Abstract
We describe the synthesis of new nitroxide-based biradical, triradical, and tetraradical compounds and the evaluation of their performance as paramagnetic dopants in dynamic nuclear polarization (DNP) experiments in solid state nuclear magnetic resonance (NMR) spectroscopy with magic-angle spinning (MAS). Under our experimental conditions, which include temperatures in the 25–30 K range, a 9.4 T magnetic field, MAS frequencies of 6.2–6.8 kHz, and microwave irradiation at 264.0 GHz from a 800 mW extended interaction oscillator source, the most effective compounds are triradicals that are related to the previously-described compound DOTOPA-TEMPO (see Thurber et al., 2010), but have improved solubility in glycerol/water solvent near neutral pH. Using these compounds at 30 mM total nitroxide concentration, we observe DNP enhancement factors of 92–128 for cross-polarized 13C NMR signals from 15N,13C-labeled melittin in partially protonated glycerol/water, and build-up times of 2.6–3.8 s for 1H spin polarizations. Net sensitivity enhancements with biradical and tetraradical dopants, taking into account absolute 13C NMR signal amplitudes and build-up times, are approximately 2–4 times lower than with the best triradicals.
Keywords: Magic-angle spinning, Cross-effect DNP, Hyperpolarization, Electron paramagnetic resonance
1. Introduction
Dynamic nuclear polarization (DNP), an effect in which irradiation of electron paramagnetic resonance (EPR) transitions with microwaves leads to enhanced polarizations of nuclear spins [1,2], has become a promising avenue for enhancement of nuclear magnetic resonance (NMR) signals in a variety of contexts. For solid state NMR experiments at low temperatures, Griffin and coworkers have shown that DNP can occur with high efficiency in high magnetic fields when samples of interest are paramagnetically doped with nitroxide-based radicals, principally through the cross-effect DNP mechanism [3,4]. Cross-effect DNP depends on energy-conserving three-spin transitions, in which oppositely polarized, coupled pairs of electron spins whose EPR frequencies differ by the NMR frequency exchange spin states simultaneously with a nuclear spin flip. The dependence of cross-effect DNP on the existence of pairs of coupled electron spins motivated Griffin and coworkers to develop nitroxide biradical compounds, such as TOTAPOL [5–7], for DNP-enhanced solid state NMR. Additional biradical compounds with different solubility properties, geometries, and EPR properties have been introduced subsequently [8– 16]. Biradicals are now widely used in solid state NMR experiments on organic, biological, and inorganic systems [17–26].
In the course of our own DNP experiments, we discovered a triradical compound called DOTOPA-TEMPO (henceforth abbreviated as DOTOPA) that produces large DNP enhancements of 1H NMR signals and 1H–13C cross-polarized 13C NMR signals under our experimental conditions [27–30], namely at temperatures below 30 K, in a 9.4 T magnetic field, in frozen glycerol/water solutions, with relatively low microwave powers, and both with and without magic-angle spinning (MAS). Under these specific conditions, with equal nitroxide concentrations, DOTOPA-doped samples exhibited both larger NMR signals and more rapid build-up of these signals than did TOTAPOL-doped samples [29].
In this paper, we report the synthesis of a variety of DOTOPA-based triradical compounds with improved solubility near neutral pH, along with the results of DNP measurements using these compounds. We also compare the performance of DOTOPA-based compounds with several related biradical and tetraradical compounds. Of the 11 compounds tested, the DOTOPA-based compounds provide the largest absolute enhancements of cross-polarized 13C NMR sensitivity under our experimental conditions.
