Abstract
The Cueva del Azufre in Tabasco, Mexico, is a nutrient-rich cave and its inhabitants need to cope with high levels of dissolved hydrogen sulfide and extreme hypoxia. One of the successful colonizers of this cave is the poeciliid fish Poecilia mexicana, which has received considerable attention as a model organism to examine evolutionary adaptations to extreme environmental conditions. Nonetheless, basic ecological data on the endemic cave molly population are still missing; here we aim to provide data on population densities, size class compositions and use of different microhabitats. We found high overall densities in the cave and highest densities at the middle part of the cave with more than 200 individuals per square meter. These sites have lower H2S concentrations compared to the inner parts where most large sulfide sources are located, but they are annually exposed to a religious harvesting ceremony of local Zoque people called La Pesca. We found a marked shift in size/age compositions towards an overabundance of smaller, juvenile fish at those sites. We discuss these findings in relation to several environmental gradients within the cave (i.e., differences in toxicity and lighting conditions), but we also tentatively argue that the annual fish harvest during a religious ceremony (La Pesca) locally diminishes competition (and possibly, cannibalism by large adults), which is followed by a phase of overcompensation of fish densities.
Keywords: Cave fish, Extremophile teleosts, Fisheries, Rotenone, Overcompensation
Introduction
Cave fishes are emerging as model systems to study regressive evolutionary processes like the reduction of eyes and pigmentation that typically accompany the colonization of caves by previously surface-dwelling species (Romero & Green, 2005; Jeffery, 2009). For example, the characid Astyanax mexicanus is a model organism for EvoDevo studies of cave evolution (Wilkens, 1988; Jeffery, 2001; Jeffery, 2009). The cave form of a Mexican live-bearing fish, the so-called cave molly (Poecilia mexicana; Gordon & Rosen, 1962) has adapted to the vastly divergent ecological conditions inside a South Mexican sulfide cave, the Cueva del Azufre (also referred to as Cueva Villa Luz or Cueva de las Sardinas; Parzefall, 1993; Parzefall, 2001) Cave environments are usually energy limited compared to photosynthetically based epigean habitats (Hüppop, 2005) and fish densities reported for several different cave systems are low, with often less than one individual per m2 (Trajano, 2001). In contrast, the Cueva del Azufre is a sulfidic, nutrient-rich habitat due to chemoautotrophic primary production through sulfide oxidizing bacteria that utilize the abundant hydrogen sulfide in the cave (Hose & Pisarowicz, 1999; Colaço, Dehairs & Desbruyeres, 2002; Summers Engel, 2005). Hydrogen sulfide is acutely toxic to most metazoans and leads to extreme hypoxia in the water (Evans, 1967; Bagarinao, 1992). Beside the Cueva del Azufre, few other sulfurous chemoautotrophic cave-ecosystem are described, such as Movile in Romania (Sarbu, Kane & Kinkle, 1996), Frasassi in Italy (Flot, Wörheide & Dattagupta, 2010) and Ayyalon in Israel (Por, 2007). All of these caves are inhabited by invertebrates—many of them endemic to the caves—that exploit this unusual food web. The Cueva del Azufre is the only known chemoautotrophic cave ecosystem which is inhabited by a vertebrate species (Plath & Tobler, 2010). However, due to its toxicity, hydrogen sulfide requires energetically costly behavioral (i.e., actively avoiding microhabitats with high levels of toxicity) and physiological adaptations (various forms of detoxification) by animals exposed to it (Tobler et al., 2009; Riesch, Plath & Schlupp, 2010). As a result of the simultaneous action of two strong selective forces (permanent darkness and hydrogen sulfide), locally adapted P. mexicana populations in the Cueva del Azufre system have received considerable scientific interest. The cave molly differs from its surface-dwelling ancestors in a distinct set of morphological, physiological, behavioral, and life-history traits; e.g., cave mollies have reduced eye size and reduced pigmentation, and females have a reduced fecundity combined with an increase in individual offspring size (Parzefall, 2001; Tobler et al., 2008a; Riesch, Plath & Schlupp, 2010; Tobler et al., 2011b). Although the cave molly has been established as a model to examine evolutionary adaptations to extreme environmental conditions, population densities have not yet been quantified in the Cueva del Azufre system, which makes interpretation of some of the ecological and evolutionary data difficult with regards to how they influence long-term stability of the systems and population dynamics.
The Cueva del Azufre drains into the El Azufre, a sulfidic surface creek, which eventually joins the Río Oxolotán. The Cueva del Azufre and El Azufre differ dramatically in the composition of fish communities compared to adjacent non-sulfidic surface habitats. Poecilia mexicana occurs as the single dominant species in both systems. Only one further fish species, the predatory cichlid ‘Cichlasoma’ salvini occurs in the upper parts of the El Azufre, but only in small numbers. In downstream areas of the El Azufre where H2S in not measurable, Heterandria bimaculata and Xiphophorus hellerii (Poeciliidae), Astyanax aeneus (Characidae) as well as ‘Cichlasoma’ salvini and Thorichtys helleri (Cichlidae) occur (Plath & Tobler, 2010). In surrounding non-sulfidic surface habitats, diverse fish communities can be found, often dominated by cichlid and poeciliid species (Tobler et al., 2006; Plath & Tobler, 2010). In the Clear Creek, a small stream that is directly connected to El Azufre, H. bimaculata occurs at a high abundance together with small numbers of X. hellerii and P. mexicana. A reduced species diversity and dominance of a few specialists have been documented from other caves (Trajano, 2001) and other sulfidic habitats (Tobler et al., 2008c).
Little is known about anthropogenic disturbances on the population ecology of P. mexicana inhabiting the Cueva del Azufre. Today, the system is increasingly influenced by a growing number of visitors which reach their peak during a traditional annual ceremony of the local indigenous Zoque people named ‘La Pesca’. The Cueva del Azufre is sacred to the Zoque people, and once a year, on the first Sunday of Easter week, the Zoque enter the cave and introduce rotenone- and deguelin-containing barbasco roots (Lonchocarpus sp., Fabaceae) into the water. Rotenone is an inhibitor of the mitochondrial complex-I of the respiratory chain, causing reduced cellular respiration (Singer & Ramsay, 1994). Barbasco is introduced into the water in the middle portion of the cave, therefore only downstream cave chambers are affected (Fig. 1). Capture of poisoned cave fish is facilitated by the anesthetic effect of barbasco, as narcotized fish are flushed out of the cave, where they are harvested using wooden baskets, and afterwards cooked and eaten as part of a religious ceremony honoring the Rain Gods (Tobler et al., 2011a). The yield of the annual harvest is considered to be indicative of the quality of the subsequent crop harvest (Hose & Pisarowicz, 1999; Tobler et al., 2011a). Annual harvests amount to several thousand individuals, and the ceremony is likely to have taken place for centuries (Hose & Pisarowicz, 1999), so it is likely to act as a strong selective force on P. mexicana populations annually exposed to it.
