Abstract
Importance
Retinal ganglion cells are known to express estrogen receptors and prior studies have suggested an association between postmenopausal hormone (PMH) use and decreased intraocular pressure, suggesting that PMH use may decrease the risk for primary open-angle glaucoma (POAG).
Objective
To determine whether the use of three different classes of PMH affect the risk for POAG.
Design
Retrospective longitudinal cohort analysis.
Setting
Claims data from enrollees in a U.S. managed-care plan for ≥4 years in which enrollees had ≥2 visits to an eye-care provider during 2001–2009.
Participants
Women ≥50 years.
Exposure(s) for observational studies
The amount of PMH medications containing estrogen only (E), those containing estrogen + progesterone (E+P), and medications containing estrogen + androgen (E+A), as captured from outpatient pharmacy claims over a 4-year period.
Main Outcome Measures
Hazard ratios (HR) for developing incident POAG with 95% confidence intervals (CI).
Results
Of 152,163 eligible enrollees, 2925 (1.9%) developed POAG. After adjustment for confounding factors, each additional month of E was associated with a 0.4% reduced risk for POAG (HR=0.996, [CI, 0.993–0.999], p=0.02). The risk for POAG did not differ with each additional month of E+P (HR=0.994, [CI, 0.987–1.001], p=0.08) or E+A (HR=0.999 [CI, 0.988–1.011], p=0.89) use.
Conclusion
Use of PMH preparations containing estrogen may help reduce the risk for POAG. If prospective studies confirm the findings of this analysis, novel treatments for this sight-threatening condition may follow.
Evidence suggests that sex hormones may play a role in the development of glaucoma.1–7 Retinal ganglion cells (RGCs) express estrogen receptors,1 and in a rat model of retinal ischemia, oral estrogen administration had a protective effect on RGCs that was mediated by these receptors.2 Several clinic-based studies have shown that postmenopausal hormone (PMH) use, which usually consists of oral preparations containing estrogen, estrogen and progesterone, or estrogen along with an androgen, is associated with a modestly reduced intraocular pressure (IOP).3–7 These data suggest that sex hormones may influence the development of open-angle glaucoma (OAG) through the lowering of IOP or protecting RGCs. However, although some population-based studies have found a trend toward a decreased risk of OAG with PMH use, the relationship noted in those studies was not statistically significant.8,9 Only a secondary analysis from the Nurses’ Health Study (NHS) found that women using estrogen and progesterone had a significantly lower risk of high-tension OAG, compared with nonusers of PMH.10
Benefits of PMH use include improvements in menopause-related vaginal and vasomotor symptoms and a decreased risk of fractures secondary to osteoporosis. However in 2005, based largely on results from the Women’s Health Initiative (WHI),11,12 the U.S. Preventive Services Task Force (USPSTF) recommended against routine and extended PMH use due to safety concerns including increased risks for heart disease, breast cancer, venous thromboembolism, stroke, and dementia.13 Interestingly, a 10-year follow-up of the WHI Estrogen-Alone Trial noted complete reversal of the adverse associations between conjugated equine estrogens and systemic diseases among women with previous hysterectomy, while the protective effect against breast cancer persisted.14
Despite favorable results in the most recent WHI Estrogen-Alone Trial follow-up,14 PMH use has declined since the USPSTF recommendations.15 Further evidence of an association between PMH and POAG would add support to the targeting of declining estrogen levels in the treatment of POAG in women. Using a large, nationwide health care claims database containing detailed medical records for more than 150,000 women, aged older than 50 years, each in the plan ≥ 4 years, we sought to evaluate the potential association between PMH use and development of POAG. The size of the sample allows us to investigate whether the risk for POAG differs according to the type of hormonal therapy prescribed: estrogen only (E), estrogen plus progesterone (E+P), or estrogen plus androgens (E+A), relative to nonusers of PMH.