2. Materials and methods
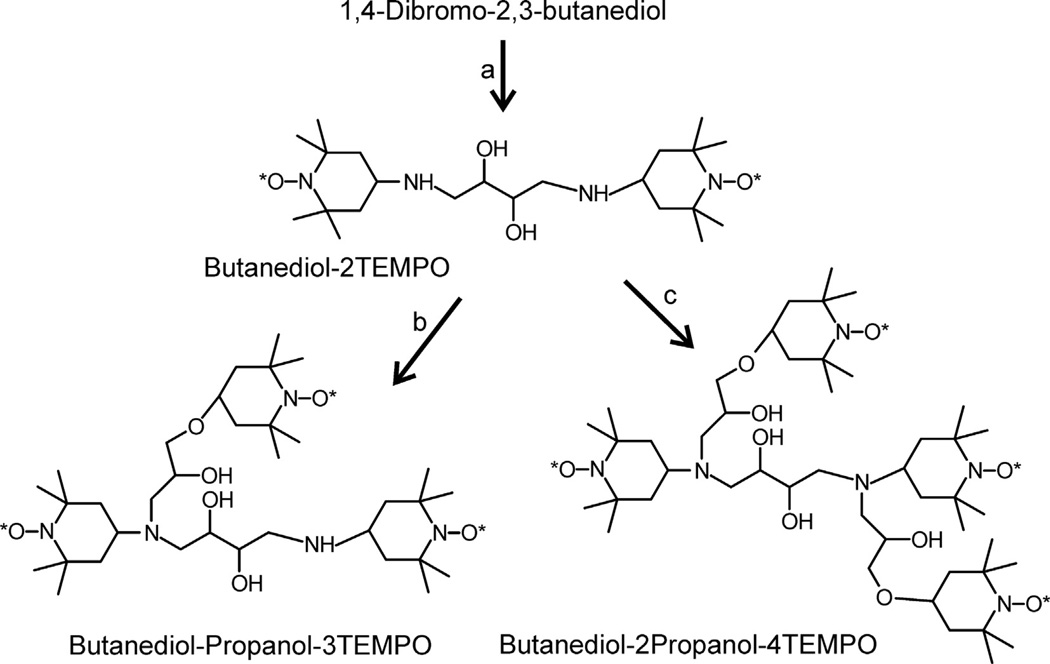
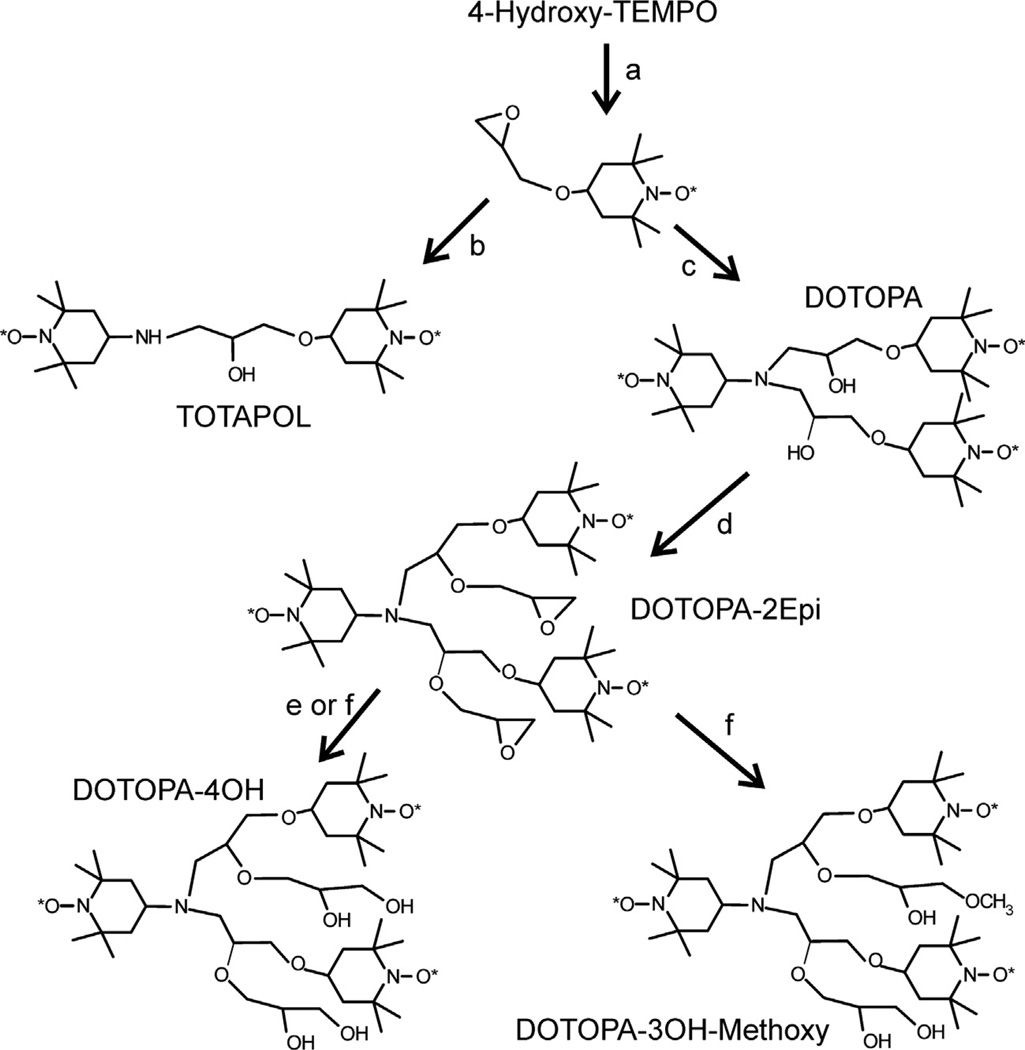
Syntheses of oligoradicals, starting with 1,3-dibromo-2-propanol, 4-hydroxy-TEMPO, and 1,4-dibromo-2,3-butanediol are summarized in Schemes 1–3, respectively. 4-Amino-TEMPO, 4-hydroxy-TEMPO, 1,3-dibromo-2-propanol, and 1,3-butadiene were purchased from Sigma–Aldrich. 4-(2,3-Epoxypropoxy)-TEMPO, TOTAPOL, and DOTOPA were synthesized as previously described [29]. In all, three biradicals (TOTAPOL, TOTAPOL-NH and Butanediol-2TEMPO), six triradicals (DOTOPA, DOTOPA-Ethanol, DOTOPA-NH, DOTOPA-4OH, DOTOPA-3OH-Methoxy, and Butanediol-Propanol-3TEMPO) and two tetraradicals (Butanediol-2Propanol-4TEMPO and 3Propanol-4TEMPO) were prepared and tested. Oligoradicals were purified by silica gel chromatography (130–270 mesh, 60 Å pore size, Sigma–Aldrich). Chemical structures and the absence of reduction of nitroxides were verified by electrospray-ionization/time-of-flight mass spectrometry (Waters Micromass LCT Premier mass spectrometer). IUPAC nomenclature for these compounds and additional details of their syntheses are given in Supplementary Information.
Scheme 1.
(a) 4-Amino-TEMPO (2.2 eq) in 0.25 N NaOH (2.0 eq) at room temperature (RT) overnight (85% yield, , m/z 399.3). (b) 2-bromoethanol (1.0 eq) in 0.25 N NaOH (1.0 eq) at RT overnight (60% yield, , m/z 443.3). (c) 4-(2,3-epoxypropoxy)-TEMPO (1.2 eq), lithium perchlorate (1.0 eq) in CH3CN at RT overnight (85% yield, , m/z 671.5). (d) 4-(2,3-epoxypropoxy)-TEMPO (1.1 eq), lithium perchlorate (1.0 eq) in CH3CN at RT overnight (5% yield, , m/z 627.4). (e) 4-(2,3-epoxypropoxy)-TEMPO (2.2 eq), lithium perchlorate (2.1 eq) in CH3CN at RT overnight (80% yield, , m/z 855.5).
Scheme 3.
(a) 4-Amino-TEMPO (2.2 eq) in 0.25 N NaOH (2.0 eq) at RT overnight (85% yield, , m/z 429.3). (b) 4-(2,3-epoxypropoxy)-TEMPO (1.1 eq), lithium perchlorate (1.0 eq) in CH3CN at RT overnight (15% yield, , m/z 657.5). (c) 4-(2,3-epoxypropoxy)-TEMPO (2.2 eq), lithium perchlorate (2.0 eq) in CH3CN at RT overnight (40% yield, , m/z 885.6).
As shown in Scheme 2, DOTOPA-4OH was prepared with two different synthetic approaches. EPR and DNP results presented below correspond to the approach labeled f. The approach labeled e, in which erbium (III) triflate was used to catalyze the hydrolysis of epoxide rings [31], produced a higher yield. However, the DNP efficiency for DOTOPA-4OH produced by approach e was found to be lower, and the EPR spectrum showed a less pronounced multiplet structure. We tentatively attribute these differences in magnetic resonance properties to differences in stereochemistry, leading to differences in conformational distributions.
Scheme 2.
(a) Epichlorohydrin (25 eq), tetrabutylammonium hydrogen sulfate (0.136 g, 4 mol%) in 50% w/w aqueous NaOH (10 ml) at RT overnight. (b) 4-amino-TEMPO (1.1 eq), lithium perchlorate (1.0 eq) in CH3CN at RT overnight (80% yield, , m/z 400.3). (c) 4-amino-TEMPO (0.5 eq), lithium perchlorate (1.0 eq) in CH3CN at RT overnight (85% yield, , m/z 628.4). (d) epichlorohydrin (25.0 eq), tetrabutylammonium hydrogen sulfate (2.0 eq) in 50% NaOH (10 ml) at RT overnight (85% yield, , m/z 740.5). (e) erbium (III) triflate (1.1 eq) in H2O:CH3CN (1:1) at 55–60 °C for 7 days (80% yield, m/z 776.4). (f) CH3OH:1 N KOH (1:1) at 55–60 °C for 7 days (40% yield, m/z 790.6. Under this condition, DOTOPA-4OH could be isolated with a yield of 40%).
