Abstract
Vascular endothelial cells (ECs) form the inner lining of all blood vessels from the largest artery and veins, viz., the aorta and venae cavae, respectively, to the capillaries that connect the arterial and venous systems. Because these two major conducting systems of the cardiovasculature differ functionally, it is not surprising that the physical makeup of arteries and veins, including the ECs that line their lumina, are also distinct. Although few would argue that the local environment contributes to the differences between arteries and veins, recent evidence has shown that the specification of arterial and venous identity is largely genetically determined.
Keywords: Vascular endothelial cells, Specification, ephrinB2, EphB4, VEGF, Notch
Introduction
The vascular endothelium is derived from the mesoderm and forms the inner layer of the entire vasculature in both blood vessels and lymphatic vessels. It is a highly dynamic organ system that participates in a variety of physiological processes, including the development and remodeling of the vasculature, the control of vascular tone and blood fluidity, and the trafficking of blood cells and nutrients. Furthermore, the endothelium is involved in a number of pathological conditions, such as atherosclerosis and cancer (via tumor angiogenesis). Once considered a homogeneous cell population that functions as a passive physical barrier between blood and tissue, endothelial cells (ECs) are now recognized to be quite “heterogeneous”. Thus, despite their common developmental origins, ECs are not identical; they differ widely in morphology and function as one traverses the vascular tree. For instance, ECs of the smallest arteries or arterioles are longer and narrower than their counterparts in the smallest veins or venules. Functionally, one of the major roles of arteriolar ECs is the control of vascular tone, whereas post-capillary venular ECs are the primary site of leukocyte trafficking.
This chapter provides an in-depth review of the structural and functional differences between the arterial and venous circulatory systems. In addition, we present a comprehensive summary of the current views on the determination of arterial-venous EC identity with an emphasis on genetic, biochemical, and biomechanical contributions. Finally, we consider the interesting questions that remain in this important area.
Blood vessel structure
The cardiovascular system is composed of the heart, blood, and the vasculature, which includes arteries, veins, and capillaries (Sherwood 2007). Arteries and veins are classified on the basis of their size and location within the vascular tree. The largest artery in the body, the aorta, originates from the left ventricle of the heart, branches into large arteries, and then into smaller arteries, which reach their target tissues or organs, where they branch into the smallest-diameter arteries known as arterioles. On the venous side of circulation, the smallest vessels, called venules, merge to form small veins that exit the organs and progressively increase in size to form the large veins until finally, they converge on the two largest veins, the venae cavae. The superior vena cava, originating from the upper trunk of the body, and the inferior vena cava, receiving blood from the lower trunk, both empty directly into the right atrium of the heart. The smallest vessels are the capillaries, which connect arterioles to venules. With the exception of the capillaries, all blood vessels have the same basic structure: an outer layer or tunica externa, a middle layer or tunica media, and an inner tunica intima. However, there are some important differences between arterial and venous blood vessels that will be discussed here (Bloom and Fawcett 1994; Gartner and Hiatt 1994).
Anatomical differences between arteries and veins
Generally speaking, veins are larger in diameter than arteries but have much thinner walls (Fig. 1). The functional relevance of these two key differences is addressed below. The tunica externa, more commonly known as the tunica adventitia, is the outermost layer of vessels and is composed mainly of connective tissue that lends structural support to the vessel. Although collagen fibers predominate, elastic fibers are also present in both arteries and veins, regardless of their size. In the larger arteries and veins, the tunica adventitia includes its own blood supply called the vasa vasorum. In addition, this layer occasionally contains longitudinally oriented smooth muscle cells (SMCs). The difference in the tunica adventitia between arteries and veins is its thickness relative to the overall thickness of the vessel wall; in veins and venules, this layer constitutes the majority of the vessel wall, whereas in arteries and arterioles, it represents only about half the total thickness.
Fig. 1.
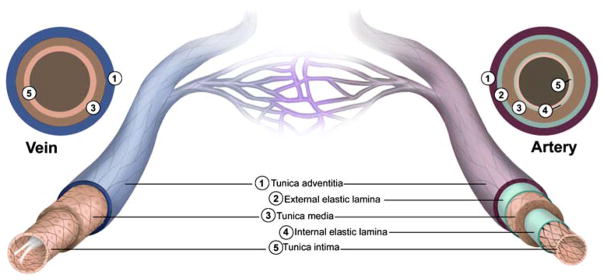
Structural features of the vasculature. All blood vessels within the branches of the vascular tree, except the capillaries, are composed of a tunica intima that is lined with endothelial cells (ECs), a tunica media that contains SMCs and elastic fibers, and a tunica adventitia made up of fibrous connective tissue. Arterial blood vessels (dark red/purple) are characterized by long narrow ECs that are aligned in the direction of blood flow, multiple layers of smooth muscle cells, and in the case of muscular arteries, elastic fibers that are arranged in two distinct bands (inner and outer elastic laminae). In contrast, venous blood vessels (blue) are lined with rounder non-aligned ECs, lack elastic laminae, and most possess valves that project into their lumen
The next layer, moving inward toward the vessel lumen, is the tunica media. It is characterized by the presence of circumferentially oriented SMCs, which provide muscular support to the vessel, and elastic fibers, which provide the basis for the vessel’s ability to stretch. In arteries of all sizes, the tunica media is typically the thickest layer and is comprised of multiple layers of SMCs and a large amount of elastic material. The elastic fibers are arranged in a thick outer band called the external elastic lamina. Although both large and small veins contain SMCs and elastic fibers, the tunica media of the venous circulation is not as clearly defined and organized as in the arterial counterparts. Furthermore, in venules, the medial layer is virtually nonexistent. As a result of these differences, the walls of veins are thinner and less rigid than those of arteries; veins thus hold a larger proportion of circulating blood than arteries.
The innermost and thinnest layer of the vasculature is the tunica intima. The defining feature of the intima is the endothelium, which directly lines the vessel lumen. The tunica intima also includes supportive matrix of connective tissue composed of collagen and elastic fibers, the latter forming a distinct band called the internal elastic lamina in most arteries. One major difference between arteries and veins is the absence, in veins, of an internal elastic lamina between the tunica intima and tunica media. However, the most striking structural difference between the two sides of the circulatory system is the presence, in veins, of specialized structures called venous valves that extend into the lumen and prevent the backflow of blood.
Distinct differences exist between the arterial and venous endothelium, with variation in both their size and shape across the vascular tree. ECs are typically flat, ranging from 0.2 μm in height at the periphery to 3 μm at the nucleus (Florey 1966). Arterial ECs are generally thicker than those in veins, with the exception of ECs of high endothelial venules (HEVs), which have been described as plump and cuboidal (for a review, see Girard and Springer 1995). Arterial ECs are long and narrow or ellipsoidal, a reflection of their alignment in the direction of undisturbed blood flow. Venous ECs are short and wide, as blood flow rates in the venous circulation are significantly lower than in the arterial circulation. Physiological venous and arterial shear stress levels are typically 1–5 dynes/cm2 and 10–40 dynes/cm2, respectively.
Another important characteristic of the endothelium is its intercellular junctions, which mediate both intercellular adhesion and communication (for a review, see Bazzoni and Dejana 2004). The two major kinds of junctions that mediate intercellular adhesion are tight junctions, also known as zona occludens, and adherens junctions, or zona adhaerens. A third type of junction, gap junctions, mediates cell-cell communication; these junctions are organized into channel-like structures called connexons, which are composed of proteins called connexins (Cxs). The endothelium expresses three Cxs: Cx37 (Reed et al. 1993), Cx40 (Bruzzone et al. 1993), and Cx43 (Pepper et al. 1992). The expression and organization of junctions varies throughout the vascular tree, corresponding to the site-specific functional requirements of the specific vessel. The intercellular junctions in arteries of all calibers are much tighter than those in veins (Simionescu et al. 1976). Post-capillary venules, in particular, exhibit loosely or poorly organized tight junctions (Simionescu et al. 1975).
Functional differences between arteries and veins
The role of blood vessels, other than the capillaries, is to serve as a conduit for the delivery of deoxygenated blood from the heart to the lungs, where the exchange of oxygen and carbon dioxide occurs at the level of the alveolus and its associated capillary network, and the return of oxygenated blood to the heart, which distributes the blood to all the tissues of the body. Whereas arteries specifically carry blood “away” from the heart, veins carry blood “toward” the heart. Generally speaking, arterial vessels contain oxygenated blood, and venous vessels carry blood that is low in oxygen. The sole exception is the pulmonary vascular system where the pulmonary arteries take deoxygenated blood to the lungs and the pulmonary veins return oxygenated blood from the lungs to the left atrium of the heart.
