Abstract
Exposing tissues to extreme high or low temperature leads to burns. Burned animals sustain several types of damage, from the disruption of the tissue to degeneration of axons projecting through muscle and skin. Such damage causes pain due to both inflammation and axonal degeneration (neuropathic-like pain). Thus, the approach to cure and alleviate the symptoms of burns must be twofold: rebuilding the tissue that has been destroyed and alleviating the pain derived from the burns. While tissue regeneration techniques have been developed, less is known on the treatment of the induced pain. Thus, appropriate animal models are necessary for the development of the best treatment for pain induced in burned tissues. We have developed a methodology in the zebrafish aimed to produce a new animal model for the study of pain induced by burns. Here we show that two events linked to the onset of burn-induced inflammation and neuropathic-like pain in mammals, degeneration of axons innervating the affected tissues and over-expression of specific genes in sensory tissues, are conserved from zebrafish to mammals.
Keywords: thermal nociception, inflammation, neuropathic pain, burns, markers genes, animal model, zebrafish
INTRODUCTION
Severe tissue burns are the source of great distress for the subjects affected. On the one hand there is psychological downside of irreparable cosmetic problems due to the disruption of skin. On the other hand, the continuous suffering from inflammatory and neuropathic-like pain that derive from the burned area (Fauerbach et al., 2005; Schneider et al., 2006; Wong and Turner, 2010).
While, the biology and medicine of tissue transplants and building in vitro new skin tissue from stem cells precursors is well developed (Priya et al., 2008), pain caused by burns has not followed the same path. This may be due in part to the paucity of animal models available for such studies. Animal in vivo experimentation, above all in the field of pain and nociception, presents ethical and scientific limitations. Rats and mice are considered the most useful animal models in this context, being suitable for genetic manipulation and having a large choice of mutants already available (Nozaki-Taguchi and Yaksh, 1998; Yregård et al., 2001; Wang et al 2005; Mogil and Basbaum, 2011; Shields et al., 2012). Studies of skin burns in animal models showed how axons innervating the tissue could be severely affected either in the short-term period directly by the burns (deCamara et al., 1982; Summer et al., 2007), or by the scar tissue, that can heavily affect axons growth in the long-term (Summer et al., 2007; Morellini et al., 2012). In addition, it has been shown that macrophages migrate to the damaged tissues and neurons and their behavior is indicative of inflammation and related pain states (Summer et al., 2008). This type of pain is frequently resistant to opioid treatment. Thus, the type of pain produced by tissue burns shows strong resemblance with inflammatory pain but also with neuropathic pain, leading researchers to coin the term “phantom skin” for the pain induced by skin burns (Atchison et al., 1991).
Numerous reports have shown that, in response to neuropathic pain and inflammation, the synthesis of several genes is de-novo induced or augmented in mammals. These factors are not simply markers of neuropathic and inflammatory pain. They also play key roles in sensitization and neuropathic pain in primary nociceptors and in the spinal cord (Ru-Rong Ji and Strichartz 2004). Some of the factors up-regulated in response to neuropathic pain and inflammation include Jun family factors, (Herdegen et al., 1991a; Herdegen et al., 1991b; Leah et al., 1991; Jenkins and Hunt, 1991;). c-Jun in turn activates the expression of Vasoactive Intestinal Peptide (VIP) (Mulderry and Dobson., 1996; Son et al., 2007) as well as other neuropeptides. Peripheral nerve injury induces higher expression levels of Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) in dorsal root ganglia (Zhang et al., 1996; Mabuchi et al., 2004) and Brain-Derived Neurotrophic Factor (BDNF) in dorsal root ganglia (Tonra et al., 1998, Li et al., 1999; Zhou et al., 2000; Fukuoka et al., 2001; Obata et al., 2003; Obata et al., 2006) and in spinal cord (Miletic and Miletic, 2002, Obata and Noguchi, 2006; Geng et al., 2010). After nerve lesions, the ubiquitous oncogene marker, c-fos, is also highly induced in mammal spinal dorsal horn neurons within 0.5/2.0 hours after the stimulation (Hunt et al., 1987; Herdegen et al., 1991a; Herdegen et al., 1991b; Coggeshall, 2005). From now on, we will refer to these genes as pain markers genes (PMGs)
The non-mammalian vertebrate zebrafish has been used to model many human diseases and is potentially a good animal model for pain and nociception studies (Sneddon LU. 2009; Gonzalez-Nunez V, Rodriguez RE. 2009; Malafoglia et al., 2013). Here we present data showing that zebrafish larvae treated with high temperatures present the characteristics of mammalian animal model suffering of tissue burns and pain suggesting that these mechanisms are evolutionarily conserved and that zebrafish could be suitable for pain studies.
MATERIALS AND METHODS
-Fish Husbandry
We used zebrafish larvae from various wild type strains (AB, Brass, Tübingen) and heterozygous larvae for the gene trap line nsftpl006Gt (Balciunie et al., 2013). Animals were maintained in a 14h/10h light/dark cycle following standard husbandry protocols (Westerfield, 1993). Embryos were obtained by natural mating in mating cages and collected in Petri dishes with embryo medium. They were cultured at 28.5°C in the incubator, with 1x phenylthiourea (PTU) in embryo medium to stop the formation of pigment. The developmental stages of the embryos were determined days post fertilization (dpf). All procedures involving zebrafish were conducted in accordance with Institutional Animal Care and Use Committee (IACUC) policies. Every effort was made to minimize the number of zebrafish used.
-Extreme High temperature test
We moved batches of 20 5dpf larvae from a beaker with embryo medium at 28°C to another beaker at 48°C, for 5 seconds, and the back to the beaker with embryo medium at 28°C. We collected the treated larvae at specific time points after the test: T0.5, T2, T4, T6, T24 (numbers express hours after stimulus). We used 5dpf sibling larvae unexposed to the shock as negative control.
For each time point samples were fixed in 4% paraformaldehyde/PBS (PFA) at 4°C overnight and then stored in methanol at −20°C. Then, we used these fish for whole-mount in situ hybridization (WISH) analysis, for FM1-43 staining or for qRT-PCR.
-FM1-43 staining
Lateral line hair cells of 5dpf zebrafish larvae were labeled by incubation in 7 mM FM1-43 dye (INVITROGEN) for 5 min in embryo medium, followed by 3 washes in fresh embryo medium (Prober et al 2008). Larvae were anesthetized in embryo medium containing 0.02% Tricaine (Sigma-Aldrich, St. Louis, MO) for live imaging. Animals were imaged using an AXIO IMAGER. A1 fluorescence microscope (ZEISS), and analyzed with the software SPOT Advanced.
-Cloning and probe preparation
Total RNA was extracted from 5dpf zebrafish larvae (AB strain) using Quiagen RNeasy Mini Kit, accordingly to the manufacturer instructions. Total RNA (1µg) was reverse transcribed to cDNA using the SuperScript® III First-Strand Synthesis System kit (Invitrogen).
We designed gene specific primers for each PMG cloning by finding their sequences in the databases. c-fos (DQ003339.2) primer pair: forward 5’-ttgcagtggatggtccagc-3’, reverse 5’-aggtagtgacgatctctggg-3’ for a 438bp amplification fragment; c-jun (DQ003340.1) primer pair: forward 5’-agcgatatcctcacttctcc-3’, reverse 5’-tttcctcttccggcatttgg-3’ for a 582bp fragment; vip (NM_001114553.2) primer pair: forward 5’-aatgcttgtgcggaacggc-3’, reverse 5’-aacgtgtcggatccaaggc-3’ for a 677bp fragment; pacap1b (NM_214715.2) primer pair: forward 5’-ataaaagtgaaggggttgaaccg-3’, reverse 5’-acaaataagcaaatcgacgtcc-3’ for a 683bp fragment; bdnf (NM_131595.2) primer pair: forward 5’-ttgcatgagagctgcgcc-3’, reverse 5’-aaccgccagccgatcttcc-3’ for a 717bp fragment.
We cloned the RT-PCR products by TA based cloning into pCRII TOPO, we used TOPO TA Cloning® Kit Dual Promoter (with pCR®II-TOPO® vector) with One Shot® TOP10F’ Chemically Competent E. coli following the kit instructions (Invitrogen).
-In situ hybridization
Samples in 100% methanol were rehydrated by rinsing in an increasing dilution of methanol/PBS and permeabilized with Proteinase K (10µg/ml) for 30 minutes at RT. Then we followed the protocol as in (Bellipanni et al., 2010). The staining reaction was stopped at the same time for control and experimental samples by fixation with 4% PFA. For longer preservation the larvae are kept in 75% glycerol in 1xPBT. The images were captured with stereo-microscope SMZ800 (Nikon), and analyzed with the software NIS-Elements BR3.0 (Nikon).
