Letter to the Editor
Recent reports have emphasized the importance of the microenvironment in the development and pathophysiology of malignancies. While most of these studies investigated interactions between stromal and neoplastic cells, hypoxia has emerged as another component of the microenvironment. While hypoxia in solid tumors is well studied, it is unclear what role, if any, it plays in the physiology of hematologic neoplasias. Lymphomas in this context may resemble solid tumors, but the hypoxic sanctuary for leukemias is thought to be the bone marrow. Hypoxia within the bone marrow of healthy individuals and cancer patients is of additional interest as it may affect hematopoietic progenitors and their differentiation1 as well as bone metastases
We and others have reported direct measurements of bone marrow pO2 by a simple and minimally invasive method using conventional gas assay methodology routinely used clinically for the measurement of pO2 2,3. Thus, the average bone marrow pO2 ranged from 48.0 – 54.9 mmHg. In our previous study we found that simulation of this physiological hypoxia induces profound changes on the biology of acute myeloid leukemia (AML) cells, in particular expression and function of the chemokine receptor CXCR4, as well as changes in cell signaling, especially in the MAP-Kinase pathway3. This notion is of special interest as constitutive activation of the MAP-Kinase pathway has been linked to the development of AML4 due to its critical function in proliferation, survival and differentiation, and its prognostic importance in AML5.
We therefore aimed to investigate the relationship between physiological hypoxia and its influence on MAP-Kinase activation. In a first step, we examined potential differences in the levels of bone marrow hypoxia in patients with AML with active disease and in remission. Therefore, bone marrow aspirates were collected from AML patients who underwent routine bone marrow aspiration in heparinized syringes. The samples, comprising ~2 mL of aspirate, were collected immediately after aspiration and analyzed within 5 – 10 minutes after aspiration for pO2 by the Portable Clinical Analyzer (i-STAT corporation, Princeton) with G3+ cartridges (Abbott Inc., NJ). We compared the oxygen content of AML bone marrows (n=7) with that of patients in complete remission (CR, n=12). Although pO2 in AML bone marrows tended to be lower (41.3±11.2 mmHg) than in CR marrows (48.8±15.9 mmHg), this difference was not significant and may in fact reflect anemia in AML patients (average Hb: 9.5±1.9 g/dL vs. 12.0±2.1 g/dL in the CR patients, p<0.01). One patient was available for analysis at diagnosis and at CR. At both time points, pO2 of the bone marrow was 38.0 mmHg, with an initial cellularity of 100% (50% blasts), decreasing to 30% (1% blasts, CR). Analysis according to high risk (complex aberrant karyotype and/or FLT3 ITD) versus intermediate risk groups (normal and intermediate karyotype) showed no significant difference between these groups (median 36.5±14.2 mmHg vs. 40.0±3.5 mmHg, p=0.29).
This data suggests that the infiltration of the bone marrow by leukemic blasts (as high as 80% in our patient group) does not have a significant impact on bone marrow oxygen levels. This can be explained by the anatomic-histological characteristics of the bone marrow cavity: this tissue consists, besides of fibrous stroma, of arterioles, venoles and, most importantly, of sinusoids6, making it an organ extremely well supplied with blood (and thus oxygen). It is important however to point out that these results do not rule out the existence of more hypoxic niches which have been described in murine bone marrows, and are believed to be the sites in which hematopoietic stem cells home7. Our analysis reports the average O2 content of ca. 2 mL of aspirated bone marrow, which may contain oxygen-rich and oxygen-low regions. Indirect measurements previously reported in animals may have a potential limitation in that prior to analysis the animals were sacrificed leading to decreased perfusion and thus widespread hypoxia, including the bone marrow. Therefore, the possibility of post mortem artifacts in areas with little or no perfusion/oxygen reserve (e.g. areas distant from vessels) cannot be ruled out.