The function of the endothelium varies depending on the blood vessel type and location within the vasculature (for a review, see Aird 2007). Vasomotor tone, or vascular tone, is an arterial function that occurs primarily at the level of the arterioles. Vascular tone refers to the degree of vessel constriction relative to a maximally dilated state and is determined by a balance between the local vasodilator and vasoconstrictor molecules. Vasodilators such as nitric oxide, bradykinin, and adenosine cause SMCs in the tunica media of the arteriole to relax, resulting in vessel dilation, reduced resistance to blood flow, and lower blood pressure. Vasoconstrictors, including endothelin and angiotensin II, contract SMC, increase vascular resistance, and raise blood pressure.
Veins, specifically post-capillary venules, are the primary site of permeability during inflammation (Majno et al. 1961). The vascular leakage observed at these sites in response to agonists such as histamine and serotonin is widely believed to be mediated by the formation of interendothelial gaps (Majno and Palade 1961). One major challenge to this view is the recent concept that clusters of vesicles and vacuoles, called vesiculo-vacuolar organelles (VVOs), are responsible for the extravasation of macromolecules (Feng et al. 1996, 1997). Whatever the mechanism, the selective expression of histamine and serotonin receptors, the absence of well-organized tight junctions, and the abundance of VVOs in post-capillary venules are specializations that likely contribute to the highly permeable nature of these vessels.
Leukocyte trafficking, which includes the attachment, rolling, firm adhesion, and transmigration of circulating leukocytes from the blood into the underlying tissues, also occurs almost exclusively in post-capillary venules. In most cases, interactions between the members of the selectin family of adhesion molecules, namely E-selectin and P-selectin, on the ECs and their glycoprotein ligands on leukocytes mediate the initial attachment and rolling. The firm adhesion of leukocytes, on the other hand, is mediated primarily by interactions between members of the endothelial immunoglobulin superfamily (IgSF), which includes ICAM-1 and VCAM-1, and leukocyte integrins, such as LFA-1 and α4β1/VLA-4. Although less well understood than the other steps in this classical multi-step cascade, the transmigration of leukocytes appears likely to involve PECAM-1/CD31 (Muller et al. 1993), CD99 (Schenkel et al. 2002), and the junctional adhesion molecule-1 (JAM-1; Ostermann et al. 2002). The fact that the venous vasculature has a lower flow rate, thinner walls, and fewer tight junctions than arterial vessels would seem to be the primary reasons why post-capillary venules are well-suited for leukocyte trafficking.
Arteriovenous EC specification
During embryonic development specific cell lineages are determined or specified. For ECs, this “specification” begins with the mesoderm-derived progenitor cell, the hemangioblast, which gives rise to hematopoietic stem cells, angioblasts, and possibly SMCs (D’Amore 2000; Yamashita et al. 2000). ECs are also known to arise from circulating bone-marrow-derived endothelial precursor cells (Asahara et al. 1997; Schatteman et al. 2007). Regardless of their origin, the differentiation of ECs is an important step in blood vessel formation (i.e., vasculogenesis and angiogenesis) and is regulated by both intrinsic and extrinsic factors.
Intrinsic factors
Until recently, the structural and functional differences between arteries and veins described above were thought to be determined solely by physiological cues, such as the direction and rate of blood flow, blood pressure, and blood oxygenation. However, compelling experimental evidence indicates that arteriovenous (AV) specification is mediated by developmental expression of genes that encode signaling molecules, including ligands, receptors, and transcription factors. Most significant has been the demonstration that the Eph family transmembrane ligand, ephrinB2, and its putative receptor tyrosine kinase, Eph receptor B4 (EphB4), are developmentally expressed by arteries and veins, respectively, prior to the assembly of blood vessels and onset of blood circulation (Wang et al. 1998). Since that pioneering work, the list of genes expressed specifically by arteries (e.g., Cx40) or veins (e.g., endomucin, Fig. 2) has rapidly expanded (Table 1). Although these genes collectively represent the known molecular markers of arterial and venous endothelium, the roles of many with regards to AV specification, if any, have yet to be elucidated.
Fig. 2.
Endomucin is selectively expressed by veins but not arteries. Diaminobenzidine immunohistochemical staining shows endomucin expression in a mouse pancreatic vein (arrow). Expression in the adjacent artery (arrowhead) is absent. Scale bar, 50 μm
Table 1.
Molecular markers of the vascular endothelium
Extrinsic factors
Much of what is known about extracellular signaling pathways in AV specification comes from studies in zebrafish. In zebrafish, angioblasts originate in the lateral plate mesoderm (LPM) and make their way to the midline where they coalesce to form the dorsal aorta (DA) and posterior cardinal vein (PCV; Zhong et al. 2001; Fig. 3). Lineage tracking studies have revealed that angioblasts within the LPM are specified to either an arterial or venous fate (Zhong et al. 2001).
Fig. 3.
Model of arteriovenous EC specification during embryonic development. Angioblasts derived from the lateral plate mesoderm (LPM) are specified into either arterial or venous ECs prior to migration to the midline where they form the dorsal aorta (DA) and posterior cardinal vein (PCV). Specification is initiated by the expression of sonic hedgehog (Shh) by the notochord (Nc) and floor plate of the neural tube (NT), with Shh then acting on the adjacent somites (S) to induce the expression of vascular endothelial growth factor (VEGF). In arterial-fated angioblasts, VEGF signaling via a VEGF receptor 2 (VEGFR2)/neuropilin 1 (NP-1) complex leads to the downstream activation of Notch and ERK signaling and the subsequent expression of the arterial marker ephrinB2. Foxc1 and Foxc2 (Foxc1/2) are transcriptional factors that control the expression of Notch components and therefore also promote arterial EC identity. In venous-fated angioblasts, COUP-TFII blocks NP-1 expression, which prevents Notch and ERK activation, and the venous marker EphB4 is expressed. Activation of the PI3K/AKT pathway also blocks ERK, which represses arterial EC fate. Adapted from Lamont and Childs (2006) and Lawson and Weinstein (2002)
Sonic hedgehog
The current model for the regulation of arterial cell fate, based on experimental findings in zebrafish and mice, suggests that Sonic hedgehog (Shh) initiates the cascade. Shh, the secreted protein product of the sonic you (syu) gene (Schauerte et al. 1998), is expressed in the notochord and floor plate of the developing zebrafish embryo (Krauss et al. 1993) at the 10-somite stage (10 ss) when vascular endothelial growth factor (VEGF) is expressed by the somites, and Flk1-positive angioblasts are migrating to the midline (Odenthal et al. 1996; Fig. 3). A role for Shh in vascular development was first identified in a screen for cardiovascular system mutants (Chen et al. 1996); further analysis revealed defects in trunk circulation (Chen et al. 1996) and in the patterning of the somites (van Eeden et al. 1996).
A more precise role for Shh in arterial EC differentiation was identified when syu mutant embryos were noted to lack the artery-specific marker, ephrin-B2a, the zebrafish homolog of ephrinB2 (Lawson et al. 2002). Additionally, ephrin-B2a was demonstrated not to be expressed in the vasculature of wild-type embryos exposed to cyclopamine, an inhibitor of Shh signaling (Cooper et al. 1998; Incardona et al. 1998). ECs were not absent in the syu mutants, as the ETS-domain transcription factor Fli-1, an endothelial marker (Brown et al. 2000), and fms-related tyrosine kinase 4 (Flt4), a venous marker (Lawson et al. 2002), were both present in the vessel that formed in lieu of a dorsal aorta and posterior cardinal vein (Brown et al. 2000). Conversely, overexpression of Shh in zebrafish embryos led to the formation of ectopic ephrin-B2a-positive (arterial) vessels in the region of the PCV (Lawson et al. 2002). Earlier work on mouse models demonstrated that Shh promoted neovascular growth in the cornea and in ischemic hind-limbs; this appeared to be attributable, in part, to the induction of VEGF and other angiogenic factors (Pola et al. 2001). As subsequently shown, syu mutant zebrafish embryos fail to express VEGF mRNA in the hypochord or somites, but VEGF expression can be rescued by microinjecting Shh mRNA (Lawson et al. 2002).
