Abstract
BACKGROUND AND PURPOSE
Vasopressin V1B receptor antagonists may be effective for the treatment of depression and anxiety and the objective of this study was to characterize the pharmacological profiles of two newly synthesized arginine vasopressin receptor 1B (V1B receptor) antagonists, TASP0233278 and TASP0390325.
EXPERIMENTAL APPROACH
We investigated the in vitro profiles of TASP0233278 and TASP0390325. In addition, the effect of TASP0390325 on the increase in plasma adrenocorticotropic hormone (ACTH) levels induced by corticotropin-releasing factor (CRF)/desmopressin (dDAVP) was investigated. We also investigated the antidepressant and anxiolytic profiles of TASP0233278 and TASP0390325 in animal models.
KEY RESULTS
Both TASP0233278 and TASP0390325 showed a high affinity and potent antagonist activity for V1B receptors. Oral administration of TASP0390325 antagonized the increase in plasma ACTH levels induced by CRF/dDAVP in rats, indicating that TASP0390325 blocks the anterior pituitary V1B receptor in vivo. Oral administration of TASP0233278 or TASP0390325 also exerted antidepressant effects in two models of depression (a forced swimming test and an olfactory bulbectomy model). Moreover, TASP0233278 improved depressive-like behaviour induced by repeated treatment with corticosterone, a model that has been shown to be resistant to treatment with currently prescribed antidepressants. In addition to depression models, TASP0233278 or TASP0390325 exerted anxiolytic effects in several anxiety models (social interaction, elevated plus-maze, stress-induced hyperthermia, separation-induced ultrasonic vocalization and sodium lactate-induced panic-like responses in panic-prone rats).
CONCLUSION
TASP0233278 and TASP0390325 are potent and orally active V1B receptor antagonists with antidepressant and anxiolytic activities in rodents.
Keywords: arginine vasopressin, V1B receptor antagonist, depression, anxiety, HPA axis
Introduction
The dysfunction of the hypothalamic–pituitary–adrenal (HPA) axis, which is caused by exposure to chronic stress, has been reported in patients with major depressive disorder (MDD) and panic disorder (PD) (Holsboer et al., 1984; Roy-Byrne et al., 1986). Patients with MDD also exhibit increased levels of CSF corticotropin-releasing factor (CRF) (Nemeroff et al., 1984), the primary hypothalamic factor driving stress-induced adrenocorticotropic hormone (ACTH) secretion from the anterior pituitary.
In addition to CRF, arginine vasopressin (AVP), a cyclic nonapeptide, has been considered to be a primary factor in the regulation of HPA axis activity (Aguilera and Rabadan-Diehl, 2000). AVP is linked to the activation of the HPA axis, and the expression level of AVP in the paraventricular nucleus is increased by both acute and repeated stress (Berkenbosch et al., 1989; Aguilera and Rabadan-Diehl, 2000). Moreover, clinically, both the plasma AVP levels and the level of AVP immunoreactivity in the paraventricular nucleus are elevated in patients with MDD, compared with healthy controls (Purba et al., 1996; van Londen et al., 1997), and AVP release is significantly correlated with anxiety symptom responses in healthy subjects challenged with an anxiogenic cholecystokinin-B agonist (Abelson et al., 2001). Thus, AVP seems to be a key component in stress-related disorders, such as depression and anxiety (for reviews, see Scott and Dinan, 1998; Zelena, 2012).
AVP binds specifically to the V1A, V1B and V2 receptors (for receptor nomenclature see Alexander et al., 2013) to exert its physiological effects (Antoni, 1984; Lolait et al., 1992; Morel et al., 1992). Of these, the V1B receptor is of interest in light of its implications in emotional processes, based on the following findings. V1B receptor mRNA is expressed in the majority of anterior pituitary corticotrophs that secret ACTH; within the brain, significant populations can be found within the hypothalamus and limbic brain regions, which regulate stress and affect (Morel et al., 1992; Lolait et al., 1995). The involvement of V1B receptors in stress and emotional states was highlighted in reports that a V1B receptor antagonist, SSR149415, exhibits antidepressant and anxiolytic effects in several animal models of depression and anxiety, particularly under high-stress conditions (Griebel et al., 2002; Serradeil-Le Gal et al., 2002; Overstreet and Griebel, 2005). Collectively, the majority of previous evidence suggests that V1B receptor antagonists may be effective for the treatment of depression and anxiety. However, because SSR149415 has been reported to have an additional potent antagonist activity for the oxytocin (OT) receptor (Griffante et al., 2005), the antidepressant and anxiolytic potential of V1B receptor blockade needs to be further explored using other V1B receptor antagonists, including ones that display a high selectivity for the V1B receptor.
Recently, we synthesized two non-peptide V1B receptor antagonists, (4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxyphenyl)-2-oxo-2,3-dihydro-1H-indol-3-yl]-4-fluoro-N,N-dimethyl-L-prolinamide (TASP0233278) and 2-[2-(3-chloro-4-fluorophenyl)-6-[3-(morpholin-4-yl)propoxy]-4-oxopyrido[2,3-d]pyrimidin-3(4H)-yl]-N-isopropylacetamide hydrochloride (TASP0390325), which have different scaffolds (Figure 1). Using these V1B receptor antagonists, we have provided additional evidence that V1B receptor antagonists exhibit antidepressant and anxiolytic effects in several animal models, presumably through the blockade of the anterior pituitary V1B receptor. We further investigated the potential of V1B receptor antagonists in animal models that mimic pathological conditions.
Figure 1.
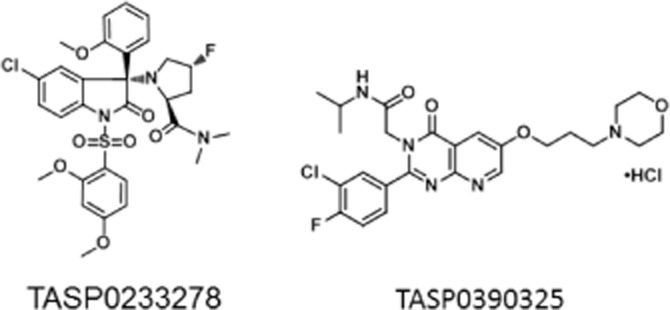
Chemical structures of TASP0233278 and TASP0390325.
Methods
Animals
Male Sprague-Dawley (SD) rats (211–246 g) were used for membrane preparation from the rat anterior pituitaries, the forced swimming test (FST), the elevated plus-maze (EPM) test, an ACTH secretion study, and the examination of locomotor activity. Male SD rats (250–330 g) were also used for the social interaction (SI) test and the panic-like responses. Male Wistar rats (301–404 g) were used for the olfactory bulbectomy model. Male SD rats (beginning of the experiments, 74–87 g) were used for repeated corticosterone (CORT) treatments. After treatment with CORT or saline for 21 consecutive days, the rats (225–313 g) were subjected to a FST. Male Institute of Cancer Research mice (25–32 g) were used for the stress-induced hyperthermia (SIH) test and to evaluate locomotor activity. Male SD rat pups (21–30 g) were used for the separation-induced ultrasonic vocalization (SIV). The total number of animals used was 678 (the number of animals in each experimental group was 4 to 10). All the animals were purchased from Charles River (Yokohama, Japan) and were maintained under a controlled temperature (23 ± 3°C) and humidity (50 ± 20%) with a 12 h light/dark cycle (lights on at 07:00). Food and water were available ad libitum except during the test. All the studies were conducted in accordance with Taisho Pharmaceutical Co., Ltd. Animal Care Committee. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (McGrath et al., 1997; Kilkenny et al., 2011).
Chemicals
TASP0233278 and TASP0390325 were synthesized at Taisho Medicinal Research Laboratories (Saitama, Japan). [Arg8]-Vasopressin (AVP) was purchased from Peptide Institute, Inc. (Osaka, Japan). OT acetate salt hydrate, [deamino-Pen1, O-Me-Tyr2, Arg8]-Vasopressin [dPTyr(Me)AVP], CORT, l-allylglycine (l-AG), and sodium lactate were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Fluvoxamine, chlordiazepoxide (CDP), and diazepam were purchased from Wako Pure Chemical Industries, Ltd. (Osaka, Japan). [3H]-AVP (2431 GBq·mmol−1) and [3H]-OT (1676 GBq·mmol−1) were purchased from PerkinElmer, Inc. (St. Waltham, MA, USA). For the in vitro studies, TASP0233278, TASP0390325 and all the other chemicals were dissolved in DMSO. For the in vivo studies, TASP0233278 was suspended in 5% cremophor EL/0.03 M HCl. TASP0390325 or CDP was suspended in 0.5% methylcellulose 400. Fluvoxamine was dissolved in saline. Diazepam was dissolved in saline with 0.3% polyoxyethylene glycol sorbitan monooleate (Tween 80). CORT was suspended in saline with 0.3% Tween 80.
