Abstract
Chimeric antigen receptor (CAR) modified T cells have emerged as powerful tools for controlling leukemias. We recently showed that anti-CD123 CAR-expressing cytokine-induced killer T cell treatment is an effective immunotherapeutic approach to eradicate Acute Myeloid Leukemia (AML) cells. Here, we discuss how this genetically modified cell-based strategy could be relevant to the field of AML therapeutics.
Keywords: CAR-T cells, Leukemic stem cells, anti-CD123, AML, immunotherapy
Acute myeloid leukemia (AML) is presently associated with a relapse rate of 50%, with a 5-y overall survival of only ~35–40%, daunting statistics challenging scientific research to identify novel treatment options. In this regard, adoptive immunotherapy with chimeric antigen receptor (CAR)-engineered T cells represents a fascinating biotechnological tool to advance the frontiers of modern cancer treatment. Recent evidence of the efficacy of this immunotherapy-based strategy has been provided in the clinical setting of chronic lymphoid leukemia.1 In brief, CARs are artificial T-cell receptors composed of a monoclonal-antibody (mAb) derived, extracellular-antigen-binding domain fused to an intracellular-TCR signal transduction region. Thus, CAR engineered T cells specifically target a cancer cell surface antigen by exploiting the antigen binding properties of monoclonal antibodies to eliminate malignant cells by activating T-cell mediated effector functions (Fig. 1).
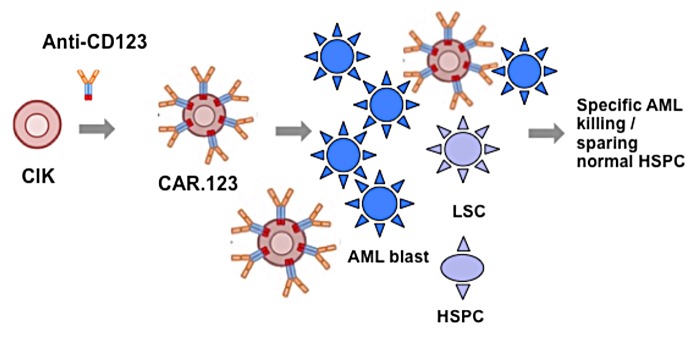
Figure 1. The strategy to eradicate AML cells via chimeric antigen-receptor (CAR) immunotherapy. Chimeric antigen receptor (CAR) engineered cytokine-induced killer (CIK) T cells specifically target acute myeloid leukemia (AML) surface antigen by exploiting the antigen binding properties of CD123-CAR activating CIK cytotoxic effector functions to specifically eliminate malignant cells while sparing the normal hematopoietic stem/progenitor (HSPC) counterpart.
In the last years accumulating evidence has provided support for the hypothesis that one of the mechanisms underlying the high rate of therapeutic failure may be the existence of AML leukemic stem cells (AML-LSCs), a rare, quiescent population residing within the osteoblastic niche of the bone marrow and resistant to chemotherapeutic drugs.2 Thus, with the aim to eradicate the AML disease by CARs, the target antigen should be expressed also at the level of the AML-LSC compartment.
