Abstract
Background
Recent evidence suggests that de novo donor specific antibodies (dnDSA) are associated with antibody mediated rejection (ABMR) and graft failure following kidney transplantation. The effects of induction immunosuppression on dnDSA are unknown.
Methods
The study population comprised 114 consecutive moderately sensitized (positive DSA and negative flow crossmatch) recipients who received deceased donor renal transplants between December 2009 and November 2011. Patients were divided in 2 groups based on induction immunosuppression: antithymocyte globulin (ATG) (n=85), or basiliximab (n=29) and were followed up for 36 months.
Results
Patients in the ATG group received a mean dose of 4.98 mg/kg ± 7.9 mg/kg, had a significantly higher PRA and received more plasmapheresis and IVIG at the time of transplant. The incidence of dnDSA (p = 0.02, HR=0.33, 95% CI 0.09 to 1.24) and ABMR (p = 0.001, HR=0.9, 95% CI 0.04 to 0.87) was significantly lower in the ATG group. In multivariate regression analyses, ATG induction was the single most important variable associated with both ABMR and dnDSA.
Conclusions
In moderately sensitized deceased donor renal transplant recipients, induction with ATG is associated with a reduction in the occurrence of dnDSA and ABMR when compared with basiliximab.
Keywords: donor-specific antibody, antibody mediated rejection, thymoglobulin, basiliximab, sensitization
INTRODUCTION
Preformed human leukocyte antigen (HLA)-donor specific antibodies (DSA) represent a significant obstacle to transplantation. In support of this observation, pre-transplant DSA significantly increased the risk for antibody mediated rejection and increased graft failure by 76% despite a negative flow cytometry crossmatch result (1). In addition to preformed DSA, de novo DSA were associated with poor transplant outcomes (2–4). The average annual incidence of dnDSA was reported as 3–4% after the first year post-transplant. Wiebe and colleagues found mean time to appearance of dnDSA was 4.6 years post-transplant (4). Independent risk factors included young age, non-adherence to immunosuppressant medications, acute cellular rejection and HLA-DRB1 antigen mismatch. Development of dnDSA post-transplant is associated with antibody-mediated rejection (ABMR) and increased risk of graft loss (3,4,5–9). As a result, post-transplant monitoring of DSA is becoming increasingly recognized as standard-of-care in renal transplant recipients (10).
Only two randomized clinical trials have evaluated the effects of ATG as induction immunosuppression in sensitized patients (11,12). In both studies, ATG (Thymo®, Genzyme, Cambridge, MA; Thymoglobulin®, IMTIX Pasteur-Mérieux-Connaught, Lyon, France) induction demonstrated beneficial short-term effects on acute rejection and graft function and survival in sensitized patients. However, there is limited information on the relationship between induction immunosuppression, dnDSA and ABMR. The purpose of this study was to evaluate the association of induction immunosuppression agents ATG and basiliximab with the incidence of dnDSA and ABMR in moderately sensitized deceased donor kidney transplant recipients.
RESULTS
Baseline characteristics
A total of 29 patients (25.5%) received basiliximab while 85 patients (74.5%) received ATG. Patients were followed for 36 months. Patients in the ATG group received a total of 4.98 mg/kg ±7.9 mg/kg, had a higher peak panel reactive antibody (PRA) level (39% vs. 22%, p=0.03) as well as greater utilization of plasmapheresis/IVIG (55% vs. 17%, p=0.0008) (Table 1) suggesting that patients in this group were more highly sensitized. All other pre-transplant risk factors were similar between the two groups including age, gender, ethnicity, retransplant status, HLA mismatch, and pretransplant DSA (Table 1). Discharge creatinine and tacrolimus levels were not statistically different between the two groups.
Table 1.