Membrane preparation from rat anterior pituitaries
Membranes prepared from male SD rat anterior pituitaries were used to evaluate the affinities of the tested compounds for rat V1B receptors. The tissues were homogenized in homogenization buffer [10 mM HEPES (pH 7.4), 250 mM sucrose, 10 mM MgCl2, 1 mM EDTA, 100 μM PMSF and protein inhibitor cocktail (cOmplete; Roche Applied Science, Penzberg, Germany)], and the homogenate was centrifuged at 190× g for 5 min at 4°C. The supernatant was centrifuged at 48 000× g for 20 min at 4°C, and the resulting pellets were suspended in homogenization buffer. These steps were repeated twice, and the membranes obtained were suspended in a small volume of homogenization buffer. Aliquots of the membranes were stored at −80°C until used.
Binding studies for V1B, V1A, V2 and OT receptors
Human V1B, V1A and V2 receptor-expressing 1321-N1 cell membranes were purchased from PerkinElmer, Inc. Human OT receptor-expressing Chem-1 cell membranes were purchased from Millipore (Billerica, MA, USA). Membrane preparations from cells or rat tissues were suspended in assay buffer [50 mM Tris-HCl (pH 7.4), 10 mM MgCl2 and 0.1% BSA]. The reactions with the test compounds were started by the addition of [3H]-AVP (final concentration, 0.4 nM) for human and rat V1B receptor, human V1A receptor, human V2 receptor, or [3H]-OT (final concentration, 0.5 nM) for human OT receptor, and the reaction mixtures were incubated for 60 min at room temperature. The reaction was terminated by rapid filtration under vacuum through a UniFilter GF/C microplate presoaked with 0.3% polyethyleneimine using a UniFliter96 harvester (PerkinElmer, Inc.). The filters were washed three times with about 0.3 mL of 50 mM Tris-HCl buffer containing 10 mM MgCl2, and the filter-bound radioactivity level was assessed. Non-specific binding was determined in the presence of 10 μM AVP (for V1B, V1A and V2 receptors) or 10 μM OT (for OT receptors).
AVP-induced [Ca2+]i assay in CHO-K1 cells expressing human V1B receptors
CHO-K1 cells stably expressing human V1B receptors seeded in 96-well black/clear bottom plates were incubated with loading buffer [10 mM HEPES-buffered HBSS (pH 7.4) containing 0.1% BSA, 0.25 mg·mL−1 amaranth, 1.25 mM probenecid, 0.02% pluronic F-127 and 1.5 μM Fluo-4/AM] for 1 h in a 5% CO2 incubator. The loading buffer was switched to 10 mM HEPES-buffered HBSS (pH 7.4) containing 0.1% BSA, 0.25 mg·mL−1 amaranth, 1.25 mM probenecid, and several concentrations of the test compounds, and the cells were then incubated for 30 min in a 5% CO2 incubator. The maximum ratio of fluorescence emission evoked by 2.5 nM AVP-induced increase in [Ca2+]i was measured using the Functional Drug Screening System (Hamamatsu Photonics, Shizuoka, Japan). When the concentration-response data for AVP-induced increase in [Ca2+]i was determined, AVP was added in the absence of the test compounds. When the effects of TASP0233278 and TASP0390325 were investigated, the maximum ratio of fluorescence emission was measured immediately after addition of the compound.
CRF/desmopressin (dDAVP)-induced increase in plasma ACTH in rats
Rats were anaesthetized with pentobarbital (40 mg·kg−1, i.p.). The depth of anaesthesia was assessed by monitoring the response to pinching the hindleg of the anaesthetized rats using forceps. Experts confirmed the response of rats, and we used rats with no response to pinching for the operation. The catheter was inserted into the right jugular vein. Two days after catheter implantation, CRF (0.3 μg·kg−1) and dDAVP (0.5 mg·kg−1) were injected through the vein catheter 50 and 60 min after the administration of TASP0390325 (0.3 and 1 mg·kg−1). Injections of CRF and dDAVP were performed at a flow rate of 0.2 mL·min−1 using infusion pumps (Harvard Apparatus, Holliston, MA, USA). In the control group, PBS containing 0.1% BSA was injected instead of CRF and dDAVP. Ten minutes after dDAVP or PBS injection, the animals were killed by decapitation. Blood was immediately centrifuged at 1200× g for 10 min at 4°C, and the plasma was collected. The plasma ACTH levels were then determined using an ACTH elisa Kit (MD Bioscience, St. Paul, MN, USA).
FST in rats
A FST was performed according to a previously reported method (Chaki et al., 2004,2004), and the effect of the test compounds was evaluated by measuring the period of immobility. Two sessions were conducted: an initial 15 min pretest, followed 24 h later by a 5 min test. TASP0233278 (0.3, 1 and 3 mg·kg−1), TASP0390325 (0.1, 0.3 and 1 mg·kg−1), or fluvoxamine (3 mg·kg−1) was administered p.o. during the period between these two sessions (right after the pretest and 1 h before the test). The test sessions were videotaped from the front of the cylinders for later scoring. Floating in the water without struggling and only making movements necessary to keep the head above the water were regarded as immobility.
Repeated CORT injection model
Rats were injected daily with CORT (20 mg·kg−1) or a vehicle for 21 days. CORT treatment was conducted according to a previously reported method (Iijima et al., 2010). The FST was performed 48 h after the final injection, and the effect of TASP0233278 was evaluated by measuring the immobility time. In the present study, the rats were tested in the swim tank only once, as we sought to assess helplessness induced by prior exposure to CORT injections, rather than prior exposure to the swim task itself. TASP0233278 (3 mg·kg−1) was administered p.o. 24 and 1 h before the FST.
Olfactory bulbectomy model
The rats were anaesthetized with pentobarbital (40 mg·kg−1, i.p.). Removal of the olfactory bulbs was performed by aspiration using a pipette, which was placed according to the rat brain atlas of Paxinos and Watson (1986), for both olfactory bulbs. Sham-operated rats were treated in the same way, including piercing of the dura mater, but their bulbs were left intact. Hyperemotionality in the olfactory bulcetomized (OB) rats was measured by scoring the emotional responses to (i) air blowing on the dorsum; (ii) a rod presented in front of the snout; (iii) resistance upon capturing and handling; and (iv) reaction upon tail pinch. These responses were graded as follows: 0, no reaction; 1, slight; 2, moderate; 3, marked; or 4, extreme response (Yamamoto et al., 1982; Shibata et al., 1984). For each emotional response, vocalization during the test was also scored and graded as follows: 0, no vocalization; 1, occasional vocalization; or 2, marked vocalization. After a 14 to 28 day postsurgical period, the emotional responses were measured. For the acute administration, TASP0390325 (0.1 and 0.3 mg·kg−1) was administered p.o. 1 h before the measurement of the emotional responses. For the chronic administration, TASP0390325 (0.1 and 0.3 mg·kg−1) was administered p.o. once daily for 14 days, and the emotional responses were measured 1 h after the final administration.
SI test in rats
This test was performed in an open-field apparatus placed in an illuminated room (300 lux). The apparatus was an open-topped vinyl chloride cage (45 × 27 × 30 cm) with a solid floor. A camera was mounted above the arena. The rats were allocated to a test pair based on weight. Both members of a pair were given the same drug treatment. In this test, the rats were placed in the test arena for a 10 min trial, and the following behaviours were scored as SI: sniffing, following, social grooming, and crawling under and over. TASP0233278 (0.1, 0.3, 1 and 3 mg·kg−1) or CDP (4 mg·kg−1) was administered p.o. 1 h before the test.
Sodium lactate-induced acute panic-like responses in rats
Sodium lactate-mediated acute panic-like responses were induced according to a previously reported method (Shekhar et al., 1996). We placed cannulae in the dorsomedial hypothalamus (DMH; coordinates in relation to bregma being 1.2 mm posterior, 2.1 mm lateral, 9.0 mm ventral) and connected them to an osmotic minipump (Alzet Model; Durect Corporation, Cupertino, CA, USA) filled with l-AG (a GAD inhibitor) solution (3.5 nmol·0.5 μL·h−1). Rats received i.v. infusions (10 mL over 15 min) of 0.5 M sodium lactate at least 5 days after the initiation of the l-AG infusions. TASP0233278 or CDP was administered p.o. 1 h before the i.v. infusion of sodium lactate, and panic-like responses, such as anxiety-like behaviour and an increased frequency of breaths, were assessed using the SI and whole-body barometric plethysmography (Buxco Research Systems, Wilmington, NC, USA) respectively. The frequency of breaths in the rats min−1 was measured in unrestrained rats using whole-body barometric plethysmography, as described previously in detail (Hamelmann et al., 1997). Briefly, rats were placed individually in the plethysmograph chambers, and the baseline respiratory rate was recorded and averaged for 5 min. The animals were then removed from the chambers, treated p.o. with TASP0233278 (1 and 3 mg·kg−1) or CDP (4 mg·kg−1), and returned to the plethysmographs. Subsequently, the respiratory rate was measured for 10 min.
