Atherosclerotic plaques contain a variety of highly thrombogenic material, such as the platelet activator collagen and the procoagulant protein tissue factor. Atherothrombosis describes the formation of a thrombus after rupture or erosion of an atherosclerotic plaque. An occlusive thrombus in coronary and carotid arteries induces myocardial infarction and stroke, respectively. Arterial clots are platelet-rich so patients with cardiovascular disease are treated prophylactically with antiplatelets drugs, such as aspirin and clopidogrel. Importantly, the recent clinical trial (ATLAS ACS 2-TIMI 51) showed that addition of low doses of the direct oral anti-factor Xa drug rivaroxaban to standard antiplatelet therapy in patients with recent acute coronary syndrome reduced death from cardiovascular causes, myocardial infarction or stroke1. However, the addition of the anticoagulant was associated with increased rates of major bleeding and intracranial hemorrhage. Indeed, an earlier trial (APPRAISE-2) with the factor Xa inhibitor apixaban was terminated prematurely because of an increase in major bleeding events2. Therefore, there is a need for a safer anticoagulant that reduces atherothrombosis without increasing bleeding.
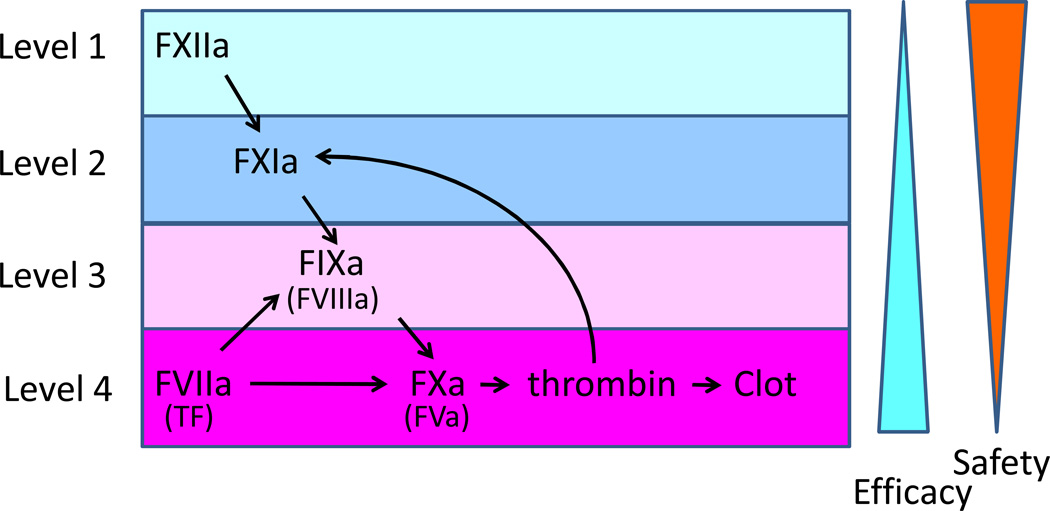
Anticoagulant drugs that are used to treat or prevent thrombosis target the common pathway of coagulation that is also required for hemostasis. However, there is a growing interest in developing safer anticoagulant drugs that target the intrinsic pathway of coagulation (factor XIIa, factor XIa and factor IXa)3. This pathway amplifies the clotting reaction but is not essential for hemostasis like the extrinsic (tissue factor and factor VIIa) and common pathways (factor Xa and thrombin) (Figure). Interestingly, two papers in this issue of Arteriosclerosis, Thrombosis and Vascular Biology show that either inhibition of factor XIIa or a reduction in factor XI reduced atherothrombosis in a mouse model4,5. These findings are significant because thrombosis is reduced without an increase in bleeding and they study thrombosis in atherosclerotic carotid arteries not healthy arteries.
Figure. Tissue factor and factor XII trigger atherothrombosis after atherosclerotic plaque rupture.
Plaque tissue factor initiates a rapid activation of the coagulation cascade whereas factor XIIa amplifies the cascade and stabilizes the thrombus. Factor XII may be activated by a variety of agonists such as collagen, nucleic acids and/or inorganic polyphosphate. One would expect differences in efficacy and safety associated with inhibition of the different levels of the intrinsic pathway versus the extrinsic and common pathways.
