Abstract
Apogossypolone (ApoG2) is a semi-synthesized derivative of gossypol. The principal objective of this study was to compare stability and toxicity between ApoG2 and gossypol, and to evaluate anti-lymphoma activity of ApoG2 in vitro and in vivo. ApoG2 shows better stability when compared with a racemic gossypol and can be better tolerated by mice compared to gossypol. ApoG2 showed significant inhibition of cell proliferation of WSU-DLCL2 and primary cells obtained from lymphoma patients, whereas it displayed no toxicity on normal peripheral blood lymphocytes. For a treatment of 72 h, the IC50 of ApoG2 was determined to be 350 nM against WSU-DLCL2 cells. Treatment with ApoG2 at 600 mg/kg resulted in significant growth inhibition of WSU-DLCL2 xenografts. When combined with CHOP, ApoG2 displayed even more complete inhibition of tumor growth. ApoG2 binds to purified recombinant Bcl-2, Mcl-1 and Bcl-XL proteins with high affinity and is shown to block the formation of heterodimers between Bcl-XL and Bim. For a treatment of 72 h, ApoG2 induced a maximum of 32% of apoptotic cell death. Western blot experiments showed that treatment with ApoG2 led to cleavage of caspase-3, caspase-9 and PARP. Moreover, pretreatment of DLCL2 cells with caspase-3, -9 and broad spectrum caspase inhibitors significantly blocked growth inhibition induced by ApoG2. In conclusion, ApoG2 effectively inhibits growth of DLCL2 cells at least partly by inducing apoptosis. It is an attractive small molecule inhibitor of the Bcl-2 family proteins to be developed further for the treatment of diffuse large cell lymphoma.
Keywords: small molecule inhibitors, Bcl-2 family of protein, diffuse large cell lymphoma, apoptosis, chemotherapy, animal model, toxicity
Introduction
Diffuse large-cell lymphoma (DLCL) accounts for 31% of all lymphomas and is the most common type of non-Hodgkin’s Lymphoma (NHL).1 Currently, the four-drug combination, cyclophosphamide-doxorubicin-vincristine-prednisone (CHOP), provides cure in 30% to 40% of unselected patients with DLCL.2 CHOP provides the cure by inducing apoptosis of cancer cells either directly or indirectly. For this reason, development of apoptosis resistance of DLCL cells to CHOP leads to most of the treatment failure.3-7 As a matter of fact, apoptosis resistance is implicated in virtually every known human malignancy.8,9 In order to overcome this challenge, many groups have targeted their research on one family of proteins, the Bcl-2 family. Proteins of the Bcl-2 family include both the ones that promote cell apoptosis (pro-apoptotic members), such as Bak, Bax, Bad, Bid, Bik and Bim, and the ones that promote cell survival (anti-apoptotic members), such as Bcl-2, Bcl-Xand Mcl-1.10-14 But they all possess at least one of four conserved motifs known as Bcl-2 homology domains (BH1 to BH4).10,15-17 Pro- and anti-apoptotic Bcl-2 family members can form heterodimers and negate each other’s function, suggesting that their relative concentration may determine whether a cell undergoes survival or death following an apoptosis stimulus.18,19 Consistent with this notion, anti-apoptotic members, such as Bcl-2 and Bcl-XL, were indeed found overexpressed in 80% of non-Hodgkin’s lymphoma and believed to be the key mediators of developing apoptotic resistance to chemotherapy.20 Structural studies have elucidated that a hydrophobic groove in anti-apoptotic members, such as Bcl-XL and Bcl-2, forms a binding pocket, into which pro-apoptotic members’ BH3 domains are able to bind.21-25 Hence, molecules that mimic pro-apoptotic BH3 domain and bind strongly to this binding pocket may be able to interfere with the formation of heterodimers between pro- and anti-apoptotic family members, render the anti-apoptotic Bcl-2 members less effective and tip the balance toward apoptosis. One class of such molecules, called non-peptidic small-molecule inhibitors (SMIs), were indeed discovered or designed and synthesized since year 2000.22 By pursuing the same strategy, our group was able to report previously promising data from preclinical studies of two SMIs, gossypol and TW-37, against diffuse large cell lymphoma.4,5 In this report, we present our studies on Apogossypolone (ApoG2), a derivative of gossypol. Gossypol is promising and is now in Phase II human clinical trials for cancer, but it is a well known toxic compound due to the two aldehyde groups in its chemical structure. We synthesized ApoG2 by removing the two aldehyde groups. By doing so, we hope to generate a compound which has reduced toxicity but retains gossypol’s anticancer activity.
The idea of developing peptide and other large molecules to inhibit anti-apoptotic family members as potential anti-cancer therapeutics has been previously explored, but none of them has proven useful in clinic so far due to certain limitations, such as poor in vivo efficacy, poor oral availability, and/or high cost.26-28 In contrast, SMIs are cell permeable organic molecules with molecular weight of less than 750 Daltons; their use in clinic appears more practical and cost effective. Moreover, one of the most promising aspects of SMIs in treating cancer is that their targets and mechanisms of action are different from conventional chemotherapeutic agents and radiation.15,29 Thus, it will be feasible to combine them with other treatments, creating a synergistic therapy, without likely development of cross-resistance or increased toxicity.
Results
ApoG2 shows improved stability under stressed conditions and can be better tolerated by mice compared to gossypol
Gossypol contains two reactive aldehyde groups in its structure (Fig. 1A). These two reactive groups form covalent Schiff ’s bases with lysine residues in proteins and have been attributed to the toxicity of gossypol in animals and in human and greatly limit the maximum dose of gossypol one can give to patients. It is expected that removal (or conversion) of these aldehyde groups will significantly reduce their toxicity. By following this rationale, we synthesized Apogossypolone (ApoG2) from gossypol (Fig. 1A).
Figure 1.
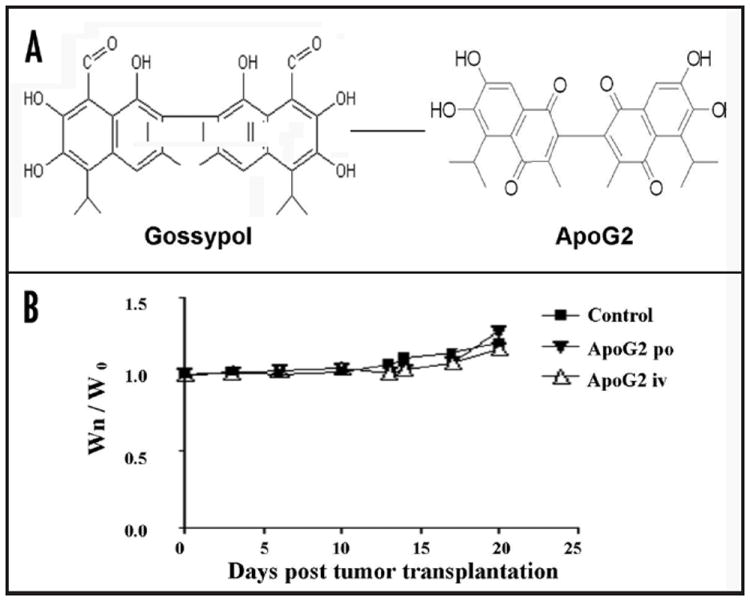
(A) Chemical structure of gossypol and Apogossypolone (ApoG2). (B) Treatment with ApoG2 at 600 mg/kg does not result in significant body weight changes between treatment groups and control group. Wn is the body weight at day (n); W0 is the body weight at day (0).