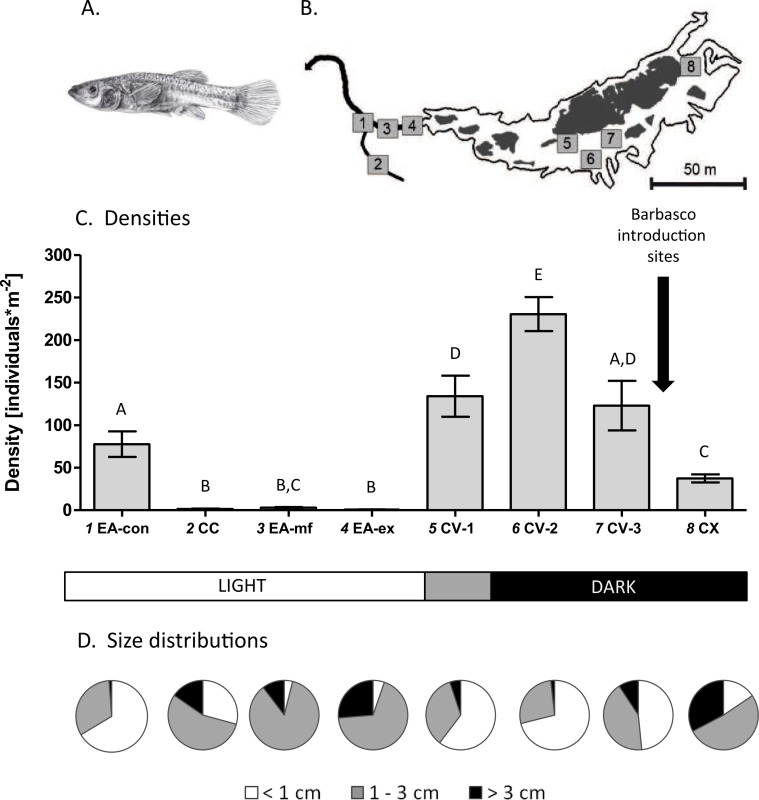
Figure 1. Study system and population densities.
(A) Drawing of a female cave molly. (B) Map of the study area showing the different sampling sites (numbers) where white areas represent water within the cave (Cueva del Azufre) and dark areas indicate dry land and bedrock. 1 EA-con, 2 CC, 3 EA-mf, 4 EA-ex, 5 CV-1, 6 CV-2, 7 CV-3, 8 CX. With the exception of sampling site CV-1 all sampling sites inside the Cueva del Azufre are completely dark. Barbasco is released annually between chamber V (CV) and chamber X (CX). Three sampling sites inside chamber V were defined (CV-1 to CV-3). Downstream of the exit of the Cueva del Azufre (EA-ex), a rather homogeneous mudflat (EA-mf) was sampled. Further sampling sites were a small non-sulfidic creek (Clear Creek; CC) and its confluence with El Azufre (EA-con). (C) Mean (±SE) densities of mollies at each sampling site. Letters above the error bars signify statistically different groups (Fisher’s LSD tests). (D) Size class compositions of mollies at the different sampling sites.
In the present study, we provide first data of local densities within different chambers of the Cueva del Azufre and adjacent El Azufre and discuss our findings with regard to environmental conditions and annual harvesting of cave mollies. We used a non-invasive technique to repeatedly assess fish densities and size-distribution patterns (as a proxy for age) inside the Cueva del Azufre (up- and downstream of the barbasco-release site) and in the sulfidic creek leaving the cave (El Azufre). Moreover, given the high structural heterogeneity of the water course inside the Cueva del Azufre with respect to water depth and flow velocity (Hose & Pisarowicz, 1999), and because Croft, Botham & Krause (2004) reported on size-specific preferences regarding water depth in another poeciliid, the Trinidadian guppy (P. reticulata), we combined our assessment of fish densities with an investigation of microhabitat use by different size classes of cave mollies.
Material and Methods
Study system
Locally adapted subterranean populations of P. mexicana (Fig. 1A) can be found in at least two different limestone caves in the vicinity of the southern Mexican city of Tapijulapa (state of Tabasco, México): the Cueva del Azufre (Gordon & Rosen, 1962) and the much smaller, non-sulfidic Cueva Luna Azufre (Tobler et al., 2008b). The sulfidic Cueva del Azufre is about 500–600 m deep and divided into 13 different cave chambers (I-XIII), with the innermost chamber being XIII (Gordon & Rosen, 1962). Several springs discharge water with high concentrations of hydrogen sulfide (H2S) into the creek draining the cave (Tobler et al., 2006). The cave creek forms a highly heterogeneous mosaic of shallow pools and backwaters that are partially divided by swift flowing riffle passages (Gordon & Rosen, 1962; Hose & Pisarowicz, 1999). While the front cave chambers receive some dim light through cracks in the ceiling, the inner parts of the cave are lightless. Consequently, (sub-)populations experience divergent selection regimes regarding light exposure, with populations from the innermost chambers living under perpetually dark conditions, whereas those from front chambers are exposed to dim sunlight through a number of cracks in the cave ceiling, so-called sky lights (Fontanier & Tobler, 2009).
Upon leaving the underground, the sulfidic creek draining the Cueva del Azufre is called ‘El Azufre’. After meandering for approximately 1.5 km, it eventually drains into the Río Oxolotán, which is part of the Río Grijalva drainage system. Despite the gradual oxidation of H2S to sulfate and elemental sulfur with increasing distance from the sulfide sources, which increases the water turbidity, and despite the influx of some smaller clear water affluents, El Azufre still has a remarkably high H2S concentration of up to ∼40 µMol (Tobler et al., 2006; Schlupp et al., 2013).
Study sites and data collection
We compared the abundance and distribution of different size classes of P. mexicana among different sampling sites along a transect starting at chamber X in the Cueva del Azufre, and following the water flow outside the cave to the confluence of El Azufre with the first freshwater influx from the Clear Creek. This transect, therefore, covered sample sites located upstream of the release point of barbasco during La Pesca (sample point in chamber X) and sites directly downstream of the release point of barbasco that are strongly affected by the annual ceremony (three sites in chamber V; CV-1, CV-2, and CV-3; Figs. 2A and 2B). Surface sites of El Azufre are also annually exposed to barbasco due to downstream effects (EA-ex, EA-mf), even though concentrations are probably considerably lower than inside the cave (Table 1). Clear creek (CC) and its confluence with EA (EA-con; Fig. 2C), on the other hand, are not influenced by barbasco.
Figure 2. Pictures of study sites.
(A) Cueva del Azufre chamber V (6 CV-2) and (B) site 7 CV-3. (C) El Azufre confluence with Clear Creek (1 EA-con).
