Abstract
Despite high rates of tobacco use during adolescence, few empirically validated smoking cessation strategies exist for adolescent smokers. Developing an understanding of the neural underpinnings of cognitive control processes in adolescent smokers, and their relationship to quit behaviors, may help advance the development of enhanced behavioral and pharmacological therapies. The current pilot study explored the relationship between brain responses during performance of the Stroop color-word interference task and reduction in tobacco use (as measured by changes in cotinine levels) in treatment-seeking adolescent smokers participating in a high-school-based smoking-cessation program. Eleven adolescent daily smokers participated in a pre-quit session during which neural activity in response to congruent and incongruent events in a Stroop task was examined using functional magnetic resonance imaging (fMRI). Changes in urine cotinine levels from pre-quit baseline to end of treatment were calculated and correlated with brain activity. Adolescents with greater activation in the inferior frontal gyrus, insula, thalamus and anterior cingulate had greater reductions in cotinine levels. The preliminary observation of a relationship between treatment outcome and neural correlates of cognitive control prior to treatment onset provides insight into individual differences in adolescent brain function that might relate importantly to treatment outcome.
Keywords: Adolescent, Stroop, cigarette, smoking, treatment
Introduction
Tobacco smoking, one of the leading precedents of a number of disease states and premature death, is primarily initiated during adolescence (Arrazola, Dube, Kaufmann, Caraballo, & Pechacek, 2011). According to the World Health Organization (WHO, 2013), if current trends continue, approximately 250 million children and teenagers who initiate smoking will die from tobacco-related diseases in adulthood. In the US alone, nearly 2000 children and adolescents begin smoking each year and more than 1/5th are current smokers by the time they leave high school. While most adolescent smokers report high interest in quitting smoking (61%; Arrazola et al., 2011), the success rates of aided and unaided quit attempts are low and range between 7–12%, and most existing treatments have limited success (Backinger & Leishchow, 2001; Grimshaw & Stanton, 2006; Sussman, 2002; Sussman, Ping, & Dent, 2006; Wiehe, Garrison, Christakis, Ebel, & Rivara, 2005). A better understanding of the neurobiological correlates of treatment outcome may facilitate the development of more effective cessation strategies.
Adolescence is a period of great developmental plasticity and growth, particularly in areas of the brain such as the prefrontal cortex that are important for higher executive functions governing cognitive control (Galvan, Hare, Parra, Penn, Voss, Glover & Casey, 2006; Giedd & Rappoport, 2010). Neural circuitry underlying cognitive control, a construct involving aspects of attention, conflict monitoring and response inhibition (Botvinick, Braver, Barch, Carter, & Cohen, 2001), has been proposed to contribute to decision-making underlying participation in addictive behaviors (Bechara, 2003). It has been proposed that during adolescence, differential neurodevelopmental trajectories of brain regions, and their interconnected circuitry, may enhance adolescent susceptibility to many high-risk behaviors, including tobacco use (Ernst, Romeo, & Andersen, 2009; Rutherford, Mayes, & Potenza, 2010; Somerville, Jones, & Casey, 2010; Chambers, Taylor, & Potenza, 2003). More specifically, tobacco use has been found to relate to deficits in neural circuitry underlying cognitive control and related processes (Galvan, Poldrack, Baker, Mcglennen, & London, 2011; Jacobsen, Slotkin, Mencl, Frost, & Pugh, 2007). Together, these data highlight the relevance of studying the neural circuitry underlying cognitive control in adolescents seeking to quit tobacco use.
This pilot study in treatment-seeking adolescent smokers is the first to explore the relationship between pre-treatment brain activation during performance of the Stroop color-word interference task and treatment-related changes in tobacco use. We chose to use the Stroop, a task that assesses cognitive control (Botvinick et al., 2001), because: 1) neural correlates of Stroop task performance have been previously investigated in adults (Peterson, Skudlarski, Gatenby, Zhang, Anderson, & Gore, 1999; Leung, Skudlarski, Gatenby, Peterson, & Gore, 2000) and adolescents (Peterson, Potenza, Wang, Zhu, Martin, Marsh, Plessen, & Yu, 2009); and, 2) both behavioral (Streeter et al., 2007) and neural (Brewer, Worhunsky, Carroll, Rounsaville, & Potenza, 2008) correlates of Stroop task performance have been linked to treatment outcomes amongst cocaine-dependent adults. In these last two studies, increased task-related activation of cortico-striatal brain regions including the ventromedial prefrontal cortex (vmPFC), cingulate cortex and striatum at treatment onset was associated with better abstinence at treatment outcome. Therefore, we hypothesized that amongst adolescent smokers pre-treatment activation of similar regions (vmPFC, cingulate and striatum) would correlate with decreases in biological measures of tobacco use. Furthermore, since the inferior frontal gyrus (IFG) has been shown to be associated with response inhibition in adolescents and adults across species (Galvan et a., 2011; Finnenberg et al., 2010), and the insula and dorsal anterior cingulate (ACC) have been linked to tobacco abstinence maintenance among adult smokers (Janes et al., 2010), we also hypothesized that Stroop-related activations in these regions would be associated with reductions in cotinine levels (a metabolite of nicotine which has a longer half life of 18–20 hours compared with that of nicotine which is 2–3 hours).
Material and Methods
All study procedures were approved by the Yale School of Medicine Human Investigations committee. Prior to initiation of any study procedures, parental consent and adolescent assent was obtained from participants aged 14–17 years and consent was obtained from adolescents who were 18 years old.