Baseline Characteristics
| Basiliximab | ATG | p | |
|---|---|---|---|
| Sample Size | 29 | 85 | - |
| Recipient age (years ± SD) | 49.7±13 | 48.5±12 | 0.4 |
| Caucasian (%) | 23 (79) | 60 (71) | 0.5 |
| Diabetic ESRD (%) | 6 (21) | 18 (21) | 0.9 |
| Female (%) | 15 (52) | 34 (40) | 0.3 |
| Retransplant (%) | 6 (20) | 29 (34) | 0.3 |
| Preemptive transplant (%) | 4 (14) | 10 (12) | 0.9 |
| Peak PRA (%) ± SD | 22±31 | 39±39 | 0.03 |
| HLA mismatch (mean ± SD) | 4.3±0.9 | 4.3±1.3 | 0.9 |
| Pretransplant DSA MFI±SD | 1090±677 | 1245±673 | 0.3 |
| PE/IVIG at transplant (%) | 5 (17) | 47 (55) | 0.0008 |
| Discharge Creatinine (mg/dL) | 3.5±2.7 | 3±2.1 | 0.3 |
| Discharge TAC concentration (ng/mL) | 5±4.4 | 7±4.6 | 0.06 |
Patient and graft outcomes
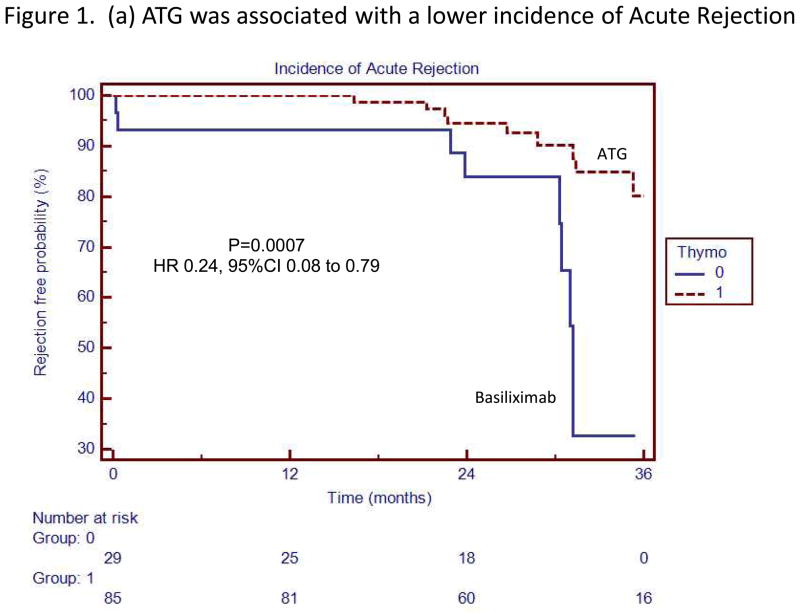
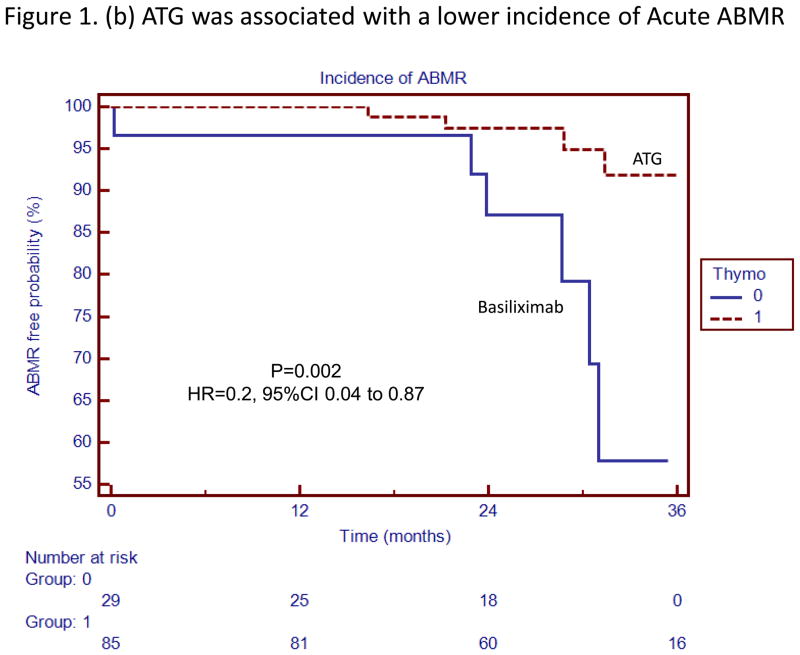
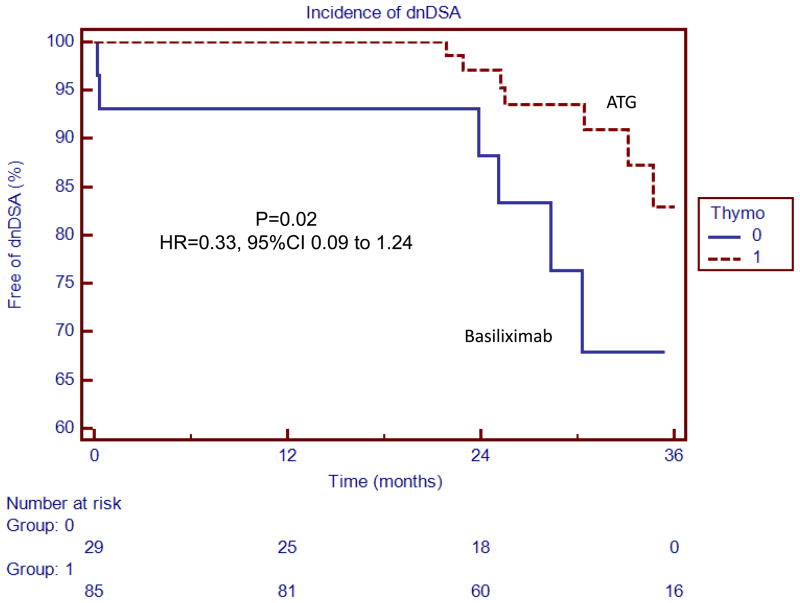
Kaplan Meier survival analyses with the associated Log-Rank tests demonstrated that the risk of acute rejection (combined cellular and antibody-mediated) (HR 0.24, 95% CI 0.08 to 0.79, p=0.0007), acute ABMR (HR=0.2, 95% CI 0.04 to 0.87, p=0.002), acute cellular rejection (HR=0.27, 95% CI 0.05 to 1.49, p=0.03) and dnDSA (HR=0.33, 95% CI 0.09 to 1.24, p=0.02) was significantly lower in patients receiving ATG induction (Figures 1 and 2).
Figure 1.
(a) ATG was associated with a lower incidence of Acute Rejection
(b) ATG was associated with a lower incidence of Acute ABMR
Thymoglobulin was associated with a lower incidence of acute rejection and acute antibody mediated rejection. The box to the right of the figures indicates patients who received ATG induction (Thymo=1 or Basiliximab Thymo=0)