Methods
Data Source
The Clinformatics DataMart (formerly the i3 InVision Data Mart) database (Ingenix, Eden Prairie, MN) contains detailed records of all beneficiaries in a managed-care network with enrollees throughout the United States. The dataset we used comprises all enrollees with ≥1 International Classification of Diseases, Ninth Revision-Clinical Modification (ICD-9-CM), code for an eye-related diagnosis (360–379.9); ≥1 Current Procedural Terminology (CPT) code for any eye-related visits, or diagnostic or therapeutic procedures (65091–68899 or 92002–92499); or any other claim submitted by an ophthalmologist or optometrist from January 1, 2001, through December 31, 2009. We had information on all these enrollees’ medical claims for ocular and nonocular conditions; sociodemographic information, including age, sex, race, education level and household net worth; and outpatient pharmacy prescriptions that were filled. Beneficiaries in the medical plan were also enrolled in the pharmacy plan. This database was used previously to study patients with glaucoma.16–18
Because the data are de-identified, the University of Michigan determined that this study was exempt from requiring Institutional Review Board approval.
Participants and Sample Selection
Patients were included in the analysis if they met these criteria: female sex, aged ≥50 years, continuous enrollment in the medical plan for ≥4 years, and ≥2 visits to an eye-care provider (ophthalmologist or optometrist). eTable 1 lists the ICD-9 and CPT codes used in the analyses. Individuals with nonincident POAG (≥1 diagnoses during a 4-year “look-back” period) were excluded. Also excluded were those assigned ICD-9-CM codes for non-POAG forms of glaucoma anytime during the study period. We also excluded persons who had breast cancer, a hysterectomy, oophorectomy, polycystic ovarian syndrome, ≥1 prescription for a selective estrogen receptor modulator (Tamoxifen citrate, Evista, Nolvadex), aromatase inhibitor (Anastrozole, Arimidex, Aromasin, Fareston, Faslodex, Femara, Teslac), or progestational agent (Crinone, Prefest, Progesterone, Prometrium, Provera) (eTable 2). All of these conditions or medications constitute relative contraindications to PMH use.
Postmenopausal Hormone Use
Individuals were classified as receiving PMH if they had ≥1 outpatient pharmacy prescription for any of the following medication classes in oral or topical forms: E, E+P, or E+A (eTable 2). Specific medications in each class were identified by using therapeutic drug class codes and American Hospital Formulary Service (AHFS) drug codes. The database contains information on the number of days for which an enrollee was supplied a given medication, thereby enabling us to quantify the amount each beneficiary was prescribed during her time in the plan.
Dependent Variable
The dependent variable for this analysis was the development of POAG. These diagnoses were identified by ICD-9-CM code 365.11. Beneficiaries were identified as developing incident POAG if they received no diagnosis of POAG during the 4-year look-back period but received a diagnosis of POAG during their subsequent time in the plan. Persons with other forms of glaucoma were excluded, as were glaucoma suspects and persons with ocular hypertension who lacked codes for POAG. A recent study showed that billing codes are >90% accurate in identifying patients with POAG, as confirmed with chart review.19
Analyses
Statistical analyses were performed by using SAS software, version 9.3 (SAS Institute, Cary, NC). Participant characteristics were summarized for the sample by using means and standard deviations for continuous variables and frequencies and percentages for categorical variables.
Cox regression with delayed entry was used to estimate the hazard for POAG associated with PMH use. We used the first 4 years of beneficiaries’ enrollment in the plan as their look back period, after which the index date marked the beginning of the follow-up period. Individuals with ≥1 diagnosis of POAG during the look-back period were considered nonincident case-patients with POAG and were excluded from the analysis. To ensure that each beneficiary had the opportunity to receive a diagnosis of POAG in the look-back period, we required all included beneficiaries to have ≥1 visit to an eye-care provider during this time period. We also required a second visit to an eye-care provider during the follow-up period, to allow for an opportunity to receive a diagnosis of POAG during that time. Beneficiaries were followed in the model from the index date until they developed POAG or were censored. Censoring occurred at the last day in the plan. The key predictor variable in our models was PMH use, which was treated as a time-dependent covariate. The number of days each beneficiary was covered by a PMH prescription was totaled over a 4-year period, from the index date to POAG onset or censoring. The look-back period ensured that enrollees had a 4-year window available to measure prior PMH exposure. For the Cox regression, we used the best subset selection method to identify covariates to include in the models.20 We evaluated for model inclusion these covariates: age at index date, race, education level, household net worth, region of residence at medical plan enrollment, urban/rural residence; the ocular comorbidities of cataract, pseudophakia or aphakia, macular degeneration, proliferative and nonproliferative diabetic retinopathy, retinal vascular occlusion; the nonocular comorbidities of diabetes mellitus, systemic hypertension, dyslipidemia, obesity, systemic hypotension, sleep apnea, migraine headache, cardiovascular disease, cerebrovascular disease, osteoporosis, dementia, and depression; and Charlson comorbidity index score (an overall health measure).21 Thirteen predictors were identified as the best-fitting explanatory variables for the outcome, including age at index date, race, household net worth, residential region, osteoporosis, retinal vascular occlusion, obesity, depression, diabetes, myocardial infarction, cataract, proliferative diabetic retinopathy, and pseudophakia/aphakia. The final multivariable model included the 13 predictors, along with use of each PMH class. For all analyses, p<0.05 was considered statistically significant.
