INTRODUCTION
Clinically managing acute endocarditis, an infection of the heart valves with a mortality rate of up to 47%, remains highly challenging and frequently unsuccessful (1, 2). The most common pathogen behind acute endocarditis is Staphylococcus aureus, followed by Streptococcus species (2, 3). Unmet clinical needs in treating acute endocarditis include: (i) more reliably diagnosing or excluding endocarditis, (ii) quickly and precisely identifying the pathogen dwelling in vegetations informing selection of antibiotics, and (iii) acquiring more quantitative data to guide decisions about surgical intervention. Currently, clinically diagnosing endocarditis relies on the modified Duke criteria (4), which combine major and minor criteria including echocardiographic imaging, clinical signs such as a new heart murmur or fever, and detection of circulating bacteria in blood cultures. Unfortunately, blood cultures can be misleading; false-negative results occur when the pathogen is present in the vegetation but not in circulation at the time of blood withdrawal, as can occur, for instance, after initiation of antibiotic therapy. The following case report from the Massachusetts General Hospital clinical service highlights this typical problem.
Case Report
A 71-year-old man with a history of mitral valve prolapse and mitral regurgitation presented with a three-week history of worsening dyspnea on exertion and fatigue. He denied fever, chills, syncope, chest pain, nausea, and vomiting. His temperature was initially 98.0 degrees F with a transient spike to 101 degrees F. His blood pressure was 109/53 mmHg with a pulse of 92 beats per minute. He had a non-painful non-pruritic rash on his lower extremities. Blood cultures were positive for Enterococcus faecalis. Transthoracic echocardiogram (TTE) showed a thickened mitral valve with mitral valve prolapse, severe mitral regurgitation, and a partially flail posterior leaflet, but no definitive vegetation. This result was judged to be unchanged from a prior echocardiography 4 months earlier. He was treated with vancomycin, ceftriaxone, and gentamycin for presumed endocarditis and discharged with a 4-week course of ampicillin and gentamycin. Clinically, the infection seemed to have cleared. Six months later, he underwent elective surgical mitral valve repair. The preoperative transesophageal echocardiogram showed no significant changes from the prior two echo studies, with no definitive evidence of vegetation. During the mitral valve repair, the surgeon observed and then resected a vegetation. Pathologic examination showed infective endocarditis with a fibrin vegetation containing Gram-positive cocci (Fig. 1). This case highlights the limitations of echocardiography: surgery was pursued because echo studies failed to indicate infection. Molecular imaging tools, by contrast, would have detected residual infection and thereby altered clinical management so that the patient could have been treated with another, possibly more specific, course of antibiotics to clear the infection before surgery.
Figure 1. Clinical Case of Recurrent Gram-positive Endocarditis.
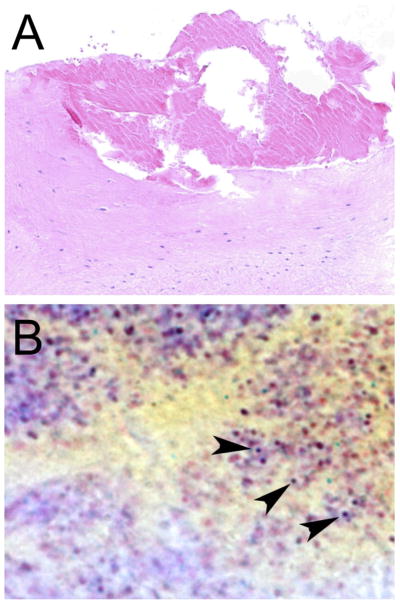
A. Hematoxylin and eosin (H&E) stained histologic section showing fibrin vegetation (top) on the mitral valve (bottom), 100× magnification. B. Tissue Gram stain showing Gram positive cocci (arrowheads) in the vegetation, 2000× magnification.
SCOPE OF THE PROBLEM AND DIAGNOSTIC GAPS
As indicated by the above case report, the clinical diagnosis of endocarditis remains difficult and partially relies on unspecific signs. Guidelines (4) recommend the use of major and minor modified Duke criteria, which include a new or changing heart murmur, fever, detection of circulating bacteria in blood cultures, predisposing conditions, Osler nodes, Janeway’s lesions, splenomegaly, and serial imaging by echo (including transesophageal echocardiograms). These serial echo studies are often done days apart to assess vegetation growth, an important factor in timing surgical intervention (5). However, none of these criteria, except for histological assessment of excised valve material, directly report on bacterial presence in cardiac vegetation. Definite diagnosis is only reached by vegetation pathology, as in the case report presented above, or through several combinations of the aforementioned criteria (two major criteria, or one major and three minor criteria, or five minor criteria)(6). Echocardiograms do not inform on the pathogen and can be false-negative in 15% of cases. Molecular imaging promises to address at least some of these hurdles, as it may provide the opportunity to quantify specific bacteria in endocarditis lesions, akin what currently is only provided by ex vivo histopathology.
Disease Origins
Endocarditis is a life-threatening inflammation of cardiac endothelium of the heart valve and surrounding tissue that results from an underlying viral, fungal, or bacterial heart infection. Endocarditis is rare in healthy individuals without congenital heart disease but can be fatal due in part to its rapid onset. Broadly, endocarditis is divided into native valve bacterial endocarditis (NBVE) and prosthetic valve bacterial endocarditis (PVBE). Coagulase-positive S. aureus and coagulase-negative S. epidermidis are the major causal pathogens of both types of endocarditis (2, 4). S. aureus endocarditis occurs in approximately 40–50% of neonatal cases and 30–40% of cases in adults 16–60 years, and it has a high mortality rate even with antibiotic therapy (2, 7). In the absence of surgery or a central line causing damage to the heart, vegetations may develop as a result of normal heart valve function (i.e. the mechanical strain of constantly opening and closing). Turbulent flow caused by congenital defects can increase localized pressure that denudes endothelial cells and consequently exposes pro-coagulant surfaces. Such damage is usually repaired quickly, but if bacteria circulate in the blood stream they may attach to the repair site, as illustrated in Fig. 2. Vegetations are predominately composed of platelets, fibrin, and actively replicating bacteria (7). In the late stage of endocarditis, vegetations can lead to valvular stenosis or insufficiency (8), pannus formation in PVBE (9), and, in advanced cases, complete valve destruction via microbe consumption (10). Compromised valve function ultimately leads to heart failure (11). With incidence rising to >15 per 100,000 patients per year, healthcare-associated endocarditis has become a major issue (4).
