Summary
Prior research has linked either basal cortisol levels or stress-induced cortisol responses to adiposity; however, it remains to be determined whether these distinct cortisol measures exert joint or independent effects. Further, it is unclear how they interact with individual and environmental characteristics to predict adiposity. The present study aims to address whether morning cortisol levels and cortisol responses to a psychosocial stressor independently and/or interactively influence body mass index (BMI) in 218 adolescents (117 female) participating in a longitudinal community study, and whether associations are moderated by sex and exposure to early maternal depression. Reports of maternal depressive symptoms were obtained in infancy and preschool. Salivary cortisol measures included a longitudinal morning cortisol measure comprising sampling points across ages 11, 13, 15, and 18 and measures of stress-induced cortisol responses assessed via the Trier Social Stress Test (TSST) at age 18. Lower morning cortisol and higher TSST cortisol reactivity independently predicted higher age 18 BMI. Morning cortisol also interacted with sex and exposure to early maternal depression to predict BMI. Specifically, girls exposed to lower levels of early maternal depression displayed a strong negative morning cortisol-BMI association, and girls exposed to higher levels of maternal depression demonstrated a weaker negative association. Among boys, those exposed to lower levels of maternal depression displayed no association, while those exposed to higher levels of maternal depression displayed a negative morning cortisol-BMI association. Results point to the independent, additive effects of morning and reactive cortisol in the prediction of BMI and suggest that exposure to early maternal depression may exert sexually dimorphic effects on normative cortisol-BMI associations.
Keywords: adiposity, adolescence, body mass index (BMI), early maternal depression, morning cortisol, obesity, reactive cortisol, sex differences
Introduction
Obesity is a global health concern with many negative short- and long-term consequences for well-being (Kraak and Story, 2010). Altered hypothalamic-pituitary-adrenal (HPA) axis activity, indexed by glucocorticoids such as cortisol, has been linked to higher adiposity through the interaction of the HPA axis with multiple neurophysiological systems, including activation of brain regions controlling food intake and hormones implicated in metabolism and appetite regulation (for a review, see Spencer and Tilbrook, 2011). Although basal cortisol levels and stress-induced cortisol responses have been separately linked to adiposity, research has yet to examine how these distinct cortisol measures work together. Furthermore, separate lines of research suggest that cortisol-adiposity associations may be influenced by the sex of the individual (Walker et al., 2000; Larsson et al., 2009) as well as prior stress exposure (Donoho et al., 2011). Understanding these complex associations may have important implications for improved identification of who is at risk of obesity as well as potential targets for intervention.
Studies of cortisol and adiposity have examined both diurnal measures and stress-induced responses of the HPA axis (Chrousos, 2000). Daily fluctuations in level of cortisol output typically follow a diurnal rhythm characterized by a surge upon awakening followed by a decline across the day. In response to a stressor, cortisol levels generally begin to increase upon encountering the stressor, peak approximately 10-30 minutes after the stressor’s onset (Foley and Kirschbaum, 2010), and then decline depending on the nature of the challenge and the individual’s resources. In general, studies of adiposity and HPA-axis functioning, both diurnal and stress-induced, have yielded mixed results (e.g., Wallerius et al., 2003; Brydon, 2011; Phillips et al., 2012). Nevertheless, many studies have linked greater adiposity to either lower morning levels of cortisol (Björntorp and Rosmond, 2000; Walker et al., 2000; Ranjit et al., 2005; Larsson et al., 2009; Kumari et al., 2010; Champaneri et al., 2013) or to higher cortisol responses to an experimental psychosocial stressor, with the latter indexed by a variety of measures including change from baseline to peak (Benson et al., 2009), total cortisol output (Epel et al., 2000), and cortisol output relative to baseline (Benson et al., 2009; Dockray et al., 2009). In our previous work examining associations of adolescent adiposity with diurnal cortisol, including morning, afternoon, and evening levels and decline over the day, we found that lower morning levels were most closely and consistently associated with higher BMI (both concurrently and longitudinally) at multiple time points across adolescence; moreover, a longitudinal measure of morning cortisol across ages 11, 13, and 15 was a stronger predictor of BMI than morning cortisol at any single age (Ruttle et al., 2013a). However, to our knowledge, no studies have considered both morning levels and stress-induced cortisol responses, leaving unexamined whether they jointly or independently predict adiposity.
The mixed findings regarding adiposity and HPA-axis functioning suggest that important individual characteristics or contextual factors may moderate those associations. In particular, there is some evidence of the moderating effects of an individual’s sex and history of stress exposure. Although the majority of cortisol-adiposity studies solely examine one sex (e.g., Björntorp and Rosmond, 2000; Epel et al., 2000; Benson et al., 2009; Brydon, 2011; Hillman et al., 2012), a few studies have considered sex differences, including two that found lower morning cortisol levels to be associated with higher adiposity in women but not men (Walker et al., 2000; Larsson et al., 2009). Further, one study of adolescent females found that a higher cortisol awakening response was more strongly associated with higher adiposity among those exposed to recent chronic school-related stress (Donoho et al., 2011).
Previous studies of the cortisol-adiposity association have not considered the potential moderating influence of early chronic stress exposure. Among the more common early life stressors, exposure to maternal depression may be particularly salient due to associated deficits in mother–child interactions and insecure attachments that can alter HPA-axis activity (Ashman et al., 2002; Brennan et al., 2008; Dougherty et al., 2011) and influence feeding behaviors (Ertel et al., 2010). We previously demonstrated that early exposure to higher maternal depressive symptoms had a particularly potent effect on later HPA-axis activity both in childhood and adolescence (Essex et al., 2002b; Essex et al., 2011; Ruttle et al., 2013b). There is also some evidence that exposure to early, chronic maternal depression is associated with increased likelihood of later overweight or obesity; however, research into adolescence is sparse and authors suggest examining moderating factors to clarify associations (Lampard et al., 2014).