CD33 antigen was among the first AML antigens to be targeted by mAbs, or immunotoxins, although, unfortunately, not proven safe due to a certain level of toxicity that has been observed in the clinical setting probably resulting from concomitant high expression of CD33 on both leukemic cells and their normal hematopoietic counterpart.3 This observation was also confirmed in our in vitro model of anti-CD33 CAR,4 prompting us to move toward a more selective targeting approach. More recently, CD123 molecule has emerged as more specific for AML cells and AML-LSCs considering that it is overexpressed by leukemic cells while being expressed at low levels by normal hematopoietic stem/progenitor cells (HSPCs).5
Cytokine-induced killer (CIK) cells represent a peculiar population to be genetically modified by CARs, both in terms of feasibility and safety profile, with no significant toxicity toward normal myelopoiesis or role in graft-vs.-host disease (GVHD). Nonetheless, CIK cells have a modest basal anti-AML activity, as observed in our previous and current trials in humans,6 such that one can envisage new strategies to improve their native killing capacity toward AML. Our initial in vitro study of CIK cells potentiated and redirected by genetic modification with anti-CD33 and anti-CD123 CARs confirmed an equal AML cytotoxic potential, with a safer profile of anti-CD123 CAR toward HSPCs.7
To further characterize the anti-AML CAR-redirected CIK cell anticancer activity, we next performed a more accurate comparison of the anti-CD33 and anti-CD123 CARs immunotherapeutic potential using in an in vivo NSG (NOD-SCID IL2Rγ−/−) mouse model. In this study, primary AML patient samples were used as targets in conditions mimicking either high leukemic blast burden and minimal residual disease (MRD) conditions. In both scenarios, both CARs were equally able to control the tumor growth without the emergence of antigen CD33- and CD123- loss variants during the treatments, as it has been recently reported in human clinical trials for CD19.CAR in the treatment of acute lymphoblastic leukemia.8 However, by performing secondary transplantation experiments and colony assays of the remaining recovered target cells, we showed that anti-CD123 CAR+CIK cells were superior to anti-CD33 CAR+ CIK cells in terms of anti-AML efficacy as well as their safety profile against HSPCs, exhibiting limited toxicity against normal long-term repopulating cells derived from umbilical cord-blood cells or normal adult bone marrow.
From our results CD123 has emerged as a potential new antigen to be targeted with the CAR approach. A recent paper by Gill et al., questions the selective effect of anti-CD123 CAR,9 showing that normal human fetal liver CD34+ cells were as sensitive to the treatment of anti-CD123 CAR as leukemic cells. This could be due to a higher CD123 expression level on human fetal liver CD34+ cells as compared with normal adult bone marrow CD34+ cells. Other possible explanations for the disparities reported between the two studies could be related to the use of different effector T cells and of diverse anti-CD123 clones from which the short-chain variable fragments (scFv) were derived for the construct.
Regardless, the evaluation of the potential “on-target but off-organ effect” on normal tissues (other than the hematopoietic compartment) expressing the target antigen must be taken into consideration. Indeed, in our study in vitro, we could observe a limited toxicity of anti-CD123 CAR-redirected CIK cells against normal tissues, such as endothelial cells and monocytes, known to express CD123 at lower levels relative to those of AML cells.
In order to ensure a translation of the anti-CD123 CAR to the clinic in the future, it may be safer to couple the CAR construct with a switch-off mechanism, such as a suicide gene strategy similar to the inducible-caspase 9 system that we previously demonstrated to be effective in rapidly eliminating effector T cells.10