Vascular endothelial growth factor
VEGF (also known as VEGF-A or vascular permeability factor) was first identified as a tumor-secreted factor that functions to increase vascular permeability in tumor vessels (Senger et al. 1986) and was later described as an endothelial mitogen and angiogenic factor (Connolly et al. 1989; Ferrara and Henzel 1989). Since that time, the roles for VEGF have grown to include the stimulation of EC migration and survival (Alon et al. 1995; Yuan et al. 1996) and the induction and maintenance of fenestrations (Roberts and Palade 1995).
VEGF signaling is mediated via its two receptor tyrosine kinases, fms-like tyrosine kinase 1 (Flt, VEGFR1; de Vries et al. 1992) and fetal liver kinase 1 (Flk1, VEGFR2; Millauer et al. 1993; Yamaguchi et al. 1993). VEGF also binds two non-tyrosine kinase co-receptors, neuropilin-1 (NRP-1, NP-1; Soker et al. 1998) and neuropilin-2 (NRP-2, NP-2; Gluzman-Poltorak et al. 2000). VEGFR2 is the main signaling receptor in the vascular endothelium, and a number of studies have indicated that VEGF signaling through VEGFR2 is required for the development of embryonic vasculature. Targeted deletion of VEGF, either homozygously or heterozygously, leads to embryonic lethality because of severe vascular abnormalities (Carmeliet et al. 1996; Ferrara et al. 1996). Similarly, VEGFR2-deficient mice die early in gestation because of a reduction in EC number and the failure to make blood vessels (Shalaby et al. 1995). VEGF was initially reported to be required for the growth and survival of ECs only during a short postnatal period (Gerber et al. 1999), and the dependence on VEGF for EC growth and survival was suggested to be gradually lost as mice reached adulthood, except for processes such as ovulation (Ferrara et al. 1998). However, the continued high expression of VEGF in virtually all adult tissues, in the absence of active angiogenesis, has led to the concept that VEGF is essential for the maintenance of the adult vasculature (Maharaj et al. 2006; Ng et al. 2001). Interestingly, VEGF has also been noted to be expressed by aortic but not venous ECs (Maharaj et al. 2006).
Studies in both mice and zebrafish have implicated VEGF signaling in the regulation of arterial differentiation. Alternative splicing of VEGF mRNA in mice generates three protein isoforms, VEGF120, VEGF164, and VEGF188, based on the number of amino acids (Shima et al. 1996). By using mice that were engineered to express single VEGF isoforms, VEGF120 and VEGF188 mice were found to have impaired retinal arterial development but normal venous and capillary vessels, whereas VEGF164 mice displayed normal retinal vasculature (Stalmans et al. 2002), indicating a specific role for VEGF in the determination of arterial identity. Consistent with this concept, overexpression of VEGF164 in cardiac muscle was shown to lead to an increase in ephrinB2-positive (arterial) and a decrease in EphB4-positive (venous) microvessels (Visconti et al. 2002). Similarly, the addition of VEGF120 or VEGF164 leads to an increase in ephrinB2 expression in ephrinB2-negative ECs (Mukouyama et al. 2002). In zebrafish, VEGF suppression by morpholinos resulted in a dramatic decrease in ephrin-B2a expression and a concomitant increase in ectopic arterial flt4 expression in the DA and defects in vascular morphology and circulation (Lawson et al. 2002).
Notch
Notch signaling is activated via interactions between Notch receptors, large highly conserved transmembrane proteins, and their ligands, Delta, Serrate, and Lag-2 (DSL). Mice possess four Notch receptors, designated Notch1–4, and five Notch ligands, Delta-like (Dll)1, Dll3, Dll4, Jagged1 (Jag1), and Jagged2 (Jag2). Although Notch signaling has well-established roles in a variety of developmental processes, including the regulation of cell fate decisions and patterning (for a review, see Artavanis-Tsakonas et al. 1999), its role in AV specification was not fully appreciated until genetic manipulation of Notch components was accomplished in mice. Notch1-deficient mice die at embryonic day 9.5 (E9.5) and exhibit severe defects in vascular morphogenesis and remodeling (Krebs et al. 2000). Likewise, Jag1 null mice are embryonic lethal and display defects in vascular remodeling (Xue et al. 1999).
Studies of the gene expression patterns of Notch components support the idea that Notch signaling is critically involved in vascular development, particularly in arterial EC differentiation. As summarized in Table 1, Notch1, Notch4, Dll4, Jag1, and Jag2 are all expressed by arterial, but not venous, ECs in mice, whereas Notch5 and DeltaC are expressed in the DA, but not the PCV, of developing zebrafish. To date, none of the Notch receptors and ligands has been identified in venous blood vessels.
The most definitive demonstration that Notch signaling is required for the establishment of AV specification comes from studies in zebrafish. Suppression of Notch activity through the expression of dominant-negative Suppressor of Hairless, which functions as an inhibitor of Notch signaling, or through breeding of mindbomb (mib) mutant embryos, which have decreased Notch activity (Jiang et al. 1996; Schier et al. 1996), leads to the reduced or failed expression of ephrinB2 (Lawson et al. 2001). Conversely, the expression of a constitutively active Notch receptor (Scheer and Campos-Ortega 1999; Scheer et al. 2001) or endothelial-specific expression of Notch5-intracellular domain results in the decreased expression of flt4 by PCV (Lawson et al. 2001). Together, these findings demonstrate that Notch signaling is not only crucial for proper AV specification, but also suppresses venous cell differentiation.
Insight into the way that Notch signaling integrates with other signaling pathways and/or factors to determine arterial EC identity has come from studies in zebrafish placing VEGF downstream of Shh and upstream of the Notch signaling pathway (Lawson et al. 2002; Fig. 3). Based on the near-identical phenotypes between VEGF morpholino-injected embryos and mib mutant embryos, VEGF and Notch signaling have been hypothesized to be involved in a common signaling cascade. The inability of VEGF121 to rescue the expression of arterial markers in mib mutant embryos indicates a requirement for Notch signaling. In contrast, Notch signaling is able to rescue arterial differentiation in the absence of VEGF, confirming that Notch signaling acts downstream of both Shh and VEGF to mediate arterial EC specification.
Transcriptional regulation in arterial specification
Despite the wealth of information on signaling pathways and the specific genes involved in AV specification, little is known about the transcriptional control of AV specification. Analysis of the human Notch4 gene promoter revealed a consensus activator protein 1 (AP-1) binding site, which was shown, by both electrophoretic mobility shift assay and quantitative chromatin immunoprecipitation in human umbilical vein endothelial cells (HUVECs), to be occupied by an AP-1 complex (Wu et al. 2005). Transfection of a Notch4 promoter-luciferase reporter construct in which the AP-1 motif was mutated resulted in a significant decrease in transcriptional activity compared with the wild-type construct in HUVECs, but not HeLa cells, suggesting that the AP-1 complex conferred EC-specific transcription of Notch4. Interestingly, the activity of AP-1 in ECs has previously been shown to be stimulated by laminar shear stress (Lan et al. 1994); this will be discussed in more detail in a later section.
Forkhead box c (Foxc) 1 and 2, two closely related members of the forkhead family of transcription factors, have been identified as regulators of arterial cell specification that lie upstream of Notch signaling (Seo et al. 2006; Fig. 3). Although the importance of forkhead factors in cardiovascular development had previously been described (Kume et al. 2001), Foxc1/Foxc2 homozygous null mouse embryos have more recently been demonstrated to display arteriovenous malformations that consist of abnormal fusion between the DA and PCV (Seo et al. 2006). In addition, ECs of these mutants exhibit either reduced or a complete lack of arterial markers, including Notch1, Notch4, Dll4, Jag1, Hey2, ephrinB2, and NP-1, whereas venous markers, such as the chicken ovalbumin upstream promoter transcription factor II (COUP-TFII) and EphB4, are normally expressed, indicating proper venous EC differentiation. Overexpression of the Foxc proteins in vitro results in the upregulation of arterial-specific genes with no apparent effect on the expression of venous markers. Together, these findings indicate that Foxc proteins have an important role in controlling the expression of Notch signaling genes and consequently are critical components in the regulation of arterial EC identity.