Results
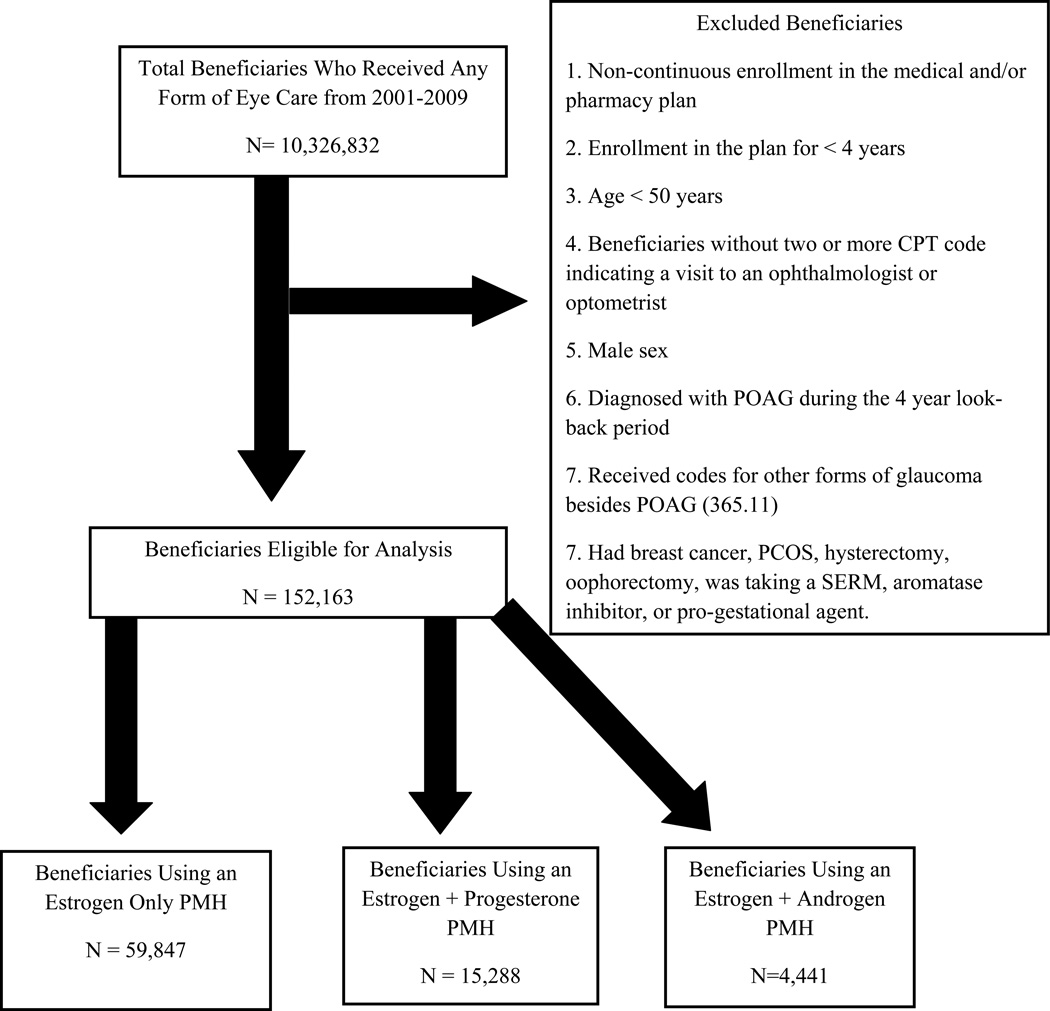
A total of 152,163 female enrollees older than 50 years met the study inclusion criteria (Figure 1). These beneficiaries were enrolled in the plan for a mean ± standard deviation (SD) of 6.4±1.5 years. Their mean age (±SD) was 65.1±8.9 years, and their racial distribution included 123,045 whites (80.9%), 6170 blacks (4.1%), 5763 Latinos (3.8%), 2502 Asian-Americans (1.6%), and 998 individuals of other races (0.7%) (Table 1). The remaining 13,685 enrollees (9.0%) were missing information on race/ethnicity. Nearly half the enrollees who met eligibility criteria (n=70,292, 46.2%) had received a prescription for ≥1 PMH medication of any class during their time in the plan. Among enrollees with PMH prescriptions, 59,847 (39.3%) had ≥1 prescription for E, 15,288 (10.1 %) for E+P, and 4,441 (2.9%) for E+A during their time in the plan. Those beneficiaries prescribed E medications had prescriptions filled for a mean of 753±719 days. Beneficiaries prescribed E+P medications had prescriptions filled for a mean of 670±594 days. Enrollees prescribed E+A medications had prescriptions filled for an average of 708±679 days. Over the course of the study, the median number of visits to eye-care providers for women who used PMH and for those who did not use PMH was 5.0.