Figure 2. Pathogenesis of S. aureus Endocarditis.
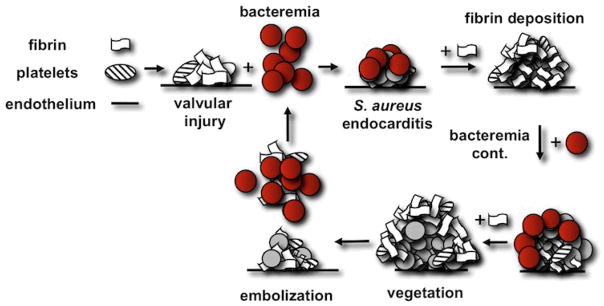
Initially, valve damage causes a small clot to form on the denuded endothelium. Next, circulating new bacteria (highlighted in red) attach, and prothrombin activators are secreted to elicit layered fibrin deposition (modified from Korzeniowski et al. (65)).
The majority of life-threatening acute endocarditis cases stem from coagulase-positive S. aureus infections, and streptococcal species are the second most prevalent trigger. More specifically, NVBE is predominantly caused by S. aureus, followed by coagulase-negative staphylococci, enterococci, and Streptococcus bovis infections (12). Although Staphylococcal infections account for the majority of cases, the frequency of Enterococcus and Streptococcus bovis isolation is more than 2-fold greater for patients with diabetes mellitus (13). In the future, next-generation sequencing efforts and whole genome analysis of endocarditis pathogens will show how they adapt during antibiotic therapies. For example, a patient with congenital heart disease was diagnosed with endocarditis, and the pathogen was sequenced from blood collected prior to and during the 12 weeks of therapy, which included rifampin, imipenem, vancomycin, and valve replacement surgery (14, 15). The resulting genomes (JH1-JH9) revealed the timing and frequency of mutations that increase antibiotic resistance and cell survival, thereby providing a glimpse into microbe evolution during disease (14, 15).
Treatment Options
Because its prognosis depends on aggressive and precise treatment, endocarditis urgently needs early, reliable diagnostics in the clinic. Intensive antibiotic therapy, typically lasting 4–6 weeks, is the first line of treatment, though surgically removing the infected valve may become necessary in aggressive cases. Timely treatment is complicated by the difficulty of both properly diagnosing endocarditis and correctly identifying the causative microorganism. The choice of antibiotics relies on blood culture results, but these can be sterile or misleading. Physician experience and estimated infection route often determine initial antibiotic selection. Subsequent treatment adjustments respond to clinical signs and rely largely on trial and error. Valve replacement surgery is a potentially life-saving treatment option, though open-heart surgery carries major risks. The purpose of surgery is to remove the vegetation and bacterial population, restore valve function and preserve cardiac output. Surgery can also reduce the risk of septic emboli, which may irreversibly damage the brain and kidneys. Emerging data show that early surgery may be beneficial, but there are no ideal criteria on which to base interventional decisions and timing (16, 17).
THE ROLE OF IMAGING
Numerous preclinical and clinical studies highlight ways molecular imaging may ameliorate diagnostic shortcomings in managing endocarditis (Table 1). The unmet clinical need to improve diagnostic options has led to preclinical and good-sized clinical studies that investigate specific imaging of heart valve infection. Most investigators used sensitive nuclear and, for preclinical studies, optical imaging techniques to detect the small and sparse targets associated with endocarditis vegetations.
Table 1.
Current Approaches to Imaging Endocarditis.
| Endocarditis Imaging Strategies | Modality | Pre-Clinical | Clinical | Mechanisms of Action/Limitations |
|---|---|---|---|---|
| Bioluminescence | Optical | ✔ | Requires a Lux operon engineered bacteria/No potential for clinical use | |
| [AF680]-ProT | Optical | ✔ | Staph. aureus binds to pathogen elaborated staphylocoagulase and von Willebrand binding protein localized to vegetation/No clinical data available | |
| [64Cu]-DPTA-ProT | PET | ✔ | ||
| [64Cu]-DOTA-labeled anti-pilin monoclonal antibody | PET | ✔ | Targets endocarditis and biofilm associated pili (Ebp) of Enterococcus faecalis/No clinical data available | |
| Radiolabeled fibrin antibodies (e.g. 111In or 99Tc) | PET, SPECT | ✔ | Deposited at the site of the vegetation or clot formation/does not distinguish pathogen from a clot | |
| Radiolabeled leukocytes (e.g. 111In or 99Tc) | ✔ | ✔ | Ex vivo labeling of host cells allows for effective tracking/does not distinguish between inflammation and infection | |
| Radiolabeled antibiotics and macrolides (e.g. 99Tc-ciprofloxacin and 3H-spiramycin) | SPECT | ✔ | ✔ | Targets bacteria through resistance mechanism/modifications may inactivate the antibiotic, thereby voiding imaging studies |
| [18F]-FDG | PET-CT | ✔ | ✔ | Marks cells with high glucose uptake/does not distinguish between inflammation and infection |
| Transthoracic echocardiogram (TTE) | Echo | ✔ | Ultrasound-based/Requires serial imaging to assess vegetation development to be effective | |
| Transesophageal echocardiogram (TEE) |
Bioluminescent Imaging of Bacteria
Light-emitting microbes are a powerful new tool for developing preclinical probes and evaluating drug efficacy. Broadly, there are three reporter classes for biolumenscent imaging (BLI): firefly (coleoptera) (18), jellyfish and sea pansies (cnidarian) (19), and bacteria (Vibrio spp. and Photorhabdus luminescens) (20). Imaging bacteria relies on the latter, but the light production is different from that used for in vivo imaging of cancer progression in animal models. BLI studies of cancer cells typically require single protein luciferase expression (e.g. lux2 under a human ubiquitin C promoter) and subsequent intraperitoneal injection with luciferase substrates (luciferin) to generate light through adenosine triphosphate (ATP) -dependent oxidation of the acid side chain of luciferin to oxyluciferin. The “glowing” luciferin is cleared rapidly from the animal, requiring re-injection for longitudinal studies. The process of generating light emission to image biolumenscent bacterial pathogens is more complicated and based on transposon insertion of a lux operon that consists of LuxABCDE genes from P. luminescens into the microbe by use of temperature-sensitive plasmids. The lux operon encodes the heterodimeric luciferase (luxA and B), long-chain fatty aldehydes (decanal), and reduced flavin mononucleotides (FMN) (luxC-E). To produce light, the luciferase converts the aliphatic-aldehyde substrate decanal, oxygen, and reduced FMN into the resulting aliphatic acid, water, FMN, and light at a wavelength of 490nm (21). The names of several bacterial strains comprise the Xen prefix, meaning originally developed by Xenogen, followed by numerical reference code. For instance, Xen 10 refers to a Streptococcus pneumonia A66.1 (22), while Xen 29 is derived from the Staphylococcus aureus ATCC 12600 parent strain (23, 24). Bacterial BLI has been used by researchers such as Xiong et al, who used bioluminescent S. aureus to monitor acute bacterial endocarditis in rats using a catheter-based injury prior to bacterial injection (23). The technique can also be used to track vegetation bacteria’s response to newly developed antibiotics. Interestingly, a recent report by Close et al. indicates the disparity between mammalian and bacterial bioluminescence production may soon be resolved through HEK 293 cells’ ability to express mammalian codon-optimized lux CDABE operon that can be used to image these cells in vivo (25). Future studies will no doubt identify additional applications for BLI techniques.