Table 1.
Sampling sites, their abbreviation code as used throughout the article, numbers of quadrants examined, and details regarding barbasco release.
| Site code | Site | Number of quadrants |
Affected by deposition of rotenone? |
Approximate distance to upstream rotenone release site [m] |
|---|---|---|---|---|
| 1 EA-con | El Azufre, confluence with clear Creek | 25 | No (only partly) | 150 |
| 2 CC | Clear Creek | 28 | No | — |
| 3 EA-mf | El Azufre, mudflat | 25 | Yes | 120 |
| 4 EA-ex | El Azufre, exit of the Cueva del Azufre | 25 | Yes | 110 |
| 5 CV-1 | Cueva del Azufre, Chamber V, site 1 | 25 | Yes | 0 |
| 6 CV-2 | Cueva del Azufre, Chamber V, site 2 | 15 | Yes | 0 |
| 7 CV-3 | Cueva del Azufre, Chamber V, site 3 | 8 | Yes | 0 |
| 8 CX | Cueva del Azufre, Chamber X | 25 | No | — |
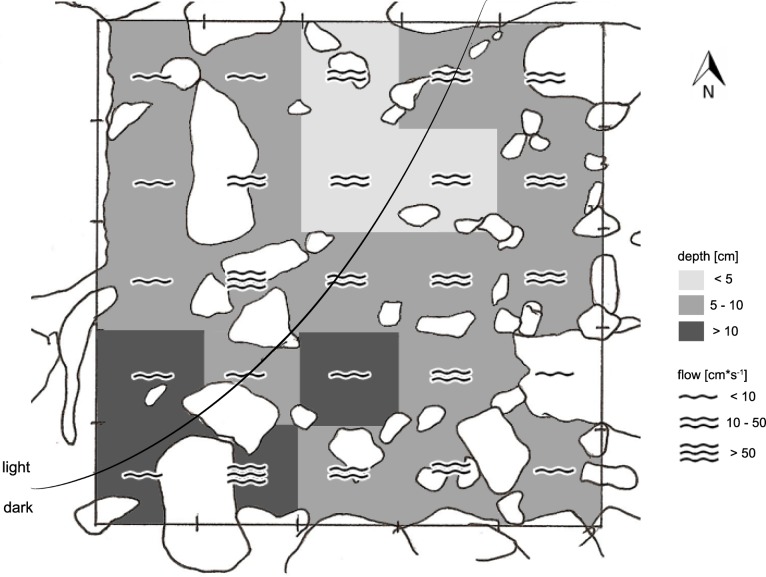
Field work was conducted in January 2010, i.e., about 9 month after the latest La Pesca ceremony in 2009 (L Arias-Rodriguez, pers. obs., 2009). At each of the eight sample sites, we defined sampling grids consisting of 50 × 50 cm quadrants with wooden sticks fixed in the ground (or stones where a grid angle fell on the shore). The number of quadrants was mostly 25 per sampling grid (i.e., 5 × 5 quadrants). In the narrow non-sulfidic surface creek (CC), however, the arrangement of quadrants was more longitudinal (4 × 7 = 28 quadrants), and in chamber V, where a particularly high degree of structural heterogeneity precluded defining larger grids, one sampling site of 5 × 5 quadrants and two smaller ones (15 and 8 quadrants, respectively) were defined (Table 1). The grids reflected the natural variation in water depth, flow velocity, and substrate types, thus covering the range of different microhabitats inhabited by mollies (an example is shown in Fig. 3).
Figure 3. Exemplary sketch of site 5 CV-1.
Showing the high degree of heterogeneity in flow regimes, water depth, substrate types, and (in this case) light regime.
Daily measurements took place between 11:00 a.m. and 4:30 p.m. Each site was visited at least 5 times (mean ± SD = 6.25 ± 1.16) on consecutive days. During the counts, we slowly approached a site while trying to avoid any movements that would cause the resident fish to flee, and we counted juveniles (<10 mm standard length (SL)), sub-adults (10–30 mm) and adults (>30 mm) in each quadrant. The observer was standing motionless at least 1.5 m downstream from the respective quadrant. Sizes were estimated qualitatively, aided by a prior training session that used wooden sticks of known size as a reference. Our definition of adults roughly followed Riesch, Plath & Schlupp (2010), who determined the mean (±SD) standard length of reproducing females to be 31.44 ± 4.40 mm (El Azufre) and 36.97 ± 4.59 mm (Cueva del Azufre, chambers V and X).
Habitat parameters were assessed after the last fish count. For each quadrant, we determined water depth using a wooden ruler stuck vertically into the water at five random locations and calculating the mean from those five measurements. Flow velocity was measured on the water surface by scoring the time a small wooden stick of about 3 cm length took to float through the whole length of a quadrant (measurement was repeated five times per quadrant and averaged across the five observations per quadrant). Mean surface flow velocity was then expressed as cm*s−1. Research followed the authorizations from CONAPESCA-DGOPA.09004.041111.3088 and Tacotalpa, Tabasco municipality.
Statistical analysis
Our first question was whether population densities differed among sampling sites. We used data for the different quadrants per site (averaged from the repeated measurements) and expressed density as total numbers of individuals per square meter. Density estimates per quadrant were used as the dependent variable in a univariate general linear model (GLM) with ‘sampling site’ as a fixed factor. We initially entered ‘mean water depth’ (F1,162 = 0.12, P = 0.98) and ‘mean flow rate’ (F1,162 = 0.22, P = 0.64) as covariates, but removed them from the final analysis since neither had a significant effect (also none of the interaction terms were significant). We used Fisher’s LSD tests for pairwise post hoc comparisons among sites.
A further question was whether size-class compositions differed among sample sites and whether distribution patterns would be stable among repeated sampling days. We used the Bray-Curtis index (Bray & Curtis, 1957) to estimate pairwise similarities among each sampling point (calculated with the R-package ‘ecodist 1.2.7’; Goslee and Urban, 2007; R Development Core Team, 2008), and used these for non-metric multidimensional scaling (‘NMDS PROXSCAL’ function in SPSS 21). To detect clusters, we used the ‘two step cluster analysis’ function based on Euclidian distances and the Bayesian information criterion. For visualization of size class compositions per site, we averaged repeated measurements of different size classes and used a mean value for each quadrant per site. By using these means we calculated the total average size class distribution per site.