Regulation of venous EC identity
The mechanisms that regulate venous EC identity are arguably less well understood than those controlling arterial identity. As such, it had become widely accepted that, in the absence of Notch signaling, venous identity is a default pathway. However, the recent observation that COUP-TFII, a member of the orphan nuclear receptor superfamily, is expressed in venous, but not arterial ECs during development, has led to the concept that vein identity may be actively specified. In the absence of COUP-TFII, venous ECs express Notch1, Jag1, ephrinB2, and NP-1 but do not fully convert into an arterial phenotype since EphB4 expression is only partially lost. On the other hand, ectopic expression of COUP-TFII in arterial ECs leads to the downregulation of the expression of NP-1 and other downstream arterial markers and to the appearance of arterial-venous fusions, in which vessels are more vein-like, as evidenced by EphB4 staining (You et al. 2005). These results suggest that the expression of COUP-TFII in venous ECs inhibits NP-1 expression, which in turn suppresses VEGF and Notch signaling, so that factors that mediate vein differentiation predominate, and vein identity is maintained (Fig. 3).
More recently, the phosphatidylinositiol-3 kinase (PI3K) signaling pathway has been implicated in the determination of venous fate (Hong et al. 2006). A small-molecule screening approach in mutant gridlock (grl) zebrafish embryos, which are characterized by insufficient arterial cells (Zhong et al. 2001) and disrupted aortic blood flow (Weinstein et al. 1995), has revealed that PI3K/AKT signaling blocks p42/44 mitogen-activated protein kinase (extracellular signal-regulated kinase; ERK) activation to promote venous specification. Specifically, the compound GS4898 inhibits PI3K such that ERK activation leads to arterial specification. Another small molecule, GS4012, restores circulation to the posterior trunk in grl mutant embryos, presumably via upregulation of VEGF expression and activation of the VEGF signaling pathway (Peterson et al. 2004). Furthermore, mosaic coexpression of dominant-negative AKT and green fluorescent protein (GFP) has been shown to lead to the localization of GFP-positive cells in the dorsal aorta, whereas coexpression of constitutively active AKT and GFP results in preferential distribution in the vein (Hong et al. 2006), providing further genetic evidence that these downstream effectors of VEGF signaling have opposing effects on the specification of arterial and venous ECs (for a review, see Zachary and Gliki 2001; Fig. 3).
Biomechanical forces and vessel plasticity
In addition to genetic determinants that are active during development, the biomechanical environment of different ECs influences their phenotype during later stages of embryonic development and in the adult. Indeed, many years ago, use of a cone-plate apparatus demonstrated that laminar shear stress influences vascular EC structure and function (Dewey et al. 1981). Microarray analysis has revealed differential gene expression by EC exposed to laminar versus turbulent flow (Garcia-Cardena et al. 2001). These in vitro findings suggest that the vascular endothelium has phenotypic plasticity.
Quail-chick grafting experiments indicate that initial AV specification, defined by the developmental expression of arterial and venous markers and the related signaling pathways, is reversible, i.e., environmental factors influence cell identity (Moyon et al. 2001a; Othman-Hassan et al. 2001). In the one study, ECs from the internal carotid artery and the vena cava of 14-day-old quail donor embryos were isolated, grafted into 3-day-old chick embryos, and detected by using the QH1 antibody (Othman-Hassan et al. 2001). In the other study, rings of dorsal aorta, carotid artery, cardinal vein, and jugular vein were taken from quail embryos at different developmental stages and were grafted into the coelom of E2 chick embryos (Moyon et al. 2001a). In both models, arterial and venous ECs from the quail embryos integrated into both arteries and veins in the chick embryos, as measured by the expression of the arterial markers ephrinB2 and NRP1 or the venous marker Tie2. This high level of plasticity in the developing vasculature was found to be lost after E7 (Moyon et al. 2001a). Therefore, in addition to physical forces, paracrine signaling from the vessel wall may be important in controlling AV specification.
Analysis of arterial-venous differentiation in the yolk sac of developing chick embryos has shown that flow regulates the expression of the arterial markers ephrinB2 and NRP1 (le Noble et al. 2004). Prior to the onset of embryonic circulation, the assembly of yolk sac vessels begins with the formation of a primary capillary plexus via vasculogenesis. Time-lapse video-microscopy has revealed that, shortly after the onset of perfusion (somite stage 21), the vitelline artery forms in the posterior arterial pole from the arterial plexus by fusion of individual capillary segments. Interestingly, not all capillary segments are integrated into the developing vessel; those that become selectively disconnected, with arterial markers downregulated, make sprouts that reconnect to the venous plexus and become reperfused.
Summary and future directions
The vascular endothelium is clearly a complex and specialized tissue composed of cells whose phenotypes are as diverse as the functions that they perform. Their phenotypic differences are reflected by the expression of unique molecular markers. Genetic programs that function during early development are responsible for initiating EC fate and ultimately blood vessel identity, but during later stages, environmental factors can influence this identity.
Although significant progress has been made in our understanding of AV specification, important questions remain. In the regulation of arterial EC identity, both Notch signaling and ERK activation have been shown to promote an arterial fate (Hong et al. 2006; Lawson et al. 2002), but whether they interact or converge on a common downstream mediator is not known. Likewise, even though the Foxc proteins have been shown to activate the Notch pathway (Seo et al. 2006), the factor(s) governing the selective activation of Notch in arterial ECs is unclear. Although the finding that COUP-TFII expression in venous ECs down-regulates Notch components and ephrinB2 has challenged the view that the venous state is a default pathway, the upstream regulators of COUP-TFII expression are unknown, as is the effect of COUP-TFII on Foxc proteins and PI3K signaling. Additionally, since the absence of COUP-TFII does not result in the complete absence of EphB4 in venous ECs, other regulators of EphB4 presumably exist.
The concept that AV specification is plastic and can be shaped by environmental cues such as flow is intriguing. Fluid shear stress has recently been shown to induce VEGF expression in osteoblasts (Thi et al. 2007) and in ECs (Goettsch et al. 2008). In addition, the PI3K and Notch signaling pathways (Wang et al. 2007) and ERK and AKT (Dimmeler et al. 1998; Sumpio et al. 2005) have also been reported to respond to shear stress. It would be interesting to know, for example, whether the pharmacological inhibition of Notch signaling in arterial ECs subjected to arterial shear stress would lead to the expression of arterial or venous markers.
Because of the high functional demands placed on the endothelium, ECs have evolved to be perhaps more dynamic than other cell types. Their ability to adapt to their environment is a reflection of their capacity to sense extracellular cues. Much attention has been directed to the study of shear-stress-responsive genes, such as KLF2, ET-1, and eNOS (for a review, see Groenendijk et al. 2007), and the description of mechanosensory complexes (Hierck et al. 2008; Tzima et al. 2005). Primary cilia, long known to be expressed by ECs (Bystrevskaya et al. 1988), have been shown to be restricted to areas of low and disturbed blood flow (Van der Heiden et al. 2006, 2008) and to sensitize ECs to fluid shear stress (Hierck et al. 2008). These and other mechanism(s) by which ECs sense their environment represent prime areas for future investigation, especially as they pertain to the maintenance of AV cell identity.
Acknowledgments
We thank Mr. Peter Mallen for the artwork, Dr. Eric Finkelstein for Fig. 2, and Ms. Christine Bagley for her editorial assistance.
The authors are supported by NIH EY05318 and EY015435 (P.A.D.). Dr. dela Paz is supported by NRSA Institutional Research Training Grant T32 HL076115. Dr. D’Amore is a Research to Prevent Blindness Senior Scientific Investigator.
Contributor Information
Nathaniel G. dela Paz, Email: nathaniel.delapaz@schepens.harvard.edu.
Patricia A. D’Amore, Email: patricia.damore@schepens.harvard.edu.