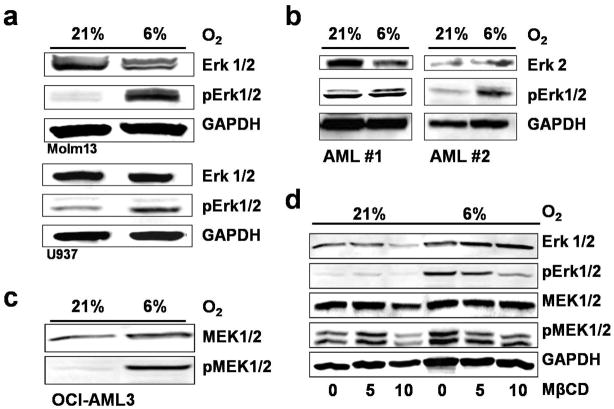
Next, we analyzed mechanisms involved in MAP-Kinase activation in AML at physiological hypoxia. To investigate the effect of physiological hypoxia on MAP-Kinase activation we exposed AML cell lines and primary AML samples to 6% O2 (pO2=45 mmHg, as has been measured in the bone marrows). For these experiments in reduced oxygen environment, the hypoxic Workstation INVIVO2 400 from Ruskinn Technology (Bridgend, UK) was used. 6% O2 induced a strong increase in the phosphorylation of Erk1/2 in AML cell lines Molm13, U937 (Figure 1a) and OCI-AML3 (average 4.4fold increase in optical density (OD) on multiple blots (n=6)) after adjustment to 6% O2 for at least 10 days as compared to their normoxic counterparts. In 2 primary samples from patients with AML, an average increase in OD in the pErk/Erk ratio of 1.7 was observed after 14–16 hours of hypoxia (Figure 1b). Viability was not different between the groups.
Figure 1. Physiologic hypoxia activates Erk in AML via MEK by utilization of lipid rafts.
(a) In AML cell lines physiological hypoxia of 6% O2 increases phosphorylation of MAP42/44 as compared to normoxia (21%) as shown by Western blotting. (b) Increase in MAP4/44 phosphorylation in two primary AML samples after 14–16hr of hypoxia. Average increase in pErk/Erk ratio by 1.8fold. (c) Upregulated Erk-Kinase MEK1/2 at 6% O2 as compared to 21% (d) Disruption of lipid rafts with Methyl-beta-Cyclodextrin leads to a dose-dependent decrease of Erk1/2 and MEK1/2 phosphorylation at 6% O2 but not at 21% O2.
As the kinase for Erk1/2 is MEK1/2, we further investigated the role of MEK1/2 in hypoxia-induced activation of MAPK 42/44. Both OCI-AML3 and Molm13 cells adjusted to 6% O2 showed increased phosphorylation of MEK1/2 as compared to 21% O2 (OCI-AML3 cells are shown in Figure 1c). In addition, treatment with 100 nM PD98059 for 1 hour led to abrogation of Erk1/2 phosphorylation in both cell lines adjusted to physiological hypoxia (data not shown). Interestingly, both Erk1/2 and MEK1/2 were upregulated within the first hour of physiological hypoxia, and remained phosphorylated for the following 24 hours. After 1 hour at 6% O2, we did not observe an increase in Hypoxia-inducible Factor (HiF) 1α, implying no role for HiF 1α in this early activation process at this pO2.
We have recently shown that physiological hypoxia and re-oxygenation of cells exposed to hypoxia alters cellular membranes, notably lipid rafts, by increasing raft cholesterol content, GM1 ganglioside expression and lipid raft structure2. To test whether MAPK phosphorylation at 6% O2 was dependent on lipid rafts, we used MβCD, an agent commonly used for the disruption of lipid rafts that removes cholesterol from the cellular membrane. Figure 1d shows the dose-dependent decrease of the MEK1/2 and Erk1/2 phosphorylation at 6% O2 but not at 21% O2. Instead, low doses of MβCD can increase Erk1/2 phosphorylation at normoxia indicating different raft functions at different levels of O2.