-Real Time-PCR (qRT-PCR)
Up regulation of the PMGs was quantitatively tested by qRT- PCRs. For each heat shock time point RNA from 10 larvae was extracted and cDNA was prepared as described above. cDNA concentration was determined by measuring the absorbance at 260nm with the spectrophotometer. The quantification of the PCR products was accomplished with a standard curve using the SYBR-Green method. SYBR-Green was included in a 2X Master Mix (Roche). We used oligonucleotides primers to amplify ~100bp fragments of each PMG using 100ng/µl of specific cDNA in each reaction. The primer pair was for c-fos-forward 5’-atctgagtgtctaagtgcc-3’, c-fos-reverse 5’-tgcttctcttgttggaggtc-3’ to amplify a 190bp fragment; for c-jun-forward 5’-attaaagccggaggaagcg-3’, c-jun-reverse 5’-actttctgcttgagctgtgc-3’ to amplify a 180bp fragment; for vip-forward 5’-ggctcttcacaagcggatac-3’, vip-reverse 5’-atcatcactgacccgctttc-3’ to obtain a 92bp fragment; for pacap1b-forward 5’-cacgcctattgggatgactt-3’, pacap1b-reverse 5’-caaaagccaggtcgcttaac-3’ to have a 89bp fragment; for bdnf-forward 5’-gagctcagcgtttgtgacag-3’, bdnf-reverse 5’-gtctggcccgacatgtctat-3’ to amplify a 80bp fragment. We tested the same gene in triplicate and we used beta-actin (actb) and glyceraldehyde 3-phosphate dehydrogenase (gadph) specific primers as standards: actb-forward 5’-tcaccaccacagccgaaag-3’, actb-reverse 5’-agaggcagcggttcccat-3’ for a product of 98bp; gadph-forward 5’-gtgtaggcgtggactgtggt-3’, gadph-reverse 5’-tgggagtcaaccaggacaaata-3’ for a 121bp fragment.
A standard curve was constructed for each PMG by serial dilution of cDNA: 25ng/µl, 50ng/µl, 100ng/µl and 200ng/µl, and it was determined that all the primers had the same efficiency. The amplification reaction took place in a Roche Light Cycler 480II System. Each reaction was performed in triplicate and each experiment had two biological replicates. The first biological replicate was tested twice from two independent preparations of cDNA. The second biological replicate was tested once for a total of 3 experimental replicates. We analyzed the results with the second derivative method, choosing the mRNA expression level of 5dpf untreated larvae to normalize our data.
-Statistics
We statistically analyzed mRNA expression level for each gene with n=9 (3 triplicates for each gene in a single experiment, for a total of 3 experiments) with one-way ANOVA (and nonparametric) analysis. Data were expressed as mean plus error standard; we used Bonferroni post-test gene expression at different time points multiple comparisons, showing significant results (*=p<0.0001). We statistically analyzed the percentage of larvae showing over-expression in each time point using Fisher’s exact test.
RESULTS
Extreme thermal (ET) noxious stimuli induce nerves damages
Zebrafish larvae are known to respond to thermal noxious stimuli, either hot or cold, by showing an increased locomotor activity (Prober et al., 2008). Embryo medium at a temperature up to 42°C passing in a flow-through system was used in an assay that tested behavioral locomotor responses of the larvae (Prober et al., 2008; Gomez Lima et al., 2012; Malafoglia et al., 2013). In this study we were interested in determining if exposure of 5dpf larvae to the highest possible temperature would mimic some of the effects of burns seen in mammals. For our purposes we used an immersion system where larvae were pooled in baskets closed at the bottom with a fine mesh net. The baskets were initially located in a floating rack and immersed in a beaker containing embryo medium at 28°C. To select the optimal temperature we tested at various temperatures starting at 35°C increasing in 5°C increments. We determined that at 50°C a large subset of larvae was severely affected with a deformed body and eventual death. Subsequent tests at 1°C intervals starting from 45°C helped us to establish the highest temperature we could use to set up the experiment, without killing the animals, was 48°C. The type of noxious heat stimulus we provide to the larvae is the highest they can tolerate; therefore it might induce extreme effects like in the case of burns in mammals. Thus, we will refer to it from now on as extreme thermal (ET) stimulus.
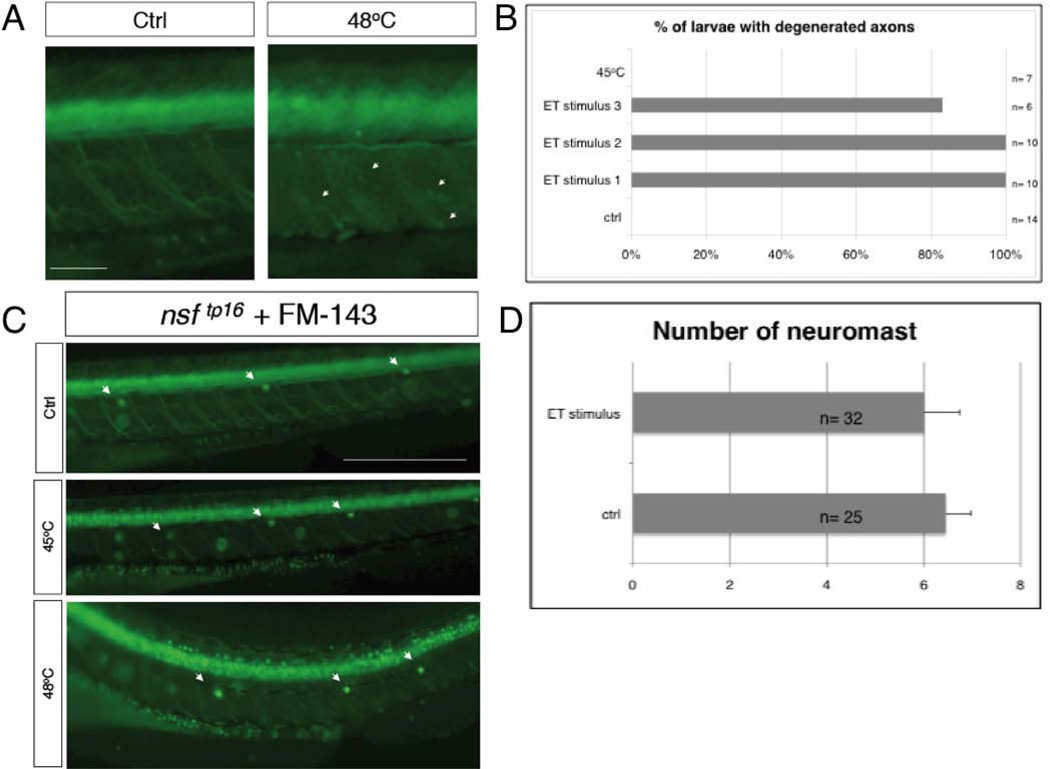
In higher vertebrates severe burns result in tissue nerves degeneration and eventually in neuropathic-like pain and inflammation (Summer et al., 2007). Following this reasoning, to check the peripheral innervations of skin and muscles, we conducted our ET assay using a neuronal Gal4 driver line (nsftpl006Gt). This gene-trap line expresses Gal4 and uas:egfp under the control of the endogenous nsf promoter gene (Wood et al., 2006; Balciunie et al., 2013). In this line egfp is expressed in a large subset of neurons of spinal cord (motoneurons), dorsal root ganglia and trigeminal ganglia. In addition this gene trap line makes visible many axonal projections (Fig. 1A). After the noxious ET stimulus at 48°C or a stimulus at 45°C we could detect two distinct categories of larvae. Exposure to 48°C produced larvae with a curved or wavy body axis and slightly deformed mouth, while exposure to 45°C resulted in larvae still looking normal. The swimming ability of the first group was dramatically reduced and the most affected larvae were not able to swim away after being stimulated by touching. Instead, these larvae showed a shaking movement. This may be the result of the neuromuscular projections and/or of muscular damage and deformation induced by the heat. Indeed, the first category had major tissue and nerve disruption including motoneuron projections and skin innervation (Fig. 1A), while the second showed no effects in any visible axons (Fig. 1C).
Figure 1. Nerve degeneration in zebrafish, after ET stimulus.
A, 5dpf nsftpl006Gt /+ larvae 2 hours after being exposed to ET treatment (48°C) (right) and control siblings (left). Arrows indicate nerves fragmentation. B, chart showing the percentage of larvae presenting at least 4 degenerating motor nerves from 3 independent experiments, ET stimulus 1-3. Control and larvae treated at 45°C show no defects. C, FM1-43 labeling of lateral line hair cells of 5dpf nsftpl006Gt /+ larvae control before the stimulus and experimental samples after the stimuli at 45°C or at 48°C. D, Average number of neuromasts labeled with FM1-43 for ET treated and untreated larvae. Error bar represent SEM. Size bar 40µm.