References
- Adams RH, Wilkinson GA, Weiss C, Diella F, Gale NW, Deutsch U, Risau W, Klein R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999;13:295–306. doi: 10.1101/gad.13.3.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahn DG, Ruvinsky I, Oates AC, Silver LM, Ho RK. tbx20, a new vertebrate T-box gene expressed in the cranial motor neurons and developing cardiovascular structures in zebrafish. Mech Dev. 2000;95:253–258. doi: 10.1016/s0925-4773(00)00346-4. [DOI] [PubMed] [Google Scholar]
- Aird WC. Phenotypic heterogeneity of the endothelium. I. Structure, function, and mechanisms. Circ Res. 2007;100:158–173. doi: 10.1161/01.RES.0000255691.76142.4a. [DOI] [PubMed] [Google Scholar]
- Alon T, Hemo I, Itin A, Pe’er J, Stone J, Keshet E. Vascular endothelial growth factor acts as a survival factor for newly formed retinal vessels and has implications for retinopathy of prematurity. Nat Med. 1995;1:1024–1028. doi: 10.1038/nm1095-1024. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Asahara T, Murohara T, Sullivan A, Silver M, van der Zee R, Li T, Witzenbichler B, Schatteman G, Isner JM. Isolation of putative progenitor endothelial cells for angiogenesis. Science. 1997;275:964–967. doi: 10.1126/science.275.5302.964. [DOI] [PubMed] [Google Scholar]
- Bazzoni G, Dejana E. Endothelial cell-to-cell junctions: molecular organization and role in vascular homeostasis. Physiol Rev. 2004;84:869–901. doi: 10.1152/physrev.00035.2003. [DOI] [PubMed] [Google Scholar]
- Bloom W, Fawcett DW. A textbook of histology. Chapman & Hall; New York: 1994. [Google Scholar]
- Brown LA, Rodaway AR, Schilling TF, Jowett T, Ingham PW, Patient RK, Sharrocks AD. Insights into early vasculogenesis revealed by expression of the ETS-domain transcription factor Fli-1 in wild-type and mutant zebrafish embryos. Mech Dev. 2000;90:237–252. doi: 10.1016/s0925-4773(99)00256-7. [DOI] [PubMed] [Google Scholar]
- Bruzzone R, Haefliger JA, Gimlich RL, Paul DL. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol Biol Cell. 1993;4:7–20. doi: 10.1091/mbc.4.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bystrevskaya VB, Lichkun VV, Antonov AS, Perov NA. An ultrastructural study of centriolar complexes in adult and embryonic human aortic endothelial cells. Tissue Cell. 1988;20:493–503. doi: 10.1016/0040-8166(88)90052-3. [DOI] [PubMed] [Google Scholar]
- Carmeliet P, Ferreira V, Breier G, Pollefeyt S, Kieckens L, Gertsenstein M, Fahrig M, Vandenhoeck A, Harpal K, Eberhardt C, Declercq C, Pawling J, Moons L, Collen D, Risau W, Nagy A. Abnormal blood vessel development and lethality in embryos lacking a single VEGF allele. Nature. 1996;380:435–439. doi: 10.1038/380435a0. [DOI] [PubMed] [Google Scholar]
- Chen JN, Haffter P, Odenthal J, Vogelsang E, Brand M, van Eeden FJ, Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Nüsslein-Volhard C. Mutations affecting the cardiovascular system and other internal organs in zebrafish. Development. 1996;123:293–302. doi: 10.1242/dev.123.1.293. [DOI] [PubMed] [Google Scholar]
- Chi JT, Chang HY, Haraldsen G, Jahnsen FL, Troyanskaya OG, Chang DS, Wang Z, Rockson SG, van de Rijn M, Botstein D, Brown PO. Endothelial cell diversity revealed by global expression profiling. Proc Natl Acad Sci USA. 2003;100:10623–10628. doi: 10.1073/pnas.1434429100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Connolly DT, Heuvelman DM, Nelson R, Olander JV, Eppley BL, Delfino JJ, Siegel NR, Leimgruber RM, Feder J. Tumor vascular permeability factor stimulates endothelial cell growth and angiogenesis. J Clin Invest. 1989;84:1470–1478. doi: 10.1172/JCI114322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper MK, Porter JA, Young KE, Beachy PA. Teratogen-mediated inhibition of target tissue response to Shh signaling. Science. 1998;280:1603–1607. doi: 10.1126/science.280.5369.1603. [DOI] [PubMed] [Google Scholar]
- D’Amore PA. Kissing cousins—evidence for a common vascular cell precursor. Nat Med. 2000;6:1323–1324. doi: 10.1038/82133. [DOI] [PubMed] [Google Scholar]
- de Vries C, Escobedo JA, Ueno H, Houck K, Ferrara N, Williams LT. The fms-like tyrosine kinase, a receptor for vascular endothelial growth factor. Science. 1992;255:989–991. doi: 10.1126/science.1312256. [DOI] [PubMed] [Google Scholar]
- Dewey CF, Jr, Bussolari SR, Gimbrone MA, Jr, Davies PF. The dynamic response of vascular endothelial cells to fluid shear stress. J Biomech Eng. 1981;103:177–185. doi: 10.1115/1.3138276. [DOI] [PubMed] [Google Scholar]
- Dimmeler S, Assmus B, Hermann C, Haendeler J, Zeiher AM. Fluid shear stress stimulates phosphorylation of Akt in human endothelial cells: involvement in suppression of apoptosis. Circ Res. 1998;83:334–341. doi: 10.1161/01.res.83.3.334. [DOI] [PubMed] [Google Scholar]
- Ekman N, Lymboussaki A, Vastrik I, Sarvas K, Kaipainen A, Alitalo K. Bmx tyrosine kinase is specifically expressed in the endocardium and the endothelium of large arteries. Circulation. 1997;96:1729–1732. doi: 10.1161/01.cir.96.6.1729. [DOI] [PubMed] [Google Scholar]
- Feng D, Nagy JA, Hipp J, Dvorak HF, Dvorak AM. Vesiculo-vacuolar organelles and the regulation of venule permeability to macromolecules by vascular permeability factor, histamine, and serotonin. J Exp Med. 1996;183:1981–1986. doi: 10.1084/jem.183.5.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng D, Nagy JA, Hipp J, Pyne K, Dvorak HF, Dvorak AM. Reinterpretation of endothelial cell gaps induced by vasoactive mediators in guinea-pig, mouse and rat: many are transcellular pores. J Physiol (Lond) 1997;504:747–761. doi: 10.1111/j.1469-7793.1997.747bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrara N, Henzel WJ. Pituitary follicular cells secrete a novel heparin-binding growth factor specific for vascular endothelial cells. Biochem Biophys Res Commun. 1989;161:851–858. doi: 10.1016/0006-291x(89)92678-8. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Carver-Moore K, Chen H, Dowd M, Lu L, O’Shea KS, Powell-Braxton L, Hillan KJ, Moore MW. Heterozygous embryonic lethality induced by targeted inactivation of the VEGF gene. Nature. 1996;380:439–442. doi: 10.1038/380439a0. [DOI] [PubMed] [Google Scholar]
- Ferrara N, Chen H, Davis-Smyth T, Gerber HP, Nguyen TN, Peers D, Chisholm V, Hillan KJ, Schwall RH. Vascular endothelial growth factor is essential for corpus luteum angiogenesis. Nat Med. 1998;4:336–340. doi: 10.1038/nm0398-336. [DOI] [PubMed] [Google Scholar]
- Florey HW. The endothelial cell. BMJ. 1966;2:487–490. doi: 10.1136/bmj.2.5512.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Cardena G, Comander J, Anderson KR, Blackman BR, Gimbrone MA., Jr Biomechanical activation of vascular endothelium as a determinant of its functional phenotype. Proc Natl Acad Sci USA. 2001;98:4478–4485. doi: 10.1073/pnas.071052598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gartner LP, Hiatt JL. Color atlas of histology. Williams & Wilkins; Baltimore: 1994. [Google Scholar]
- Gerber HP, Hillan KJ, Ryan AM, Kowalski J, Keller GA, Rangell L, Wright BD, Radtke F, Aguet M, Ferrara N. VEGF is required for growth and survival in neonatal mice. Development. 1999;126:1149–1159. doi: 10.1242/dev.126.6.1149. [DOI] [PubMed] [Google Scholar]
- Girard JP, Springer TA. High endothelial venules (HEVs): specialized endothelium for lymphocyte migration. Immunol Today. 1995;16:449–457. doi: 10.1016/0167-5699(95)80023-9. [DOI] [PubMed] [Google Scholar]