Figure 2.
ATG was associated with a lower incidence of dnDSA
Thymoglobulin was associated with a lower incidence of de novo donor-specific antibodies. The box to the right of the figures indicates patients who received ATG induction (Thymo=1 or Basiliximab Thymo=0)
At one year, patients receiving ATG induction had a lower sum dnDSA level (455±1,828 vs. 3,652±12,835 MFI, p=0.02, Table 2) while sum MFI levels (pre-existing and de novo) were similar between the two groups (Table 2). Death (2 vs. 1), graft loss (4 vs. 2), cytomegalovirus infection (11 vs. 3), BK nephropathy (7 vs. 1), one-year serum creatinine, proteinuria and estimated glomerular filtration rate (eGFR) were not different between ATG and basiliximab groups (Table 2).
Table 2.
Kidney function and DSA
| Basiliximab | ATG | |||
|---|---|---|---|---|
| Kidney function at 12M | eGFR (mL/min) ±SD | 55±17 | 56±25 | 0.9 |
| Creatinine (mg/dL) | 1.4±0.4 | 1.4±0.5 | 0.8 | |
| UPC (mg/mg) | 0.3±0.4 | 0.4±0.4 | 0.4 | |
|
| ||||
| DSA at 12M | dnDSA (MFI±SD) | 3,652±12,835 | 455±1,828 | 0.02 |
| DSA (MFI±SD) | 4,060±6,300 | 2,454±4,265 | 0.2 | |
Univariate analysis and multivariable Cox regression analyses demonstrated no association between dnDSA and recipient age, race, gender and transplant number, pretransplant DSA, peak PRA, tacrolimus level at discharge (Table 3). However, ATG induction (HR 0.16, 95% CI 0.04 to 0.5, p=0.003) and plasmapheresis/IVIG therapy at the time of transplant (HR 3.8, 95% CI 1.12 to 12.7, p=0.03) were significant predictors of dnDSA (Table 3). ATG induction was the single most important predictor of ABMR (HR 0.16, 95% CI 0.05 to 0.6, p=0.006, Table 3).