The current study builds on prior research, including our own, first by investigating the independent and joint associations of morning cortisol levels and cortisol responses to a psychosocial stressor with adiposity (i.e., body mass index; BMI) in a longitudinal community sample of 18-year-olds, and second by considering whether these cortisol-BMI associations are moderated by the individual’s sex and exposure to maternal depression across the infancy and preschool periods. We hypothesized that lower morning cortisol levels would be associated with higher BMI. We also anticipated that greater cortisol responses to the psychosocial stress paradigm, the Trier Social Stress Test (TSST), would also be associated with higher BMI; however, due to inconsistencies in the measures used across previous studies, we took an exploratory approach by examining three indices of stress-induced cortisol responses. We also anticipated moderating effects of sex and exposure to early maternal depression on cortisol-BMI associations but, due to the sparse literature, did not hypothesize the direction of these effects.
Methods
Participants
Participants are the offspring of families recruited for the Wisconsin Study of Families and Work, a longitudinal study of child development. The original sample comprised 570 women and their partners recruited during pregnancy from obstetric/gynecology clinics and a low income clinic in two Midwestern cities (Hyde et al., 1995); 560 of the women had live births and were eligible to continue in the study. At age 18, a subset of 232 adolescents participated in an intensive assessment of acute stress reactivity; 218 (117 female) had complete data on all variables included in the current study. The majority of participants (90%) identified as Caucasian. At recruitment (1990 – 1991), mothers’ ages ranged from 20 to 41 years (M = 30) and fathers’ from 20 to 55 years (M = 32), 96% were married, and the majority were well-educated (mothers: 2% < high school degree, 40% high school graduates, 41% college graduates, and 17% had post-baccalaureate education; fathers’ education levels were similar). Annual family income at the initial assessment ranged from $10,000 to over $200,000 (Mdn = $48,000). The only significant difference between the 218 participating families and non-participants on demographic variables was that fathers in the participating sub-sample were slightly older: M = 32.0 (SD = 5.47) versus M = 30.9 (SD = 4.74), t (548) = −2.52, p < .05. There were no significant differences on any other demographic or moderating (i.e., sex, early maternal depression) variables. Parents gave informed consent at each time point; child assent was obtained beginning at age 11 years. All procedures were reviewed and approved by the University of Wisconsin Institutional Review Board and were in accordance with the Declaration of Helsinki.
Measures
Adolescent Body Mass Index (BMI)
At age 18, a trained research assistant used a level and tape measure to assess adolescent height. Height measurements were repeated until two measurements were obtained within ¼ inch of each other, and the two closest readings were averaged. Weight was measured with a Health o meter EVERWeigh Lithium Electronic Scale (Sunbeam Health Division, Bridgeview, Illinois). Weight measurements were repeated until two readings within ½ pound were obtained, and the two closest readings were averaged. Adolescent body mass index (BMI) was calculated by dividing body weight in kilograms by the square of height in meters (kg/m2) and assigned a percentile adjusted for age and sex using representative data from the U.S. (Ogden et al., 2002).
Salivary Cortisol
Morning cortisol levels were assessed when adolescents were ages 11, 13, 15, and 18 years old; stress-induced cortisol responses were assessed at age 18 years. Details are provided below. All samples were collected via passive drool without the use of stimulants. At each age, saliva samples were stored in a −80 °C freezer until assayed using well-established, salivary enzymeimmunoassay kits (Salimetrics, State College, PA). All samples were assayed in duplicate. Intra-assay and inter-assay coefficients of variation for the assays were 3.5% and 5.1% respectively for age 18 and averaged 3.8 and 7.4% for the earlier assessments. The lower limit of detection for all assays was .003 μg/dL. Raw cortisol scores were log-transformed and extreme values were Winsorized to normalize distributions.
Morning cortisol
For each assessment across adolescence (ages 11, 13, 15, and 18), saliva samples were collected at home shortly after waking for 3 consecutive mornings. At each of the assessments, adolescents were instructed to provide a saliva sample immediately upon waking, prior to eating food, drinking beverages, and brushing teeth; freeze samples immediately after collection; and record collection time and medication use on collection days. Completed samples were transported to the lab on ice by research staff. A hierarchical linear model (HLM; for more information, see Bryk and Raudenbush, 1992) was employed to aggregate samples over several days and across the four assessments, taking into consideration daily variability in sampling time. Longitudinal morning cortisol values were computed by fitting a two-level HLM to the data that included within-individual variations in the intercept and slope (Level 1) and random effects representing between-individual differences (Level 2), not distinguishing between assessments or days. A predicted cortisol value was extracted for each individual, reflecting predicted average logged morning cortisol level from age 11 to 18.
Reactive cortisol
Stress-induced cortisol responses were assessed in the summer following grade 12 (age 18) with a version of the TSST (Kirschbaum et al., 1993), a well-established and highly standardized psychosocial stress paradigm consisting of 5min of free speech and 5min of mental arithmetic performed before of a panel of scrutinizing judges. To allow standardized testing in different settings, all TSSTs were administered in a portable testing booth comprising collapsible walls, folding chairs, lighting equipment, video camera and microphone atop a 1.5 × 2.4 m rug. All 218 participants engaged in TSST assessments in the late afternoon, 170 in a laboratory setting and 48 in a home setting. Following a 60min acclimation period of questionnaires and neutral-content DVDs, all participants were escorted by a research assistant to a separate room which contained the TSST testing booth where two research assistants (one male and one female) enacting the role of TSST judges were seated. After the TSST, the judges exited and the research assistant re-entered the booth and remained with the adolescent during the 60min recovery period for completion of questionnaires and saliva collection. Serial samples of saliva were collected across the visit, including at baseline (following acclimation and immediately preceding the TSST), and 0, 10, 20, 30, 45, and 60min following completion of the TSST. To parallel measures employed in previous studies, three measures of stress-induced cortisol responses were derived from these individual samples: (1) cortisol reactivity (average peak sample minus baseline); (2) area under the curve with respect to ground (AUCg), i.e., the geometric area below the cortisol response curve and above 0, which represents total cortisol output; and (3) area under the curve with respect to increase (AUCi), i.e., the geometric area below the cortisol response curve and above the individual’s baseline level, which represents change over time relative to starting level (see Pruessner et al., 2003).