- Gluzman-Poltorak Z, Cohen T, Herzog Y, Neufeld G. Neuropilin-2 is a receptor for the vascular endothelial growth factor (VEGF) forms VEGF-145 and VEGF-165. J Biol Chem. 2000;275:18040–18045. doi: 10.1074/jbc.M909259199. [DOI] [PubMed] [Google Scholar]
- Goettsch W, Gryczka C, Korff T, Ernst E, Goettsch C, Seebach J, Schnittler HJ, Augustin HG, Morawietz H. Flow-dependent regulation of angiopoietin-2. J Cell Physiol. 2008;214:491–503. doi: 10.1002/jcp.21229. [DOI] [PubMed] [Google Scholar]
- Groenendijk BC, Van der Heiden K, Hierck BP, Poelmann RE. The role of shear stress on ET-1, KLF2, and NOS-3 expression in the developing cardiovascular system of chicken embryos in a venous ligation model. Physiology (Bethesda) 2007;22:380–389. doi: 10.1152/physiol.00023.2007. [DOI] [PubMed] [Google Scholar]
- Helbling PM, Saulnier DM, Brandli AW. The receptor tyrosine kinase EphB4 and ephrin-B ligands restrict angiogenic growth of embryonic veins in Xenopus laevis. Development. 2000;127:269–278. doi: 10.1242/dev.127.2.269. [DOI] [PubMed] [Google Scholar]
- Herzog Y, Kalcheim C, Kahane N, Reshef R, Neufeld G. Differential expression of neuropilin-1 and neuropilin-2 in arteries and veins. Mech Dev. 2001;109:115–119. doi: 10.1016/s0925-4773(01)00518-4. [DOI] [PubMed] [Google Scholar]
- Hierck BP, Van der Heiden K, Alkemade FE, Van de Pas S, Van Thienen JV, Groenendijk BC, Bax WH, Van der Laarse A, Deruiter MC, Horrevoets AJ, Poelmann RE. Primary cilia sensitize endothelial cells for fluid shear stress. Dev Dyn. 2008;237:725–735. doi: 10.1002/dvdy.21472. [DOI] [PubMed] [Google Scholar]
- Hong CC, Peterson QP, Hong JY, Peterson RT. Artery/vein specification is governed by opposing phosphatidylinositol-3 kinase and MAP kinase/ERK signaling. Curr Biol. 2006;16:1366–1372. doi: 10.1016/j.cub.2006.05.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Incardona JP, Gaffield W, Kapur RP, Roelink H. The teratogenic Veratrum alkaloid cyclopamine inhibits sonic hedgehog signal transduction. Development. 1998;125:3553–3562. doi: 10.1242/dev.125.18.3553. [DOI] [PubMed] [Google Scholar]
- Jiang YJ, Brand M, Heisenberg CP, Beuchle D, Furutani-Seiki M, Kelsh RN, Warga RM, Granato M, Haffter P, Hammerschmidt M, Kane DA, Mullins MC, Odenthal J, Eeden FJ, Van Nüsslein-Volhard C. Mutations affecting neurogenesis and brain morphology in the zebrafish, Danio rerio. Development. 1996;123:205–216. doi: 10.1242/dev.123.1.205. [DOI] [PubMed] [Google Scholar]
- Kaipainen A, Korhonen J, Mustonen T, Hinsbergh VW, van Fang GH, Dumont D, Breitman M, Alitalo K. Expression of the fms-like tyrosine kinase 4 gene becomes restricted to lymphatic endothelium during development. Proc Natl Acad Sci USA. 1995;92:3566–3570. doi: 10.1073/pnas.92.8.3566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krauss S, Concordet JP, Ingham PW. A functionally conserved homolog of the Drosophila segment polarity gene hh is expressed in tissues with polarizing activity in zebrafish embryos. Cell. 1993;75:1431–1444. doi: 10.1016/0092-8674(93)90628-4. [DOI] [PubMed] [Google Scholar]
- Krebs LT, Xue Y, Norton CR, Shutter JR, Maguire M, Sundberg JP, Gallahan D, Closson V, Kitajewski J, Callahan R, Smith GH, Stark KL, Gridley T. Notch signaling is essential for vascular morphogenesis in mice. Genes Dev. 2000;14:1343–1352. [PMC free article] [PubMed] [Google Scholar]
- Kuhn A, Brachtendorf G, Kurth F, Sonntag M, Samulowitz U, Metze D, Vestweber D. Expression of endomucin, a novel endothelial sialomucin, in normal and diseased human skin. J Invest Dermatol. 2002;119:1388–1393. doi: 10.1046/j.1523-1747.2002.19647.x. [DOI] [PubMed] [Google Scholar]
- Kume T, Jiang H, Topczewska JM, Hogan BL. The murine winged helix transcription factors, Foxc1 and Foxc2, are both required for cardiovascular development and somitogenesis. Genes Dev. 2001;15:2470–2482. doi: 10.1101/gad.907301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamont RE, Childs S. MAPping out arteries and veins. Sci STKE. 2006;2006:pe39. doi: 10.1126/stke.3552006pe39. [DOI] [PubMed] [Google Scholar]
- Lan Q, Mercurius KO, Davies PF. Stimulation of transcription factors NF kappa B and AP1 in endothelial cells subjected to shear stress. Biochem Biophys Res Commun. 1994;201:950–956. doi: 10.1006/bbrc.1994.1794. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Weinstein BM. Arteries and veins: making a difference with zebrafish. Nat Rev Genet. 2002;3:674–682. doi: 10.1038/nrg888. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Scheer N, Pham VN, Kim CH, Chitnis AB, Campos-Ortega JA, Weinstein BM. Notch signaling is required for arterial-venous differentiation during embryonic vascular development. Development. 2001;128:3675–3683. doi: 10.1242/dev.128.19.3675. [DOI] [PubMed] [Google Scholar]
- Lawson ND, Vogel AM, Weinstein BM. Sonic hedgehog and vascular endothelial growth factor act upstream of the Notch pathway during arterial endothelial differentiation. Dev Cell. 2002;3:127–136. doi: 10.1016/s1534-5807(02)00198-3. [DOI] [PubMed] [Google Scholar]
- le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, Breant C, Fleury V, Eichmann A. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131:361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- Liao W, Bisgrove BW, Sawyer H, Hug B, Bell B, Peters K, Grunwald DJ, Stainier DY. The zebrafish gene cloche acts upstream of a flk-1 homologue to regulate endothelial cell differentiation. Development. 1997;124:381–389. doi: 10.1242/dev.124.2.381. [DOI] [PubMed] [Google Scholar]
- Liu C, Shao ZM, Zhang L, Beatty P, Sartippour M, Lane T, Livingston E, Nguyen M. Human endomucin is an endothelial marker. Biochem Biophys Res Commun. 2001;288:129–136. doi: 10.1006/bbrc.2001.5737. [DOI] [PubMed] [Google Scholar]
- Maharaj AS, Saint-Geniez M, Maldonado AE, D’Amore PA. Vascular endothelial growth factor localization in the adult. Am J Pathol. 2006;168:639–648. doi: 10.2353/ajpath.2006.050834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G, Palade GE. Studies on inflammation. I. The effect of histamine and serotonin on vascular permeability: an electron microscopic study. J Biophys Biochem Cytol. 1961;11:571–605. doi: 10.1083/jcb.11.3.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majno G, Palade GE, Schoefl GI. Studies on inflammation. II. The site of action of histamine and serotonin along the vascular tree: a topographic study. J Biophys Biochem Cytol. 1961;11:607–626. doi: 10.1083/jcb.11.3.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millauer B, Wizigmann-Voos S, Schnurch H, Martinez R, Moller NP, Risau W, Ullrich A. High affinity VEGF binding and developmental expression suggest Flk-1 as a major regulator of vasculogenesis and angiogenesis. Cell. 1993;72:835–846. doi: 10.1016/0092-8674(93)90573-9. [DOI] [PubMed] [Google Scholar]
- Morgan SM, Samulowitz U, Darley L, Simmons DL, Vestweber D. Biochemical characterization and molecular cloning of a novel endothelial-specific sialomucin. Blood. 1999;93:165–175. [PubMed] [Google Scholar]
- Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A. Plasticity of endothelial cells during arterial-venous differentiation in the avian embryo. Development. 2001a;128:3359–3370. doi: 10.1242/dev.128.17.3359. [DOI] [PubMed] [Google Scholar]
