Abstract
Dependence to drugs of abuse is closely associated with impulsivity, or the propensity to choose a lower, but immediate, reward over a delayed, but more valuable outcome. Here, we review clinical and preclinical studies showing that striatal dopamine signaling and D2 receptor levels – which have been shown to be decreased in addiction - directly impact impulsivity, which is itself predictive of drug self-administration. Based on these studies, we propose that the alterations in D2 receptor binding and dopamine release seen in imaging studies of addiction constitute neurobiological markers of impulsivity. Recent studies in animals also show that higher striatal dopamine signaling at the D2 receptor is associated with a greater willingness to expend effort to reach goals, and we propose that this same relationship applies to humans, particularly with respect to recovery from addiction.
Introduction
Alcohol consumption is the third largest risk factor for poor health (WHO, 2011) and globally there are an estimated 40 million problem drug users (UNODC, 2010). However, it is well known that the likelihood of developing a substance use disorder involves biological and behavioral factors that can exacerbate or mitigate this risk. Among the behaviors most closely associated with addiction are alterations in reward-driven behavior and an impaired ability to sustain goal directed behavior (Groman and Jentsch, 2012; Leyton, 2007). Thus, addiction is characterized by deficits in inhibitory control, which is an impaired capacity to appropriately inhibit thoughts or actions that lead to impulsive behaviors (Groman and Jentsch, 2012).
Impulsivity occurs in a variety of forms, which can be categorized as: 1) choice impulsivity, or acting to obtain a smaller immediate reward over a larger delayed reward: 2) impulsive action, the inability to inhibit a behavior once initiated; 3) reflection impulsivity, or action without sufficiently evaluating information; 4) attention impulsivity, the impaired ability to persist in a relevant behavior and avoid distraction (for review see (Bickel et al., 2012a; Dalley et al., 2011; de Wit, 2009; Leeman and Potenza, 2012)). Overall, studies in addiction show that most types of impulsivity are increased in substance use disorders, measured with impulsiveness scales, neuropsychological batteries, motor-inhibition tasks, and decision-making tasks (see reviews above and (Bechara et al., 2002; Bickel et al., 2007; Chambers and Potenza, 2003; Izquierdo and Jentsch, 2012). Although impulsivity occurs in a variety of manifestations, in this review we focus on choice impulsivity since this has been studied in conjunction with imaging studies of addiction. Examples of tasks that evaluate choice impulsivity include the delay discounting task, in which addicted individuals show preference for smaller, immediate rewards over larger, delayed rewards (Bickel et al., 2012b; de Wit, 2009) or the Iowa Gambling Task, in which substance abuse is associated with choices for high immediate gains despite higher future losses (Bechara et al., 2002).
The high degree of overlap between impulsivity and addiction suggest that these processes rely on overlapping neurobiological mechanisms. Indeed, there is substantial evidence that the corticostriatal system – and dopaminergic transmission in particular -is a common neurobiological substrate for these behavioral and cognitive processes. Imaging studies in human subjects show that addiction is associated with a significant decrease in striatal dopamine transmission, measured as dopamine D2 receptor binding and pre-synaptic dopamine release. Given the data in preclinical studies showing that impulsivity is associated with similar deficits in striatal dopamine signaling, the goal of this review is to address the question of whether the human imaging studies of addiction reflect the neurobiology of impulsive behavior, rather than only the effects of chronic drug exposure on brain dopamine neurotransmission.
This hypothesis is based on the following findings, described in further detail below.
Imaging studies in addiction show a decrease in D2 receptors and dopamine release in the striatum. However, this decrease is seen across addictions, independent of the substance abused, despite the fact that these drugs have their primary effect of different receptors and transporters in the brain. In addition, the magnitude of the decrease is also similar across addictions. Moreover, some non-addiction disorders, such as attention deficit disorder and obesity, which also have a significant impulsive component, show the same decrease in dopamine transmission.
Increasing data suggests that these biomarkers (low D2 receptor binding and dopamine release) may occur prior to drug exposure, in parallel with impulsivity, which then confer a vulnerability to develop addiction. Preclinical studies show that manipulating striatal dopamine signaling, particularly at the D2 receptor, evokes impulsive behavior. Furthermore, both impulsivity and low striatal dopamine transmission are predictive of drug self-administration. Importantly, these animal models are consistent with the imaging studies investigating striatal dopamine signaling, impulsivity, and addiction.
Although many studies show that addiction is associated with a reduction in striatal D2 receptors and dopamine release, two recent studies show that there is a subgroup of addicted individuals who do not express this neurobiological marker. This subgroup is distinguished by the fact that they respond to behavioral treatments for addiction. Thus, when presented with alternatives to drug use, these subjects shift their behavior away from taking drugs toward other reinforced behaviors. The fact that this subgroup does not express the biomarker seen so consistently in addiction may reflect their ability to exert cognitive control, or suppress impulsive responses, when presented with alternatives to drug use.
Animal studies of motivation, or the willingness to expend effort to obtain a goal, demonstrate that this behavior can be modulated by dopamine signaling in the ventral striatum (Salamone et al., 2007). To an extent, motivation can be thought of as the inverse of impulsivity, as the ability to resist behavior initiation, and exert greater effort in order to obtain a more valuable outcome. Preclinical studies show that increased dopamine signaling at the D2 receptor in the nucleus accumbens enhances motivation, whereas impaired D2 signaling lessens it (Salamone et al., 2007). This has been less studied in humans, but recent imaging studies suggest that this same interaction between dopamine signaling at the D2 receptor and motivation may also apply.
Positron Emission Tomography (PET) imaging
PET radiotracer imaging studies use radioactive ligands, usually agonists or antagonists, that bind to receptors in the brain. In addiction, most PET studies have imaged the dopamine D2 receptor family (D2, D3, and D4; but referred to as D2 here) of the striatum. PET imaging can also be used to image the response of the dopamine system to a stimulant challenge (such as amphetamine or methylphenidate). The method is based on animal microdialysis studies, where striatal dopamine levels are increased in response to the administration of a stimulant. Modeling this with PET, D2 receptor availability is obtained before and after the administration of a stimulant, to increase extracellular dopamine. The increase in endogenous dopamine results in fewer D2 receptors available to bind to the radiotracer (shown in figure 1), such that the PET scans provide an indirect measure of the responsiveness of the dopamine system to a pharmacologic challenge.
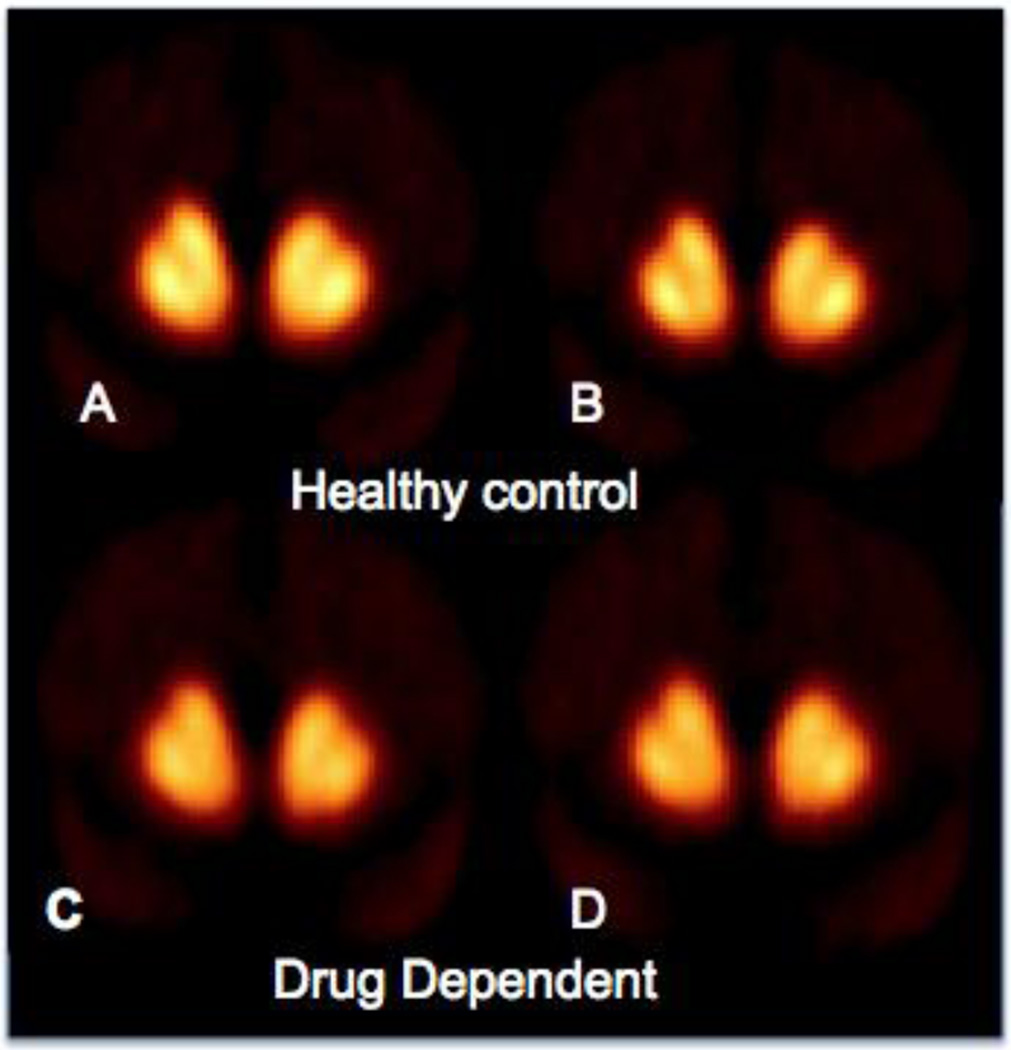
Figure 1.
PET scans in healthy controls and drug (heroin) dependent subjects The comparison of panels A and B (pre- and post-stimulant administration) in the healthy controls shows that radiotracer ([11C]raclopride) binding is reduced in the striatum following methylphenidate administration.
The heroin dependent subjects have lower D2 receptors (BPND) compared to controls in the baseline condition (panel A vs C). This group also has less radiotracer displacement (Δ BPND) following methylphenidate (panels C vs D show less change than panels A vs B). PET imaging studies of dependence to other drugs of abuse shows a similar pattern where both D2 receptor binding (BPND) and dopamine release (Δ BPND) are blunted compared to controls.
Thus, most PET imaging studies in drug and alcohol addiction use two outcome measures: 1) binding potential which is a measure of D2 receptor availability (usually BPND, which is the specific binding of the radiotracer to the receptor normalized by the non-specific binding); and 2) the percent decrease in radiotracer binding resulting from increased endogenous dopamine (ΔBPND), which provides an estimate of pre-synaptic dopamine release (Laruelle, 2000; Volkow et al., 1994).
Imaging dopamine transmission in drug and alcohol addiction
A consistent finding in imaging studies of addiction is a decrease in striatal D2 receptor binding, which may be one of the most replicated findings in human imaging research. These studies show that subjects with substance use disorders, compared to control subjects, have a decrease in the D2 receptors in the striatum on the order of about 20%. The first report was in 1990, where a study showed that cocaine abuse was associated with a decrease in D2 receptor availability in the striatum compared to controls (Volkow et al, 1990), and has been followed by a series of studies confirming this observation (Martinez et al., 2004; Martinez et al., 2011; Martinez et al., 2009; Volkow et al., 1993; Volkow et al., 1997). However, this finding is not isolated to cocaine dependence. In alcohol dependence, Hietala et al (Hietala et al., 1994) showed a decrease in D2 receptor availability in the striatum and this has now been replicated in additional studies (Heinz et al., 2005; Heinz et al., 2004; Martinez et al., 2005; Volkow et al., 1996; Volkow et al., 2002b; Volkow et al., 2007b), although some groups report a lack of a difference (shown in table 1). The finding of decreased D2 receptor binding has also been observed in methamphetamine abuse (Boileau et al., 2012; Lee et al., 2009; Volkow et al., 2001a; Wang et al., 2012), opiate dependence (Martinez et al., 2012; Wang et al., 1997b; Zijlstra et al., 2008), and tobacco dependence (Albrecht et al., 2013a; Brody et al., 2004; Brown et al., 2012; Fehr et al., 2008; Stokes et al., 2012). Notably, studies of cannabis abuse have not shown this same decrease in D2 receptor binding (Albrecht et al., 2013b; Sevy et al., 2008; Stokes et al., 2012; Urban et al., 2012).
Table 1.
