Abstract
Tumor-infiltrating lymphocytes influence colorectal cancer progression. We have recently documented that tertiary lymphoid tissue in the colorectal cancer microenvironment orchestrates lymphocyte infiltration and that tertiary lymphoid tissue and lymphocytes cooperate in a coordinated antitumor immune response to improve patient outcome. Thus, tertiary lymphoid tissue represents a potential target in the design of tailored immune-based therapeutic approaches.
Keywords: biomarkers, colorectal cancer, immunotherapy, prognosis, tertiary lymphoid tissue, tumor-infiltrating lymphocytes
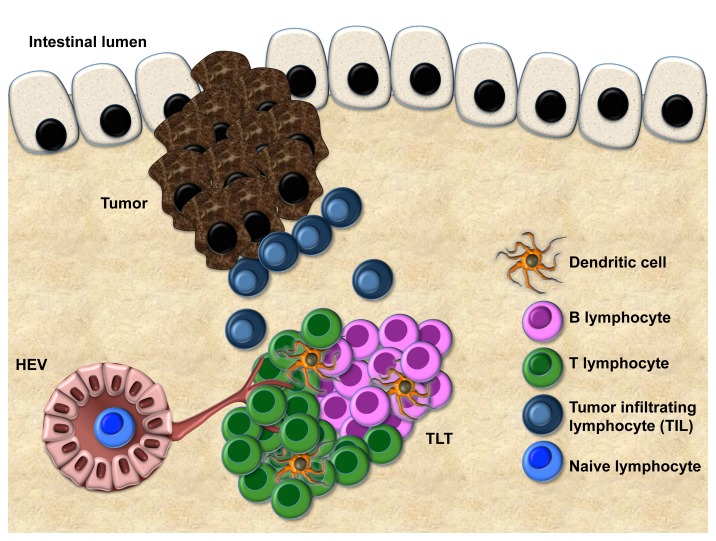
The immune system plays a pivotal role in modulating the occurrence and progression of colorectal cancer (CRC).1,2 Various prior studies have shown an association between the number of tumor-infiltrating lymphocytes (TILs) and colorectal cancer prognosis, particularly in early-stage tumors.3 Surprisingly, the molecular architecture underlying this phenomenon has yet to be identified and further, the site at which tumor antigen recognition and subsequent presentation occurs, a process critical for the activation of anticancer immune effectors, is unknown. In several pathologic contexts, activation and recruitment of tumor-specific T cells can conditionally occur extranodally in peripheral tissues, i.e., in structures defined as tertiary lymphoid tissue (TLT).4 Such ectopic TLT is a histologically distinct entity thought to act as an efficient immune site in the recruitment5 and activation of lymphocytes from the circulation (Fig. 1). This immunologic role is supported by the presence of lymphorganogenic chemokines (such as CXCL13, CCL21), which are responsible for lymphocyte recruitment and topologic segregation, and a high number of high endothelial venules (HEVs), which are required for the proper trafficking of immune mediators. The presence of TLT in the tumor milieu has been reported in several tumor types.6 Nevertheless, the occurrence of TLT in the CRC microenvironment, its functional ability to mediate recruitment of TILs, and its clinical relevance in the context of TNM staging have, so far, been largely disregarded.
Figure 1. Features of tertiary lymphoid tissue (TLT) at the tumor margin. Compartmentalized areas of T cells and B cells, dendritic cells, and a vascular network, including lymphatic vessels and high endothelial venules (HEV) are shown. The correlation between the extent of tertiary lymphoid tissue (TLT) and T-cell infiltration suggests that TLT represents a gateway for tumor-infiltrating T cells (TILs).
We therefore investigated the occurrence and clinical relevance of TLT in human CRC and the dynamics of TLT and potential TIL recruitment in a preclinical model of CRC.7 We first identified aggregates of CD3+ lymphocytes at the tumor margin, a region that also contained clusters of CD20+ B cells and CD21+ follicular dendritic cells (FDCs) and showed expression of CXCL13 and CCL21. Interestingly, these aggregates contained PNAd+ HEVs and Lyve-1+ lymphatic vessels, suggesting that TLT is endowed with the essential features of functional immune sites. Encouraged by these findings, we designed a novel standardized method for quantitative analysis of CD3+ TLT in the tumor microenvironment in a cohort of 351 stage II and III CRCs and compared the extent of TLT with the number of interspersed CD3+ TILs. In the overall cohort, the presence of TLT correlated with the density of TILs, suggesting that TLT might be involved in the recruitment of T cells to the tumor site. In particular, TLT and TILs had similar prognostic behavior and were both highly efficient in predicting disease recurrence among patients with nodal negative CRC. Notably, we showed that TLT and TIL densities were linearly correlated among patients with good prognosis, whereas there was no such relationship detected in those who experienced disease recurrence, suggesting that coordination of these immune pathways is required to direct antitumor immune responses. Additionally, in an azoxymethane/dextran sodium sulfate (AOM/DSS) murine model, adoptively transferred GFP+ splenocytes specifically homed to TLT at the tumor site, providing functional evidence that TLT mediates the recruitment of TILs to the locally inflamed tumor microenvironment. Thus, in addition to its function as a gateway for the access of TILs to the tumor microenvironment, our prognostic data imply a substantial role for TLT in the generation of the local antitumor immune response.