- Moyon D, Pardanaud L, Yuan L, Breant C, Eichmann A. Selective expression of angiopoietin 1 and 2 in mesenchymal cells surrounding veins and arteries of the avian embryo. Mech Dev. 2001b;106:133–136. doi: 10.1016/s0925-4773(01)00425-7. [DOI] [PubMed] [Google Scholar]
- Mukouyama YS, Shin D, Britsch S, Taniguchi M, Anderson DJ. Sensory nerves determine the pattern of arterial differentiation and blood vessel branching in the skin. Cell. 2002;109:693–705. doi: 10.1016/s0092-8674(02)00757-2. [DOI] [PubMed] [Google Scholar]
- Muller WA, Weigl SA, Deng X, Phillips DM. PECAM-1 is required for transendothelial migration of leukocytes. J Exp Med. 1993;178:449–460. doi: 10.1084/jem.178.2.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng YS, Rohan R, Sunday ME, Demello DE, D’Amore PA. Differential expression of VEGF isoforms in mouse during development and in the adult. Dev Dyn. 2001;220:112–121. doi: 10.1002/1097-0177(2000)9999:9999<::AID-DVDY1093>3.0.CO;2-D. [DOI] [PubMed] [Google Scholar]
- Odenthal J, Haffter P, Vogelsang E, Brand M, Eeden FJ, van Furutani-Seiki M, Granato M, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Warga RM, Allende ML, Weinberg ES, Nüsslein-Volhard C. Mutations affecting the formation of the notochord in the zebrafish, Danio rerio. Development. 1996;123:103–115. doi: 10.1242/dev.123.1.103. [DOI] [PubMed] [Google Scholar]
- Ostermann G, Weber KS, Zernecke A, Schroder A, Weber C. JAM-1 is a ligand of the beta(2) integrin LFA-1 involved in transendothelial migration of leukocytes. Nat Immunol. 2002;3:151–158. doi: 10.1038/ni755. [DOI] [PubMed] [Google Scholar]
- Othman-Hassan K, Patel K, Papoutsi M, Rodriguez-Niedenfuhr M, Christ B, Wilting J. Arterial identity of endothelial cells is controlled by local cues. Dev Biol. 2001;237:398–409. doi: 10.1006/dbio.2001.0383. [DOI] [PubMed] [Google Scholar]
- Pepper MS, Montesano R, Aoumari A, el Gros D, Orci L, Meda P. Coupling and connexin 43 expression in microvascular and large vessel endothelial cells. Am J Physiol. 1992;262:C1246–C1257. doi: 10.1152/ajpcell.1992.262.5.C1246. [DOI] [PubMed] [Google Scholar]
- Peterson RT, Shaw SY, Peterson TA, Milan DJ, Zhong TP, Schreiber SL, MacRae CA, Fishman MC. Chemical suppression of a genetic mutation in a zebrafish model of aortic coarctation. Nat Biotechnol. 2004;22:595–599. doi: 10.1038/nbt963. [DOI] [PubMed] [Google Scholar]
- Pola R, Ling LE, Silver M, Corbley MJ, Kearney M, Blake Pepinsky R, Shapiro R, Taylor FR, Baker DP, Asahara T, Isner JM. The morphogen Sonic hedgehog is an indirect angiogenic agent upregulating two families of angiogenic growth factors. Nat Med. 2001;7:706–711. doi: 10.1038/89083. [DOI] [PubMed] [Google Scholar]
- Rajantie I, Ekman N, Iljin K, Arighi E, Gunji Y, Kaukonen J, Palotie A, Dewerchin M, Carmeliet P, Alitalo K. Bmx tyrosine kinase has a redundant function downstream of angiopoietin and vascular endothelial growth factor receptors in arterial endothelium. Mol Cell Biol. 2001;21:4647–4655. doi: 10.1128/MCB.21.14.4647-4655.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed KE, Westphale EM, Larson DM, Wang HZ, Veenstra RD, Beyer EC. Molecular cloning and functional expression of human connexin37, an endothelial cell gap junction protein. J Clin Invest. 1993;91:997–1004. doi: 10.1172/JCI116321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts WG, Palade GE. Increased microvascular permeability and endothelial fenestration induced by vascular endothelial growth factor. J Cell Sci. 1995;108:2369–2379. doi: 10.1242/jcs.108.6.2369. [DOI] [PubMed] [Google Scholar]
- Schatteman GC, Dunnwald M, Jiao C. Biology of bone marrow-derived endothelial cell precursors. Am J Physiol Heart Circ Physiol. 2007;292:H1–H18. doi: 10.1152/ajpheart.00662.2006. [DOI] [PubMed] [Google Scholar]
- Schauerte HE, Eeden FJ, van Fricke C, Odenthal J, Strahle U, Haffter P. Sonic hedgehog is not required for the induction of medial floor plate cells in the zebrafish. Development. 1998;125:2983–2993. doi: 10.1242/dev.125.15.2983. [DOI] [PubMed] [Google Scholar]
- Scheer N, Campos-Ortega JA. Use of the Gal4-UAS technique for targeted gene expression in the zebrafish. Mech Dev. 1999;80:153–158. doi: 10.1016/s0925-4773(98)00209-3. [DOI] [PubMed] [Google Scholar]
- Scheer N, Groth A, Hans S, Campos-Ortega JA. An instructive function for Notch in promoting gliogenesis in the zebrafish retina. Development. 2001;128:1099–1107. doi: 10.1242/dev.128.7.1099. [DOI] [PubMed] [Google Scholar]
- Schenkel AR, Mamdouh Z, Chen X, Liebman RM, Muller WA. CD99 plays a major role in the migration of monocytes through endothelial junctions. Nat Immunol. 2002;3:143–150. doi: 10.1038/ni749. [DOI] [PubMed] [Google Scholar]
- Schier AF, Neuhauss SC, Harvey M, Malicki J, Solnica-Krezel L, Stainier DY, Zwartkruis F, Abdelilah S, Stemple DL, Rangini Z, Yang H, Driever W. Mutations affecting the development of the embryonic zebrafish brain. Development. 1996;123:165–178. doi: 10.1242/dev.123.1.165. [DOI] [PubMed] [Google Scholar]
- Seki T, Yun J, Oh SP. Arterial endothelium-specific activin receptor-like kinase 1 expression suggests its role in arterialization and vascular remodeling. Circ Res. 2003;93:682–689. doi: 10.1161/01.RES.0000095246.40391.3B. [DOI] [PubMed] [Google Scholar]
- Senger DR, Perruzzi CA, Feder J, Dvorak HF. A highly conserved vascular permeability factor secreted by a variety of human and rodent tumor cell lines. Cancer Res. 1986;46:5629–5632. [PubMed] [Google Scholar]
- Seo S, Fujita H, Nakano A, Kang M, Duarte A, Kume T. The forkhead transcription factors, Foxc1 and Foxc2, are required for arterial specification and lymphatic sprouting during vascular development. Dev Biol. 2006;294:458–470. doi: 10.1016/j.ydbio.2006.03.035. [DOI] [PubMed] [Google Scholar]
- Shalaby F, Rossant J, Yamaguchi TP, Gertsenstein M, Wu XF, Breitman ML, Schuh AC. Failure of blood-island formation and vasculogenesis in Flk-1-deficient mice. Nature. 1995;376:62–66. doi: 10.1038/376062a0. [DOI] [PubMed] [Google Scholar]
- Sherwood L. Human physiology: from cells to systems. Thomson Brooks/Cole; Belmont: 2007. [Google Scholar]
- Shima DT, Kuroki M, Deutsch U, Ng YS, Adamis AP, D’Amore PA. The mouse gene for vascular endothelial growth factor. Genomic structure, definition of the transcriptional unit, and characterization of transcriptional and post-transcriptional regulatory sequences. J Biol Chem. 1996;271:3877–3883. doi: 10.1074/jbc.271.7.3877. [DOI] [PubMed] [Google Scholar]
- Shin D, Anderson DJ. Isolation of arterial-specific genes by subtractive hybridization reveals molecular heterogeneity among arterial endothelial cells. Dev Dyn. 2005;233:1589–1604. doi: 10.1002/dvdy.20479. [DOI] [PubMed] [Google Scholar]
- Shutter JR, Scully S, Fan W, Richards WG, Kitajewski J, Deblandre GA, Kintner CR, Stark KL. Dll4, a novel Notch ligand expressed in arterial endothelium. Genes Dev. 2000;14:1313–1318. [PMC free article] [PubMed] [Google Scholar]
- Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. The microvasculature. J Cell Biol. 1975;67:863–885. doi: 10.1083/jcb.67.3.863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simionescu M, Simionescu N, Palade GE. Segmental differentiations of cell junctions in the vascular endothelium. Arteries and veins. J Cell Biol. 1976;68:705–723. doi: 10.1083/jcb.68.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smithers L, Haddon C, Jiang YJ, Lewis J. Sequence and embryonic expression of deltaC in the zebrafish. Mech Dev. 2000;90:119–123. doi: 10.1016/s0925-4773(99)00231-2. [DOI] [PubMed] [Google Scholar]