Table 3.
Risk Factors for dnDSA and Acute ABMR
| Risk Factors for dnDSA | ||||||
|---|---|---|---|---|---|---|
| Univariate | Stepwise Multivariate Regression | |||||
| Covariate | P | HR | 95% CI | P | HR | 95% CI |
| ATG | 0.003 | 0.1 | 0.02 to 0.48 | 0.003 | 0.16 | 0.04 to 0.5 |
| Age > 50 years | 0.4 | 1.8 | 0.44 to 7.05 | - | - | - |
| African American | 0.07 | 4.2 | 0.88 to 20.4 | - | - | - |
| Female | 0.3 | 1.9 | 0.55 to 6.67 | - | - | - |
| Discharge TAC level | 0.3 | 0.9 | 0.83 to 1.07 | - | - | - |
| DSA at Tx > 1,000 MFI | 0.06 | 0.18 | 0.02 to 1.05 | - | - | - |
| Peak PRA | 0.1 | 0.98 | 0.95 to 1.0 | - | - | - |
| Retransplant status | 0.08 | 5.4 | 0.81 to 36.2 | - | - | - |
| PE/IVIG at transplant | 0.001 | 28 | 3.8 to 201.9 | 3.03 | 3.8 | 1.12 to 12.7 |
| Risk Factors for Acute ABMR | ||||||
|---|---|---|---|---|---|---|
| Covariate | P | HR | 95% CI | P | HR | 95% CI |
| ATG | 0.0007 | 0.01 | 0.001 to 0.15 | 0.006 | 0.16 | 0.05 to 0.6 |
| Age > 50 years | 0.08 | 0.15 | 0.01 to 1.26 | - | - | - |
| African American | 0.1 | 4.7 | 0.74 to 30.79 | - | - | - |
| Female | 0.5 | 1.6 | 0.35 to 7.56 | - | - | - |
| Discharge TAC level | 0.1 | 1.1 | 0.96 to 1.40 | - | - | - |
| DSA at Tx > 1,000 MFI | 0.008 | 0.03 | 0.002 to 0.42 | - | - | - |
| Peak PRA | 0.1 | 1.02 | 0.99 to 1.05 | - | - | - |
| Retransplant status | 0.5 | 0.5 | 0.05 to 4.83 | - | - | - |
| PE/IVIG at transplant | 0.001 | 88.5 | 5.7 to 1369 | - | - | - |
Patients in the ATG group underwent more plasmapheresis/IVIG, but were also at a higher immunological risk at baseline, evidenced by a higher peak PRA (p=0.03). Similarly, patients receiving plasmapheresis/IVIG were more sensitized with a higher DSA at transplant (1630+/−700 vs. 850+/−360, p<0.0001).
Further analyses demonstrated that the majority (60%) of dnDSA were directed against donor class II HLA and that the sum MFI rapidly increased with time until month 6. The majority (73%) of rejection episodes were associated with HLA class II DSA. Mean time to acute ABMR was 4.6±6 months and 9 out of 11 episodes of ABMR were ABMR phenotype 1, suggesting that ATG may prevent both ABMR phenotypes.
DISCUSSION
Our study shows that the use of ATG induction in moderately sensitized deceased donor kidney transplant recipients was associated with a lower incidence of dnDSA and antibody mediated rejection. Thibaudin et al. randomized sensitized renal transplant recipients to induction with ATG or no induction, with ATG associated with a lower incidence of biopsy-proven acute rejection from 64% to 38% and increased 1-year graft survival from 76% to 89% (11). Noel et al. assessed induction with ATG versus daclizumab in high immunological risk deceased donor renal transplant recipients, finding a lower incidence of biopsy-proven acute rejection (15.0% vs. 27.2%, p=0.016) and steroid-resistant rejection (2.7% vs. 14.9%, p=0.002) in the ATG arm at one year (12). No differences were seen in one-year patient or graft survival. Limitations of these studies included lack of long-term data and information regarding the development of dnDSA or ABMR.
