Abstract
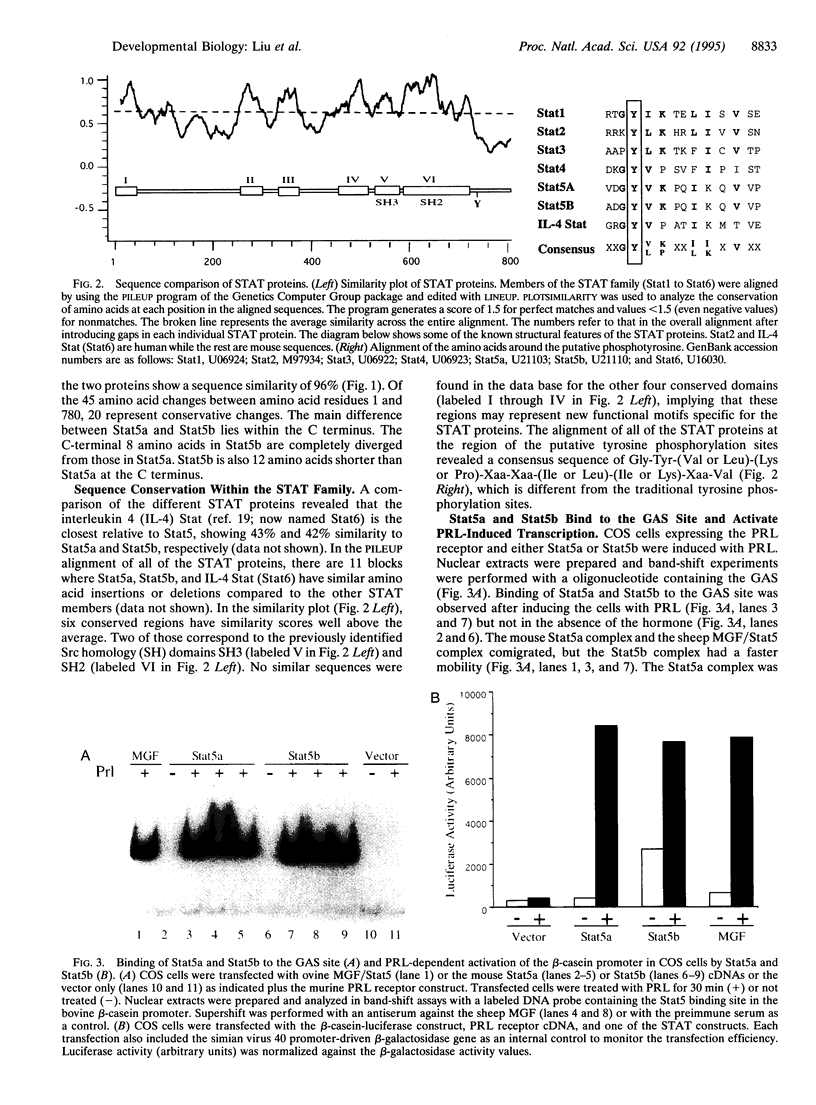
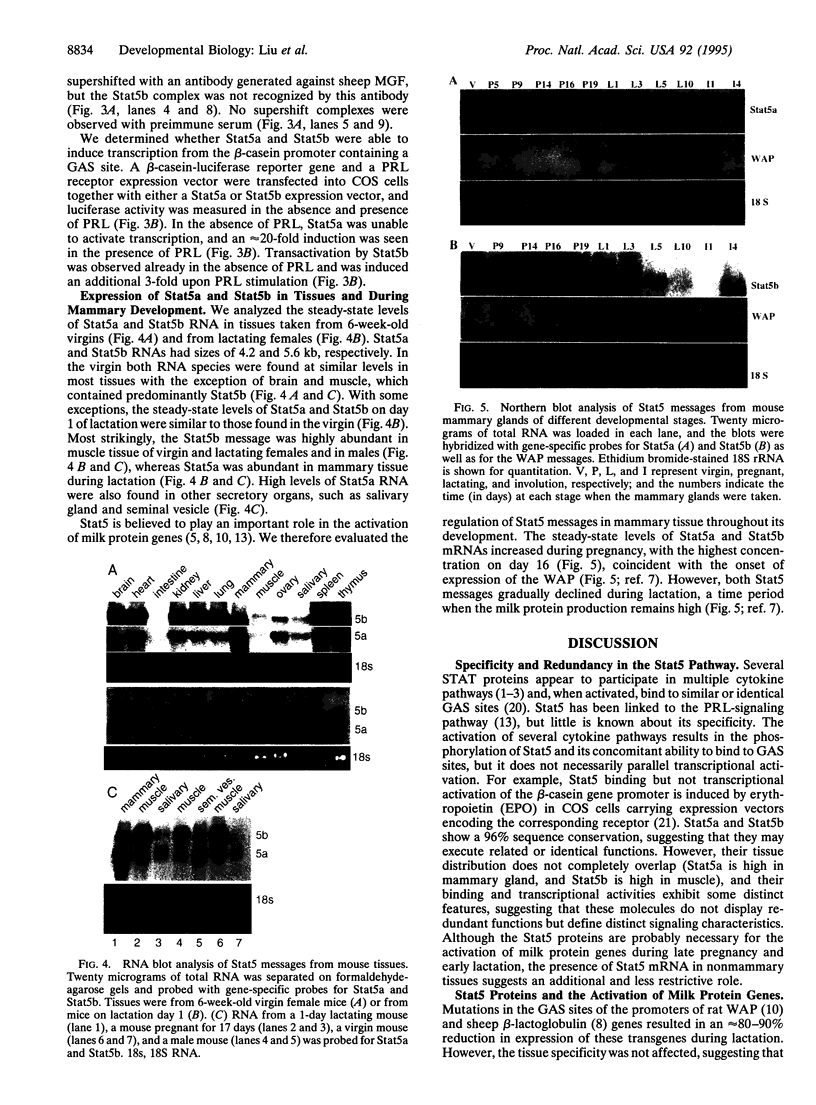
Prolactin (PRL) induces transcriptional activation of milk protein genes, such as the whey acidic protein (WAP), beta-casein, and beta-lactoglobulin genes, through a signaling cascade encompassing the Janus kinase Jak2 and the mammary gland factor (MGF; also called Stat5), which belongs to the family of proteins of signal transducers and activators of transcription (STAT). We isolated and sequenced from mouse mammary tissue Stat5 mRNA and a previously unreported member, which we named Stat5b (Stat5 is renamed to Stat5a). On the protein level Stat5a and Stat5b show a 96% sequence similarity. The 5' and 3' untranslated regions of the two mRNAs are not conserved. Stat5a comprises 793 amino acids and is encoded by a mRNA of 4.2 kb. The Stat5b mRNA has a size of 5.6 kb and encodes a protein of 786 amino acids. Both Stat5a and Stat5b recognized the GAS site (gamma-interferon-activating sequence; TTCNNNGAA) in vitro and mediated PRL-induced transcription in COS cells transfected with a PRL receptor. Stat5b also induced basal transcription in the absence of PRL. Similar levels of Stat5a and Stat5b mRNAs were found in most tissues of virgin and lactating mice, but a differential accumulation of the Stat5 mRNAs was found in muscle and mammary tissue. The two RNAs are present in mammary tissue of immature virgin mice, and their levels increase up to day 16 of pregnancy, followed by a decline during lactation. The increase of Stat5 expression during pregnancy coincides with the activation of the WAP gene.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Azam M., Erdjument-Bromage H., Kreider B. L., Xia M., Quelle F., Basu R., Saris C., Tempst P., Ihle J. N., Schindler C. Interleukin-3 signals through multiple isoforms of Stat5. EMBO J. 1995 Apr 3;14(7):1402–1411. doi: 10.1002/j.1460-2075.1995.tb07126.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdon T. G., Maitland K. A., Clark A. J., Wallace R., Watson C. J. Regulation of the sheep beta-lactoglobulin gene by lactogenic hormones is mediated by a transcription factor that binds an interferon-gamma activation site-related element. Mol Endocrinol. 1994 Nov;8(11):1528–1536. doi: 10.1210/mend.8.11.7877621. [DOI] [PubMed] [Google Scholar]
- Burdon T., Sankaran L., Wall R. J., Spencer M., Hennighausen L. Expression of a whey acidic protein transgene during mammary development. Evidence for different mechanisms of regulation during pregnancy and lactation. J Biol Chem. 1991 Apr 15;266(11):6909–6914. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Darnell J. E., Jr, Kerr I. M., Stark G. R. Jak-STAT pathways and transcriptional activation in response to IFNs and other extracellular signaling proteins. Science. 1994 Jun 3;264(5164):1415–1421. doi: 10.1126/science.8197455. [DOI] [PubMed] [Google Scholar]