Despite its obvious advantages, using these light-emitting microbes can be challenging. Photons scatter as they move from the infection site into surrounding tissues. In addition, BLI is inherently two-dimensional, though the advent of bimodal bioluminescence/X-ray systems provides better spatial information in the x/y plane and improved anatomical information. True 3D bimodal instruments are being explored but are not yet widely available (26). Another important consideration, given the lux operon’s transposon incorporation and forced microbial evolution through antibotic selection, is that the lux operon insertion site, selected solely for maximum light production per colony, is inconstant among pathogens. Additionally, BLI requires a non-naturally occurring bacterium, which means the strategy is not translatable to the clinic. These challenges and incosistencies need to be addressed for BLI to progress as an effective research tool.
Targeted Imaging of Vegetations
In our experience with a mouse model of endocarditis (Fig. 3), BLI very effectively screens animals for bacterial colonization verification prior to more involved procedures, such as targeted imaging by PET-CT. Preliminary BLI allows us to pre-select mice with definite infection for costly and time intensive experiments.
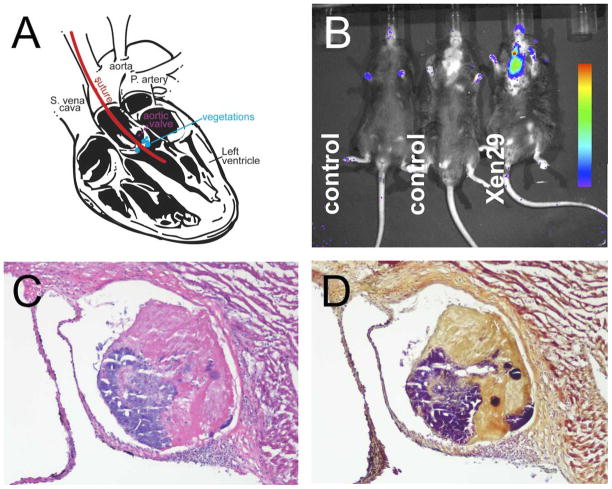
Figure 3. Bioluminescence Imaging in a Mouse Model of Endocarditis.
A. Induction involves catheter insertion followed by intravenous injection of bacteria. B. Bioluminescence imaging after infection with the S. aureus strain Xen 29. C. H&E stain of vegetation in aortic root. D. Gram stain of an adjacent section. Modified from Panizzi et al. (27)).
Advanced preclinical imaging techniques such as fluorescence molecular tomography (FMT) and positron emission tomography (PET) fused with computed tomography (FMT-CT or PET-CT, respectively) visualize novel fluorescently- or chelator-labeled prothrombin analogs in a mouse model of S. aureus endocarditis (Fig. 4A (27)). Prothrombin is a key blood-clotting zymogen, and using its analogs harnesses its high-affinity interactions. S. aureus expresses two prothrombin activators, staphylocoagulase (28, 29) and von Willebrand binding protein (30). Prothrombin analogs were generated by incubating prothrombin with recombinant N-terminal staphylocoagulase fragment fused to His6-tag (29). The resulting complex is proteolytically active and subsequently covalently inactivated with a tri-peptide thrombin inhibitor that was modified with N-succinimidyl (acetylthio)acetate, thereby adding a sulfhydryl group in the form of a protected thiol moiety. Separating the prothrombin probe analogs took advantage of the terminal His6-tag’s unique ability to function even under reducing conditions. Deprotecting the thiol produced single-site probe incorporation. Once free of the staphylocoagulase, the prothrombin analogs partially revert to the zymogen state but have no protease potential when used in vivo, so that the imaging agent has no effect on the host’s clotting system. The specific site-labeling also preserves all exosite I interaction, which is important for fibrinogen, hirudin, factor V/Va, and staphylocoagulase binding (28, 29, 31). Intravenously injecting these labeled prothrombin analogs facilitated in vivo tracking of prothrombin being sequestered from host blood and deposited into the growing fibrin layers of endocarditis vegetations (27). Noninvasive FMT/CT imaging monitored antibiotic therapy and infection relapse. Co-localizing the imaging agent with BLI signal from the bioluminescent S. aureus confirmed specificity in a given region of interest. Clinically translating this fluorescent imaging agent will depend on labeling prothrombin with PET isotopes (Fig 4B).
Figure 4. Imaging of Endocarditis with an Agent Targeted to S. aureus Produced Staphylocoagulase.
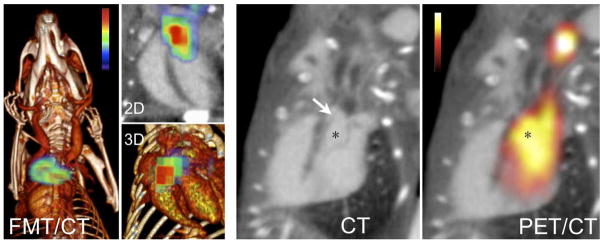
Left panels show a fluorochrome prothrombin analogon for optical imaging, right panels a 64-copper labeled PET version of the reporter. The arrow points out the suture in the left ventricular outflow tract (asterisk). Modified from Panizzi et al. (27).
Addressing the increasing incidence of enterococcus endocarditis in diabetic patients, Pinkston et al. developed a PET reporter for Enterococcus faecalis endocarditis and biofilm associated pili (Ebp). A [64Cu]-DOTA-monoclonal antibody recognizes Ebp. The authors confirmed cell binding with transmission electron microscopy and used in vivo PET-CT in rats with enterococcus endocarditis (32).