Imaging studies measuring dopamine D2 receptor binding in addiction. Most studies use Positron Emission Tomography (PET) and some used Single Photon Emission Tomography (SPET), which is indicated with an asterisk. Although the SPET studies showed no change in D2 receptor availability compared to controls, differences in imaging methodology are unlikely to explain these differences.
| Drug abused | Studies showing decreased striatal D2 receptor binding |
Studies showing no difference in striatal D2 receptor binding |
|---|---|---|
| Cocaine | (Volkow et al., 1990); (Volkow et al., 1993); (Volkow et al., 1997); (Martinez et al., 2004); (Martinez et al., 2009); (Martinez et al., 2011) | (Malison et al., 1999)* |
| Alcohol | (Hietala et al., 1994); (Volkow et al., 1996); (Volkow et al., 2002); (Heinz et al., 2004); (Heinz et al., 2005); (Martinez et al., 2005); (Volkow et al., 2007); (Rominger et al., 2012) (seen only with voxel-based - not region of interest - analysis) | (Repo et al., 1999) *; (Guardia et al., 2000) *; |
| Opiates | (Wang et al., 1997); (Zijlstra et al., 2008); (Martinez et al., 2012) | (Daglish et al., 2008) |
| Methamphetamine | (Volkow et al., 2001); (Lee et al., 2009); (Wang et al., 2012); (Boileau et al., 2012) | |
| Nicotine | (Brody et al., 2004); (Fehr et al., 2008); (Brown et al., 2012) (males only; no difference females); (Stokes et al., 2012) (Albrecht et al., 2013) |
While fewer imaging studies have measured stimulant-induced dopamine release (ΔBP), these show a blunted effect in substance use disorders compared to matched controls. Thus, substance abusers have a lower dopamine response, which most likely reflects decreased pre-synaptic dopamine release in the striatum (shown in figure 1). This finding has been shown in cocaine abuse (Malison et al., 1999; Martinez et al., 2011; Martinez et al., 2007; Volkow et al., 1997), alcohol dependence (Martinez et al., 2005; Volkow et al., 2007b), opiate dependence (Martinez et al., 2012), methamphetamine abuse (Wang et al., 2012), and cigarette smoking (Busto et al., 2009) (shown in table 2). PET studies have also shown that dependence is associated with decreased presynaptic dopamine. Cocaine abuse is associated with both decreased [18F]DOPA uptake and striatal vesicular monoamine transporter 2 (VMAT2) binding, which provide measures of pre-synaptic dopamine (Narendran et al., 2012; Wu et al., 1997). In alcohol dependence, imaging studies show a reduction in VMAT2 compared to controls (Gilman et al., 1998) although not of [18F]DOPA uptake (Heinz et al., 2005; Tiihonen et al., 1998).
Table 2.
Imaging studies measuring the percent decrease in radiotracer binding following a stimulant challenge (ΔBPND), which provides an indirect measure of pre-synaptic dopamine release. One study used Single Photon Emission Tomography (SPET), which is indicated with an asterisk. To date, no studies have shown that ΔBPND is similar to that of matched controls using a stimulant challenge to increase extracellular dopamine.
| Drug abused | Studies showing decreased dopamine release |
|---|---|
| Cocaine | (Volkow et al., 1997); (Malison et al., 1999)*; (Martinez et al., 2007); Martinez, 2011 #7360} |
| Alcohol | (Martinez et al., 2005); (Volkow et al., 2007); |
| Opiates | (Martinez et al., 2012) |
| Methamphetamine | (Wang et al., 2012) |
PET imaging of the targets of the substance abused
Although the majority of studies imaging in addiction report a decrease in D2 receptor availability and dopamine release, imaging studies of the target of the substance abused do not always show a consistent effect. Cocaine acts directly at the dopamine transporter, but imaging studies of the transporter show little effect (Malison et al., 1998; Volkow et al., 1996; Wang et al., 1997a). In fact, the dopamine transporter may be upregulated immediately following abstinence from cocaine, but returns to control levels within a few days (Malison et al., 1998). On the other hand, the decrease in D2 receptor binding is long lasting, at least 3–4 months in humans (Volkow et al., 1993) and possibly longer, up to a year in non-human primates depending on the individual (Nader et al., 2006). Imaging studies of alcohol dependence do not show a consistent change in the GABAA receptor, although this receptor is the target for treatment for alcohol withdrawal, and no consistent changes have been observed in other neurotransmitter systems (such as the serotonin, opioid, or cannabinoid systems) (for review see (Broft and Martinez, 2012)). One reason for this lack of consistency in imaging studies of alcohol dependence may be clinical factors, such as the degree of addiction or the duration of abstinence at the time of scanning. However, as with cocaine abuse, the decrease in D2 receptor availability is long lasting (at least 4 months) (Volkow et al., 2002b), and occurs independent of clinical factors, suggesting that this may be a marker for a behavioral trait associated with dependence, such as impaired inhibitory control.
Alternatively, studies in methamphetamine abusers show a consistent and long lasting decrease in the dopamine transporter, which is the main target of this drug (Chou et al., 2007; Johanson et al., 2006; McCann et al., 1998; McCann et al., 2008; Sekine et al., 2001; Volkow et al., 2001b). Imaging studies of nicotine abuse report upregulation of the nicotinic receptor (Cosgrove et al., 2009; Mukhin et al., 2008; Staley et al., 2006; Wullner et al., 2008) and an imaging study of opiate abusers showed upregulation of the mu receptor (Zubieta et al., 2000). Thus, depending on the substance abused, some studies do report alterations in the primary neurotransmitter system that is targeted by the drug of abuse. Nonetheless, the fact that D2 receptors and dopamine release are consistently seen across addictions, and to a similar extent, suggests that this may be a biomarker for a persistent underlying behavior that is common across addictions.
Imaging dopamine transmission in other disorders
In addition to studies in addiction, low D2 receptor binding and low dopamine release have been reported in other disorders. For example, both obesity and attention deficit disorder are associated with high impulsivity, measured with tasks similar to those used in addiction (Bickel et al., 2012b; Carr and Epstein, 2011; Malloy-Diniz et al., 2007). Obesity has been shown to be associated with low D2 receptor binding, and morbidly obese individuals with the lowest D2 values had the largest body mass index (de Weijer et al., 2011; Volkow et al., 2008; Wang et al., 2001), although this was not seen in one study (Steele et al., 2010). In addition, Volkow et al. (Volkow et al., 2008) showed that low striatal D2 receptor binding correlates with metabolism in prefrontal regions that are implicated in inhibitory control, and this group hypothesized that the association between D2 receptors and impulsivity is mediated partly by dopamine’s modulation of prefrontal regions. This group has also previously reported that D2 receptor availability in the striatum of healthy controls modulates eating behavior, where low D2 receptor binding correlated with a greater tendency to eat when exposed to stress (Volkow et al., 2003). Studies of bulimia, which is also associated with poor inhibitory control (Waxman, 2009), show that this disorder may also be associated with decrease D2 receptor binding and dopamine release (Broft et al., 2012), although increased dopamine release in response to a food cue in obese food bingers was reported in one study (Wang et al., 2011). Together, these findings suggest that impulsive eating and addiction share this neurobiological marker, in addition to the inability to restrain the behavior despite awareness of its negative effects (Volkow et al., 2008).
Attention deficit disorder has also been shown to be associated with a decrease in both D2 receptor binding and stimulant-induced dopamine release (Volkow et al., 2007a), and blunted dopamine release was associated with both greater symptoms of inattention and with greater drug liking (Volkow et al., 2007a; although see Rosa-Neto et al., 2005). A subsequent study of ADHD subjects showed the same finding of reduced D2 receptor binding in the ventral striatum and caudate (Volkow et al., 2009), which correlated inversely with attention. Based on these results, this group hypothesized that decreased dopamine transmission plays a role in the motivation and reward deficits seen in ADHD, which are characterized by poor response inhibition and preference for small immediate rewards over larger delayed rewards (Volkow et al., 2009). Consistent with this, recent fMRI studies report decreased activation of the ventral striatum for both immediate and delayed rewards and in adult and adolescent participants with ADHD compared with controls (Plichta et al., 2009; Scheres et al., 2007).
However, it should be noted that decreases in D2 receptor binding are not seen in pathological gamblers compared to control subjects, which also has a significant impulsive component (Boileau et al., 2013; Clark et al., 2012; Joutsa et al., 2012; Linnet et al., 2011). Nonetheless, two of these studies (Boileau et al., 2013; Clark et al., 2012) did show that low D2 binding correlated with greater impulsivity within the group of gamblers. Imaging studies of impulsive behavior in healthy controls report mixed results. While some studies found an inverse relationship between measures of impulsivity or response inhibition and D2 BPND and delta BPND, other studies showed the opposite (Buckholtz et al., 2010; Ghahremani et al., 2012; Oswald et al., 2007; Reeves et al., 2012). One reason for this discrepancy may be explained by the “inverted U” model, which proposes that optimal dopamine transmission, which is neither too low nor too high, is required for optimal response inhibition (Cools and D'Esposito, 2011; Gjedde et al., 2010; Leyton, 2007).
Taken together, these studies suggest that addiction and psychiatric disorders that are associated with impulsivity and a lack of inhibitory control share partially overlapping neurobiological mechanisms, such as low D2 receptor binding and dopamine release in the striatum.
Measures of impulsivity and dopamine transmission in addiction
Imaging studies in addiction also show that deficits in dopamine transmission correlate with increased impulsivity. Methamphetamine abuse is associated with lower D2 receptor binding compared to controls, and higher levels of impulsivity, measured with the Barratt Impulsiveness Scale (Lee et al., 2009). In addition, greater impulsivity in the methamphetamine abusers correlated with lower D2 receptor binding in the caudate and nucleus accumbens (Lee et al., 2009).
In cocaine abuse, low pre-synaptic dopamine release in the ventral striatum is associated with the choice to self-administer cocaine (Martinez et al., 2007). In this study, cocaine abusers were given the choice between smoked cocaine and money, presented as an alternative reinforcer to cocaine. The amount of money was higher than the street value of the dose of cocaine, and the dose of cocaine was very low, with minimal positive subjective effects. The results showed that low dopamine release in the ventral striatum was predictive of the choice for cocaine over money, despite the weighting towards the money. Thus, the subjects with low dopamine release made the more impulsive choice, and chose a smaller, immediate reward over a larger, delayed reward (subjects received and could spend the money only after study completion).
Dopamine transmission in addiction: association with the prefrontal cortex
Dopamine signaling in the striatum is part of a larger system that mediates impulsive behavior, which includes the prefrontal cortex (Cools, 2008; Dalley et al., 2011; George and Koob, 2010; Groman and Jentsch, 2012). Both preclinical and human imaging studies implicate these brain regions - in addition to striatum - in mediating impulsive behavior and cognitive control. Imaging studies of cocaine and methamphetamine abuse have shown that low D2 receptor binding in the striatum is associated with reduced glucose metabolism in the prefrontal cortex, including the orbito-frontal cortex and cingulate gyrus, which play a key role in impulsivity (Volkow et al., 2001a; Volkow et al., 1993). In healthy controls, dopamine depletion impairs fronto-striatal functional connectivity during set-shifting, suggesting that low dopamine may impair the control that the prefrontal cortex exhibits over the striatum in governing cognitive flexibility (Nagano-Saito et al., 2008). Given the role that these pre-frontal cortical brain regions play in modulating inhibitory control these findings suggest that dysregulation of striatal dopamine transmission affects the prefrontal cortical circuits, which act together to modulate drive and impulsivity (Dalley et al., 2011; Groman and Jentsch, 2012).
Taken together, these studies in addiction suggest that low D2 receptor availability and dopamine release in the striatum are neurobiological markers of increased impulsivity. This hypothesis is supported by various animal studies, where manipulating striatal dopamine transmission and D2 receptor signaling directly impacts impulsive behavior.
Impulsivity and vulnerability to addiction: preclinical studies
Similarly to humans, cognitive deficits have been described in animals chronically exposed to drugs of abuse (Crean et al., 2011; Porter et al., 2011) indicating that these impairments may be due to chronic drug use. In particular, exposure to drugs of abuse has been reported to produce an enhancement in impulsive-like responding in animals (Dallery and Locey, 2005; Richards et al., 1999) and in humans (de Wit, 2009). Although there are evidence that drug exposure could reduce some forms of impulsivity (Caprioli et al., 2013), animal studies aimed at identifying vulnerability factors for the development of addiction consistently reveal that impulsivity itself may influence the development of drug dependence.
Animal studies show that high impulsivity predicts the tendency to escalate drug intake. In rats, high impulsivity – measured by delay discounting – is a vulnerability factor for both alcohol (Poulos et al., 1995) and cocaine (Perry and Carroll, 2008; Perry et al., 2005) self-administration. Dalley et al. (Dalley et al., 2007) showed that excessive impulsive responding in the 5-choice Serial Reaction Time Task (5CSRTT) predicts marked escalation of cocaine self-administration compared with non-impulsive controls.
Using a similar approach, Belin et al. (Belin et al., 2008) further showed that high impulsivity predicts the development of compulsive drug-taking, whereas high reactivity to novelty predicts the propensity to initiate cocaine self-administration.
Impulsivity in animals not only confers predisposition to escalate drug self-administration but also increases vulnerability to relapse since, after a period of abstinence, impulsive animals showed enhanced reinstatement of drug-seeking responses (Everitt et al., 2008b). Similarly, enhanced impulsive responding in the 5CSRTT is predictive of enhanced nicotine self-administration and resistance to extinction (Diergaarde et al., 2008). Together, these data strongly support the view that impulsivity is an endophenotype predictive for addiction potential (Dalley et al., 2011).
Common neurobiological impairments in impulsivity and addiction
The high degree of overlap between impulsivity and addiction suggests that these processes share similar neurobiological mechanisms. In accordance, animal studies provide substantial evidence that the corticostriatal system – and dopaminergic transmission in particular - is a common neurobiological substrate.