Figure 1.
Selection of Beneficiaries for Analysis.
POAG = primary open-angle glaucoma; PMH = post-menopausal hormone replacement therapy; PCOS = Polycystic ovarian syndrome; SERM = serum estrogen receptor modulator; CPT = Current Procedural Terminology
Table 1.
Demographic Characteristics
| Women without POAG | Women with POAG | |
|---|---|---|
| Study population, n (%) | 149,238 (98.1%) | 2,925 (1.9%) |
| Mean Age ± SD | 65.1 ± 8.9 | 66.3 ± 9.2 |
| PMH use n (%) | ||
| Any PMH | 69,046 (46.3%) | 1,246 (42.6%) |
| Estrogen only | 58,820 (39.4%) | 1,027 (35.1%) |
| Estrogen + Progesterone | 14,992 (10.1%) | 296 (10.1%) |
| Estrogen + Testosterone | 4,379 (2.9%) | 62 (2.1%) |
| Race, n (%) | ||
| White | 120,806 (89.0%) | 2,239 (83.5%) |
| Black | 5,967 (4.4%) | 203 (7.6%) |
| Latino | 5,603 (4.1%) | 160 (6.0%) |
| Asian-American | 2,448 (1.8%) | 54 (2.0%) |
| Other | 971 (0.7%) | 27 (1.0%) |
| Education, n (%) | ||
| < High School | 1,414 (1.0%) | 39 (1.4%) |
| High School Diploma | 46,349 (32.1%) | 934 (32.9%) |
| Some College | 58,384 (40.4%) | 1,162 (40.9%) |
| College Graduate | 37,961 (26.3%) | 699 (24.6%) |
| Advanced degree | 418 (0.3%) | 5 (0.2%) |
| Household net worth, n (%) | ||
| <$25K | 7,203 (5.1%) | 169 (6.2%) |
| $25K–$75K | 6,506 (4.6%) | 136 (5.0%) |
| $75K–$150K | 13,954 (10.0%) | 313 (11.4%) |
| $150K–$500 K | 64,261 (45.8%) | 1,230 (44.8%) |
| >$500K | 48,248 (34.4%) | 898 (32.7%) |
POAG, Primary Open-angle glaucoma; PMH, post-menopausal hormone replacement therapy; SD = standard deviation
Primary Open Angle Glaucoma Development
Over all, 2925 individuals (1.9%) had an incident POAG diagnosis during their time in the plan. The mean (±SD) age of those with incident POAG was 66.3±9.2 years, compared with 65.1±8.9 years for those not developing POAG (Table 1). Relative to whites, blacks (adjusted HR, 1.72 [1.48–2.01], p<0.0001) and Latinos (adjusted HR, 1.41 [1.19–1.67], p<0.0001) had an increased hazard for POAG. Relative to beneficiaries with a household net worth <$25,000, beneficiaries with a household net worth of $150–500,000 or >$500,000 had a decreased hazard for POAG (adjusted HRs, 0.83 [0.70–0.98], p=0.03, and 0.79 [0.66–0.94], p=0.007, respectively) (Table 2).
Table 2.