Previously, imaging of endocardial fibrin deposition by pathogenic bacteria relied on fibrin-targeted antibodies labeled with either 111Indium (111In) (33, 34) or Technetium (99Tc) (35). This approach detected endocarditis in rabbits after catheter injury of the valves followed by inoculation with Streptococcus sanguinis (35). The fibrin-targeted antibodies also partially inhibited vegetation development (35). Other strategies used labeled immune or host cells (i.e. platelets or granulocytes) to track immune cells deposit at the infection site (36, 37). While quite sensitive, these strategies do not discriminate infection, let alone the characteristics of a specific pathogen. Using pooled anti-staphylococcus antibodies derived from endocarditis patients resulted in limited accumulation of imaging tracer in vegetations (38). Modified antibiotics may also detect bacterial infections. For instance, vancomycin was conjugated to a near-infrared fluorochrome for fluorescence imaging (39) or to iron oxide nanoparticles detectable by MRI (40). Effective vegetation penetration was noted for 3H-spiramycin (41), a macrolide antibiotic that inhibits bacterial protein synthesis. Future imaging approaches that could determine antibiotic susceptibility or resistance of microbes in vegetations would provide a powerful tool for the choice of appropriate antibiotics. Other preclinical imaging agents, such as stannous pyrophosphate (Sn2P2O7) (99Tc-PYP) (42) or a synthetic zinc (II) coordination complex that targets bacterial cells’ anionic surfaces (43, 44), rely on surface charge differences between the pathogens and mammalian tissue. Imaging strategies using labeled annexin V, which binds phosphatidylserine on apoptotic cells, and activated platelets have also been used in experimental endocarditis (45).
Future Smart Probes for Imaging Endocarditis
There are a number potentially useful targeted imaging probes that have yet to be tested in endocarditis. One interesting example is an imaging agent that is taken up by bacteria via the maltodextrin transport pathway. That mammalian host cells do not express this transporter may drastically reduce background uptake of the maltohexaose-conjugated fluorochrome reporter (46). Another recently-developed fluorescent imaging agent specifically targets S. aureus via a nuclease cleaved probe (47). This activatable reporter relies on unquenching a fluorochrome when a target sequence, which is specific for the nuclease produced by the bacterium but not by host cells, is cleaved. While this probe may enable multispectral parallel detection of several pathogens, the optical technology it uses makes clinical translation for non-invasive imaging challenging. These promising new targeting methods warrant preclinical testing in an endocarditis model.
Clinical endocarditis imaging
When endocarditis is suspected, TTE is mandatory and often supplemented by TEE. TTE efficacy is limited by fat and bones in the chest wall, lung interference, and, especially in patients with infected prosthetic valves, the presence of artificial heart valves (48). By comparison, TEE provides higher-resolution images of aortic anatomy and is less hindered by air in the lungs. In a series of 118 patients with endocarditis confirmed by either surgery or autopsy, TEE was >3.5-fold better at detecting cardiac abscesses than TTE (49). Although TEE is more effective than TTE, neither method provides specific information beyond anatomy and valve function. Additionally, their efficacy often depends on tracking changes in anatomy and valve function over several imaging sessions. In 262 patients with suspected endocarditis, 6 patients had at least 6 TTEs and 4 patients had at least 4 TEEs (50). Serial echo is estimated to waste 120 million dollars annually (5, 50).
The lack of specific diagnostic imaging agents for endocarditis limits clinical diagnosis of S. aureus endocarditis. Existing methods such as echocardiography are very valuable but can be hampered by ambiguous results. Acute endocarditis may develop rapidly – within two weeks –but for effective diagnosis, the clinician must wait until the vegetation of bacteria, fibrin and platelets is large enough to be detected by echocardiogram. By this time, treatment may be less effective as bacteria wall themselves off with fibrin-platelet shields (17, 18). In addition to making diagnoses quicker and more accurate, specialized endocarditis imaging agents could improve clinician’s ability to monitor disease progression and administer effective antibiotic therapy.
Clinical studies have begun exploring PET imaging for endocarditis with methods used to detect other bacterial infections. For example, PET imaging18F-FDG labeled leukocytes shows leukocyte accumulation without reporting on specific pathogens (51). Other researchers are investigating single-photon emission computerized tomography (SPECT)-based imaging agents. Patients’ leukocytes are isolated, incubated ex vivo with a chelated 99Tc or 111In, and re-injected to determine sites of inflammation associated with endocarditis or pannus formation (52–54). In one example, infection sites identified by SPECT were true positive in 35 of 40 patients patients with significant inflammatory disease components, including later stages and abscesses (52). Although promising, leukocyte tracking may not adequately identify small vegetations, particularly in cases where biofilm-forming pathogens are seeded on prosthetic devices. Similar limitations may apply to 99Tc-labeled leukocyte antagonists (55, 56), the human neutrophil peptide HNP-1 (57), platelet GPIIb/IIIa receptor antagonists (58, 59), and anti-granulocyte monoclonal antibodies (60, 61). Despite these challenges, diagnosis with PET or SPECT isotope-labeled antibiotics remains an attractive option for clinical imaging. In a large study on 99Tc-ciprofloxacin efficacy in detecting infection sites, endocarditis was identified with 100% specificity, using blood culture results as the gold standard, in 26 patients (62).
18F-FDG PET/CT imaging is currently the most promising option for clinical molecular imaging of endocarditis. An image example provided in Fig. 5 shows the isotope-labeled glucose analogon trapped in lesions, likely in cells with high glucose uptake. In endocarditis, these are probably leukocytes that accumulate at the site of infection. Several 18F-FDG PET/CT clinical trials have yielded promising results. For instance, 18F-FDG appears to be particularly helpful in detecting endocarditis in patients with artificial heart valves. Endocarditis is frequent among these patients, but the prosthetic valves make diagnostic echocardiography less effective. In a study by Saby et al., among 13 patients with proven endocarditis, PET/CT imaging was positive in 12 patients whereas echocardiography was positive in only 7 patients (63). A larger study of 345 patients revealed that adding 18F-FDG PET/CT imaging to the clinical management of 115 endocarditis patients decreased morbidity and mortality. Mortality in the 18F-FDG PET/CT group was 19%, compared to 32% in the control group (64). These studies indicate that 18F-FDG PET imaging should already be considered in current clinical care, especially in difficult or ambiguous cases. However, the method also has disadvantages, including alack of specificity for bacterial presence and potential background uptake of 18F-FDG in myocardium. In addition, freshly operated valves will return false positive results due to post-surgical inflammation in the healing heart.