Our first analysis detected pronounced variation in population densities and size distributions (see results) and thus, we decided to analyze potential effects of water depth and flow velocity (i.e., microhabitat choice) in a site-wise fashion. We focused on sites inside the Cueva del Azufre (CV-1, CV-3 and CX) where (a) fish densities were sufficiently high and (b) sufficient variability of those environmental variables was found to allow for a meaningful analysis. All other sites were excluded from this analysis. For each site, fish density per quadrant was entered as the dependent variable in repeated measures (rm) GLMs with ‘size class’ (three levels) as the repeated measurement. We grouped water depth (<5 cm, 5–10 cm, >10 cm) and flow velocity (<10 cm*s−1, 10–50 cm*s−1, >50 cm*s−1) into three classes each and used these habitat parameters as fixed factors. However, neither the main factor ‘flow velocity’ nor any interaction term involving ‘flow velocity’ had a significant effect in any of the three site-specific models (CV-1: F4,38 = 1.27, P = 0.30; CV-3: F2,4 = 3.28, P = 0.14; CX: F2,20 = 0.44, P = 0.65), and so we subsequently removed this term from all models.
Results
Local population densities
When comparing mean densities per quadrant across sites we detected a significant difference among sampling sites (GLM; F7,164 = 32.49, P < 0.001; Fig. 1B). Post hoc pairwise LSD tests found most pairwise comparisons to be statistically significant; qualitatively, densities increased from surface sites (mean ± SE across sites = 21.0 ± 5.0 individuals*m−2) towards the cave (119.5 ± 12.7 individuals*m−2). Also, sites downstream of the barbasco release-site (chamber V; 162.3 ± 16.1 individuals*m−2) had considerably higher densities than the site in chamber X that lies upstream of the release-site (37.4 ± 4.8 individuals*m−2).
Differences in size-class compositions
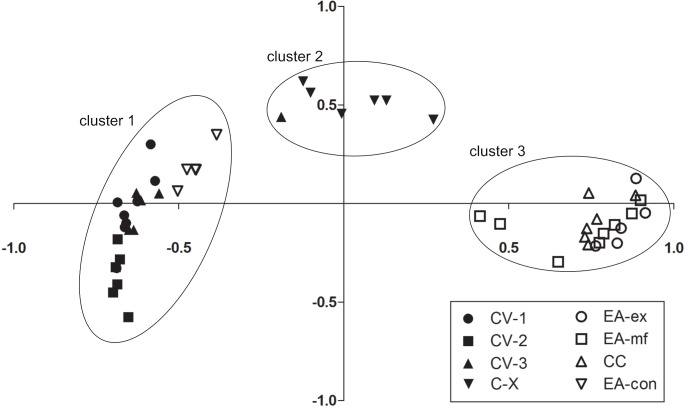
The NMDS based on Bray-Curtis similarities found data from repeated sampling days to cluster together, suggesting that the observed size-class compositions were stable over the period of this study (Fig. 4). There were three distinct clusters, and in only one case was a single sampling day of a given sampling site assigned to the ‘wrong’ cluster. The first cluster comprised the three sample sites in cave chamber V and EA-con. Samples had high overall densities and were composed mostly of small individuals. Cluster two comprised the rearmost cave site CX. Samples in this cluster were characterized by intermediate densities but a particularly high proportion of large individuals. Cluster three comprised all surface sites except EA-con and was characterized by overall low densities and mostly intermediate-sized fish (Fig. 4).
Figure 4. Differences in size-class compositions of Poecilia mexicana in the Cueva del Azufre system.
Non-metric Multi-Dimensional Scaling (NMDS) plots based on Bray-Curtis similarities for each sampling site and day.
Microhabitat use of different size classes
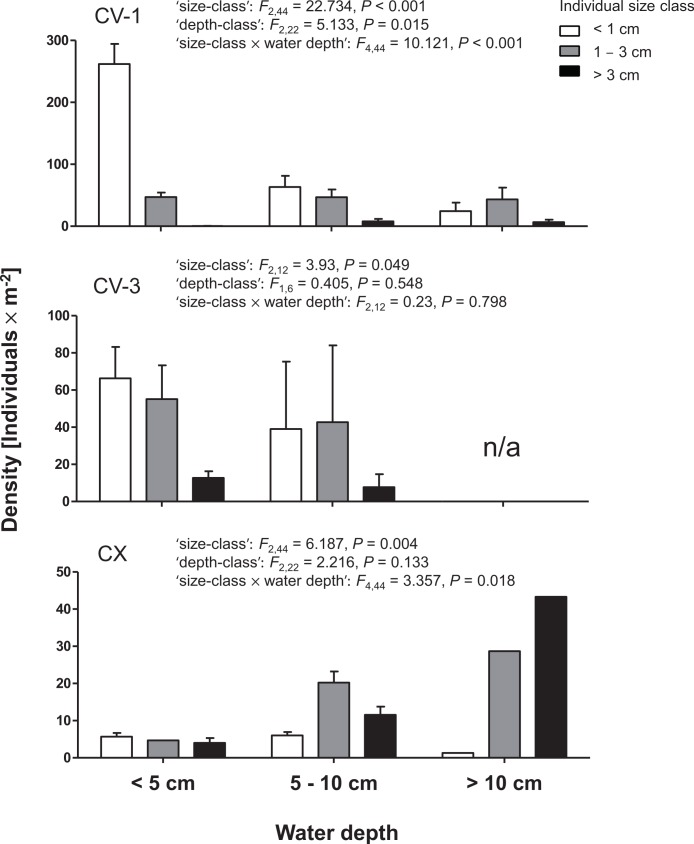
In the rmGLMs treating the different size-classes as the repeated measurement, the interaction term ‘size-class × water-depth’ had a significant effect for two of the three sampling sites included in this analysis—notably, those sites with the most variation in water depth (Fig. 5). This result is indicative of differences in microhabitat use among different size classes of cave mollies: generally, larger fish were found in deeper areas, whereas smaller fish resided in shallow parts. A significant main effect of the repeated measurement (‘size class’) in all three analyses confirms the overabundance of small-sized fish in cave chamber V, and of large-bodied fish in chamber X (Fig. 5).
Figure 5. Population densities of cave mollies in the Cueva del Azufre.
Mean (± SE) densities of mollies, categorized in three size classes (<1 cm, white, 1–3 cm, gray, and >3 cm, black) in three water depths (<5 cm, 5–10 cm, and >10 cm). Results of rmGLMs are inserted. Note the different y-axis scales. Error bars are given if more than one sampling grid of a given depth class was present within the sampling site.
Discussion
We provide detailed information on population densities of cave-adapted P. mexicana in the Cueva del Azufre. Repeated measurement in different cave chambers uncovered very stable patterns of high densities, confirming qualitative estimates provided by Parzefall (1993). Density estimates of P. mexicana in the cave were extraordinarily high and exceed those of other cave fishes, which are usually low, with often less than one individual per m2 (Trajano, 2001). Furthermore, densities were higher inside the cave compared to adjacent surface populations.