For electron paramagnetic resonance (EPR) measurements, oligoradicals were dissolved in acetonitrile at concentrations of 0.5 mM. Measurements were performed at room temperature, using a Bruker Elexsys X-band EPR spectrometer and dielectric ring resonator. Spectra were recorded with 1 G field modulation at 100 kHz, a 10 G/s field sweep rate, and 0.16 mW microwave power.
For solid state NMR measurements, oligoradicals were first dissolved in perdeuterated dimethyl sulfoxide (d6-DMSO) at high concentration (230–690 mM). Extinction coefficients in DMSO at the nitroxide radical absorption peak (440–460 nm) were determined by titration with ascorbic acid, monitored by the UV-visible absorption, and found to be 11 cm−1 M−1 (based on nitroxide concentration) [29,32]. The 26-residue peptide melittin [33–36] was synthesized and purified by standard Fmoc solid-phase synthesis and reverse-phase HPLC methods, with uniform 15N,13C-labeling of Pro14, Ala15, Leu16, and Ile17. (The isotopically labeled peptide is henceforth called PALI-melittin.) Solutions of PALI-melittin at 5 mM were prepared in partially deuterated glycerol/water, using perdeuterated, 13C-depleted glycerol, D2O, and H2O in a 57:33:10 volume ratio, buffered with 25 mM phosphate at pH 7.4. Oligoradicals were added to achieve final nitroxide concentrations of 30 mM (i.e., dopant concentrations of 15 mM, 10 mM, or 7.5 mM for biradicals, triradicals, or tetraradicals, respectively). Small quantities of D2O were added as required to maintain equal 1H concentrations in all samples.
NMR measurements were performed at 9.39 T (400.9 MHz and 100.8 MHz 1H and 13C NMR frequencies, respectively), using a Bruker Avance III spectrometer and the home-built, low-temperature DNP MAS NMR probe described previously [27,37]. The quasi-optical system for transmission and circular polarization of microwave irradiation was as previously described [27,29], except that an extended interaction oscillator (EIO) (Communications & Power Industries, Inc.), with output power of approximately 0.8W at 264.0 GHz, was used as the microwave source. Microwaves were transmitted from the EIO to the quasi-optical polarizing system through a threaded aluminum tube (13 mm inner diameter, 0.5 mm thread pitch, 94 cm length). Sample temperatures were determined from spin–lattice relaxation times of 79Br in KBr contained in a glass capsule placed in the MAS rotor along with the sample [38]. For 1H NMR measurements, the nuclear spin polarization was first destroyed with several (3–5) 90° pulses. After the polarization build-up delay, the NMR signal was detected with a single 20° pulse to avoid saturating the receiver pre-amplifier of the spectrometer. The peak intensity of the Fourier transform was used as the measure of the 1H signal strength, to reduce the effect of background 1H NMR signals. For cross-polarized 13C NMR measurements, an 800 µs contact time and 40 kHz 13C radio-frequency field strength were used, with two dummy scans to establish a steady state before signal acquisition with 70 kHz 1H decoupling with two-pulse phase modulation [39]. The integral of the aliphatic region of the 13C spectrum was used as the measure of cross-polarized 13C signal strength. DNP build-up times (τDNP) were determined from the dependence of cross-polarized 13C signals on the polarization build-up delay after saturation with three 1H 90° pulses separated by 10 ms. 13C and 1H NMR signal strengths reported in Table 1 are corrected for small differences in the measurement temperatures (assuming a 1/T dependence of the signals) and in the recycle delay or polarization build-up times relative to τDNP. DNP enhancements of cross-polarized 13C (εC) and 1H (εH) NMR signals in Table 1 are ratios of signal strengths with and without microwave irradiation.
Table 1.
Summary of DNP measurements on paramagnetically doped, PALI-melittin solutions in frozen d6-glycerol/D2O/H2O (57:33:10 ratio by volume) at pH 7.4. Total nitroxide concentrations are 30 mM. DNP enhancements of cross-polarized 13C (εC) and 1H (εH) NMR signals are ratios with and without 800 mW microwave irradiation. 13C signals are total aliphatic signal areas. 1H signals are the peak intensities in the spectra. 1H NMR and microwave frequencies are 400.9 MHz and 264.0 GHz, respectively. Solubilities are estimated from optical absorption spectra of solutions after ultracentrifugation to remove aggregated dopants.
| Oligoradical dopant | εC(±5%) | 13C signal (±25%; arb. units) | τDNP (±15%; s) |
13 C signal/ (±25%) |
εH (±5%) |
1H signal (±25%; arb. units) | Temperature (±2; K) |
MAS frequency (kHz) |
Solubility |
|---|---|---|---|---|---|---|---|---|---|
| DOTOPA-Ethanol | 102 | 2.9 | 3.8 | 1.48 | 92 | 4.7 | 26 | 6.7 | ≥10 mM |
| DOTOPA-NH | 128 | 2.3 | 3.1 | 1.29 | 92 | 3.8 | 26 | 6.8 | ≥10 mM |
| DOTOPA-4OH | 92 | 1.6 | 2.6 | 0.99 | 97 | 3.7 | 25 | 6.5 | ≥10 mM |
| DOTOPA | 100 | 2.0 | 4.5 | 0.92 | 84 | 4.9 | 25 | 6.7 | 4 mM |
| DOTOPA-3OH-Methoxy | 94 | 1.6 | 3.7 | 0.83 | 79 | 3.3 | 25 | 6.8 | ≥10 mM |
| 3Propanol-4TEMPO | 51 | 2.4 | 9.6 | 0.78 | 49 | 4.0 | 29 | 6.7 | 2 mM |
| Butanediol-Propanol-3TEMPO | 50 | 1.0 | 2.6 | 0.59 | 56 | 2.7 | 27 | 6.2 | ≥10 mM |
| TOTAPOL-NH | 63 | 1.5 | 7.4 | 0.55 | 68 | 2.0 | 25 | 6.5 | <15 mM |
| Butanediol-2Propanol-4TEMPO | 49 | 1.1 | 4.2 | 0.53 | 41 | 3.9 | 23 | 6.6 | 4 mM |
| TOTAPOL | 46 | 0.7 | 3.7 | 0.38 | 44 | 2.2 | 26 | 6.6 | ≥15mM |
| Butanediol-2TEMPO | 66 | 0.8 | 3.9 | 0.38 | 62 | 2.5 | 26 | 6.6 | ≥15mM |
After low-temperature DNP measurements, samples were unloaded from MAS rotors at room temperature and subjected to ultracentrifugation (270,000g, 60 min) to pellet any aggregated dopant. Optical absorption spectra (Fig. S1) were recorded with a NanoDrop 1000 spectrophotometer. The intensity of the nitroxide absorption peak at 460 nm was used to estimate concentrations of soluble dopant, after subtraction of background absorption due to PALI-melittin and DMSO. Three dopants were found to have solubilities below the target value of 30 mM nitroxide, namely 3Propanol-4TEMPO, Butanediol-2Propanol-4TEMPO, and DOTOPA.