Figure 5. Clinical 18FDG PET/CT Imaging.
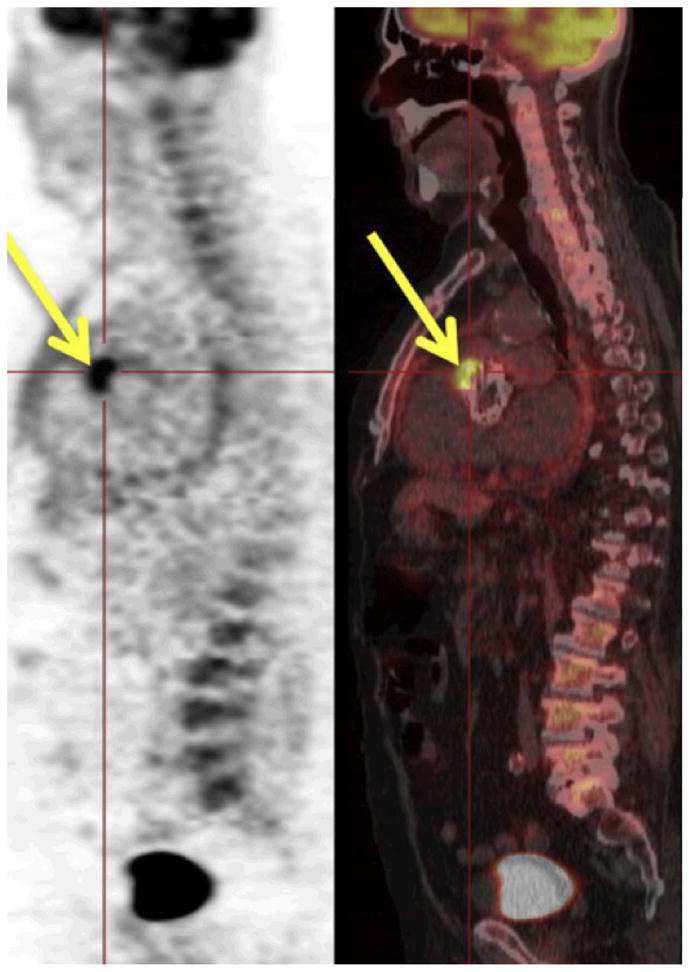
PET/CT in a patient with a definite diagnosis of prosthetic valve endocarditis. The high uptake (yellow arrows) is observed at the level of the anterior aortic annulus. From Saby et al. (63).
FUTURE DIRECTIONS
Methods that specifically detect infectious pathogens are the most promising developmental direction for imaging endocarditis. At this point, however, it is unclear which methods or affinity ligands will be most suitable for clinical translation. In this review, we described work using antibodies targeted to specific bacterial strains, molecules that attach to virulence factors secreted by S. aureus, isotope-labeled antibiotics, and agents that are taken up and metabolized by bacteria but not human host cells. If translated, any of these approaches could diagnose endocarditis and detect infectious embolisms, which are applications with clear clinical utility, as well as track local bacterial response to antibiotics, which would be useful in both drug trials and clinical cases where individual response is unclear. One challenge for researchers going forward is that imaging agents targeting endocarditis that can identify a specific bacterial strain may miss other pathogens or mixed infections. Possibly, multimodal clinical molecular imaging, such as PET/MRI, could solve this problem via a dual reporter approach that pairs a broadly sensitive but unspecific agent with a second agent that precisely detects a suspected prevalent strain.
ACTION PLANS
Clinically developing advanced molecular imaging tools for endocarditis will be expensive. Despite the serious clinical need posed by endocarditis, its specialized molecular imaging agents will be a niche indication. Consequently, the pharmaceutical industry may have little interest in developing and marketing molecular imaging systems for endocarditis. Academic centers and public funding agencies will likely have to carry forward this important work, which will include i) development and validation of new pathogen-specific probes targeting the top endocarditis pathogens, ii) discovery of pathogenic mechanisms that can serve as imaging targets, iii) implementation of technologies that are capable of specific and cost effective endocarditis detection, and iv) testing of dual reporter approach for multimodal clinical molecular imaging, such as PET/MRI.
Acknowledgments
This work was supported by the National Institutes of Health through grants provided by the National Heart, Lung, and Blood Institute, R00HL094533 (to P.P.) & R01HL114477 (to P.P. and M.N.), the National Institute of Allergy and Infectious Diseases grant 2R44AI085840-02 (to P.P.).