Multivariable Analysisa: Risk Factors for Primary Open-Angle Glaucoma
| Risk Factor | Adjusted Hazard Ratio [95% Confidence Interval] |
P-Value |
|---|---|---|
| Raceb | ||
| Black | 1.72 [1.48–2.01] | <0.0001 |
| Latino | 1.41 [1.19–1.67] | <0.0001 |
| Asian-American | 1.17 [0.89–1.55] | 0.26 |
| Other | 1.41 [0.95–2.12] | 0.09 |
| Household Net Worthc | ||
| $25–75,000 | 0.88 [0.70–1.11] | 0.28 |
| $75–150,000 | 0.92 [0.76–1.12] | 0.42 |
| $150–500,000 | 0.83 [0.70–0.98] | 0.03 |
| >$500,000 | 0.79 [0.66–0.94] | 0.007 |
| PMH Used | ||
| Estrogen Only | 0.996 [0.993–0.999] | 0.02 |
| Estrogen + Progesterone | 0.994 [0.987–1.001] | 0.08 |
| Estrogen + Androgen | 0.999 [0.988–1.011] | 0.89 |
Multivariable regression analysis controlled for the following variables through a best subset selection process: age at index date, race, household net worth, region of residence, osteoporosis, retinal vascular occlusion, obesity, depression, diabetes mellitus, myocardial infarction, cataract, proliferative diabetic retinopathy, pseudophakia/aphakia, and the use of each class of PMH.
Compared to whites
Compared to <$25,000
1 month of PMH consumption
HR, Hazard Ratio; CI, Confidence Interval; POAG, Primary Open-Angle Glaucoma; PMH, post-menopausal hormone use
The percentages of women taking E, E+P, and E+A who developed POAG were 1.7%,1.9%, and 1.4%, respectively. By comparison, 2.1% of those using no PMH developed POAG. After adjustment for age alone, women using E or E+P had a slightly decreased hazard for POAG. Each additional month of use reduced the risk by 0.6% for E alone (adjusted HR, 0.994, [0.991–0.997] p=0.0003) and by 1.2% for E+P (adjusted HR, 0.988, [0.982–0.995], p=0.0007), relative to PMH nonusers. E+A use had no association with POAG (adjusted HR, 0.994, [CI, 0.983–1.006], p=0.33).
In the multivariable analysis, after adjustment for age plus other sociodemographic factors and ocular and systemic comorbidities, the association between E use and POAG was statistically significant. Every additional month of E use reduced the hazard for POAG by 0.4% (adjusted HR, 0.996, [0.993–0.999], p=0.02). We found no association between E+P (adjusted HR, 0.994, [CI, 0.987–1.001], p=0.08) or E+A (adjusted HR, 0.999, [CI, 0.988–1.011], p=0.89) use and POAG (Table 2).
Discussion
In our analysis of more than 150,000 U.S. women, a lower proportion of PMH users than nonusers developed POAG. After adjustment for age and other possible confounding factors, women prescribed E had a 0.4%-per-month reduction in POAG risk and those prescribed E+P had a 0.6%-per-month reduction.
Several other studies have assessed the relationship between PMH use and risk for glaucoma. The Rotterdam Study8 and the Blue Mountain Eye Study (BMES)9 each showed up to a 50% reduced risk of OAG among users of PMH; neither of these study findings was found to be statistically significant, which may have been due to their relatively small sample sizes of those experiencing the outcome. A secondary analysis of data from the NHS10 found that the use of medications containing E+P was associated with a reduction in POAG risk in a subset of women with elevated IOP, a finding that was statistically significant. One population-based study that did not demonstrate an association between PMH use and risk of POAG was the Los Angeles Latino Eye Study (LALES).22 Direct comparison of these various studies with one another and with ours can be challenging as the sample populations differ with respect to sociodemographic characteristics, such as age and race/ethnicity, both of which are key factors which themselves are known to affect the risk of developing POAG. Although in our study only PMH containing E alone significantly reduced the risk for POAG, other population-based studies did not collect data on the type of PMH used, as they simply asked women whether they had used PMH previously. Differences in study design and ways of identifying OAG development may also explain why some, but not all, of these studies found a reduced risk for glaucoma among PMH users. Collectively, the findings of our analyses, when viewed along with some others, offer evidence that sex hormones may indeed affect the likelihood of developing glaucoma in a subset of women.