3. Results
3.1. EPR spectroscopy of oligoradicals
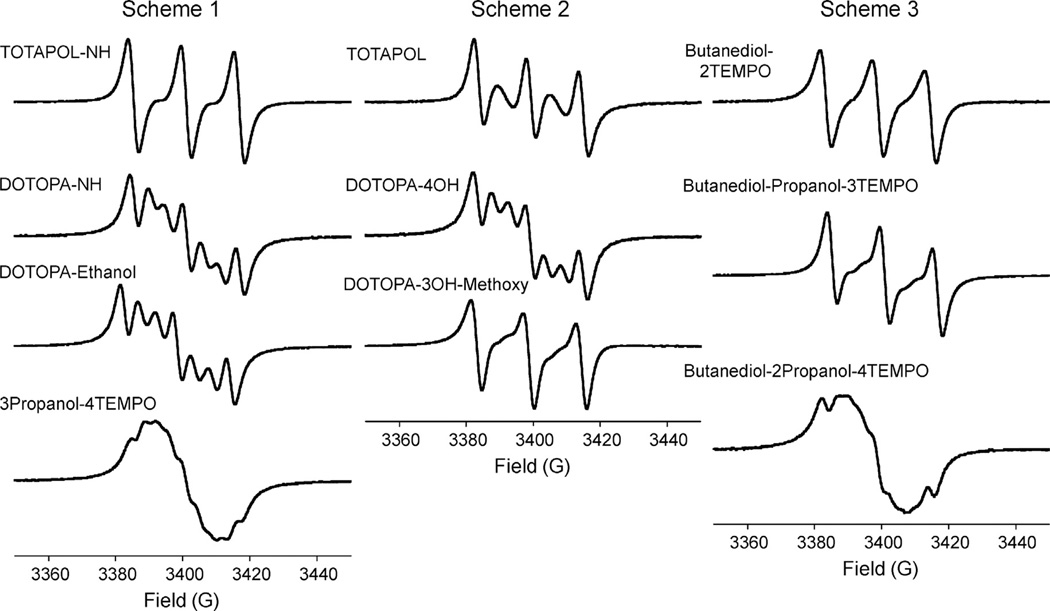
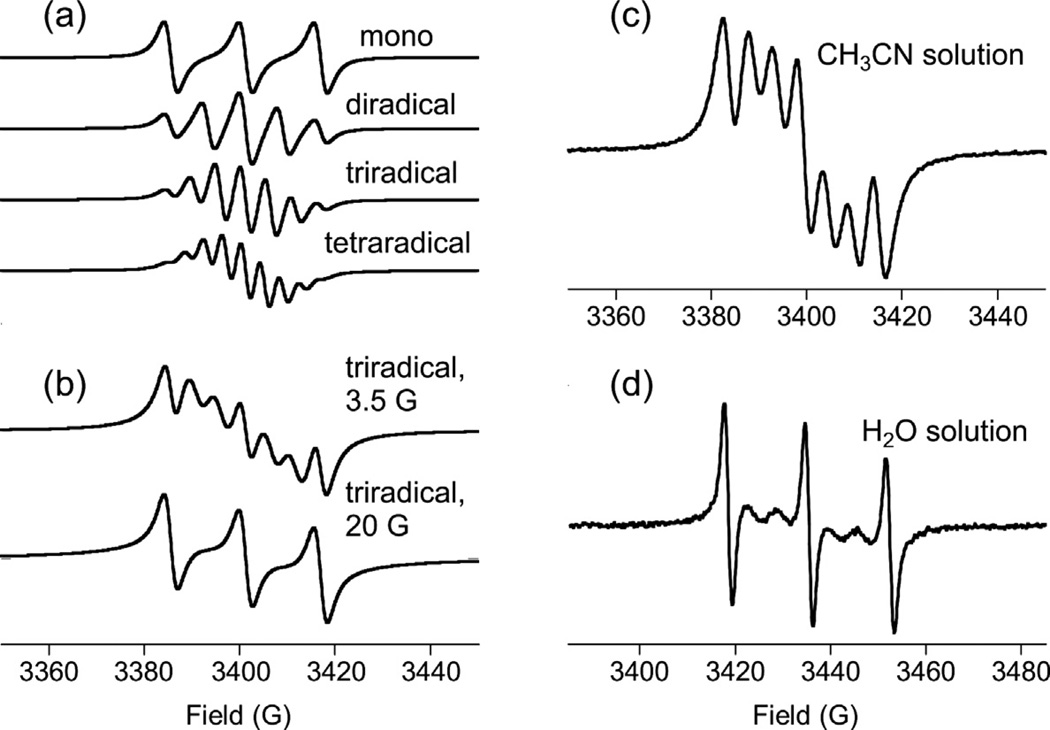
Fig. 1 shows derivative-mode EPR spectra of all oligoradicals in acetonitrile (except DOTOPA, for which EPR spectra are shown in Fig. 2d). Ideally, the spectrum of a rapidly tumbling, flexible oligoradical containing n nitroxide moieties in isotropic solution should be a multiplet of 2n + 1 equally spaced lines, with splittings of A/n, and with relative area ratios of 1:2:3:2:1 for biradicals, 1:3:6:7:6:3:1 for triradicals, and 1:4:10:16:19:16:10:4:1 for tetraradicals. This prediction assumes that the electron spin on each nitroxide has hyperfine coupling A to the unpolarized spin-1 14N nucleus of that nitroxide, and that electron spins exchange very rapidly through collisions among nitroxide moieties, causing each electron spin to experience a net hyperfine field equal to the average of the n hyperfine fields. Ideal derivative spectra are shown in Fig. 2a.
Fig. 1.
X-band EPR spectra of 0.5 mM solutions of the indicated nitroxide oligoradicals, synthesized according to Schemes 1–3, in acetonitrile at room temperature. Spectra are displayed in the usual derivative mode.
Fig. 2.
(a) Calculated X-band EPR lineshapes for radicals containing 1–4 nitroxide moieties, assuming fast spin exchange among all nitroxides, a 15.7 G hyperfine coupling to the 14N nucleus in each nitroxide, and a 5 G Lorentzian linewidth (full width at half maximum) for each EPR transition. (b) Calculated lineshapes for triradicals with additional exchange broadening characterized by the parameter Γ (see text), with Γ = 3.5 G and Γ = 20 G. These lineshapes illustrate the effects of intermediate rates of spin exchange on the EPR multiplet patterns. (c and d) Experimental X-band EPR spectra of 0.5 mM solutions of DOTOPA in acetonitrile or water.