Abbreviations
- TTE
Transthoracic echocardiogram
- TEE
Transesophageal echocardiogram
- FMT-CT or PET-CT
respectively, fluorescence molecular tomography (FMT) or positron emission tomography (PET) fused with computed tomography
- SPECT
single-photon emission computed tomography
- NBVE
native valve bacterial endocarditis
- PVBE
prosthetic valve bacterial endocarditis
- BLI
biolumenscent imaging
- DOTA
1,4,7,10-tetraazacyclododecane-1,4,7,10-tetraacetic acid
- 111In
111Indium
- 99Tc
Technetium
- Sn2P2O7) (99Tc-PYP
stannous pyrophosphate
- MAb
monoclonal antibody
- 18F-FDG
2-deoxy-2-(18F)fluoro-D-glucose
References
- 1.Bayer AS, Bolger AF, Taubert KA, Wilson W, Steckelberg J, Karchmer AW, et al. Diagnosis and management of infective endocarditis and its complications. Circulation. 1998;98:2936–48. doi: 10.1161/01.cir.98.25.2936. [DOI] [PubMed] [Google Scholar]
- 2.Mylonakis E, Calderwood SB. Infective endocarditis in adults. N Engl J Med. 2001;345:1318–30. doi: 10.1056/NEJMra010082. [DOI] [PubMed] [Google Scholar]
- 3.Fowler VG, Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, et al. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA: the journal of the American Medical Association. 2005;293:3012–21. doi: 10.1001/jama.293.24.3012. [DOI] [PubMed] [Google Scholar]
- 4.Baddour LM, Wilson WR, Bayer AS, Fowler VG, Jr, Bolger AF, Levison ME, et al. Infective endocarditis: diagnosis, antimicrobial therapy, and management of complications: a statement for healthcare professionals from the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association: endorsed by the Infectious Diseases Society of America. Circulation. 2005;111:e394–434. doi: 10.1161/CIRCULATIONAHA.105.165564. [DOI] [PubMed] [Google Scholar]
- 5.Cabell CH, Fowler VG., Jr Repeated echocardiography after the diagnosis of endocarditis: too much of a good thing? Heart. 2004;90:975–6. doi: 10.1136/hrt.2003.031328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li JS, Sexton DJ, Mick N, Nettles R, Fowler VG, Jr, Ryan T, et al. Proposed modifications to the Duke criteria for the diagnosis of infective endocarditis. Clinical infectious diseases: an official publication of the Infectious Diseases Society of America. 2000;30:633–8. doi: 10.1086/313753. [DOI] [PubMed] [Google Scholar]
- 7.Durack DT. Infective and noninfective endocarditis. The Heart: Arteries and Veins. 1990:1230–55. [Google Scholar]
- 8.Sacks PV, Lakier JB, Barlow JB. Severe aortic stenosis produced by bacterial endocarditis. British medical journal. 1969;3:97–8. doi: 10.1136/bmj.3.5662.97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pibarot P, Dumesnil JG. Prosthetic heart valves: selection of the optimal prosthesis and long-term management. Circulation. 2009;119:1034–48. doi: 10.1161/CIRCULATIONAHA.108.778886. [DOI] [PubMed] [Google Scholar]
- 10.Slabbekoorn M, Horlings HM, van der Meer JT, Windhausen A, van der Sloot JA, Lagrand WK. Left-sided native valve Staphylococcus aureus endocarditis. The Netherlands journal of medicine. 2010;68:341–7. [PubMed] [Google Scholar]
- 11.Tiong IY, Novaro GM, Jefferson B, Monson M, Smedira N, Penn MS. Bacterial endocarditis and functional mitral stenosis: a report of two cases and brief literature review. Chest. 2002;122:2259–62. doi: 10.1378/chest.122.6.2259. [DOI] [PubMed] [Google Scholar]
- 12.Cecchi E, Imazio M, Trinchero R. The changing face of infective endocarditis. Heart (British Cardiac Society) 2006;92:1365–6. doi: 10.1136/hrt.2006.092635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Olmos C, Vilacosta I, Pozo E, Fernandez C, Sarria C, Lopez J, Ferrera C, Maroto L, Gonzalez I, Vivas D, et al. Prognostic implications of diabetes in patients with left-sided endocarditis: findings from a large cohort study. Medicine. 2014;93:114–119. doi: 10.1097/MD.0000000000000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sieradzki K, Leski T, Dick J, Borio L, Tomasz A. Evolution of a vancomycin-intermediate Staphylococcus aureus strain in vivo: multiple changes in the antibiotic resistance phenotypes of a single lineage of methicillin-resistant S. aureus under the impact of antibiotics administered for chemotherapy. Journal of clinical microbiology. 2003;41:1687–93. doi: 10.1128/JCM.41.4.1687-1693.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mwangi MM, Wu SW, Zhou Y, Sieradzki K, de Lencastre H, Richardson P, et al. Tracking the in vivo evolution of multidrug resistance in Staphylococcus aureus by whole-genome sequencing. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:9451–6. doi: 10.1073/pnas.0609839104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tleyjeh IM, Steckelberg JM, Georgescu G, Ghomrawi HM, Hoskin TL, Enders FB, et al. The association between the timing of valve surgery and 6-month mortality in left-sided infective endocarditis. Heart (British Cardiac Society) 2008;94:892–6. doi: 10.1136/hrt.2007.118968. [DOI] [PubMed] [Google Scholar]
- 17.Greenspon AJ, Prutkin JM, Sohail MR, Vikram HR, Baddour LM, Danik SB, et al. Timing of the most recent device procedure influences the clinical outcome of lead-associated endocarditis results of the MEDIC (Multicenter Electrophysiologic Device Infection Cohort) Journal of the American College of Cardiology. 2012;59:681–7. doi: 10.1016/j.jacc.2011.11.011. [DOI] [PubMed] [Google Scholar]
- 18.de Wet JR, Wood KV, DeLuca M, Helinski DR, Subramani S. Firefly luciferase gene: structure and expression in mammalian cells. Molecular and cellular biology. 1987;7:725–37. doi: 10.1128/mcb.7.2.725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart RC, Matthews JC, Hori K, Cormier MJ. Renilla reniformis bioluminescence: luciferase-catalyzed production of nonradiating excited states from luciferin analogues and elucidation of the excited state species involved in energy transfer to Renilla green fluorescent protein. Biochemistry. 1979;18:2204–10. doi: 10.1021/bi00578a011. [DOI] [PubMed] [Google Scholar]
