H, Takeda M, et al. Connexin43 hemichannel-mediated regulation of connexin43. PLoS ONE. 2013;8:e58057. doi: 10.1371/journal.pone.0058057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu HT, Toychiev AH, Takahashi N, Sabirov RZ, Okada Y. Maxi-anion channel as a candidate pathway for osmosensitive ATP release from mouse astrocytes in primary culture. Cell Res. 2008;18:558–565. doi: 10.1038/cr.2008.49. [DOI] [PubMed] [Google Scholar]
- Liu N, Zhuang S. Tissue protective and anti-fibrotic actions of suramin: new uses of an old drug. Curr Clin pharmacol. 2011b;6:137–142. doi: 10.2174/157488411796151174. [DOI] [PubMed] [Google Scholar]
- Liu N, Tolbert E, Pang M, Ponnusamy M, Yan H, Zhuang S. Suramin inhibits renal fibrosis in chronic kidney disease. J Am Soc Nephrol. 2011a;22:1064–1075. doi: 10.1681/ASN.2010090956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu N, He S, Tolbert E, Gong R, Bayliss G, Zhuang S. Suramin alleviates glomerular injury and inflammation in the remnant kidney. PLoS ONE. 2012;7:e36194. doi: 10.1371/journal.pone.0036194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu D, Soleymani S, Madakshire R, Insel PA. ATP released from cardiac fibroblasts via connexin hemichannels activates profibrotic P2Y2 receptors. FASEB Journal. 2012;26:2580–2591. doi: 10.1096/fj.12-204677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCain DF, Wu L, Nickel P, Kassack MU, Kreimeyer A, Gagliardi A, et al. Suramin derivatives as inhibitors and activators of protein-tyrosine phosphatases. J Biol Chem. 2004;279:14713–14725. doi: 10.1074/jbc.M312488200. [DOI] [PubMed] [Google Scholar]
- McGeary RP, Bennett AJ, Tran QB, Cosgrove KL, Ross BP. Suramin: clinical uses and structure-activity relationships. Mini Rev Med Chem. 2008;8:1384–1394. doi: 10.2174/138955708786369573. [DOI] [PubMed] [Google Scholar]
- Menestrina G, Bashford CL, Pasternak CA. Pore-forming toxins: experiments with S. aureus alpha-toxin, C. perfringens theta-toxin and E. coli haemolysin in lipid bilayers, liposomes and intact cells. Toxicon. 1990;28:477–491. doi: 10.1016/0041-0101(90)90292-f. [DOI] [PubMed] [Google Scholar]
- Muller DJ, Hand GM, Engel A, Sosinsky GE. Conformational changes in surface structures of isolated connexin 26 gap junctions. EMBO J. 2002;21:3598–3607. doi: 10.1093/emboj/cdf365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H. Stimulation of extracellular signal-regulated kinase pathway by suramin with concomitant activation of DNA synthesis in cultured cells. J Pharmacol Exp Ther. 2004;308:744–753. doi: 10.1124/jpet.103.058230. [DOI] [PubMed] [Google Scholar]
- Nakazawa K, Inoue K, Ito K, Koizumi S, Inoue K. Inhibition by suramin and reactive blue 2 of GABA and glutamate receptor channels in rat hippocampal neurons. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:202–208. doi: 10.1007/BF00169334. [DOI] [PubMed] [Google Scholar]
- North RA. Molecular physiology of P2X receptors. Physiol Rev. 2002;82:1013–1067. doi: 10.1152/physrev.00015.2002. [DOI] [PubMed] [Google Scholar]
- Okada SF, O'Neal WK, Huang P, Nicholas RA, Ostrowski LE, Craigen WJ, et al. Voltage-dependent anion channel-1 (VDAC-1) contributes to ATP release and cell volume regulation in murine cells. J Gen Physiol. 2004;124:513–526. doi: 10.1085/jgp.200409154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill ER, Sakowska MM, Laver DR. Regulation of the calcium release channel from skeletal muscle by suramin and the disulfonated stilbene derivatives DIDS, DBDS, and DNDS. Biophys J. 2003;84:1674–1689. doi: 10.1016/S0006-3495(03)74976-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Penuela S, Bhalla R, Gong XQ, Cowan KN, Celetti SJ, Cowan BJ, et al. Pannexin 1 and pannexin 3 are glycoproteins that exhibit many distinct characteristics from the connexin family of gap junction proteins. J Cell Sci. 2007;120((Pt 21)):3772–3783. doi: 10.1242/jcs.009514. [DOI] [PubMed] [Google Scholar]