Early Maternal Depressive Symptoms
Maternal depression symptoms were measured in infancy (child ages 1, 4, and 12 months) and preschool (child ages 3.5 and 4.5 years) by the Center for Epidemiologic Studies—Depression scale (CES-D; Radloff, 1977), a 20-item measure assessing frequency of depressive symptomology on a 4-point response scale (ranging from 0 = “rarely or none of the time” to 3 = “most or all of the time”) with possible overall scores ranging from 0 to 60. Scores were averaged within each time period (i.e., infancy and preschool) and then averaged across the two periods. The internal consistency of the items within the scale was good to excellent across all assessments (αs > 0.85).
Control Variables
Three control variables previously shown to influence cortisol and/or obesity were considered: early pubertal development (Matchock et al., 2007; Ruttle et al., 2013a); adolescent depression (Goodman and Whitaker, 2002; Ruttle et al., 2011); and use of oral contraceptives (Bouma et al., 2009). The location where the TSST was conducted (i.e., laboratory versus home) was also included as a potential confound.
Early pubertal development
When youth were 11 years old, mothers and youth completed a self-administered Tanner stage measure based on descriptions and line drawings (Morris and Udry, 1980); mothers also completed the Pubertal Development Scale (PDS; Petersen et al., 1988). PDS ratings were converted to the Tanner metric and then averaged with mother Tanner ratings before averaging with child ratings (Shirtcliff et al., 2012).
Adolescent depression symptoms
Symptoms of depression were assessed using self-report during the initial acclimation period of the TSST visit via an adolescent version (Burk et al., 2011) of the MacArthur Health and Behavior Questionnaire (Boyce et al., 2002; Essex et al., 2002a). For each of 15 items, adolescents decided which of two statements was most like them (e.g., “I feel like crying most days/I don’t feel like crying most days”) and rated whether that statement was really like me, mostly like me, or sort of like me, resulting in items that were scored from 1 to 6 depending on which item-half was endorsed and to what degree. The depression scale is computed as a mean and had excellent internal consistency in this sample (α = .90).
Oral contraceptives
Use of oral contraceptives was dummy coded as −0.5 for absence and +0.5 for presence. There was no use of any other medication with known effects on HPA-axis functioning or adiposity (e.g., oral glucocorticoids).
TSST location
The location where the TSST was conducted in the standardized portable “booth” was dummy coded as −0.5 for laboratory and +0.5 for home.
Statistical Analysis
Descriptive statistics were examined for each cortisol sample across the TSST; these were used to confirm the expected overall pattern of response (i.e., rise from baseline followed by post-peak decline) and to identify the time point with the highest average cortisol value, which was then used in the computation of the cortisol reactivity measure. Descriptive statistics (mean, standard deviation) and partial correlations (controlling on early pubertal status, adolescent depression symptoms, use of oral contraceptives, and location of the TSST) among all of the major variables were also examined. Additional descriptive statistics were obtained to better understand the BMI distribution and preponderance of clinical levels of maternal depression. Major research questions were addressed via hierarchical multiple regression analysis, with each continuous predictor variable centered at its mean and dichotomous variables coded as −.5 and +.5. To address the question of the associations of morning cortisol levels and stress-induced cortisol responses with BMI, the two cortisol variables and their interaction, together with the control variables, were entered into the model in the first step. In addition, to adequately control on any influence of the location of the TSST (laboratory vs. home) on the cortisol-BMI associations, two-way multiplicative interaction terms of TSST location with each of the cortisol variables were included. Next, to investigate possible moderating effects of sex and exposure to early maternal depression, these two variables were added to the model in the second step, together with four two-way interactions of maternal depression or sex by each of the two cortisol variables, and the two three-way interactions of sex by maternal depression by each of the cortisol variables. To examine differences in associations between measures of stress-induced cortisol responses and BMI, separate regressions were conducted using those stress-induced cortisol-response measures that were significantly associated with BMI in partial correlations. For comparability with cross-sectional studies, secondary regression analyses were run that substituted morning cortisol from age 18 (the age when stress-induced TSST cortisol measures were also assessed) for the longitudinal morning cortisol measure.
Results
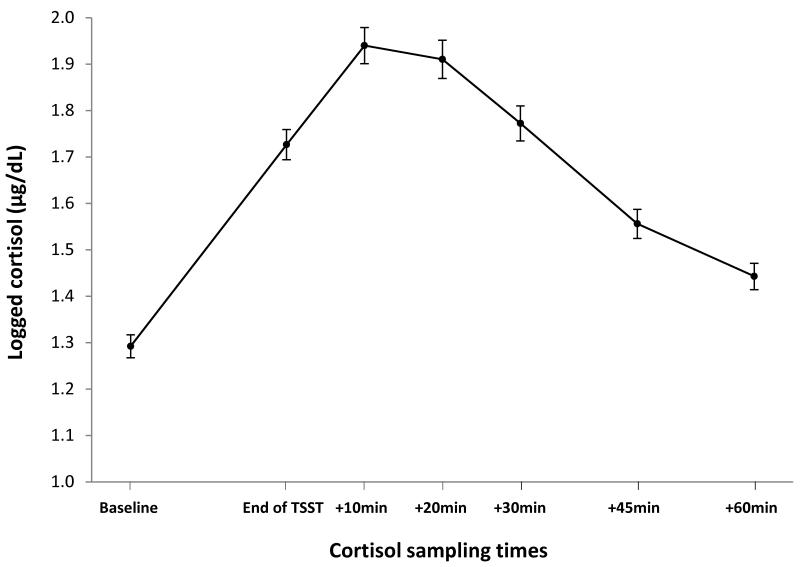
As shown in Figure 1, results indicate that the TSST induced an overall cortisol response in this sample of adolescents. The TSST cortisol reactivity score was computed as the cortisol score at 10min post-TSST (i.e., the overall peak) minus baseline.
Figure 1.
Mean (SE) log-transformed cortisol levels across the TSST paradigm.
