Abstract
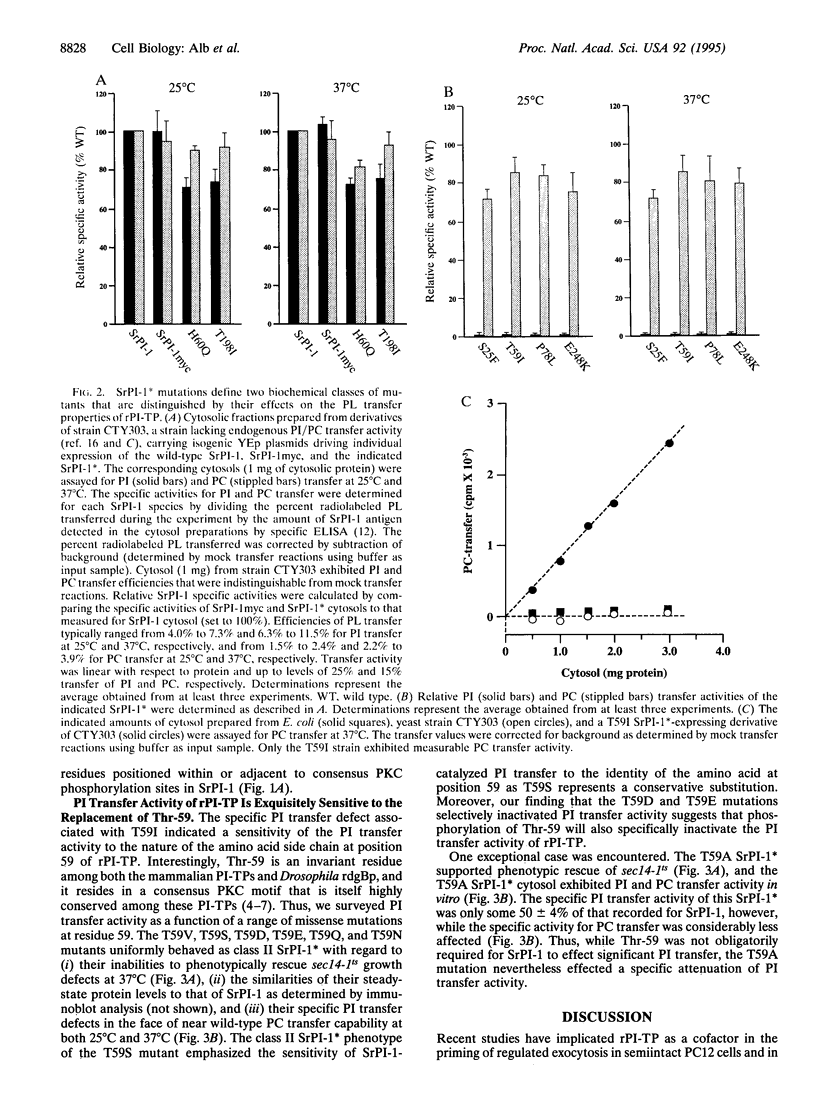
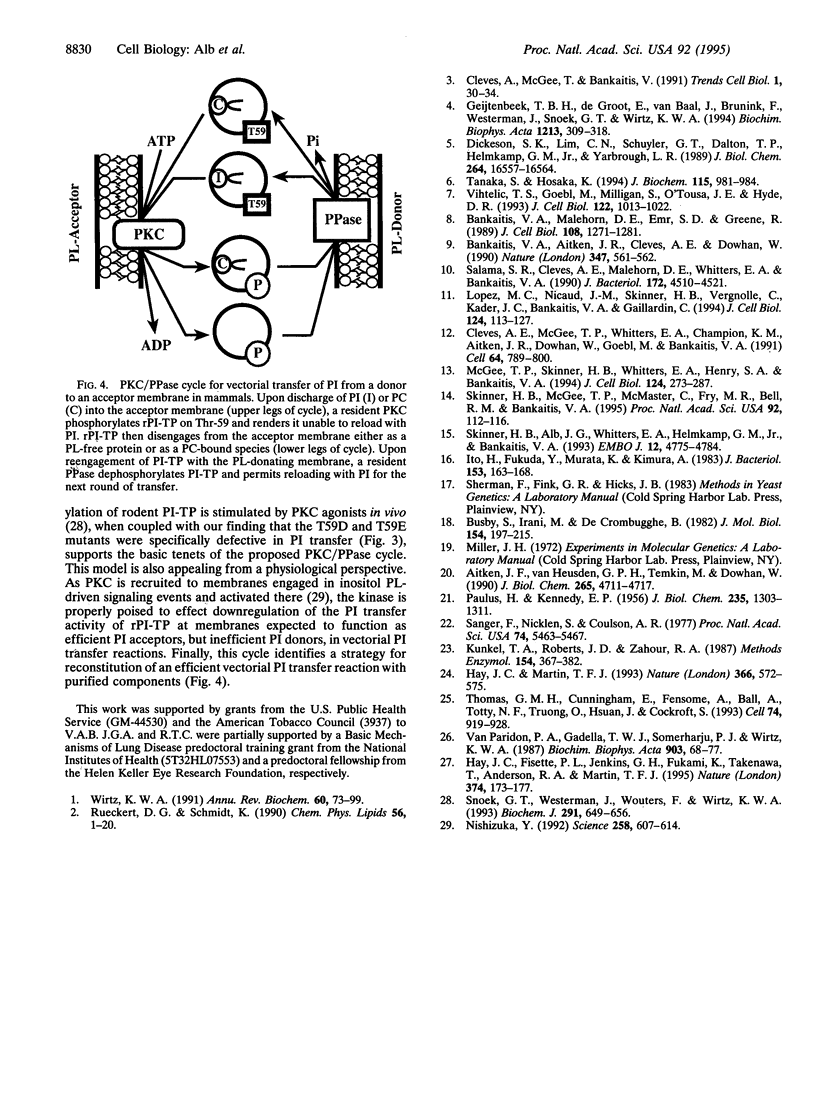
The mammalian phosphatidylinositol/phosphatidylcholine transfer proteins (PI-TPs) catalyze exchange of phosphatidylinositol (PI) or phosphatidylcholine (PC) between membrane bilayers in vitro. We find that Ser-25, Thr-59, Pro-78, and Glu-248 make up a set of rat (r) PI-TP residues, substitution of which effected a dramatic reduction in the relative specific activity for PI transfer activity without significant effect on PC transfer activity. Thr-59 was of particular interest as it is a conserved residue in a highly conserved consensus protein kinase C phosphorylation motif in metazoan PI-TPs. Replacement of Thr-59 with Ser, Gln, Val, Ile, Asn, Asp, or Glu effectively abolished PI transfer capability but was essentially silent with respect to PC transfer activity. These findings identify rPI-TP residues that likely cooperate to form a PI head-group binding/recognition site or that lie adjacent to such a site. Finally, the selective sensitivity of the PI transfer activity of rPI-TP to alteration of Thr-59 suggests a mechanism for in vivo regulation of rPI-TP activity.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aitken J. F., van Heusden G. P., Temkin M., Dowhan W. The gene encoding the phosphatidylinositol transfer protein is essential for cell growth. J Biol Chem. 1990 Mar 15;265(8):4711–4717. [PubMed] [Google Scholar]
- Bankaitis V. A., Aitken J. R., Cleves A. E., Dowhan W. An essential role for a phospholipid transfer protein in yeast Golgi function. Nature. 1990 Oct 11;347(6293):561–562. doi: 10.1038/347561a0. [DOI] [PubMed] [Google Scholar]
- Bankaitis V. A., Malehorn D. E., Emr S. D., Greene R. The Saccharomyces cerevisiae SEC14 gene encodes a cytosolic factor that is required for transport of secretory proteins from the yeast Golgi complex. J Cell Biol. 1989 Apr;108(4):1271–1281. doi: 10.1083/jcb.108.4.1271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busby S., Irani M., Crombrugghe B. Isolation of mutant promoters in the Escherichia coli galactose operon using local mutagenesis on cloned DNA fragments. J Mol Biol. 1982 Jan 15;154(2):197–209. doi: 10.1016/0022-2836(82)90060-2. [DOI] [PubMed] [Google Scholar]
- Cleves A. E., McGee T. P., Whitters E. A., Champion K. M., Aitken J. R., Dowhan W., Goebl M., Bankaitis V. A. Mutations in the CDP-choline pathway for phospholipid biosynthesis bypass the requirement for an essential phospholipid transfer protein. Cell. 1991 Feb 22;64(4):789–800. doi: 10.1016/0092-8674(91)90508-v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cleves A., McGee T., Bankaitis V. Phospholipid transfer proteins: a biological debut. Trends Cell Biol. 1991 Jul;1(1):30–34. doi: 10.1016/0962-8924(91)90067-j. [DOI] [PubMed] [Google Scholar]