ApoG2 was first subjected to stability tests. In these tests, ApoG2 was compared with a racemic gossypol. The spectral purity of these two compounds was evaluated by using an HPLC system equipped with UV detector. ApoG2 and racemic gossypol are stable in the solid states upon storing in amber glass containers and standing at room temperature for three weeks (Table 1). Stress tests showed that ApoG2 and racemic gossypol remained almost intact when they were exposed to normal light for two hours (Table 1). We also tested their stability under conditions of 0.1 N HCl, 0.1 N NaOH or 30% H2O2. As illustrated in Table 1, spectral purity of ApoG2 in 0.1 N HCl, 0.1 N NaOH or 30% H2O2 remains above 95%. In contrast, spectral purity values of the racemic gossypol degraded to a range of 10.4 ± 0.2% to 15.8 ± 0.4% under the same conditions.
Table 1.
Stability tests of ApoG2 in comparison with a racemic gossypol (n = 2)
| Spectral purity (%) in solid state in amber glass container at room temperature | ||
|---|---|---|
| ApoG2 | Racemic gossypol | |
| 0 h | 100.0 ± 0 | 100.0 ± 0 |
| 4 days | 99.7 ± 0.5 | 99.4 ± 0.1 |
| 7 days | 99.9 ± 0.2 | 99.8 ± 0.1 |
| 2 weeks | 100.0 ± 0.1 | 99.9 ± 0.1 |
| 3 weeks | 100.0 ± 0.1 | 99.8 ± 0.2 |
| Spectral purity (%) under stressed conditions | ||
| ApoG2 | Racemic gossypol | |
| Reference | 98.0 ± 0.4 | 92.6 ± 0.6 |
| 0.1 N HCI, 5 mm | 96.5 ± 0.4 | 15.8 ± 0.4 |
| 0.1 N NaOH, 5 mm | 96.3 ± 0.4 | 10.4 ± 0.2 |
| 30% H2O2, 5 mm | 97.3 ± 0.2 | 14.5 ± 0.4 |
| Normal light, 2 h | 95.2 ± 0.2 | 92.3 ± 0.2 |
Note: Spectral purities were evaluated using an HPLC system equipped with UV detector. n: number of replicates.
Next, we compared the toxicities of ApoG2 and gossypol in mice. The maximal tolerated dose (MTD) of ApoG2 was evaluated in non-tumor-bearing SCID mice using two different routes of administrations, iv and po. In both routes of administration, ApoG2 was well tolerated in mice up to 800 mg/kg (160 mg/kg per day for 5 days). Animals at this dose displayed no gross signs of toxicity, such as significant (>5%) weight loss (Fig. 1B) or lethargic behavior. In comparison, the MTD for gossypol was determined to be 120 mg/kg, given in three divided dosages daily of 40 mg/kg per iv injection. Animals at this does experienced weight loss of <5% (data not shown), but displayed certain signs of toxicity, such as scruffy and rough hair as well as lethargic behavior. Dosages of gossypol at daily iv injection of 40 mg/kg for 4 days or daily iv injection of 60 mg/kg for three days are lethal to the animals. Kitada et al. reported recently that treatment with gossypol caused a great deal of hepatotoxicity and gastrointestinal toxicity in normal Balb/c mice compared to treatment with vehicle control or Apogossypol, which is another derivative of gossypol.34 In our studies, sections of mouse liver, small and large intestines were subjected to H & E staining and revealed no abnormality among vehicle control, ApoG2 and gossypol treated groups (data not shown).
ApoG2 inhibits cell growth of WSU-DLCL2 in vitro
Anti-cell proliferation activity of ApoG2 was investigated with two procedures: Trypan Blue cell counting and MTT assay. In the cell counting study, cells were treated with ApoG2 over a period of up to 96 h, stained with Trypan Blue and counted. As shown in Figure 2A, treatment with ApoG2 resulted in a dose-, and time-dependent inhibition of cell proliferation. ApoG2 inhibited growth of WSU-DLCL2 cells by approximately 50% at a concentration of 350 nM; at a concentration of 1000 nM, ApoG2 inhibited cell growth by over 95%. In the MTT assay, cells were treated with ApoG2 for 72 h. Results from MTT assay were quite consistent with that from cell counting (Fig. 2B). Based on these two studies, we estimate that the 50% growth inhibition concentration (IC50) of ApoG2 on WSU-DLCL2 cells is approximately 350 nM.
Figure 2.
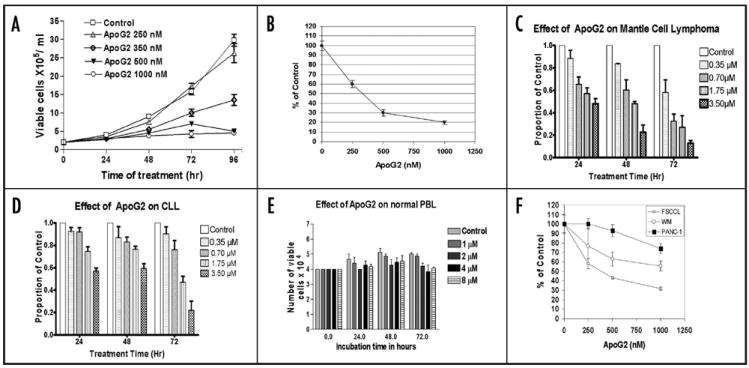
In vitro cell growth inhibition effect of ApoG2. Cell growth was monitored either by Trypan Blue cell counting (A, C–E) or by MTT assay (B). (A and B) WSU-DLCL2. (C) primary cells of mantle cell Lymphoma. (D) primary cells of chronic B-cell lymphocytic leukemia. (E) normal peripheral blood mononuclear cells. (F) FSCCL and WM cell growth was monitored by Trypan blue cell counting, whereas PANC-1 was monitored by MTT assay.
For the purpose of finding out whether ApoG2 could extend its effect of growth inhibition to primary lymphoma cells that were never cultured in vitro, we isolated, cultured and treated cells from two patients with 0–3.5 μM of ApoG2 for 72 h. As shown in Figure 2C and D, ApoG2 showed significant cytotoxic effect on these two primary lymphoma cells (mantle cell lymphoma and chronic lymphocytic leukemia) at a concentration of 350 nM or higher. On the other hand, when normal peripheral blood lymphocytes were treated with ApoG2, no major cytotoxicity was exhibited at a concentration as high as 8 μM and exposure time of 72 h (Fig. 2E).
ApoG2 inhibits the growth of WSU-DLCL2 in SCID mouse xenografts
Because ApoG2 was well tolerated in mice up to 800 mg/kg (160 mg/kg per day for 5 days), we could not determine the MTD of ApoG2 in SCID mice due to the availability of the compound. At a dose of 600 mg/kg (120 mg/kg daily for 5 days) whether iv or po, ApoG2 alone was enough to result in a growth inhibition of mouse xenografts that is comparable with that resulted from treatment with CHOP (Fig. 3A and B). For this reason, we chose this dose in all subsequent efficacy trials. When ApoG2 was administered in conjunction with CHOP, it was more significant that a more complete growth inhibition was achieved after one cycle of treatment (Fig. 3A and B).