Variation in population densities can be explained by different factors affecting cave molly population dynamics; e.g., environmental heterogeneity may contribute to population differences. The highest H2S concentrations (>300 µM) are found in parts of chamber X, where most large sulfide sources are located, while concentrations in chamber V are lower (2–32 µM), as H2S is increasingly bound with oxygen with increasing distance from the sulfide sources (Tobler et al., 2006). However, ecotoxicological experiments repeatedly found small adults to have higher H2S-resistance than large-bodied adults, possibly reflecting senescence effects or size-specific thresholds regarding the rate of sulfide influx to the body to oxidation (Tobler et al., 2011b; Plath et al., 2013; Riesch et al., 2014a). Hence, we would expect fish in chamber X to actually be smaller than fish from chamber V if different H2S concentrations were the main driver of population differences.
Beside different H2S concentrations, the sites within the cave differ in the presence of light. Whereas chamber V receives some dim light through cracks in the ceiling, the inner parts of the cave are lightless. Photophobic behavior is a factor that has been proposed to promote the colonization of perpetually dark caves and the choice of microhabitat (Poulson, 1964; Barr Jr, 1968). While photophobic behavior has been reported in several cavefishes (Wilkens, 1988; Camassa, 2001; Wilkens, 2001; Timmermann & Plath, 2009), photophilic behavior was found in both surface and cave forms of P. mexicana (Parzefall et al., 2007). In theory, this photophilic behavior could lead to an accumulation of fish in chamber V (sites 5–7) compared to chamber X (site 8) if fish moved between cave chambers but were less likely to return to dark sites, but this line of argumentation is not compatible with the observation of small-scale genetic structure among different cave chambers (Plath et al., 2007).
The different light regimes may also affect trophic interactions since the deep and lightless parts of the cave depend solely on chemoautotrophic primary production, while organic matter can enter through cracks in chamber V, and then provide the basis for detritivore animal communities that constitute an additional food source in other cave systems (Hüppop, 2005). Nevertheless, more research is needed on the extent to which these few sky lights might indeed provide significant influx of additional nutrients, because stomach content analysis of cave mollies, for example, does not so far strongly support such a notion (Tobler et al., 2009).
Furthermore, cave chambers may differ in predation regimes. Inside the Cueva del Azufre, aquatic water bugs of the genus Belostoma prey upon cave mollies and Belostoma prefer large over small cave mollies as prey (Tobler, Schlupp & Plath, 2007; Tobler, Franssen & Plath, 2008; Plath et al., 2011). Mark-recapture analysis found individual densities of water bugs to be approximately one individual per m2 in chamber V (Tobler, Schlupp & Plath, 2007), and while empirical data are as yet lacking, observational evidence over several years of field work suggests that densities are much lower in the innermost chambers.
Belostoma predation, however, might explain microhabitat use of different size classes of cave mollies. Belostoma are typically found on rocks at the water’s edge (Tobler, Franssen & Plath, 2008), and so large cave mollies—being preferred by the water bugs (Plath et al., 2011)—could use deeper parts of the water column to avoid predation risk. The preference for large size-classes was confirmed for another belostomatid preying on mosquitofish (Schumann, Cavallaro & Hoback, 2012). On the other hand, small fish could avoid filial cannibalism, which is known from other poeciliids (Loekle, Madison & Christian, 1982; Nesbit & Meffe, 1993), by using shallow parts of the water column that exclude large mature fish.
One factor that most likely influences population dynamics is the annual ‘La Pesca’ ceremony. The ceremony leads to a strong temporary reduction of local fish densities in those cave chambers that are situated downstream of the barbasco release site (Tobler et al., 2011a). Our study was conducted approximately nine months after the last ceremony, but given rather long generation times in P. mexicana (roughly 3–6 months for males and 7–10 months for females from birth until reaching maturation under common-garden rearing conditions; Riesch et al., 2014b), we predicted to find lower (sub-)population densities and especially fewer large-bodied individuals downstream of the site in the Cueva del Azufre where barbasco is annually released. Instead, while fish densities were generally high in the cave, they were highest downstream of the barbasco release site. However, sample sites affected by the release of barbasco had population structures that were strongly shifted towards an overabundance of the smallest size classes (i.e., juveniles). These patterns were stable when repeated samplings from subsequent days were compared.
Migration within the Cueva del Azufre is unidirectional, out of the cave, and migration among different cave chambers occurs only to a small extent, which results in population genetic differentiation, as shown based on nuclear microsatellites (Plath et al., 2007), and is also reflected in morphological differences among fish from different cave chambers (Fontanier & Tobler, 2009). Hence, re-colonization of the affected sites from other parts of the cave (i.e., source–sink dynamics) is unlikely, and the observed recovery of the respective populations likely represents an autochthonous effect. After the temporal decline in population density following La Pesca, the surviving individuals benefit from reduced intraspecific resource competition. Detritus and green algae are the dominant food sources of surface-dwelling P. mexicana from non-sulfidic streams, while diets of conspecifics in the sulfidic surface and cave streams are dominated by chemoautotrophic (sulfur) bacteria and aquatic invertebrates (like larvae of the dipteran Goeldichironomus fulvipilus and small snails; (Roach, Tobler & Winemiller, 2011)). In particular, access to invertebrate prey could be favored not only by the absence of competing fish species, but especially by temporarily relaxed competition among the surviving adult P. mexicana. Generally, relaxed competition translates into higher growth rates, faster maturation, and increased adult fecundity (Stearns, 1976), which may lead to stage-specific biomass overcompensation, thereby compensating for the removal of individuals from the population (Werner & Gilliam, 1984; de Roos et al., 2007; Schröder, Persson & de Roos, 2009). This idea received support from empirical harvesting experiments that found the negative relationship between adult mortality and abundance/density to be reversed if mortality does not affect a certain portion of the population. Experimental studies on laboratory populations of the poeciliid fish Heterandria formosa showed that biomass of the juvenile size class increased in response to intermediate adult mortality rates (Schröder, Persson & de Roos, 2009). Another study showed that a pathogen outbreak in a wild perch population (Perca fluviatilis) was followed by a biomass overcompensation of the juvenile stage as a result of increased adult mortality. Age-specific adult fecundity and body mass of one- and two year old perch increased after the disease outbreak, suggesting that increased adult mortality released perch from competition and cannibalism, thereby increasing somatic and reproductive growth (Ohlberger et al., 2011). In the Cueva del Azufre, the stage-specific biomass overcompensation may lead to increasing population densities, based on temporarily increased adult fecundity that leads to high numbers of juvenile fish. This would result in cave molly populations regaining the high densities seen before La Pesca, again leading to increased competition. This is consistent with earlier observations of cave mollies showing reduced body condition (measured, e.g., as fat content) compared to fish from surface sites (Riesch, Plath & Schlupp, 2010; Riesch, Plath & Schlupp, 2011). Hence, human-induced selection and predation by Belostoma ought to have very similar effects on the populations exposed to them. We are inclined to argue, however, that the relative influence of Belostoma predation is considerably lower than the effects of the massive annual fish harvest. Previous reports of increased rotenone-resistance in fish from chamber V, but not chamber X (Tobler et al., 2011a), confirm that La Pesca undoubtedly has a strong selective influence on populations annually exposed to it.