Participants
Adolescent smokers were recruited from local Connecticut high schools to participate in a smoking cessation research study using procedures similar to our earlier work (Cavallo et al., 2007; Krishnan-Sarin et al., 2006). Adolescents had to report smoking at least five cigarettes per day for the past six months, with quantitative urine cotinine levels of 350 ng/ml or higher (Graham Massey Analytical Labs, Shelton, CT), and had to be seeking treatment. The participants in the current study (n=11) were a subgroup who chose to participate in the fMRI study from a larger group of 82 smokers who participated in a smoking cessation trial (Krishnan-Sarin et al., 2012) all of whom were offered the option of completing the fMRI study.
All participants completed questionnaires assessing demographic information, smoking history as well as nicotine dependence (modified Fagerstrom Tolerance Questionnaire; mFTQ; Prokhorov, Pallonen, Fava, Ding, & Niaura, 1996). The Diagnostic Interview Schedule Children Predictive Scale (DPS; Lucas et al., 2001) and an evaluation by a clinical psychologist were used to assess current DSM-IV Axis I disorders (First, Spitzer, Gibbon, & Williams, 2002) and exclude those with a current diagnosis of generalized anxiety disorder, major depressive disorder, or a substance dependence disorder (other than nicotine dependence). We also excluded those with any significant current medical condition, endorsing a suicidal/homicidal risk or using any psychotropic medications (including anxiolytics and antidepressants). Other exclusionary criteria included color-blindness or pregnancy and conditions that are contra-indicated to fMRI scanning.
fMRI Stroop Task
A week to ten days prior to initiating the treatment program, all participants performed the fMRI Stroop color-word interference task as described in the “supplemental materials” section and in our previous publications (Brewer et al., 2008; Devito, Worhunsky, Carroll, Rounsaville, Kober, & Potenza, 2012).
Smoking Cessation Intervention and Other Procedures
All interventions were guided and supervised using manuals based on our previous work (Cavallo et al., 2007; Krishnan-Sarin et al., 2006). Briefly, all participants scheduled a quit date which was the start of a four-week treatment period and received a 45-minute “preparation to quit” session, 4–7 days prior to their quit date, during which motivational and cognitive behavioral strategies were used to emphasize the risks of continued smoking and benefits of quitting and teach strategies to initiate cigarette abstinence on quit day. At the end of this session, adolescents were randomly assigned to receive one of three treatment conditions for four weeks following quit date: Cognitive Behavioral Therapy (CBT) alone (no abstinence reinforcement), Contingent Reinforcement for abstinence (Contingency Management - CM) alone, or CBT+CM. Those in the CM conditions could receive up to $262 over the four weeks if they remained abstinent [as assessed daily using CO levels (Vitalograph Breath CO, Bedfont, MA0 and semiquantitative urine cotinine readings (NicAlert Immunoassay Test Strips, Jant Pharmacal Corporation, Encino, CA)]. All groups also received payments ($5–$20) for attending weekly assessment appointments and completing CBT sessions. Payments for attendance were chosen to ensure fairly equivalent total incentives across groups (and minimize the possibility of differences in outcome being related to incentive amounts) and were as follows: 1) CM alone group: $5 at each weekly appointment for completing assessments, 2) CM + CBT group: $5 at each weekly appointment for completing assessments and $5 for attending CBT sessions, 3) CBT alone group: $20 at each weekly appointment for completing assessments and $20 for attending CBT sessions.
Participants also received monetary compensation ($75) for completing the fMRI session. The primary outcome was end of treatment quantitative urine cotinine levels (Graham Massey Analytical labs, Shelton, CT).
Data Analyses - 2.4.1 fMRI Data Acquisition and Analyses
Images were obtained with a Siemens TIM Trio 3T MRI system (Siemens AG, Erlangen, Germany) and analyzed using methods described in our previous publications (Brewer et al., 2008;Devito et al., 2012; Kober et al., 2010) and in the “supplemental materials” section.
Changes in Cotinine Levels and Stroop-related Activity
Percent changes in cotinine levels were calculated by subtracting end-of-treatment cotinine levels from the baseline. Per guidelines provided by Mermelstein and colleagues (Mermelstein et al., 2002), the two non-completers were considered to be smoking and therefore have no changes in cotinine levels. Correlations between differences in blood-oxygen-level-dependent (BOLD) signal-change during incongruent versus congruent conditions and percent change in cotinine levels during treatment were assessed using voxel-wise correlational analyses across the whole brain, employing a family-wise-error correction.
Results
Participants
Participants were 11 treatment-seeking adolescents (4 female; 9 Caucasian, 1 Hispanic, 1 African American) with a mean age of 17 years (SD=1.12) (Table 1) participating in a school-based smoking-cessation trial (Krishnan-Sarin et al., 2012). When compared to the 71 adolescents who did not participate in the fMRI study, the 11 participating adolescents did not differ on number of cigarettes/day [14.1 (SD=5.2) versus 12.22 (SD=4.99)] or average modified Fagerstrom scores [5.4 (SD=1.2) versus 5.4 (SD=1.8)], but did have higher baseline urine cotinine levels [1315 (SD=786) versus 1091 (SD=205) ng/ml, p<0.05]. While none of the participants met criteria for any non-nicotine substance abuse or dependence, 6 reported marijuana use and 7 reported alcohol use (see Table 1). Of the 11 participants, eight completed the four-week intervention program, one quit but discontinued treatment after the first week and two discontinued treatment prior to quit day. There were no demographic differences between completers and non-completers, or in end-of-treatment cotinine levels between treatment modalities.