Numerous studies have analyzed the role of different clotting factors in mouse models of arterial thrombosis. The most popular is the ferric chloride carotid artery thrombosis model. Topical application of ferric chloride induces oxidative damage of the vessel wall and formation of an occlusive thrombosis that is monitored using a flow probe6. Components of the extrinsic (tissue factor) and the intrinsic (FXI and FXII) coagulation pathways contribute to clot formation7–9. The extrinsic and intrinsic pathways also play a role in thrombosis in other mouse models10,11. However, the major limitation of these studies is that healthy vessels in young, healthy mice are injured rather diseased vessels.
Feeding Apoe−/− mice a Western-type diet for 18–20 weeks induces atherosclerotic lesions in the aortic arch and carotid arteries. One study showed increased thrombosis in atherosclerotic carotid arteries of Apoe−/− mice indicating that these carotid arteries are more thrombotic compared with healthy carotid arteries12. However, it is rare for these lesions to spontaneously rupture. Importantly, the Heemskerk group recently developed a mouse model of acute atherothrombosis13. Localized rupture of atherosclerotic plaques in carotid arteries is induced in vivo by ultrasound. Subsequent formation of thrombi is visualized by two-photon laser scanning microscropy.
The two new studies used different approaches to analyze the role of the intrinsic coagulation pathway in atherothrombosis. Kuijpers and colleagues4 determined the effect of inhibition of either factor VIIa (extrinsic pathway) or factor XIIa (intrinsic pathway) on atherothrombosis. Interestingly, inhibition of the factor VIIa with an active-site inhibited factor VIIa (FVIIai) reduced early thrombosis formation (0.5 minutes after plaque rupture) without affecting stability as measured by embolization. In contrast, inhibition of factor XIIa with either corn trypsin inhibitor or r-HA-infestin-4 did not affect the initial formation of the thrombus but reduced the size of the thrombus at a later time (2 and 10 minutes after plaque rupture), and increased embolization suggesting that this reduced the stability of the thrombus. Similarly, van Montfoort and colleagues5 showed that lowering factor XI levels using an antisense oligonucleotide did not reduce thrombus formation at 1 and 3 minutes after plaque rupture but did reduce thrombus formation at 5 and 10 minutes. Furthermore, the number of fluorescent platelets shed from the thrombus was increased in mice with a lower level of factor XI. Finally, administration of the factor XI antisense oligonucleotide did not affect the tail bleeding time. An earlier study14 found no role of factor XIIa in an in vitro model of atherothrombosis that used human atheromatous plaque material. However, this model may not accurately reproduce the complexity of thrombus formation in vivo. Taken together, these two studies indicate that both tissue factor/factor VIIa and factor XIIa triggered coagulation contribute to atherothrombosis in this mouse model (Figure).
How do the tissue factor/factor VIIa and factor XII pathways interact during atherothrombosis? Interestingly, factor XII and factor XI have been shown to play a role in tissue factor- induced thrombosis models in baboons and a tissue factor-induced pulmonary embolization in mice15–16. These results suggest that after plaque rupture tissue factor is the initial trigger that rapidly activates the coagulation cascade. This is followed by factor XIIa-dependent amplification of the clotting cascade. The delayed activation of factor XII Factor XII is likely mediated by constituents of the plaque, such as collagen, nucleic acids and polyphosphates. Importantly, inhibition of factor XIIa should be safer than anticoagulants that target factor Xa or thrombin because factor XIIa is not required for hemostasis. Similarly, factor XI deficiency is not associated with major bleeding and would be a relatively safe target. Further studies are needed to directly compare the effect of inhibiting factor XIIa versus factor XIa.
Acknowledgements
I would like to thank A. Phil Owens III for helpful comments and funding from the National Institutes of Health (HL006350).