EPM test in rats
This test was conducted according to a previously reported method (Chaki et al., 2004,2004). Rats were placed in a 40 cm tall, 20 cm wide cylindrical plastic container containing 25 cm of water maintained at 25°C for 2 min. Rats were then removed from the tank and allowed to recover for a further 5 min before the EPM test was performed. The apparatus consisted of a plus-shaped maze, elevated 50 cm from the floor, with two opposite open arms, each 50 × 10 cm, crossed at right angles by two arms of the same dimensions, but enclosed by 40 cm high walls with an open roof. In addition, a 1 cm high edge made of Plexiglas surrounded the open arms to prevent falls. The illumination measured at the centre of the maze was 40 lux. The amount of time spent in the open arms of the maze was recorded using a video tracking system (Muromachi Kikai, Tokyo, Japan). Rats were naive to the apparatus. We previously demonstrated that diazepam (3 mg·kg−1, p.o.) reversed the reduction of time spent in open arm under identical conditions (data not shown). TASP0233278 (0.3, 1 and 3 mg·kg−1) was administered p.o. 1 h before the swim stress.
SIV of rat pups
The procedure was adapted from that described by Olivier et al. (1998). On the day of the experiment, rat pups were injected i.p. with TASP0233278 (3, 10 and 30 mg·kg−1) or fluvoxamine (1 mg·kg−1). Immediately following the injection, each pup was returned to its mother and littermates until the test. Thirty minutes later, each pup was individually placed in a 500 mL glass beaker lined with filter paper (φ70 mm) located below a microphone in a sonically isolated box (60 × 70 × 60 cm). The number of vocalizations during the 5 min test period was detected using a UMA2 amplifier (Muromachi Kikai, Tokyo, Japan), and a sound pressure of 60 dB was set as the threshold. At the end of the test, the pups were returned to their mothers and littermates.
SIH test in mice
The test was performed according to a previously reported method (Spooren et al., 2002). In this test, the rectal temperature was recorded in singly housed mice at two consecutive time points (T1 and T2), which were interspaced by a defined time interval. Because the value of the second temperature recording exceeded the value of the initial measure (mirroring the stress reaction), the difference between the two core temperatures reflects SIH. Body temperature was measured in each mouse twice, at t = 0 min (T1) and t = +15 min (T2). The difference in temperature (T2 – T1) was considered to reflect the SIH. The rectal temperature was measured using a thermometer (BWT-100; Bio Research Center, Nagoya, Japan). TASP0233278 (3, 10 and 30 mg·kg−1), TASP0390325 (3, 10 and 30 mg·kg−1), or diazepam (1 mg·kg−1) was administered p.o. 1 h before the test (T1).
Spontaneous locomotor activity in rats and mice
Rats or mice were housed individually in transparent acrylic cages (47 × 28.5 × 29.5 cm for rats, φ30 cm × 30 cm for mice), and spontaneous locomotor activity was recorded for 30 min using a SCANET apparatus (Neuroscience Inc., Tokyo, Japan) placed in a sound-proof box. TASP0233278 (3, 10 and 30 mg·kg−1) or TASP0390325 (3, 10 and 30 mg·kg−1) was administered p.o. 1 h before the test.
Statistical analysis
All the data were analysed using a one-way anova or two-way anova. Subsequent comparisons between the vehicle and treatment groups were performed using Student's t-test or Dunnett's test. All the data were analysed using SAS System Version 9.2 (SAS Institute Japan, Ltd., Tokyo, Japan).
Results
Affinities and antagonist activities of TASP0233278 and TASP0390325 for V1B receptors
The in vitro profiles of TASP0233278 and TASP0390325 are summarized in Table 1. Both TASP0233278 and TASP0390325 dose-dependently inhibited [3H]-AVP binding to recombinant human V1B receptors, with IC50 values of 4.85 (95% confidential interval; 3.36–6.99) and 2.72 (95% confidential interval; 2.05–3.59) nM respectively (Table 1, Figure 2). In addition, both TASP0233278 and TASP0390325 also inhibited [3H]-AVP binding to rat anterior pituitary membranes, with IC50 values of 3.98 (95% confidential interval; 3.00–5.28) and 2.22 (95% confidential interval; 1.68–2.94) nM, respectively (Table 1, Figure 2), indicating that no difference between species exists. The antagonist activities of TASP0233278 and TASP0390325 at the human V1B receptor were assessed by quantifying the inhibition of AVP-increased [Ca2+]i in CHO-K1 cells stably expressing human V1B receptors. In CHO-K1 cells stably expressing human V1B receptors, AVP concentration-dependently increased [Ca2+]i with EC50 value of 1.74 nM (Figure 3A). TASP0233278 and TASP0390325 potently attenuated the 2.5 nM AVP-induced increase in [Ca2+]i, with IC50 values of 19.0 (95% confidential interval; 12.7–28.3) and 20.2 (95% confidential interval; 14.1–28.8) nM, respectively (Table 1, Figure 3B), while they did not change the basal [Ca2+]i up to 10 μM (data not shown).
Table 1.
In vitro profiles of TASP0233278 and TASP0390325
| IC50 (nM) | ||||||
|---|---|---|---|---|---|---|
| [3H]-AVP binding | [3H]-OT binding | [Ca2+]i | ||||
| – | hV1BR | rV1BR (pituitary) | hV1AR | hV2R | hOTR | hV1BR |
| TASP0233278 | 4.85 | 3.98 | 286 | 511 | 45.8 | 19.0 |
| TASP0390325 | 2.72 | 2.22 | >10 000 | >10 000 | >10 000 | 20.2 |
| AVP | 0.467 | 0.864 | 0.591 | 0.998 | – | – |
| OT | – | – | – | – | 0.567 | – |
| dPTry(Me)AVP | – | – | – | – | – | 2310 |
Data represent mean value obtained from three separate experiments, each done in duplicate.
Figure 2.
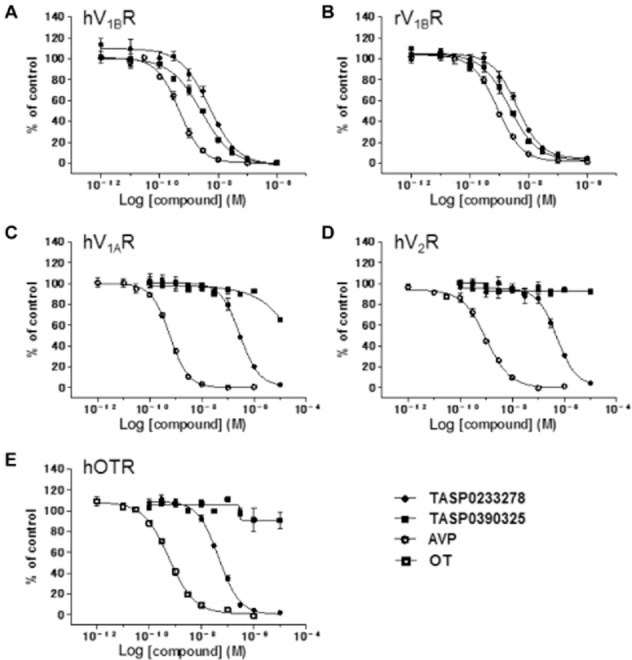
Inhibition of radioligand-specific binding to V1B, V1A, V2 and OT receptors by TASP0233278 and TASP0390325. Effect of TASP0233278, TASP0390325, AVP and OT was determined by [3H]-AVP binding to human V1B receptors (A), rat V1B receptors (B), human V1A (C) or V2 (D) receptors, and by [3H]-OT binding to human OT receptors (E). Data represent mean value obtained from three separate experiments.
Figure 3.
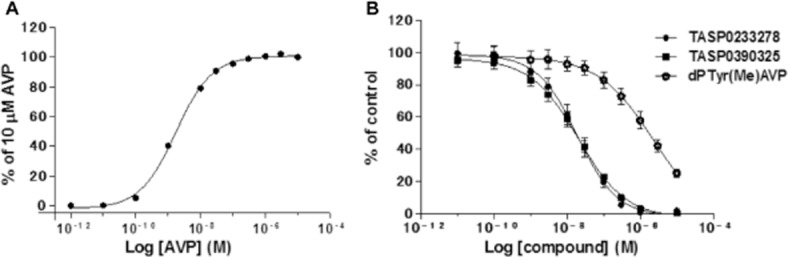
Concentration–response curve of AVP-induced increase in [Ca2+]i in CHO-K1 cells stably expressing human V1B receptors (A) and inhibition of AVP-induced increase in [Ca2+]i by TASP0233278 and TASP0390325 (B). Data represent mean value obtained from two (A) and three separate experiments (B).
Selectivity of TASP0233278 and TASP0390325
The selectivity of TASP0233278 and TASP0390325 over other vasopressin receptor subtypes (V1A and V2 receptors) and OT receptos was determined using [3H]-AVP binding to human V1A and V2 receptors and [3H]-OT binding to human OT receptors respectively. TASP0233278 weakly inhibited ligand binding to human V1A and V2 receptors, and human OT receptors, with IC50 values of 286, 511 and 45.8 nM respectively (Table 1, Figure 2). In contrast, TASP0390325 did not exhibit an affinity for any of these receptors, even at 10 μM. Moreover, TASP0390325 did not show a significant affinity for 85 other molecules, including receptors, transporters and ion channels at 10 μM (data not shown). Thus, TASP0390325 is selective for V1B receptors, while TASP0233278 shows weak affinities for V1A, V2 and OT receptors, in addition to V1B receptors.