Figure 3.
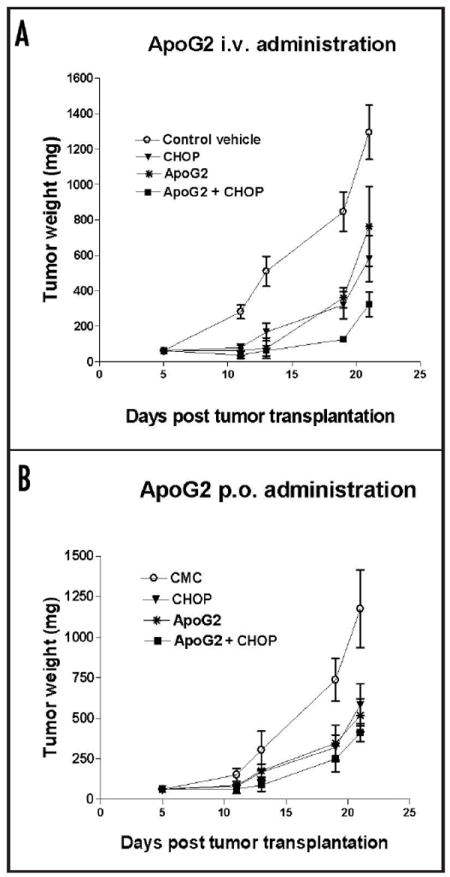
Effect of ApoG2 on SCID mouse tumor xenograft. ApoG2 was administered by either oral or iv route. (A) ApoG2 or ApoG2 and CHOP combination were administered to the animal by iv route. (B) ApoG2 or ApoG2 and CHOP combination were administered to the animal by oral route.
ApoG2 binds to purified recombinant human Bcl-2 and Mcl-1 targets with higher affinity compared to gossypol
For the purpose of finding out the functional mechanism of ApoG2, we first compared the binding affinity of ApoG2 and (-)-gossypol for their intended pharmacologic targets (Bcl-2, Bcl-XL) and Mcl-1) by performing a fluorescence polarization assay, which was described previously.4,22 Each binding experiment was performed for a total of three times (n = 3). Competitive binding experiments determined that ApoG2 binds to recombinant Bcl-2, Mcl-1 and Bcl-XL proteins with Ki values of 35 ± 14 nM (Fig. 4A), 25 ± 10 nM and 660 ± 62 nM, respectively. In comparison, (-)-gossypol was determined to bind to Bcl-2, Mcl-1 and Bcl-XL proteins with Ki values of 260 ± 30 nM (Fig. 4A), 170 ± 10 nM, and 480 ± 40 nM, respectively.
Figure 4.
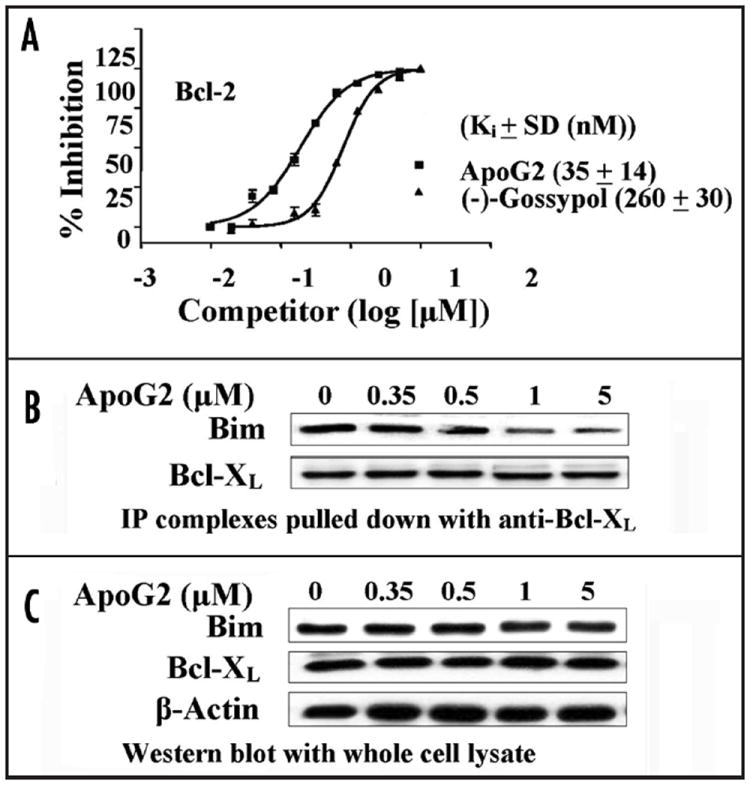
(A) Plotting of competitive binding of ApoG2 and (-)-gossypol to purified recombinant Bcl-2 protein. (B) Treatment of WSU-DLCL2 with ApoG2 interferes with the formation of heterodimers between Bcl-XL and Bim. Co-immunoprecipitation with anti-Bcl-XL antibody; Western blot with anti-Bim and anti-Bcl-XL antibodies. (C) Western blot of whole cell lysate with anti-Bim and anti-Bcl-XL antibodies. β-actin was probed to show loading of equal amount of protein lysate.
ApoG2 interferes with the formation of heterodimers between anti-apoptotic and pro-apoptotic Bcl-2 family members
Gossypol was able to interact with anti-apoptotic Bcl-2 family members to sequester them from forming dimers with pro-apoptotic members. ApoG2 was designed to retain this capability. As shown in Figure 4B, ApoG2 blocked the formation of heterodimers between Bcl-XL and Bim in a concentration-dependent manner. On the other hand, it did not affect significantly the protein levels of Bcl-XL or Bim at concentrations up to 5 μM (Fig. 4C). We wish to point out that there may be more heterodimers between pro- and anti-apoptotic members that can be affected by the treatment with ApoG2. What we show in Figure 4B is just a demonstration.
ApoG2 induces apoptotic WSU-DLCL2 cell death
Because ApoG2 was expected to target proteins in the apoptotic pathway and was found to be able to block the interactions between proand anti-apoptotic Bcl-2 family members, we were prompted to investigate whether ApoG2 is able to induce apoptotic cell death of WSU-DLCL2. Following treatment with ApoG2 at concentrations of 0 μm, 1.0 μm, 2.0 μm and 4.0 μm, cells were collected, stained with AO/EtBr, and counted. As illustrated in Figure 5A, treatment with ApoG2 resulted in significant increase of the number of apoptotic cells at 24-, 48- and 72-h time points. For example, treatment with 4.0 μm of ApoG2 for 24, 48 and 72 h resulted in 10, 17 and 32% of apoptotic cells respectively compared to control of approximately 3, 4 and 7% respectively. In seeking a second experimental procedure to confirm our AO/EtBr staining data, cell apoptosis was also examined by staining with Annexin V-PI. Following 24 h of treatment of ApoG2 at concentrations of 0 μm, 1.0 μM, 2.0 μM, 4.0 μM and 8.0 μM, cells were stained with Annexin V-PI and subjected to flow cytometry analysis. Data acquired from this experiment was consistent with that from our AO/EtBr experiment. As shown in Figure 5B, treatment with ApoG2 at concentrations of 0 μm, 1.0 μM, 2.0 μM, 4.0 μM and 8.0 μM resulted in 5%, 10%, 11%, 12% and 15% apoptotic cell death, respectively.