- 20.Frackman S, Anhalt M, Nealson KH. Cloning, organization, and expression of the bioluminescence genes of Xenorhabdus luminescens. Journal of bacteriology. 1990;172:5767–73. doi: 10.1128/jb.172.10.5767-5773.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Demidova TN, Gad F, Zahra T, Francis KP, Hamblin MR. Monitoring photodynamic therapy of localized infections by bioluminescence imaging of genetically engineered bacteria. Journal of photochemistry and photobiology B, Biology. 2005;81:15–25. doi: 10.1016/j.jphotobiol.2005.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Francis KP, Yu J, Bellinger-Kawahara C, Joh D, Hawkinson MJ, Xiao G, et al. Visualizing pneumococcal infections in the lungs of live mice using bioluminescent Streptococcus pneumoniae transformed with a novel gram-positive lux transposon. Infection and immunity. 2001;69:3350–8. doi: 10.1128/IAI.69.5.3350-3358.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Xiong YQ, Willard J, Kadurugamuwa JL, Yu J, Francis KP, Bayer AS. Real-time in vivo bioluminescent imaging for evaluating the efficacy of antibiotics in a rat Staphylococcus aureus endocarditis model. Antimicrob Agents Chemother. 2005;49:380–7. doi: 10.1128/AAC.49.1.380-387.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kadurugamuwa JL, Sin L, Albert E, Yu J, Francis K, DeBoer M, et al. Direct continuous method for monitoring biofilm infection in a mouse model. Infection and immunity. 2003;71:882–90. doi: 10.1128/IAI.71.2.882-890.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Close DM, Patterson SS, Ripp S, Baek SJ, Sanseverino J, Sayler GS. Autonomous bioluminescent expression of the bacterial luciferase gene cassette (lux) in a mammalian cell line. PloS one. 2010;5:e12441. doi: 10.1371/journal.pone.0012441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cronin M, Akin AR, Collins SA, Meganck J, Kim JB, Baban CK, et al. High resolution in vivo bioluminescent imaging for the study of bacterial tumour targeting. PloS one. 2012;7:e30940. doi: 10.1371/journal.pone.0030940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panizzi P, Nahrendorf M, Figueiredo JL, Panizzi J, Marinelli B, Iwamoto Y, et al. In vivo detection of Staphylococcus aureus endocarditis by targeting pathogen-specific prothrombin activation. Nat Med. 2011;17:1142–6. doi: 10.1038/nm.2423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Friedrich R, Panizzi P, Fuentes-Prior P, Richter K, Verhamme I, Anderson PJ, et al. Staphylocoagulase is a prototype for the mechanism of cofactor-induced zymogen activation. Nature. 2003;425:535–9. doi: 10.1038/nature01962. [DOI] [PubMed] [Google Scholar]
- 29.Panizzi P, Friedrich R, Fuentes-Prior P, Kroh HK, Briggs J, Tans G, et al. Novel fluorescent prothrombin analogs as probes of staphylocoagulase-prothrombin interactions. J Biol Chem. 2006;281:1169–78. doi: 10.1074/jbc.M507955200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kroh HK, Panizzi P, Bock PE. Von Willebrand factor-binding protein is a hysteretic conformational activator of prothrombin. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:7786–91. doi: 10.1073/pnas.0811750106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bock PE, Panizzi P, Verhamme IM. Exosites in the substrate specificity of blood coagulation reactions. J Thromb Haemost. 2007;5(Suppl 1):81–94. doi: 10.1111/j.1538-7836.2007.02496.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pinkston KL, Singh KV, Gao P, Wilganowski N, Robinson H, Ghosh S, Azhdarinia A, Sevick-Muraca EM, Murray BE, Harvey BR. Targeting pili in enterococcal pathogenesis. Infection and immunity. 2014;82:1540–1547. doi: 10.1128/IAI.01403-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Knight LC, Maurer AH, Ammar IA, Shealy DJ, Mattis JA. Evaluation of indium-111-labeled anti-fibrin antibody for imaging vascular thrombi. J Nucl Med. 1988;29:494–502. [PubMed] [Google Scholar]
- 34.Rosebrough SF, Grossman ZD, McAfee JG, Kudryk BJ, Subramanian G, Ritter-Hrncirik CA, et al. Thrombus imaging with indium-111 and iodine-131-labeled fibrin-specific monoclonal antibody and its F(ab′)2 and Fab fragments. J Nucl Med. 1988;29:1212–22. [PubMed] [Google Scholar]
- 35.Yokota M, Basi DL, Herzberg MC, Meyer MW. Anti-fibrin antibody binding in valvular vegetations and kidney lesions during experimental endocarditis. Microbiol Immunol. 2001;45:699–707. doi: 10.1111/j.1348-0421.2001.tb01304.x. [DOI] [PubMed] [Google Scholar]
- 36.Riba AL, Thakur ML, Gottschalk A, Andriole VT, Zaret BL. Imaging experimental infective endocarditis with indium-111-labeled blood cellular components. Circulation. 1979;59:336–43. doi: 10.1161/01.cir.59.2.336. [DOI] [PubMed] [Google Scholar]
- 37.Segal AW, Arnot RN, Thakur ML, Lavender JP. Indium-111-labelled leucocytes for localisation of abscesses. Lancet. 1976;2:1056–8. doi: 10.1016/s0140-6736(76)90969-7. [DOI] [PubMed] [Google Scholar]
- 38.Huang JT, Raiszadeh M, Sakimura I, Montgomerie JZ, Harwig JF. Detection of bacterial endocarditis with technetium-99m-labeled antistaphylococcal antibody. J Nucl Med. 1980;21:783–6. [PubMed] [Google Scholar]
- 39.van Oosten M, Schafer T, Gazendam JA, Ohlsen K, Tsompanidou E, de Goffau MC, et al. Real-time in vivo imaging of invasive- and biomaterial-associated bacterial infections using fluorescently labelled vancomycin. Nature communications. 2013;4:2584. doi: 10.1038/ncomms3584. [DOI] [PubMed] [Google Scholar]
- 40.Lee H, Sun E, Ham D, Weissleder R. Chip-NMR biosensor for detection and molecular analysis of cells. Nature medicine. 2008;14:869–74. doi: 10.1038/nm.1711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cremieux AC, Vallois JM, Maziere B, Ottaviani M, Bouvet A, Carbon C, et al. 3H-spiramycin penetration into fibrin vegetations in an experimental model of streptococcal endocarditis. The Journal of antimicrobial chemotherapy. 1988;22(Suppl B):127–33. doi: 10.1093/jac/22.supplement_b.127. [DOI] [PubMed] [Google Scholar]
- 42.Riba AL, Downs J, Thakur ML, Gottschalk A, Andriole VT, Zaret BL. Technetium-99m stannous pyrophosphate imaging of experimental infective endocarditis. Circulation. 1978;58:111–9. doi: 10.1161/01.cir.58.1.111. [DOI] [PubMed] [Google Scholar]