In summary, we found remarkable fish densities of more than 200 individuals per m2 in some parts of the cave. While other selective forces certainly also need to be considered, we argue that the annual La Pesca has major effects on the population ecology and evolutionary trajectory of cave mollies. We are aware of potential caveats of this line of argument, as not all differences reported here may be due to the annual La Pesca ceremony. Nevertheless, from a conservational point of view, knowledge about whether and how human activities affect teleost populations is especially pertinent in the case of locally adapted populations that are endemic to a small area. Therefore, we recommend a repeated sampling before and after La Pesca in order to demonstrate the influence of the ritual. While human influences on highly endemic, locally adapted populations (or, in terms of conservation biology, evolutionary significant units; Moritz, 1994) generally are to be evaluated as highly problematic, management plans for cave mollies ought to consider the important role La Pesca plays in the religion of the local human population. Carried out in the traditional way, fish populations in downstream cave chambers can obviously recover after the ceremony. However, we wish to highlight the necessity to critically review that those practices do not affect deeper parts of the cave and that no commercially available, more efficient fish toxins will be employed in the future.
Supplemental Information
Acknowledgments
We thank J Dzienko, N Karau, A Oranth and S Stadler for their help in the field. Artwork (drawing of a cave molly female (Fig. 1A)) was prepared by M Ziege.
Funding Statement
The present study was financially supported by the research funding program “LOEWE–Landes-Offensive zur Entwicklung Wissenschaftlich-ökonomischer Exzellenz” of the Hessian Ministry of Higher Education, Research, and the Arts. Financial support also came from the Deutsche Forschungsgemeinschaft (PL 470/1-2) and from the Herrmann-Willkomm-Stiftung. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests. David Bierbach is an employee of the Leibniz-Institute of Freshwater Ecology and Inland Fisheries; Jonas Jourdan is an employee of the Biodiversity and Climate Research Centre.
Author Contributions
Jonas Jourdan analyzed the data, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
David Bierbach analyzed the data, reviewed drafts of the paper.
Rüdiger Riesch reviewed drafts of the paper.
Angela Schießl and Adriana Wigh performed the experiments.
Lenin Arias-Rodriguez performed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Jeane Rimber Indy contributed reagents/materials/analysis tools.
Sebastian Klaus analyzed the data, contributed reagents/materials/analysis tools.
Claudia Zimmer performed the experiments, analyzed the data, reviewed drafts of the paper.
Martin Plath conceived and designed the experiments, performed the experiments, analyzed the data, reviewed drafts of the paper.
Animal Ethics
The following information was supplied relating to ethical approvals (i.e., approving body and any reference numbers):
The methodology used in our study was a non-invasive technique. The study system was not influenced, and data recoding was based exclusively on visual observations.
Field Study Permissions
The following information was supplied relating to field study approvals (i.e., approving body and any reference numbers):
Current research was authorized by CONAPESCA-DGOPA.09004.041111.3088 and the Tacotalpa, Tabasco municipality.
References
- Bagarinao (1992).Bagarinao T. Sulfide as an environmental factor and toxicant: tolerance and adaptations in aquatic organisms. Aquatic Toxicology. 1992;24:21–62. doi: 10.1016/0166-445X(92)90015-F. [DOI] [Google Scholar]
- Barr Jr (1968).Barr TC., Jr Cave ecology and the evolution of troglobites. Evolutionary Biology. 1968;2:35–102. [Google Scholar]
- Bray & Curtis (1957).Bray JR, Curtis JT. An ordination of the upland forest communities of southern Wisconsin. Ecological Monographs. 1957;27:325–349. doi: 10.2307/1942268. [DOI] [Google Scholar]
- Camassa (2001).Camassa MM. Responses to light in epigean and hypogean populations of Gambusia affinis (Cyprinodontiformes:Poeciliidae) Environmental Biology of Fishes. 2001;62:115–118. doi: 10.1023/A:1011872509199. [DOI] [Google Scholar]
- Colaço, Dehairs & Desbruyeres (2002).Colaço A, Dehairs F, Desbruyeres D. Nutritional relations of deep-sea hydrothermal fields at the Mid-Atlantic Ridge: a stable isotope approach. Deep Sea Research Part I: Oceanographic Research Papers. 2002;49:395–412. doi: 10.1016/S0967-0637(01)00060-7. [DOI] [Google Scholar]
- Croft, Botham & Krause (2004).Croft DP, Botham MS, Krause J. Is sexual segregation in the guppy, Poecilia reticulata, consistent with the predation risk hypothesis? Environmental Biology of Fishes. 2004;71:127–133. doi: 10.1007/s10641-004-0092-5. [DOI] [Google Scholar]
- de Roos et al. (2007).de Roos AM, Schellekens T, van Kooten T, van de Wolfshaar KE, Claessen D, Persson L. Food-dependent growth leads to overcompensation in stage-specific Biomass when mortality increases: the influence of maturation versus reproduction regulation. American Naturalist. 2007;170:E59–E76. doi: 10.1086/520119. [DOI] [PubMed] [Google Scholar]
- Evans (1967).Evans CL. The toxicity of hydrogen sulphide and other sulphides. Quarterly Journal of Experimental Physiology and Cognate Medical Sciences. 1967;52:231–248. doi: 10.1113/expphysiol.1967.sp001909. [DOI] [PubMed] [Google Scholar]
- Flot, Wörheide & Dattagupta (2010).Flot J-F, Wörheide G, Dattagupta S. Unsuspected diversity of Niphargus amphipods in the chemoautotrophic cave ecosystem of Frasassi, central Italy. BMC Evolutionary Biology. 2010;10:171. doi: 10.1186/1471-2148-10-171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fontanier & Tobler (2009).Fontanier ME, Tobler M. A morphological gradient revisited: cave mollies vary not only in eye size. Environmental Biology of Fishes. 2009;86:285–292. doi: 10.1007/s10641-009-9522-3. [DOI] [Google Scholar]