Table 1.
Characteristics of Adolescent participants
| Full sample | CBT alone | CM alone | CM+CBT | |
|---|---|---|---|---|
| N | 11 | 4 | 3 | 4 |
| Male/Female | 7/4 | 2/2 | 2/1 | 3/1 |
| Age (SD) | 17.0 (1.1) | 16.5 (1.5) | 17.0 (0.9) | 17.5 (1.2) |
| Cigarettes/day | 12.22 (4.99) | 12.8 (4.0) | 11.8 (3.5) | 12.0 (5.0) |
| Baseline Cotinine | 1315 (786) | 1280 (450) | 1340 (834) | 1325 (720) |
| Fagerstrom scores | 5.4 (1.8) | 5.2 (2.5) | 5.6 (1.1) | 5.4 (1.5) |
| Marijuana use | N=6 10.8 (7.1) days/past month [0.5 joints/each day of use] |
N=2 10.2 (4.3) days/past month |
N=2 11.5 (8.5) days/past month |
N=2 10.7(6.8) days/past month |
| Alcohol use | N=7 1.7 (0.9) days/past month [4.2 (0.3) drinks/drinking day] |
N=3 1.8 (1.1) days/past month |
N=1 1.6 days/past month |
N=3 1.7 (0.9) days/past month |
| Treatment Completers | N=8 | N=3 | N=2 | N=3 |
Cotinine Levels
End-of-treatment cotinine levels were significantly reduced in all completers from baseline levels of 1250 (SD=814) to 259 (SD=104) ng/ml (t=3.18, p<0.05), with three participants having levels of 0 ng/ml.
Stroop Behavioral Performance
A paired-samples t-test showed a significant difference in reaction times between congruent and incongruent stimuli (t=-3.19, p=.01). Average reaction time to congruent stimuli was significantly shorter (M=446.56msec, SD=90.57) than the average reaction time to incongruent stimuli (M=533.13msec, SD=169.19), consistent with greater interference during incongruent trials. The mean number of errors per incongruent Stroop run was 2.34 (SD=1.64), representing an overall error frequency of 6.7%. Behavioral measures (reaction times to congruent and incongruent stimuli and Stroop error rates) did not correlate with baseline smoking (mean cigarettes/day) or percent change in cotinine levels during treatment (both p>0.05).
fMRI Results
fMRI Stroop Effect
The main effect of Stroop trials is described in Table 2A. Consistent with other fMRI studies of the Stroop-effect (Carter & Van Veen, 2007), the contrast of incongruent versus congruent trials showed increased activity in areas including the bilateral IFG, insula, dorsal ACC, medial prefrontal cortex (mPFC) and subcortical regions including the striatum and thalamus (Supplemental Figure 1). Baseline levels of smoking (cigarettes/day, cotinine levels) correlated with Stroop-related activations (shown in “Supplemental Results” section).
Table 2.
| A: Main effects (incongruent versus congruent stimuli) on the Stroop Task | ||||||||
|---|---|---|---|---|---|---|---|---|
| MNI Coordinates |
||||||||
| Stroop Main Effect Contrast |
Structure | BA | Left/ Right |
x | y | z | k | T |
| Incongruent> Congruent | Anterior Cingulate/ Insula/Caudate/Thalamus/ Middle Frontal Gyrus | 32 | L | 0 | 27 | 36 | 4536 | 13.03 |
| Inferior Parietal Lobule/ Supramarginal Gyrus | 40 | R | 45 | −30 | 51 | 979 | 10.67 | |
| Inferior Frontal Gyrus/ Middle Frontal Gyrus | R | 45 | 27 | −3 | 1587 | 10.33 | ||
| Inferior Parietal Lobule | 40 | L | −51 | −39 | 27 | 724 | 8.66 | |
| Superior Frontal Gyrus/ Orbital Gyrus/ Medial Frontal Gyrus | 8 | L | −18 | 36 | 48 | 1883 | −11.45 | |
| Posterior Cingulate/ Middle Temporal Gyrus/ Precentral Gyrus/ Paracentral Lobule/ Postcentral Gyrus | 23 | L | −9 | −57 | 12 | 2669 | −9.36 | |
| Middle Temporal Gyrus | 21 | L | −51 | 9 | −27 | 447 | −7.19 | |
| Superior Temporal Gyrus | 22 | R | 69 | −27 | 6 | 460 | −5.03 | |
| B: Stroop main effect correlations with percent change in cotinine levels | ||||||||
|---|---|---|---|---|---|---|---|---|
| MNI Coordinates | ||||||||
| Stroop Main Effect Contrast |
Structure | BA | Left/ Right |
x | y | z | k | Peak R-value |
| Incongruent> Congruent | Medial Frontal Gyrus/ Anterior Cingulate/ vmPFC | 9 | R | 9 | 36 | 33 | 280 | 0.886 |
| Insula/ Inferior Frontal Gyrus | 13 | R | 6 | −18 | −15 | 298 | 0.857 | |