References
- 1.Mega JL, Brauwald E, Wiviott SD, et al. Rivaroxaban in patients with a recent acute coronary syndrome. N Engl J Med. 2012;366:9–19. doi: 10.1056/NEJMoa1112277. [DOI] [PubMed] [Google Scholar]
- 2.Alexander JH, Lopes RD, James S, et al. Apixaban with antiplatelet therapy after acute coronary syndrome. N Engl J Med. 2011;365:699–708. doi: 10.1056/NEJMoa1105819. [DOI] [PubMed] [Google Scholar]
- 3.Muller F, Gailani D, Renne T. Factor XI and factor XII as antithrombotic targets. Curr Opin Hematol. 2011;18:349–355. doi: 10.1097/MOH.0b013e3283497e61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kuijpers M, van der Meijden P, Feijge M, Mattheiij NJA, May F, Govers-Riemslag J, Meijers JCM, Heemskerk J, Renne T, Cosemans J. Factor XII regulates the pathological process of thrombus formation on rupture plaques. Arterioscler Thromb Vasc Biol. doi: 10.1161/ATVBAHA.114.303315. in press. [DOI] [PubMed] [Google Scholar]
- 5.Van Montfoort M, Kuijpers M, Knaup V, Bhanot S, Monia B, Roelofs, Heemskerk J, Meijers JCM. Inhibition of factor XI prevents pathological thrombus formation an acutely ruptured atherosclerotic plaques. Arterioscler Thromb Vasc Biol. doi: 10.1161/ATVBAHA.114.303209. in press. [DOI] [PubMed] [Google Scholar]
- 6.Westrick RJ, Winn ME, Eitzman DT. Murine models of vascular thrombosis. Arterioscler Thromb Vasc Biol. 2007;27:2079–2093. doi: 10.1161/ATVBAHA.107.142810. [DOI] [PubMed] [Google Scholar]
- 7.Rosen ED, Gailani D, Castellino FJ. FXI is essential for thrombus formation following FeCl3-induced injury of the carotid artery in the mouse. Thromb Haemost. 2002;87:774–776. [PubMed] [Google Scholar]
- 8.Wang X, Cheng Q, Xu L, Feuerstein GZ, Has MY, Smith PL, Seiffert DA, Schumacher WA, Ogletree ML, Gailani D. Effects of factor IX or factor XI deficiency on ferric chloride-induced carotid artery occlusion in mice. J Thromb Haemost. 2005;3:695–702. doi: 10.1111/j.1538-7836.2005.01236.x. [DOI] [PubMed] [Google Scholar]
- 9.Wang L, Miller C, Swarthout RF, Rao M, Mackman N, Taubman MB. Vascular smooth muscle-derived tissue factor is critical for arterial thrombosis after ferric chloride-induced injury. Blood. 2009;113:705–713. doi: 10.1182/blood-2007-05-090944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Day SM, Reeve JL, Pedersen B, Farris DM, Myers DD, Im M, Wakefield TW, Mackman N, Fay WP. Macrovascular thrombosis is driven by tissue factor derived primarily from the blood vessel wall. Blood. 2005;105:192–198. doi: 10.1182/blood-2004-06-2225. [DOI] [PubMed] [Google Scholar]
- 11.Renne T, Pozgajova M, Gruner S, Schuh K, Pauer HU, Burfeind P, Gailani D, Nieswandt B. Defective thrombus formation in mice lacking factor XII. J Exp Med. 2005;202:271–281. doi: 10.1084/jem.20050664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Westrick RJ, Bodary PF, Xu Z, Shen YC, Broze GJ, Eitzman DT. Deficiency of tissue factor pathway inhibitor promotes atherosclerosis and thrombosis in mice. Circulation. 2001;103:3044–3046. doi: 10.1161/hc2501.092492. [DOI] [PubMed] [Google Scholar]
- 13.Kuijpers MJE, Gilio K, Reitsma S, Nergiz-Unal R, Prinzen L, Heenman S, Lutgens E, van Zandvoort AMJ, Nieswandt B, Oude Egbrink GA, Heemskerk JWM. J Thromb Haemost. 2008;7:152–161. doi: 10.1111/j.1538-7836.2008.03186.x. [DOI] [PubMed] [Google Scholar]
- 14.Reininger AJ, Bernlochner I, Penz SM, Ranvanat C, Smethurst P, Farndale RW, Gachet C, Brandl R, Seiss W. A 2-step mechanism of arterial thrombosis formation induced by human atherosclerotic plaques. J Am Coll Cardiol. 2010;55:1147–1158. doi: 10.1016/j.jacc.2009.11.051. [DOI] [PubMed] [Google Scholar]
- 15.Gruber A, Hanson SR. Factor XI-dependence of surface- and tissue factor-initiated thrombus propagation on primates. Blood. 2003;102:953–955. doi: 10.1182/blood-2003-01-0324. [DOI] [PubMed] [Google Scholar]
- 16.Cheng Q, Tucker EI, Pine MS, Sisler I, Matafonov A, Sun M-F, White-Adams TC, Smith SA, Hanson SR, McCarty OJT, Renne T, Gruber A, Gailani D. Blood. 2010;116:3981–3989. doi: 10.1182/blood-2010-02-270918. [DOI] [PMC free article] [PubMed] [Google Scholar]