Zebrafish larvae are able to regenerate axotomized innervation in a timely fashion when the nerve soma remains intact and this regeneration begins already at 24h following axotomy (Martin et al., 2010; Rosenberg et al., 2012). We followed single larvae with the most severe phenotype obtained after ET stimulus to check if axons were able to regenerate. In the 4 larvae we followed we saw that axon-disruption persisted at 6h after the stimulus, but at 24h after the stimulus larvae showed signs of recovery and 2 days later were swimming normally (data not shown), suggesting that motor nerve somata were not destroyed by our assay.
These data clearly indicate that the extreme thermal noxious stimulus we used induces a temporary degeneration of motor innervation.
Quantitative analysis of gene expression in zebrafish larvae after ET stimulus
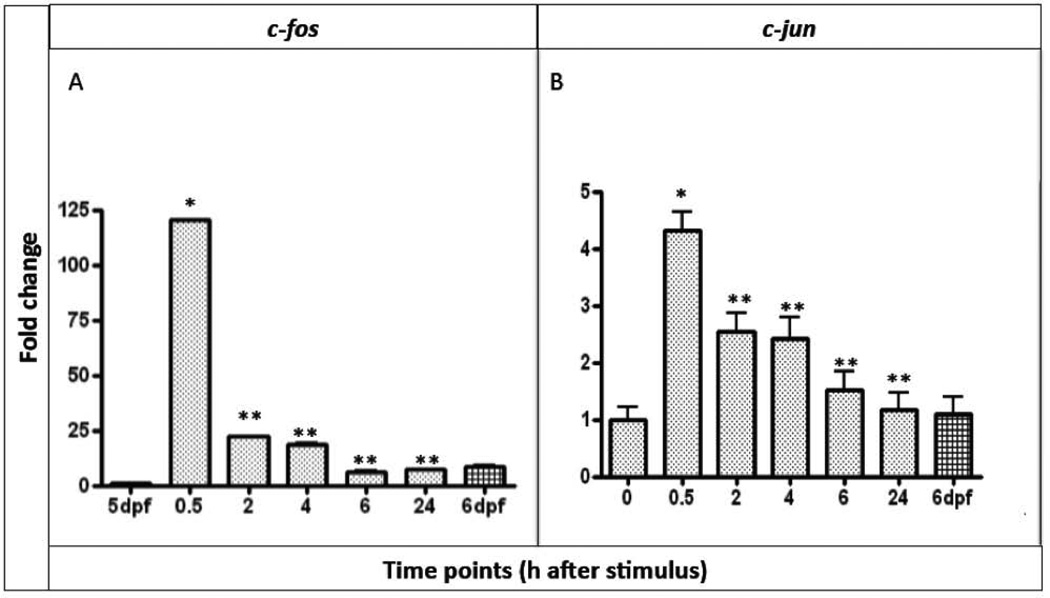
Studies in mammals have identified a set of genes that are activated after neuropathic and inflammatory pain. We decided to test if a subset of these factors, c-jun, c-fos, vip, pacap1b and bdnf, that we called PMGs (pain marker genes), was activated after noxious ET stimuli in zebrafish larvae. We first compared the expression levels of the PMGs in control and experimental larvae by real time RT-PCR (qRT-PCR). The purpose of such test was to determine quantitatively if the expression level of any of these markers was effectively up-regulated in the larvae exposed to noxious temperature and if our choice of time intervals was appropriate. We tested up to 5 time points: 30’min; 2h; 4h; 6h and 24h after stimulus, and used 5 and 6 day untreated larvae as controls. The early responsive genes c-fos and c-jun exhibited higher levels of mRNA expression already after 30’ from the thermal nociceptive stimulation followed by a consistent decrease in expression at later time points. Expression of c-fos (Fig. 2A) showed the earliest and strongest enhancement 30’ after noxious heat stimulus and it was activated ~140 fold more than the control sample at 5dpf, which we used for normalization. At the following time points (2h and 4h after stimulus) c-fos expression level decreased dramatically but was still 20-25 fold higher than the control. At 6h and 24h after the stimulus the levels were even lower but still above the levels of expression of c-fos in the control larvae at 5dpf. However, when compared to 6dpf untreated control larvae the levels of expression of c-fos at 6h and 24h after the stimulus were the same of the other control.
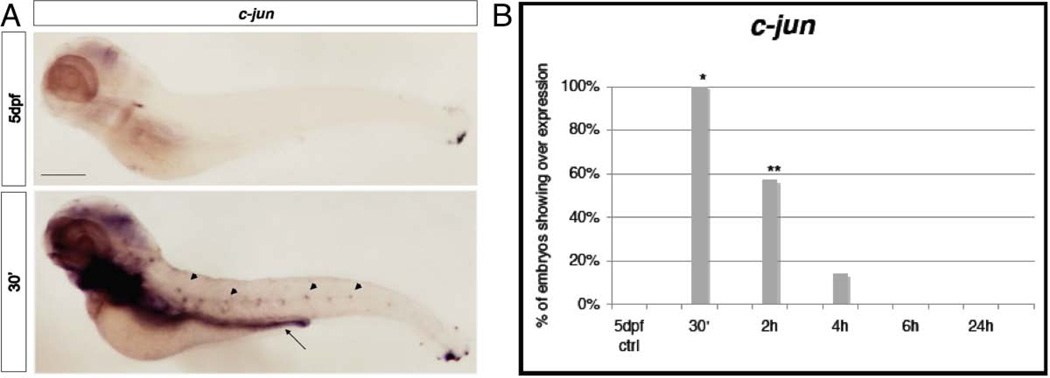
Figure 2. Quantitative analysis of c-fos and c-jun expression.
A, quantification of c-fos and B, c-jun mRNA expression in 5dpf larvae after 30’, 2h, 4h, 6h and 24h from the ET treatment. We used mRNA expression level of 5dpf untreated larvae to normalize our data. n=9 per gene, data are expressed as mean ± SEM; Bonferroni post-test gene expression at different time points comparisons: * = p<0.0001 between T0 and T0.5; **= p<0.0001 between T0.5 and T2, T4, T6, T24
After 30’ from noxious heat stimulus c-jun transcription (Figure 2B) was also rapidly activated, but only by ~4 fold. This initial activation decreased about 25% in 2-4h after stimulus. At 6h and 24h the expression levels had returned to the control levels.
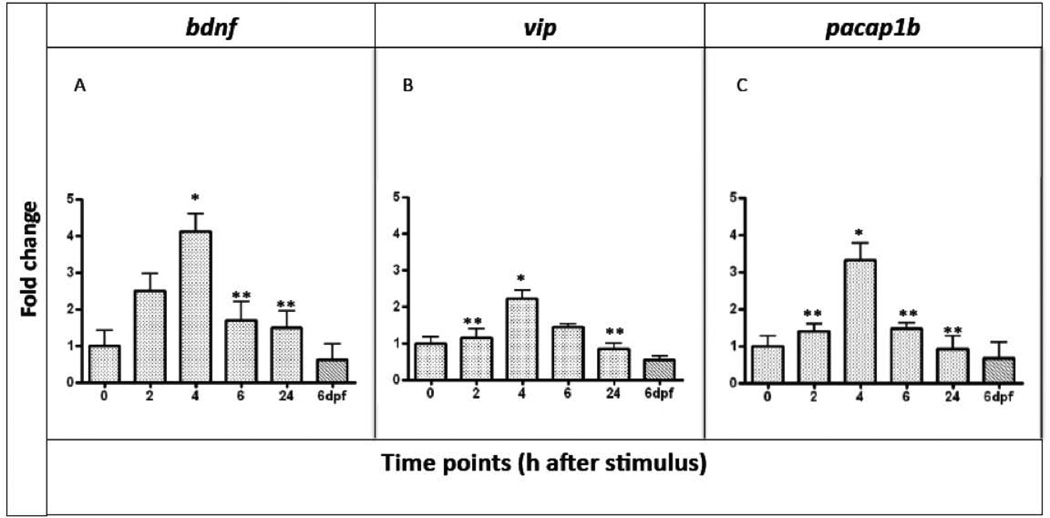
The expression levels of vip, pacap1b and bdnf (Figure 3ABC) were analyzed starting at 2h after the stimulus. In all cases the mRNA levels peaked at 4h after the heat stimulus and were close to the normal physiological level at the earlier and later time points (2h and 24h).