Descriptive statistics and partial correlations of all major variables are presented in Table 1. Results reveal a negative association between BMI and adolescent morning cortisol and positive associations of BMI with TSST cortisol reactivity and AUCi, with cortisol reactivity demonstrating the strongest association with BMI. There was no association of BMI with TSST AUCg. To understand the clinical relevance of BMI and maternal depression scores, additional descriptive statistics were obtained. Examination of obesity categories revealed that 6.9% (n = 15) of adolescents were lower weight (<15th percentile), 69.7% (n = 152) were normal weight (15th to 84th percentile), 12.8% (n = 28) were overweight (85th to 94th percentile) and 10.6% (n = 23) were obese (≥ 95th percentile) according to age and gender norms. Using the established cut point of 16 on the CES-D (CES-D; Radloff, 1977), 17.4% (n = 38) of mothers met criteria for depression at least once during the infancy period, 17.4% (n = 38) met criteria at least once during the preschool period, and 9.2% (n = 20) met criteria at least once during both developmental periods.
Table 1. Descriptive Statistics and Partial Correlations among Outcome and Predictor Variables.
| Descriptive Statistics | Partial correlations | |||||||
|---|---|---|---|---|---|---|---|---|
| Variables | Mean | SD | Morning cortisol |
TSST cortisol reactivity |
TSST cortisol AUCg |
TSST cortisol AUCi |
Sex | Early maternal depression |
| 1. BMI (percentile) | 60.49 | 28.42 | −.16* | .21** | .02 | .17* | −.00 | .05 |
| 2. Morning cortisol | 1.22 | 0.02 | −.02 | .11 | −.03 | .19** | −.01 | |
| 3. TSST cortisol reactivity | 0.65 | 0.62 | .62*** | .96*** | .01 | −.04 | ||
| 4. TSST cortisol AUCg | 155.00 | 39.94 | .62*** | −.13* | .02 | |||
| 5. TSST cortisol AUCi | 33.55 | 40.88 | .05 | −.03 | ||||
| 6. Sex | 0.54 | 0.50 | −.07 | |||||
| 7. Early maternal depression | 6.83 | 5.41 | ||||||
p ≤ .05;
p ≤ .01;
p ≤ .001
Note: Correlations use centered predictors and partial out the effect of early pubertal development, age 18 depression symptoms, age 18 oral contraceptive usage, and TSST location. Sex was coded as −0.5 for males and +0.5 for females.
Associations between Morning and Stress-Induced Cortisol and BMI
Initial regression analyses focused on morning cortisol and TSST cortisol reactivity, as the latter was the stress-induced cortisol measure most strongly linked to BMI. Results revealed significant main effects of both morning cortisol and TSST cortisol reactivity with BMI (Table 2, Step 1). Specifically, as hypothesized, lower morning cortisol levels and higher TSST cortisol reactivity were both significantly associated with higher BMI. There was no significant interactive effect of morning cortisol × TSST cortisol reactivity.
Table 2. Prediction of Body Mass Index (Percentile) at Age 18.
| Step 1 | Step 2 | |||||
|---|---|---|---|---|---|---|
| Predictors | β | t | p | β | t | p |
| Cortisol Variables | ||||||
| Morning cortisol | −.24 | −2.94 | .004 | −.28 | −3.32 | .001 |
| TSST cortisol reactivity | .20 | 2.64 | .009 | .18 | 2.36 | .02 |
| Morning cortisol × TSST cortisol reactivity | −.09 | −1.39 | .17 | −.13 | −1.81 | .07 |
| Potential Moderating Variables | ||||||
| Sex | .05 | .62 | .54 | |||
| Early maternal depression | −.04 | −.53 | .59 | |||
| Sex × early maternal depression | .15 | 1.93 | .06 | |||
| Sex × morning cortisol | −.13 | −1.91 | .06 | |||
| Sex × TSST cortisol reactivity | −.00 | −.03 | .98 | |||
| Early maternal depression × morning cortisol | −.04 | −.44 | .66 | |||
| Early maternal depression × TSST cortisol reactivity | .00 | .04 | .96 | |||
| Sex × early maternal depression × morning cortisol | .18 | 2.34 | .02 | |||
| Sex × early maternal depression × TSST cortisol reactivity | −.06 | −.86 | .39 | |||
| R2 = .148 (p < .001) | R2 = .202 (p <.001) | |||||
Early pubertal development, age 18 depression symptoms, age 18 oral contraceptive usage, TSST location, and two two-way interactions between TSST location × Cortisol variables were included as control variables; the only control variable significantly associated with BMI was TSST Location (β = −.14; p = .04). Bold values indicate significance.
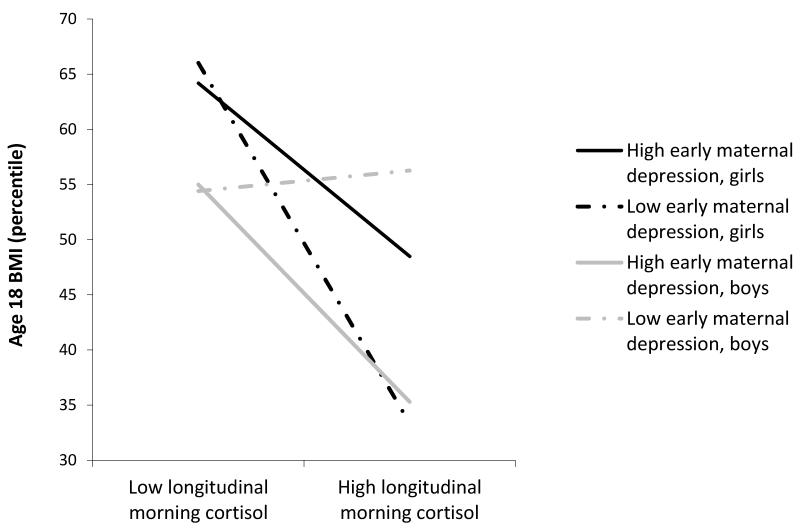
Next, when the potential moderators were considered, both the morning and reactive cortisol variables remained significant predictors of BMI and a significant three-way sex × maternal depression × morning cortisol interaction emerged (Table 2, Step 2). To probe this interaction, we ran the regression analyses separately for girls and boys. Results revealed that the interaction of maternal depression × longitudinal morning cortisol was marginally significant for both sexes but in opposite directions – that is, positive for girls (β = .17, t = 1.82, p = .07) and negative for boys (β = −.19, t = −1.63, p = .11). As shown in Figure 2, girls exposed to lower levels of maternal depression demonstrated a strong negative association between morning cortisol and BMI (i.e., lower morning cortisol was associated with higher BMI and higher morning cortisol was associated with lower BMI) and girls exposed to higher levels of maternal depression displayed a weaker negative association. Boys exposed to lower levels of maternal depression displayed no association between morning cortisol and BMI, whereas boys exposed to higher levels of maternal depression demonstrated a negative morning cortisol-BMI association, parallel to that of girls exposed to high levels of maternal depression.