- Dickeson S. K., Lim C. N., Schuyler G. T., Dalton T. P., Helmkamp G. M., Jr, Yarbrough L. R. Isolation and sequence of cDNA clones encoding rat phosphatidylinositol transfer protein. J Biol Chem. 1989 Oct 5;264(28):16557–16564. [PubMed] [Google Scholar]
- Geijtenbeek T. B., de Groot E., van Baal J., Brunink F., Westerman J., Snoek G. T., Wirtz K. W. Characterization of mouse phosphatidylinositol transfer protein expressed in Escherichia coli. Biochim Biophys Acta. 1994 Aug 4;1213(3):309–318. doi: 10.1016/0005-2760(94)00063-8. [DOI] [PubMed] [Google Scholar]
- Hay J. C., Fisette P. L., Jenkins G. H., Fukami K., Takenawa T., Anderson R. A., Martin T. F. ATP-dependent inositide phosphorylation required for Ca(2+)-activated secretion. Nature. 1995 Mar 9;374(6518):173–177. doi: 10.1038/374173a0. [DOI] [PubMed] [Google Scholar]
- Hay J. C., Martin T. F. Phosphatidylinositol transfer protein required for ATP-dependent priming of Ca(2+)-activated secretion. Nature. 1993 Dec 9;366(6455):572–575. doi: 10.1038/366572a0. [DOI] [PubMed] [Google Scholar]
- Ito H., Fukuda Y., Murata K., Kimura A. Transformation of intact yeast cells treated with alkali cations. J Bacteriol. 1983 Jan;153(1):163–168. doi: 10.1128/jb.153.1.163-168.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kunkel T. A., Roberts J. D., Zakour R. A. Rapid and efficient site-specific mutagenesis without phenotypic selection. Methods Enzymol. 1987;154:367–382. doi: 10.1016/0076-6879(87)54085-x. [DOI] [PubMed] [Google Scholar]
- Lopez M. C., Nicaud J. M., Skinner H. B., Vergnolle C., Kader J. C., Bankaitis V. A., Gaillardin C. A phosphatidylinositol/phosphatidylcholine transfer protein is required for differentiation of the dimorphic yeast Yarrowia lipolytica from the yeast to the mycelial form. J Cell Biol. 1994 Apr;125(1):113–127. doi: 10.1083/jcb.125.1.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGee T. P., Skinner H. B., Whitters E. A., Henry S. A., Bankaitis V. A. A phosphatidylinositol transfer protein controls the phosphatidylcholine content of yeast Golgi membranes. J Cell Biol. 1994 Feb;124(3):273–287. doi: 10.1083/jcb.124.3.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- PAULUS H., KENNEDY E. P. The enzymatic synthesis of inositol monophosphatide. J Biol Chem. 1960 May;235:1303–1311. [PubMed] [Google Scholar]
- Rueckert D. G., Schmidt K. Lipid transfer proteins. Chem Phys Lipids. 1990 Nov;56(1):1–20. doi: 10.1016/0009-3084(90)90083-4. [DOI] [PubMed] [Google Scholar]