- Gouilleux F., Pallard C., Dusanter-Fourt I., Wakao H., Haldosen L. A., Norstedt G., Levy D., Groner B. Prolactin, growth hormone, erythropoietin and granulocyte-macrophage colony stimulating factor induce MGF-Stat5 DNA binding activity. EMBO J. 1995 May 1;14(9):2005–2013. doi: 10.1002/j.1460-2075.1995.tb07192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouilleux F., Wakao H., Mundt M., Groner B. Prolactin induces phosphorylation of Tyr694 of Stat5 (MGF), a prerequisite for DNA binding and induction of transcription. EMBO J. 1994 Sep 15;13(18):4361–4369. doi: 10.1002/j.1460-2075.1994.tb06756.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groner B., Altiok S., Meier V. Hormonal regulation of transcription factor activity in mammary epithelial cells. Mol Cell Endocrinol. 1994 Apr;100(1-2):109–114. doi: 10.1016/0303-7207(94)90288-7. [DOI] [PubMed] [Google Scholar]
- Hou J., Schindler U., Henzel W. J., Ho T. C., Brasseur M., McKnight S. L. An interleukin-4-induced transcription factor: IL-4 Stat. Science. 1994 Sep 16;265(5179):1701–1706. doi: 10.1126/science.8085155. [DOI] [PubMed] [Google Scholar]
- Ihle J. N., Kerr I. M. Jaks and Stats in signaling by the cytokine receptor superfamily. Trends Genet. 1995 Feb;11(2):69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- Kordon E. C., McKnight R. A., Jhappan C., Hennighausen L., Merlino G., Smith G. H. Ectopic TGF beta 1 expression in the secretory mammary epithelium induces early senescence of the epithelial stem cell population. Dev Biol. 1995 Mar;168(1):47–61. doi: 10.1006/dbio.1995.1060. [DOI] [PubMed] [Google Scholar]
- Li S., Rosen J. M. Distal regulatory elements required for rat whey acidic protein gene expression in transgenic mice. J Biol Chem. 1994 May 13;269(19):14235–14243. [PubMed] [Google Scholar]
- Li S., Rosen J. M. Glucocorticoid regulation of rat whey acidic protein gene expression involves hormone-induced alterations of chromatin structure in the distal promoter region. Mol Endocrinol. 1994 Oct;8(10):1328–1335. doi: 10.1210/mend.8.10.7854350. [DOI] [PubMed] [Google Scholar]
- Li S., Rosen J. M. Nuclear factor I and mammary gland factor (STAT5) play a critical role in regulating rat whey acidic protein gene expression in transgenic mice. Mol Cell Biol. 1995 Apr;15(4):2063–2070. doi: 10.1128/mcb.15.4.2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKnight R. A., Spencer M., Dittmer J., Brady J. N., Wall R. J., Hennighausen L. An Ets site in the whey acidic protein gene promoter mediates transcriptional activation in the mammary gland of pregnant mice but is dispensable during lactation. Mol Endocrinol. 1995 Jun;9(6):717–724. doi: 10.1210/mend.9.6.8592517. [DOI] [PubMed] [Google Scholar]