The beneficial effects of ATG on acute rejection and dnDSA appeared relatively late, suggesting that the mechanism by which ATG prevents the development of donor specific antibodies is mediated via inhibition of both primary and secondary immune responses. For example, evidence suggests that ATG induces complement-independent apoptosis of naïve plasma B cells and plasma cells in vitro (13). It is also possible that ATG inhibits the secondary immune response by memory B cells, via T cell inhibition. A recent study by Ayasoufi noted that administration of ATG a week prior to transplant was substantially superior in inhibiting anti-donor T cell responses than at the time of transplant, suggesting that ATG has the ability to target preexisting donor-reactive memory T cells (14). Importantly, ATG induces Tregs following immune reconstitution, indicating that rATG therapy may also suppress B cells and DSA generation via activation of Tregs (16–18). Further in vitro and clinical studies are needed to investigate these specific mechanisms.
In our study, plasmapheresis/IVIG was associated with a greater risk of dnDSA. Rather than being a cause of dnDSA or ABMR, it is more likely that this treatment was utilized more in higher-risk patients, and that the protective effects of ATG were independent of plasmapheresis/IVIG. In support of this hypothesis, the analysis of ABMR in patients who did not undergo plasmapheresis/IVIG revealed that lymphocyte depletion was associated with a significantly lower incidence of ABMR. The converse was also true; in all patients who underwent plasmapheresis/IVIG at the time of transplant, the incidence of ABMR was lower with ATG. Our study further highlights the importance of monitoring for DSA and dnDSA in moderately sensitized patients (10,13). Recent consensus guidelines surrounding the testing and clinical management of HLA antibodies in transplantation advocate measurement of DSA and protocol biopsies during the first 3 months posttransplant in high-risk (desensitized or DSA positive/XM negative) patients (10). They suggest monitoring DSA during the first month posttransplant in intermediate-risk (history of DSA but currently negative) patients, and biopsy if DSA present. The need for serial DSA screening is recognized to determine time to onset of dnDSA before graft dysfunction, protocol biopsies at first appearance of dnDSA with documented pathologic correlation and clinical trials assessing prevention of the production of DSA.
Observational studies hold inherent limitations of which the reader should be aware and the single center design limits conclusions that may be drawn. However, our findings suggest that in moderately sensitized deceased donor renal transplant recipients, induction with thymoglobulin is associated with a reduction in the incidence of dnDSA and ABMR when compared with basiliximab. Randomized clinical trials with long term follow up and mechanistic studies are needed to determine the effect of antithymocyte globulin induction on donor specific B cells, plasma cells and patient and graft outcomes.
MATERIALS AND METHODS
Patient population and induction therapy
Approval was obtained from the UW Institutional Review Board and Human Subjects Committee (M2010-1296). The study population consisted of 114 consecutive moderately sensitized patients who received deceased donor kidney transplants at the institution between December 2009 and November 2011. Patients were classified as moderately sensitized if they exhibited a negative flow cytometric crossmatch despite the presence of DSA mean florescence intensity (MFImax) values by single antigen bead testing between 500–4,000 at the time of transplantation (15). The desensitization protocol for this group of patients included plasmapheresis and IVIG (100 mg/kg) and induction immunosuppression with ATG 5–6 mg/kg (rabbit anti-human thymocyte immunoglobulin, Thymoglobulin®, Sanofi). A subgroup of patients received induction therapy with basiliximab 20 mg on POD0 and POD3 (Simulect®, Novartis), based on individual provider preference. All patients received a 100 mg IV bolus of dexamethasone intraoperatively, 50 mg IV on postoperative day 1, and tapered to prednisone 30 mg daily at time of discharge.