- Peoples RW, Li C. Inhibition of NMDA-gated ion channels by the P2 purinoceptor antagonists suramin and reactive blue 2 in mouse hippocampal neurones. Br J Pharmacol. 1998;124:400–408. doi: 10.1038/sj.bjp.0701842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu F, Dahl G. A permeant regulating its permeation pore: inhibition of pannexin 1 channels by ATP. Am J Physiol Cell Physiol. 2009;296:C250–C2505. doi: 10.1152/ajpcell.00433.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quist AP, Rhee SK, Lin H, Lal R. Physiological role of gap-junctional hemichannels. Extracellular calcium-dependent isosmotic volume regulation. J Cell Biol. 2000;148:1063–1074. doi: 10.1083/jcb.148.5.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter K, Kiefer KP, Grzesik BA, Clauss WG, Fronius M. Hydrostatic pressure activates ATP-sensitive K+ channels in lung epithelium by ATP release through pannexin and connexin hemichannels. FASEB J. 2014;28:45–55. doi: 10.1096/fj.13-229252. [DOI] [PubMed] [Google Scholar]
- Sabirov RZ, Dutta AK, Okada Y. Volume-dependent ATP-conductive large-conductance anion channel as a pathway for swelling-induced ATP release. J Gen Physiol. 2001;118:251–266. doi: 10.1085/jgp.118.3.251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saez JC, Schalper KA, Retamal MA, Orellana JA, Shoji KF, Bennett MV. Cell membrane permeabilization via connexin hemichannels in living and dying cells. Exp Cell Res. 2010;316:2377–2389. doi: 10.1016/j.yexcr.2010.05.026. [DOI] [PubMed] [Google Scholar]
- Scheckenbach KE, Crespin S, Kwak BR, Chanson M. Connexin channel-dependent signaling pathways in inflammation. J Vasc Res. 2011;48:91–103. doi: 10.1159/000316942. [DOI] [PubMed] [Google Scholar]
- Shi L, Xu M, Liu J, Zhang Z, Bao Z, Wang Y, et al. K(ATP) channels are involved in regulatory volume decrease in rat cardiac myocytes. Physiol Res. 2009;58:645–652. doi: 10.33549/physiolres.931594. [DOI] [PubMed] [Google Scholar]
- Sim JA, Broomhead HE, North RA. Ectodomain lysines and suramin block of P2X1 receptors. J Biol Chem. 2008;283:29841–29846. doi: 10.1074/jbc.M802523200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skals M, Jorgensen NR, Leipziger J, Praetorius HA. α-Hemolysin from Escherichia coli uses endogenous amplification through P2X receptor activation to induce hemolysis. PNAS. 2009;106:4030–4035. doi: 10.1073/pnas.0807044106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto F, Lambrecht G, Nickel P, Stuhmer W, Busch AE. Antagonistic properties of the suramin analogue NF023 at heterologously expressed P2X receptors. Neuropharmacology. 1999;38:141–149. doi: 10.1016/s0028-3908(98)00158-0. [DOI] [PubMed] [Google Scholar]
- St Aubin CN, Zhou JJ, Linsdell P. Identification of a second blocker binding site at the cytoplasmic mouth of the cystic fibrosis transmembrane conductance regulator chloride channel pore. Mol Pharmacol. 2007;71:1360–1368. doi: 10.1124/mol.106.031732. [DOI] [PubMed] [Google Scholar]
- Stout CE, Costantin JL, Naus CC, Charles AC. Intercellular calcium signaling in astrocytes via ATP release through connexin hemichannels. J Biol Chem. 2002;277:10482–10488. doi: 10.1074/jbc.M109902200. [DOI] [PubMed] [Google Scholar]
- Stratmann J, Scheer J, Ryan CA. Suramin inhibits initiation of defense signaling by systemin, chitosan, and a beta-glucan elicitor in suspension-cultured Lycopersicon peruvianum cells. Proc Natl Acad Sci U S A. 2000;97:8862–8867. doi: 10.1073/pnas.97.16.8862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan KA, Kim B, Buzdon M, Feldman EL. Suramin disrupts insulin-like growth factor-II (IGF-II) mediated autocrine growth in human SH-SY5Y neuroblastoma cells. Brain Res. 1997;744:199–206. doi: 10.1016/S0006-8993(96)01078-5. [DOI] [PubMed] [Google Scholar]