Figure 2.
Three-way interaction of sex, early maternal depression, and longitudinal morning cortisol predicting age 18 BMI. Maternal depression and morning cortisol scores are plotted at 1SD above and below the mean.
When the regression model was rerun with TSST cortisol AUCi in place of cortisol reactivity, the main effect of TSST cortisol AUCi was weaker than for TSST cortisol reactivity (β = .14, t = 1.82, p = .07). Otherwise, as expected, the main effect of morning cortisol (β = −.29, t = −3.44, p = .001) and the three-way sex × maternal depression × morning cortisol interaction (β = .20, t = 2.57, p = .011) remained significant, and all other interactions remained non-significant.
As secondary analyses, both the cortisol reactivity and cortisol AUCi regression models were rerun substituting age 18 morning cortisol for the longitudinal morning cortisol measure. In both models, main effects of age 18 morning cortisol and corresponding three-way interactions were in the same direction as the effects identified for the longitudinal morning cortisol measure, but were weaker predictors of BMI (cortisol reactivity model main effect: β = −.24, t = −2.80, p = .006; three-way interaction: β = .15, t = 1.88, p = .06; cortisol AUCi model main effect: β = −.25, t = −2.84 p = .005; three-way interaction: β = .16, t = 2.03, p = .04).
Discussion
Prior studies have found associations between higher adiposity and either lower morning cortisol levels (Björntorp and Rosmond, 2000; Walker et al., 2000; Ranjit et al., 2005; Larsson et al., 2009; Kumari et al., 2010; Champaneri et al., 2013) or greater stress-induced cortisol responses (Epel et al., 2000; Benson et al., 2009; Dockray et al., 2009). The present study extends this work by examining—for the first time, to our knowledge—both morning cortisol levels and stress-induced cortisol responses in the same model. In a longitudinal community sample of 18 year olds, we found lower morning cortisol (an HLM-derived measure that averaged levels across ages 11, 13, 15, and 18) and greater TSST cortisol reactivity (i.e., 10min post-TSST minus baseline) to be associated with higher BMI, independent of one another. This finding suggests that the effects of these distinct, uncorrelated cortisol measures are additive and account for independent portions of variance in explaining BMI. Furthermore, when these cortisol variables were substituted with the age 18 morning (for longitudinal morning) or TSST AUCi (for TSST reactivity), similar but weaker associations with BMI were identified; these results suggest that more stable measures of basal HPA-axis functioning and measures reflecting the reactivity rather than recovery portion of the stress response may be most closely associated with BMI.
The present study also extends prior research by examining whether sex and/or maternal depressive symptoms moderated the observed cortisol-BMI associations. Results revealed no moderation of the association between stress-induced cortisol responses and BMI. However, moderation was observed for the association of morning cortisol with BMI: a significant three-way interaction was found for sex × maternal depression × morning cortisol, revealing that the anticipated negative association between longitudinal morning cortisol and BMI was not consistent for all participants. Specifically, girls exposed to lower levels of maternal depression displayed a strong negative association between morning cortisol and BMI, and this association was weaker among girls exposed to higher levels of early maternal depression. Conversely, boys exposed to lower levels of early maternal depression demonstrated no morning cortisol-BMI association, whereas boys exposed to higher levels of maternal depression displayed a negative association similar in magnitude to girls exposed to higher levels of maternal depression.
These findings of moderation suggest that exposure to maternal depression may alter the normative associations between cortisol and BMI in a sexually dimorphic manner. Despite some research on the links between various stressors, cortisol, and obesity (Pasquali, 2012), the effects of early life stress on cortisol-obesity associations and how such associations may differ for males and females remain largely unexamined. Two studies examining sex differences in cortisol-BMI associations found negative associations between morning cortisol and adiposity in females but not in males (Walker et al., 2000; Larsson et al., 2009), which parallels the gender-specific associations observed in the present study between morning cortisol and BMI among adolescents exposed to lower levels of maternal depressive symptoms. Moreover, one may anticipate sex differences in a variety of mental and physical health conditions associated with HPA axis functioning, given the extensive interplay between the systems governing adrenal and gonadal hormones (Pasquali, 2012). While this is the first study examining the influence of maternal depression on cortisol-BMI associations, previous research has shown inconsistent associations between maternal depression and adiposity (Lampard et al., 2014), suggesting that maternal depression may work in combination with other factors, such as stress physiology, to influence adiposity.
Although the present study was not designed to examine the mechanisms through which altered HPA axis activity influences obesity, nor the underlying mechanisms through which these associations may vary by sex or exposure to early life maternal depression, we and other researchers have speculated about some of these associations. Considerable preclinical and clinical research has identified several pathways through which increased reactive cortisol may influence obesity (Bjorntorp, 1996; Wajchenberg, 2000). Suggested mechanisms include impairment of the activity of growth hormone, pituitary gonadotropin, and thyroid stimulating hormone (Chrousos, 2000) as well as antagonizing the effects of insulin, breaking down lipids, and increasing blood glucose levels (Stulnig, 2004), all of which can contribute to weight gain and promote fat accumulation. Mechanisms underlying the association between low morning cortisol levels and increased adiposity are less clear but it has been suggested that lower basal cortisol levels may be reflective of HPA axis down-regulation due to earlier chronic exposure to high levels of endogenously released basal and/or stress responsive cortisol (Champaneri, 2013). It is also worth considering that lower morning levels and higher stress-induced cortisol responses may be independently associated with increased BMI because these two physiological patterns are linked to different subtypes of obesity (e.g., lower morning levels may be related to peripheral adiposity whereas increased cortisol reactivity may be related to visceral adiposity); however, this possibility has yet to be examined. Additionally, given that various genetic polymorphisms linked to obesity have also been found to be associated with increased or decreased cortisol sensitivity (see Nieuwenhuizen and Rutters, 2008), it may be that genetic factors play a role in the differential contribution of lower morning and higher reactive cortisol levels. Although the mechanisms through which sex and maternal depression influence cortisol-adiposity associations remains unclear, research has shown sexually dimorphic effects of early life stress (including maternal depression) in neuroendocrine functioning (Ruttle et al., 2013b), sex differences in associations between early life stress, cortisol, brain functioning, and mental health (Burghy et al., 2012), and sex differences in cortisol-obesity associations (Walker et al., 2000; Larsson et al., 2009).