Maintenance immunosuppression and viral prophylaxis
Maintenance immunosuppression consisted of a three-drug regimen of prednisone, tacrolimus, and mycophenolate sodium. No corticosteroid withdrawal, avoidance, or minimization was pursued in these patients. Prednisone dose at 1-month post transplant was 5–10 mg per day. Tacrolimus target level was 7–11 ng/ml at discharge. Donor cytomegalovirus (CMV) serostatus positive/recipient negative patients received six month of valganciclovir, along with any CMV positive donor or recipient patients who received ATG. Patients received three months of treatment with low dose acyclovir if donor and recipient cytomegalovirus serologies were negative. Basiliximab induction recipients with positive CMV serostatus received prophylaxis with high dose acyclovir. All patients received 1 year of pneumocystis jirovecii pneumonia prophylaxis with sulfamethoxazole-trimethoprim or an alternative agent if documented sulfa allergy.
Determination of de novo DSA
HLA antibodies were identified using LabScreen Single Antigen Beads (One Lambda, Canoga Park, CA) and were analyzed at baseline, along with 1 week, 3 months, 6 months and 12 months post-transplant. Specificities were assigned using multiple criteria consistent with consensus guidelines, including patterns of epitope reactivity, MFI value, assay background, and individual bead performance. A dnDSA was defined as an antibody that was not detectable pretransplant and appeared post-transplant. For patients exhibiting multiple dnDSA, the sum of the highest MFI value for each specificity was considered for analysis.
Diagnosis of rejection
All episodes of rejection were biopsy proven based on indication biopsies and were evaluated according to Banff 97 classification updated in 2010 (19).
Statistical analysis
Baseline characteristics and outcomes between patients receiving basiliximab or ATG induction were compared. For categorical data, Fisher’s exact test or Chi-squared test were used, where appropriate. Continuous numerical data was analyzed using Student’s t test. Survival analyses for ABMR and dnDSA were completed by the Kaplan-Meier method. Univariate and multivariable stepwise Cox regression analyses were performed to determine the risk factors associated with ABMR and dnDSA. Data are described as mean values with standard deviation. All p-values of less than 0.05 were considered statistically significant. MedCalc Software (Acacialaan 22, B-8400 Ostend, Belgium) was used for the analyses.
Abbreviations
- ABMR
mixed + pure antibody mediated rejection
- ATG
Anti thymocyte globulin
- DSA
donor specific antibody
- dnDSA
de novo donor specific antibodies
- MFI
mean fluorescence intensity
- PRA
panel reactive antibody
Footnotes
-
Marissa M. Brokhof, PharmD, BCPS
Participated in research design, in the writing of the paper, in the performance of the research and in data analysis
No conflicts of interest
600 Highland Avenue, Madison, WI 53792
-
Hans W. Sollinger, MD, PhD, FACS
Participated in the writing of the paper.
No conflicts of interest
BX7375, Clinical Science Center-H4, 600 Highland Avenue, Madison, WI 53792
-
David R. Hager, PharmD, BCPS, CNSC
Participated in research design and in the writing of the paper.
No conflicts of interest
600 Highland Avenue, Madison, WI 53792
-
Brenda L. Muth, RN, MS, ACNP
Participated in research design, in the writing of the paper, in the performance of the research and in data analysis.
No conflicts of interest.
5143 MFCB, 1685 Highland Avenue, Madison, WI 53705
-
John D. Pirsch, MD
Participated in the writing of the paper.
No conflicts of interest
600 Highland Avenue, Madison, WI 53792
-
Luis A. Fernandez, MD
Participated in the writing of the paper.
No conflicts of interest
BX7375, Clinical Science Center-H4, 600 Highland Avenue, Madison, WI 53792
-
Janet M. Bellingham, MD
Participated in the writing of the paper.
No conflicts of interest
BX7375, Clinical Science Center-H4, 600 Highland Avenue, Madison, WI 53792
-
Joshua D. Mezrich, MD
Participated in the writing of the paper.
No conflicts of interest
BX7375, Clinical Science Center-H4, 600 Highland Avenue, Madison, WI 53792
-
David P. Foley, MD
Participated in the writing of the paper.
No conflicts of interest
BX7375, Clinical Science Center-H4, 600 Highland Avenue, Madison, WI 53792
-
Anthony M. D’Alessandro, MD
Participated in the writing of the paper.