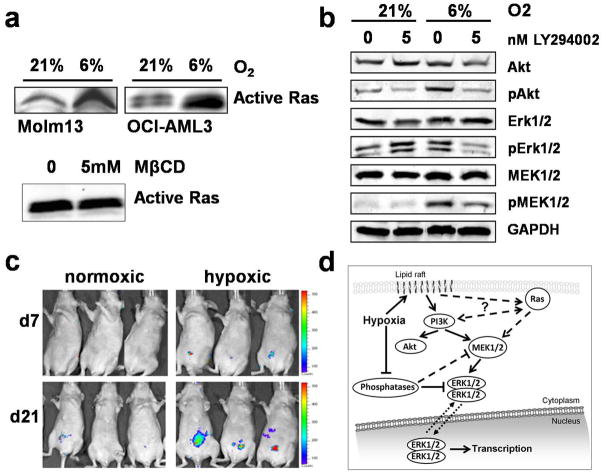
The canonical upstream-located regulator of the MEK/Erk signal cascade is Ras, which is located at the inner leaflet of the cellular membrane. We therefore hypothesized that physiological hypoxia increases MAPK activity by increasing GTP-bound Ras. Ras activation was assayed with a Ras activation assay (Cytoskeleton, Denver, CO) and performed according to the manufacturer’s protocol. In brief, active, GTP-bound Ras was pulled-down using the Ras-binding domain of Raf1 in the form a GST fusion protein bound to colored gluthation beads. After immunoprecipitation, the beads were subjected to Western blotting. Figure 2a shows indeed higher levels of GTP-bound Ras, i.e. in its active form, at physiological hypoxia, both in Molm13 and OCI-AML3 cells. To further examine pathways involved in activation of MEK/Erk, we used the pan-Src-family inhibitor PP2. Interestingly, we did not observe an effect on MAPK phosphorylation at 6% O2 and accordingly, phosphorylation of Ras activator Src was dramatically decreased at 6% O2 (data not shown). This data implies that under physiological hypoxia Ras is either activated independently of Src or that activation of MAPK might be independent of increased Ras activation. We therefore tested the effect of MβCD on Ras activity. While MβCD decreased MAPK phosphorylation (as shown above), it had no effect on Ras activity (Figure 2a). This data indicates that Ras may be of minor importance for increased MAPK activity at 6% O2.
Figure 2. Hypoxic activation of MAP-Kinases depends on PI3K but not Ras and results in accelerated engraftment in a murine leukemia.
(a) Pull-down of active, GTP-bound Ras shows a significantly higher amount of base-line activated Ras at 6% O2 as compared to normoxia. Methyl-beta-Cyclodextrin at 15 mM, a concentration effective in decreasing Erk1/2 and MEK1/2 activity at 6% O2, has no effect on active Ras. (b) PI3K inhibition with LY294002 reduces phosphorylation of MEK1/2 and Erk1/2 at physiological hypoxia (6%), but not at 21% O2. AKT phosphorylation at S473 (Cell Signaling Technology, Beverly, MA) was effectively inhibited at 6% and 21% O2. (c) Adjustment of Molm13 cells to physiological hypoxia leads to faster engraftment in a nude mouse model. Left panel: 3 representative mice from each group (total n=8 per group); right panel: the statistical significant difference in bioluminescence is lost 4 weeks after tail vein injection (n=8 in each group). (d) Proposed mechanism of MAP-Kinase activation at physiological hypoxia. For details see text.
Recent reports have linked MAPK activation to lipid rafts8 with one proposed mechanism being a PI3K-dependent activation of MEK/Erk independent of Ras/Raf1. We therefore hypothesized that PI3K inhibition could inhibit Erk1/2 and MEK1/2 activation at physiological hypoxia but not at normoxia. To test this hypothesis, we investigated the effect of PI3K inhibition with LY294002 on MEK/Erk and Akt activation at 6% and 21% O2. Figure 2b shows the effect of LY294002 on OCI-AML3 cells. Whereas exposure to 5 nM LY294002 for 1 hour leads to a decrease of phosphorylation of Akt (at S473) at both 21% and 6% O2, it does not inhibit MAPK activation at 21% O2 (but rather increases Erk1/2 phosphorylation in OCI-AML3 cells). At physiological hypoxia however, both Erk1/2 and MEK1/2 activity is diminished by PI3K inhibition. Relative changes in OD of multiple experiments (n=3) were as follows: 5 nM LY249002 decreased Akt phosphorylation by 50% at normoxia and by 60% at physiological hypoxia; at 21% O2 the average decrease on MEK1/2 phosphorylation was 10% and 40% increase for Erk1/2, while at physiologic hypoxia a 30% decrease was observed for both kinases.