In practice, the limit of very rapid exchange is not usually achieved. Fluctuations of hyperfine fields associated with exchange then contribute to the transverse relaxation times of the electron spins, and hence to the EPR linewidths. Multiplet components arising from oligoradicals in which all 14N nuclei have the same spin state (i.e., mj = 1, mj = 0, or mj = −1 for all j, where mj is the 14N spin quantum number for the jth nitroxide) do not have hyperfine fluctuations and are not broadened. These three lines remain pronounced in the derivative spectra, while other lines are broadened and attenuated to varying degrees, as is apparent in Fig. 1. Fig. 2b shows simulated EPR spectra for a triradical in which effects of intermediate exchange are modeled by an additional linewidth contribution for each multiplet component equal to [(m1 – mave)2 + (m2 – mave)2 + (m3 – mave)2](3Γ/2), where mave = (m1 + m2 + m3)/3. This model assumes random exchange among nitroxides and an EPR linewidth proportional to the mean squared hyperfine field fluctuation. Comparison of Fig. 2b with Fig. 1 shows that this model adequately explains variations among the EPR spectra of triradicals. Further support comes from the dependence of the EPR spectrum of DOTOPA on solvent, as shown in Fig. 2c and d. The greater viscosity of water apparently leads to slower exchange, and hence a larger value of Γ.
From a practical standpoint, results in Fig. 1 and Fig. 2 show that EPR spectra alone are not always sufficient to prove that a triradical compound actually contains three non-reduced nitroxides, as the spectra of triradicals can resemble spectra of monoradicals or biradicals when exchange is not sufficiently rapid. In our hands, mass spectrometry is a more definitive test for the absence of reduced nitroxides in oligoradicals. Examples of mass spectra that illustrate this point are shown in Fig. S2.
3.2. Evaluation of DNP performance
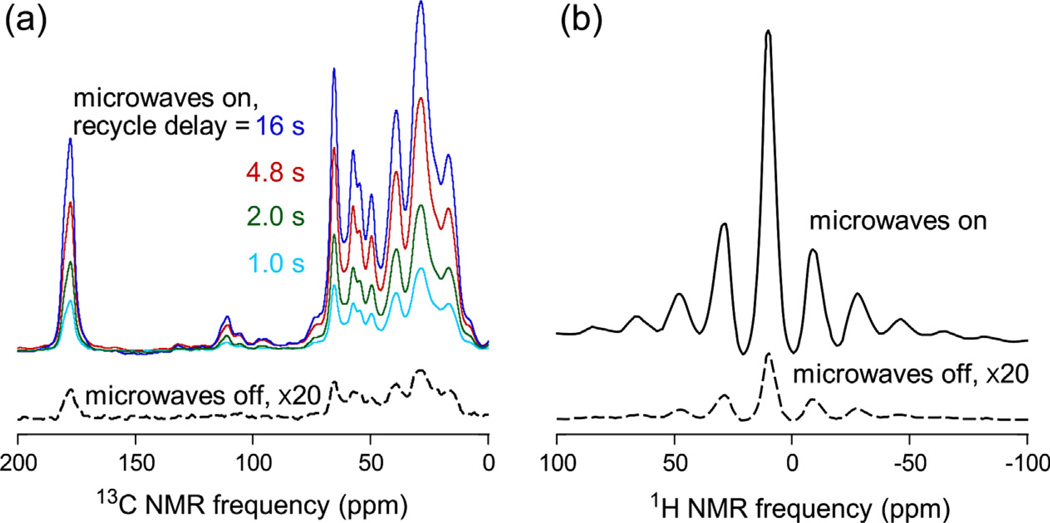
Fig. 3 shows examples of 13C and 1H NMR spectra of a doped PALI-melittin solution in partially-protonated glycerol/water at low temperature and with MAS. In Fig. 3a, the build-up of cross-polarized 13C NMR signals with increasing recycle delay under continuous microwave irradiation allows us to determine τDNP. Comparisons of spectra obtained with and without microwave irradiation allow us to determine εC and εH. The 1H NMR spectrum of the frozen solution contains a series of MAS sidebands because 1H nuclei in the solvent are relatively dilute, so that 1H–1H magnetic dipole–dipole couplings are averaged out even at MAS frequencies below 7 kHz.
Fig. 3.
(a) Cross-polarized 13C NMR spectra of 5 mM PALI-melittin (uniformly 15N,13C-labeled at Pro14, Ala15, Leu16, and Ile17) in partially protonated glycerol/water with 10 mM DOTOPA-Ethanol at 26 K and 6.7 kHz MAS frequency. Spectra are shown as a function of the recycle delay with microwaves on, and with a recycle delay of 4.8 s with microwaves off. Vertical scales are adjusted to account for differences in the number of scans, which was 4 or 16 for spectra with microwaves on and 128 for the spectrum with microwaves off. (b) 1H NMR spectra of the same sample under the same conditions. Each spectrum was obtained in a single scan with a 32 s delay after saturation of 1H spin polarization.
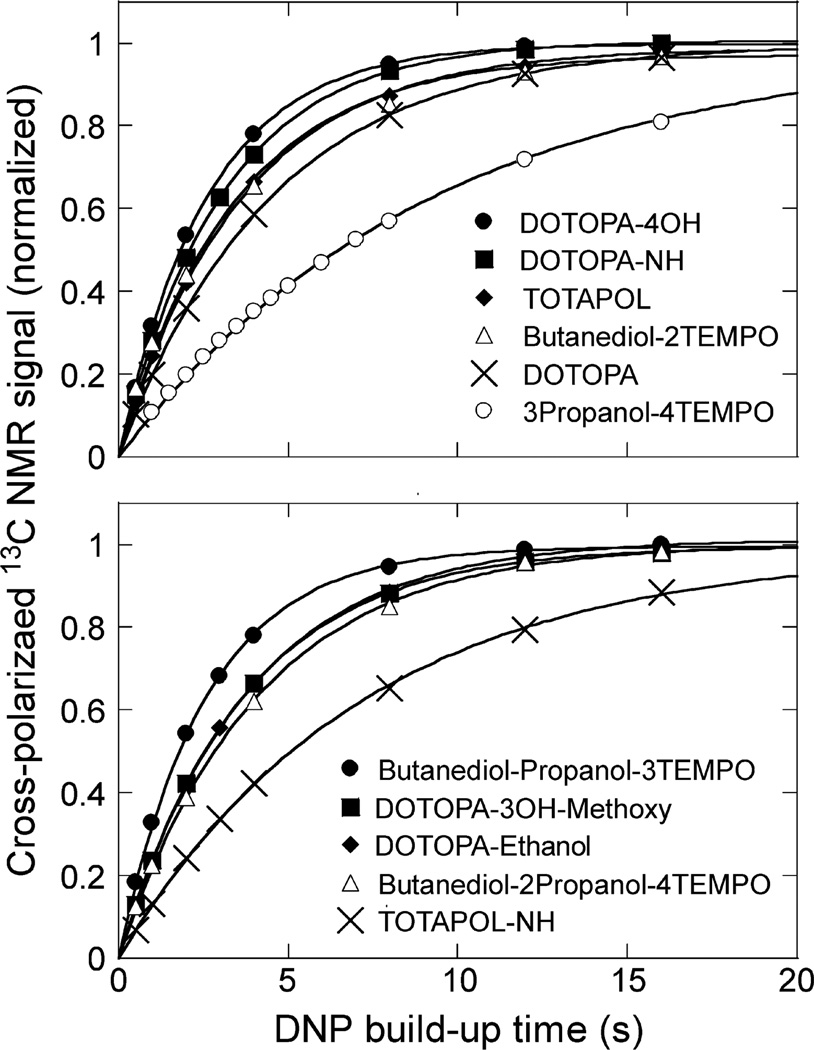
DNP build-up curves for all 11 oligoradicals, obtained under similar experimental conditions, are shown in Fig. 4. With total nitroxide concentrations of 30 mM, most oligoradicals produce values of τDNP in the 2.6–4.5 s range at sample temperatures in the 23–29 K range. Two compounds, the biradical TOTAPOL-NH and the tetraradical 3Propanol-4TEMPO, produce significantly larger values of τDNP, most likely due to their poor solubilities.