It is well established that solid tumors recruit infiltrating immune cells in their milieu with a wide degree of heterogeneity according to the organ of origin and the stage of disease, thus contributing to the complexity of the cancer microenvironment.8 The positive prognostic ability of tumor-infiltrating immune cells in CRC provides a rationale for the design of immunotherapeutic strategies aiming to increase the presence of activated TILs. Our work adds a critical player to this stage by revealing, for the first time, that the presence of TLT favors recruitment and activation of immune cells in the CRC milieu. Importantly, we demonstrate that the TLT plays a vital role in mediating the dynamics of T-cell recruitment to the tumor microenvironment. Defective tumor homing of effector T cells is a major obstacle to the efficacy of current immunotherapeutic approaches.9 In this regard, neogenesis of TLT in the tumor microenvironment could represent a vehicle for the host immune system to deliver an effective burden of T cells to tumor areas that may not be otherwise accessible.
The American Joint Committee on Cancer (AJCC)/Union for International Cancer Control (UICC) TNM classification is the only standardized method currently available for clinicians to stratify cancer patients according to their potential survival. Our analysis of the clinical relevance of TLT has revealed that, although possessing features of active immune sites and, thus may be potentially useful for defining tumor progression, its efficacy in mediating the antitumor response appears to be reduced in nodal positive (stage III) CRC. In this setting, our data implicating TLT as a clinically relevant feature of the anticancer immune landscape in early-stage CRC argue for the integration of TNM classification with quantification of immune cells in the tumor microenvironment in a revised TNM-Immune (TNM-I) staging prognostic system in the clinic.10 In addition, although innovative therapies seeking to boost the host immune response remain highly sought after for the treatment of CRC, nodal metastasis seems to be a critical variable determining the antitumor efficacy of the adaptive antitumor response. Failure of the immune components to predict prognosis might result from the presence of an immunosuppressive microenvironment or the selection of immune markers that do not reflect the ongoing host immune response to control tumor growth. In the latter scenario, the role of the humoral immune subset within the structural organization of TLT, and whether it could represent a prognostic and functionally relevant feature in the generation of antitumor immune responses, remains to be determined. Further efforts to standardize methods for TLT detection in the tumor microenvironment, coupled with assessment of its prognostic relevance, will provide a potential screening platform amenable to the identification of subclasses of patients with active and functional antitumor immune response pathways that may presage clinically detectable tumor rejection. Therefore, the design of novel immunotherapies should take into account the complexity of the prognostic nature of the immune microenvironment together with clinical disease staging to tailor treatments for patients who are most likely to respond positively.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Giuseppe Di Caro is supported by the fellowship “Post-doctoral Fellowships -anno 2014,” from Fondazione Umberto Veronesi. Federica Marchesi is supported by the Italian Ministry of University and Research FIRB grant (RBAP11H2R9 to A.M.). This work was supported by Humanitas Clinical and Research Center (grant Translation in Medical Oncology 2013 to F.M.) and by the Italian Association for Cancer Research (grant MFAG11677 to F.M.).
Glossary
Abbreviations:
- CRC
colorectal cancer
- FDC
follicular dendritic cell
- HEV
high endothelial venule
- TIL
tumor-infiltrating lymphocyte
- TLT
tertiary lymphoid tissue
Citation: Di Caro G, Marchesi F. Tertiary Lymphoid Tissue, a T cell gateway in the tumor microenvironment. OncoImmunology 2014; 3:e28850; 10.4161/onci.28850
References
- 1.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–44. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 2.Di Caro G, Marchesi F, Galdiero MR, Grizzi F. Immune mediators as potential diagnostic tools for colorectal cancer: from experimental rationale to early clinical evidence. Expert Rev Mol Diagn. 2014;14:387–99. doi: 10.1586/14737159.2014.900443. [DOI] [PubMed] [Google Scholar]
- 3.Pagès F, Kirilovsky A, Mlecnik B, Asslaber M, Tosolini M, Bindea G, Lagorce C, Wind P, Marliot F, Bruneval P, et al. In situ cytotoxic and memory T cells predict outcome in patients with early-stage colorectal cancer. J Clin Oncol. 2009;27:5944–51. doi: 10.1200/JCO.2008.19.6147. [DOI] [PubMed] [Google Scholar]
- 4.Aloisi F, Pujol-Borrell R. Lymphoid neogenesis in chronic inflammatory diseases. Nat Rev Immunol. 2006;6:205–17. doi: 10.1038/nri1786. [DOI] [PubMed] [Google Scholar]
- 5.de Chaisemartin L, Goc J, Damotte D, Validire P, Magdeleinat P, Alifano M, Cremer I, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011;71:6391–9. doi: 10.1158/0008-5472.CAN-11-0952. [DOI] [PubMed] [Google Scholar]
- 6.Goc J, Fridman WH, Sautès-Fridman C, Dieu-Nosjean MC. Characteristics of tertiary lymphoid structures in primary cancers. Oncoimmunology. 2013;2:e26836. doi: 10.4161/onci.26836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Di Caro G, Bergomas F, Grizzi F, Doni A, Bianchi P, Malesci A, Laghi L, Allavena P, Mantovani A, Marchesi F. Occurrence of tertiary lymphoid tissue is associated with T-cell infiltration and predicts better prognosis in early-stage colorectal cancers. Clin Cancer Res. 2014;20:2147–58. doi: 10.1158/1078-0432.CCR-13-2590. [DOI] [PubMed] [Google Scholar]
- 8.Di Caro G, Marchesi F, Laghi L, Grizzi F. Immune cells: plastic players along colorectal cancer progression. J Cell Mol Med. 2013;17:1088–95. doi: 10.1111/jcmm.12117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gajewski TF, Schreiber H, Fu YX. Innate and adaptive immune cells in the tumor microenvironment. Nat Immunol. 2013;14:1014–22. doi: 10.1038/ni.2703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taube JM. Emerging Immunologic Biomarkers: Setting the (TNM-Immune) Stage. Clin Cancer Res. 2014;20:2023–5. doi: 10.1158/1078-0432.CCR-14-0328. [DOI] [PMC free article] [PubMed] [Google Scholar]