Abnormalities in the structure and function of striatal and prefrontal regions in animals are associated with greater impulsivity (Dalley et al., 2011; Groman and Jentsch, 2012). Lesions of the orbitofrontal cortex promote perseverative responding, whereas lesions of the infralimbic cortex enhance premature responding (Chudasama et al., 2003). Lesions of the medial striatum, a main projection area, produce both of these behavioral changes (Rogers et al., 2001). Impulsive behavior, characterized by a persistent deficit in the animals’ ability to choose a large delayed reward over a smaller immediate one, have been described after lesions of the nucleus accumbens core (Cardinal et al., 2001).
Corticostriatal function highly relies on dopaminergic transmission from mesencephalic dopaminergic neurons. Cognitive control deficits are often associated with alterations in dopamine transmission in prefrontal cortex and striatum (Arnsten et al., 1994; Cai and Arnsten, 1997; Cools, 2008; Cools and D'Esposito, 2011; Landau et al., 2009; Vernaleken et al., 2007) and dopamine depletion in those brain areas impairs cognitive control (Clarke et al., 2011; Collins et al., 2000; Crofts et al., 2001; O'Neill and Brown, 2007). More specifically, preclinical studies indicate that alterations in dopamine transmission in the striatum mediate impulsivity, at least in part, since the amphetamine-induced increase in impulsivity is attenuated by local depletion of dopamine in the striatum (Baunez and Robbins, 1999; Cole and Robbins, 1989).
Dopaminergic dysfunction has been largely proposed to be a main factor for the development of addiction. The dopamine pathway strongly modulates the reinforcing and motivational properties of rewards and the motivational aspects of behavior (Berridge, 2007; Salamone and Correa, 2012; Schultz, 2006; Wise, 2008) and is implicated in disorders that involve abnormal reward seeking and taking. Dopamine levels are decreased in animals chronically exposed to drugs of abuse (Castner et al., 2000; Kirkland Henry et al., 2009; Lee et al., 2011; Maisonneuve et al., 1995; Melega et al., 2008; Segal and Kuczenski, 1992; Sorg et al., 1997). Even though chronic exposure to drugs can decrease dopamine, lower dopamine transmission before drug use could be a vulnerability factor for the further development of addiction. Indeed, alcohol-naïve mice (George et al., 1995) or rats (Gongwer et al., 1989; Quintanilla et al., 2007) selectively bred to prefer ethanol display decreased basal dopamine levels in the nucleus accumbens, and alcohol preference in these animals is reversed by direct dopamine receptor activation (George et al., 1995).
D2 receptor-dependent dopamine transmission in animals is involved in a range of cognitive processes that are impaired in addiction, such as motivation (Salamone and Correa, 2012) and behavioral flexibility (Groman and Jentsch, 2012). Similarly, impulsivity in animals correlates with reduced D2 receptor availability in the striatum. Using PET and autoradiography, previous studies have shown that D2 receptor availability is reduced in the nucleus accumbens of impulsive rats (Dalley et al., 2007; Jupp et al., 2013; Caprioli et al., 2013). In monkeys, D2 receptor availability is inversely correlated with impulsive responses (Czoty et al., 2010). Strikingly, animals with low D2 receptor levels in the striatum prior to drug or alcohol exposure subsequently display greater cocaine or alcohol self-administration (Czoty et al., 2004; Dalley et al., 2007; McBride et al., 1993; Morgan et al., 2002; Nader et al., 2006; Stefanini et al., 1992). Besson et al. (Besson et al., 2013) recently provided evidence that the decrease in striatal D2 receptor binding in impulsive animals is related to decreased mRNA expression in the nucleus accumbens shell as well as in the VTA. Moreover, peripheral administration of D2 receptor antagonist increases impulsivity (Wade et al., 2000) as does intra-accumbens shell administration (Besson et al., 2010). Finally, knock-down of D2 receptor in the striatum produces addiction-like, compulsive eating in mice (Johnson and Kenny, 2010), whereas its overexpression attenuates alcohol consumption and cocaine self-administration (Thanos et al., 2008; Thanos et al., 2005; Thanos et al., 2004; Thanos et al., 2001). Importantly, similarly to dopamine transmission, drug exposure worsens the decrease in D2 receptor mRNA levels throughout the striatum in both high and low impulsive animals (Besson et al., 2013).
These data support the idea that dysfunction of the D2 receptor dependent transmission in the striatum represents a common mechanism underlying impulsivity and addiction, and that these neurobiological alterations are - at least in part - predictive of the development of addiction. To an extent, studies in humans are in agreement. Imaging studies of non-addicted individuals show that low striatal D2 receptor availability is predictive of a pleasurable response to intravenous stimulant administration, while high binding was associated with an aversive experience (Volkow et al., 1999; Volkow et al., 2002a). Insofar as pleasurable experience with a drug is a potential risk for addiction (Davidson et al., 1993; Volkow et al., 2002a), these results suggest that low D2 receptor binding may confer vulnerability. Similar results were seen in a study of subjects with a family history of alcohol or cocaine abuse who have higher D2 receptor availability compared to those without this risk factor, suggesting that high D2 receptor binding may serve as a marker for resilience (Volkow et al., 2006a; Volkow et al., 2006b). More recently, Casey et al. (Casey et al., In Revision) showed that non-dependent young adults at high risk for addiction (family history of addiction and experience with drugs of abuse) have lower presynaptic dopamine release compared to controls, suggesting that this may be associated with an increased risk of future addiction. Similar results have been reported in the IMAGEN trial, which showed that adolescents with the potential for problematic substance use display greater risk taking and lower striatal activation (in response to the monetary incentive delay reward task) relative to comparison subjects (Schneider et al., 2012). Another IMAGEN study using the same fMRI task showed that activation of the ventral striatum in response to reward anticipation was lower in adolescent smokers compared to controls, even in subjects who had smoked less than 10 occasions, suggesting that blunted activation occurs beyond an effect that could be attributed just to nicotine exposure (Peters et al., 2011). Since the monetary incentive delay task has been proposed to depend - at least in part - on striatal dopamine function (Knutson and Gibbs, 2007; Schott et al., 2008), these findings suggest that low striatal dopamine signaling is associated with an increased risk of addiction.
Overall, these studies in animals and humans indicate that low D2 receptor levels and low dopamine signaling correspond with impulsive behavior, which leads to a preference for small immediate rewards and increased drug self-administration. However, as described above, animal studies have also shown that drug exposure itself can both further increase impulsive behavior and decrease dopamine release and D2 receptor binding, suggesting that a feedback loop may exist where low striatal dopamine provokes impulsivity and increased drug-taking, which in turn further reduces dopamine signaling and response inhibition. This model is depicted in figure 2.
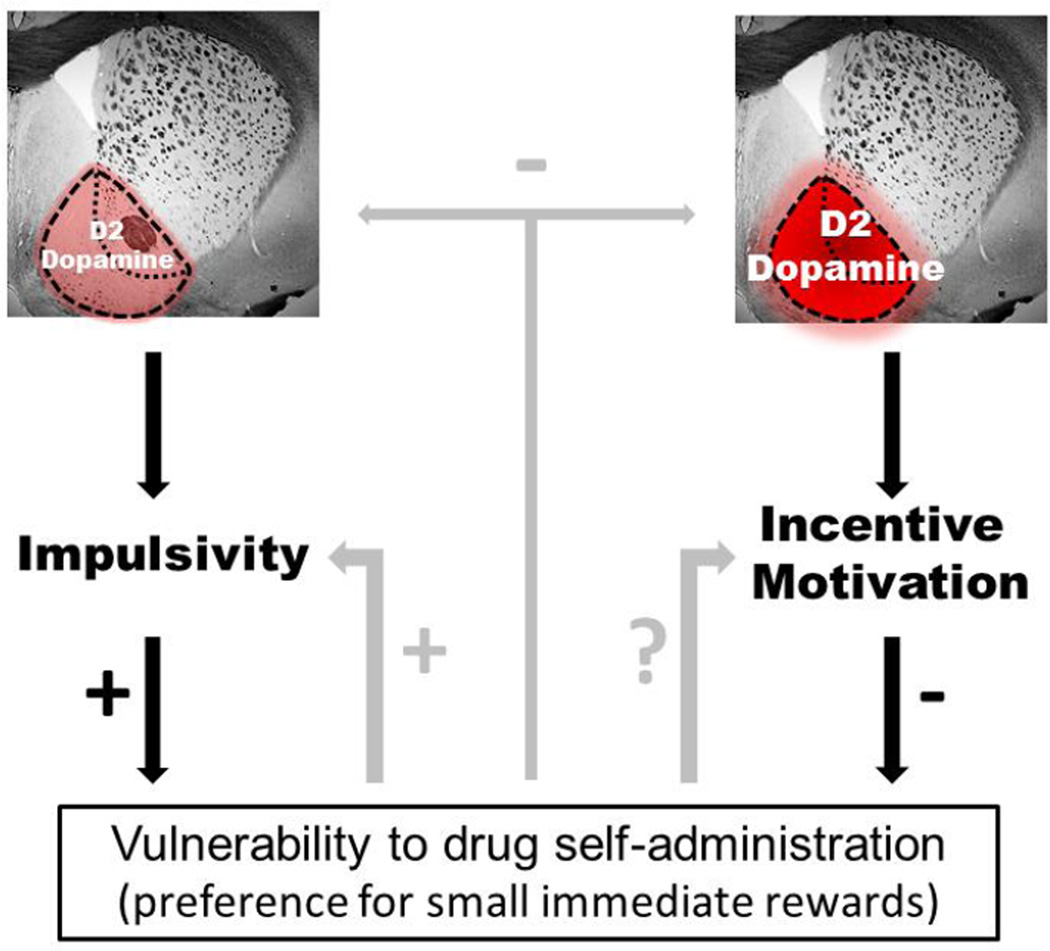
Figure 2.
Theoretical model based on human and animal data. Low D2 receptor levels and dopamine transmission in the ventral striatum (left) lead to impulsive behavior, including the choice for smaller, immediate rewards over larger, but delayed or more effortful, rewards, which may represent an underlying behavioral pattern seen in addiction. In contrast, higher D2 receptor levels and increased dopamine transmission in the ventral striatum (right) correlate with increased incentive motivation, which could shift the choice towards more effortful but bigger outcomes – such as successful treatment – over smaller, immediate reward – such as drug consumption. In accordance with this hypothesis, imaging studies in humans show that subjects that respond to treatment have higher transmission at the D2 receptor compared to those who continue to take drugs.
Studies in animals show that drug exposure itself can also impact on impulsivity as well as D2 receptor levels and dopamine transmission, suggesting that the decrease in dopamine transmission and D2 receptor levels in addiction can be both a predictor and a consequence of drug use. Thus, continued drug exposure could further diminish D2 receptor signaling and increase. Whether drug self-administration impairs incentive motivation is not currently known.
Dopamine transmission and incentive motivation
As opposed to impulsivity, increasing striatal dopamine release and D2 receptor levels in the nucleus accumbens in particular, facilitates incentive motivation, or the “willingness to expend effort to reach a goal” (Salamone and Correa, 2012; Salamone et al., 2007). The mesoaccumbens dopamine pathway mediates a variety of rewarding properties of reinforcers including the modulation of motivation (Schultz, 2006; Wise, 2008). There is substantial evidence that mesoaccumbens dopamine directly modulates effort expenditure based upon a cost/benefit computation (Salamone and Correa, 2012; Salamone et al., 2007). Consistent with this view, dopamine release in the nucleus accumbens increases during instrumental performance (Ostlund et al., 2011; Segovia et al., 2011). Moreover, increases in dopamine release facilitate operant responding, whereas dopamine depletion impairs operant responding in reinforcement schedules with high work requirements (Palmiter, 2008; Salamone et al., 2007). Moreover, dopamine antagonism in the nucleus accumbens shifts choice away from more effortful toward less effortful reward-seeking behavior (Salamone et al., 2007).
D2 dependent dopamine signaling in particular has been shown to play a major role in goal-directed behaviors. Genetic deletion (Tran et al., 2002) or pharmacologic blockade (Salamone et al., 2007) of D2 receptors impair operant responding, in particular the willingness to work for a reward. In addition, we have recently shown that D2 receptor overexpression in the ventral – but not dorsal - striatum selectively enhances willingness to expend effort to obtain a preferred outcome over a smaller, but less effortful reward (Trifilieff et al., 2013). In other words, higher dopamine transmission and D2-dependent dopamine signaling in the nucleus accumbens are associated with greater willingness to work for larger but more effortful rewards, while lower dopamine and D2 receptor activity shifts animal’s choice towards less effortful and smaller immediate rewards (Figure 2).
These findings are consistent with studies showing that the potentiation and inhibition of dopamine signaling alters cost/benefit decision making in human volunteers. In a PET study of control subjects, Treadway et al (Treadway et al., 2012) showed a positive association between striatal dopamine release (left caudate) and the willingness to expend effort to obtain a larger reward over a smaller reward. Moreover, experimentally-induced decrease in dopamine transmission reduces effort expenditure for drug-rewards (Barrett et al., 2008; Venugopalan et al., 2011).
