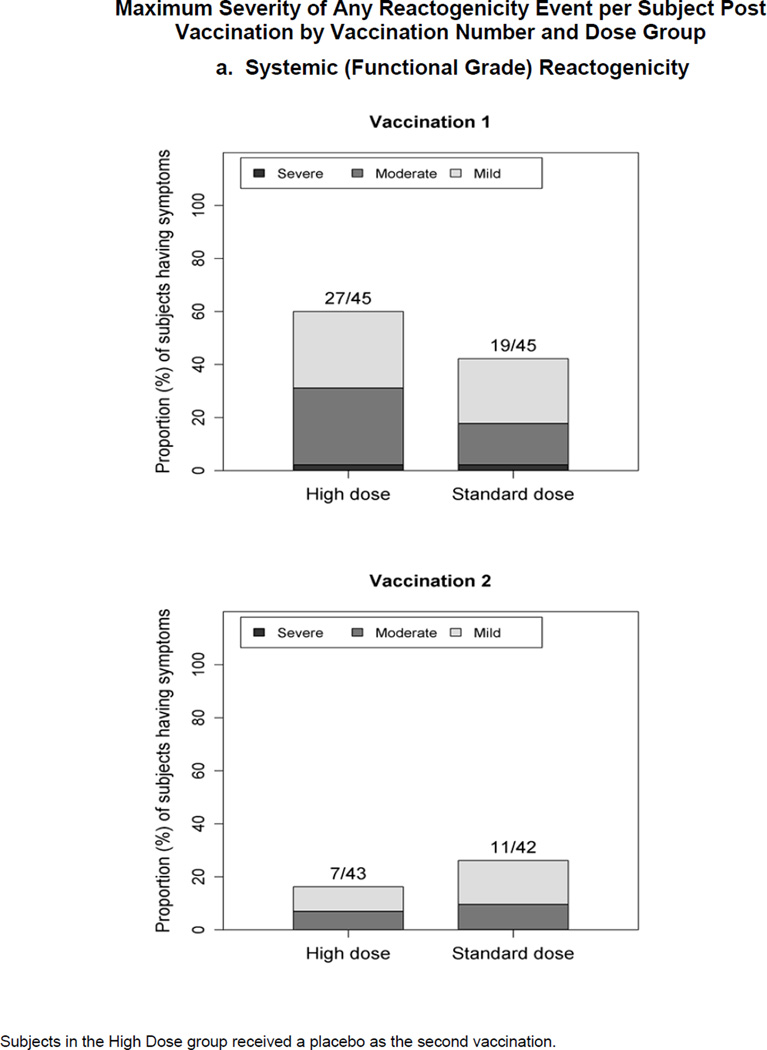
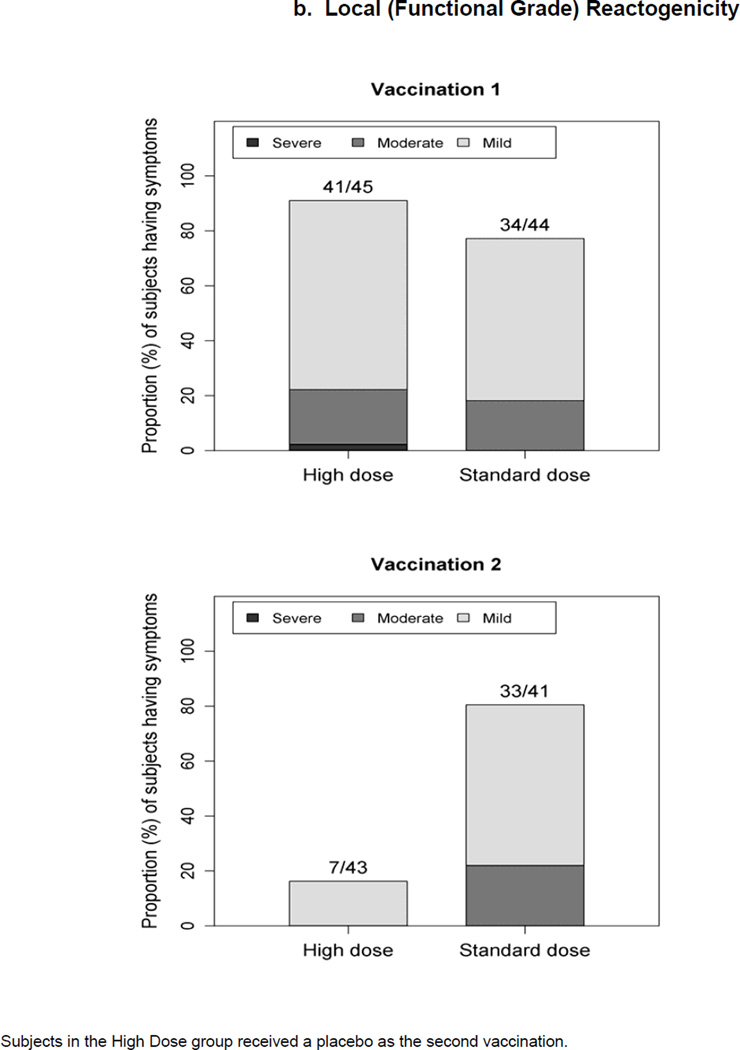
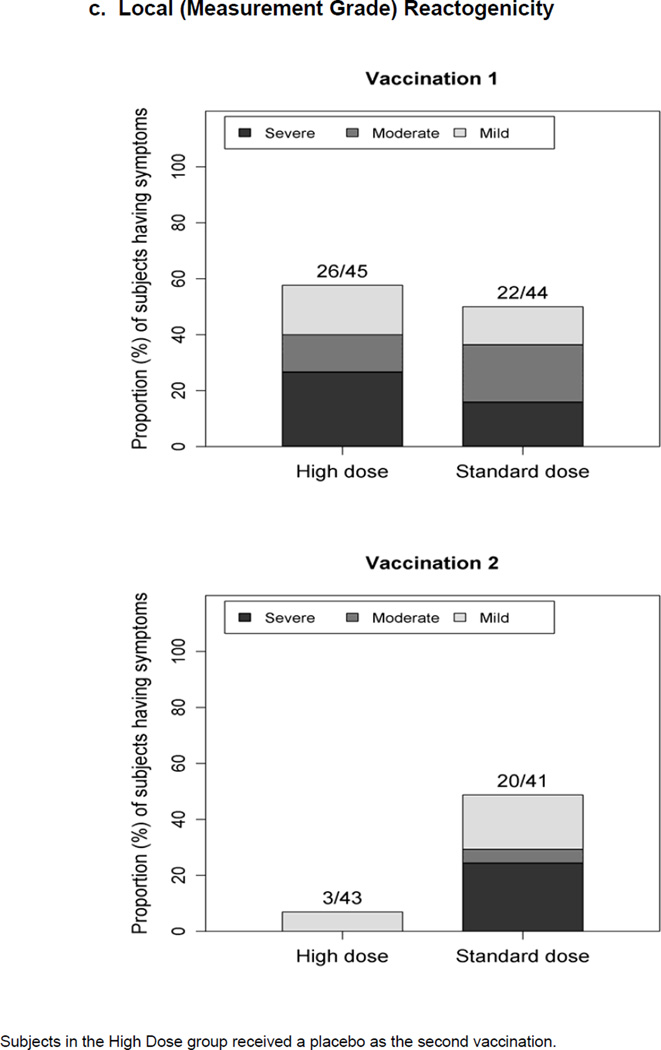
Figure 1.
Maximum severity grade of any reactogenicity event collected per subject in the HD and SD groups for 15 days (Days 0–14) after each vaccination. Erythema and Induration were measured and graded as mild (<15 mm), moderate (15–30 mm) or severe (>30 mm). Other AEs including pain at the injection site, redness, swelling (induration was assessed by the clinical staff), muscle aches, chills, headache, nausea, feeling tired, underarm pain, underarm swelling, itchiness at vaccination site, change in appetite, and joint pain were graded using a functional scale of mild (present but easily tolerated), moderate (able to tolerate routine activity with effort), and severe (unable to continue routine activity). Fever grading scale for oral temperature (°C) was mild 38.0 – 39.0, moderate >39.0 – 40.0, severe >40.0; fever is captured under systemic reactogenicity. Figure 1.a. a) Systemic (functional grade) reactogenicity; b) Local (functional grade) reactogenicity; c) Local (measurement grade) reactogenicity.