Effects of TASP0390325 on CRF/dDAVP-induced plasma ACTH in rats
AVP and analogues of AVP (e.g. dDAVP) potentiate the effect of CRF on the stimulation of ACTH secretion through V1B receptors. In vivo receptor antagonism of the anterior pituitary V1B receptor with TASP0390325 was evaluated by measuring the inhibition of CRF/dDAVP-induced increases in plasma ACTH in rats. The vehicle-treated group showed a significant increase in the plasma ACTH levels, compared with the control group, indicating that injections of CRF and dDAVP elevated the plasma ACTH levels (Figure 4). TASP0390325 significantly antagonized the increase in the plasma ACTH level at a dose of 1 mg·kg−1 (Figure 4). In contrast, TASP0390325 (0.3 and 1.0 mg·kg−1) itself did not significantly affect basal ACTH levels (vehicle, 49.3 ± 11.7 pg·mL−1; 0.3 mg·kg−1, 37.4 ± 9.6 pg·mL−1; 1 mg·kg−1, 16.8 ± 4.8 pg·mL−1) [F(2, 19) = 3.04, P = 0.07].
Figure 4.
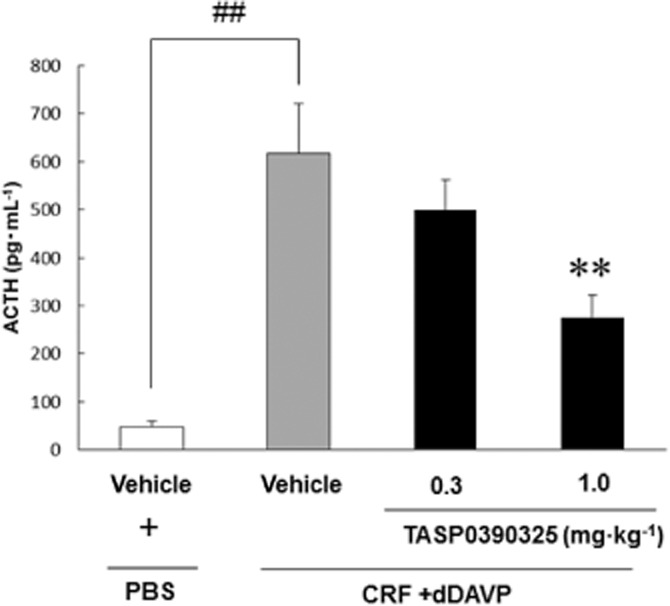
Effect of TASP0390325 on the increase in plasma ACTH levels induced by CRF/dDAVP injections in rats. The vertical axis shows the concentration of ACTH (pg·mL−1). All data are expressed as the mean ± SEM (n = 7–8). ##P < 0.01 indicates significant effects compared with the vehicle-treated PBS group (Student's t-test). **P < 0.01 indicates significant effects compared with the vehicle-treated CRF/dDAVP group (Dunnett's test).
Effects of TASP0233278 and TASP0390325 during the FST in rats
Fluvoxamine (3 mg·kg−1) significantly reduced the immobility time in the FST in rats (Figure 5A and B). Likewise, both TASP0233278 [F(3, 28) = 5.41, P < 0.01] and TASP0390325 [F(3, 36) = 6.01, P < 0.01] significantly reduced the immobility time in the FST in rats (Figure 5A and B).
Figure 5.
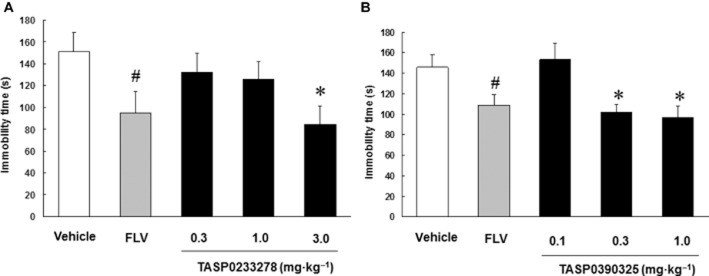
Effects of TASP0233278 (A) and TASP0390325 (B) in the FST in rats. The vertical axis shows the immobility time (s). All data are expressed as the mean ± SEM (n = 8–10). FLV, fluvoxamine. (A) One-way anova: [F(3, 28) = 5.41, P < 0.01]. (B) One-way anova: [F(3, 36) = 6.01, P < 0.01]. #P < 0.05 indicates significant effects compared with the vehicle (Student's t-test). *P < 0.05 indicates significant effects compared with the vehicle (Dunnett's test).
Effect of TASP0233278 on the repeated CORT injections-increased immobility time during the FST in rats
Repeated CORT injections significantly increased the immobility time in the FST in rats, compared with a vehicle-treated group. A two-way anova revealed a significant interaction between the repeated CORT treatment and TASP0233278 (3 mg·kg−1) [F(1, 36) = 5.81, P < 0.05]. Thus, TASP0233278 (3 mg·kg−1) significantly reduced the increase in the immobility time induced by the repeated CORT injections (Figure 6). TASP0233278 (3 mg·kg−1) did not affect the immobility time in the vehicle-treated group (Figure 6).
Figure 6.
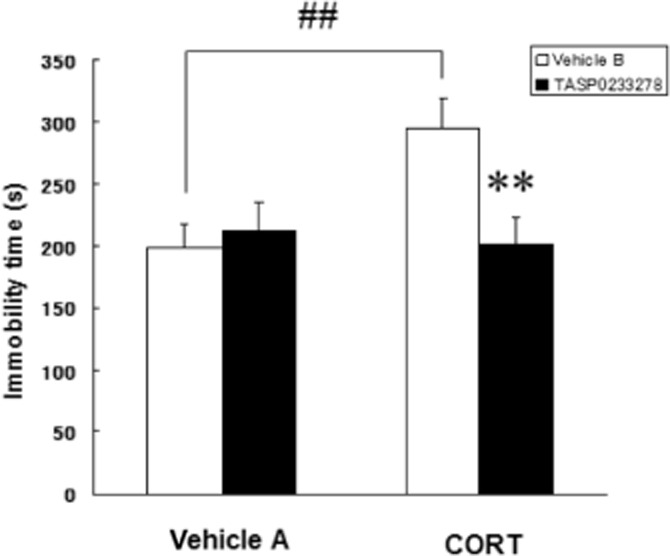
Effect of TASP0233278 on immobility time, during the FST, induced by repeated CORT injections in rats. The vertical axis shows the immobility time (s). vehicle A: 0.3% Tween 80, vehicle B: 5% cremophor EL/0.03 M HCl. All data are expressed as the mean ± SEM (n = 10). Two-way anova: [F(1, 36) = 5.81, P < 0.05]. ##P < 0.01 indicates significant effects compared with the vehicle A (Student's t-test). **P < 0.01 indicates significant effects compared with the CORT-treated vehicle B (Student's t-test).
Effects of TASP0390325 on olfactory bulbectomy-induced hyperemotionality
Acute treatment with TASP0390325 [F(3, 31) = 0.41, P = 0.75] did not affect hyperemotionality in OB rats (Figure 7A). Chronic treatment (14 days) with TASP0390325 (0.3 mg·kg−1) [F(3, 31) = 9.3, P < 0.01] significantly reduced hyperemotionality in OB rats (Figure 7B).
Figure 7.
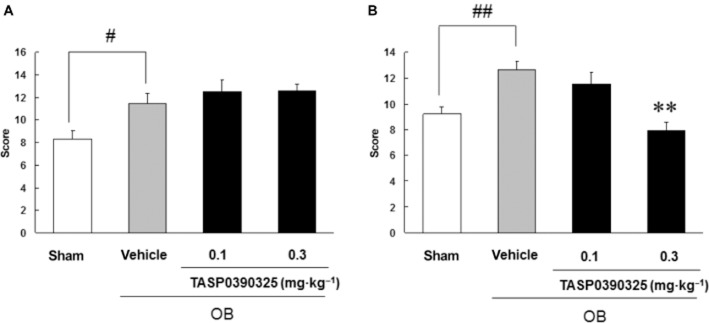
Effects of acute administration (A) or a 14 day repeated administration (B) of TASP0390325 on the hyperemotionality in the olfactory bulbectomized rats. The vertical axis shows the emotional response score. All data are expressed as the mean ± SEM (n = 8–10). (A) Two-way anova: [F(3, 31) = 0.41, P = 0.75] (B) Two-way anova: [F(3, 31) = 9.3, P < 0.01]. #P < 0.05 and ##P < 0.01 indicate significant effects compared with sham-operated group (Student's t-test). **P < 0.01 indicates significant effects compared with the vehicle-treated group (Dunnett's test).
Effect of TASP0233278 on the SI test in rats
CDP (4 mg·kg−1) significantly prolonged the SI of paired unfamiliar rats (Figure 8). Likewise, TASP0233278 significantly increased the SI time [F(4, 35) = 5.69, P < 0.01] (Figure 8).
Figure 8.