Longer exposure to estrogen has been postulated to protect the optic nerve from OAG in women compared with men until menopause, when endogenous estrogen exposure becomes similar between the sexes. Women, on average, live longer than men and thus have a longer opportunity to experience glaucoma-related visual impairment after estrogen protection of the optic nerve ceases. The Rotterdam study23 found that early menopause was associated with an increased risk for OAG, and Pasquale and colleagues10 showed that among NHS participants older than 65 years, the incidence of OAG was decreased with older age at menopause. Unfortunately, no consistent billing code exists for menopause status, thus we could not assess the potential effect of this variable on OAG risk; conceivably, the inability to account for menopause status may have affected the results.
We also explored the possibility of a dose-response effect and found that each additional month of E or E+P use further reduced the risk for POAG. Assuming a linear relationship between PMH exposure and POAG, we found that use of E or E+P continuously for 4 years yielded a hazard reduction of 18% and 26%, respectively (data not shown). In the NHS—which captured participants’ medication use for as long as 20 years and, to our knowledge, is the only study that quantified PMH use for longer than our study—women’s risk for POAG was reduced by 42% with use of E+P, although more than 5 years of use was not associated with an additional protective effect.10
Others have demonstrated that the impact of PMH use on glaucoma risk may be genotype specific or may depend on gene-environment interactions. For example, Kang and colleagues24 evaluated the relationship between certain nitric oxide synthase (NOS3) single-nucleotide-polymorphisms, sex, PMH use, and POAG. They found that, over all, the different NOS3 polymorphisms were not associated with POAG. However, for women with certain NOS3 genotypes, PMH therapy reduced the risk of the high-tension variant of POAG.
While the inverse association between E or E+P use and POAG risk could be due to chance, the mounting biological data linking declining sex hormones to POAG—including estrogen protection of RGCs from death in an animal model of retinal ischemia2 and clinic-based studies in which PMH use is associated with IOP reduction—suggest the association is valid, although further confirmation is needed. Evidence that estrogen protects against other neuronal diseases in animal models and humans is compelling. For example, when estrogen therapy has been initiated close to menopause onset, denying neurons a critical period without estrogen, women’s risks for Alzheimer disease, Parkinson disease, and cognitive decline decrease.25–28 Our subsample of E users (n=59,847) was considerably larger than the E+P (n=15,288) and E+A (n=4,441) groups, and this may partially explain our finding of statistical significance for the E−alone users only, not the other PMH-using groups. (The results regarding E+P users approached statistical significance.)
Our study has several limitations. First, we relied on claims data—a source that lacks information on clinical parameters (e.g., IOP, extent of visual field loss, central corneal thickness, age at menopause, duration of endogenous estrogen exposure, and parity). By using billing data instead of clinical data, we may have included some patients who were erroneously assigned POAG-related codes or excluded others whose true diagnosis of POAG was not coded. However, such coding errors would be problematic only if a differential misclassification of glaucoma status existed between PMH users and nonusers, which we consider to be unlikely. Second, because all patients had health insurance, our findings may be nongeneralizable to uninsured persons, who tend to disproportionately comprise racial minorities and socioeconomically disadvantaged persons. Third, we did not consider patients’ possible lack of adherence to prescribed PMH therapies. However, if many recipients of PMH prescriptions were nonadherent, the results would be biased toward the null hypothesis. If all enrollees prescribed PMH were fully adherent, it is likely the risk of POAG would be reduced even more than what we report. Finally, PMH users may be more health conscious than other enrollees are and more prone to seek eye care—although this would bias the results toward an increased risk for POAG diagnosis with PMH use, which is not what we found.
To our knowledge, this is the largest study to assess a potential relationship between PMH use and glaucoma. Claims data allowed us to more accurately capture PMH exposure, by using pharmacy records rather than relying on patients’ self-report. Our study also benefits from a patient sample that is more diverse than those of population-based studies limited to particular geographic regions and the available supply of willing participants. In addition, our numbers were ample to explore the relationship between several classes of PMH, and to account for several key potential confounding variables.
Over all, these findings, along with results of other population-based studies, suggest that PMH use may generally affect women’s risk for glaucoma. More research is needed to better understand the complex relationship between PMH use and glaucoma. Additional work should also further explore whether the risk for POAG is affected only by E alone, or by any PMH class. Ongoing studies exploring how estrogen may affect apoptosis of the RGC layer may ultimately lead to the identification of novel mechanisms by which sex hormones can affect POAG or novel, non-oral routes of PMH administration that may further decrease the risk for POAG.