- Gordon & Rosen (1962).Gordon MS, Rosen DE. A cavernicolous form of the poeciliid fish Poecilia sphenops from Tabasco, Mexico. Copeia. 1962;2:360–368. doi: 10.2307/1440903. [DOI] [Google Scholar]
- Goslee and Urban (2007).Goslee SC, Urban DL. The ecodist package for dissimilarity-based analysis of ecological data. Journal of Statistical Software. 2007;22(7):1–19. [Google Scholar]
- Hose & Pisarowicz (1999).Hose LD, Pisarowicz JA. Cueva de Villa Luz, Tabasco, Mexico: reconnaissance study of an active sulfur spring cave and ecosystem. Journal of Cave and Karst Studies. 1999;61:13–21. [Google Scholar]
- Hüppop (2005).Hüppop K. Adaptation to low food. In: Culver DC, White WB, editors. Encyclopedia of caves. Amsterdam: Elsevier; 2005. pp. 4–10. [Google Scholar]
- Jeffery (2001).Jeffery WR. Cavefish as a model system in evolutionary developmental biology. Developmental Biology. 2001;231:1–12. doi: 10.1006/dbio.2000.0121. [DOI] [PubMed] [Google Scholar]
- Jeffery (2009).Jeffery WR. Regressive evolution in Astyanax cavefish. Annual Review of Genetics. 2009;43:25–47. doi: 10.1146/annurev-genet-102108-134216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loekle, Madison & Christian (1982).Loekle DM, Madison DM, Christian JJ. Time dependency and kin recognition of cannibalistic behavior among poeciliid fishes. Behavioral and Neural Biology. 1982;35:315–318. doi: 10.1016/S0163-1047(82)90749-X. [DOI] [Google Scholar]
- Moritz (1994).Moritz C. Defining ‘evolutionarily significant units’ for conservation. Trends in Ecology & Evolution. 1994;9:373–374. doi: 10.1016/0169-5347(94)90057-4. [DOI] [PubMed] [Google Scholar]
- Nesbit & Meffe (1993).Nesbit DH, Meffe GK. Cannibalism frequencies in wild populations of the eastern mosquitofish (Gambusia holbrooki: Poeciliidae) in South Carolina. Copeia. 1993;3:867–870. doi: 10.2307/1447254. [DOI] [Google Scholar]
- Ohlberger et al. (2011).Ohlberger J, Langangen Ø, Edeline E, Claessen D, Winfield IJ, Stenseth NC, Vøllestad LA. Stage-specific biomass overcompensation by juveniles in response to increased adult mortality in a wild fish population. Ecology. 2011;92:2175–2182. doi: 10.1890/11-0410.1. [DOI] [PubMed] [Google Scholar]
- Parzefall (1993).Parzefall J. Behavioral ecology of cave-dwelling fishes. In: Pitcher TJ, editor. Behavior of teleost fishes. London: Chapman & Hall; 1993. pp. 573–606. [Google Scholar]
- Parzefall (2001).Parzefall J. A review of morphological and behavioural changes in the cave molly, Poecilia mexicana, from Tabasco, Mexico. Environmental Biology of Fishes. 2001;62:263–275. doi: 10.1023/A:1011899817764. [DOI] [Google Scholar]
- Parzefall et al. (2007).Parzefall J, Kraus C, Tobler M, Plath M. Photophilic behaviour in surface- and cave- dwelling Atlantic mollies Poecilia mexicana (Poeciliidae) Journal of Fish Biology. 2007;71:1225–1231. doi: 10.1111/j.1095-8649.2007.01581.x. [DOI] [Google Scholar]
- Plath et al. (2007).Plath M, Hauswaldt JS, Moll K, Tobler M, De Leon FJG, Schlupp I, Tiedemann R. Local adaptation and pronounced genetic differentiation in an extremophile fish, Poecilia mexicana, inhabiting a Mexican cave with toxic hydrogen sulphide. Molecular Ecology. 2007;16:967–976. doi: 10.1111/j.1365-294X.2006.03212.x. [DOI] [PubMed] [Google Scholar]
- Plath et al. (2013).Plath M, Pfenninger M, Lerp H, Riesch R, Eschenbrenner C, Slattery PA, Bierbach D, Herrmann N, Schulte M, Arias-Rodriguez L, Indy JR, Passow C, Tobler M. Genetic differentiation and selection against migrants in evolutionarily replicated extreme environments. Evolution. 2013;67:2647–2661. doi: 10.1111/evo.12133. [DOI] [PubMed] [Google Scholar]
- Plath et al. (2011).Plath M, Riesch R, Culumber Z, Streit B, Tobler M. Giant water bug (Belostoma sp.) predation on a cave fish (Poecilia mexicana): effects of female body size and gestational state. Evolutionary Ecology Research. 2011;13:133–144. [Google Scholar]
- Plath & Tobler (2010).Plath M, Tobler M. Subterranean fishes of Mexico (Poecilia mexicana, Poeciliidae) In: Trajano E, Bichuette ME, Kapoor BG, editors. The biology of subterranean fishes. Enfield, NH: Science Publishers; 2010. pp. 283–332. [Google Scholar]
- Por (2007).Por FD. Ophel: a groundwater biome based on chemoautotrophic resources. The global significance of the Ayyalon cave finds, Israel. Hydrobiologia. 2007;592:1–10. doi: 10.1007/s10750-007-0795-2. [DOI] [Google Scholar]
- Poulson (1964).Poulson TL. Animals in aquatic environments: animals in caves. In: Bill DB, editor. Handbook of physiology. Washington, D.C.: American Physiological Society; 1964. pp. 749–771. [Google Scholar]
- R Development Core Team (2008).R Development Core Team 2008. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria.
- Riesch, Plath & Schlupp (2010).Riesch R, Plath M, Schlupp I. Toxic hydrogen sulfide and dark caves: life-history adaptations in a livebearing fish (Poecilia mexicana, Poeciliidae) Ecology. 2010;91:1494–1505. doi: 10.1890/09-1008.1. [DOI] [PubMed] [Google Scholar]
- Riesch, Plath & Schlupp (2011).Riesch R, Plath M, Schlupp I. Toxic hydrogen sulphide and dark caves: pronounced male life-history divergence among locally adapted Poecilia mexicana (Poeciliidae) Journal of Evolutionary Biology. 2011;24:596–606. doi: 10.1111/j.1420-9101.2010.02194.x. [DOI] [PubMed] [Google Scholar]
- Riesch et al. (2014a).Riesch R, Plath M, Schlupp I, Tobler M, Langerhans RB. Colonisation of toxic environments drives predictable life-history evolution in livebearing fishes (Poeciliidae) Ecology Letters. 2014a;17:65–71. doi: 10.1111/ele.12209. [DOI] [PubMed] [Google Scholar]
- Riesch et al. (2014b).Riesch R, Reznick DN, Plath M, Schlupp I. 2014b. The impact of permanent darkness and food availability on life histories of surface- and cave-dwelling Atlantic mollies (Poecilia mexicana). Submitted.