Overall, the present study’s findings highlight the complex associations between HPA axis functioning, sex, early exposure to maternal depression, and BMI. Understanding the underlying processes and mechanisms will require additional human as well as preclinical studies. Long-term prospective studies beginning early in life with repeated measures of stress exposure, HPA axis functioning and adiposity are necessary in order to establish directionality between cortisol-BMI associations. This is important because increased cortisol has been implicated as both cause and consequence of weight gain in healthy adults, i.e., administration of corticotropin-releasing hormone increased cortisol levels and prompted increased food consumption, and intentional weight gain resulted in increased cortisol levels (for a review, see Foss and Dyrstad, 2011). Furthermore, additional human studies are warranted to identify the role of psychosocial mechanisms in certain cortisol-obesity associations. For example, given the cultural stigmatization of obesity and idealization of thin female bodies and lean, muscular male bodies (Halliwell and Diedrichs, 2012), obese adolescents –especially those who have internalized dominant appearance ideals (Bearman et al., 2006) – may be more likely to demonstrate strong stress responses under the intense evaluative scrutiny of the TSST. While human studies may be able to address some of the remaining unanswered questions, preclinical studies are needed to fully elucidate the underlying biological mechanisms through which cortisol influences obesity. As an initial step to better understanding the neurophysiological mechanisms underlying cortisol-obesity associations in preclinical populations, studies should seek to identify parallel associations between adiposity and basal, diurnal, and reactive cortisol in both humans and animals, as the methods traditionally employed to test cortisol-obesity associations in these populations typically differ (Dallman et al., 2006).
By examining multiple measures of HPA axis functioning in relation to BMI in a community-based, adolescent sample, the present study serves as a stepping stone for future research. Our sample size was substantially larger than many studies of adolescents and adults (Epel et al., 2000; e.g., Benson et al., 2009; Dockray et al., 2009; Brydon, 2011; exceptions include Hillman et al., 2012; Phillips et al., 2012). Moreover, we examined associations in both males and females, explored key individual and contextual factors as potential moderators, and included important control variables. However, this study is not without limitations. The relative racial and ethnic homogeneity limits the generalizability of our findings. Also, although BMI is a commonly employed method of measuring adiposity, the results may not necessarily extend to other measures (e.g., waist circumference, visceral/subcutaneous adipose tissue), which may better reflect various obesity phenotypes that are often influenced by different underlying processes and that vary by sex (Pasquali, 2012). Further, the current study does not allow us to infer directionality or temporal sequencing of the observed effects, thus long-term developmental studies examining both cortisol and adiposity over time are necessary. Additionally, exactly how both diurnal and reactive cortisol independently contribute to BMI remains unclear; further research is needed to identify the mechanisms that connect HPA-axis function and adiposity in typically developing human populations and improve our understanding of the pathogenesis of obesity. Together, such information will improve our ability to identify individuals at risk of becoming overweight, suggest new foci for prevention/intervention efforts, and ultimately contribute to the attenuation of the negative economic, emotional, and physical health impacts of obesity.
Acknowledgements
The authors wish to express their appreciation to the participating families, the staff of the Wisconsin Study of Families and Work, and Dr. Elizabeth Shirtcliff for providing consultation on the cortisol measures.
Funding
This research was supported by NIH grants R01-MH044340, P50-MH052354, P50-MH069315, P50-MH084051, R21-MH082705, and UL1-RR025011; the HealthEmotions Research Institute and the Robert Wood Johnson Foundation Health & Society Scholars Program, both at the University of Wisconsin–Madison; and the John D. and Catherine T. MacArthur Foundation Research Network on Psychopathology and Development. MJS was also supported by the Clinical and Translational Science Award (CTSA) program, through the NIH National Center for Advancing Translational Sciences (NCATS), grant UL1TR000427. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. Partial support for PLR was provided by the Canadian Institutes of Health Research Post-doctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest
The authors declare that they have no conflicts of interest.
Contributors
M.H. Klein, M.J. Slattery, N.H. Kalin, J.M. Armstrong, & M.J. Essex designed the study and wrote the protocol. P.L. Ruttle managed the literature searches and analyses. P.L. Ruttle & M.J. Essex undertook the statistical analysis, and P.L. Ruttle wrote the first draft of the manuscript. All authors contributed to and have approved the final manuscript.