BA = Brodman’s Area
ROI = Region of Interest
k = cluster size in voxels
positive T values indicate regions in which greater activation is seen in response to incongruent versus congruent stimuli; negative T values indicate regions in which greater activation is seen in response to congruent versus incongruent stimuli
BA = Brodman’s Area
ROI = Region of Interest
k = cluster size in voxels
vmPFC= ventromedial prefrontal cortex
Correlations Between Changes in Cotinine Levels and fMRI Stroop Effect
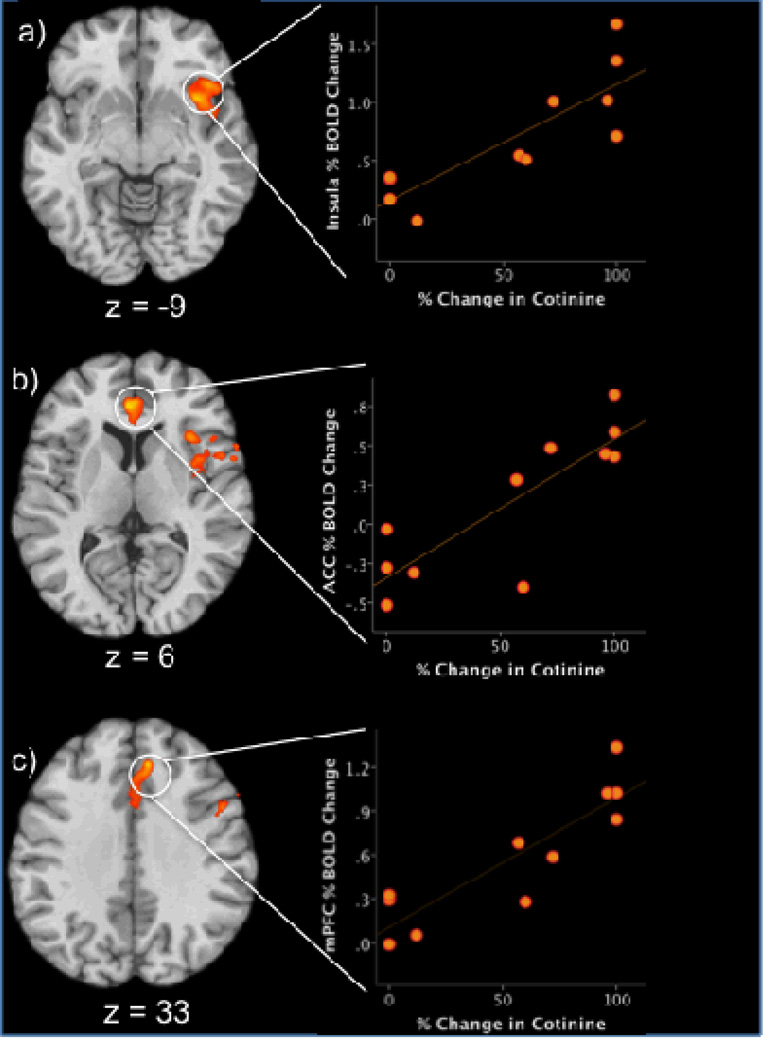
Whole-brain correlations were performed between percent change in cotinine levels from baseline to the end of study and pre-treatment Stroop activity (incongruent vs. congruent trials; Supplemental Figure 2; Figure 1; Table 2B). Percent change in cotinine levels was positively correlated with Stroop-effect activity in the: 1) right insula extending to the right IFG (Figure 1a); 2) dorsomedial frontal gyrus (Figure 1c) extending ventrally to the ACC (Figure 1b); and, 3) right midbrain extending to the thalamus (Supplemental Figure 2). Percent change in cotinine levels was negatively correlated with activation in the: 1) right superior frontal gyrus; and, 2) the posterior cingulate extending to the parahippocampal gyrus (Supplemental Figure 2).
Figure 1.
Significant correlations between Stroop related activation and percent changes in cotinine levels (left side of figure). Orange/yellow indicates areas of positive correlations between % decrease in cotinine levels and increased % BOLD signal changes in the incongruent versus congruent contrast. Axial slices demonstrate the correlation with a cluster in the right insula (z=−9), ACC (z=6) and dorsal mPFC (z=33). Numbers indicate z-axis MNI coordinates. Right side of the brain is on the right. Scatterplots (right side of figure) demonstrate the distribution of individual scores in the correlation between % change in cotinine levels and % BOLD signal change in A) Insula, B) ACC, and C) medal prefrontal cortex, during Stroop task performance in the adolescent smoking group (n = 11).
BOLD = blood-oxygenation-level-dependent
MNI = Montreal Neurological Institute
ACC=anterior cingulate cortex
mPFC= medial prefrontal cortex
r = Pearson correlation coefficient
Discussion
This pilot investigation examined the relationship between pre-treatment regional brain activation during a cognitive control task in treatment-seeking adolescent smokers. We observed that reduction in tobacco use, as measured by changes in urine cotinine levels, was related to pre-treatment Stroop-related brain activations. As in previous studies of adults and adolescents (Leung et al., 2000; Peterson et al., 2009, Devito et al., 2012; Worhunsky et al., 2013), fMRI Stroop effect was associated with activation of brain regions including dorsolateral prefrontal, ventrolateral prefrontal, insular and anterior cingulate cortices. Greater activation in the IFG, ACC and insula, but not in the vmPFC or striatum, was associated with greater reduction in cotinine levels (Brewer et al., 2008). Activity in other regions including dorsal mPFC, thalamus, posterior cingulate, cerebellum and parahippocampus was also related to treatment outcome. These preliminary results indicate that adolescents showing greater Stroop-related activation of cognitive control circuitry prior to behavioral therapy may be better able to decrease or quit their smoking. One attractive explanation for this finding is that they may be able to do so by more successfully exerting cognitive control in situations that might interfere with their quit effort. Alternatively, these activation patterns may reflect a greater capacity to incorporate elements of behavioral therapies and future studies (including pre-/post-treatment measurement powered to investigate each therapy) are needed to examine these possibilities.