In attention deficit disorder, deficits in motivation are associated with lower D2 dopamine receptors in the ventral striatum measured with PET (Volkow et al., 2011). In this study, trait motivation was assessed using the Achievement scale, which measures characteristics such as drive and persistence, and the level of D2 receptors in the nucleus accumbens of ADHD subjects positively correlated with trait motivation (Volkow et al., 2011). Similarly, Tomer et al. (Tomer et al., 2008) showed that higher D2 receptor availability in the left striatum is associated with greater positive incentive motivation in healthy controls.
Incentive Motivation and treatment response in addiction
As described above, when presented with the choice to obtain money or self-administer cocaine, cocaine-abusing subjects with blunted dopamine release chose the immediate, but smaller reward over the larger but delayed reward (Martinez et al., 2007). Similar results have been shown with respect to motivation, where treatment-seeking cocaine dependent subjects underwent PET scans prior to behavioral treatment (Martinez et al., 2011). The treatment used positive reinforcement (monetary vouchers) as incentive for abstinence from cocaine (contingency management) (Higgins et al., 2003). The results showed that the cocaine-abusing subjects with higher D2 receptor levels and higher dopamine release in the ventral striatum responded to treatment, while those with lower levels did not (Martinez et al., 2011). Similar results were seen in a study of methamphetamine abusers, where subjects were scanned with [11C]raclopride to obtain BPND and delta BPND, prior to enrollment in treatment. The results showed that the methamphetamine-abusing subjects who successfully maintained abstinence had higher values for both D2 receptor binding and dopamine release, compared to methamphetamine abusers who did not respond to treatment (Wang et al., 2012).
Thus in both studies, the subjects with the higher D2 binding in the striatum and higher dopamine release showed less impulsive behavior and chose the larger, delayed reward (money and/or treatment) over a smaller, immediate reward (drug). Importantly, in both studies the subjects who responded to treatment did not differ from the control group in measures of D2 receptors levels or dopamine release. In other words, substance dependent subjects with intact dopamine transmission were able to expend effort to obtain a larger goal, which was their pursuit of recovery from addiction. Moreover, in the study of cocaine abusers, the subjects who responded to treatment had a higher socioeconomic status compared to those who relapsed (Barratt Simplified Measure of Social Status: treatment responders 34.1 ± 9.2; non-responders 27.1 ± 4.5, p = 0.01), and actually did not differ from the control group (34.8 ± 7.9, p = 0.8). This suggests that the cocaine dependent subjects who respond to treatment have better psychosocial function compared to those who relapse, and may have a greater ability, or motivation, to obtain certain non-drug rewards (such as education and employment).
Increasing signaling at the D2 receptor as a potential target for treatment?
We have reviewed the human and animal data showing that low striatal dopamine transmission, measured as low D2 receptor binding and low pre-synaptic dopamine release, is associated with increased choice impulsivity, which correlates with a preference for small immediate rewards over larger rewards requiring greater effort. These factors may precede addiction, and could serve as a risk factor for substance use disorders. However, animal studies also show that drug exposure itself can increase impulsivity and decrease D2 receptor binding and dopamine release. Thus, we propose a model where low D2 receptor signaling coincides with impulsivity, both of which can be exacerbated by drug exposure, with a progressive increase in further drug use. Alternatively, incentive motivation, or the willingness to exert greater effort to obtain a more valuable outcome correlates with increased signaling at the D2 receptor (Martinez et al., 2011; Trifilieff et al., 2013) (Figure 2).
From these studies we suggest that low D2 receptor signaling in the ventral striatum serves as a neurobiological correlate of impulsive and motivated behavior, and that increased impulsivity is generally accompanied by impaired incentive motivation. Thus, we propose that increasing D2 receptor signaling in the ventral striatum may serve as a treatment strategy for addiction, perhaps by reducing choice impulsivity and enhancing motivation. While non-selective dopamine agonists have, at best, limited efficacy for cocaine addiction (Amato et al., 2011; Perez-Mana et al., 2011), a recent study showed that levodopa/carbidopa was most effective when combined with a behavioral treatment that uses positive reinforcement to shift behavior (Schmitz et al., 2008), suggesting that the pharmacologic increase in dopamine signaling is most effective when combined with treatment that addresses impulsivity and motivation. Alternatively, future treatments that selectively activate the postsynaptic D2 receptor of the ventral striatum might be required. Given the generally opposing effects of pharmacological compounds on presynaptic and postsynaptic D2 receptors, this would require a functionally-selective compound that simultaneously antagonizes presynaptic D2 receptors while acting as an agonist post-synaptically. The development of such functionally-selective compounds has been proven to be feasible (Mottola et al., 2002) and is therefore a promising strategy for the treatment of addiction.
A question raised by this model is the issue of whether increasing motivation in addicted individuals would increase their motivation to obtain more drug vs motivation for alternative non-drug rewards. To our knowledge, this question remains unknown, and it would be important for future studies in addiction to address this question. This hypothesis could be tested in animals previously exposed to drug self-administration using a competing behavioral approach in which the animal is given the choice between low dose drug consumption and alternative non-drug reward – such as intense sweetness (Lenoir et al. 2007; 2013), which has been hypothesized to be highly relevant to model addiction in animals (Ahmed, 2005; Ahmed et al., 2013). We hypothesize that increasing D2 receptor signaling in the nucleus accumbens of animals with a history of drug self-administration could shift their behavior away from dug use and towards non-drug reward.
Limitations
There are various limitations to this hypothesis. Some of these will require further studies in both human and animal subjects. The limitations include more precise determination of the types of impulsivity that are affected in addiction, the effects of enhancing incentive motivation on addictive behaviors, the bidirectional effect of manipulating striatal dopamine on impulsivity, and a better understanding of the roles of the different striatal subdivisions in addiction. These are each summarized below.
We have used “impulsivity” to largely mean “acting to obtain a smaller, immediate reward over expending effort to obtain a larger, delayed reward”. However, while there are different forms of impulsive behaviors (Bickel et al., 2012a; Dalley et al., 2011; de Wit, 2009; Leeman and Potenza, 2012), we included various types in the term impulsivity, particularly in discussing the human studies. The main reason for this is that subtypes of impulsive behaviors altered in the context of addiction are not as clearly defined in human imaging studies as they are in other studies. Additionally, we did not specifically address the role of compulsive behavior in drug-taking, since the human studies were not fully designed to separate impulsive and compulsive drug taking. As stated by Izquierdo and Jentsch (Izquierdo and Jentsch, 2012), controlled decision-making requires inhibitory control over the automatic response system, which may reflect either impulsive or compulsive behavior. Future studies in substance abusing humans should investigate these components of decision-making, as it has been done in animal models (Everitt et al., 2008a; Izquierdo and Jentsch, 2012). In addition, previous authors have proposed that impulsivity results from impaired higher cognitive processes that rely on the integrity of brain regions, such as the prefrontal cortex, in addition to the striatum (Dalley et al., 2011; Groman and Jentsch, 2012). In this review, we have focused on impulsivity and dopamine signaling in the striatum, since most studies of addiction have imaged this particular neurotransmitter system. While other receptor systems also mediate aspects of impulsive behavior and likely play a role in addiction, such as the D1 receptor or serotonin system, which plays a crucial role in impulsive action, these have been discussed elsewhere and should be considered in future studies (Dalley and Roiser, 2012; Kirby et al., 2011).
In this review we have only briefly addressed the likelihood that dopamine transmission in the striatum has an inverted U effect, where increasing dopamine benefits individuals with low dopamine transmission, but can aggravate impulsive behaviors in individuals with high dopamine function (Leyton, 2007). A two factor model has been proposed by Leyton (Leyton, 2007) which asserts that low dopamine correlates with an inability to sustain goal directed behavior, a preference for small immediate rewards, and impaired decision making. This model also proposes that abnormally high dopamine is associated with novelty seeking, premature responding and behavioral disinhibition. The data presented here are in agreement with this model. However, we have only focused on the behavioral correlates of low dopamine, and refer the reader to the review of Leyton (Leyton, 2007) for full discussion of the two factor model.
Throughout this review we have mainly focused on dopamine signaling in the ventral striatum due to the imaging data in addiction implicating this brain region. In addition, preclinical studies largely show that impulsive behavior correlates closely with dopamine signaling at the D2 receptor in this striatal subdivision. However, these preclinical studies also show that drug-induced neuroadaptations initially involve the ventral striatum, but that long-term drug exposure progressively involves the more dorsal regions of the striatum (Belin et al., 2009; Everitt et al., 2008b; Porrino et al., 2004). As extensively described by previous authors, these alterations might reflect a progression from ventral to dorsal domains of the striatum that accompanies the switch from controlled to compulsive drug seeking and drug-taking behaviors (Belin et al., 2009; Everitt et al., 2008b; Izquierdo and Jentsch, 2012). Imaging studies support this theory, and show increasing involvement of ventral-to-dorsal striatum in addiction and that reversal learning, which measures flexibility of a response, is closely associated with D2 receptor signaling in the dorsal striatum (Izquierdo and Jentsch, 2012). Furthermore, recent studies in stimulant dependent individuals show that these subjects make more perseverative errors compared to both controls and subjects with obsessive compulsive disorder, and that dopamine agonist administration normalized perseverative responding in these subjects (Ersche et al., 2011; Ersche et al., 2008). This topic has been reviewed in detail previously and the reader is referred to these publications (Belin et al., 2009; Everitt et al., 2008a; Groman and Jentsch, 2012; Izquierdo and Jentsch, 2012).
Conclusion
We have suggested that increased impulsivity is characterized by a decrease in D2 receptor signaling in the ventral striatum and generally accompanied by impaired incentive motivation, or a reduced willingness to expend effort for larger but more effortful reward, which, in the context of human addiction, would be a non-drug goal. The data from animal models of impulsive behavior and incentive motivation are remarkably consistent with the human data with respect to striatal dopamine signaling. Preclinical studies have shown that overexpressing postsynaptic D2 receptors in the ventral striatum increases incentive motivation, in particular the willingness to work for more effortful reward over smaller but immediate outcome (Trifilieff et al., 2013). Notably, a similar manipulation results in decreased drug intake (Thanos et al., 2008; Thanos et al., 2005; Thanos et al., 2004; Thanos et al., 2001) and, as mentioned above, cocaine-abusing subjects with higher D2 receptor levels in the ventral striatum showed better response to treatment (Martinez et al., 2011; Wang et al., 2012). These findings suggest that increasing D2 receptor signaling may both reduce drug-self-administration and increase an individual’s willingness to work for a non-drug reinforcer and therefore facilitate treatment. However, to date, this has not been shown in animal models of substance abuse and will require further investigation.
Highlights.
Dependence to drugs of abuse is associated with impulsivity.
We review studies showing that dopamine and D2 receptor levels impact impulsivity.
Alterations in dopamine and D2 signaling in addiction are markers of impulsivity.