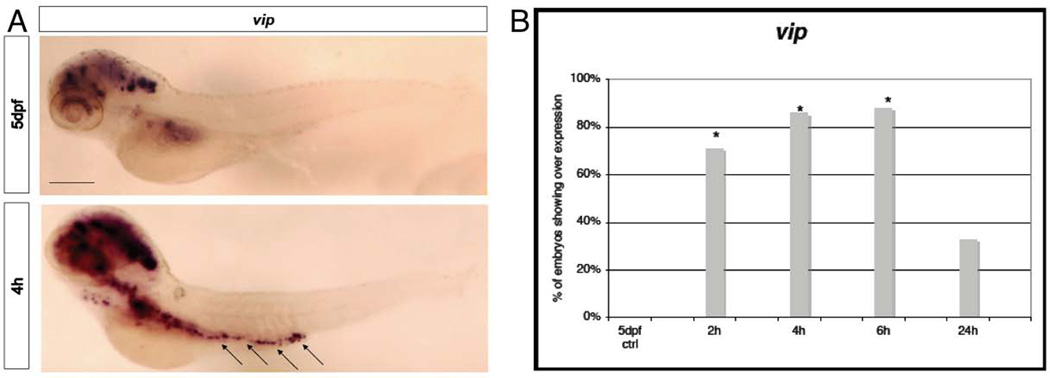
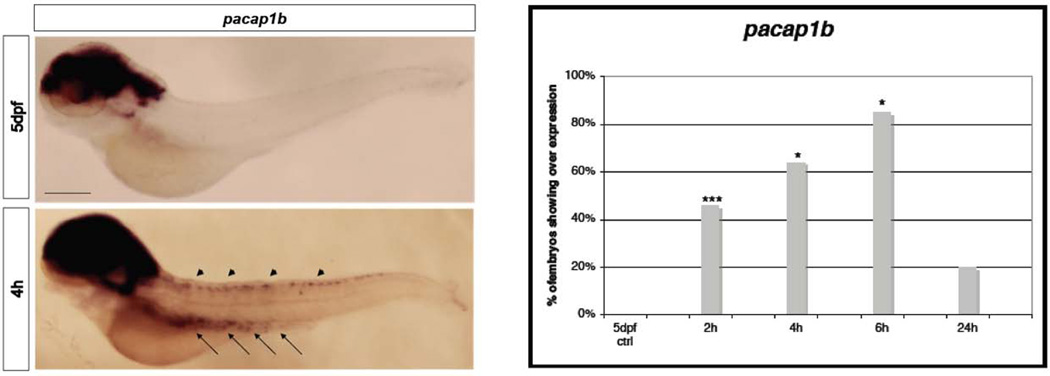
Figure 3. Quantitative analysis of bdnf, vip and pacap1b mRNAs expression.
A, bdnf; B, vip; C, pacap1b in 5dpf larvae after 2h, 4h, 6h and 24h from the ET treatment. We used mRNA level of 5dpf untreated larvae to normalize our data. n=9 per gene, data are expressed as mean ± SEM; Bonferroni post-test gene expression at different time points comparisons:* = p<0.0001 between T0 and T4; **= p<0.0001 between T4 and T2, T6, T24
These data clearly indicate that the noxious ET stimulus induces the PMG expression, that this induction is time-dependent and that the time points we chose for our analysis were appropriate. In fact, after the stimulus we see a dynamic behavior: first an increase, then a decrease to normal levels of expression at 24h.
Localization of PMG expression in noxious heat treated zebrafish larvae
The results from qRT-PCR are not sufficient to test our original hypothesis, which proposes that PMGs expression in mammals in response to neuropathic pain and inflammation is evolutionary conserved in the chordate lineage from teleosts to humans. In fact such expression may be caused by several other factors such cell stress and apoptosis. However, it has been shown that the expression of these factors is closely dependent on noxious stimuli when the expression is localized in specialized tissues, like centers functioning in pain transduction and sensing like the dorsal spinal cord and the dorsal root ganglia, (Ji and Strichartz 2004; Coggeshall, 2005; Malafoglia et al., 2013). Thus, we needed to determine if there is a similar pattern in the zebrafish. We addressed this point by analyzing the expression patterns of each PMG by WISH at the same time points used for the qRT-PCR. Consistent with the relative qRT-PCR results we found that, after the ET stimulus, all the PMGs are up-regulated. In addition, some of the PMGs were up-regulated in regions predicted to be involved in noxious signal transduction. Specifically, we found that PMGs were up-regulated in specific sensory systems like the spinal cord and brain, but not in dorsal root ganglia. In addition, we found some of the PMGs expressed in single cells surrounding the gut that may be enteric neurons and in the neuromasts of the lateral line.
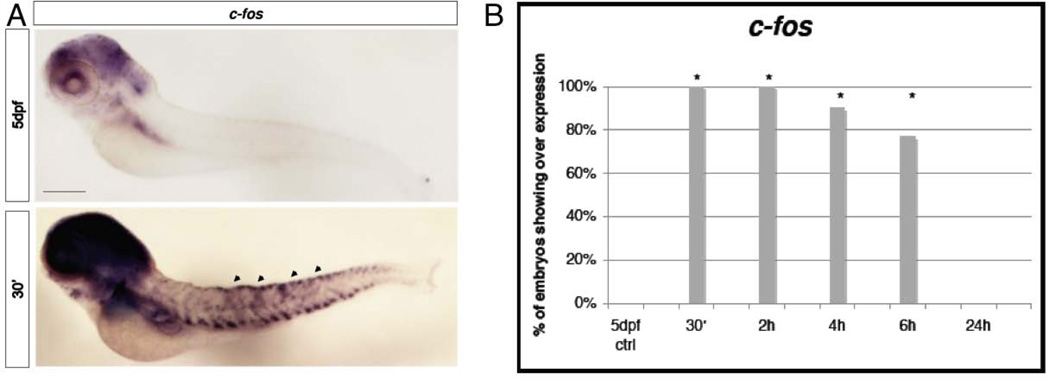
Starting from 30’ after the stimulus we analyzed the expression of c-fos that is usually localized at the level of the hindbrain, the trigeminal ganglia, the spinal cord neurons, the telencephalon, the diencephalon and a small population of blood cells (Thiesse et al., 2001). In our assay c-fos appeared to be enriched at the spinal cord and in the brain relative to the control (Fig. 4A). This result was particularly clear because, when c-fos expression in discrete cells of the dorsal spinal cord of treated larvae were fully stained, the control larvae still lacked coloration. Interestingly, such up-regulation persisted until 6h after the stimulus (Fig. 4B), a time in which the qRT-PCR levels were already markedly reduced. This suggests that this specific expression is unrelated to the high levels of induction of c-fos seen 30min after the stimulus by qRT-PCR. Moreover, expression in treated larvae analyzed 24 hours after the stimulus returned to normal levels (identical to the untreated controls) not presenting anymore the high levels of expression in the brain and spinal cord. In figure 5A we show c-jun WISH analysis. c-jun is normally present in the brain, gill, liver and muscular system of zebrafish (Gonzalez et al, 2006). The treated larvae did not show up-regulation in the spinal cord or dorsal root ganglia. However, in our assay c-jun appeared to be up-regulated beginning at 30’ after the stimulus at the level of the gut, in which we saw a homogeneous coloration, and in lateral spots that could be neuromasts of the lateral line. This expression reached its peak, in terms of percentage of larvae presenting such coloration, at 4h and returned to normal levels at 6h (Figure 5A and 5B). Neuromasts of the lateral line are very sensitive neurosensory structures that could have been affected by the ET treatment. Therefore, to determine if the noxious ET treatment could have a major effect on the neuromasts, we decided to look more carefully at this structure after ET stimulation. Before the stimulus we labeled the neuromasts of the sensory lateral line of wild type (or nsftpl006Gt line) 5dpf zebrafish larvae by exposing them to the dye FM-143 (Gale et al., 2001; Meyers et al., 2003; Prober et al., 2008) and observed and counted the neuromasts before and 1 hour after the treatment. Using this methodology we did not find a statistical significantly effect of the ET treatment on neuromast number or organization (Fig 1C arrowheads and 1D). To determine if the noxious ET assay had a delayed effect on the neuromasts we analyzed the larvae a second time, 2-3 hours after the stimulus. Again we found no effect (data not shown).
Figure 4. WISH analysis of c-fos.
A, Expression a t 30’ after the ET treatment compared with the physiological c-fos expression in 5dpf untreated larvae. Treated larvae present c-fos high expression at the level of the spinal cord and brain (arrowheads). Size bar 100µm. B, Chart indicates the percentage of larvae showing expression of c-fos in the spinal cord of 5dpf larvae at 30’, 2h, 4h, 6h and 24h from the ET treatment. * p≤0.001 (Fisher’s exact test).
Figure 5. WISH analysis of c-jun.
A, Expression at 30’ after the ET treatment, at the level of the gut (arrow) with homogeneous coloration and in the neuromasts of the lateral line (arrowheads) compared with the normal expression in the 5dpf untreated larvae. Size bar 100µm. B, Chart indicates the percentage of larvae showing expression of c-jun in the gut and neuromasts of 5dpf larvae at 30’, 2h, 4h, 6h and 24h from the ET treatment. * p≤0.001; ** Is p≤0.002 (Fisher’s exact test).