References
- Ashman SB, Dawson G, Panagiotides H, Yamada E, Wilkinson CW. Stress hormone levels of children of depressed mothers. Dev. Psychopathol. 2002;14:333–349. doi: 10.1017/s0954579402002080. [DOI] [PubMed] [Google Scholar]
- Bearman SK, Presnell K, Martinez E, Stice E. The skinny on body dissatisfaction: a longitudinal study of adolescent girls and boys. J. Youth Adolesc. 2006;35:229–241. doi: 10.1007/s10964-005-9010-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benson S, Arck PC, Tan S, Mann K, Hahn S, Janssen OE, Schedlowski M, Elsenbruch S. Effects of obesity on neuroendocrine, cardiovascular, and immune cell responses to acute psychosocial stress in premenopausal women. Psychoneuroendocrinology. 2009;34:181–189. doi: 10.1016/j.psyneuen.2008.08.019. [DOI] [PubMed] [Google Scholar]
- Björntorp P, Rosmond R. Obesity and cortisol. Nutrition. 2000;16:924–936. doi: 10.1016/s0899-9007(00)00422-6. [DOI] [PubMed] [Google Scholar]
- Bouma EMC, Riese H, Ormel J, Verhulst FC, Oldehinkel AJ. Adolescents’ cortisol responses to awakening and social stress; effects of gender, menstrual phase and oral contraceptives. The TRAILS study. Psychoneuroendocrinology. 2009;34:884–893. doi: 10.1016/j.psyneuen.2009.01.003. [DOI] [PubMed] [Google Scholar]
- Boyce WT, Essex MJ, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. I: Exploring the “headwaters” of early life morbidities. J. Am. Acad. Child Adolesc. Psychiatry. 2002;41:580–587. doi: 10.1097/00004583-200205000-00016. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Pargas R, Walker EF, Green P, Newport DJ, Stowe ZN. Maternal depression and infant cortisol: influences of timing, comorbidity and treatment. J. Child Psychol. Psychiatry. 2008;49:1099–1107. doi: 10.1111/j.1469-7610.2008.01914.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brydon L. Adiposity, leptin and stress reactivity in humans. Biol. Psychol. 2011;86:114–120. doi: 10.1016/j.biopsycho.2010.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryk AS, Raudenbush SW. Hierarchical Linear Models: Applications and Data Analysis Methods, Advanced Qualitative Techniques in the Social Sciences. Sage Publications; Thousand Oaks, CA: 1992. [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, Fox ME, Hayes AS, Kalin NH, Essex MJ, Davidson RJ, Birn RM. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nat. Neurosci. 2012;15:1736–1741. doi: 10.1038/nn.3257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burk LR, Armstrong JM, Park J-H, Zahn-Waxler C, Klein MH, Essex MJ. Stability of early identified aggressive victim status in elementary school and associations with later mental health problems and functional impairments. J. Abnorm. Child Psychol. 2011;39:225–238. doi: 10.1007/s10802-010-9454-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Champaneri S, Xu X, Carnethon MR, Bertoni AG, Seeman T, DeSantis AS, Diez Roux A, Shrager S, Golden SH. Diurnal salivary cortisol is associated with body mass index and waist circumference: the Multiethnic Study of Atherosclerosis. Obesity. 2013;21:E56–63. doi: 10.1002/oby.20047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chrousos GP. The role of stress and the hypothalamic-pituitary-adrenal axis in the pathogenesis of the metabolic syndrome: neuro-endocrine and target tissue-related causes. Int. J. Obes. Relat. Metab. Disord. 2000;24(Suppl 2):S50–55. doi: 10.1038/sj.ijo.0801278. [DOI] [PubMed] [Google Scholar]
- Dallman MF, Pecoraro NC, La Fleur SE, Warne JP, Ginsberg AB, Akana SF, Laugero KC, Houshyar H, Strack AM, Bhatnagar S, Bell ME. Glucocorticoids, chronic stress, and obesity. Prog. Brain Res. 2006;153:75–105. doi: 10.1016/S0079-6123(06)53004-3. [DOI] [PubMed] [Google Scholar]
- Dockray S, Susman EJ, Dorn LD. Depression, cortisol reactivity, and obesity in childhood and adolescence. J. Adolesc. Health. 2009;45:344–350. doi: 10.1016/j.jadohealth.2009.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donoho CJ, Weigensberg MJ, Emken BA, Hsu JW, Spruijt-Metz D. Stress and abdominal fat: preliminary evidence of moderation by the cortisol awakening response in Hispanic peripubertal girls. Obesity. 2011;19:946–952. doi: 10.1038/oby.2010.287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dougherty LR, Klein DN, Rose S, Laptook RS. Hypothalamic-pituitary-adrenal axis reactivity in the preschool-age offspring of depressed parents: moderation by early parenting. Psychol. Sci. 2011;22:650–658. doi: 10.1177/0956797611404084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epel ES, McEwen B, Seeman T, Matthews K, Castellazzo G, Brownell KD, Bell J, Ickovics JR. Stress and body shape: stress-induced cortisol secretion is consistently greater among women with central fat. Psychosom. Med. 2000;62:623–632. doi: 10.1097/00006842-200009000-00005. [DOI] [PubMed] [Google Scholar]
- Ertel KA, Koenen KC, Rich-Edwards JW, Gillman MW. Maternal depressive symptoms not associated with reduced height in young children in a US prospective cohort study. PLoS ONE. 2010;5:e13656. doi: 10.1371/journal.pone.0013656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Essex MJ, Boyce WT, Goldstein LH, Armstrong JM, Kraemer HC, Kupfer DJ. The confluence of mental, physical, social, and academic difficulties in middle childhood. II: Developing the MacArthur Health and Behavior Questionnaire. J. Am. Acad. Child Adolesc. Psychiatry. 2002a;41:588–603. doi: 10.1097/00004583-200205000-00017. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Klein MH, Cho E, Kalin NH. Maternal stress beginning in infancy may sensitize children to later stress exposure: effects on cortisol and behavior. Biol. Psychiatry. 2002b;52:776–784. doi: 10.1016/s0006-3223(02)01553-6. [DOI] [PubMed] [Google Scholar]
- Essex MJ, Shirtcliff EA, Burk LR, Ruttle PL, Klein MH, Slattery MJ, Kalin NH, Armstrong JM. Influence of early life stress on later hypothalamic-pituitary- adrenal axis functioning and its covariation with mental health symptoms: a study of the allostatic process from childhood into adolescence. Dev. Psychopathol. 2011;23:1039–1058. doi: 10.1017/S0954579411000484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foley P, Kirschbaum C. Human hypothalamus-pituitary-adrenal axis responses to acute psychosocial stress in laboratory settings. Neurosci. Biobehav. Rev. 2010;35:91–96. doi: 10.1016/j.neubiorev.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Foss B, Dyrstad SM. Stress in obesity: cause or consequence? Med. Hypotheses. 2011;77:7–10. doi: 10.1016/j.mehy.2011.03.011. [DOI] [PubMed] [Google Scholar]