- Mui A. L., Wakao H., O'Farrell A. M., Harada N., Miyajima A. Interleukin-3, granulocyte-macrophage colony stimulating factor and interleukin-5 transduce signals through two STAT5 homologs. EMBO J. 1995 Mar 15;14(6):1166–1175. doi: 10.1002/j.1460-2075.1995.tb07100.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittius C. W., Hennighausen L., Lee E., Westphal H., Nicols E., Vitale J., Gordon K. A milk protein gene promoter directs the expression of human tissue plasminogen activator cDNA to the mammary gland in transgenic mice. Proc Natl Acad Sci U S A. 1988 Aug;85(16):5874–5878. doi: 10.1073/pnas.85.16.5874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittius C. W., Sankaran L., Topper Y. J., Hennighausen L. Comparison of the regulation of the whey acidic protein gene with that of a hybrid gene containing the whey acidic protein gene promoter in transgenic mice. Mol Endocrinol. 1988 Nov;2(11):1027–1032. doi: 10.1210/mend-2-11-1027. [DOI] [PubMed] [Google Scholar]
- Shamay A., Pursel V. G., Wall R. J., Hennighausen L. Induction of lactogenesis in transgenic virgin pigs: evidence for gene and integration site-specific hormonal regulation. Mol Endocrinol. 1992 Feb;6(2):191–197. doi: 10.1210/mend.6.2.1569963. [DOI] [PubMed] [Google Scholar]
- Standke G. J., Meier V. S., Groner B. Mammary gland factor activated by prolactin on mammary epithelial cells and acute-phase response factor activated by interleukin-6 in liver cells share DNA binding and transactivation potential. Mol Endocrinol. 1994 Apr;8(4):469–477. doi: 10.1210/mend.8.4.7519723. [DOI] [PubMed] [Google Scholar]
- Taniguchi T. Cytokine signaling through nonreceptor protein tyrosine kinases. Science. 1995 Apr 14;268(5208):251–255. doi: 10.1126/science.7716517. [DOI] [PubMed] [Google Scholar]
- Topper Y. J., Freeman C. S. Multiple hormone interactions in the developmental biology of the mammary gland. Physiol Rev. 1980 Oct;60(4):1049–1106. doi: 10.1152/physrev.1980.60.4.1049. [DOI] [PubMed] [Google Scholar]
- Wakao H., Gouilleux F., Groner B. Mammary gland factor (MGF) is a novel member of the cytokine regulated transcription factor gene family and confers the prolactin response. EMBO J. 1994 May 1;13(9):2182–2191. doi: 10.1002/j.1460-2075.1994.tb06495.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y. C., Ciarletta A. B., Temple P. A., Chung M. P., Kovacic S., Witek-Giannotti J. S., Leary A. C., Kriz R., Donahue R. E., Wong G. G. Human IL-3 (multi-CSF): identification by expression cloning of a novel hematopoietic growth factor related to murine IL-3. Cell. 1986 Oct 10;47(1):3–10. doi: 10.1016/0092-8674(86)90360-0. [DOI] [PubMed] [Google Scholar]