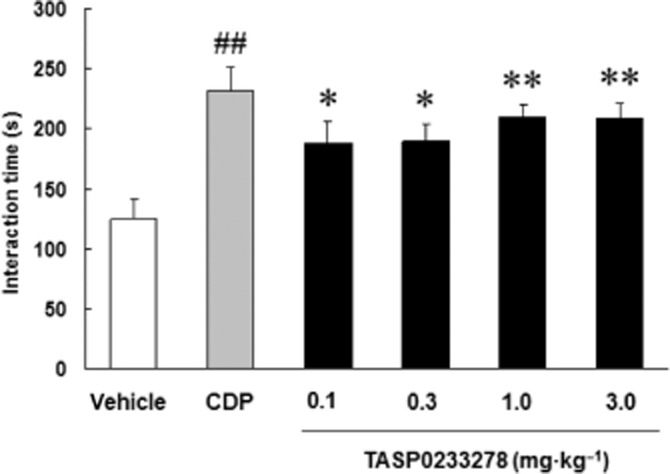
Effect of TASP0233278 in the SI test in rats. The vertical axis shows the interaction time (s). All data are expressed as the mean ± SEM (n = 8). One-way anova: [F(4, 35) = 5.69, P < 0.01]. ##P < 0.01 indicates significant effects compared with the vehicle (Student's t-test). *P < 0.05 and **P < 0.01 indicate significant effects compared with the vehicle (Dunnett's test).
Effects of TASP0233278 on sodium lactate-induced panic-like responses in rats
After 6 days of l-AG infusion into the DMH, the infusion of sodium lactate induced a significant increase in anxiety-like behaviour, as measured by a decreased interaction time in the SI test, as well as a significant increase in the respiratory rate ( Figure 9A and B). Thus, the infusion of sodium lactate induces panic-like responses. CDP (4 mg·kg−1) significantly blocked the lactate-induced decrease in the SI test (Figure 9A). Likewise, TASP0233278 (3 mg·kg−1) [F(2, 15) = 5.60, P < 0.05] significantly blocked the lactate-induced decrease in SI (Figure 9A). In addition to the attenuation of anxiety-like behaviour, CDP (4 mg·kg−1) significantly blocked the lactate-induced increase in the respiratory rate (Figure 9B). TASP0233278 [F(2, 12) = 3.47, P = 0.06] had a tendency to counteract the lactate-induced increase in the respiratory rate (Figure 9B). TASP0233278 (3 mg·kg−1) and CDP (4 mg·kg−1) did not affect the respiratory rate in the saline-treated group (data not shown).
Figure 9.
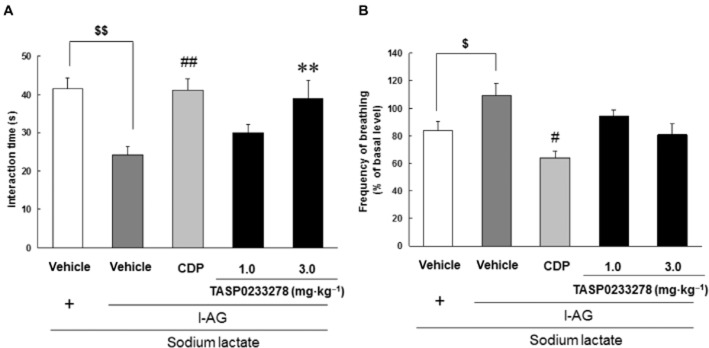
Effects of TASP0233278 on sodium lactate (0.5 M)-induced panic-like responses in rats. (A) Indicates the SI test. The vertical axis shows the interaction time (s). All data are expressed as the mean ± SEM (n = 5–7). (A) Two-way anova: [F(2, 15) = 5.60, P < 0.05]. $$P < 0.01 indicates significant effects compared with the saline-treated vehicle (Student's t-test). ##P < 0.01 indicates significant effects compared with l-AG-treated vehicle (Student's t-test), **P < 0.01 indicates significant effects compared with l-AG-treated vehicle (Dunnett's test). (B) Indicates the respiratory rate. The vertical axis shows the frequency of breathing (% of basal level). All data are expressed as the mean ± SEM (n = 4–6). (B) Two-way anova: [F(2, 12) = 3.47, P = 0.06]. $P < 0.05 indicates significant effects compared with the saline-treated vehicle (Student's t-test). #P < 0.05 indicates significant effects compared with l-AG-treated vehicle (Student's t-test).
Effect of TASP0233278 on anxiety-like behaviour in the EPM test in rats
Exposure to swim stress for 2 min significantly reduced the time spent in the open arms in the EPM task, showing that the rats were more anxious (Figure 10). TASP0233278 [F(3, 27) = 4.30, P < 0.05] reversed the anxiety induced by swim stress at 1 mg·kg−1 (Figure 10). We previously demonstrated that diazepam (3 mg·kg−1) reversed stress-induced changes under identical conditions (data not shown).
Figure 10.
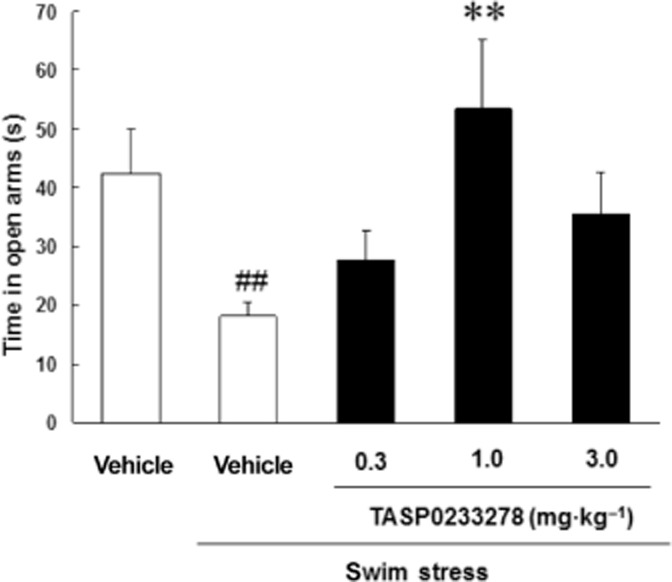
Effect of TASP0233278 on swim stress-induced reduction in time spent on open arms in EPM task in rats. The vertical axis shows the time in open arms (s). All data are expressed as the mean ± SEM (n = 8). One-way anova: [F(3, 27) = 4.30, P < 0.05]. ##P < 0.01 indicates significant effects compared with the vehicle-treated non-stress group (Student's t-test); **P < 0.01 indicates significant effects compared with vehicle-treated stress group (Dunnett's test).
Effect of TASP0233278 on SIV of rat pups
Fluvoxamine (1 mg·kg−1) significantly reduced ultrasonic vocalization induced by the separation of pups from their mother and littermates ( Figure 11). TASP0233278 [F(3, 36) = 2.5, P = 0.07] had a tendency to reduce ultrasonic vocalization, compared with vehicle groups (Figure 11).
Figure 11.
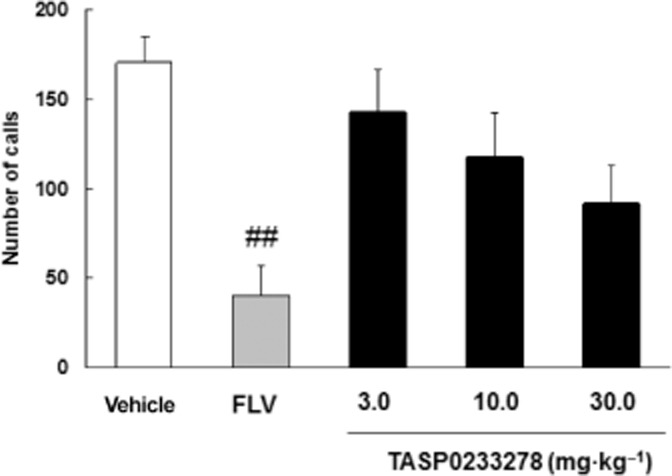
Effect of TASP0233278 on SIV in rat pups. The vertical axis shows the number of calls. All data are expressed as the mean ± SEM (n = 10). FLV, fluvoxamine. One-way anova: [F(3, 36) = 2.5, P = 0.07]. ##P < 0.01 indicates significant effects compared with vehicle (Student's t-test).
Effects of TASP0233278 and TASP0390325 in the SIH test in mice
Diazepam (1 mg·kg−1) attenuated SIH (Figure 12). TASP0233278 [F(3, 32) = 8.28, P < 0.01] also significantly reduced SIH at doses of 10 and 30 mg·kg−1 (Figure 12A). In contrast, TASP0233278 had no effect on the basal rectal temperatures (T1) at any of the doses tested (Figure 12A). TASP0390325 [F(3, 35) = 3.67, P < 0.05] significantly reduced SIH at doses of 3 and 30 mg·kg−1 (Figure 12B). TASP0390325 also had no effect on the basal rectal temperatures (T1) at any of the doses tested (Figure 12B).
Figure 12.