No conflicts of interest
BX7375, Clinical Science Center-H4, 600 Highland Avenue, Madison, WI 53792
-
Jon S. Odorico, MD
Participated in the writing of the paper.
No conflicts of interest
BX7375, Clinical Science Center-H4, 600 Highland Avenue, Madison, WI 53792
-
Maha A. Mohamed, MD
Participated in the writing of the paper.
No conflicts of interest
5142 MFCB, 1685 Highland Avenue, Madison, WI 53705
-
Vijay Vidyasagar, MD
Participated in the writing of the paper.
No conflicts of interest
5142 MFCB, 1685 Highland Avenue, Madison, WI 53705
-
Thomas M. Ellis, PhD
Participated in analytic tools and in the writing of the paper.
No conflicts of interest
D4/212 CSC, 600 Highland Avenue, Madison, WI 53792
-
Dixon B. Kaufman, MD, PhD, FACS
Participated in the writing of the paper.
No conflicts of interest.
H5/701A, 1685 Highland Avenue, Madison, WI 51703
-
Arjang Djamali, MD, MS, FASN
Participated in research design, in the writing of the paper, in the performance of research and in data analysis.
No conflicts of interest.
5142 MFCB, 1685 Highland Avenue, Madison, WI 53705
References
- 1.Mohan S, Palanisamy A, Tsapepas D, Tanriover B, Crew RJ, Dube G, et al. Donor-Specific Antibodies Adversely Affect Kidney Allograft Outcomes. J Am Soc Nephrol. 2012;23:2061–2071. doi: 10.1681/ASN.2012070664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Everly MJ, Rebellato LM, Haisch CE, Ozawa M, Parker K, Briley KP, et al. Incidence and impact of de novo donor-specific alloantibody in primary renal allografts. Transplantation. 2013;95(3):410–417. doi: 10.1097/TP.0b013e31827d62e3. [DOI] [PubMed] [Google Scholar]
- 3.Lachmann N, Terasaki PI, Budde K, Liefeldt L, Kahl A, Reinke P, et al. Anti-human leukocyte antigen and donor-specific antibodies detected by luminex posttransplant serve as biomarkers for chronic rejection of renal allografts. Transplantation. 2009;87:1505–1513. doi: 10.1097/TP.0b013e3181a44206. [DOI] [PubMed] [Google Scholar]
- 4.Wiebe C, Gibson IW, Blydt-Hansen TD, Karpinski M, Ho J, Storsley LJ, et al. Evolution and clinical pathologic correlations of de novo donor-specific HLA antibody post kidney transplant. Am J Transplant. 2012;12:1157–1167. doi: 10.1111/j.1600-6143.2012.04013.x. [DOI] [PubMed] [Google Scholar]
- 5.Lefaucheur C, Suberbielle-Boissel C, Hill CS, Nochy D, Andrade J, Antoine C, et al. Clinical relevance of preformed HLA donor-specific antibodies in kidney transplantation. Am J Transplant. 2008;8(2):324–331. doi: 10.1111/j.1600-6143.2007.02072.x. [DOI] [PubMed] [Google Scholar]
- 6.Cooper JE, Gralla J, Cagle L, Goldberg R, Chan L, Wiseman AC. Inferior kidney allograft outcomes in patients with de novo donor-specific antibodies are due to acute rejection episodes. Transplantation. 2011;91(10):1103–1109. doi: 10.1097/TP.0b013e3182139da1. [DOI] [PubMed] [Google Scholar]
- 7.Hidalgo LG, Campbell PM, Sis B, Einecke G, Mengel M, Chang J, et al. De novo donor-specific antibody at the time of kidney transplant biopsy associates with microvascular pathology and late graft failure. Am J Transplant. 2009;9:2532–2541. doi: 10.1111/j.1600-6143.2009.02800.x. [DOI] [PubMed] [Google Scholar]
- 8.Worthington JE, Martin S, Al-Husseini DM, Dyer PA, Johnson RW. Posttransplantation production of donor HLA-specific antibodies as a predictor of renal transplant outcome. Transplantation. 2003;75:1034–1040. doi: 10.1097/01.TP.0000055833.65192.3B. [DOI] [PubMed] [Google Scholar]