The findings from the present pilot study show both similarities to and differences from findings from other drug-using populations. For example, although pre-treatment Stroop-related brain activation was related to better biologically measured treatment outcomes in the present study, different brain regions (the vmPFC, striatum and more posterior aspects of the cingulate) were implicated in a study of cocaine-dependent adults (Brewer et al., 2008). However, subsequent analysis of the cocaine-dependent adults using independent component analysis related activations of many of the brain regions identified in the current study (including dorsal ACC, mPFC, insula, IFG and thalamus) to networks linked to treatment outcome (Worhunsky et al., 2013). Additionally, increased activation of similar brain regions has been observed during performance of a cognitive control task in a separate study of cocaine-abusing adults and linked with treatment outcome (Connollya, Foxec, Nierenberge, Shpanerc, & Garavan, 2012), suggesting that these regions contribute to cognitive control and outcome more broadly.
Interestingly, amongst female adult smokers, relatively increased pre-treatment activation of the insula and dorsal ACC in response to smoking-related cues was observed in those who later slipped or relapsed to smoking (Janes et al., 2010). Furthermore, relatively diminished functional connectivity between the insula and ACC was observed in the slip/lapse group, and the degree of smoking-cue-related activation in the insula and ACC correlated with smoking-related attentional biases. These findings and others (Peterson et al., 1999; Janes et al., 2010; Wexler, 2001) suggest that some smokers might be better able to engage similar brain regions for attentional rather than motivational purposes when presented with smoking-related visual cues (Janes et al., 2010b). Thus, in the present study, relatively greater Stroop-related engagement of ACC and related neurocircuitry was implicated in attentional and impulse control aspects of Stroop performance (Petersen et al., 1999). These results also suggest possible greater disengagement of regions like the posterior cingulate implicated in default mode processing. Both congruent and incongruent conditions were associated with relatively diminished activity in this network; however, significantly greater decreases were noted in the incongruent condition. In this way, better cognitive control may be linked to improved treatment outcome in adolescent smokers. These findings resonate with those from other studies of drug dependence in which relatively increased activation of attentional circuitry during cognitive control processing and relatively decreased activation of similar regions during reward processing are linked to better outcomes (Brewer et al., 2008; Jia et al., 2011), consistent with the notion that “top-down” and “bottom-up” processes may compete for recruitment of overlapping networks (Potenza, Sofuoglu, Carroll, & Rounsaville, 2011). Although currently speculative, these hypotheses warrant further testing and consideration in development of behavioral and pharmacological treatments for smoking cessation. For example, from a psychosocial perspective, teaching adolescents how to attend to cues and control impulses both at the conscious and subconscious levels using cognitive remediation strategies may warrant examination (Janes et al., 2010; Wexler, 2011). From a pharmacological perspective, medications that alter the function of these brain regions may help with treatment of addictions (Potenza et al., 2011).
From a developmental perspective, preclinical evidence suggests that the frontal regions of the adolescent brain are particularly susceptible to nicotine, and that changes induced by nicotine exposure may lead to cognitive deficits that persist into adulthood (Counotte et al., 2008; Schochet, Kelley, & Landry, 2005). Clinical evidence from adult smokers supports the existence of cognitive deficits, especially in deprived smokers, and nicotine appears to increase task-related neural activity in deprived but not in active smokers (Newhouse, Potter, Dumas, & Thiel, 2011). In contrast, the influence of tobacco use and deprivation on cognitive deficits in adolescents is still controversial (Colby et al., 2010; Dinn, Aycicegi, & Harris, 2004; Jacobsen, Krystal, Mencl, Westerveld, Frost, & Pugh, 2005; Zack, Belsito, Scher, Eissenberg, & Corrigall, 2001). However, limited existing evidence suggests that adolescent tobacco users have deficits in the neural circuitry related to attention and memory processes (Jacobsen et al., 2007) as well as in executive-function-related processes (Galvan et al., 2011). We observed that during incongruent relative to congruent trials adolescent smokers experienced enhanced activity in frontal regions like the bilateral IFG, dorsal ACC, mPFC and subcortical regions including the striatum and thalamus. Since our study did not include nonsmokers, we cannot draw any conclusions about tobacco-specific deficits in neural responses on the Stroop task. However, Galvan and colleagues (2011) have reported that while adolescent smokers and nonsmokers did not differ in neural responses to a Stop-Signal task, heavier smoking was associated with greater cortical activation. It is important to note that adolescent smokers in our study were not deprived from cigarettes for a prolonged period of time, suggesting that effects observed were probably not related to nicotine abstinence. Future studies should examine the influence of tobacco smoking and abstinence on cognitive control processes and their neural underpinnings in adolescent smokers.
An important limitation of our study is the small sample size; thus conclusions should be drawn tentatively. Future studies with larger samples may replicate these findings or have greater power to detect efficacies related to specific individual characteristics or therapeutic influences. Because of the limited sample size, we were unable to examine the effects of the different treatment modalities being tested in our intervention or examine fully relationships with other tobacco use parameters. Neural mechanisms underlying the efficacies of specific therapies have been proposed (e.g., see Feldstein-Ewing et al, 2011 for proposed neural mechanisms underlying motivational interventions), and the current study lays the foundation for similar studies investigating CBT and CM in youth. We did not collect any developmental variables (e.g. measures of pubertal maturation) and were therefore not able to examine developmental differences in responses. We also did not consistently obtain self-reports or biochemical tests of tobacco or other substance use immediately prior to the scan. Future studies need to control such potential confounding variables. Furthermore, while this small sample precluded meaningful investigation of potential influences of other substances, larger samples could allow for analyses that investigate directly the relationships with other measures of substance use, as we have done in other samples (Yip et al., 2013). Despite these limitations, a significant strength of our study is the inclusion of a well-characterized sample of adolescent smokers and the use of biochemical measures to evaluate reduction in tobacco use.