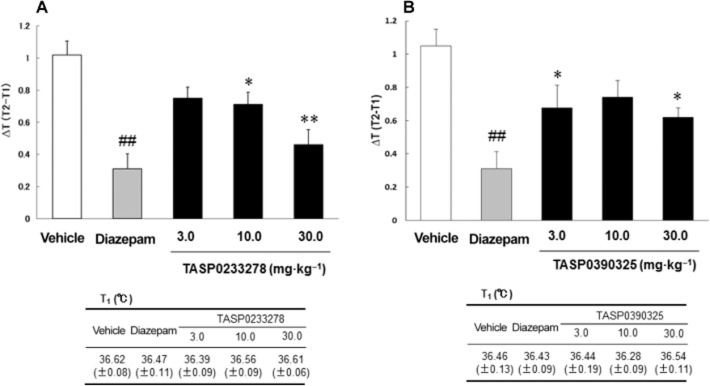
Effects of TASP0233278 (A) and TASP0390325 (B) on SIH in mice. The upper panels show ΔT (T2-T1) in the SIH test. The lower panels show basal rectal temperatures (T1). All data are expressed as the mean ± SEM (n = 9–12). (A) One-way anova: [F(3, 32) = 8.28, P < 0.01]. (b) One-way anova: [F(3, 35) = 3.67, P < 0.05]. ##P < 0.01 indicates significant effects compared with vehicle (Student's t-test). *P < 0.05 and **P < 0.01 indicate significant effects compared with vehicle (Dunnett's test).
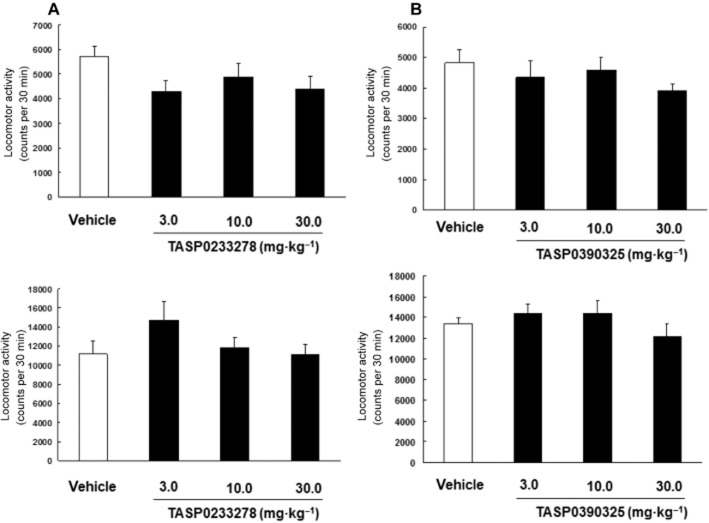
Effects of TASP0233278 and TASP0390325 on locomotor activity in rats and mice
TASP0233278 did not affect the spontaneous locomotor activity in rats [F(3, 27) = 1.85, P = 1.85] and mice [F(3, 27) = 1.85, P = 1.85] (Figure 13A). TASP0390325 also did not affect the spontaneous locomotor activity in rats [F(3, 28) = 0.86, P = 0.47] and mice [F(3, 35) = 1.1, P = 0.36] (Figure 13B). In addition, TASP0390325 did not affect the spontaneous locomotor activity in rats at lower doses (counts 30 min−1: vehicle, 5402.2 ± 464.5; 0.3 mg·kg−1, 5862.3 ± 480.1; 1 mg·kg−1, 5446.2 ± 284.5) [F(2, 27) = 0.37, P = 0.70].
Figure 13.
Effects of TASP0233278 (A) and TASP0390325 (B) on the locomotor activity in rats (upper panels) and mice (lower panels). The vertical axis displays the locomotor activity. All data are expressed as the mean ± SEM (n = 8–10). (A) One-way anova: rats [F(3, 27) = 1.85, P = 1.85] and mice [F(3, 27) = 1.85, P = 1.85]. (B) One-way anova: [F(3, 28) = 0.86, P = 0.47] and mice [F(3, 35) = 1.1, P = 0.36].
Discussion and conclusions
In the present study, we further demonstrated that V1B receptor blockade exhibited antidepressant and anxiolytic effects in a wide range of animal models using two newly synthesized V1B receptor antagonists that have different scaffolds, thereby strengthening the assumption that V1B receptors are involved in the pathophysiology of depression and anxiety.
Both TASP0233278 and TASP0390325 displayed a high affinity and potent antagonist activity for human and rat V1B receptors. Of these, TASP0233278 has the same scaffold as SSR149415, while TASP0390325 was derived from a scaffold different from that of SSR149415. Thus, TASP0233278 showed weak affinities for other vasopressin receptor subtypes, similar to SSR149415, while TASP0390325 was highly selective for the V1B receptor. Of note, we observed in a preliminary study that TASP0390325 shifted the concentration–response curve of AVP-induced increase in [Ca2+]i rightward without changing the maximal response (data not shown), indicating that TASP0390325 is a competitive antagonist. In addition, our pharmacokinetic studies revealed that both compounds were detected in the pituitary at reasonable concentrations following oral administration (data not shown). Moreover, we confirmed that orally administered TASP0390325 blocked V1B receptor in the anterior pituitary in vivo by demonstrating that TASP0390325 antagonized the CRF/dDAVP-induced increase in plasma ACTH level in rats, which also raises the possibility that this compound may block HPA axis activation. It should be noted that TASP0390325 exerted the effect at a lower dose compared with SSR149415, although TASP0390325 has a similar affinity and antagonist activity for rat V1B receptors as SSR149414 (Serradeil-Le Gal et al., 2002), indicating improved pharmacokinetic profiles. Using these compounds as pharmacological tools, we explored the efficacy of V1B receptor antagonists in animal models of depression and anxiety.
The antidepressant potentials of TASP0233278 and TASP0390325 were examined in two widely used depression models, the FST and the olfactory bulbectomy model. Both TASP0233278 and TASP0390325 elicited antidepressant-like effects in the FST. In addition, TASP0390325 reduced the hyperemotionality induced by an olfactory bulbectomy after 14 days of repeated dosing. The present results are consistent with a previous report that a V1B receptor antagonist, SSR149415, had antidepressant-like effects in these animal models (Griebel et al., 2003; 2005,; Iijima and Chaki, 2007). Of note, TASP0390325 exhibited an antidepressant effect at doses similar to those required for the blockade of V1B receptors in the anterior pituitary in vivo, suggesting that TASP0390325 exerted its effect through the blockade of anterior pituitary V1B receptors. Moreover, both compounds did not affect locomotor activity at doses much higher than those required to exert the antidepressant effects, indicating that the antidepressant effects are not attributed to altered locomotion.
The present findings that two different V1B receptor antagonists with different scaffolds, including one with a high selectivity for V1B receptors, exhibited antidepressant effects in animal models strengthen the assumption, which was mostly based on SSR149415 data, that V1B receptor blockade exerts antidepressant effects.
In addition to the models mentioned earlier, the antidepressant effect of TASP0233278 was further evaluated in a repeated CORT treatment model of rats using immobility time in the FST as an endpoint. This model represents a depression model associated with a severe impairment of HPA axis function and has been shown to be resistant to treatment with clinically prescribed antidepressants (Iijima et al., 2010). TASP0233278 counteracted the increase in the immobility time induced by repeated CORT injections, suggesting that V1B receptor antagonists might be an effective treatment for depressed patients, particularly those with severely impaired HPA axis function.
The anxiolytic activities of TASP0233278 and TASP0390325 were examined in four anxiety models (EPM, SI, SIH, SIV), all of which have been used to evaluate the anxiolytic potential of compounds. TASP0233278 and/or TASP0390325 exhibited anxiolytic effects in these anxiety models. SSR149415 has been reported to display anxiolytic effects in anxiety models that were similar or identical to those used in the present study, although TASP0233278 and TASP0390325 exerted more potent effects than SSR149415 (Serradeil-Le Gal et al., 2002; Griebel et al., 2003; Iijima and Chaki, 2005; Shimazaki et al., 2006). Therefore, the present results support the previous findings that a V1B receptor antagonist exhibits anxiolytic effects. In the anxiety models used in the present study, anxiety-like behaviours or events are induced by various stress (forced swim, encounter with unfamiliar species in a lightly lit arena, anticipation of uncomfortable insult, and separation from their mother and littermates); thus, these models involve high-stress conditions. Indeed, increased blood ACTH levels have been reported in these models (File and Clarke, 1980; Appenrodt et al., 1999; Heinrichs et al., 2002; Iijima and Chaki, 2005). Given that V1B receptor antagonists including SSR149415 have been reported to reduce AVP- or stress-induced elevations in ACTH secretion to a significant degree (Serradeil-Le Gal et al., 2002; Craighead et al., 2008), V1B receptor antagonists may reduce stress-induced anxiety by attenuating the stress-induced increase in ACTH level. We also reported that the anxiolytic effect of SSR149415 was no longer observed in hypophysectomized rats in the SI test (Shimazaki et al., 2006), suggesting that the blockade of HPA axis activity may be responsible for the effects of V1B receptor antagonists. Thus, this finding also supports the hypothesis that the regulation of HPA axis activity is involved in the anxiolytic effect of V1B receptor antagonists. However, a previous report also indicated that the basolateral amygdala is involved in the anxiolytic-like effects of SSR149415 in the EPM test (Salome et al., 2006). The sites of action of V1B receptor antagonists may possibly depend on the paradigms that are being tested. Alternatively, the V1A receptor, which is abundantly expressed in the brain, could be involved in the action of centrally injected SSR149415. Further studies are required to evaluate the sites of action underlying the anxiolytic effects of V1B receptor antagonists. One drawback of the present study was that most of the anxiolytic effects were induced with a less selective compound TASP0233278, because of limited amounts of the compound available, and the roles of V1A and OT receptors in anxiety and the involvement of these receptors in the actions of TASP0233278 cannot be fully ruled out. Therefore, the role of V1B receptors in anxiolytic potential remains to be further tested with more selective V1B receptor antagonists.