- 9.Terasaki PI, Ozawa M. Predicting kidney graft failure by HLA antibodies: a prospective trail. Am J Transplant. 2004;4:438–443. doi: 10.1111/j.1600-6143.2004.00360.x. [DOI] [PubMed] [Google Scholar]
- 10.Tait BD, Susal C, Gebel HM, Nickerson PW, Zachary AA, Claas FH, et al. Consensus guidelines on the testing and clinical management issues associated with HLA and non-HLA antibodies in transplantation. Transplantation. 2013;95(1):19–47. doi: 10.1097/TP.0b013e31827a19cc. [DOI] [PubMed] [Google Scholar]
- 11.Thibaudin D, Alamartine E, de Filippis JP, Diab N, Laurent B, Berthoux F. Advantages of antithymocyte globulin induction in sensitized kidney recipients: a randomized prospective study comparing induction with and without antithymocyte globulin. Nephrol Dial Transplant. 1998;13:711–715. doi: 10.1093/ndt/13.3.711. [DOI] [PubMed] [Google Scholar]
- 12.Noel C, Abramowicz D, Durand D, Mourad G, Lang P, Kessler M, et al. Daclizumab versus antithymocyte globulin in high-immunological-risk renal transplant recipients. J Am Soc Nephrol. 2009;20(6):1385–1392. doi: 10.1681/ASN.2008101037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zand MS, Vo T, Huggins J, Felgar R, Liesveld J, Pellegrin T, et al. Polyclonal rabbit antithymocyte globulin triggers B-cell and plasma cell apoptosis by multiple pathways. Transplantation. 2005;79(11):1507–1515. doi: 10.1097/01.tp.0000164159.20075.16. [DOI] [PubMed] [Google Scholar]
- 14.Ayasoufi K, Yu H, Fan R, Wang X, Williams J, Valujskikh A. Pretransplant antithymocyte globulin has increased efficacy in controlling donor-reactice memory T cells in mice. Am J Transplant. 2013;13:589–599. doi: 10.1111/ajt.12068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niederhaus SV, Muth B, Lorentzen DF, Wai P, Pirsch JD, Samaniego-Picota M, et al. Luminex-based desensitization protocols: The University of Wisconsin initial experience. Transplantation. 2011;92(1):12–17. doi: 10.1097/TP.0b013e31821c93bb. [DOI] [PubMed] [Google Scholar]
- 16.Todeschini M, Cortinovis M, Perico N, Poli F, Innocente A, Cavinato RA, et al. In Kidney Transplant Patients, Alemtuzumab but Not Basiliximab/Low-Dose Rabbit Anti-Thymocyte Globulin Induces B Cell Depletion and Regeneration, Which Associates with a High Incidence of De Novo Donor-Specific Anti-HLA Antibody Development. Journal of immunology. 2013;191(5):2818–2828. doi: 10.4049/jimmunol.1203261. [DOI] [PubMed] [Google Scholar]
- 17.Krystufkova E, Sekerkova A, Striz I, Brabcova I, Girmanova E, Viklicky O. Regulatory T cells in kidney transplant recipients: the effect of induction immunosuppression therapy. Nephrology, dialysis, transplantation : official publication of the European Dialysis and Transplant Association - European Renal Association. 2012;27(6):2576–2582. doi: 10.1093/ndt/gfr693. [DOI] [PubMed] [Google Scholar]
- 18.Gurkan S, Luan Y, Dhillon N, Allam SR, Montague T, Bromberg JS, et al. Immune reconstitution following rabbit antithymocyte globulin. American journal of transplantation : official journal of the American Society of Transplantation and the American Society of Transplant Surgeons. 2010;10(9):2132–2141. doi: 10.1111/j.1600-6143.2010.03210.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, et al. Banff ’09 Meeting Report: antibody mediated graft deterioration and implementation of banff working groups. Am J Transplant. 2010;10:464–471. doi: 10.1111/j.1600-6143.2009.02987.x. [DOI] [PubMed] [Google Scholar]