In goldfish, the mRNA for vip is usually expressed in the brain and intestine (Tse et al., 2002). In zebrafish VIP is localized in nitrergic neurons and fibers of the neuro-enteric system (Olsson et al., 2008; Uyttebroek et al., 2010; Shepherd and Eisen, 2011). Interestingly, these neurons are involved in gut related neuropathies in humans (Furness, 2000; Furness, 2012). After the noxious ET stimulus there was clearly a strong enhancement of expression with a salt and pepper pattern along the gut line in cells that may belong to the neuro-enteric system of the zebrafish and in particular to the nitrergic neurons, where vip is normally present (Figure 6A). As an interesting correlation to our finding, high levels of VIP have been reported in plasma and rectal mucosa of patients suffering of irritable bowel syndrome (Simrén et al., 2001; Palsson et al., 2004) and increased number of VIP-neurons has been found in sub-mucosal plexus in non-inflamed rectum of patients suffering for Crohn’s disease (Schneider et al., 2001). Moreover, c-jun up-regulation in the whole gut region after 30min from the stimulus (Fig 5A arrowhead, Fig5B), and vip expression in the neuro-enteric system (Figure 6A and B) after 2-6h, nicely correlates with the findings in mammals that induction of c-jun determines vip expression in rat sensory neurons (Mulderry and Dobson. 1996; Son et al., 2007).
Figure 6. WISH analysis of vip.
A, Expression at 4h after the stimulus in spots along the gut (arrows), that might be enteric neurons compared with a 5dpf control larva. Size bar 100µm. B, Chart indicates the percentage of larvae showing expression of vip in the gut of 5dpf larvae at 2h, 4h, 6h and 24h from the ET treatment. * p≤0.001; (Fisher’s exact test).
In zebrafish two pacap genes have been identified and both found to be duplicated (Fradinger and Sherwood, 2000; Wang et al., 2003). Transcripts for the gene pacap1b have been detected at the level of the telencephalon, the diencephalon, the rhombencephalon, in neurons of dorsal part of the spinal cord (Alexandre et al., 2011) and in neurons of the neuro-enteric system (Olsson et al., 2008; Uyttebroek et al., 2010). In our treated larvae we found stronger expression in single cells of the spinal cord and gut. As in the case of c-fos and vip, and consistent with the reported expression pattern, these could be neurons of the dorsal spinal cord and neurons of the neuro-enteric system, where pacap1b is normally expressed at lower levels (Figure 7A). In the brain there was also an increased but more diffuse expression of pacap1b. Again, the higher percentage of larvae expressing pacap1b and vip in putative neurons were seen at 6h after the ET stimulus (Fig 6AB and 7AB), a stage in which the over-all increase of expression levels, seen by qRT-PCR, were returning to normal. Moreover, about 25% of the larvae analyzed 24h after the stimulus, when the mRNAs levels analyzed by qRT-PCR were indistinguishable from the controls, showed expression of pacap1b and vip in the spinal cord and putative enteric neurons. This suggests that a subset of the larvae exposed to ET stimulus may show persistent activation of pathways linked with pain perception.
Figure 7. WISH analysis of pacap1b.
A, Expression at 4h after the stimulus, in the spinal cord (arrowheads), brain and in spots along the gut(arrows), that might be enteric neurons, compared with 5dpf control. Size bar 100µm. B, Chart indicates the percentage of larvae showing expression of pacap1b in the spinal cord and gut of 5dpf larvae at 2h, 4h, 6h and 24h from the ET treatment. * p≤0.001; *** Is p≤0.01 (Fisher’s exact test).
Normal bdnf expression is found in the brain specifically in the telencephalon, the peripheral olfactory organ and the cranial ganglion (Thisse et al. 2004). This was the only PMG showing over-expression after the thermal stimulus just within the same regions stained in the control larvae. The higher levels of expression were restricted between 4h and 6h after stimulus (Figure S1 A and B).
All our WISH analyses were conducted so that both control and experimental larvae were stained for the same time. Thus, together these data indicate that the majority of the PMGs we chose for our analysis are up-regulated after the ET stimulus in a specific subset of neurosensory cells of the zebrafish in a restricted timeline and at specific loci.
DISCUSSION
We have established a noxious thermal assay for 5dpf zebrafish larvae that produces effects similar to tissue burns in higher vertebrates. After the ET assay, axons innervating the trunk musculature and skin of 5dpf zebrafish larvae appeared dramatically affected, with large regions presenting axonal degeneration. In mammals, axon degeneration is known to produce post-burn neuropathic-like pain (Fauerbach et al., 2005; Schneider et al., 2006; Wong and Turner, 2010), and expression of pro-inflammatory cytokines that induce an inflammatory response in the organism (Summer et al., 2008). In addition, these burn effects will probably induce macrophage activation and migration to the site of injury (Yregård et al., 2001; Shields et al., 2012; Rosenberg et al., 2012). Inflammation and neuropathic pain have been shown to activate a list of factors in key neuromodulatory regions in mammals, like dorsal root ganglia, spinal cord neurons and neurons of the brain. We have analyzed quantitatively and qualitatively the expression levels of the transcripts of a set of these genes identified in previous studies in mammals that respond to noxius stimuli (c-fos, c-jun, vip, pacap1b and bdnf) (Ru-Rong Ji and Strichartz 2004; Coggeshall, 2005). c-Jun and c-Fos are members of the immediate-early gene family (Herdegen and Leah, 1998). These factors are involved in a variety of cellular processes including proliferation and survival, differentiation, growth, apoptosis, response to cell stress, cell migration and transformation (Loebrich and Nedivi 2009; Pérez-Cadahía et al., 2011). VIP is a potent peptide having many functions such as stimulating exocrine and endocrine secretion, moderating smooth muscle relaxation, regulating circadian rhythms and neuro-modulation (Tse et al, 2002). Nerve injury determines vip induction in dorsal root ganglia and dorsal horn of the spinal cord in rat (Ma and Bisby, 1998). It has been found that vip is under a regulatory pathway controlled by c-JUN, and neuropathic pain induction of c-jun results into a following induction of vip in cultured sensory neurons and in dorsal root ganglia in rat (Mulderry and Dobson, 1996; Son et al., 2007). PACAP belongs to the same super-family of neurotrophic factor of VIP (the glucagon/secretin superfamily) and it has a wide range of functions as neuromodulator, neurotrophin, smooth muscle relaxant (Dickinson and Fleetwood-Walker, 1999; Fradinger et al., 2003; Krueckl et al., 2003); PACAP also stimulates the growth hormone release from the pituitary cells (Parker et al., 1997, Wong et al., 1998). In human there are two biologically active PACAP forms, PACAP27 and PACAP38, where the second one is a C-terminal extension of PACAP27 (Miyata et al., 1989; Miyata et al., 1990). Both VIP and PACAP have an excitatory effect in spinal cord function (Dickinson and Fleetwood-Walker, 1999). In fish, two pacap genes were identified and both have been duplicated (Fradinger and Sherwood, 2000; Wang et al., 2003). BDNF is a neurotrophin (brain-derived neurotrophic factor) that supports the survival of existing neurons and stimulates the differentiation and the growth of new neurons. Thus, all these factors respond to several types of biological events including noxious stimuli, and in this last case they are not simply markers for neuropathic pain and inflammation, instead they appear to be actively involved in the modulation of these mechanisms.
The noxious ET assay causes axon degeneration and inflammation, but is probably also inducing a variety of other effects in the zebrafish, including cell stress and apoptosis. Thus, it is very difficult to determine unambiguously the effect that leads to the activation of the genes we have studied. For instance, in our quantitative analysis the PMGs showed a rapid response to the noxious heat, in particular c-fos and c-jun, with an up-regulation by 30’ after the stimulus. At later time points, their expression levels decreased to the control levels. These quantitative data let us determine if and when the over-expression of these factors occurs after the ET stimulus. However, they do not discriminate between the various cellular and tissue processes induced by the assay and leading to PMG induction. To clarify this point we conducted WISH to analyze the expression of PMGs. If generic stress status or apoptosis were the source of PMGs expression we would expect to see their expression diffused across many cellular types and tissues, in a timeline strictly correlated with the qRT-PCR profile. Alternatively, if the axon degeneration and related inflammatory states lead to PMGs expression, we would also expect up-regulation of PMGs in restricted areas of the larvae, especially in sensory tissues. Indeed, WISH analysis brought additional and relevant information to our study. In fact, we determined that PMGs are up-regulated in sensory tissues of zebrafish larvae. Moreover, this specific expression is still persistent after the peak of induction seen by qRT-PCR, meaning that the specific expression in neurosensory tissues is temporally distinct from the bulk of generic gene induction.
Taken together, these results restrict the possible cellular/tissue responses that may lead to PMG over expression, suggesting that the persistent activation of these factors in neurosensory structures may be determined by degeneration of motor axons and skin innervation and/or the resulting inflammatory state. In the future, it would be interesting to study the effect of single neuron ablation on the expression in the spinal cord of c-fos and pacap1b as well as determining cytokine levels and macrophage localization during the same time window that we have studied. Moreover, these tests and the use of specific anti-inflammatory drugs would help to better define the roles of inflammation and axon degeneration in determining PMG up-regulation.