In addition to animal models of anxiety disorder, we examined the effect of TASP0233278 on sodium lactate-induced panic-like responses in rats. One of the most consistent abnormalities in PD is a susceptibility to panic attacks in response to normally innocuous stimuli, such as sodium lactate (Liebowitz et al., 1986) and the inhalation of 7.5% CO2 (Gorman et al., 1994). An animal model of panic attack has been previously reported to exhibit anxiety and panic-like responses following exposure to i.v. sodium lactate (Shekhar et al., 1996; Shekhar and Keim, 1997). In the present study, TASP0233278 as well as a benzodiazepine, CDP, counteracted the lactate-induced reduction in SI and the increase in respiratory frequency in panic-prone rats. In light of the present results and those of a previous report showing that a CRF1 receptor antagonist also blocked the lactate-induced behaviour and cardiovascular responses (Shekhar et al., 2011), compounds that act on the HPA axis may be useful for the treatment of PD.
Recent clinical studies have indicated that SSR149415 failed to satisfy the efficacy requirements in patients with MDD and generalized anxiety disorder (Griebel et al., 2012). The reason for these failures was not fully understood. The authors claimed that the SSR149415 doses that were used were not high enough; higher doses of SSR149415 were needed to block HPA axis activation in a CRF test. Of note, both TASP0233278 and TASO0390325 exerted antidepressant effects in animal models at lower doses compared with SSR149415 (Serradeil-Le Gal et al., 2002), which may be ascribed to improved pharmacokinetic profiles. Another plausible reason is the selectivity of SSR149415, as the antagonist activity of SSR149415 at OT receptors might counteract the antidepressant and anxiolytic effects of V1B receptor antagonists (Chaviaras et al., 2010; Ring et al., 2010). Moreover, based on the present results and previous reports, V1B receptor antagonists, but not monoaminergic antidepressants, may directly affect the abnormalities of the HPA axis through the blockade of V1B receptors in the anterior pituitary. Thus, among patients with MDD and anxiety disorders, V1B receptor antagonists may be effective in individuals who exhibit abnormalities in HPA axis function.
In conclusion, the present studies provided further evidence that the blockade of V1B receptors produces antidepressant- and anxiolytic-like effects in various rodent models (summarized in Table 2) and that V1B receptor antagonists may be useful for the treatment of depression and some anxiety disorders. In addition, TASP0233278 and TASP0390325 should provide useful tools to further elucidate the effectiveness of V1B receptor antagonists for the treatment of depression and anxiety disorders as well as the neuronal mechanisms underlying the antidepressant and anxiolytic effects of V1B receptor antagonists.
Table 2.
The effects of TASP0233278 and TASP0390325 in depression/anxiety models
| Model | TASP0233278 (MED) | TASP0390325 (MED) |
|---|---|---|
| Forced swimming (rat) | 3 mgkg–1 | 0.3 mgkg–1 |
| Repeated CORT injections (rat) | 3 mgkg–1 | NT |
| Olfactory bulbectomy (rat) | NT | 0.3 mgkg–1 |
| SI (rat) | 0.1 mgkg–1 | NT |
| Acute panic-like responses (rat) | 3 mgkg–1 (SI) / no effect (RR) | NT |
| EPM (swim stress) (rat) | 1 mgkg–1 | NT |
| SIV (rat pups) | No effect | NT |
| SIH (mice) | 10 mgkg–1 | 3 mgkg–1 |
MED, minimum effective dose; NT, not tested.
Glossary
- AVP
arginine vasopressin
- CDP
chlordiazepoxide
- CORT
corticosterone
- CRF
corticotropin-releasing factor
- dDAVP
desmopressin
- EPM
elevated plus-maze
- FST
forced swimming test
- HPA
hypothalamic-pituitary-adrenal
- MDD
major depressive disorder
- OT
oxytocin
- PD
panic disorder
- SIH
stress-induced hyperthermia
- SIV
separation-induced ultrasonic vocalization
Conflict of interest
None.
References
- Abelson JL, Le Melledo J, Bichet DG. Dose response of arginine vasopressin to the CCK-B agonist pentagastrin. Neuropsychopharmacology. 2001;24:161–169. doi: 10.1016/S0893-133X(00)00182-2. [DOI] [PubMed] [Google Scholar]
- Aguilera G, Rabadan-Diehl C. Vasopressinergic regulation of the hypothalamic–pituitary–adrenal axis: implications for stress adaptation. Regul Pept. 2000;96:23–29. doi: 10.1016/s0167-0115(00)00196-8. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ, CGTP Collaborators The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Antoni FA. Novel ligand specificity of pituitary vasopressin receptors in the rat. Neuroendocrinology. 1984;39:186–188. doi: 10.1159/000123976. [DOI] [PubMed] [Google Scholar]
- Appenrodt E, Kroning G, Schwarzberg H. Increased plasma ACTH in rats exposed to the elevated plus-maze is independent of the pineal gland. Psychoneuroendocrinology. 1999;24:833–838. doi: 10.1016/s0306-4530(99)00040-2. [DOI] [PubMed] [Google Scholar]
- Berkenbosch F, De Goeij DC, Tilders FJ. Hypoglycemia enhances turnover of corticotropin-releasing factor and of vasopressin in the zona externa of the rat median eminence. Endocrinology. 1989;125:28–34. doi: 10.1210/endo-125-1-28. [DOI] [PubMed] [Google Scholar]
- Chaki S, Nakazato A, Kennis L, Nakamura M, Mackie C, Sugiura M, et al. Anxiolytic- and antidepressant-like profile of a new CRF1 receptor antagonist, R278995/CRA0450. Eur J Pharmacol. 2004;485:145–158. doi: 10.1016/j.ejphar.2003.11.032. [DOI] [PubMed] [Google Scholar]
- Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, et al. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004;46:457–467. doi: 10.1016/j.neuropharm.2003.10.009. [DOI] [PubMed] [Google Scholar]
- Chaviaras S, Mak P, Ralph D, Krishnan L, Broadbear JH. Assessing the antidepressant-like effects of carbetocin, an oxytocin agonist, using a modification of the forced swimming test. Psychopharmacology (Berl) 2010;210:35–43. doi: 10.1007/s00213-010-1815-x. [DOI] [PubMed] [Google Scholar]
- Craighead M, Milne R, Campbell-Wan L, Watson L, Presland J, Thomson FJ, et al. Characterization of a novel and selective V1B receptor antagonist. Prog Brain Res. 2008;170:527–535. doi: 10.1016/S0079-6123(08)00440-8. [DOI] [PubMed] [Google Scholar]
- File SE, Clarke A. Intraventricular ACTH reduces social interaction in male rats. Pharmacol Biochem Behav. 1980;12:711–715. doi: 10.1016/0091-3057(80)90154-9. [DOI] [PubMed] [Google Scholar]
- Gorman JM, Papp LA, Coplan JD, Martinez JM, Lennon S, Goetz RR, et al. Anxiogenic effects of CO2 and hyperventilation in patients with panic disorder. Am J Psychiatry. 1994;151:547–553. doi: 10.1176/ajp.151.4.547. [DOI] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Serradeil-Le Gal C, Wagnon J, Pascal M, Scatton B, et al. Anxiolytic- and antidepressant-like effects of the non-peptide vasopressin V1b receptor antagonist, SSR149415, suggest an innovative approach for the treatment of stress-related disorders. Proc Natl Acad Sci U S A. 2002;99:6370–6375. doi: 10.1073/pnas.092012099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griebel G, Simiand J, Stemmelin J, Gal CS, Steinberg R. The vasopressin V1b receptor as a therapeutic target in stress-related disorders. Curr Drug Targets CNS Neurol Disord. 2003;2:191–200. doi: 10.2174/1568007033482850. [DOI] [PubMed] [Google Scholar]
- Griebel G, Stemmelin J, Gal CS, Soubrie P. Non-peptide vasopressin V1b receptor antagonists as potential drugs for the treatment of stress-related disorders. Curr Pharm Des. 2005;11:1549–1559. doi: 10.2174/1381612053764797. [DOI] [PubMed] [Google Scholar]
- Griebel G, Beeské S, Stahl SM. The vasopressin V(1b) receptor antagonist SSR149415 in the treatment of major depressive and generalized anxiety disorders: results from 4 randomized, double-blind, placebo-controlled studies. J Clin Psychiatry. 2012;73:1403–1411. doi: 10.4088/JCP.12m07804. [DOI] [PubMed] [Google Scholar]