Figure 5.

ApoG2 induces WSU-DLCL2 cell apoptosis. (A) Acridine Orange/Ethidium Bromide staining. Cells were treated with 0.5 to 4 μM of ApoG2. At 24, 48 and 72 h time points, cells were collected and stained with AO/EtBr, followed by cell counting under fluorescent microscope. Cells seen in orange or light orange were regarded as apoptotic; cells in green or light green were taken as viable. (B) AnnexinV-PI staining. After treatment with ApoG2 at indicated concentrations for 24 h, cells were collected and stained with Annexin V and PI, followed by flow-cytometry analysis to determine the percentage of apoptotic cells.
ApoG2- induced cell growth inhibition depends at least partly on the activation of caspase-3 and caspase-9
In order to confirm that ApoG2 induces apoptosis in WSU-DLCL2 cells, we performed immunoblotting analysis of some of the proteins which are markers of cell apoptosis, such as activated caspase-3 and caspase-9, and cleaved poly (ADP-ribose) polymerase (PARP). As demonstrated in Figure 6A, treatment with ApoG2 indeed resulted in the activation of cleavages of caspase-3, caspase-9 and PARP. For the purpose of finding out whether ApoG2-induced cell growth inhibition depends on the activities of caspase-3 and caspase-9, we treated DLCL2 cells with caspase-family inhibitor, Z-VAD-FMK, caspase-9 inhibitor, Z-LEHD-FMK and caspase-3 inhibitor, Z-DEVD-FMK. The caspase inhibitors showed no effects of growth inhibition themselves (Fig. 6B). But all three inhibitors significantly blocked growth inhibition induced by treatment with ApoG2 (Fig. 6B). Treatment with 1000 nM of ApoG2 resulted in an approximately 80% of growth inhibition, which was reduced to approximately 40% of growth inhibition by each of the three caspase inhibitors (Fig. 6B). Thus, we speculate that ApoG2 induces DLCL2 cell growth inhibition at least partly by inducing caspase-3 and caspase-9 dependent apoptosis.
Figure 6.
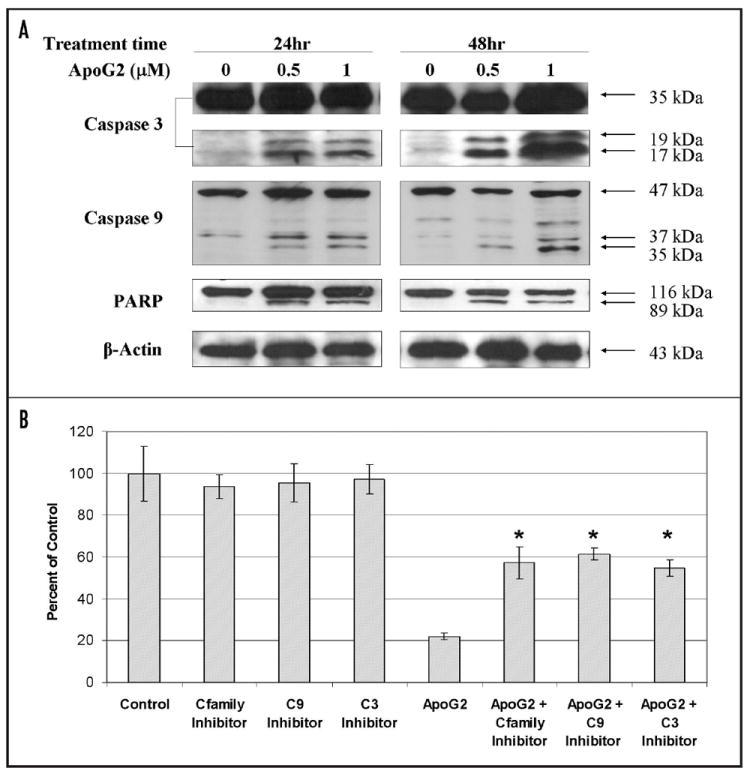
(A) Activation of cleavage of caspase-3, caspase-9 and PARP. Whole cell lysate was used for Western blot analysis. β-actin was probed to show equal loading of total protein. (B) DLCL2 cells treated with caspase family inhibitor (Cfamily Inhibitor), Z-VAD-FMK, caspase-9 inhibitor (C9 Inhibitor), Z-LEHDFMk, caspase-3 inhibitor (C3 Inhibitor), Z-DEVD-FMK, and/or ApoG2 (1000 nM). * indicates p < 0.05 when ApoG2 and caspase inhibitor combined treatment compared with corresponding either caspase alone treatment or ApoG2 alone treatment.
Discussion
Apogossypolone (ApoG2) is a semi-synthesized cell permeable non-peptidic small molecule inhibitor, expected to target a broad spectrum of anti-apoptotic Bcl-2 family members (pan-Bcl-2 inhibitor), including Bcl-2, Bcl-XL and Mcl-1. Our report is the first one exploring the anti-lymphoma activity of ApoG2 against diffuse large cell lymphoma. Data presented in this paper is consistent with the following statements: (1) ApoG2 is more stable than its parental compound gossypol; (2) MTD studies indicate that ApoG2 is less toxic than gossypol; (3) in our in vitro studies against WSU-DLCL2 cell line, IC50 value of ApoG2 was estimated to be 350 nM for a treatment of 72 h, which is a marked improvement over (-)-gossypol’s estimated IC50 value of well over 1000 nM in the same cell line;5 (4) treatment with ApoG2 can result in significant growth inhibition of WSU-DLCL2 SCID xenografts. When combined with CHOP, a more complete inhibition of xenograft tumor growth can be achieved.
The two aldehyde groups of gossypol have been attributed to its toxicity in animals and in human and greatly limit the maximum dose one can give to patients. It is expected that derivatives produced from gossypol by removal or conversion of these aldehyde groups shall have significantly reduced toxicity, while at the same time retain gossypol’s anti-cancer activity or become more potent than gossypol. It is quite interesting that Kitada et al. reported their findings very recently on the comparison of Apogossypol and gossypol.34 In their in vitro studies against Bcl-2 expressing B-cells, they determined the medial lethal dose (LD50) of Apogossypol and gossypol to be 3–5 μM and 7.5–10 μM, respectively. In their in vivo studies using a Bcl-2 transgenic mouse model, they formulated Apogossypol and gossypol in 100% sesame oil and administered the compounds at MTD of 60 μM/kg (which is approximately 31 mg/kg) to the animals orally (daily × 5 days × 3 weeks). In comparison, our group determined the MTD of gossypol at 120 mg/kg by formulating the compound in a DMSO-containing cocktail and administering it to the animals by three daily iv injections at equal does of 40 mg/kg. They compared the toxicities of Apogossypol and gossypol in normal female Balb/c mice and demonstrated that treatment with gossypol caused a great deal of hepatotoxicity and gastrointestinal toxicity to the animals compared to treatment with vehicle control or Apogossypol. In our studies, however, we did not observe similar toxicities among vehicle control, ApoG2 and gossypol treated groups as evidenced by H & E staining of sections of mouse liver, small and large intestines. We believe it might be explained by the fact that Kitada et al. treated the animals at a higher dose of gossypol for a much longer period of time (120 μM/kg, which is approximately 62 mg/kg, for five days daily po × 3 weeks). In comparison, we treated the animals with gossypol by only three daily iv injections at equal does of 40 mg/kg.