In our study, c-jun is particularly interesting. We think that the induction of c-jun expression in the gut of zebrafish larvae could be indicative of painful conditions. There are at least two, not mutually exclusive, explanations of how gut epithelia may be affected by our noxious ET stimuli: first the 5dpf larvae we used are extremely tiny and heat could quickly pass from the body surface into the interior of the zebrafish. Alternatively, the treatment may have caused the forced ingestion of hot water and subsequent stimulation of the gut epithelia. We think that the expression of c-jun in the gut could have neurosensory relevance, because it precedes by about 2-3 hours the increase of vip and pacap1b mRNA levels in putative enteric neurons, suggesting that c-Jun may control vip and pacap1b expression in that tissue. This hierarchical genetic cascade has been shown in dorsal root ganglia of rat models for neuropathic pain (Mulderry and Dobson, 1996; Son et al., 2007), suggesting that this mechanism may be conserved during the evolution from teleosts to mammals. In addition, in Crohn’s disease and irritable bowel syndrome the number of VIP positive neurons and VIP blood-levels, respectively, are increased. Further studies will be necessary to finally demonstrate this genetic pathway in zebrafish gut and if our approach could model such diseases in zebrafish.
In our assay we also found that pacap1b mRNA levels were increased in the spinal cord a few hours after the onset of c-fos expression and in putative enteric neurons after c-jun expression. The mammalian pacap promoter/enhancer region contains sequence motifs homologous to cAMP response element (CRE), TPA response element, and growth hormone factor-1 binding site (Yamamoto et al., 1998). Thus, pacap, as well as c-fos, contains a CRE element. However, no cis-regulatory sequences for c-Jun and c-Fos have yet been identified in the pacap promoter. Further studies on zebrafish pacap1b promoter-enhancer elements would be very informative regarding the possible direct or indirect role of c-Jun and c-Fos in controlling pacap1b expression in our assay.
Interestingly, we do not see induction of any of these factors in the dorsal root ganglia. This could be due to the relative early developmental stage at which we conducted our assay, reflecting an incomplete development of the dorsal root ganglions cell types (Raible et al., 1992; An et al., 2002; McGraw et al., 2008). Alternatively, our noxious ET test may have destroyed dorsal root ganglia neurons.
In conclusion, we have established a new assay of nociception in zebrafish larvae that induces effects similar to post-burn neuropathic pain as seen in mammals. The temporally restricted localization of some PMGs in sensory structures of the spinal cord strongly suggests that their roles in neuropathic pain modulation may be conserved from zebrafish to mammal. In addition, the over expression of c-jun, vip and pacap1b in putative enteric neurons of the gut may be the result of an additional nociceptive mechanism that we have stimulated with our treatment and which indicates a genetic hierarchy in this response. Thus, we think that with this work we have provided evidence supporting the use of zebrafish larvae to study cellular and genetic networks related to neuropathic and/or inflammatory pain symptoms in mammals.
Supplementary Material
Figure S1: WISH analysis of bdnf. A, Expression after the stimulus, in the brain. Size bar 100µm. B, Chart indicates the percentage of larvae showing expression of pacap1b in the brain of 5dpf larvae at 2h, 4h, 6h and 24h from the ET treatment. * p≤0.001; *** Is p≤0.01 (Fisher’s exact test).
ACKNOWLEDGMENTS
We are grateful to Prof. Ray Habbas and his lab members for the use of the stereo-microscope SMZ800 (Nikon), and analysis of images with the software NIS-Elements BR3.0 (Nikon). We thank Dr. Bruce Bryant for the kind gift of the chemical FM1-43 and for helpful comments on the manuscript.
REFERENCES
- Alexandre D, Alonzeau J, Bill BR, Ekker SC, Waschek JA. Expression analysis of PAC1- R and PACAP genes in zebrafish embryos. J Mol Neurosci. 2011;43:94–100. doi: 10.1007/s12031-010-9397-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- An M, Luo R, Henion PD. Differentiation and maturation of zebrafish dorsal root ganglia and sympathetic ganglion neurons. J Comp Neurol. 2002;446:267–275. doi: 10.1002/cne.10214. [DOI] [PubMed] [Google Scholar]
- Atchison NE, Osgood PF, Carr DB, Szyfelbein SK. Pain during burn dressing change in children: Relationship to burn area, depth and analgesic regimens. Pain. 1991;47:41–45. doi: 10.1016/0304-3959(91)90009-M. [DOI] [PubMed] [Google Scholar]
- Bellipanni G, Murakami T, Weinberg ES. Molecular dissection of Otx1 functional domains in the zebrafish embryo. J Cell Physiol. 2010;222:286–293. doi: 10.1002/jcp.21944. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE. Fos nociception the dorsal horn. Prog Neurobiol. 2005;77:299–352. doi: 10.1016/j.pneurobio.2005.11.002. [DOI] [PubMed] [Google Scholar]
- De Camara DL, Raine TJ, London MD, Robson MC, Heggers JP. Progression of thermal injury: A morphologic study. Plast Reconstr Surg. 1982;69:491–499. doi: 10.1097/00006534-198203000-00016. [DOI] [PubMed] [Google Scholar]
- Dickinson T, Fleetwood-Walker SM. VIP and PACAP: Very important in pain? Trends Pharmacol Sci. 1999;20:324–329. doi: 10.1016/s0165-6147(99)01340-1. [DOI] [PubMed] [Google Scholar]
- Fauerbach JA, Lezotte D, Hills RA, Cromes GF, Kowalske K, de Lateur BJ, Goodwin CW, Blakeney P, Herndon DN, Wiechman SA, Engrav LH, Patterson DR. Burden of burn: A norm-based inquiry into the influence of burn size and distress on recovery of physical and psychosocial function. J Burn Care Rehabil. 2005;26:21–32. doi: 10.1097/01.bcr.0000150216.87940.ac. [DOI] [PubMed] [Google Scholar]
- Fradinger EA, Sherwood NM. Characterization of the gene encoding both growth hormone-releasing hormone (GRF) and pituitary adenylate cyclase-activating polypeptide (PACAP) in the zebrafish. Mol Cell Endocrinol. 2000;165:211–219. doi: 10.1016/s0303-7207(00)00251-3. [DOI] [PubMed] [Google Scholar]
- Fukuoka T, Kondo E, Dai Y, Hashimoto N, Noguchi K. Brain-derived neurotrophic factor increases in the uninjured dorsal root ganglion neurons in selective spinal nerve ligation model. J Neurosci. 2001;21:4891–4900. doi: 10.1523/JNEUROSCI.21-13-04891.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furness JB. Types of neurons in the enteric nervous system. J Auton Nerv Syst. 2000;81:87–96. doi: 10.1016/s0165-1838(00)00127-2. [DOI] [PubMed] [Google Scholar]
- Furness JB. The enteric nervous system and neurogastroenterology. Nat Rev Gastroenterol Hepatol. 2012;9:286–294. doi: 10.1038/nrgastro.2012.32. [DOI] [PubMed] [Google Scholar]
- Gale JE, Marcotti W, Kennedy HJ, Kros CJ, Richardson GP. FM 1–43 dye behaves as a permeant blocker of the hair-cell mechanotransducer channel. J Neurosci. 2001;21:7013–7025. doi: 10.1523/JNEUROSCI.21-18-07013.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geng SJ, Liao FF, Dang WH, Ding X, Liu XD, Cai J, Han JS, Wan Y, Xing GG. Contribution of the spinal cord BDNF to the development of neuropathic pain by activation of the NR2B-containing NMDA receptors in rats with spinal nerve ligation. Exp Neurol. 2010;222:256–266. doi: 10.1016/j.expneurol.2010.01.003. [DOI] [PubMed] [Google Scholar]
- Gomez Lima M, Maximino C, de Jesus Oliveira Batista E, Matos Oliveira KR, Herculano AM. Nocifensive behavior in adult and larval zebrafish. In: Zebrafish Protocols for Neurobehavioral Research. Neuromethods. 2012;66:153–166. [Google Scholar]