At present, in the scenario of AML immunotherapy, Phase I clinical trials targeting CD123 by mAbs and immunotoxins (Clinical Trials.gov ID NCT 004401739 and NCT 00397579) have registered only minor clinical responses, suggesting the need to develop more powerful strategies. In this regard, together with the development of more powerful mAbs (Fc-optimized or new bi- or even tri-specific antibodies), the CAR approach could offer several advantages compared with mAbs, showing a more efficient biodistribution and improved synergism with the immune system through the release of cytokines. Moreover, a potential development of long-lasting cell-mediated immune responses could offer the possibility to durably control the disease overtime. Additionally, the persistence and functional activity of CAR-redirected T cells can be tuned and improved by the addition of the CD28OX40 co-stimulatory domain generating third generation CARs, as demonstrated in our recent publication.11
In conclusion, an anti-CD123 CAR-based strategy, eventually coupled with a suicide gene system, could truly represent a major advancement in the field of AML, particularly for high-risk transplanted patients in the context of minimal residual disease, or as an alternative biological treatment for older patients where standard aggressive chemoradiotherapy approaches are not applicable.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Citation: Tettamanti S, Biondi A, Biagi E, Bonnet D. CD123 AML targeting by chimeric antigen receptors: a novel magic bullet for AML therapeutics?. OncoImmunology 2014; 3:e28835; 10.4161/onci.28835
References
- 1.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ishikawa F, Yoshida S, Saito Y, Hijikata A, Kitamura H, Tanaka S, Nakamura R, Tanaka T, Tomiyama H, Saito N, et al. Chemotherapy-resistant human AML stem cells home to and engraft within the bone-marrow endosteal region. Nat Biotechnol. 2007;25:1315–21. doi: 10.1038/nbt1350. [DOI] [PubMed] [Google Scholar]
- 3.Larson RA, Sievers EL, Stadtmauer EA, Löwenberg B, Estey EH, Dombret H, Theobald M, Voliotis D, Bennett JM, Richie M, et al. Final report of the efficacy and safety of gemtuzumab ozogamicin (Mylotarg) in patients with CD33-positive acute myeloid leukemia in first recurrence. Cancer. 2005;104:1442–52. doi: 10.1002/cncr.21326. [DOI] [PubMed] [Google Scholar]
- 4.Marin V, Pizzitola I, Agostoni V, Attianese GM, Finney H, Lawson A, Pule M, Rousseau R, Biondi A, Biagi E. Cytokine-induced killer cells for cell therapy of acute myeloid leukemia: improvement of their immune activity by expression of CD33-specific chimeric receptors. Haematologica. 2010;95:2144–52. doi: 10.3324/haematol.2010.026310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jordan CT, Upchurch D, Szilvassy SJ, Guzman ML, Howard DS, Pettigrew AL, Meyerrose T, Rossi R, Grimes B, Rizzieri DA, et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia. 2000;14:1777–84. doi: 10.1038/sj.leu.2401903. [DOI] [PubMed] [Google Scholar]
- 6.Introna M, Borleri G, Conti E, Franceschetti M, Barbui AM, Broady R, Dander E, Gaipa G, D’Amico G, Biagi E, et al. Repeated infusions of donor-derived cytokine-induced killer cells in patients relapsing after allogeneic stem cell transplantation: a phase I study. Haematologica. 2007;92:952–9. doi: 10.3324/haematol.11132. [DOI] [PubMed] [Google Scholar]
- 7.Tettamanti S, Marin V, Pizzitola I, Magnani CF, Giordano Attianese GM, Cribioli E, Maltese F, Galimberti S, Lopez AF, Biondi A, et al. Targeting of acute myeloid leukaemia by cytokine-induced killer cells redirected with a novel CD123-specific chimeric antigen receptor. Br J Haematol. 2013;161:389–401. doi: 10.1111/bjh.12282. [DOI] [PubMed] [Google Scholar]
- 8.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gill S, Tasian SK, Ruella M, Shestova O, Li Y, Porter DL, et al. Efficacy against human acute myeloid leukemia and myeloablation of normal hematopoiesis in a mouse model using chimeric antigen receptor-modified T cells. Blood. Blood. 2014 doi: 10.1182/blood-2013-09-529537. Forthcoming. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marin V, Cribioli E, Philip B, Tettamanti S, Pizzitola I, Biondi A, Biagi E, Pule M. Comparison of different suicide-gene strategies for the safety improvement of genetically manipulated T cells. Hum Gene Ther Methods. 2012;23:376–86. doi: 10.1089/hgtb.2012.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pizzitola I, Anjos-Afonso F, Rouault-Pierre K, Lassailly F, Tettamanti S, Spinelli O, Biondi A, Biagi E, Bonnet D. Chimeric antigen receptors against CD33/CD123 antigens efficiently target primary acute myeloid leukemia cells in vivo. Leukemia. 2014 doi: 10.1038/leu.2014.62. [Forthcoming] [DOI] [PubMed] [Google Scholar]