Taken together, this data shows that physiological hypoxia induced robust phosphorylation of Erk. Importantly, our data suggest that activation of the MEK/Erk signal cascade under physiological hypoxia is not controlled by canonical Ras/Raf1 signaling, but is promoted, at least in part, by PI3K (which also promotes Akt phosphorylation). The role – or lack thereof – of Ras in this mechanism remains to be further elucidated. While we found increased amounts of active Ras in physiological hypoxia, we did not find any consequences of MβCD on Ras activity despite decreased MAP-Kinase activity. Altogether these data suggests that physiological hypoxia regulates MEK/Erk and Akt activation via lipid rafts independent from Ras/Raf1.
Figure 2d summarizes our findings and proposes a possible mechanism for activation of the MEK/Erk pathway under physiological hypoxia. In this model, physiological hypoxia increases membrane cholesterol which promotes lipid raft formation (as previously shown2), which in turn leads to activation of PI3K, consequently activating both MEK/Erk and Akt. The connection between PI3K and lipid rafts has already been suggested9 in normoxia and interactions between the PI3K pathway and the MEK/Erk signaling cascade are well established. In addition, the possibility that PI3K functions as an alternative activator of MEK/Erk under physiological conditions shortcutting Ras could also provide additional explanation as to why Ras inhibition, most notably by farnesyl transferase inhibitors, has not fulfilled its promise in clinical studies that their preclinical efficacy testing, performed in normoxia, suggested. Furthermore, inhibition of FLT-3-signaling in AML patients with FLT-3-ITD with Sorafenib did not result in consistent inhibition of pERK, as was expected10.
Next we examined the functional consequences of MAPK activation on AML cells adjusted to hypoxic conditions. No significant differences in growth kinetics were found between normoxic cells and adjusted cells grown in physiological hypoxia in vitro [OCI-AML: estimated doubling time 24 hours (21%O2) vs. 23 hours (6% O2); Molm13: estimated doubling time 26 hours 24 hours (6% O2)]. To investigate whether activation of MAPK imparts AML cells with a more aggressive phenotype in vivo, we utilized a murine leukemia model to compare engraftment kinetics of hypoxia-adjusted cells to normoxic cells. Molm13 cells stably expressing a dual renilla luciferase-GFP reporter were kept under normoxic or hypoxic conditions for 10 days. Prior to tail-vein injection of 2 × 106 cells into athymic nude mice (Five-week old 01B74 athymic nude (nu/nu) mice (NCI, Frederick, MD), n=8 per group), MAPK activation was confirmed via Western blotting. The result is depicted in Figure 2c. Mice transplanted with cells adjusted to physiological hypoxia engrafted significantly faster (day 7: 2fold [p<0.05], day 21: 4fold [p<0.01]) as measured by bioluminescence imaging using the IVIS200 Imaging System (Xenogen/Caliper Life Science, Hopkinton, MA). This difference was lost 4 weeks after transplantation, and no difference in survival in the hypoxia-transplanted group as compared to controls was seen.
Certainly this faster engraftment cannot be tied to increased MEK/Erk and Akt signaling alone, as we have previously shown that adjustment of cells to 6% O2 also affects CXCR4 expression, a chemokine receptor responsible for trafficking of lymphocytes and homing of stem cells. Moreover, physiological hypoxia did not only alter CXCR4 expression but also the response to its natural ligand SDF-12. These may impact homing and engraftment of leukemic cells reflected in initially increased tumor mass. Over time, previously normoxic cells will assume a hypoxic phenotype, this eliminating the initial growth and homing advantage of pre-injection hypoxic cells, or alternatively, hypoxic cells might lose their growth advantage over time.