It should be noted that we are treating cells with doses of drug 10- to 30-fold higher than the inhibition constant (Ki) values determined in competitive binding assays. The Ki values of ApoG2 for competing with BH3 peptide for binding to Bcl-2, Mcl-1 and Bcl-XL are obtained from the Cheng-Prusoff equation:35
where IC50 is ApoG2 concentration at which a 50% inhibition is achieved, D is the concentration of the binding substrate, and Kd is the substrate concentration (in the absence of inhibitor) at which the velocity of the reaction is half-maximal. Our competitive binding experiments determined that ApoG2 binds to recombinant Bcl-2, Mcl-1 and Bcl-XL proteins with Ki values of 35 ± 14 (Fig. 1B), 25 ± 10 and 660 ± 62 nM, respectively. Based on the equation, it is obvious that the IC50 values are always higher than the Ki value (because Kd/(Kd + D) is always less than 1.0). In the case of competing for binding with Bcl-2, for example, the IC50 of ApoG2 is ~350 nM, which is 10-fold higher than its Ki value. Therefore, the drug concentrations we used in our in vitro experiments are in good agreement with the Ki values determined from our binding experiments.
Since the pioneering studies by Wang et al. in 2000,22 over a dozen non-peptidic BH3 mimetic SMIs have been identified and are now at various preclinical and/or clinical stages of development.4,15,22,23,33,36-39 Gossypol is one of the SMIs and is now in Phase II human clinical trials. Although it has been studied extensively for its anticancer activity since 1980s, most of the work related to gossypol-induced cell death has focused on its DNA fragmentation and cell cycle arrest effects.40-42 Little was known about gossypol’s mechanism of inducing cell apoptosis until recent studies revealed that it binds to Bcl-2 family proteins via the BH3 binding pocket to promote cell death.43 As expected, ApoG2 retains gossypol’s capability of inducing apoptotic cell death. However, it is reasonable to speculate that although ApoG2 may function as bone fide BH3 mimetics and bind to Bcl-2 family members to induce cell apoptosis, it may also causes cell death through other pathways. In other words, ApoG2’s function may not depend totally on Bcl-2 protein. This may explain the observation that the level of WSU-DLCL2 cell apoptotic death (treatment for 72 h with 4 μM of ApoG2 results in 32% of apoptosis) does not add up to the level of cell growth inhibition (over 80% inhibition at a concentration of 1 μM). Our speculation is further strengthened by our evaluation of growth inhibition effects of ApoG2 on three other tumor cell lines: FSCCL, WM and PANC-1. FSCCL is a low grade follicular small cleaved cell lymphoma line with high level of Bcl-2;44 WM is a Waldenstrom’s macroglobulinemia line with very low level of Bcl-2;45 while PANC-1 is a pancreatic cell line with very low level of Bcl-2 as well.46 As shown in Figure 2F, ApoG2 induces growth inhibition on all three cell lines, even though WM and PANC-1 have low level of Bcl-2 protein. It appears though, that ApoG2 is more effective against cells with high level of Bcl-2, such as DLCL2 and FSCCL. More studies devoted to investigate other functional mechanisms utilized by ApoG2 to inhibit cell growth are now actively underway in our lab.
The main challenge in developing a therapeutic agent is that it needs to show efficacy in vivo. By applying a SCID mouse xenograft model, we were able to show that ApoG2 by itself was effective in inhibiting tumor growth in the animal. In addition, when administered in combination with CHOP, ApoG2 achieved a more complete tumor growth inhibition. In our studies, ApoG2 was well tolerated by the animals for doses up to 800 mg/kg whether administered orally or by iv injection. Jia et al. reported recently on the comparison of the pharmacokinetic and metabolic profiling among gossypol, Apogossypol and Apogossypol hexaacetate.47 A few of their findings are: (1) gossypol and Apogossypol exhibited similar stability in various species of plasma; (2) gossypol’s bioavailability when given orally is 12.2–17.6%; (3) Apogossypol shows superior blood concentrations over time compared to gossypol, probably due to slower clearance of the compound. In our studies, we had determined the serum concentration of ApoG2 in mice as 5.0 μM (unbound) when given iv at 80 mg/kg per day for five days. In order to get a better pharmacokinetic and metabolic profiling of ApoG2, we will need further studies. However, we believe that results of our in vivo studies indicate very good stability and bioavailability of ApoG2.
In summary, results presented in this paper demonstrate that ApoG2 has improved stability and potency compared to its parental compound gossypol. It exerts at least part of its cell growth inhibition by inducing cell apoptosis. It is very effective in inhibiting tumor growth in SCID mouse model either by itself or in conjunction with CHOP, while it is well tolerated by the animals. Our studies suggest that ApoG2 represents a promising agent that should be developed for the treatment of non-Hodgkin’s lymphoma in the clinic. Although our studies are quite limited, we feel that they warrant further preclinical development of ApoG2 in a wider sampling of not only diffuse large cell lymphoma, but also other types of lymphoma.
Materials and Methods
Cell culture, reagents, patient-derived lymphoma cells, and normal peripheral blood lymphocytes (PBL)
The WSU-DLCL2 cell line was established in our laboratory and has high levels of Bcl-2 and Bcl-XL protein expression.30 Cells were plated in 24-well culture plates (Costar, Cambridge, MA) at a density of 2 × 105 viable cells per ml per well. Triplicate wells were treated with 0–1000 nM of ApoG2. Plates were incubated at 37°C in a humidified incubator with 5% CO2. All cultures were monitored throughout the experiment by cell counting every 24 h for four days using 0.4% Trypan Blue stain (Life Technologies, Grand Island, NY) and a hemacytometer.
Fresh lymphoma cells obtained from mantle cell lymphoma and chronic lymphocytic leukemia were used to assess the cytotoxic effect of ApoG2. Similarly, PBL obtained from a healthy donor were used to assay the cytotoxic effect of ApoG2 on normal human lymphocytes. Cells were plated in 24-well culture plates at a density of 4 × 104 viable cells per ml per well. Triplicate wells were treated with ApoG2. Plates were incubated at 37°C in a humidified incubator with 5% CO2. All cultures were monitored throughout the experiment by cell counting every 24 h for four days using 0.4% Trypan Blue stain and a hemacytometer.
Caspase-3 inhibitor, Z-DEVD-FMK, caspase-9 inhibitor, Z-LEHD-FMK, and caspase-family inhibitor, Z-VAD-FMK were purchased from Biovision (Mountain View, CA, USA). ApoG2 was synthesized by removing gossypol’s two aldehyde groups and was kept in a desiccator. For treating cell culture, it was dissolved in DMSO and used fresh; for treating animals orally (po), it was mixed with carboxymethylcellulose sodium (CMC); for treating animals intravenously (iv), it was dissolved in a cocktail containing 2% DMSO (v/v). When normal animals were injected with this DMSO-containing cocktail, no gross signs of toxicity, such as weight loss, were observed. Statistical analysis was done using the t-test (two tailed) with 95% confidence intervals between treated and untreated samples. p < 0.05 was used to indicate statistical significance.