Fig. 4.
Build-up of cross-polarized 13C NMR signals as a function of the recycle delay for oligoradical dopants presented in Table 1. Signals are areas of aliphatic regions of PALI-melittin spectra, as in Fig. 3a. Lines are fits to the function 1 − exp(−t/τDNP).
Results of all DNP measurements are summarized in Table 1. Values of εC range from 46 to 128, with εH ≈ εC as expected. DNP-enhanced 13C NMR signals (all measured with recycle delays approximately equal to 1.25τDNP) vary over a somewhat larger range, by a factor of four, indicating that factors other than εC influence the 13C NMR signal strengths. Such factors may include variations in the extent to which the oligoradicals attenuate cross-polarized 13C MAS NMR signals in the absence of microwaves, through a combination of paramagnetic ‘‘bleaching’’ effects [40–42] and perturbation of the 1H spin polarization from thermal equilibrium by cross-effect relaxation with non-equilibrium electron spin polarization differences under MAS [43].
The limited solubility of DOTOPA in glycerol/water near neutral pH was one of our primary motivations for synthesis of the various DOTOPA derivatives. As indicated in Table 1, DOTOPA-4OH, DOT-OPA-3OH-Methoxy, DOTOPA-Ethanol, and DOTOPA-NH have apparent solubilities above 10 mM at pH 7.4, leading to values of εC similar to the value for DOTOPA and to smaller values of τDNP. The DNP-enhanced signal-to-noise ratio for cross-polarized 13C NMR signals acquired in a fixed measurement time is proportional to the absolute 13C NMR signal per scan (not necessarily the value of εC) and inversely proportional to . As shown in Table 1, DOTOPA-Ethanol and DOTOPA-NH are the preferred triradical dopants from this standpoint.
Under the specific conditions of our experiments, and with identical total nitroxide concentrations, DOTOPA-based triradicals yield larger values of εC and better net 13C NMR sensitivities than do biradicals, including TOTAPOL, TOTAPOL-NH, and Butanediol-2TEMPO. Tetraradicals offer no advantages over triradicals.
3.3. Dependences on MAS frequency and temperature
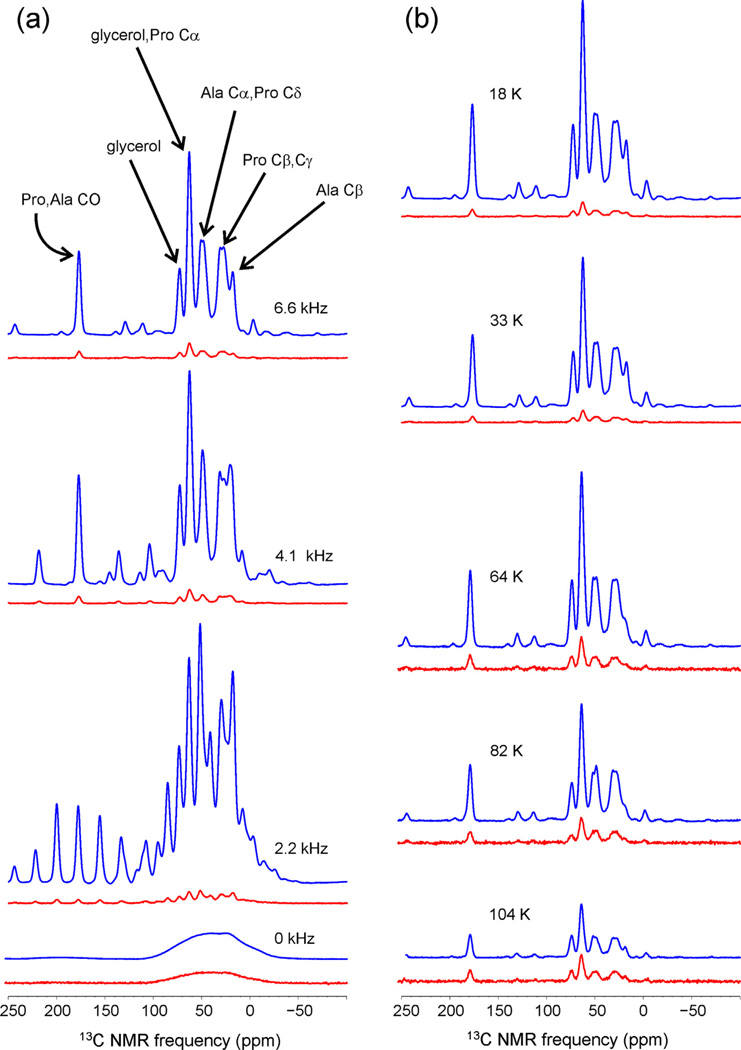
To test the performance of triradical dopants under other experimental conditions, experiments were performed on a frozen solution of uniformly 15N,13C-labeled l-proline and l-alanine (50 mM each) in partially deuterated glycerol/water (same composition as in PALI-melittin solutions) containing 10 mM DOTOPA-Ethanol. 13C NMR spectra are shown in Fig. 5 and results are summarized in Table 2. No significant differences were observed in DNP enhancements of 13C NMR signals from l-proline, l-alanine, and glycerol. As expected [43], DNP enhancements and build-up rates under MAS are much greater than in the absence of MAS and are largest at relatively low MAS frequencies. Enhancements are reduced at higher temperatures, presumably due to reduced electron spin–lattice relaxation times, but are still significant above 80 K even though our experiments use only 0.8W of microwave power.
Fig. 5.