- Gonzalez P, Baudrimont M, Boudou A, Bordineaud JP. Comparative effects of direct cadmium contamination on gene expression in gills, liver, skeletal muscles and brain of the zebrafish (Danio rerio) Biometal. 2006;19:225–235. doi: 10.1007/s10534-005-5670-x. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Nunez V, Rodriguez RE. The Zebrafish: A model to study the endogenous mechanisms of pain. ILAR J. 2009;50:373–386. doi: 10.1093/ilar.50.4.373. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Leah JD. Inducible and constitutive transcription factors in the mammalian nervous system: Control of gene expression by Jun, Fos and Krox, and CREB/ ATF proteins. Brain Res Brain Res Rev. 1998;28:370–490. doi: 10.1016/s0165-0173(98)00018-6. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Tölle TR, Bravo R, Zieglgänsberger W, Zimmermann M. Sequential expression of JUN B, JUN D and FOS B proteins in rat spinal neurons: Cascade of transcriptional operations during nociception. Neurosci Lett. 1991a;129:221–224. doi: 10.1016/0304-3940(91)90466-7. [DOI] [PubMed] [Google Scholar]
- Herdegen T, Kovary K, Leah J, Bravo R. Specific temporal and spatial distribution of JUN, FOS, and KROX-24 proteins in spinal neurons following noxious transsynaptic stimulation. J Comp Neurol. 1991b;313:178–191. doi: 10.1002/cne.903130113. [DOI] [PubMed] [Google Scholar]
- Hirayama J, Cardone L, Doi M, Sassone-Corsi P. Common pathways in circadian and cell cycle clocks: Light-dependent activation of Fos/AP-1 in zebrafish controls CRY-1a and WEE-1. Proc Natl Acad Sci USA. 2005;102:10194–10199. doi: 10.1073/pnas.0502610102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt SP, Pini A, Evan G. Induction of c-fos-like protein in spinal cord neurons following sensory stimulation. Nature. 1987;328:632–634. doi: 10.1038/328632a0. [DOI] [PubMed] [Google Scholar]
- Jenkins R, Hunt SP. Long-term increase in the levels of c-jun mRNAand jun protein-like immunoreactivity in motor and sensory neurons following axon damage. Neurosci Lett. 1991;129:107–110. doi: 10.1016/0304-3940(91)90731-8. [DOI] [PubMed] [Google Scholar]
- Krueckl SL, Fradinger EA, Sherwood NM. Developmental changes in the expression of growth hormone-releasing hormone and pituitary adenylate cyclase-activating polypeptide in zebrafish. J Comp Neurol. 2003;455:396–405. doi: 10.1002/cne.10494. [DOI] [PubMed] [Google Scholar]
- Leah JD, Herdegen T, Bravo R. Selective expression of Jun proteins following axotomy and axonal transport block in peripheral nerves in the rat: Evidence for a role in the regeneration process. Brain Res. 1991;566:198–207. doi: 10.1016/0006-8993(91)91699-2. [DOI] [PubMed] [Google Scholar]
- Li WP, Xian C, Rush RA, Zhou XF. Upregulation of brain-derived neurotrophic factor and neuropeptide Y in the dorsal ascending sensory pathway following sciatic nerve injury in rat. Neurosci Lett. 1999;260:49–52. doi: 10.1016/s0304-3940(98)00958-6. [DOI] [PubMed] [Google Scholar]
- Loebrich S, Nedivi E. The function of activity-regulated genes in the nervous system. Physiol Rev. 2009;89:1079–1103. doi: 10.1152/physrev.00013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W, Bisby MA. Partial and complete sciatic nerve injuries induce similar increases of neuropeptide Y and vasoactive instestinal peptide immunoreactivities in primary sensory neurons and their central projections. Neuroscience. 1998;86:1217–1234. doi: 10.1016/s0306-4522(98)00068-2. [DOI] [PubMed] [Google Scholar]
- Mabuchi T, Shintani N, Matsumura S, Okuda-Ashitaka E, Hashimoto H, Muratani T, Minami T, Baba A, Ito S. Pituitary adenylate cyclase-activating polypeptide is required for the development of spinal sensitization and induction of neuropathic pain. J Neurosci. 2004;24:7283–7291. doi: 10.1523/JNEUROSCI.0983-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malafoglia V, Bryant B, Raffaeli W, Giordano A, Bellipanni G. The zebrafish as a model for nociception studies. J Cell Physiol. 2013;228:1956–1966. doi: 10.1002/jcp.24379. [DOI] [PubMed] [Google Scholar]
- Martin SM, O’Brien GS, Portera-Cailliau C, Sagasti A. Wallerian degeneration of zebrafish trigeminal axons in the skin is required for regeneration and developmental pruning. Development. 2010;137:3985–3994. doi: 10.1242/dev.053611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGraw HF, Nechiporuk A, Raible DW. Zebrafish dorsal root ganglia neural precursor cells adopt a glial fate in the absence of neurogenin1. J Neurosci. 2008;28:12558–12569. doi: 10.1523/JNEUROSCI.2079-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyers JR, MacDonald RB, Duggan A, Lenzi D, Standaert DG, Corwin JT, Corey DP. Lighting up the senses: FM 1-43 loading of sensory cells through nonselective ion channels. J Neurosci. 2003;23:4054–4065. doi: 10.1523/JNEUROSCI.23-10-04054.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletic G, Miletic V. Increases in the concentration of brain derived neurotrophic factor in the lumbar spinal dorsal horn are associated with pain behavior following chronic constriction injury in rats. Neurosci Lett. 2002;319:137140. doi: 10.1016/s0304-3940(01)02576-9. [DOI] [PubMed] [Google Scholar]
- Miyata A, Arimura A, Dahl RR, Minamino N, Uehara A, Jiang L, Culler MD, Coy DH. Isolation of a novel 38 residue-hypothalamic polypeptide which stimulates adenylate cyclase in pituitary cells. Biochem Biophys Res Commun. 1989;164:567–574. doi: 10.1016/0006-291x(89)91757-9. [DOI] [PubMed] [Google Scholar]
- Miyata A, Jiang L, Dahl RD, Kitada C, Kubo K, Fujino M, Minamino N, Arimura A. Isolation of a neuropeptide corresponding to the N-terminal 27 residues of the pituitary adenylate cyclase activating polypeptide with 38 residues (PACAP38) Biochem Biophys Res Commun. 1990;170:643–648. doi: 10.1016/0006-291x(90)92140-u. [DOI] [PubMed] [Google Scholar]
- Mogil JS, Basbaum AI. Introducing the Biennial review of pain. Pain. 2011;152:S1. doi: 10.1016/j.pain.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Morellini NM, Fear MW, Rea S, West AK, Wood FM, Dunlop SA. Burn injury has a systemic effect on reinnervation of skin and restoration of nociceptive function. Wound Repair Regen. 2012;20:367–377. doi: 10.1111/j.1524-475X.2012.00787.x. [DOI] [PubMed] [Google Scholar]
- Mulderry PK, Dobson SP. Regulation of VIP and other neuropeptides by c-Jun in sensory neurons: Implications for the neuropeptide response to axotomy. Eur J Neurosci. 1996;8:2479–2491. doi: 10.1111/j.1460-9568.1996.tb01542.x. [DOI] [PubMed] [Google Scholar]
- Nozaki-Taguchi N, Yaksh TL. A novel model of primary and secondary hyperalgesia after mild thermal injury in the rat. Neurosci Lett. 1998;254:25–28. doi: 10.1016/s0304-3940(98)00648-x. [DOI] [PubMed] [Google Scholar]
- Obata K, Noguchi K. BDNF in sensory neurons and chronic pain. Neurosci Res. 2006;55:1–10. doi: 10.1016/j.neures.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Fukuoka T, Yi D, Tokunaga A, Hashimoto N, Yoshikawa H, Noguchi K. Contribution of injured and uninjured dorsal root ganglion neurons to pain behavior and the changes in gene expression following chronic constriction injury of the sciatic nerve in rats. Pain. 2003;101:6577. doi: 10.1016/s0304-3959(02)00296-8. [DOI] [PubMed] [Google Scholar]
- Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, Fukuoka T, Tokunaga A, Noguchi K. The effect of site and type of nerve injury on the expression of brainderived neurotrophic factor in the dorsal root ganglion and on neuropathic pain behavior. Neuroscience. 2006;137:961–970. doi: 10.1016/j.neuroscience.2005.10.015. [DOI] [PubMed] [Google Scholar]
- Olsson C, Holmberg A, Holmgren S. Development of enteric and vagal innervation of the zebrafish (Danio rerio) gut. J Comp Neurol. 2008;508:756–770. doi: 10.1002/cne.21705. [DOI] [PubMed] [Google Scholar]
- Palsson OS, Morteau O, Bozymsky EM, Woosley JT, Sartor RB, Davies MJ, Johnson DA, Turner MJ, Whitehead WE. Elevated vasoactive intestinal peptide concentrations in patients with irritable bowel syndrome. Dig Dis Sci. 2004;49:1236–1243. doi: 10.1023/b:ddas.0000037818.64577.ef. [DOI] [PubMed] [Google Scholar]