WSU-DLCL2 xenografts
Mouse xenografts were established as described previously.4 Basically, four-week-old female ICR-SCID mice were obtained from Taconic Laboratory (Germantown, NY). The mice were adapted and WSU-DLCL2 xenografts were developed as described previously.31 Each mouse received 107 WSU-DLCL2 cells (in serum-free RPMI 1640) subcutaneously (sc) in each flank area. When sc tumors developed to ~1,500 mg, mice were euthanized and tumors dissected and mechanically dissociated into single-cell suspensions. Mononuclear cells were separated by Ficoll-Hypaque density centrifugation and washed twice with RPMI 1640. These cells were subjected to phenotypic analysis for comparison with the established tumor cell line to insure the human origin and its stability. After formation of sc tumors, serial propagation was accomplished by excising the tumors, trimming extraneous material, and cutting the tumors into fragments of roughly equal weight of 25 mg, which are transplanted sc using a 12-gauge trocar into the flanks of a new group of mice.
ApoG2 in vivo efficacy test
Mice bearing tumor xenografts were checked three times per week for tumor development. When tumor xenografts developed into palpable tumors, groups of five animals bearing bilateral tumors were removed randomly and assigned to six different treatment groups as follows: (1) ApoG2 iv at 120 mg/kg per day for five days; (2) ApoG2 po at 120 mg/kg per day for five days; (3) CHOP one injection at maximum tolerated dose (MTD) as determined previously;4 (4) ApoG2 iv at 120 mg/kg per day for five days plus CHOP one injection; (5) ApoG2 po at 120 mg/kg per day for five days plus CHOP one injection; and (6) control. Mice were monitored frequently for measuring size of tumors, recording changes in body weight, and examining of any side effects of the drugs. SC tumors were measured three times per week. Tumor weight was estimated by a standard equation used in our laboratory: tumor weight (mg) = (A × B2)/2, where A and B are the length and width (in mm) of the tumor, respectively.4
Fluorescence polarization-based binding assay for recombinant Bcl-2, Bcl-XL and Mcl-1 protein
This experiment was carried out as described previously.4,22 Briefly, 5-carboxyfluorecein was coupled to the N-terminus of a peptide, GQVGRQLAIIGDDINR, derived from the BH3 domain of Bak (Flu-BakBH3), which is able to bind to Bcl-2, Bcl-XL and Mcl-1 with high-affinity. Recombinant human Bcl-2, Bcl-XL and Mcl-1 proteins were prepared as described.32 The binding of Flu-BakBH3 to Bcl-2, Bcl-XL and Mcl-1 proteins was measured on a LS-50 luminescence spectrometer equipped with polarizers using a dual path length quartz cell (500 ml) (Perkin-Elmer). The fluorophore was excited with vertical polarized light at 480 nm (excitation slit width 15 nm), and the polarization value of the emitted light was observed through vertical and horizontal polarizers at 530 nm (emission slit width 15 nm). The binding affinity of each compound for Bcl-2, Bcl-XL and Mcl-1 proteins was assessed by determining the ability of different concentrations of the compound to inhibit Flu-BakBH3 binding.
Co-immunoprecipitation of pro- and anti-apoptotic complexes and Western blot
Primary antibodies specific for Bcl-XL and Bim were obtained from Santa Cruz biotechnology (Santa Cruz, CA). Co-immunoprecipitation was carried out as described previously.4 Basically, WSU-DLCL2 cells were lysed on ice in RIPA lysis buffer (0.5 ml per 1 × 107 cells/100 mm dish) containing 20 mM HEPES, 250 mM NaCl, 2 mM EDTA, 0.5 mM EGTA, 1 mM DTT, 2 mM sodium orthovanadate, 1% NP-40, and 10 μg/ml aprotinin, 10 μg/ml leupeptin, 10 μg/ml PMSF and 0.5 mg/ml of benzamidine) for 30 min. After pelleting the cellular debris by centrifugation at 10,000 × g for 20 min at 4°C, supernatant was collected and protein content was quantified with the BCA method.33 Cell lysate equivalent to 100 μg of total protein was pre-cleared with 10 μl of protein A and G agarose mixture (1:1), followed by incubation with 2 μg of Bcl-XL, or Bim antibody overnight at 4°C on a rotary rotor. In the next morning, 20 μl of protein A and G agarose mixture (1:1) in cold RIPA lysis buffer was added and incubated for additional three hours at 4°C. Immunoprecipitates were collected by centrifugation at 5000 rpm for one minute and washed three times (300 μl/each) in cold lysis buffer. After final wash, immunoprecipitates were resuspended in 2 × SDS protein sample loading buffer, boiled for five minutes, resolved with 12% SDS-PAGE and electroblotted to hybond C-extra membranes (Amersham Life Science, Arlington Heights, IL). Membranes were subsequently immunoblotted with antibodies to human Bcl-XL and Bim (Santa Cruz, CA), or cytochrome C, PARP, Caspase 3, Caspase 8 and Caspase 9 (Cell Signaling, Danvers, MA).
Acridine orange/ethidium bromide (AO/EtBr) cell counting and flow-cytometry analysis of annexin V-propidium iodide (PI) staining
WSU-DLCL2 cells were seeded in 24-well culture clusters (Costar, Cambridge, MA) at a density of 2 × 105 viable cells per ml per well and exposed to 0~1000 nM of ApoG2 for 48 h, 72 h and 96 h. At each time point cells were collected by centrifugation and resuspended in 25 μl of PBS. 1 μl of AO/EtBr mix was added into each sample followed by analysis under fluorescent microscope. Cells seen in orange or light orange were counted as apoptotic, cells in green or light green were counted as viable. Data was analyzed by using GraphPad Prism 4.03 software. For flow-cytometry analysis, WSU-DLCL2 cells were seeded in T-25 cell culture flask (Costar, Cambridge, MA) at a density of 105 viable cells/ml and exposed to ApoG2 for 24 h. 5 × 105 cells from each treatment were collected and stained by Annexin V-PI reagent using Annexin V-FITC Apoptosis Detection Kit (BioVision, Mountain View, CA) by following manufacturer’s instructions and further analyzed by Flow cytometry with wave length at Ex = 488 nm, Em = 530 nm.
Acknowledgments
This work was supported by a Leukemia and Lymphoma Society grant 6028-8 (Mohammad R.M.), by National Institutes of Health grants R01 CA109389 (Mohammad R.M.) and P30 CA22453-20 (Wang S.), and by a Department of Defense Breast Cancer Program grant BC0009140 (Wang S.).