- Griffante C, Green A, Curcuruto O, Haslam CP, Dickinson BA, Arban R. Selectivity of d[Cha4]AVP and SSR149415 at human vasopressin and oxytocin receptors: evidence that SSR149415 is a mixed vasopressin V1b/oxytocin receptor antagonist. Br J Pharmacol. 2005;146:744–751. doi: 10.1038/sj.bjp.0706383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamelmann E, Schwarze J, Takeda K, Oshiba A, Larsen GL, Irvin CG, et al. Noninvasive measurement of airway responsiveness in allergic mice using barometric plethysmography. Am J Respir Crit Care Med. 1997;156:766–775. doi: 10.1164/ajrccm.156.3.9606031. [DOI] [PubMed] [Google Scholar]
- Heinrichs SC, De Souza EB, Schulteis G, Lapsansky JL, Grigoriadis DE. Brain penetrance, receptor occupancy and antistress in vivo efficacy of a small molecule corticotropin releasing factor type I receptor selective antagonist. Neuropsychopharmacology. 2002;27:194–202. doi: 10.1016/S0893-133X(02)00299-3. [DOI] [PubMed] [Google Scholar]
- Holsboer F, Haack D, Gerken A, Vecsei P. Plasma dexamethasone concentrations and differential suppression response of cortisol and corticosterone in depressives and controls. Biol Psychiatry. 1984;19:281–291. [PubMed] [Google Scholar]
- Iijima M, Chaki S. Separation-induced ultrasonic vocalization in rat pups: further pharmacological characterization. Pharmacol Biochem Behav. 2005;82:652–657. doi: 10.1016/j.pbb.2005.11.005. [DOI] [PubMed] [Google Scholar]
- Iijima M, Chaki S. An arginine vasopressin V1b antagonist, SSR1494154 elicits antidepressant-like effects in an olfactory bulbectomy model. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:622–627. doi: 10.1016/j.pnpbp.2006.12.008. [DOI] [PubMed] [Google Scholar]
- Iijima M, Ito A, Kurosu S, Chaki S. Pharmacological characterization of repeated corticosterone injection-induced depression model in rats. Brain Res. 2010;1359:75–80. doi: 10.1016/j.brainres.2010.08.078. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments – the ARRIVE guidelines. J Cereb Blood Flow Metab. 2011;31:991–993. doi: 10.1038/jcbfm.2010.220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liebowitz MR, Gorman JM, Fyer A, Dillon D, Levitt M, Klein DF. Possible mechanisms for lactate's induction of panic. Am J Psychiatry. 1986;143:495–502. doi: 10.1176/ajp.143.4.495. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, O'Carroll AM, McBride OW, Konig M, Morel A, Brownstein MJ. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature. 1992;357:336–339. doi: 10.1038/357336a0. [DOI] [PubMed] [Google Scholar]
- Lolait SJ, O'Carroll AM, Mahan LC, Felder CC, Button DC, Young WS, 3rd, et al. Extrapituitary expression of the rat V1b vasopressin receptor gene. Proc Natl Acad Sci U S A. 1995;92:6783–6787. doi: 10.1073/pnas.92.15.6783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Londen L, Goekoop JG, van Kempen GM, Frankhuijzen-Sierevogel AC, Wiegant VM, van der Velde EA, et al. Plasma levels of arginine vasopressin elevated in patients with major depression. Neuropsychopharmacology. 1997;17:284–292. doi: 10.1016/S0893-133X(97)00054-7. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel A, O'Carroll AM, Brownstein MJ, Lolait SJ. Molecular cloning and expression of a rat V1a arginine vasopressin receptor. Nature. 1992;356:523–526. doi: 10.1038/356523a0. [DOI] [PubMed] [Google Scholar]
- Nemeroff CB, Widerlov E, Bissette G, Walleus H, Karlsson I, Eklund K, et al. Elevated concentrations of CSF corticotropin-releasing factor-like immunoreactivity in depressed patients. Science. 1984;226:1342–1344. doi: 10.1126/science.6334362. [DOI] [PubMed] [Google Scholar]
- Olivier B, Molewijk HE, van der Heyden JA, van Oorschot R, Ronken E, Mos J, et al. Ultrasonic vocalizations in rat pups: effects of serotonergic ligands. Neurosci Biobehav Rev. 1998;23:215–227. doi: 10.1016/s0149-7634(98)00022-0. [DOI] [PubMed] [Google Scholar]
- Overstreet DH, Griebel G. Antidepressant-like effects of the vasopressin V1b receptor antagonist SSR149415 in the Flinders Sensitive Line rat. Pharmacol Biochem Behav. 2005;82:223–227. doi: 10.1016/j.pbb.2005.07.021. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 2nd edn. Sydney, Australia: Academic Press; 1986. [Google Scholar]
- Purba JS, Hoogendijk WJ, Hofman MA, Swaab DF. Increased number of vasopressin- and oxytocin-expressing neurons in the paraventricular nucleus of the hypothalamus in depression. Arch Gen Psychiatry. 1996;53:137–143. doi: 10.1001/archpsyc.1996.01830020055007. [DOI] [PubMed] [Google Scholar]
- Ring RH, Schechter LE, Leonard SK, Dwyer JM, Platt BJ, Graf R, et al. Receptor and behavioral pharmacology of WAY-267464, a non-peptide oxytocin receptor agonist. Neuropharmacology. 2010;58:69–77. doi: 10.1016/j.neuropharm.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Roy-Byrne PP, Uhde TW, Post RM, Gallucci W, Chrousos GP, Gold PW. The corticotropin-releasing hormone stimulation test in patients with panic disorder. Am J Psychiatry. 1986;143:896–899. doi: 10.1176/ajp.143.7.896. [DOI] [PubMed] [Google Scholar]
- Salome N, Stemmelin J, Cohen C, Griebel G. Differential roles of amygdaloid nuclei in the anxiolytic- and antidepressant-like effects of the V1b receptor antagonist, SSR149415, in rats. Psychopharmacology (Berl) 2006;187:237–244. doi: 10.1007/s00213-006-0424-1. [DOI] [PubMed] [Google Scholar]
- Scott LV, Dinan TG. Vasopressin and the regulation of hypothalamic–pituitary–adrenal axis function: implications for the pathophysiology of depression. Life Sci. 1998;62:1985–1998. doi: 10.1016/s0024-3205(98)00027-7. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Wagnon J, Simiand J, Griebel G, Lacour C, Guillon G, et al. Characterization of (2S,4R)-1-[5-chloro-1-[(2,4-dimethoxyphenyl)sulfonyl]-3-(2-methoxy-phenyl)-2-oxo-2,3-dihydro-1H-indol-3-yl]-4-hydroxy-N,N-dimethyl-2-pyrrolidine carboxamide (SSR149415), a selective and orally active vasopressin V1b receptor antagonist. J Pharmacol Exp Ther. 2002;300:1122–1130. doi: 10.1124/jpet.300.3.1122. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Keim SR. The circumventricular organs form a potential neural pathway for lactate sensitivity: implications for panic disorder. J Neurosci. 1997;17:9726–9735. doi: 10.1523/JNEUROSCI.17-24-09726.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shekhar A, Keim SR, Simon JR, McBride WJ. Dorsomedial hypothalamic GABA dysfunction produces physiological arousal following sodium lactate infusions. Pharmacol Biochem Behav. 1996;55:249–256. doi: 10.1016/s0091-3057(96)00077-9. [DOI] [PubMed] [Google Scholar]
- Shekhar A, Johnson PL, Fitz SD, Nakazato A, Chaki S, Steckler T, et al. A selective, non-peptide CRF receptor 1 antagonist prevents sodium lactate-induced acute panic-like responses. Int J Neuropsychopharmacol. 2011;14:355–365. doi: 10.1017/S1461145710001355. [DOI] [PubMed] [Google Scholar]
- Shibata S, Nakanishi H, Watanabe S, Ueki S. Effects of chronic administration of antidepressants on mouse-killing behavior (muricide) in olfactory bulbectomized rats. Pharmacol Biochem Behav. 1984;21:225–230. doi: 10.1016/0091-3057(84)90219-3. [DOI] [PubMed] [Google Scholar]
- Shimazaki T, Iijima M, Chaki S. The pituitary mediates the anxiolytic-like effects of the vasopressin V1B receptor antagonist, SSR149415, in a social interaction test in rats. Eur J Pharmacol. 2006;543:63–67. doi: 10.1016/j.ejphar.2006.06.032. [DOI] [PubMed] [Google Scholar]
- Spooren WP, Schoeffter P, Gasparini F, Kuhn R, Gentsch C. Pharmacological and endocrinological characterisation of stress-induced hyperthermia in singly housed mice using classical and candidate anxiolytics (LY314582, MPEP and NKP608) Eur J Pharmacol. 2002;435:161–170. doi: 10.1016/s0014-2999(01)01562-x. [DOI] [PubMed] [Google Scholar]
- Yamamoto T, Shibata S, Ueki S. Effects of locus coeruleus stimulation on muricide in olfactory bulbectomized and raphe lesioned rats. Jpn J Pharmacol. 1982;32:845–853. doi: 10.1254/jjp.32.845. [DOI] [PubMed] [Google Scholar]
- Zelena D. Vasopressin in health and disease with a focus on affective disorders. Cent Nerv Syst Agents Med Chem. 2012;12:286–303. doi: 10.2174/187152412803760609. [DOI] [PubMed] [Google Scholar]