- Taouil K, Hannaert P. Evidence for the involvement of K+ channels and K(+)-Cl- cotransport in the regulatory volume decrease of newborn rat cardiomyocytes. Pflugers Arch. 1999;439:56–66. doi: 10.1007/s004249900117. [DOI] [PubMed] [Google Scholar]
- Thompson RJ, Zhou N, MacVicar BA. Ischemia opens neuronal gap junction hemichannels. Science. 2006;312:924–927. doi: 10.1126/science.1126241. [DOI] [PubMed] [Google Scholar]
- Voogd TE, Vansterkenburg EL, Wilting J, Janssen LH. Recent research on the biological activity of suramin. Pharmacol Rev. 1993;45:177–203. [PubMed] [Google Scholar]
- Walev I, Martin E, Jonas D, Mohamadzadeh M, Muller-Klieser W, Kunz L, et al. Staphylococcal alpha-toxin kills human keratinocytes by permeabilizing the plasma membrane for monovalent ions. Infect Immun. 1993;61:4972–4979. doi: 10.1128/iai.61.12.4972-4979.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Jackson DG, Dahl G. The food dye FD&C Blue no. 1 is a selective inhibitor of the ATP release channel Panx1. J Gen Physiol. 2013;141:649–656. doi: 10.1085/jgp.201310966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley JS, Chen R, Jamieson GP. The ATP4- receptor-operated channel (P2Z class) of human lymphocytes allows Ba2+ and ethidium+ uptake: inhibition of fluxes by suramin. Arch Biochem Biophys. 1993;305:54–60. doi: 10.1006/abbi.1993.1392. [DOI] [PubMed] [Google Scholar]
- Yao J, Huang T, Fang X, Chi Y, Zhu Y, Wan Y, et al. Disruption of gap junctions attenuates aminoglycoside-elicited renal tubular cell injury. Br J Pharmacol. 2010;160:2055–2068. doi: 10.1111/j.1476-5381.2010.00860.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao H-B, Yu N, Fleming CR. Gap junctional hemichannel-mediated ATP release and hearing controls in the inner ear. Proc Natl Acad Sci U S A. 2005;102:18724–18729. doi: 10.1073/pnas.0506481102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhuang S, Lu B, Daubert RA, Chavin KD, Wang L, Schnellmann RG. Suramin promotes recovery from renal ischemia/reperfusion injury in mice. Kidney Int. 2009;75:304–311. doi: 10.1038/ki.2008.506. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Effects of knockdown of Cx43 on cellular uptake of EtBr following removal of extracellular Ca2+. NRK cells were treated with either control siRNA or siRNA against Cx43 for 48 h. Thereafter, cells were exposed to normal or Ca2+-free culture medium that contained 10 μM EtBr 15 min. The cellular uptake of EtBr was photographed (magnification, ×320).
Figure S2 Hypotonic stress-induced disruption of membrane integrity. NRK cells were pre-incubated with 10 μM calcein for 1 h. After that, they were exposed to distilled water for the indicated time intervals. Cell membrane integrity was evaluated by the retention of fluorescent dye calcein inside the cells under microscope (A; magnification, ×160) or the fluorescent intensity of the remaining cells under a fluorescent reader (B). Results in B are expressed as % of fluorescence relative to normal control (mean ± SE, n = 4). *P < 0.05 compared with untreated control.
Figure 3 Suppression of low Ca2+-induced activation of Akt by suramin and PPADS. NRK cells were pretreated with or without 300 μM suramin or 10 μM PPADS for 30 min before exposing to Ca2+-free culture medium for an additional 5 min. Cellular proteins were extracted and subjected to Western analysis for phosphorylation of Akt and β-actin. Note the similar potency of PPADS and suramin on Ca2+ deprivation-induced activation of Akt.
Table S1 Effects of suramin on cell membrane receptors, channels and adhesive molecules.