References
- 1.Armitage JO, Weisenburger DD. New approach to classifying non-Hodgkin’s lymphoma: clinical features of the major histological subtypes. J Clin Oncol. 1998;6:278–95. doi: 10.1200/JCO.1998.16.8.2780. [DOI] [PubMed] [Google Scholar]
- 2.Elias L, Portlock CS, Rosenberg SA. Combination chemotherapy of diffuse histocytic lymphoma with cyclonphosphamide, Adriamycin, vincristine and prednisone (CHOP) Cancer. 1978;42:1705–10. doi: 10.1002/1097-0142(197810)42:4<1705::aid-cncr2820420408>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 3.collins-Burow B, Santos ES. Ritaximab and its role as maintenance therapy in non-Hodgkin lymphoma. Expert Rev Anticancer Ther. 2007;7:257–73. doi: 10.1586/14737140.7.3.257. [DOI] [PubMed] [Google Scholar]
- 4.Mohammad RM, Goustin AS, Aboukameel A, Chen B, Banerjee S, Wang G, Nikolovska-Coleska Z, Wang SM, Al-Katib AM. Preclinical studies of TW-37, a new non-peptide small-molecule inhibitor of Bcl-2, in diffuse large cell lymphoma xenograft model reveal drug action on both Bcl-2 and Mcl-1. Clin Cancer Res. 2007;13:2226–35. doi: 10.1158/1078-0432.CCR-06-1574. [DOI] [PubMed] [Google Scholar]
- 5.Mohammad RM, Wang SM, Aboukameel A, Chen B, Wu X, Chen J, Al-Katib AM. Preclinical studies of a nonpeptidic small-molecule inhibitor of Bcl-2 and Bcl-XL [(-)-gossypol] against diffuse large cell lymphoma. Mol Cancer Ther. 2005;4:13–21. [PubMed] [Google Scholar]
- 6.Jabbour E, Chalhoub B, Suzan F, Aloulou S, Cainap C, Toumi N, Fermé C, Carde P, Ribrag V. Outcome of elderly patients with aggressive non-Hodgkin’s lymphoma refractory to or relapsing after first-line CHOP or CHOP-like chemotherapy: a low probability of cure. Leuk Lymphoma. 2004;45:1391–4. doi: 10.1080/10428190310001653736. [DOI] [PubMed] [Google Scholar]
- 7.Cerny T, Borisch B, Introna M, Johnson P, Rose AL. Mechanism of action of retuximab. Anticancer Drugs. 2002;13:3–10. doi: 10.1097/00001813-200211002-00002. [DOI] [PubMed] [Google Scholar]
- 8.Reed JC. Regulation of apoptosis by bcl-2 family proteins and its role in cancer and chemoresistance. Curr Opin Oncol. 1995;7:541–6. doi: 10.1097/00001622-199511000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Kelekar A, Thompson CB. Bcl-2-family proteins: the role of the BH3 domain in apoptosis. Trends Cell Biol. 1998;8:324–30. doi: 10.1016/s0962-8924(98)01321-x. [DOI] [PubMed] [Google Scholar]
- 10.Adams JM, Cory S. The Bcl-2 protein family: arbiters of cell survival. Science. 1998;281:1322–6. doi: 10.1126/science.281.5381.1322. [DOI] [PubMed] [Google Scholar]
- 11.Reed JC. Bcl-2 family proteins. Oncogene. 1998;17:3225–36. doi: 10.1038/sj.onc.1202591. [DOI] [PubMed] [Google Scholar]
- 12.Fernandez-Luna JL. Apoptosis regulators as target for cancer therapy. ClinTransl Oncol. 2007;9:555–62. doi: 10.1007/s12094-007-0103-7. [DOI] [PubMed] [Google Scholar]
- 13.Zhang L, Ming L, Yu J. BH3 mimetic to improve cancer therapy; mechanisms and examples. Drug Resist Update. 2007;10:207–17. doi: 10.1016/j.drup.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Enings KE, Wiggins CM, Cook SJ. Bim and the pro-survival Bcl-2 proteins: opposites attract, ERK repels. Cell Cycle. 2007;6:2236–40. doi: 10.4161/cc.6.18.4728. [DOI] [PubMed] [Google Scholar]
- 15.Mohammad RM, Anshu G, Goustin AS. Small-molecule inhibitors of Bcl-2 family proteins as therapeutic agents in Cancer. Recent Patents on Anti-Cancer Drug Discovery. 2008;3:25–36. doi: 10.2174/157489208783478676. [DOI] [PubMed] [Google Scholar]
- 16.Roset R, Ortet L, Gil-Gomez G. Role of Bcl-2 family members on apoptosis: what we have learned from knock-out mice. Front Biosci. 2007;12:4722–30. doi: 10.2741/2421. [DOI] [PubMed] [Google Scholar]
- 17.Verma YK, Gangenahall GU, Singh VK, Gupta P, Chandra R, sharma RK, Raj HG. Cell death regulation by B-cell lymphoma proteins. Apoptosis. 2006;11:459–71. doi: 10.1007/s10495-006-5702-1. [DOI] [PubMed] [Google Scholar]
- 18.Johnstone RW, Ruefli AA, Lowe SW. Apoptosis: a link between cancer genetics and chemotherapy. Cell. 2002;108:153–64. doi: 10.1016/s0092-8674(02)00625-6. [DOI] [PubMed] [Google Scholar]
- 19.Oltuai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–19. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 20.Buolamwini JK. Novel anticancer drug discovery. Current Opin Chem Biol. 1999;3:500–9. doi: 10.1016/S1367-5931(99)80073-8. [DOI] [PubMed] [Google Scholar]
- 21.Enyedy IJ, Ling Y, Nacro K, Tomita Y, Wu X, Cao Y, Guo R, Li B, Zhu X, Huang Y, Long YQ, Roller PP, Yang D, Wang SM. Discovery of small-molecule inhibitors of Bcl-2 through structure-based computer screening. J Med Chem. 2001;44:4313–24. doi: 10.1021/jm010016f. [DOI] [PubMed] [Google Scholar]
- 22.Wang JL, Liu D, Zhang ZJ, Shan S, Han X, Srinivasula SM, Croce CM, Alnemri ES, Huang Z. Structure-based discovery of an organic compound that binds Bcl-2 protein and induces apoptosis of tumor cells. Proc Natl Acad Sci USA. 2000;97:7124–9. doi: 10.1073/pnas.97.13.7124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muchmore SW, Sattle M, Liang H, Meadows RP, Harlan JE, Yoon HS, Nettesheim D, Chang BS, Thompson CB, Wong SL, Ng SL, Fesik SW. X-ray and NMR structure of human Bcl-XL, an inhibitor of programmed cell death. Nature. 1996;381:335–41. doi: 10.1038/381335a0. [DOI] [PubMed] [Google Scholar]
- 24.Aritomi M, Kunishima N, Inohara N, Ishibashi Y, Ohta S, Morikawa K. Implications for the function of the Bcl-2 protein family. J Biol Chem. 1997;272:27886–92. doi: 10.1074/jbc.272.44.27886. [DOI] [PubMed] [Google Scholar]
- 25.Sattler M, Liang H, Nettesheim D, Meadows RP, Harlan JE, Eberstadt M, Yoon HS, Shuker SB, Chang BS, Minn AJ, Thompson CB, Fesik SW. Structure of Bcl-XL-Bak peptide complex: recognition between regulators of apoptosis. Science. 1997;275:983–6. doi: 10.1126/science.275.5302.983. [DOI] [PubMed] [Google Scholar]