Striatal dopamine signaling at the D2 receptor is associated with treatment response.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Literature Cited
- Ahmed SH. Imbalance between drug and non-drug reward availability: a major risk factor for addiction. Eur J Pharmacol. 2005;526:9–20. doi: 10.1016/j.ejphar.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Ahmed SH, Lenoir M, Guillem K. Neurobiology of addiction versus drug use driven by lack of choice. Curr Opin Neurobiol. 2013 doi: 10.1016/j.conb.2013.01.028. [DOI] [PubMed] [Google Scholar]
- Albrecht DS, Kareken DA, Yoder KK. Effects of smoking on D/D striatal receptor availability in alcoholics and social drinkers. Brain imaging and behavior. 2013a doi: 10.1007/s11682-013-9233-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albrecht DS, Skosnik PD, Vollmer JM, Brumbaugh MS, Perry KM, Mock BH, Zheng QH, Federici LA, Patton EA, Herring CM, Yoder KK. Striatal D(2)/D(3) receptor availability is inversely correlated with cannabis consumption in chronic marijuana users. Drug Alcohol Depend. 2013b;128:52–57. doi: 10.1016/j.drugalcdep.2012.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amato L, Minozzi S, Pani PP, Solimini R, Vecchi S, Zuccaro P, Davoli M. Dopamine agonists for the treatment of cocaine dependence. Cochrane database of systematic reviews. 2011:CD003352. doi: 10.1002/14651858.CD003352.pub3. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Cai JX, Murphy BL, Goldman-Rakic PS. Dopamine D1 receptor mechanisms in the cognitive performance of young adult and aged monkeys. Psychopharmacology (Berl) 1994;116:143–151. doi: 10.1007/BF02245056. [DOI] [PubMed] [Google Scholar]
- Baunez C, Robbins TW. Effects of dopamine depletion of the dorsal striatum and further interaction with subthalamic nucleus lesions in an attentional task in the rat. Neuroscience. 1999;92:1343–1356. doi: 10.1016/s0306-4522(99)00065-2. [DOI] [PubMed] [Google Scholar]
- Bechara A, Dolan S, Hindes A. Decision-making and addiction (part II): myopia for the future or hypersensitivity to reward? Neuropsychologia. 2002;40:1690–1705. doi: 10.1016/s0028-3932(02)00016-7. [DOI] [PubMed] [Google Scholar]
- Belin D, Jonkman S, Dickinson A, Robbins TW, Everitt BJ. Parallel and interactive learning processes within the basal ganglia: relevance for the understanding of addiction. Behav Brain Res. 2009;199:89–102. doi: 10.1016/j.bbr.2008.09.027. [DOI] [PubMed] [Google Scholar]
- Belin D, Mar AC, Dalley JW, Robbins TW, Everitt BJ. High impulsivity predicts the switch to compulsive cocaine-taking. Science. 2008;320:1352–1355. doi: 10.1126/science.1158136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge KC. The debate over dopamine's role in reward: the case for incentive salience. Psychopharmacology (Berl) 2007;191:391–431. doi: 10.1007/s00213-006-0578-x. [DOI] [PubMed] [Google Scholar]
- Besson M, Belin D, McNamara R, Theobald DE, Castel A, Beckett VL, Crittenden BM, Newman AH, Everitt BJ, Robbins TW, Dalley JW. Dissociable control of impulsivity in rats by dopamine d2/3 receptors in the core and shell subregions of the nucleus accumbens. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2010;35:560–569. doi: 10.1038/npp.2009.162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Besson M, Pelloux Y, Dilleen R, Theobald DE, Lyon A, Belin-Rauscent A, Robbins TW, Dalley JW, Everitt BJ, Belin D. Cocaine modulation of fronto-striatal expression of zif268, D2 and 5-HT2c receptors in high and low impulsive rats. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Gatchalian KM, McClure SM. Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology (Berl) 2012a;221:361–387. doi: 10.1007/s00213-012-2689-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Jarmolowicz DP, Mueller ET, Koffarnus MN, Gatchalian KM. Excessive discounting of delayed reinforcers as a trans-disease process contributing to addiction and other disease-related vulnerabilities: emerging evidence. Pharmacol Ther. 2012b;134:287–297. doi: 10.1016/j.pharmthera.2012.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bickel WK, Miller ML, Yi R, Kowal BP, Lindquist DM, Pitcock JA. Behavioral and neuroeconomics of drug addiction: competing neural systems and temporal discounting processes. Drug Alcohol Depend 90 Suppl. 2007;1:S85–S91. doi: 10.1016/j.drugalcdep.2006.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boileau I, Payer D, Chugani B, Lobo D, Behzadi A, Rusjan PM, Houle S, Wilson AA, Warsh J, Kish SJ, Zack M. The D2/3 dopamine receptor in pathological gambling: a positron emission tomography study with [(11) C]-(+)-propyl-hexahydro-naphtho-oxazin and [(11) C]raclopride. Addiction. 2013;108:953–963. doi: 10.1111/add.12066. [DOI] [PubMed] [Google Scholar]
- Boileau I, Payer D, Houle S, Behzadi A, Rusjan PM, Tong J, Wilkins D, Selby P, George TP, Zack M, Furukawa Y, McCluskey T, Wilson AA, Kish SJ. Higher binding of the dopamine D3 receptor-preferring ligand [11C]-(+)-propyl-hexahydro-naphtho-oxazin in methamphetamine polydrug users: a positron emission tomography study. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:1353–1359. doi: 10.1523/JNEUROSCI.4371-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brody AL, Olmstead RE, London ED, Farahi J, Meyer JH, Grossman P, Lee GS, Huang J, Hahn EL, Mandelkern MA. Smoking-induced ventral striatum dopamine release. Am J Psychiatry. 2004;161:1211–1218. doi: 10.1176/appi.ajp.161.7.1211. [DOI] [PubMed] [Google Scholar]
- Broft A, Martinez D. Neurochemical Imaging of Addictive Disorders. In: Gründer G, editor. Molecular Imaging in the Clinical Neurosciences. Aachen Germany: Spinger; 2012. pp. 249–271. [Google Scholar]
- Broft A, Shingleton R, Kaufman J, Liu F, Kumar D, Slifstein M, Abi-Dargham A, Schebendach J, Van Heertum R, Attia E, Martinez D, Walsh BT. Striatal dopamine in bulimia nervosa: a PET imaging study. The International journal of eating disorders. 2012;45:648–656. doi: 10.1002/eat.20984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AK, Mandelkern MA, Farahi J, Robertson C, Ghahremani DG, Sumerel B, Moallem N, London ED. Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. The international journal of neuropsychopharmacology / official scientific journal of the Collegium Internationale Neuropsychopharmacologicum. 2012;15:989–994. doi: 10.1017/S1461145711001957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckholtz JW, Treadway MT, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Shelby ES, Smith CE, Kessler RM, Zald DH. Dopaminergic network differences in human impulsivity. Science. 2010;329:532. doi: 10.1126/science.1185778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busto UE, Redden L, Mayberg H, Kapur S, Houle S, Zawertailo LA. Dopaminergic activity in depressed smokers: a positron emission tomography study. Synapse. 2009;63:681–689. doi: 10.1002/syn.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai JX, Arnsten AF. Dose-dependent effects of the dopamine D1 receptor agonists A77636 or SKF81297 on spatial working memory in aged monkeys. J Pharmacol Exp Ther. 1997;283:183–189. [PubMed] [Google Scholar]
- Caprioli D, Hong YT, Sawiak SJ, Ferrari V, Williamson DJ, Jupp B, Adrian Carpenter T, Aigbirhio FI, Everitt BJ, Robbins TW, Fryer TD, Dalley JW. Baseline-Dependent Effects of Cocaine Pre-Exposure on Impulsivity and D Receptor Availability in the Rat Striatum: Possible Relevance to the Attention-Deficit Hyperactivity Syndrome. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2013 doi: 10.1038/npp.2013.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardinal RN, Pennicott DR, Sugathapala CL, Robbins TW, Everitt BJ. Impulsive choice induced in rats by lesions of the nucleus accumbens core. Science. 2001;292:2499–2501. doi: 10.1126/science.1060818. [DOI] [PubMed] [Google Scholar]
- Carr KA, Epstein LH. Relationship between food habituation and reinforcing efficacy of food. Learning and motivation. 2011;42:165–172. doi: 10.1016/j.lmot.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey K, C B, MV C, GB B, A D, M L. In Revision. Reduced dopamine response to amphetamine in subjects at ultra high risk for addiction. doi: 10.1016/j.biopsych.2013.08.033. [DOI] [PubMed] [Google Scholar]
- Castner SA, al-Tikriti MS, Baldwin RM, Seibyl JP, Innis RB, Goldman-Rakic PS. Behavioral changes and [123I]IBZM equilibrium SPECT measurement of amphetamine-induced dopamine release in rhesus monkeys exposed to subchronic amphetamine. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;22:4–13. doi: 10.1016/S0893-133X(99)00080-9. [DOI] [PubMed] [Google Scholar]
- Chambers RA, Potenza MN. Neurodevelopment, impulsivity, and adolescent gambling. Journal of gambling studies / co-sponsored by the National Council on Problem Gambling and Institute for the Study of Gambling and Commercial Gaming. 2003;19:53–84. doi: 10.1023/a:1021275130071. [DOI] [PubMed] [Google Scholar]
- Chou YH, Huang WS, Su TP, Lu RB, Wan FJ, Fu YK. Dopamine transporters and cognitive function in methamphetamine abuser after a short abstinence: A SPECT study. Eur Neuropsychopharmacol. 2007;17:46–52. doi: 10.1016/j.euroneuro.2006.05.002. [DOI] [PubMed] [Google Scholar]
- Chudasama Y, Passetti F, Rhodes SE, Lopian D, Desai A, Robbins TW. Dissociable aspects of performance on the 5-choice serial reaction time task following lesions of the dorsal anterior cingulate, infralimbic and orbitofrontal cortex in the rat: differential effects on selectivity, impulsivity and compulsivity. Behav Brain Res. 2003;146:105–119. doi: 10.1016/j.bbr.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Clark L, Stokes PR, Wu K, Michalczuk R, Benecke A, Watson BJ, Egerton A, Piccini P, Nutt DJ, Bowden-Jones H, Lingford-Hughes AR. Striatal dopamine D(2)/D(3) receptor binding in pathological gambling is correlated with mood-related impulsivity. Neuroimage. 2012;63:40–46. doi: 10.1016/j.neuroimage.2012.06.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke HF, Hill GJ, Robbins TW, Roberts AC. Dopamine, but not serotonin, regulates reversal learning in the marmoset caudate nucleus. J Neurosci. 2011;31:4290–4297. doi: 10.1523/JNEUROSCI.5066-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cole BJ, Robbins TW. Effects of 6-hydroxydopamine lesions of the nucleus accumbens septi on performance of a 5-choice serial reaction time task in rats: implications for theories of selective attention and arousal. Behav Brain Res. 1989;33:165–179. doi: 10.1016/s0166-4328(89)80048-8. [DOI] [PubMed] [Google Scholar]
- Collins P, Wilkinson LS, Everitt BJ, Robbins TW, Roberts AC. The effect of dopamine depletion from the caudate nucleus of the common marmoset (Callithrix jacchus) on tests of prefrontal cognitive function. Behav Neurosci. 2000;114:3–17. doi: 10.1037//0735-7044.114.1.3. [DOI] [PubMed] [Google Scholar]
- Cools R. Role of dopamine in the motivational and cognitive control of behavior. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2008;14:381–395. doi: 10.1177/1073858408317009. [DOI] [PubMed] [Google Scholar]
- Cools R, D'Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cosgrove KP, Batis J, Bois F, Maciejewski PK, Esterlis I, Kloczynski T, Stiklus S, Krishnan-Sarin S, O'Malley S, Perry E, Tamagnan G, Seibyl JP, Staley JK. beta2-Nicotinic acetylcholine receptor availability during acute and prolonged abstinence from tobacco smoking. Arch Gen Psychiatry. 2009;66:666–676. doi: 10.1001/archgenpsychiatry.2009.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crean RD, Crane NA, Mason BJ. An evidence based review of acute and long-term effects of cannabis use on executive cognitive functions. J Addict Med. 2011;5:1–8. doi: 10.1097/ADM.0b013e31820c23fa. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crofts HS, Dalley JW, Collins P, Van Denderen JC, Everitt BJ, Robbins TW, Roberts AC. Differential effects of 6-OHDA lesions of the frontal cortex and caudate nucleus on the ability to acquire an attentional set. Cereb Cortex. 2001;11:1015–1026. doi: 10.1093/cercor/11.11.1015. [DOI] [PubMed] [Google Scholar]
- Czoty PW, Gage HD, Nader MA. Differences in D2 dopamine receptor availability and reaction to novelty in socially housed male monkeys during abstinence from cocaine. Psychopharmacology (Berl) 2010;208:585–592. doi: 10.1007/s00213-009-1756-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czoty PW, Morgan D, Shannon EE, Gage HD, Nader MA. Characterization of dopamine D1 and D2 receptor function in socially housed cynomolgus monkeys self-administering cocaine. Psychopharmacology (Berl) 2004;174:381–388. doi: 10.1007/s00213-003-1752-z. [DOI] [PubMed] [Google Scholar]