- Parker DB, Power ME, Swanson P, Rivier J, Sherwood NM. Exon skipping in the gene encoding pituitary adenylate cyclase-activating polypeptide in salmon alters the expression of two hormones that stimulated growth hormone release. Endocrinology. 1997;138:414–423. doi: 10.1210/endo.138.1.4830. [DOI] [PubMed] [Google Scholar]
- Pérez-Cadahía B, Drobic B, Davie JR. Activation and function of immediate-early genes in the nervous system. Biochem Cell Biol. 2011;89:61–73. doi: 10.1139/O10-138. [DOI] [PubMed] [Google Scholar]
- Priya SG, Jungvid H, Kumar A. Skin tissue engineering for tissue repair and regeneration. Tissue Eng Part B Rev. 2008;14:105–118. doi: 10.1089/teb.2007.0318. [DOI] [PubMed] [Google Scholar]
- Prober DA, Zimmerman S, Myers BR, McDermott BM, Jr, Kim SH, Caron S, Rihel J, Solnica-Krezel L, Julius D, Hudspeth Aj, Schier AF. Zebrafish TRPA1 channels are required for chemosensation but not for thermosensation or mechanosensory hair cell function. J Neurosci. 2008;28:1010210110. doi: 10.1523/JNEUROSCI.2740-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raible DW, Wood A, Hodsdon W, Henion PD, Weston JA, Eisen JS. Segregation and early dispersal of neural crest cells in the embryonic zebrafish. Dev Dyn. 1992;195:29–42. doi: 10.1002/aja.1001950104. [DOI] [PubMed] [Google Scholar]
- Rosenberg AF, Wolman MA, Franzini-Armstrong C, Granato M. In vivo nervemacrophage interactions following peripheral nerve injury. J Neurosci. 2012;32:3898–3909. doi: 10.1523/JNEUROSCI.5225-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ru-Rong Ji, Gary Strichartz. Cell signaling and the genesis of neuropathic pain. Sci STKE. 2004;(252):reE14. doi: 10.1126/stke.2522004re14. [DOI] [PubMed] [Google Scholar]
- Schneider J, Jehle EC, Starlinger MJ, Neunlist M, Michel K, Hoppe S, Schemann M. Neurotransmitter coding of enteric neurones in the submucous plexus is changed in non-inflamed rectum of patients with Crohn’s disease. Neurogastroenterol Motil. 2001;13:255–264. doi: 10.1046/j.1365-2982.2001.00265.x. [DOI] [PubMed] [Google Scholar]
- Schneider JC, Harris NL, El Shami A, Sheridan RL, Schulz JT, III, Bilodeau ML, Ryan CM. A descriptive review of neuropathic-like pain after burn injury. J Burn Care Res. 2006;27:524–528. doi: 10.1097/01.BCR.0000226019.76946.5D. [DOI] [PubMed] [Google Scholar]
- Shepherd I, Eisen J. Development of the zebrafish enteric nervous system. Methods Cell Biol. 2011;101:143–160. doi: 10.1016/B978-0-12-387036-0.00006-2. 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields SD, Cheng X, Uçeyler N, Sommer C, Dib-Hajj SD, Waxman SG. Sodium channel Na(v)1.7 is essential for lowering heat pain threshold after burn injury. J Neurosci. 2012;32:10819–10832. doi: 10.1523/JNEUROSCI.0304-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simrén M, Abrahamsson H, Björnsson ES. An exaggerated sensory component of the gastrocolonic response in patients with irritable bowel syndrome. Gut. 2001;48:20–27. doi: 10.1136/gut.48.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sneddon LU. Pain perception in fish: Indicators and endpoints. ILAR J. 2009;50:338–342. doi: 10.1093/ilar.50.4.338. [DOI] [PubMed] [Google Scholar]
- Son SJ, Lee KM, Jeon SM, Park ES, Park KM, Cho HJ. Activation of transcription factor c-jun in dorsal root ganglia induces VIP and NPY upregulation and contributes to the pathogenesis of neuropathic pain. Exp Neurol. 2007;204:467–472. doi: 10.1016/j.expneurol.2006.09.020. [DOI] [PubMed] [Google Scholar]
- Summer GJ, Puntillo KA, Miaskowski C, Green PG, Levine JD. Burn injury pain: The continuing challenge. J Pain. 2007;8:533–548. doi: 10.1016/j.jpain.2007.02.426. [DOI] [PubMed] [Google Scholar]
- Summer GJ, Romero-Sandoval EA, Bogen O, Dina OA, Khasar SG, Levine JD. Proinflammatory cytokines mediating burn-injury pain. Pain. 2008;135:98–107. doi: 10.1016/j.pain.2007.05.012. [DOI] [PubMed] [Google Scholar]
- Thisse B, Pflumio S, Furthauer M, Loppin B, Heyer V, Degrave A, Woehl R, Lux A, Steffan T, Charbonnier XQ, Thisse C. Expression of the zebrafish genome during embryogenesis. 2001 ZFIN ID: ZDB-PUB-010810-1. [Google Scholar]
- Tonra JR, Curtis R, Wong V, Cliffer KD, Park JS, Timmes A, Nguyen T, Lindsay RM, Acheson A, DiStefano PS. Axotomy upregulates the anterograde transport and expression of brain- derived neurotrophic factor by sensory neurons. J Neurosci. 1998;18:4374–4383. doi: 10.1523/JNEUROSCI.18-11-04374.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tse DL, Pang RT, Wong AO, Chan SM, Vaundry H, Chow BK. Identification of a potential receptor for both peptide histidine isoleucine and peptide histidine valine. Endocrinology. 2002;143:1327–1336. doi: 10.1210/endo.143.4.8714. [DOI] [PubMed] [Google Scholar]
- Uyttebroek L, Shepherd IT, Harrisson F, Hubens G, Blust R, Timmermans JP, Van Nassauw L. Neurochemical coding of enteric neurons in adult and embryonic zebrafish (Danio rerio) J Comp Neurol. 2010;518:4419–4438. doi: 10.1002/cne.22464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Wong AO, Ge W. Cloning, regulation of messenger ribonucleic acid expression, and function of a new isoform of pituitary adenylate cyclase-activating polypeptide in the zebrafish ovary. Endocrinology. 2003;144:4799–4810. doi: 10.1210/en.2003-0501. [DOI] [PubMed] [Google Scholar]
- Wang S, Lim G, Yang L, Zeng Q, Sung B, Jeevendra Martyn JA, Mao J. A rat model of unilateral hindpaw burn injury: Slowly developing rightwards shift of the morphine dose–response curve. Pain. 2005;116:87–95. doi: 10.1016/j.pain.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Westerfield M. The zebrafish book: A guide for the laboratory use of zebrafish (Brachydanio rerio) Eugine: University of Oregon Press; 1993. [Google Scholar]
- Wong L, Turner L. Treatment of post-burn neuropathic pain: Evaluation of pregablin. Burns. 2010;36:769–772. doi: 10.1016/j.burns.2009.05.018. [DOI] [PubMed] [Google Scholar]
- Wong AO, Leung MY, Shea WL, Tse LY, Chang JP, Chow BK. Hypophysiotropic action of a pituitary adenylate cyclase-activating polypeptide (PACAP) in the goldfish; immunohistochemical demonstration of PACAP in the pituitary, PACAP stimulation of growth hormone release from pituitary cells, and molecular cloning of pituitary type I PACAP receptor. Endocrinology. 1998;139:3465–3479. doi: 10.1210/endo.139.8.6145. [DOI] [PubMed] [Google Scholar]
- Woods IG, Lyons DA, Voas MG, Pogoda HM, Talbot WS. nsf is essential for organization of myelinated axons in zebrafish. Curr Biol. 2006;16:636–648. doi: 10.1016/j.cub.2006.02.067. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Hashimoto H, Hagihara N, Nishino A, Fujita T, Matsuda T, Baba A. Cloning and characterization of the mouse pituitary adenylate cyclase-activating polypeptide (PACAP) gene. Gene. 1998;211:63–69. doi: 10.1016/s0378-1119(98)00110-3. [DOI] [PubMed] [Google Scholar]
- Yregård L, Löwhagen PH, Cassuto J, Nilsson U, Lindblom L, Räntfors J, Tarnow P. A new technique for the analysis of endogenous mediators released following thermal injury. Burns. 2001;27:9–16. doi: 10.1016/s0305-4179(00)00077-2. [DOI] [PubMed] [Google Scholar]
- Zhang YZ, Hannibal J, Zhao Q, Moller K, Danielsen N, Fahrenkrug J, Sundler F. Pituitary adenylate cyclase activating peptide expression in the rat dorsal root ganglia: Upregulation after peripheral nerve injury. Neuroscience. 1996;74:1099–1110. doi: 10.1016/0306-4522(96)00168-6. [DOI] [PubMed] [Google Scholar]
- Zhou J, Yu Y, Tang Z, Shen Y, Xu L. Differential expression of mRNAs of GDNF family in the striatum following 6-OHDA-induced lesion. Neuroreport. 2000;11:3289–3293. doi: 10.1097/00001756-200009280-00048. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: WISH analysis of bdnf. A, Expression after the stimulus, in the brain. Size bar 100µm. B, Chart indicates the percentage of larvae showing expression of pacap1b in the brain of 5dpf larvae at 2h, 4h, 6h and 24h from the ET treatment. * p≤0.001; *** Is p≤0.01 (Fisher’s exact test).