In summary, this exploratory and preliminary investigation suggests that adolescent smokers who exhibited at treatment onset greater Stroop-related activation in brain regions implicated in cognitive control were more successful at reducing tobacco use. Future studies should replicate these results in larger samples, examine the concurrent influence of variations in smoking levels and investigate directly the neural mechanisms underlying the efficacies of specific behavioral therapies. Neuroimaging research is typically challenging to conduct in adolescents, and this preliminary study provides initial novel, informative, proof-of-concept data that contribute importantly as a “next step” in the process of understanding the neural correlates of behavioral treatments for adolescent smokers. By continuing and expanding upon this line of research, it is anticipated that behavioral therapies may be refined and targeted through an improved understanding of how behavioral interventions change brain function and lead to diminishment or cessation of addictive behaviors in adolescents.
Supplementary Material
Acknowledgments
Sources of Support
Preparation of this manuscript was supported by NIH grants P50 AA15632, P50 DA009241, RL1AA017539, P20 DA027844, R01 DA020908, K01 DA027750, UL1 RR024139 and K12 DA00167.
References
- 1.Arrazola A, Dube S, Kaufmann R, Caraballo R, Pechacek T. Tobacco Use Among Middle and High School Students United States, 2000 – 2009. Morbidity and Mortality Weekly Report. 2011;59(33):1063–1068. [PubMed] [Google Scholar]
- 2.Backinger CL, Leischow SJ. Advancing the Science of Adolescent Tobacco Use Cessation. American Journal of Health Behavior. 2001;25(3):183–190. doi: 10.5993/ajhb.25.3.4. [DOI] [PubMed] [Google Scholar]
- 3.Bechara A. Risky Business: Emotion, Decision-Making, and Addiction. Journal of Gambling Studies. 2003;19(1):23–51. doi: 10.1023/a:1021223113233. [DOI] [PubMed] [Google Scholar]
- 4.Botvinick MM, Braver TS, Barch DM, Carter CS, Cohen JD. Conflict monitoring and cognitive control. Psychological Review. 2001;108(3):624–652. doi: 10.1037/0033-295x.108.3.624. [DOI] [PubMed] [Google Scholar]
- 5.Brewer JA, Worhunsky PD, Carroll KM, Rounsaville BJ, Potenza MN. Pretreatment Brain Activation During Stroop Task Is Associated with Outcomes in Cocaine-Dependent Patients. Biological Psychiatry. 2008;64(11):998–1004. doi: 10.1016/j.biopsych.2008.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Carter C, Van Veen V. Anterior cingulate cortex and conflict detection: an update of theory and data. Cognitive, Affective and Behavioral Neuroscience. 2007;7(4):367–379. doi: 10.3758/cabn.7.4.367. [DOI] [PubMed] [Google Scholar]
- 7.Cavallo D, Cooney J, Duhig A, Smith A, Liss T, McFetridge A, Babuscio T, Nich C, Carroll K, Rounsaville B, Krishnan Sarin S. Combining cognitive behavioral therapy with contingency management for smoking cessation in adolescent smokers: a preliminary comparison of two different CBT formats. American Journal on Addictions. 2007;16(6):468–474. doi: 10.1080/10550490701641173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chambers A, Taylor JR, Potenza MN. Developmental Neurocircuitry of Motivation in Adolescence: A Critical Period of Addiction Vulnerability. American Journal of Psychiatry. 2003;160:1041–1052. doi: 10.1176/appi.ajp.160.6.1041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Colby S, Leventhal A, Brazil L, Lewis Esquerre J, Stein LAR, Rohsenow D, Monti P, Niaura R. Smoking abstinence and reinstatement effects in adolescent cigarette smokers. Nicotine and Tobacco Research. 2010;12(1):19–28. doi: 10.1093/ntr/ntp167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connollya CG, Foxec JJ, Nierenberge J, Shpanerc M, Garavan H. The neurobiology of cognitive control in successful cocaine abstinence. Drug and Alcohol Dependence. 2012;121:45–53. doi: 10.1016/j.drugalcdep.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Counotte DS, Spijker S, Van de Burgwal LH, Hogenboom F, Schoffelmeer ANM, De Vries TJ, Smit AB, Pattij T. Long-Lasting Cognitive Deficits Resulting from Adolescent Nicotine Exposure in Rats. Neuropsychopharmacology. 2008;34(2):299–306. doi: 10.1038/npp.2008.96. [DOI] [PubMed] [Google Scholar]
- 12.Devito E, Worhunsky P, Carroll K, Rounsaville B, Kober H, Potenza M. A preliminary study of the neural effects of behavioral therapy for substance use disorders. Drug and Alcohol Dependence. 2012;122(3):228–235. doi: 10.1016/j.drugalcdep.2011.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dinn W, Aycicegi A, Harris C. Cigarette smoking in a student sample: neurocognitive and clinical correlates. Addictive Behaviors. 2004;29(1):107–126. doi: 10.1016/j.addbeh.2003.07.001. [DOI] [PubMed] [Google Scholar]