- Soker S, Takashima S, Miao HQ, Neufeld G, Klagsbrun M. Neuropilin-1 is expressed by endothelial and tumor cells as an isoform-specific receptor for vascular endothelial growth factor. Cell. 1998;92:735–745. doi: 10.1016/s0092-8674(00)81402-6. [DOI] [PubMed] [Google Scholar]
- Stalmans I, Ng YS, Rohan R, Fruttiger M, Bouche A, Yuce A, Fujisawa H, Hermans B, Shani M, Jansen S, Hicklin D, Anderson DJ, Gardiner T, Hammes HP, Moons L, Dewerchin M, Collen D, Carmeliet P, D’Amore PA. Arteriolar and venular patterning in retinas of mice selectively expressing VEGF isoforms. J Clin Invest. 2002;109:327–336. doi: 10.1172/JCI14362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sumpio BE, Yun S, Cordova AC, Haga M, Zhang J, Koh Y, Madri JA. MAPKs (ERK1/2, p38) and AKT can be phosphorylated by shear stress independently of platelet endothelial cell adhesion molecule-1 (CD31) in vascular endothelial cells. J Biol Chem. 2005;280:11185–11191. doi: 10.1074/jbc.M414631200. [DOI] [PubMed] [Google Scholar]
- Thi MM, Iacobas DA, Iacobas S, Spray DC. Fluid shear stress upregulates vascular endothelial growth factor gene expression in osteoblasts. Ann N Y Acad Sci. 2007;1117:73–81. doi: 10.1196/annals.1402.020. [DOI] [PubMed] [Google Scholar]
- Thompson MA, Ransom DG, Pratt SJ, MacLennan H, Kieran MW, Detrich HW, 3rd, Vail B, Huber TL, Paw B, Brownlie AJ, Oates AC, Fritz A, Gates MA, Amores A, Bahary N, Talbot WS, Her H, Beier DR, Postlethwait JH, Zon LI. The cloche and spadetail genes differentially affect hematopoiesis and vasculogenesis. Dev Biol. 1998;197:248–269. doi: 10.1006/dbio.1998.8887. [DOI] [PubMed] [Google Scholar]
- Tian H, McKnight SL, Russell DW. Endothelial PAS domain protein 1 (EPAS1), a transcription factor selectively expressed in endothelial cells. Genes Dev. 1997;11:72–82. doi: 10.1101/gad.11.1.72. [DOI] [PubMed] [Google Scholar]
- Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, Cao G, DeLisser H, Schwartz MA. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437:426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- Van der Heiden K, Groenendijk BC, Hierck BP, Hogers B, Koerten HK, Mommaas AM, Gittenberger-de Groot AC, Poelmann RE. Monocilia on chicken embryonic endocardium in low shear stress areas. Dev Dyn. 2006;235:19–28. doi: 10.1002/dvdy.20557. [DOI] [PubMed] [Google Scholar]
- Van der Heiden K, Hierck BP, Krams R, Crom R, de Cheng C, Baiker M, Pourquie MJ, Alkemade FE, DeRuiter MC, Gittenberger-de Groot AC, Poelmann RE. Endothelial primary cilia in areas of disturbed flow are at the base of atherosclerosis. Atherosclerosis. 2008;196:542–550. doi: 10.1016/j.atherosclerosis.2007.05.030. [DOI] [PubMed] [Google Scholar]
- van Eeden FJ, Granato M, Schach U, Brand M, Furutani-Seiki M, Haffter P, Hammerschmidt M, Heisenberg CP, Jiang YJ, Kane DA, Kelsh RN, Mullins MC, Odenthal J, Warga RM, Allende ML, Weinberg ES, Nüsslein-Volhard C. Mutations affecting somite formation and patterning in the zebrafish, Danio rerio. Development. 1996;123:153–164. doi: 10.1242/dev.123.1.153. [DOI] [PubMed] [Google Scholar]
- van Kempen MJ, Jongsma HJ. Distribution of connexin37, connexin40 and connexin43 in the aorta and coronary artery of several mammals. Histochem Cell Biol. 1999;112:479–486. doi: 10.1007/s004180050432. [DOI] [PubMed] [Google Scholar]
- Villa N, Walker L, Lindsell CE, Gasson J, Iruela-Arispe ML, Weinmaster G. Vascular expression of Notch pathway receptors and ligands is restricted to arterial vessels. Mech Dev. 2001;108:161–164. doi: 10.1016/s0925-4773(01)00469-5. [DOI] [PubMed] [Google Scholar]
- Visconti RP, Richardson CD, Sato TN. Orchestration of angiogenesis and arteriovenous contribution by angiopoietins and vascular endothelial growth factor (VEGF) Proc Natl Acad Sci USA. 2002;99:8219–8224. doi: 10.1073/pnas.122109599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang HU, Chen ZF, Anderson DJ. Molecular distinction and angiogenic interaction between embryonic arteries and veins revealed by ephrin-B2 and its receptor Eph-B4. Cell. 1998;93:741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- Wang XL, Fu A, Raghavakaimal S, Lee HC. Proteomic analysis of vascular endothelial cells in response to laminar shear stress. Proteomics. 2007;7:588–596. doi: 10.1002/pmic.200600568. [DOI] [PubMed] [Google Scholar]
- Weinstein BM, Stemple DL, Driever W, Fishman MC. Gridlock, a localized heritable vascular patterning defect in the zebrafish. Nat Med. 1995;1:1143–1147. doi: 10.1038/nm1195-1143. [DOI] [PubMed] [Google Scholar]
- Wu J, Iwata F, Grass JA, Osborne CS, Elnitski L, Fraser P, Ohneda O, Yamamoto M, Bresnick EH. Molecular determinants of NOTCH4 transcription in vascular endothelium. Mol Cell Biol. 2005;25:1458–1474. doi: 10.1128/MCB.25.4.1458-1474.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue Y, Gao X, Lindsell CE, Norton CR, Chang B, Hicks C, Gendron-Maguire M, Rand EB, Weinmaster G, Gridley T. Embryonic lethality and vascular defects in mice lacking the Notch ligand Jagged1. Hum Mol Genet. 1999;8:723–730. doi: 10.1093/hmg/8.5.723. [DOI] [PubMed] [Google Scholar]
- Yamaguchi TP, Dumont DJ, Conlon RA, Breitman ML, Rossant J. Flk-1, an flt-related receptor tyrosine kinase is an early marker for endothelial cell precursors. Development. 1993;118:489–498. doi: 10.1242/dev.118.2.489. [DOI] [PubMed] [Google Scholar]
- Yamashita J, Itoh H, Hirashima M, Ogawa M, Nishikawa S, Yurugi T, Naito M, Nakao K, Nishikawa S. Flk1-positive cells derived from embryonic stem cells serve as vascular progenitors. Nature. 2000;408:92–96. doi: 10.1038/35040568. [DOI] [PubMed] [Google Scholar]
- You LR, Lin FJ, Lee CT, DeMayo FJ, Tsai MJ, Tsai SY. Suppression of Notch signalling by the COUP-TFII transcription factor regulates vein identity. Nature. 2005;435:98–104. doi: 10.1038/nature03511. [DOI] [PubMed] [Google Scholar]
- Yuan F, Chen Y, Dellian M, Safabakhsh N, Ferrara N, Jain RK. Time-dependent vascular regression and permeability changes in established human tumor xenografts induced by an anti-vascular endothelial growth factor/vascular permeability factor antibody. Proc Natl Acad Sci USA. 1996;93:14765–14770. doi: 10.1073/pnas.93.25.14765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan L, Moyon D, Pardanaud L, Breant C, Karkkainen MJ, Alitalo K, Eichmann A. Abnormal lymphatic vessel development in neuropilin 2 mutant mice. Development. 2002;129:4797–4806. doi: 10.1242/dev.129.20.4797. [DOI] [PubMed] [Google Scholar]
- Zachary I, Gliki G. Signaling transduction mechanisms mediating biological actions of the vascular endothelial growth factor family. Cardiovasc Res. 2001;49:568–581. doi: 10.1016/s0008-6363(00)00268-6. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Rosenberg M, Mohideen MA, Weinstein B, Fishman MC. Gridlock, an HLH gene required for assembly of the aorta in zebrafish. Science. 2000;287:1820–1824. doi: 10.1126/science.287.5459.1820. [DOI] [PubMed] [Google Scholar]
- Zhong TP, Childs S, Leu JP, Fishman MC. Gridlock signalling pathway fashions the first embryonic artery. Nature. 2001;414:216–220. doi: 10.1038/35102599. [DOI] [PubMed] [Google Scholar]