- Roach, Tobler & Winemiller (2011).Roach KA, Tobler M, Winemiller KO. Hydrogen sulfide, bacteria, and fish: a unique, subterranean food chain. Ecology. 2011;92:2056–2062. doi: 10.1890/11-0276.1. [DOI] [PubMed] [Google Scholar]
- Romero & Green (2005).Romero A, Green S. The end of regressive evolution: examining and interpreting the evidence from cave fishes. Journal of Fish Biology. 2005;67:3–32. doi: 10.1111/j.0022-1112.2005.00776.x. [DOI] [Google Scholar]
- Sarbu, Kane & Kinkle (1996).Sarbu SM, Kane TC, Kinkle BK. A chemoautotrophically based cave ecosystem. Science. 1996;272:1953–1955. doi: 10.1126/science.272.5270.1953. [DOI] [PubMed] [Google Scholar]
- Schlupp et al. (2013).Schlupp I, Colston TJ, Joachim BL, Riesch R. Translocation of cave fish (Poecilia mexicana) within and between natural habitats along a toxicity gradient. Ecology of Freshwater Fish. 2013;22:228–233. doi: 10.1111/eff.12017. [DOI] [Google Scholar]
- Schröder, Persson & de Roos (2009).Schröder A, Persson L, de Roos AM. Culling experiments demonstrate size-class specific biomass increases with mortality. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:2671–2676. doi: 10.1073/pnas.0808279106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumann, Cavallaro & Hoback (2012).Schumann DA, Cavallaro MC, Hoback WW. Size selective predation of fish by Hydrophilis triangularis (Coleoptera: Hydrophilidae) and Lethocerus americanus (Hemiptera: Belostomatidae) Journal of the Kansas Entomological Society. 2012;85:155–159. doi: 10.2317/JKES120116.2.1. [DOI] [Google Scholar]
- Singer & Ramsay (1994).Singer TP, Ramsay RR. The reaction sites of rotenone and ubiquinone with mitochondrial NADH dehydrogenase. Biochimica Et Biophysica Acta-Bioenergetics. 1994;1187:198–202. doi: 10.1016/0005-2728(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Stearns (1976).Stearns SC. Life-history tactics: a review of the ideas. Quarterly Review of Biology. 1976;51:3–47. doi: 10.1086/409052. [DOI] [PubMed] [Google Scholar]
- Summers Engel (2005).Summers Engel A. Chemoautotrophy. In: Culver DC, White WB, editors. Encyclopedia of caves. Amsterdam: Elsevier; 2005. pp. 90–102. [Google Scholar]
- Timmermann & Plath (2009).Timmermann M, Plath M. Phototactic response and light sensitivity in an epigean and a hypogean population of a barb (Garra barreimiae, Cyprinidae) Aquatic Ecology. 2009;43:539–547. doi: 10.1007/s10452-008-9173-z. [DOI] [Google Scholar]
- Tobler et al. (2011a).Tobler M, Culumber Z, Plath M, Winemiller K, Rosenthal G. An indigenous religious ritual selects for resistance to a toxicant in a livebearing fish. Biology Letters. 2011a;7:229–232. doi: 10.1098/rsbl.2010.0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobler et al. (2011b).Tobler M, Palacios M, Chapman LJ, Mitrofanov I, Bierbach D, Plath M, Arias-Rodriguez L, de Leon FJG, Mateos M. Evolution in extreme environments: replicated phenotypic differentiation in livebearing fish inhabiting sulfidic springs. Evolution. 2011b;65:2213–2228. doi: 10.1111/j.1558-5646.2011.01298.x. [DOI] [PubMed] [Google Scholar]
- Tobler, Franssen & Plath (2008).Tobler M, Franssen CM, Plath M. Male-biased predation of a cave fish by a giant water bug. Naturwissenschaften. 2008;95:775–779. doi: 10.1007/s00114-008-0382-z. [DOI] [PubMed] [Google Scholar]
- Tobler et al. (2008a).Tobler M, DeWitt TJ, Schlupp I, García de León FJ, Herrmann R, Feulner PG, Tiedemann R, Plath M. Toxic hydrogen sulfide and dark caves: phenotypic and genetic divergence across two abiotic environmental gradients in Poecilia mexicana. Evolution. 2008a;62:2643–2659. doi: 10.1111/j.1558-5646.2008.00466.x. [DOI] [PubMed] [Google Scholar]
- Tobler et al. (2008b).Tobler M, Riesch R, de Leon FJG, Schupp I, Plath M. New and morphologically distinct population of cavernicolous Poecilia mexicana (Poeciliidae:Teleostei) Environmental Biology of Fishes. 2008b;82:101–108. doi: 10.1007/s10641-007-9258-x. [DOI] [Google Scholar]
- Tobler et al. (2008c).Tobler M, Riesch R, Garcíade León F, Schlupp I, Plath M. Two endemic and endangered fishes, Poecilia sulphuraria (Alvarez, 1948) and Gambusia eurystoma Miller, 1975 (Poeciliidae, Teleostei) as only survivors in a small sulphidic habitat. Journal of Fish Biology. 2008c;72:523–533. doi: 10.1111/j.1095-8649.2007.01716.x. [DOI] [Google Scholar]
- Tobler et al. (2009).Tobler M, Riesch RW, Tobler CM, Plath M. Compensatory behaviour in response to sulphide-induced hypoxia affects time budgets, feeding efficiency, and predation risk. Evolutionary Ecology Research. 2009;11:935–948. [Google Scholar]
- Tobler et al. (2006).Tobler M, Schlupp I, Heubel KU, Riesch R, de Leon FJG, Giere O, Plath M. Life on the edge: hydrogen sulfide and the fish communities of a Mexican cave and surrounding waters. Extremophiles. 2006;10:577–585. doi: 10.1007/s00792-006-0531-2. [DOI] [PubMed] [Google Scholar]
- Tobler, Schlupp & Plath (2007).Tobler M, Schlupp I, Plath M. Predation of a cave fish (Poecilia mexicana, Poeciliidae) by a giant water-bug (Belostoma, Belostomatidae) in a Mexican sulphur cave. Ecological Entomology. 2007;32:492–495. doi: 10.1111/j.1365-2311.2007.00892.x. [DOI] [Google Scholar]
- Trajano (2001).Trajano E. Ecology of subterranean fishes: an overview. Environmental Biology of Fishes. 2001;62:133–160. doi: 10.1023/A:1011841913569. [DOI] [Google Scholar]
- Werner & Gilliam (1984).Werner EE, Gilliam JF. The ontogenetic niche and species interactions in size-structured populations. Annual Review of Ecology and Systematics. 1984;15:393–425. doi: 10.1146/annurev.es.15.110184.002141. [DOI] [Google Scholar]
- Wilkens (1988).Wilkens H. Evolution and genetics of epigean and cave Astyanax fasciatus (Characidae, Pisces) Evolutionary Biology. 1988;23:271–367. [Google Scholar]
- Wilkens (2001).Wilkens H. Convergent adaptations to cave life in the Rhamdia laticauda catfish group (Pimelodidae, Teleostei) Environmental Biology of Fishes. 2001;62:251–261. doi: 10.1023/A:1011897805681. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.