- 26.Morris MJ, Tong WP, Cordon-Cardo C, Drobnjak M, Kelly WK, Slovin SF, Terry KL, Siedlecki K, Swanson P, Rafi M, Dipaola RS, Rosen N, Scher HI. Phase I trial of Bcl-2 antisense oligonucleotide (G3139) administered by continuous intravenous infusion in patients with advanced cancer. Clin Cancer Res. 2002;8:679–83. [PubMed] [Google Scholar]
- 27.Piche A, Grim J, Rancourt C, Gómez-Navarro J, Reed JC, Curiel DT. Modulation of Bcl-2 protein levels by an intracellular anti-Bcl-2 single-chain antibody increases drug-induced cytotoxicity in the breast cell line MCF-7. Cancer Res. 1998;58:2134–40. [PubMed] [Google Scholar]
- 28.Wang JL, Zhang ZJ, Choksi S, Shan S, Lu Z, Croce CM, Alnemri ES, Korngold R, Huang Z. Cell permeable Bcl-2 binding peptides: a chemical approach to apoptosis induction in tumor cells. Cancer Res. 2000;60:1498–502. [PubMed] [Google Scholar]
- 29.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, Bruncko M, Deckwerth TL, Dinges J, Hajduk PJ, Joseph MK, Kitada S, Korsmeyer SJ, Kunzer AR, Letai A, Li C, Mitten MJ, Nettesheim DG, Ng S, Nimmer PM, O’Connor JM, Oleksijew A, Petros AM, Reed JC, Shen W, Tahir SK, Thompson CB, Tomaselli KJ, Wang B, Wendt MD, Zhang H, Fesik SW, Rosenberg SH. An inhibitor of Bcl-2 family proteins induces regression of solid tumors. Nature. 2005;435:677–81. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 30.Al-katib AM, Smith MR, Kamanda WS, Pettit GR, Hamdan M, Mohamed AN, Chelladurai B, Mohammad RM. Bryostatin1 downregulates mdr1 and potentiates vincristine cytotoxicity in diffuse large cell lymphoma xenografts. Clin Cancer Res. 1998;4:1305–14. [PubMed] [Google Scholar]
- 31.Mohammad RM, Wall NR, Dutcher JA, Al-Katib AM. The addition of Bryostatin 1 to cyclophosphamide, doxorubicin, vincristine and prednisone (CHOP) chemotherapy improves response in a CHOP-resistant human diffuse large cell lymphoma xenograft model. Clin Cancer Res. 2000;6:4950–6. [PubMed] [Google Scholar]
- 32.Wang G, Nikolovska-Colesk Z, Yang CY, Wang R, Tang G, Guo J, Shangary S, Qiu S, Gao W, Yang D, Meagher J, Stuckey J, Krajewski K, Jiang S, Roller PP, Abaan HO, Tomita Y, Wang SM. Structure-based design of potent small-molecule inhibitors of anti-apoptotic Bcl-2 proteins. J Med Chem. 2006;49:6139–42. doi: 10.1021/jm060460o. [DOI] [PubMed] [Google Scholar]
- 33.Smith PK, Krohn RI, Hermanosn GT, Mallia AK, Gartner FH, Provenzano MD, Fujimoto EK, Goeke NM, Olson BJ, Klenk DC. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985;150:76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- 34.Kitada S, Kress CL, Krajewska M, Jia L, Pellecchia M, Reed JC. Bcl-2 antagonist ApoGossypol (NSC736630) displays single-agent activity in Bcl-2 transgenic mice and has superior efficacy with less toxicity compared to Gossypol (NSC19048) Blood. 2008;111:3211–19. doi: 10.1182/blood-2007-09-113647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 36.Trudel S, Li ZH, Rauw J, Tiedemann RE, Wen XY, Stewart AK. Preclinical studies of the pan-Bcl inhibitor Obatoclax (GX015-070) in multiple myeloma. Blood. 2007;109:5430–8. doi: 10.1182/blood-2006-10-047951. [DOI] [PubMed] [Google Scholar]
- 37.Kaneko M, Nakashima T, Uosaki Y, Hara M, Ikeda S, Kanda Y. Synthesis of tetrocarcin derivatives with specific inhibitory activity towards Bcl-2 functions. Bioorg Med Chem Lett. 2001;11:887–90. doi: 10.1016/s0960-894x(01)00094-4. [DOI] [PubMed] [Google Scholar]
- 38.Zhang YH, Bhunia A, Wan KF, Lee MC, Chan SL, Yu VC, Mok YK. Chelerythrine and sanguinarine dock at distinct sites on Bcl-XL that are not the classic BH3 binding cleft. J Mol Biol. 2006;364:536–49. doi: 10.1016/j.jmb.2006.09.023. [DOI] [PubMed] [Google Scholar]
- 39.Chen M, Xing D, Chen T, Zhang L. Bim involvement in Bax activation during UV irradiation-induced apoptosis. Biochem Biophys Res Commun. 2007;358:559–65. doi: 10.1016/j.bbrc.2007.04.167. [DOI] [PubMed] [Google Scholar]
- 40.Jarvis WD, Turner AJ, Povirk LF, Traylor RS, Grant S. Induction of apoptotic DNA fragmentation and cell death in HL-60 human promyelocytic leukemia cells by pharmacological inhibitors of protein kinase C. Cancer Res. 1994;54:1707–14. [PubMed] [Google Scholar]
- 41.Shelley MD, Hartley L, Groundwater PW, Fish RG. Structure-activity studies on gossypol in tumor cell lines. Anticancer Drugs. 2000;11:209–16. doi: 10.1097/00001813-200003000-00009. [DOI] [PubMed] [Google Scholar]
- 42.Teng CS. Gossypol-induced apoptotic DNA fragmentation correlates with inhibited protein kinase C activity in spermatocytes. Contraception. 1995;52:389–95. doi: 10.1016/0010-7824(95)00227-8. [DOI] [PubMed] [Google Scholar]
- 43.Kitada S, Leone M, Sareth S, Zhai D, Reed JC, Pellecchia M. Discovery, characterization, and structure-activity relationships studies of proapoptotic polyphenols targeting B-cell lymphocyte/leukemia-2 proteins. J Med Chem. 2003;46:4259–64. doi: 10.1021/jm030190z. [DOI] [PubMed] [Google Scholar]
- 44.Mohammad RM, Mohamed AN, Smith MR, Jawadi NS, Al-Katib A. A unique EBV-negative low grade lymphoma line (WSU-FSCCL) exhibiting both t(14;18) and t(8;11) Cancer Genet Cytogenet. 1993;70:62–7. doi: 10.1016/0165-4608(93)90132-6. [DOI] [PubMed] [Google Scholar]
- 45.Al-Katib AM, Mensah-Osman E, Aboukameel A, Mohammad RM. The Wayne State University Waldenstrom’s Macroglobulinemia preclinical model for Waldenstrom’s macro-globulinemia. Semin Oncol. 2003;30:313–7. doi: 10.1053/sonc.2003.50043. [DOI] [PubMed] [Google Scholar]
- 46.Fahy BN, Schlieman MG, Mortenson MM, Virudachalam S, Bold RJ. Targeting BCL-2 overexpression in various human malignancies through NFkappaB inhibition by the proteasome inhibitor bortezomib. Cancer Chemother Pharmacol. 2005;56:46–54. doi: 10.1007/s00280-004-0944-5. [DOI] [PubMed] [Google Scholar]
- 47.Jia L, Coward LC, Kerstner-Wood CD, Cork RL, Gorman GS, Noker PE, Kitada S, Pellecchia M, Reed JC. Comparison of pharmacokinetic and metabolic profiling among gossypol, apogossypol and apogossypol hexaacetate. Cancer Chemther Pharmacol. 2008;61:63–73. doi: 10.1007/s00280-007-0446-3. [DOI] [PubMed] [Google Scholar]