- Goodman E, Whitaker RC. A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics. 2002;110:497–504. doi: 10.1542/peds.110.3.497. [DOI] [PubMed] [Google Scholar]
- Halliwell E, Diedrichs PC. Influence of the media. In: Rumsey N, Harcourt D, editors. The Oxford handbook of the psychology of appearance. Oxford University Press; New York: 2012. pp. 217–238. [Google Scholar]
- Hillman JB, Dorn LD, Loucks TL, Berga SL. Obesity and the hypothalamic-pituitary-adrenal axis in adolescent girls. Metab. Clin. Exp. 2012;61:341–348. doi: 10.1016/j.metabol.2011.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyde JS, Klein MH, Essex MJ, Clark R. Maternity leave and women’s mental health. Psychol. Women Q. 1995;19:257–285. [Google Scholar]
- Kirschbaum C, Pirke KM, Hellhammer DH. The “Trier Social Stress Test”: a tool for investigating psychobiological stress responses in a laboratory setting. Neuropsychobiology. 1993;28:76–81. doi: 10.1159/000119004. [DOI] [PubMed] [Google Scholar]
- Kraak VI, Story M. A public health perspective on healthy lifestyles and public-private partnerships for global childhood obesity prevention. J. Am. Diet. Assoc. 2010;110:192–200. doi: 10.1016/j.jada.2009.10.036. [DOI] [PubMed] [Google Scholar]
- Kumari M, Chandola T, Brunner E, Kivimaki M. A nonlinear relationship of generalized and central obesity with diurnal cortisol secretion in the Whitehall II study. J. Clin. Endocrinol. Metab. 2010;95:4415–4423. doi: 10.1210/jc.2009-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lampard AM, Franckle RL, Davison KK. Maternal depression and childhood obesity: A systematic review. Prev. Med. 2014;59:60–67. doi: 10.1016/j.ypmed.2013.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson CA, Gullberg B, Råstam L, Lindblad U. Salivary cortisol differs with age and sex and shows inverse associations with WHR in Swedish women: a cross-sectional study. BMC Endocr. Disord. 2009;9:16. doi: 10.1186/1472-6823-9-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matchock RL, Dorn LD, Susman EJ. Diurnal and seasonal cortisol, testosterone, and DHEA rhythms in boys and girls during puberty. Chronobiol. Int. 2007;24:969–990. doi: 10.1080/07420520701649471. [DOI] [PubMed] [Google Scholar]
- Morris NM, Udry JR. Validation of a self-administered instrument to assess stage of adolescent development. J. Youth Adolesc. 1980;9:271–280. doi: 10.1007/BF02088471. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuizen AG, Rutters F. The hypothalamic-pituitary-adrenal-axis in the regulation of energy balance. Physiol. Behav. 2008;94:169–177. doi: 10.1016/j.physbeh.2007.12.011. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- Pasquali R. The hypothalamic-pituitary-adrenal axis and sex hormones in chronic stress and obesity: pathophysiological and clinical aspects. Ann. N. Y. Acad. Sci. 2012;1264:20–35. doi: 10.1111/j.1749-6632.2012.06569.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen AC, Crockett LJ, Richards M, Boxer A. A self-report measure of pubertal status: reliability, validity, and initial norms. J. Youth Adolesc. 1988;17:117–133. doi: 10.1007/BF01537962. [DOI] [PubMed] [Google Scholar]
- Phillips AC, Roseboom TJ, Carroll D, de Rooij SR. Cardiovascular and cortisol reactions to acute psychological stress and adiposity: cross-sectional and prospective associations in the Dutch Famine Birth Cohort Study. Psychosom. Med. 2012;74:699–710. doi: 10.1097/PSY.0b013e31825e3b91. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, Hellhammer DH. Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology. 2003;28:916–931. doi: 10.1016/s0306-4530(02)00108-7. [DOI] [PubMed] [Google Scholar]
- Radloff LS. The CES-D Scale: a self-report depression scale for research in the general population. Appl. Psychol. Meas. 1977;1:385–401. [Google Scholar]
- Ranjit N, Young EA, Raghunathan TE, Kaplan GA. Modeling cortisol rhythms in a population-based study. Psychoneuroendocrinology. 2005;30:615–624. doi: 10.1016/j.psyneuen.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Ruttle PL, Javaras KN, Klein MH, Armstrong JM, Burk LR, Essex MJ. Concurrent and longitudinal associations between diurnal cortisol and body mass index across adolescence. J. Adolesc. Health. 2013a;52:731–737. doi: 10.1016/j.jadohealth.2012.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Armstrong JM, Klein MH, Essex MJ. Neuroendocrine coupling across adolescence and the longitudinal influence of early life stress. Dev. Psychobiol. 2013b doi: 10.1002/dev.21138. Advance online publication. doi:10.1002/dev.21138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruttle PL, Shirtcliff EA, Serbin LA, Ben-Dat Fisher D, Stack DM, Schwartzman AE. Disentangling psychobiological mechanisms underlying internalizing and externalizing behaviors in youth: longitudinal and concurrent associations with cortisol. Horm. Behav. 2011;59:123–132. doi: 10.1016/j.yhbeh.2010.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirtcliff EA, Allison AL, Armstrong JM, Slattery MJ, Kalin NH, Essex MJ. Longitudinal stability and developmental properties of salivary cortisol levels and circadian rhythms from childhood to adolescence. Dev. Psychobiol. 2012;54:493–502. doi: 10.1002/dev.20607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spencer SJ, Tilbrook A. The glucocorticoid contribution to obesity. Stress. 2011;14:233–246. doi: 10.3109/10253890.2010.534831. [DOI] [PubMed] [Google Scholar]
- Walker BR, Soderberg S, Lindahl B, Olsson T. Independent effects of obesity and cortisol in predicting cardiovascular risk factors in men and women. J. Intern. Med. 2000;247:198–204. doi: 10.1046/j.1365-2796.2000.00609.x. [DOI] [PubMed] [Google Scholar]
- Wallerius S, Rosmond R, Ljung T, Holm G, Björntorp P. Rise in morning saliva cortisol is associated with abdominal obesity in men: a preliminary report. J. Endocrinol. Invest. 2003;26:616–619. doi: 10.1007/BF03347017. [DOI] [PubMed] [Google Scholar]