- Dallery J, Locey ML. Effects of acute and chronic nicotine on impulsive choice in rats. Behav Pharmacol. 2005;16:15–23. doi: 10.1097/00008877-200502000-00002. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Everitt BJ, Robbins TW. Impulsivity, compulsivity, and top-down cognitive control. Neuron. 2011;69:680–694. doi: 10.1016/j.neuron.2011.01.020. [DOI] [PubMed] [Google Scholar]
- Dalley JW, Fryer TD, Brichard L, Robinson ES, Theobald DE, Laane K, Pena Y, Murphy ER, Shah Y, Probst K, Abakumova I, Aigbirhio FI, Richards HK, Hong Y, Baron JC, Everitt BJ, Robbins TW. Nucleus accumbens D2/3 receptors predict trait impulsivity and cocaine reinforcement. Science. 2007;315:1267–1270. doi: 10.1126/science.1137073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalley JW, Roiser JP. Dopamine, serotonin and impulsivity. Neuroscience. 2012;215:42–58. doi: 10.1016/j.neuroscience.2012.03.065. [DOI] [PubMed] [Google Scholar]
- Davidson ES, Finch JF, Schenk S. Variability in subjective responses to cocaine: initial experiences of college students. Addict Behav. 1993;18:445–453. doi: 10.1016/0306-4603(93)90062-e. [DOI] [PubMed] [Google Scholar]
- de Weijer BA, van de Giessen E, van Amelsvoort TA, Boot E, Braak B, Janssen IM, van de Laar A, Fliers E, Serlie MJ, Booij J. Lower striatal dopamine D2/3 receptor availability in obese compared with non-obese subjects. EJNMMI research 1, 37. 2011 doi: 10.1186/2191-219X-1-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Wit H. Impulsivity as a determinant and consequence of drug use: a review of underlying processes. Addict Biol. 2009;14:22–31. doi: 10.1111/j.1369-1600.2008.00129.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diergaarde L, Pattij T, Poortvliet I, Hogenboom F, de Vries W, Schoffelmeer AN, De Vries TJ. Impulsive choice and impulsive action predict vulnerability to distinct stages of nicotine seeking in rats. Biol Psychiatry. 2008;63:301–308. doi: 10.1016/j.biopsych.2007.07.011. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Abbott S, Craig KJ, Muller U, Suckling J, Ooi C, Shabbir SS, Clark L, Sahakian BJ, Fineberg NA, Merlo-Pich EV, Robbins TW, Bullmore ET. Response perseveration in stimulant dependence is associated with striatal dysfunction and can be ameliorated by a D(2/3) receptor agonist. Biol Psychiatry. 2011;70:754–762. doi: 10.1016/j.biopsych.2011.06.033. [DOI] [PubMed] [Google Scholar]
- Ersche KD, Roiser JP, Robbins TW, Sahakian BJ. Chronic cocaine but not chronic amphetamine use is associated with perseverative responding in humans. Psychopharmacology (Berl) 2008;197:421–431. doi: 10.1007/s00213-007-1051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2008a;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everitt BJ, Belin D, Economidou D, Pelloux Y, Dalley JW, Robbins TW. Review. Neural mechanisms underlying the vulnerability to develop compulsive drug-seeking habits and addiction. Philos Trans R Soc Lond B Biol Sci. 2008b;363:3125–3135. doi: 10.1098/rstb.2008.0089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehr C, Yakushev I, Hohmann N, Buchholz HG, Landvogt C, Deckers H, Eberhardt A, Klager M, Smolka MN, Scheurich A, Dielentheis T, Schmidt LG, Rosch F, Bartenstein P, Grunder G, Schreckenberger M. Association of low striatal dopamine D2 receptor availability with nicotine dependence similar to that seen with other drugs of abuse. Am J Psychiatry. 2008;165:507–514. doi: 10.1176/appi.ajp.2007.07020352. [DOI] [PubMed] [Google Scholar]
- George O, Koob GF. Individual differences in prefrontal cortex function and the transition from drug use to drug dependence. Neurosci Biobehav Rev. 2010;35:232–247. doi: 10.1016/j.neubiorev.2010.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George SR, Fan T, Ng GY, Jung SY, O'Dowd BF, Naranjo CA. Low endogenous dopamine function in brain predisposes to high alcohol preference and consumption: reversal by increasing synaptic dopamine. J Pharmacol Exp Ther. 1995;273:373–379. [PubMed] [Google Scholar]
- Ghahremani DG, Lee B, Robertson CL, Tabibnia G, Morgan AT, De Shetler N, Brown AK, Monterosso JR, Aron AR, Mandelkern MA, Poldrack RA, London ED. Striatal dopamine D(2)/D(3) receptors mediate response inhibition and related activity in frontostriatal neural circuitry in humans. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:7316–7324. doi: 10.1523/JNEUROSCI.4284-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilman S, Koeppe RA, Adams KM, Junck L, Kluin KJ, Johnson-Greene D, Martorello S, Heumann M, Bandekar R. Decreased striatal monoaminergic terminals in severe chronic alcoholism demonstrated with (+)[11C]dihydrotetrabenazine and positron emission tomography. Annals of neurology. 1998;44:326–333. doi: 10.1002/ana.410440307. [DOI] [PubMed] [Google Scholar]
- Gjedde A, Kumakura Y, Cumming P, Linnet J, Moller A. Inverted-U-shaped correlation between dopamine receptor availability in striatum and sensation seeking. Proc Natl Acad Sci U S A. 2010;107:3870–3875. doi: 10.1073/pnas.0912319107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gongwer MA, Murphy JM, McBride WJ, Lumeng L, Li TK. Regional brain contents of serotonin, dopamine and their metabolites in the selectively bred high- and low-alcohol drinking lines of rats. Alcohol. 1989;6:317–320. doi: 10.1016/0741-8329(89)90089-x. [DOI] [PubMed] [Google Scholar]
- Groman SM, Jentsch JD. Cognitive control and the dopamine D(2)-like receptor: a dimensional understanding of addiction. Depress Anxiety. 2012;29:295–306. doi: 10.1002/da.20897. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Buchholz HG, Grunder G, Kumakura Y, Cumming P, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation of alcohol craving with striatal dopamine synthesis capacity and D2/3 receptor availability: a combined [18F]DOPA and [18F]DMFP PET study in detoxified alcoholic patients. Am J Psychiatry. 2005;162:1515–1520. doi: 10.1176/appi.ajp.162.8.1515. [DOI] [PubMed] [Google Scholar]
- Heinz A, Siessmeier T, Wrase J, Hermann D, Klein S, Grusser SM, Flor H, Braus DF, Buchholz HG, Grunder G, Schreckenberger M, Smolka MN, Rosch F, Mann K, Bartenstein P. Correlation between dopamine D(2) receptors in the ventral striatum and central processing of alcohol cues and craving. Am J Psychiatry. 2004;161:1783–1789. doi: 10.1176/appi.ajp.161.10.1783. [DOI] [PubMed] [Google Scholar]
- Hietala J, West C, Syvalahti E, Nagren K, Lehikoinen P, Sonninen P, Ruotsalainen U. Striatal D2 dopamine receptor binding characteristics in vivo in patients with alcohol dependence. Psychopharmacology (Berl) 1994;116:285–290. doi: 10.1007/BF02245330. [DOI] [PubMed] [Google Scholar]
- Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, Dantona RL, Anthony S. Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psychiatry. 2003;60:1043–1052. doi: 10.1001/archpsyc.60.9.1043. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Jentsch JD. Reversal learning as a measure of impulsive and compulsive behavior in addictions. Psychopharmacology (Berl) 2012;219:607–620. doi: 10.1007/s00213-011-2579-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanson CE, Frey KA, Lundahl LH, Keenan P, Lockhart N, Roll J, Galloway GP, Koeppe RA, Kilbourn MR, Robbins T, Schuster CR. Cognitive function and nigrostriatal markers in abstinent methamphetamine abusers. Psychopharmacology (Berl) 2006;185:327–338. doi: 10.1007/s00213-006-0330-6. [DOI] [PubMed] [Google Scholar]
- Johnson PM, Kenny PJ. Dopamine D2 receptors in addiction-like reward dysfunction and compulsive eating in obese rats. Nat Neurosci. 2010;13:635–641. doi: 10.1038/nn.2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutsa J, Johansson J, Niemela S, Ollikainen A, Hirvonen MM, Piepponen P, Arponen E, Alho H, Voon V, Rinne JO, Hietala J, Kaasinen V. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. Neuroimage. 2012;60:1992–1999. doi: 10.1016/j.neuroimage.2012.02.006. [DOI] [PubMed] [Google Scholar]
- Kirby LG, Zeeb FD, Winstanley CA. Contributions of serotonin in addiction vulnerability. Neuropharmacology. 2011;61:421–432. doi: 10.1016/j.neuropharm.2011.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkland Henry P, Davis M, Howell LL. Effects of cocaine self-administration history under limited and extended access conditions on in vivo striatal dopamine neurochemistry and acoustic startle in rhesus monkeys. Psychopharmacology (Berl) 2009;205:237–247. doi: 10.1007/s00213-009-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knutson B, Gibbs SE. Linking nucleus accumbens dopamine and blood oxygenation. Psychopharmacology (Berl) 2007;191:813–822. doi: 10.1007/s00213-006-0686-7. [DOI] [PubMed] [Google Scholar]
- Landau SM, Lal R, O'Neil JP, Baker S, Jagust WJ. Striatal dopamine and working memory. Cereb Cortex. 2009;19:445–454. doi: 10.1093/cercor/bhn095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laruelle M. Imaging synaptic neurotransmission with in vivo binding competition techniques: a critical review. J Cereb Blood Flow Metab. 2000;20:423–451. doi: 10.1097/00004647-200003000-00001. [DOI] [PubMed] [Google Scholar]
- Lee B, London ED, Poldrack RA, Farahi J, Nacca A, Monterosso JR, Mumford JA, Bokarius AV, Dahlbom M, Mukherjee J, Bilder RM, Brody AL, Mandelkern MA. Striatal dopamine D2/d3 receptor availability is reduced in methamphetamine dependence and is linked to impulsivity. J Neurosci. 2009;29:14734–14740. doi: 10.1523/JNEUROSCI.3765-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Parish CL, Tomas D, Horne MK. Chronic cocaine administration reduces striatal dopamine terminal density and striatal dopamine release which leads to drug-seeking behaviour. Neuroscience. 2011;174:143–150. doi: 10.1016/j.neuroscience.2010.11.055. [DOI] [PubMed] [Google Scholar]
- Leeman RF, Potenza MN. Similarities and differences between pathological gambling and substance use disorders: a focus on impulsivity and compulsivity. Psychopharmacology (Berl) 2012;219:469–490. doi: 10.1007/s00213-011-2550-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leyton M. Conditioned and sensitized responses to stimulant drugs in humans. Prog Neuropsychopharmacol Biol Psychiatry. 2007;31:1601–1613. doi: 10.1016/j.pnpbp.2007.08.027. [DOI] [PubMed] [Google Scholar]
- Linnet J, Moller A, Peterson E, Gjedde A, Doudet D. Dopamine release in ventral striatum during Iowa Gambling Task performance is associated with increased excitement levels in pathological gambling. Addiction. 2011;106:383–390. doi: 10.1111/j.1360-0443.2010.03126.x. [DOI] [PubMed] [Google Scholar]
- Maisonneuve IM, Ho A, Kreek MJ. Chronic administration of a cocaine “binge” alters basal extracellular levels in male rats: an in vivo microdialysis study. J Pharmacol Exp Ther. 1995;272:652–657. [PubMed] [Google Scholar]
- Malison RT, Best SE, van Dyck CH, McCance EF, Wallace EA, Laruelle M, Baldwin RM, Seibyl JP, Price LH, Kosten TR, Innis RB. Elevated striatal dopamine transporters during acute cocaine abstinence as measured by [123I] beta-CIT SPECT. Am J Psychiatry. 1998;155:832–834. doi: 10.1176/ajp.155.6.832. [DOI] [PubMed] [Google Scholar]
- Malison RT, Mechanic KY, Klummp H, Baldwin RM, Kosten TR, Seibyl JP, Innis RB. Reduced amphetamine-stimulated dopamine release in cocaine addicts as measured by [123I]IBZM SPECT. Journal of Nuclear Medicine. 1999;40:110P. [Google Scholar]
- Malloy-Diniz L, Fuentes D, Leite WB, Correa H, Bechara A. Impulsive behavior in adults with attention deficit/ hyperactivity disorder: characterization of attentional, motor and cognitive impulsiveness. Journal of the International Neuropsychological Society : JINS. 2007;13:693–698. doi: 10.1017/S1355617707070889. [DOI] [PubMed] [Google Scholar]
- Martinez D, Broft A, Foltin RW, Slifstein M, Hwang DR, Huang Y, Perez A, Frankle WG, Cooper T, Kleber HD, Fischman MW, Laruelle M. Cocaine dependence and D2 receptor availability in the functional subdivisions of the striatum: relationship with cocaine-seeking behavior. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2004;29:1190–1202. doi: 10.1038/sj.npp.1300420. [DOI] [PubMed] [Google Scholar]
- Martinez D, Carpenter KM, Liu F, Slifstein M, Broft A, Friedman AC, Kumar D, Van Heertum R, Kleber HD, Nunes E. Imaging dopamine transmission in cocaine dependence: link between neurochemistry and response to treatment. Am J Psychiatry. 2011;168:634–641. doi: 10.1176/appi.ajp.2010.10050748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Gil R, Slifstein M, Hwang DR, Huang Y, Perez A, Kegeles L, Talbot P, Evans S, Krystal J, Laruelle M, Abi-Dargham A. Alcohol dependence is associated with blunted dopamine transmission in the ventral striatum. Biol Psychiatry. 2005;58:779–786. doi: 10.1016/j.biopsych.2005.04.044. [DOI] [PubMed] [Google Scholar]