Cross-polarized 13C NMR spectra of uniformly 15N,13C-labeled l-proline and l-alanine in partially protonated glycerol/water with 10 mM DOTOPA-Ethanol. Vertical scales in all spectra are directly comparable, except that spectra recorded without microwave irradiation (red) are scaled up by a factor of 8 relative to spectra recorded with microwave irradiation (blue), and all spectra recorded above 60 K are additionally scaled up by a factor of 4. (a) Dependence on MAS frequency, with temperatures in the 17–22 K range. (b) Dependence on temperature, with MAS frequencies in the 6.55–6.70 kHz range. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Table 2.
Summary of DNP measurements on a solution of uniformly 15N,13C-labeled L-proline and L-alanine (50 mM each) in frozen d6-glycerol/D2O/H2O (57:33:10 ratio by volume) at pH 7.4, containing 10 mM DOTOPA-Ethanol.
| MAS frequency (kHz) | Temperature (K) | εC | 13C signal (arb. units) | τDNP (s) | 13C signal/ | εH | 1H signal (arb. units) |
|---|---|---|---|---|---|---|---|
| 0.00 | 21 | 20 | 1.0 | 31 | 0.19 | – | – |
| 2.20 | 17 | 155 | 4.6 | 7.3 | 1.72 | – | – |
| 4.10 | 20 | 128 | 3.2 | 6.6 | 1.25 | – | – |
| 6.60 | 22 | 102 | 2.1 | 6.0 | 0.85 | – | – |
| 6.70 | 18 | 107 | 1.9 | 6.2 | 0.77 | 95 | 4.8 |
| 6.60 | 33 | 98 | 1.4 | 6.2 | 0.58 | 92 | 3.7 |
| 6.60 | 64 | 43 | 0.39 | 5.0 | 0.18 | 44 | 1.2 |
| 6.55 | 82 | 35 | 0.27 | 5.2 | 0.12 | 33 | 0.85 |
| 6.70 | 104 | 17 | 0.12 | 4.3 | 0.06 | 15 | 0.35 |
4. Discussion
Since the original development of biradical dopants for DNP in solid state NMR [5–7], a variety of nitroxide-based biradicals have been described, with differences in DNP performance attributable to differences in electron–electron distance, electron spin–lattice relaxation time (T1e), relative orientation of nitroxide groups, solubility, or other properties [8–16]. The optimal choice of dopant certainly depends on the nature of the sample under investigation and may also depend on measurement conditions such as temperature, magnetic field strength, microwave power, and MAS frequency. Under the specific conditions of our experiments, we find that triradical dopants derived from DOTOPA provide larger net DNP enhancements of cross-polarized 13C MAS NMR signals than the biradicals listed in Table 1, at least for soluble peptides such as melittin.
It should be noted that melittin contains a large fraction of methyl-bearing residues (13 residues out of 26) and exists primarily as a tetramer under our experimental conditions [36]. Thus, the observed DNP enhancements for PALI-melittin (largest εC = 128) may be affected by the intrinsic 1H spin–lattice relaxation rate [37] within the melittin tetramer. Larger enhancements may be observed in frozen solutions of small molecules, monomeric peptides, or proteins with a lower density of methyl groups. It should also be noted that the EIO microwave source used in the current experiments produces DNP enhancements that are up to 7.5 times larger than the enhancement reported previously for PALI-melittin when a 30 mW microwave source was used [27].
Several factors may affect the relative DNP enhancements from dopants that contain different numbers of nitroxide groups. First, increasing the number of nitroxides per molecule increases the number of close electron–electron pairs. Numerical simulations have suggested that only about half of randomly oriented nitroxide biradicals are effective for cross-effect DNP [43], even when electron–electron distances do not vary, with the remaining biradicals contributing little to the net DNP enhancement due to unfavorable electron g-tensor orientations or other factors. When each electron has two strongly coupled partners within a triradical, the probability of effective cross-effect DNP is increased. The apparent inferiority of tetraradicals in our experiments, relative to triradicals, may be due to the relatively poor solubility of the tetraradicals. Additionally, electron–electron distances in the tetraradicals may be longer than in the DOTOPA-based triradicals, reducing the intrinsic DNP build-up rate. Second, strong cross-effect DNP occurs when the spin polarization of one electron in a pair is saturated by microwave irradiation, while the second electron remains polarized [43]. Thus, cross-effect DNP not only requires strongly coupled electron pairs, but also requires that microwave-induced saturation not spread rapidly among electrons by spin/spectral diffusion. We speculate that triradicals may represent an optimal balance of these requirements, while spectral diffusion may become more rapid in tetraradicals. Third, the values of T1e may vary among nitroxide-based dopants.
In conclusion, we find that DOTOPA-based triradical compounds are efficient polarizing agents for DNP-enhanced solid state NMR under MAS at low temperatures. The new compounds have good solubilities near neutral pH, and will therefore be useful in future studies of a wide variety of biomolecular systems.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health. We thank Dr. Kan-Nian Hu for helpful discussions regarding the synthesis and properties of oligoradicals and Dr. John R. Lloyd for assistance with mass spectrometry.
Appendix A. Supplementary material
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.jmr.2014.05.002.