- 14.Ernst M, Romeo RD, Andersen SL. Neurobiology of the development of motivated behaviors in adolescence: A window into a neural systems model. Pharmacology Biochemistry and Behavior. 2009;93(3):199–211. doi: 10.1016/j.pbb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 15.Feldstein-Ewing FW, Filbey FM, Hendershot CS, McEarchern AD, Hutchinson KE. Proposed model of the neurobiological mechanisms underlying psychosocial alcohol interventions: the example of motivational interviewing. Journal of Studies on Alcohol and Drugs. 2011;72(6):903–916. doi: 10.15288/jsad.2011.72.903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fineberg N, Potenza M, Chamberlain S, Berlin H, Menzies L, Bechara A, Sahakian B, Robbins T, Bullmore E, Hollander E. Probing compulsive and impulsive behaviors, from animal models to endophenotypes; a narrative review. Neuropsychopharmacology. 2010;35:591–604. doi: 10.1038/npp.2009.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.First MB, Spitzer RL, Gibbon M, Williams JBW. New York State Psychiatric Institute; 2002. Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition With Psychotic Screen (SCID-I/P W/ PSY SCREEN) [Google Scholar]
- 18.Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier Development of the Accumbens Relative to Orbitofrontal Cortex Might Underlie Risk-Taking Behavior in Adolescents. The Journal of Neuroscience. 2006;26(25):6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Galvan A, Poldrack RA, Baker CM, McGlennen KM, London ED. Neural Correlates of Response Inhibition and Cigarette Smoking in Late Adolescence. Neuropsychopharmacology. 2011;36(5):970–978. doi: 10.1038/npp.2010.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Giedd JN, Rapoport JL. Structural MRI of Pediatric Brain Development: What Have We Learned and Where Are We Going? Neuron. 2010;67(5):728–734. doi: 10.1016/j.neuron.2010.08.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Grimshaw G, Stanton A. Tobacco cessation interventions for young people. The Cochrane Collaboration. 2006;4:1–51. doi: 10.1002/14651858.CD003289.pub4. [DOI] [PubMed] [Google Scholar]
- 22.Jacobsen L, Krystal J, Mencl WE, Westerveld M, Frost S, Pugh K. Effects of smoking and smoking abstinence on cognition in adolescent tobacco smokers. Biological Psychiatry. 2005;57(1):56–66. doi: 10.1016/j.biopsych.2004.10.022. [DOI] [PubMed] [Google Scholar]
- 23.Jacobsen L, Slotkin T, Mencl WE, Frost S, Pugh K. Gender-specific effects of prenatal and adolescent exposure to tobacco smoke on auditory and visual attention. Neuropsychopharmacology. 2007;32(12):2453–2464. doi: 10.1038/sj.npp.1301398. [DOI] [PubMed] [Google Scholar]
- 24.Janes AC, Pizzagalli DA, Richardt S, Frederick BD, Chuzi S, Pachas G, Culhane MA, Holmes AJ, Fava M, Evins AE, Kaufman MJ. Brain Reactivity to Smoking Cues Prior to Smoking Cessation Predicts Ability to Maintain Tobacco Abstinence. Biological Psychiatry. 2010;67(8):722–729. doi: 10.1016/j.biopsych.2009.12.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Janes AC, Pizzagalli DA, Richardt S, Frederick BdB, Holmes AJ, Sousa J, Fava M, Evins AE, Kaufman MJ. Neural Substrates of Attentional Bias for Smoking-Related Cues: An fMRI Study. Neuropsychopharmacology. 2010b;35(12):2339–2345. doi: 10.1038/npp.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jia Z, Worhunsky PD, Carroll KM, Rounsaville BJ, Stevens MC, Pearlson GD, Potenza MN. An Initial Study of Neural Responses to Monetary Incentives as Related to Treatment Outcome in Cocaine Dependence. Biological Psychiatry. 2011;70(6):553–560. doi: 10.1016/j.biopsych.2011.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kober H, Mende-Siedlecki P, Kross EF, Weber J, Mischel W, Hart CL, Ochsner KN. Prefrontal-striatal pathway underlies cognitive regulation of craving. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(33):14811–14816. doi: 10.1073/pnas.1007779107. 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krishnan Sarin S, Duhig A, McKee S, McMahon T, Liss T, McFetridge A, Cavallo D. Contingency management for smoking cessation in adolescent smokers. Experimental and clinical psychopharmacology. 2006;14(3):306–310. doi: 10.1037/1064-1297.14.3.306. [DOI] [PubMed] [Google Scholar]
- 29.Krishnan-Sarin S, Cavallo DA, Cooney JL, Schepis TS, Kong G, Liss AK, Liss AK, McMahon T, Nich C, Babuscio T, Carroll KM, Rounsaville B. Contingency Management and Cognitive Behavioral Therapy for smoking cessation in adolescent smokers. Colleges of Problems on Drug Dependence. 2012 [Google Scholar]
- 30.Leung HC, Skudlarski P, Gatenby JC, Peterson BS, Gore JC. An event-related functional MRI study of the stroop color word interference task. Cerebral Cortex. 2000;10(6):552–560. doi: 10.1093/cercor/10.6.552. [DOI] [PubMed] [Google Scholar]