In conclusion, our data demonstrate that physiological oxygen conditions in the bone marrow microenvironment activate the MEK/Erk signal cascade in the absence of activating mutations by a lipid raft- and PI3K-regulated mechanism, surprisingly independent of Ras/Raf1. Cells adjusted to hypoxia showed a more rapid engraftment in-vivo. These observations stress the importance of studying AML cells under physiological conditions, in particular at physiological hypoxia and caution against translating signaling pathways established at unphysiological normoxic conditions, in fact “hyperoxic” conditions, to physiologic tumor hypoxia.
Acknowledgments
The authors would like to thank Seshagiri Duvvuri, Twee Tsao, Teresa McQueen and Leslie Calvert for technical support.
This work was in part supported by a grant from the Deutsche Forschungsgesellschaft (Grant FI 1487 to M.F.) and supported by grants from the National Cancer Institute (PO1 CA055164, P30 CA016672) and the Paul and Mary Haas Chair in Genetics (to MA).
References
- 1.Cipolleschi MG, Dello Sbarba P, Olivotto M. The role of hypoxia in the maintenance of hematopoietic stem cells. Blood. 1993;82:2031–2037. [PubMed] [Google Scholar]
- 2.Fiegl M, Samudio I, Clise-Dwyer K, Burks JK, Mnjoyan Z, Andreeff M. CXCR4 expression and biologic activity in acute myeloid leukemia are dependent on oxygen partial pressure. Blood. 2009;113:1504–1512. doi: 10.1182/blood-2008-06-161539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison JS, Rameshwar P, Chang V, Bandari P. Oxygen saturation in the bone marrow of healthy volunteers. Blood. 2002;99:394. doi: 10.1182/blood.v99.1.394. [DOI] [PubMed] [Google Scholar]
- 4.Towatari M, Iida H, Tanimoto M, Iwata H, Hamaguchi M, Saito H. Constitutive activation of mitogen-activated protein kinase pathway in acute leukemia cells. Leukemia. 1997;11:479–484. doi: 10.1038/sj.leu.2400617. [DOI] [PubMed] [Google Scholar]
- 5.Kornblau SM, Tibes R, Qiu YH, Chen W, Kantarjian HM, Andreeff M, Coombs KR, Mills GB. Functional proteomic profiling of AML predicts response and survival. Blood. 2009;113:154–64. doi: 10.1182/blood-2007-10-119438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kiel MJ, Morrison SJ. Uncertainty in the niches that maintain haematopoietic stem cells. Nat Rev Immunol. 2008;8:290–301. doi: 10.1038/nri2279. [DOI] [PubMed] [Google Scholar]
- 7.Parmar K, Mauch P, Vergilio JA, Sackstein R, Down JD. Distribution of hematopoietic stem cells in the bone marrow according to regional hypoxia. Proc Natl Acad Sci. 2007;104:5431–5436. doi: 10.1073/pnas.0701152104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen X, Resh MD. Activation of mitogen-activated protein kinase by membrane-targeted Raf chimeras is independent of raft localization. J Biol Chem. 2001;276:34617–34623. doi: 10.1074/jbc.M103995200. [DOI] [PubMed] [Google Scholar]
- 9.Peres C, Yart A, Perret B, Salles JP, Raynal P. Modulation of phosphoinositide 3-kinase activation by cholesterol level suggests a novel positive role for lipid rafts in lysophosphatidic acid signalling. FEBS Lett. 2003;534:164–168. doi: 10.1016/s0014-5793(02)03832-2. [DOI] [PubMed] [Google Scholar]
- 10.Ravandi F, Cortes JE, Jones D, Faderl S, Garcia-Manero G, Konopleva MY, O’Brien S, Estrov Z, Borthakur G, Thomas D, Pierce SR, Brandt M, Byrd A, Bekele BN, Pratz K, Luthra R, Levis M, Andreeff M, Kantarjian HM. Phase I/II Study of Combination Therapy With Sorafenib, Idarubicin, and Cytarabine in Younger Patients With Acute Myeloid Leukemia. J Clin Oncol. 2010 Mar 8; doi: 10.1200/JCO.2009.25.4888. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]