Acknowledgments
Grant support: National Eye Institute K23 Mentored Clinician Scientist Award (JDS: 1K23EY019511-01); American Glaucoma Society Clinician Scientist Grant (JDS), Blue Cross Blue Shield of Michigan Foundation (JDS and PANC), Research to Prevent Blindness (DCM and JDS); Heed Foundation Fellowship (PANC); National Eye Institute Core Grant EY00703; National Eye Institute RO1 EY015473 (LRP); Harvard Glaucoma Center of Excellence, the Margolis Fund (LRP); and the Arthur Ashley Foundation (LRP).
The funding organizations had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Joshua D. Stein, MD, MS, had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Presented in part at American Glaucoma Society Annual Meeting, New York, NY, March 3, 2012, and American Academy of Ophthalmology Annual Meeting, Chicago, IL, November 12, 2012.
eTable 1 and eTable 2 are available for online viewing only.
References
- 1.Munaut C, Lambert V, Noel A, et al. Presence of oestrogen receptor type beta in human retina. Br J Ophthalmol. 2001 Jul;85(7):877–882. doi: 10.1136/bjo.85.7.877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Russo R, Cavaliere F, Watanabe C, et al. 17Beta-estradiol prevents retinal ganglion cell loss induced by acute rise of intraocular pressure in rat. Prog Brain Res. 2008;173:583–590. doi: 10.1016/S0079-6123(08)01144-8. [DOI] [PubMed] [Google Scholar]
- 3.Uncu G, Avci R, Uncu Y, Kaymaz C, Develioglu O. The effects of different hormone replacement therapy regimens on tear function, intraocular pressure and lens opacity. Gynecol Endocrinol. 2006 Sep;22(9):501–505. doi: 10.1080/09513590600917919. [DOI] [PubMed] [Google Scholar]
- 4.Sator MO, Joura EA, Frigo P, et al. Hormone replacement therapy and intraocular pressure. Maturitas. 1997 Sep;28(1):55–58. doi: 10.1016/s0378-5122(97)00060-1. [DOI] [PubMed] [Google Scholar]
- 5.Affinito P, Di Spiezio Sardo A, Di Carlo C, et al. Effects of hormone replacement therapy on ocular function in postmenopause. Menopause. 2003 Sep-Oct;10(5):482–487. doi: 10.1097/01.GME.0000063568.84134.35. [DOI] [PubMed] [Google Scholar]
- 6.Altintas O, Caglar Y, Yuksel N, Demirci A, Karabas L. The effects of menopause and hormone replacement therapy on quality and quantity of tear, intraocular pressure and ocular blood flow. Ophthalmologica. 2004 Mar-Apr;218(2):120–129. doi: 10.1159/000076148. [DOI] [PubMed] [Google Scholar]
- 7.Tint NL, Alexander P, Tint KM, Vasileiadis GT, Yeung AM, Azuara-Blanco A. Hormone therapy and intraocular pressure in nonglaucomatous eyes. Menopause. 2010 Jan-Feb;17(1):157–160. doi: 10.1097/gme.0b013e3181b82fb4. [DOI] [PubMed] [Google Scholar]
- 8.Hulsman CA, Westendorp IC, Ramrattan RS, et al. Is open-angle glaucoma associated with early menopause? The Rotterdam Study. Am J Epidemiol. 2001 Jul 15;154(2):138–144. doi: 10.1093/aje/154.2.138. [DOI] [PubMed] [Google Scholar]
- 9.Lee AJ, Mitchell P, Rochtchina E, Healey PR. Female reproductive factors and open angle glaucoma: the Blue Mountains Eye Study. Br J Ophthalmol. 2003 Nov;87(11):1324–1328. doi: 10.1136/bjo.87.11.1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pasquale LR, Rosner BA, Hankinson SE, Kang JH. Attributes of female reproductive aging and their relation to primary open-angle glaucoma: a prospective study. J Glaucoma. 2007 Oct-Nov;16(7):598–605. doi: 10.1097/IJG.0b013e318064c82d. [DOI] [PubMed] [Google Scholar]
- 11.Rossouw JE, Anderson GL, Prentice RL, et al. Risks and benefits of estrogen plus progestin in healthy postmenopausal women: principal results From the Women's Health Initiative randomized controlled trial. JAMA. 2002 Jul 17;288(3):321–333. doi: 10.1001/jama.288.3.321. [DOI] [PubMed] [Google Scholar]
- 12.Anderson GL, Limacher M, Assaf AR, et al. Effects of conjugated equine estrogen in postmenopausal women with hysterectomy: the Women's Health Initiative randomized controlled trial. JAMA. 2004 Apr 14;291(14):1701–1712. doi: 10.1001/jama.291.14.1701. [DOI] [PubMed] [Google Scholar]