- 31.Lucas CP, Zhang H, Fisher PW, Shaffer D, Regier DA, Narrow WE, Bourdon K, Dulcan MK, Canino G, Rubio Stipec M, Lahey BB, Friman P. The DISC Predictive Scales (DPS): efficiently screening for diagnoses. Journal of the American Academy of Child and Adolescent Psychiatry. 2001;40(4):443–449. doi: 10.1097/00004583-200104000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Mermelstein R, Colby S, Patten C, Prokhorov A, Brown R, Myers M, Adelman W, Hudmon K, McDonald P. Methodological issues in measuring treatment outcome in adolescent smoking cessation studies. Nicotine and Tobacco Research. 2002;4(4):395–403. doi: 10.1080/1462220021000018470. [DOI] [PubMed] [Google Scholar]
- 33.Newhouse P, Potter A, Dumas J, Thiel C. Functional brain imaging of nicotinic effects on higher cognitive processes. Biochemical Pharmacology. 2011;82(8):943–951. doi: 10.1016/j.bcp.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Peterson BS, Skudlarski P, Gatenby JC, Zhang H, Anderson AW, Gore JC. An fMRI study of Stroop word-color interference: evidence for cingulate subregions subserving multiple distributed attentional systems. Biological Psychiatry. 1999;45(10):1237–1258. doi: 10.1016/s0006-3223(99)00056-6. [DOI] [PubMed] [Google Scholar]
- 35.Peterson B, Potenza M, Wang Z, Zhu H, Martin A, Marsh R, Plessen K, Yu S. An FMRI study of the effects of psychostimulants on default-mode processing during Stroop task performance in youths with ADHD. The American Journal of Psychiatry. 2009;166(11):1286–1294. doi: 10.1176/appi.ajp.2009.08050724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Potenza Marc N, Sofuoglu M, Carroll Kathleen M, Rounsaville Bruce J. Neuroscience of Behavioral and Pharmacological Treatments for Addictions. Neuron. 2011;69(4):695–712. doi: 10.1016/j.neuron.2011.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prokhorov AV, Pallonen UE, Fava JL, Ding L, Niaura R. Measuring nicotine dependence among high-risk adolescent smokers. Addictive Behaviors. 1996;21(1):117–127. doi: 10.1016/0306-4603(96)00048-2. [DOI] [PubMed] [Google Scholar]
- 38.Rutherford HJV, Mayes LC, Potenza MN. Neurobiology of Adolescent Substance Use Disorders: Implications for Prevention and Treatment. Child and Adolescent Psychiatric Clinics of North America. 2010;19(3):479–492. doi: 10.1016/j.chc.2010.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schochet TL, Kelley AE, Landry CF. Differential expression of arc mRNA and other plasticity-related genes induced by nicotine in adolescent rat forebrain. Neuroscience. 2005;135(1):285–297. doi: 10.1016/j.neuroscience.2005.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72(1):124–133. doi: 10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Streeter CC, Terhune DB, Whitfield TH, Gruber S, Sarid-Segal O, Silveri MM, Tzilos G, Afshar M, Rouse ED, Tian H, Renshaw PF, Ciraulo DA, Yurgelun-Todd DA. Performance on the Stroop Predicts Treatment Compliance in Cocaine-Dependent Individuals. Neuropsychopharmacology. 2007;33(4):827–836. doi: 10.1038/sj.npp.1301465. [DOI] [PubMed] [Google Scholar]
- 42.Sussman S. Effects of sixty six adolescent tobacco use cessation trials and seventeen prospective studies of self-initiated quitting. Tobacco Induced Diseases. 2002;1(1):35–81. doi: 10.1186/1617-9625-1-1-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sussman S, Ping S, Dent C. A meta-analysis of teen cigarette smoking cessation. Health Psychology. 2006;25(5):549–557. doi: 10.1037/0278-6133.25.5.549. [DOI] [PubMed] [Google Scholar]
- 44.Wexler BE. Functional magnetic resonance imaging of cocaine craving. The American Journal of Psychiatry. 2001;158(1):86–95. doi: 10.1176/appi.ajp.158.1.86. [DOI] [PubMed] [Google Scholar]
- 45.Wexler BE. Computerized Cognitive Remediation Treatment for Substance Abuse Disorders. Biological Psychiatry. 2011;69(3):197–198. doi: 10.1016/j.biopsych.2010.11.016. [DOI] [PubMed] [Google Scholar]
- 46.Wiehe SE, Garrison MM, Christakis DA, Ebel BE, Rivara FP. A systematic review of school-based smoking prevention trials with long-term follow-up. Journal of Adolescent Health. 2005;36(3):162–169. doi: 10.1016/j.jadohealth.2004.12.003. [DOI] [PubMed] [Google Scholar]
- 47.Worhunsky P, Stevens M, Carroll K, Rounsaville B, Calhoun V, Pearlson G, Potenza M. Functional Brain Networks Associated with Cognitive Control, Cocaine Dependence and Treatment Outcome. Psychology of Addictive Behaviors. 2013 doi: 10.1037/a0029092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.World Health Organization. Tobacco Free Initiative - Tobacco and Youth. 2013 Available at http://www.who.int/tobacco/control/populations/youth/en/index.html.
- 49.Yip SW, Lacadie C, Xu J, Worhunsky PD, Fulbright RK, Constable RT, Potenza MN. Reduced genual corpus collosal white matter integrity in pathological gambling and its relationship to alcohol abuse or dependence. World Journal of Biological Psychiatry. 2013 doi: 10.3109/15622975.2011.568068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zack M, Belsito L, Scher R, Eissenberg T, Corrigall WA. Effects of abstinence and smoking on information processing in adolescent smokers. Psychopharmacology. 2001;153(2):249–257. doi: 10.1007/s002130000552. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.