- 43.Leevy WM, Gammon ST, Jiang H, Johnson JR, Maxwell DJ, Jackson EN, et al. Optical imaging of bacterial infection in living mice using a fluorescent near-infrared molecular probe. Journal of the American Chemical Society. 2006;128:16476–7. doi: 10.1021/ja0665592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leevy WM, Gammon ST, Johnson JR, Lampkins AJ, Jiang H, Marquez M, et al. Noninvasive optical imaging of Staphylococcus aureus bacterial infection in living mice using a Bis-dipicolylamine-Zinc(II) affinity group conjugated to a near-infrared fluorophore. Bioconjug Chem. 2008;19:686–92. doi: 10.1021/bc700376v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rouzet F, Hernandez MD, Hervatin F, Sarda-Mantel L, Lefort A, Duval X, et al. Technetium 99m-Labeled annexin V scintigraphy of platelet activation in vegetations of experimental endocarditis. Circulation. 2008 doi: 10.1161/CIRCULATIONAHA.107.718114. [DOI] [PubMed] [Google Scholar]
- 46.Ning X, Lee S, Wang Z, Kim D, Stubblefield B, Gilbert E, et al. Maltodextrin-based imaging probes detect bacteria in vivo with high sensitivity and specificity. Nature materials. 2011;10:602–7. doi: 10.1038/nmat3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hernandez FJ, Huang L, Olson ME, Powers KM, Hernandez LI, Meyerholz DK, et al. Noninvasive imaging of Staphylococcus aureus infections with a nuclease-activated probe. Nature medicine. 2014 doi: 10.1038/nm.3460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Appelbe A, Martin RP. Transesophageal Echocardigraphy. In: Schlant RC, Alexander RW, editors. Hurst’s the Heart: Arteries and Veins. 8. New York: 1994. pp. 2253–66. [Google Scholar]
- 49.Sawada SG, Segar DS, Ryan T, Brown SE, Dohan AM, Williams R, et al. Echocardiographic detection of coronary artery disease during dobutamine infusion. Circulation. 1991;83:1605–14. doi: 10.1161/01.cir.83.5.1605. [DOI] [PubMed] [Google Scholar]
- 50.Vieira ML, Grinberg M, Pomerantzeff PM, Andrade JL, Mansur AJ. Repeated echocardiographic examinations of patients with suspected infective endocarditis. Heart (British Cardiac Society) 2004;90:1020–4. doi: 10.1136/hrt.2003.025585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dumarey N, Egrise D, Blocklet D, Stallenberg B, Remmelink M, del Marmol V, et al. Imaging infection with 18F-FDG-labeled leukocyte PET/CT: initial experience in 21 patients. J Nucl Med. 2006;47:625–32. [PubMed] [Google Scholar]
- 52.Erba PA, Conti U, Lazzeri E, Sollini M, Doria R, De Tommasi SM, et al. Added value of 99mTc-HMPAO-labeled leukocyte SPECT/CT in the characterization and management of patients with infectious endocarditis. J Nucl Med. 2012;53:1235–43. doi: 10.2967/jnumed.111.099424. [DOI] [PubMed] [Google Scholar]
- 53.de Vries EF, Roca M, Jamar F, Israel O, Signore A. Guidelines for the labelling of leucocytes with (99m)Tc-HMPAO. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. European journal of nuclear medicine and molecular imaging. 2010;37:842–8. doi: 10.1007/s00259-010-1394-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Roca M, de Vries EF, Jamar F, Israel O, Signore A. Guidelines for the labelling of leucocytes with (111)In-oxine. Inflammation/Infection Taskgroup of the European Association of Nuclear Medicine. European journal of nuclear medicine and molecular imaging. 2010;37:835–41. doi: 10.1007/s00259-010-1393-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rennen HJ, Laverman P, van Eerd JE, Oyen WJ, Corstens FH, Boerman OC. PET imaging of infection with a HYNIC-conjugated LTB4 antagonist labeled with F-18 via hydrazone formation. Nucl Med Biol. 2007;34:691–5. doi: 10.1016/j.nucmedbio.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 56.Bleeker-Rovers CP, Rennen HJ, Boerman OC, Wymenga AB, Visser EP, Bakker JH, et al. 99mTc-labeled interleukin 8 for the scintigraphic detection of infection and inflammation: first clinical evaluation. J Nucl Med. 2007;48:337–43. [PubMed] [Google Scholar]
- 57.Welling MM, Nibbering PH, Paulusma-Annema A, Hiemstra PS, Pauwels EK, Calame W. Imaging of bacterial infections with 99mTc-labeled human neutrophil peptide-1. J Nucl Med. 1999;40:2073–80. [PubMed] [Google Scholar]
- 58.Oyen WJ, Boerman OC, Brouwers FM, Barrett JA, Verheugt FW, Ruiter DJ, et al. Scintigraphic detection of acute experimental endocarditis with the technetium-99m labelled glycoprotein IIb/IIIa receptor antagonist DMP444. European journal of nuclear medicine. 2000;27:392–9. doi: 10.1007/s002590050521. [DOI] [PubMed] [Google Scholar]
- 59.Brouwers FM, Oyen WJ, Boerman OC, Barrett JA, Verheugt FW, Corstens FH, et al. Evaluation of Tc-99m-labeled glycoprotein IIb/IIIa receptor antagonist DMP444 SPECT in patients with infective endocarditis. Clinical nuclear medicine. 2003;28:480–4. doi: 10.1097/01.RLU.0000067508.82824.75. [DOI] [PubMed] [Google Scholar]
- 60.Morguet AJ, Munz DL, Ivancevic V, Werner GS, Sandrock D, Bokemeier M, et al. Immunoscintigraphy using technetium-99m-labeled anti-NCA-95 antigranulocyte antibodies as an adjunct to echocardiography in subacute infective endocarditis. Journal of the American College of Cardiology. 1994;23:1171–8. doi: 10.1016/0735-1097(94)90607-6. [DOI] [PubMed] [Google Scholar]
- 61.Sarwar M, Higuchi T, Tomiyoshi K, Inoue T, Oriuchi N, Kbalil A, et al. 99mTc-labeled chimeric anti-NCA 95 antigranulocyte monoclonal antibody for bone marrow imaging. Radiation medicine. 1998;16:391–7. [PubMed] [Google Scholar]
- 62.Britton KE, Wareham DW, Das SS, Solanki KK, Amaral H, Bhatnagar A, et al. Imaging bacterial infection with (99m)Tc-ciprofloxacin (Infecton) Journal of clinical pathology. 2002;55:817–23. doi: 10.1136/jcp.55.11.817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Saby L, Laas O, Habib G, Cammilleri S, Mancini J, Tessonnier L, et al. Positron emission tomography/computed tomography for diagnosis of prosthetic valve endocarditis: increased valvular 18F-fluorodeoxyglucose uptake as a novel major criterion. Journal of the American College of Cardiology. 2013;61:2374–82. doi: 10.1016/j.jacc.2013.01.092. [DOI] [PubMed] [Google Scholar]
- 64.Vos FJ, Bleeker-Rovers CP, Kullberg BJ, Adang EM, Oyen WJ. Cost-effectiveness of routine (18)F-FDG PET/CT in high-risk patients with gram-positive bacteremia. J Nucl Med. 2011;52:1673–8. doi: 10.2967/jnumed.111.089714. [DOI] [PubMed] [Google Scholar]
- 65.Korzeniowski O, Kaye D. Infective endocarditis. In: EB, editor. Heart Disease A Textbook of Cardiovascular Medicine. Philadelphia: 1992. pp. 1078–105. [Google Scholar]