- 13.Calonge N. Hormone replacement therapy for the prevention of chronic conditions in post345 menopausal women recommendation statement. [Accessed Accessed August 8, 2012]; http://www.uspreventiveservicestaskforce.org/uspstf/uspspmho.htm. [Google Scholar]
- 14.LaCroix AZ, Chlebowski RT, Manson JE, et al. Health outcomes after stopping conjugated equine estrogens among postmenopausal women with prior hysterectomy: a randomized controlled trial. JAMA. 2011 Apr 6;305(13):1305–1314. doi: 10.1001/jama.2011.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kumle M. Declining breast cancer incidence and decreased HRT use. Lancet. 2008 Aug 23;372(9639):608–610. doi: 10.1016/S0140-6736(08)61255-6. [DOI] [PubMed] [Google Scholar]
- 16.Stein JD, Kim DS, Mundy KM, et al. The association between glaucomatous and other causes of optic neuropathy and sleep apnea. Am J Ophthalmol. 2011 Dec;152(6):989–998. e983. doi: 10.1016/j.ajo.2011.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Newman-Casey PA, Talwar N, Nan B, Musch DC, Stein JD. The relationship between components of metabolic syndrome and open-angle glaucoma. Ophthalmology. 2011 Jul;118(7):1318–1326. doi: 10.1016/j.ophtha.2010.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stein JD, Kim DS, Niziol LM, et al. Differences in rates of glaucoma among Asian Americans and other racial groups, and among various Asian ethnic groups. Ophthalmology. 2011 Jun;118(6):1031–1037. doi: 10.1016/j.ophtha.2010.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muir KW, Gupta CK, Gill P, Stein JD. Accuracy of International Classification of Disease (ICD-9-CM) billing codes for ophthalmologic conditions. Arch Ophthalmol. doi: 10.1001/jamaophthalmol.2013.577. [in press]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hosmer DW, Jovanovic B, Lemeshow S. Best subsets logistic regression. Biometrics. 1989;45(4):1265–1270. [Google Scholar]
- 21.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 22.Doshi V, Ying-Lai M, Azen SP, Varma R. Sociodemographic, family history, and lifestyle risk factors for open-angle glaucoma and ocular hypertension. The Los Angeles Latino Eye Study. Ophthalmology. 2008 Apr;115(4):639–647 e632. doi: 10.1016/j.ophtha.2007.05.032. [DOI] [PubMed] [Google Scholar]
- 23.Dielemans I, Vingerling JR, Wolfs RC, Hofman A, Grobbee DE, de Jong PT. The prevalence of primary open-angle glaucoma in a population-based study in The Netherlands. The Rotterdam Study. Ophthalmology. 1994 Nov;101(11):1851–1855. doi: 10.1016/s0161-6420(94)31090-6. [DOI] [PubMed] [Google Scholar]
- 24.Kang JH, Wiggs JL, Rosner BA, et al. Endothelial nitric oxide synthase gene variants and primary open-angle glaucoma: interactions with sex and postmenopausal hormone use. Invest Ophthalmol Vis Sci. 2010 Feb;51(2):971–979. doi: 10.1167/iovs.09-4266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Scott E, Zhang QG, Wang R, Vadlamudi R, Brann D. Estrogen neuroprotection and the critical period hypothesis. Front Neuroendocrinol. 2012 Jan;33(1):85–104. doi: 10.1016/j.yfrne.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocca WA, Grossardt BR, Shuster LT. Oophorectomy, menopause, estrogen treatment, and cognitive aging: clinical evidence for a window of opportunity. Brain Res. 2011 Mar 16;1379:188–198. doi: 10.1016/j.brainres.2010.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer's disease. Front Neuroendocrinol. 2009 Jul;30(2):239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.MacLennan AH, Henderson VW, Paine BJ, et al. Hormone therapy, timing of initiation, and cognition in women aged older than 60 years: the REMEMBER pilot study. Menopause. 2006 Jan-Feb;13(1):28–36. doi: 10.1097/01.gme.0000191204.38664.61. [DOI] [PubMed] [Google Scholar]