- Martinez D, Greene K, Broft A, Kumar D, Liu F, Narendran R, Slifstein M, Van Heertum R, Kleber HD. Lower level of endogenous dopamine in patients with cocaine dependence: findings from PET imaging of D(2)/D(3) receptors following acute dopamine depletion. Am J Psychiatry. 2009;166:1170–1177. doi: 10.1176/appi.ajp.2009.08121801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez D, Narendran R, Foltin RW, Slifstein M, Hwang DR, Broft A, Huang Y, Cooper TB, Fischman MW, Kleber HD, Laruelle M. Amphetamine-induced dopamine release: markedly blunted in cocaine dependence and predictive of the choice to self-administer cocaine. Am J Psychiatry. 2007;164:622–629. doi: 10.1176/ajp.2007.164.4.622. [DOI] [PubMed] [Google Scholar]
- Martinez D, Saccone PA, Liu F, Slifstein M, Orlowska D, Grassetti A, Cook S, Broft A, Van Heertum R, Comer SD. Deficits in dopamine D(2) receptors and presynaptic dopamine in heroin dependence: commonalities and differences with other types of addiction. Biol Psychiatry. 2012;71:192–198. doi: 10.1016/j.biopsych.2011.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride WJ, Chernet E, Dyr W, Lumeng L, Li TK. Densities of dopamine D2 receptors are reduced in CNS regions of alcohol-preferring P rats. Alcohol. 1993;10:387–390. doi: 10.1016/0741-8329(93)90025-j. [DOI] [PubMed] [Google Scholar]
- McCann U, Wong D, Yokoi F, Villemagne V, Dannals R, Ricaurte G. Reduced striatal dopamine transporter density in abstinent methamphetamine and methcathinone users: evidence from positron emission tomography studies with [11C]WIN-35,428. J Neurosci. 1998;18:8417–8422. doi: 10.1523/JNEUROSCI.18-20-08417.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCann UD, Kuwabara H, Kumar A, Palermo M, Abbey R, Brasic J, Ye W, Alexander M, Dannals RF, Wong DF, Ricaurte GA. Persistent cognitive and dopamine transporter deficits in abstinent methamphetamine users. Synapse. 2008;62:91–100. doi: 10.1002/syn.20471. [DOI] [PubMed] [Google Scholar]
- Melega WP, Jorgensen MJ, Lacan G, Way BM, Pham J, Morton G, Cho AK, Fairbanks LA. Long-term methamphetamine administration in the vervet monkey models aspects of a human exposure: brain neurotoxicity and behavioral profiles. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2008;33:1441–1452. doi: 10.1038/sj.npp.1301502. [DOI] [PubMed] [Google Scholar]
- Morgan D, Grant KA, Gage HD, Mach RH, Kaplan JR, Prioleau O, Nader SH, Buchheimer N, Ehrenkaufer RL, Nader MA. Social dominance in monkeys: dopamine D2 receptors and cocaine self-administration. Nat Neurosci. 2002;5:169–174. doi: 10.1038/nn798. [DOI] [PubMed] [Google Scholar]
- Mottola DM, Kilts JD, Lewis MM, Connery HS, Walker QD, Jones SR, Booth RG, Hyslop DK, Piercey M, Wightman RM, Lawler CP, Nichols DE, Mailman RB. Functional selectivity of dopamine receptor agonists. I. Selective activation of postsynaptic dopamine D2 receptors linked to adenylate cyclase. J Pharmacol Exp Ther. 2002;301:1166–1178. doi: 10.1124/jpet.301.3.1166. [DOI] [PubMed] [Google Scholar]
- Mukhin AG, Kimes AS, Chefer SI, Matochik JA, Contoreggi CS, Horti AG, Vaupel DB, Pavlova O, Stein EA. Greater nicotinic acetylcholine receptor density in smokers than in nonsmokers: a PET study with 2-18F–FA-85380. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49:1628–1635. doi: 10.2967/jnumed.108.050716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nader MA, Morgan D, Gage HD, Nader SH, Calhoun TL, Buchheimer N, Ehrenkaufer R, Mach RH. PET imaging of dopamine D2 receptors during chronic cocaine self-administration in monkeys. Nat Neurosci. 2006;9:1050–1056. doi: 10.1038/nn1737. [DOI] [PubMed] [Google Scholar]
- Nagano-Saito A, Leyton M, Monchi O, Goldberg YK, He Y, Dagher A. Dopamine depletion impairs frontostriatal functional connectivity during a set-shifting task. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2008;28:3697–3706. doi: 10.1523/JNEUROSCI.3921-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narendran R, Lopresti BJ, Martinez D, Mason NS, Himes M, May MA, Daley DC, Price JC, Mathis CA, Frankle WG. In vivo evidence for low striatal vesicular monoamine transporter 2 (VMAT2) availability in cocaine abusers. Am J Psychiatry. 2012;169:55–63. doi: 10.1176/appi.ajp.2011.11010126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Neill M, Brown VJ. The effect of striatal dopamine depletion and the adenosine A2A antagonist KW-6002 on reversal learning in rats. Neurobiol Learn Mem. 2007;88:75–81. doi: 10.1016/j.nlm.2007.03.003. [DOI] [PubMed] [Google Scholar]
- Ostlund SB, Wassum KM, Murphy NP, Balleine BW, Maidment NT. Extracellular dopamine levels in striatal subregions track shifts in motivation and response cost during instrumental conditioning. J Neurosci. 2011;31:200–207. doi: 10.1523/JNEUROSCI.4759-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oswald LM, Wong DF, Zhou Y, Kumar A, Brasic J, Alexander M, Ye W, Kuwabara H, Hilton J, Wand GS. Impulsivity and chronic stress are associated with amphetamine-induced striatal dopamine release. Neuroimage. 2007;36:153–166. doi: 10.1016/j.neuroimage.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Palmiter RD. Dopamine signaling in the dorsal striatum is essential for motivated behaviors: lessons from dopamine-deficient mice. Ann N Y Acad Sci. 2008;1129:35–46. doi: 10.1196/annals.1417.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez-Mana C, Castells X, Vidal X, Casas M, Capella D. Efficacy of indirect dopamine agonists for psychostimulant dependence: a systematic review and meta-analysis of randomized controlled trials. J Subst Abuse Treat. 2011;40:109–122. doi: 10.1016/j.jsat.2010.08.012. [DOI] [PubMed] [Google Scholar]
- Perry JL, Carroll ME. The role of impulsive behavior in drug abuse. Psychopharmacology (Berl) 2008;200:1–26. doi: 10.1007/s00213-008-1173-0. [DOI] [PubMed] [Google Scholar]
- Perry JL, Larson EB, German JP, Madden GJ, Carroll ME. Impulsivity (delay discounting) as a predictor of acquisition of IV cocaine self-administration in female rats. Psychopharmacology (Berl) 2005;178:193–201. doi: 10.1007/s00213-004-1994-4. [DOI] [PubMed] [Google Scholar]
- Peters J, Bromberg U, Schneider S, Brassen S, Menz M, Banaschewski T, Conrod PJ, Flor H, Gallinat J, Garavan H, Heinz A, Itterman B, Lathrop M, Martinot JL, Paus T, Poline JB, Robbins TW, Rietschel M, Smolka M, Strohle A, Struve M, Loth E, Schumann G, Buchel C. Lower ventral striatal activation during reward anticipation in adolescent smokers. Am J Psychiatry. 2011;168:540–549. doi: 10.1176/appi.ajp.2010.10071024. [DOI] [PubMed] [Google Scholar]
- Plichta MM, Vasic N, Wolf RC, Lesch KP, Brummer D, Jacob C, Fallgatter AJ, Gron G. Neural hyporesponsiveness and hyperresponsiveness during immediate and delayed reward processing in adult attention-deficit/hyperactivity disorder. Biol Psychiatry. 2009;65:7–14. doi: 10.1016/j.biopsych.2008.07.008. [DOI] [PubMed] [Google Scholar]
- Porrino LJ, Lyons D, Smith HR, Daunais JB, Nader MA. Cocaine self-administration produces a progressive involvement of limbic, association, and sensorimotor striatal domains. J Neurosci. 2004;24:3554–3562. doi: 10.1523/JNEUROSCI.5578-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter JN, Olsen AS, Gurnsey K, Dugan BP, Jedema HP, Bradberry CW. Chronic cocaine self-administration in rhesus monkeys: impact on associative learning, cognitive control, and working memory. J Neurosci. 2011;31:4926–4934. doi: 10.1523/JNEUROSCI.5426-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulos CX, Le AD, Parker JL. Impulsivity predicts individual susceptibility to high levels of alcohol self-administration. Behav Pharmacol. 1995;6:810–814. [PubMed] [Google Scholar]
- Quintanilla ME, Bustamante D, Tampier L, Israel Y, Herrera-Marschitz M. Dopamine release in the nucleus accumbens (shell) of two lines of rats selectively bred to prefer or avoid ethanol. Eur J Pharmacol. 2007;573:84–92. doi: 10.1016/j.ejphar.2007.06.038. [DOI] [PubMed] [Google Scholar]
- Reeves SJ, Polling C, Stokes PR, Lappin JM, Shotbolt PP, Mehta MA, Howes OD, Egerton A. Limbic striatal dopamine D2/3 receptor availability is associated with non-planning impulsivity in healthy adults after exclusion of potential dissimulators. Psychiatry Res. 2012;202:60–64. doi: 10.1016/j.pscychresns.2011.09.011. [DOI] [PubMed] [Google Scholar]
- Richards JB, Sabol KE, de Wit H. Effects of methamphetamine on the adjusting amount procedure, a model of impulsive behavior in rats. Psychopharmacology (Berl) 1999;146:432–439. doi: 10.1007/pl00005488. [DOI] [PubMed] [Google Scholar]
- Rogers RD, Baunez C, Everitt BJ, Robbins TW. Lesions of the medial and lateral striatum in the rat produce differential deficits in attentional performance. Behav Neurosci. 2001;115:799–811. doi: 10.1037//0735-7044.115.4.799. [DOI] [PubMed] [Google Scholar]
- Rosa-Neto P, Lou HC, Cumming P, Pryds O, Karrebaek H, Lunding J, Gjedde A. Methylphenidate-evoked changes in striatal dopamine correlate with inattention and impulsivity in adolescents with attention deficit hyperactivity disorder. Neuroimage. 2005;25:868–876. doi: 10.1016/j.neuroimage.2004.11.031. [DOI] [PubMed] [Google Scholar]
- Salamone JD, Correa M. The mysterious motivational functions of mesolimbic dopamine. Neuron. 2012;76:470–485. doi: 10.1016/j.neuron.2012.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salamone JD, Correa M, Farrar A, Mingote SM. Effort-related functions of nucleus accumbens dopamine and associated forebrain circuits. Psychopharmacology (Berl) 2007;191:461–482. doi: 10.1007/s00213-006-0668-9. [DOI] [PubMed] [Google Scholar]
- Scheres A, Milham MP, Knutson B, Castellanos FX. Ventral striatal hyporesponsiveness during reward anticipation in attention-deficit/hyperactivity disorder. Biol Psychiatry. 2007;61:720–724. doi: 10.1016/j.biopsych.2006.04.042. [DOI] [PubMed] [Google Scholar]
- Schmitz JM, Mooney ME, Moeller FG, Stotts AL, Green C, Grabowski J. Levodopa pharmacotherapy for cocaine dependence: choosing the optimal behavioral therapy platform. Drug Alcohol Depend. 2008;94:142–150. doi: 10.1016/j.drugalcdep.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider S, Peters J, Bromberg U, Brassen S, Miedl SF, Banaschewski T, Barker GJ, Conrod P, Flor H, Garavan H, Heinz A, Ittermann B, Lathrop M, Loth E, Mann K, Martinot JL, Nees F, Paus T, Rietschel M, Robbins TW, Smolka MN, Spanagel R, Strohle A, Struve M, Schumann G, Buchel C. Risk taking and the adolescent reward system: a potential common link to substance abuse. Am J Psychiatry. 2012;169:39–46. doi: 10.1176/appi.ajp.2011.11030489. [DOI] [PubMed] [Google Scholar]
- Schott BH, Minuzzi L, Krebs RM, Elmenhorst D, Lang M, Winz OH, Seidenbecher CI, Coenen HH, Heinze HJ, Zilles K, Duzel E, Bauer A. Mesolimbic functional magnetic resonance imaging activations during reward anticipation correlate with reward-related ventral striatal dopamine release. J Neurosci. 2008;28:14311–14319. doi: 10.1523/JNEUROSCI.2058-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schultz W. Behavioral theories and the neurophysiology of reward. Annu Rev Psychol. 2006;57:87–115. doi: 10.1146/annurev.psych.56.091103.070229. [DOI] [PubMed] [Google Scholar]
- Segal DS, Kuczenski R. In vivo microdialysis reveals a diminished amphetamine-induced DA response corresponding to behavioral sensitization produced by repeated amphetamine pretreatment. Brain Res. 1992;571:330–337. doi: 10.1016/0006-8993(92)90672-v. [DOI] [PubMed] [Google Scholar]
- Segovia KN, Correa M, Salamone JD. Slow phasic changes in nucleus accumbens dopamine release during fixed ratio acquisition: a microdialysis study. Neuroscience. 2011;196:178–188. doi: 10.1016/j.neuroscience.2011.07.078. [DOI] [PubMed] [Google Scholar]
- Sekine Y, Iyo M, Ouchi Y, Matsunaga T, Tsukada H, Okada H, Yoshikawa E, Futatsubashi M, Takei N, Mori N. Methamphetamine-related psychiatric symptoms and reduced brain dopamine transporters studied with PET. Am J Psychiatry. 2001;158:1206–1214. doi: 10.1176/appi.ajp.158.8.1206. [DOI] [PubMed] [Google Scholar]
- Sevy S, Smith GS, Ma Y, Dhawan V, Chaly T, Kingsley PB, Kumra S, Abdelmessih S, Eidelberg D. Cerebral glucose metabolism and D2/D3 receptor availability in young adults with cannabis dependence measured with positron emission tomography. Psychopharmacology (Berl) 2008;197:549–556. doi: 10.1007/s00213-008-1075-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorg BA, Davidson DL, Kalivas PW, Prasad BM. Repeated daily cocaine alters subsequent cocaine-induced increase of extracellular dopamine in the medial prefrontal cortex. J Pharmacol Exp Ther. 1997;281:54–61. [PubMed] [Google Scholar]