- Salama S. R., Cleves A. E., Malehorn D. E., Whitters E. A., Bankaitis V. A. Cloning and characterization of Kluyveromyces lactis SEC14, a gene whose product stimulates Golgi secretory function in Saccharomyces cerevisiae. J Bacteriol. 1990 Aug;172(8):4510–4521. doi: 10.1128/jb.172.8.4510-4521.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H. B., Alb J. G., Jr, Whitters E. A., Helmkamp G. M., Jr, Bankaitis V. A. Phospholipid transfer activity is relevant to but not sufficient for the essential function of the yeast SEC14 gene product. EMBO J. 1993 Dec;12(12):4775–4784. doi: 10.1002/j.1460-2075.1993.tb06166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner H. B., McGee T. P., McMaster C. R., Fry M. R., Bell R. M., Bankaitis V. A. The Saccharomyces cerevisiae phosphatidylinositol-transfer protein effects a ligand-dependent inhibition of choline-phosphate cytidylyltransferase activity. Proc Natl Acad Sci U S A. 1995 Jan 3;92(1):112–116. doi: 10.1073/pnas.92.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snoek G. T., Westerman J., Wouters F. S., Wirtz K. W. Phosphorylation and redistribution of the phosphatidylinositol-transfer protein in phorbol 12-myristate 13-acetate- and bombesin-stimulated Swiss mouse 3T3 fibroblasts. Biochem J. 1993 Apr 15;291(Pt 2):649–656. doi: 10.1042/bj2910649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka S., Hosaka K. Cloning of a cDNA encoding a second phosphatidylinositol transfer protein of rat brain by complementation of the yeast sec14 mutation. J Biochem. 1994 May;115(5):981–984. doi: 10.1093/oxfordjournals.jbchem.a124448. [DOI] [PubMed] [Google Scholar]
- Thomas G. M., Cunningham E., Fensome A., Ball A., Totty N. F., Truong O., Hsuan J. J., Cockcroft S. An essential role for phosphatidylinositol transfer protein in phospholipase C-mediated inositol lipid signaling. Cell. 1993 Sep 10;74(5):919–928. doi: 10.1016/0092-8674(93)90471-2. [DOI] [PubMed] [Google Scholar]
- Van Paridon P. A., Gadella T. W., Jr, Somerharju P. J., Wirtz K. W. On the relationship between the dual specificity of the bovine brain phosphatidylinositol transfer protein and membrane phosphatidylinositol levels. Biochim Biophys Acta. 1987 Sep 18;903(1):68–77. doi: 10.1016/0005-2736(87)90156-8. [DOI] [PubMed] [Google Scholar]
- Vihtelic T. S., Goebl M., Milligan S., O'Tousa J. E., Hyde D. R. Localization of Drosophila retinal degeneration B, a membrane-associated phosphatidylinositol transfer protein. J Cell Biol. 1993 Sep;122(5):1013–1022. doi: 10.1083/jcb.122.5.1013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wirtz K. W. Phospholipid transfer proteins. Annu Rev Biochem. 1991;60:73–99. doi: 10.1146/annurev.bi.60.070191.000445. [DOI] [PubMed] [Google Scholar]