- Staley JK, Krishnan-Sarin S, Cosgrove KP, Krantzler E, Frohlich E, Perry E, Dubin JA, Estok K, Brenner E, Baldwin RM, Tamagnan GD, Seibyl JP, Jatlow P, Picciotto MR, London ED, O'Malley S, van Dyck CH. Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006;26:8707–8714. doi: 10.1523/JNEUROSCI.0546-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele KE, Prokopowicz GP, Schweitzer MA, Magunsuon TH, Lidor AO, Kuwabawa H, Kumar A, Brasic J, Wong DF. Alterations of central dopamine receptors before and after gastric bypass surgery. Obesity surgery. 2010;20:369–374. doi: 10.1007/s11695-009-0015-4. [DOI] [PubMed] [Google Scholar]
- Stefanini E, Frau M, Garau MG, Fadda F, Gessa GL. Alcohol-prefering rats have fewer dopamine D2 receptors in the limbic area. Alcohol Alcohol. 1992;27 [PubMed] [Google Scholar]
- Stokes PR, Egerton A, Watson B, Reid A, Lappin J, Howes OD, Nutt DJ, Lingford-Hughes AR. History of cannabis use is not associated with alterations in striatal dopamine D2/D3 receptor availability. Journal of psychopharmacology. 2012;26:144–149. doi: 10.1177/0269881111414090. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Umegaki H, Volkow ND. D2R DNA transfer into the nucleus accumbens attenuates cocaine self-administration in rats. Synapse. 2008;62:481–486. doi: 10.1002/syn.20523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Rivera SN, Weaver K, Grandy DK, Rubinstein M, Umegaki H, Wang GJ, Hitzemann R, Volkow ND. Dopamine D2R DNA transfer in dopamine D2 receptor-deficient mice: effects on ethanol drinking. Life Sci. 2005;77:130–139. doi: 10.1016/j.lfs.2004.10.061. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Taintor NB, Rivera SN, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R, Fowler JS, Gatley SJ, Wang GJ, Volkow ND. DRD2 gene transfer into the nucleus accumbens core of the alcohol preferring and nonpreferring rats attenuates alcohol drinking. Alcohol Clin Exp Res. 2004;28:720–728. doi: 10.1097/01.alc.0000125270.30501.08. [DOI] [PubMed] [Google Scholar]
- Thanos PK, Volkow ND, Freimuth P, Umegaki H, Ikari H, Roth G, Ingram DK, Hitzemann R. Overexpression of dopamine D2 receptors reduces alcohol self-administration. J Neurochem. 2001;78:1094–1103. doi: 10.1046/j.1471-4159.2001.00492.x. [DOI] [PubMed] [Google Scholar]
- Tiihonen J, Vilkman H, Rasanen P, Ryynanen OP, Hakko H, Bergman J, Hamalainen T, Laakso A, Haaparanta-Solin M, Solin O, Kuoppamaki M, Syvalahti E, Hietala J. Striatal presynaptic dopamine function in type 1 alcoholics measured with positron emission tomography. Mol Psychiatry. 1998;3:156–161. doi: 10.1038/sj.mp.4000365. [DOI] [PubMed] [Google Scholar]
- Tomer R, Goldstein RZ, Wang GJ, Wong C, Volkow ND. Incentive motivation is associated with striatal dopamine asymmetry. Biological psychology. 2008;77:98–101. doi: 10.1016/j.biopsycho.2007.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran AH, Tamura R, Uwano T, Kobayashi T, Katsuki M, Matsumoto G, Ono T. Altered accumbens neural response to prediction of reward associated with place in dopamine D2 receptor knockout mice. Proc Natl Acad Sci U S A. 2002;99:8986–8991. doi: 10.1073/pnas.132284599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Buckholtz JW, Cowan RL, Woodward ND, Li R, Ansari MS, Baldwin RM, Schwartzman AN, Kessler RM, Zald DH. Dopaminergic mechanisms of individual differences in human effort-based decision-making. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2012;32:6170–6176. doi: 10.1523/JNEUROSCI.6459-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trifilieff P, Feng B, Urizar E, Winiger V, Ward RD, Taylor KM, Martinez D, Moore H, Balsam PD, Simpson EH, Javitch JA. Increasing dopamine D2 receptor expression in the adult nucleus accumbens enhances motivation. Mol Psychiatry. 2013 doi: 10.1038/mp.2013.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UNODC. Drug Statistics and Trends. World Drug Report 2010. 2010 [Google Scholar]
- Urban NB, Slifstein M, Thompson JL, Xu X, Girgis RR, Raheja S, Haney M, Abi-Dargham A. Dopamine release in chronic cannabis users: a [11c]raclopride positron emission tomography study. Biol Psychiatry. 2012;71:677–683. doi: 10.1016/j.biopsych.2011.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vernaleken I, Buchholz HG, Kumakura Y, Siessmeier T, Stoeter P, Bartenstein P, Cumming P, Grunder G. 'Prefrontal' cognitive performance of healthy subjects positively correlates with cerebral FDOPA influx: an exploratory [18F]-fluoro-L-DOPA-PET investigation. Hum Brain Mapp. 2007;28:931–939. doi: 10.1002/hbm.20325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N. Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. Am J Psychiatry. 2001a;158:2015–2021. doi: 10.1176/appi.ajp.158.12.2015. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Chang L, Wang GJ, Fowler JS, Franceschi D, Sedler M, Gatley SJ, Miller E, Hitzemann R, Ding YS, Logan J. Loss of dopamine transporters in methamphetamine abusers recovers with protracted abstinence. J Neurosci. 2001b;21:9414–9418. doi: 10.1523/JNEUROSCI.21-23-09414.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wang GJ, Hitzemann R, Logan J, Schlyer DJ, Dewey SL, Wolf AP. Decreased dopamine D2 receptor availability is associated with reduced frontal metabolism in cocaine abusers. Synapse. 1993;14:169–177. doi: 10.1002/syn.890140210. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Fowler JS, Wolf AP, Schlyer D, Shiue CY, Alpert R, Dewey SL, Logan J, Bendriem B, Christman D, et al. Effects of chronic cocaine abuse on postsynaptic dopamine receptors. Am J Psychiatry. 1990;147:719–724. doi: 10.1176/ajp.147.6.719. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G-J, Fowler JS, Logan J, Schlyer D, Hitzemann R, Lieberman J, Angrist B, Pappas N, MacGregor R, Burr G, Cooper T, Wolf AP. Imaging endogenous dopamine competition with [11C]raclopride in the human brain. Synapse. 1994;16:255–262. doi: 10.1002/syn.890160402. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Begleiter H, Porjesz B, Fowler JS, Telang F, Wong C, Ma Y, Logan J, Goldstein R, Alexoff D, Thanos PK. High levels of dopamine D2 receptors in unaffected members of alcoholic families: possible protective factors. Arch Gen Psychiatry. 2006a;63:999–1008. doi: 10.1001/archpsyc.63.9.999. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Gifford A, Hitzemann R, Ding YS, Pappas N. Prediction of reinforcing responses to psychostimulants in humans by brain dopamine D2 receptor levels. Am J Psychiatry. 1999;156:1440–1443. doi: 10.1176/ajp.156.9.1440. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Gatley SJ, Hitzemann R, Chen AD, Dewey SL, Pappas N. Decreased striatal dopaminergic responsiveness in detoxified cocaine-dependent subjects. Nature. 1997;386:830–833. doi: 10.1038/386830a0. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Logan J, Hitzemann R, Ding YS, Pappas N, Shea C, Piscani K. Decreases in dopamine receptors but not in dopamine transporters in alcoholics. Alcoholism, Clinical & Experimental Research. 1996;20:1594–1598. doi: 10.1111/j.1530-0277.1996.tb05936.x. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Fowler JS, Thanos P, Logan J, Gatley SJ, Gifford A, Ding YS, Wong C, Pappas N. Brain DA D2 receptors predict reinforcing effects of stimulants in humans: replication study. Synapse. 2002a;46:79–82. doi: 10.1002/syn.10137. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM. Evaluating dopamine reward pathway in ADHD: clinical implications. JAMA : the journal of the American Medical Association. 2009;302:1084–1091. doi: 10.1001/jama.2009.1308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Fowler JS, Jayne B, Telang F, Logan J, Ding YS, Gatley SJ, Hitzemann R, Wong C, Pappas N. Effects of alcohol detoxification on dopamine D2 receptors in alcoholics: a preliminary study. Psychiatry Res. 2002b;116:163–172. doi: 10.1016/s0925-4927(02)00087-2. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Maynard L, Jayne M, Fowler JS, Zhu W, Logan J, Gatley SJ, Ding YS, Wong C, Pappas N. Brain dopamine is associated with eating behaviors in humans. The International journal of eating disorders. 2003;33:136–142. doi: 10.1002/eat.10118. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn J, Telang F, Solanto MV, Fowler JS, Logan J, Ma Y, Schulz K, Pradhan K, Wong C, Swanson JM. Depressed dopamine activity in caudate and preliminary evidence of limbic involvement in adults with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry. 2007a;64:932–940. doi: 10.1001/archpsyc.64.8.932. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Newcorn JH, Kollins SH, Wigal TL, Telang F, Fowler JS, Goldstein RZ, Klein N, Logan J, Wong C, Swanson JM. Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. Mol Psychiatry. 2011;16:1147–1154. doi: 10.1038/mp.2010.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Childress AR, Jayne M, Ma Y, Wong C. Cocaine cues and dopamine in dorsal striatum: mechanism of craving in cocaine addiction. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2006b;26:6583–6588. doi: 10.1523/JNEUROSCI.1544-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Logan J, Jayne M, Ma Y, Pradhan K, Wong C. Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci. 2007b;27:12700–12706. doi: 10.1523/JNEUROSCI.3371-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Wang GJ, Telang F, Fowler JS, Thanos PK, Logan J, Alexoff D, Ding YS, Wong C, Ma Y, Pradhan K. Low dopamine striatal D2 receptors are associated with prefrontal metabolism in obese subjects: possible contributing factors. Neuroimage. 2008;42:1537–1543. doi: 10.1016/j.neuroimage.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wade TR, de Wit H, Richards JB. Effects of dopaminergic drugs on delayed reward as a measure of impulsive behavior in rats. Psychopharmacology (Berl) 2000;150:90–101. doi: 10.1007/s002130000402. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Geliebter A, Volkow ND, Telang FW, Logan J, Jayne MC, Galanti K, Selig PA, Han H, Zhu W, Wong CT, Fowler JS. Enhanced striatal dopamine release during food stimulation in binge eating disorder. Obesity. 2011;19:1601–1608. doi: 10.1038/oby.2011.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Smith L, Volkow ND, Telang F, Logan J, Tomasi D, Wong CT, Hoffman W, Jayne M, Alia-Klein N, Thanos P, Fowler JS. Decreased dopamine activity predicts relapse in methamphetamine abusers. Mol Psychiatry. 2012;17:918–925. doi: 10.1038/mp.2011.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Fischman M, Foltin R, Abumrad NN, Logan J, Pappas NR. Cocaine abusers do not show loss of dopamine transporters with age. Life Sci. 1997a;61:1059–1065. doi: 10.1016/s0024-3205(97)00614-0. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Fowler JS, Logan J, Abumrad NN, Hitzemann RJ, Pappas NS, Pascani K. Dopamine D2 receptor availability in opiate-dependent subjects before and after naloxone-precipitated withdrawal. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1997b;16:174–182. doi: 10.1016/S0893-133X(96)00184-4. [DOI] [PubMed] [Google Scholar]
- Wang GJ, Volkow ND, Logan J, Pappas NR, Wong CT, Zhu W, Netusil N, Fowler JS. Brain dopamine and obesity. Lancet. 2001;357:354–357. doi: 10.1016/s0140-6736(00)03643-6. [DOI] [PubMed] [Google Scholar]
- Waxman SE. A systematic review of impulsivity in eating disorders. European eating disorders review : the journal of the Eating Disorders Association. 2009;17:408–425. doi: 10.1002/erv.952. [DOI] [PubMed] [Google Scholar]
- WHOOrganization WH, editor. Who news: Alcohol. 2011. [Google Scholar]
- Wise RA. Dopamine and reward: the anhedonia hypothesis 30 years on. Neurotox Res. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JC, Bell K, Najafi A, Widmark C, Keator D, Tang C, Klein E, Bunney BG, Fallon J, Bunney WE. Decreasing striatal 6-FDOPA uptake with increasing duration of cocaine withdrawal. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 1997;17:402–409. doi: 10.1016/S0893-133X(97)00089-4. [DOI] [PubMed] [Google Scholar]
- Wullner U, Gundisch D, Herzog H, Minnerop M, Joe A, Warnecke M, Jessen F, Schutz C, Reinhardt M, Eschner W, Klockgether T, Schmaljohann J. Smoking upregulates alpha4beta2* nicotinic acetylcholine receptors in the human brain. Neurosci Lett. 2008;430:34–37. doi: 10.1016/j.neulet.2007.10.011. [DOI] [PubMed] [Google Scholar]
- Zijlstra F, Booij J, van den Brink W, Franken IH. Striatal dopamine D2 receptor binding and dopamine release during cue-elicited craving in recently abstinent opiate-dependent males. Eur Neuropsychopharmacol. 2008;18:262–270. doi: 10.1016/j.euroneuro.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Zubieta J, Greenwald MK, Lombardi U, Woods JH, Kilbourn MR, Jewett DM, Koeppe RA, Schuster CR, Johanson CE. Buprenorphine-induced changes in mu-opioid receptor availability in male heroin-dependent volunteers: a preliminary study. Neuropsychopharmacology : official publication of the American College of Neuropsychopharmacology. 2000;23:326–334. doi: 10.1016/S0893-133X(00)00110-X. [DOI] [PubMed] [Google Scholar]