References
- 1.Overhauser AW. Polarization of nuclei in metals. Phys. Rev. 1953;92:411–415. [Google Scholar]
- 2.Carver TR, Slichter CP. Experimental verification of the overhauser nuclear polarization effect. Phys. Rev. 1956;102:975–980. [Google Scholar]
- 3.Kessenikh AV, Lushchikov VI, Manenkov AA, Taran YV. Proton polarization in irradiated polyethylenes. Sov. Phys. Sol. State. 1963;5:321–329. [Google Scholar]
- 4.Farrar CT, Hall DA, Gerfen GJ, Inati SJ, Griffin RG. Mechanism of dynamic nuclear polarization in high magnetic fields. J. Chem. Phys. 2001;114:4922–4933. [Google Scholar]
- 5.Song CS, Hu KN, Joo CG, Swager TM, Griffin RG. TOTAPOL: a biradical polarizing agent for dynamic nuclear polarization experiments in aqueous media. J. Am. Chem. Soc. 2006;128:11385–11390. doi: 10.1021/ja061284b. [DOI] [PubMed] [Google Scholar]
- 6.Hu KN, Yu HH, Swager TM, Griffin RG. Dynamic nuclear polarization with biradicals. J. Am. Chem. Soc. 2004;126:10844–10845. doi: 10.1021/ja039749a. [DOI] [PubMed] [Google Scholar]
- 7.Hu KN, Song C, Yu HH, Swager TM, Griffin RG. High-frequency dynamic nuclear polarization using biradicals: a multifrequency EPR lineshape analysis. J. Chem. Phys. 2008;128 doi: 10.1063/1.2816783. [DOI] [PubMed] [Google Scholar]
- 8.Dane EL, Maly T, Debelouchina GT, Griffin RG, Swager TM. Synthesis of a BDPA-TEMPO Biradical. Org. Lett. 2009;11:1871–1874. doi: 10.1021/ol9001575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuki Y, Maly T, Ouari O, Karoui H, Le Moigne F, Rizzato E, Lyubenova S, Herzfeld J, Prisner T, Tordo P, Griffin RG. Dynamic nuclear polarization with a rigid biradical. Angew. Chem. Int. Edit. 2009;48:4996–5000. doi: 10.1002/anie.200805940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ysacco C, Rizzato E, Virolleaud MA, Karoui H, Rockenbauer A, Le Moigne F, Siri D, Ouari O, Griffin RG, Tordo P. Properties of dinitroxides for use in dynamic nuclear polarization (DNP) Phys. Chem. Chem. Phys. 2010;12:5841–5845. doi: 10.1039/c002591g. [DOI] [PubMed] [Google Scholar]
- 11.Dane EL, Corzilius B, Rizzato E, Stocker P, Maly T, Smith AA, Griffin RG, Ouari O, Tordo P, Swagert TM. Rigid orthogonal bis-TEMPO biradicals with improved solubility for dynamic nuclear polarization. J. Org. Chem. 2012;77:1789–1797. doi: 10.1021/jo202349j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kiesewetter MK, Corzilius B, Smith AA, Griffin RG, Swager TM. Dynamic nuclear polarization with a water-soluble rigid biradical. J. Am. Chem. Soc. 2012;134:4537–4540. doi: 10.1021/ja212054e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Macholl S, Johannesson H, Ardenkjaer-Larsen JH. Trityl biradicals and C-13 dynamic nuclear polarization. Phys. Chem. Chem. Phys. 2010;12:5804–5817. doi: 10.1039/c002699a. [DOI] [PubMed] [Google Scholar]
- 14.Zagdoun A, Casano G, Ouari O, Lapadula G, Rossini AJ, Lelli M, Baffert M, Gajan D, Veyre L, Maas WE, Rosay M, Weber RT, Thieuleux C, Coperet C, Lesage A, Tordo P, Emsley L. A Slowly relaxing rigid biradical for efficient dynamic nuclear polarization surface-enhanced NMR spectroscopy: expeditious characterization of functional group manipulation in hybrid materials. J. Am. Chem. Soc. 2012;134:2284–2291. doi: 10.1021/ja210177v. [DOI] [PubMed] [Google Scholar]
- 15.Liu YP, Villamena FA, Rockenbauer A, Song YG, Zweier JL. Structural factors controlling the spin-spin exchange coupling: EPR spectroscopic studies of highly asymmetric trityl-nitroxide biradicals. J. Am. Chem. Soc. 2013;135:2350–2356. doi: 10.1021/ja311571v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zagdoun A, Casano G, Ouari O, Schwarzwalder M, Rossini AJ, Aussenac F, Yulikov M, Jeschke G, Coperet C, Lesage A, Tordo P, Emsley L. Large molecular weight nitroxide biradicals providing efficient dynamic nuclear polarization at temperatures up to 200 K. J. Am. Chem. Soc. 2013;135:12790–12797. doi: 10.1021/ja405813t. [DOI] [PubMed] [Google Scholar]
- 17.Rossini AJ, Zagdoun A, Lelli M, Canivet J, Aguado S, Ouari O, Tordo P, Rosay M, Maas WE, Coperet C, Farrusseng D, Emsley L, Lesage A. Dynamic nuclear polarization enhanced solid-state NMR spectroscopy of functionalized metal-organic frameworks. Angew. Chem. Int. Edit. 2012;51:123–127. doi: 10.1002/anie.201106030. [DOI] [PubMed] [Google Scholar]
- 18.Ravera E, Corzilius B, Michaelis VK, Rosa C, Griffin RG, Luchinat C, Bertini I. Dynamic nuclear polarization of sedimented solutes. J. Am. Chem. Soc. 2013;135:1641–1644. doi: 10.1021/ja312553b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Takahashi H, Ayala I, Bardet M, De Paepe G, Simorre JP, Hediger S. Solidstate NMR on bacterial cells: selective cell wall signal enhancement and resolution improvement using dynamic nuclear polarization. J. Am. Chem. Soc. 2013;135:5105–5110. doi: 10.1021/ja312501d. [DOI] [PubMed] [Google Scholar]
- 20.Wang T, Park YB, Caporini MA, Rosay M, Zhong LH, Cosgrove DJ, Hong M. Sensitivity-enhanced solid-state NMR detection of expansin’s target in plant cell walls. Proc. Natl. Acad. Sci. USA. 2013;110:16444–16449. doi: 10.1073/pnas.1316290110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ong YS, Lakatos A, Becker-Baldus J, Pos KM, Glaubitz C. Detecting substrates bound to the secondary multidrug efflux pump EmrE by DNP-enhanced solid-state nmr. J. Am. Chem. Soc. 2013;135:15754–15762. doi: 10.1021/ja402605s. [DOI] [PubMed] [Google Scholar]
- 22.Lafon O, Thankamony ASL, Kobayashi T, Carnevale D, Vitzthum V, Slowing II, Kandel K, Vezin H, Amoureux JP, Bodenhausen G, Pruski M. Mesoporous silica nanoparticles loaded with surfactant: low temperature magic angle spinning C-13 and Si-29 NMR enhanced by dynamic nuclear polarization. J. Phys. Chem. C. 2013;117:1375–1382. [Google Scholar]
- 23.Gruning WR, Rossini AJ, Zagdoun A, Gajan D, Lesage A, Emsley L, Coperet C. Molecular-level characterization of the structure and the surface chemistry of periodic mesoporous organosilicates using DNP-surface enhanced NMR spectroscopy. Phys. Chem. Chem. Phys. 2013;15:13270–13274. doi: 10.1039/c3cp00026e. [DOI] [PubMed] [Google Scholar]
- 24.Gelis I, Vitzthum V, Dhimole N, Caporini MA, Schedlbauer A, Carnevale D, Connell SR, Fucini P, Bodenhausen G. Solid-state NMR enhanced by dynamic nuclear polarization as a novel tool for ribosome structural biology. J. Biomol. NMR. 2013;56:85–93. doi: 10.1007/s10858-013-9721-2. [DOI] [PubMed] [Google Scholar]
- 25.Andreas LB, Barnes AB, Corzilius B, Chou JJ, Miller EA, Caporini M, Rosay M, Griffin RG. Dynamic nuclear polarization study of inhibitor binding to the M2(18–60) proton transporter from influenza A. Biochemistry. 2013;52:2774–2782. doi: 10.1021/bi400150x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Akbey U, Altin B, Linden A, Ozcelik S, Gradzielski M, Oschkinat H. Dynamic nuclear polarization of spherical nanoparticles. Phys. Chem. Chem. Phys. 2013;15:20706–20716. doi: 10.1039/c3cp53095g. [DOI] [PubMed] [Google Scholar]
- 27.Thurber KR, Potapov A, Yau WM, Tycko R. Solid state nuclear magnetic resonance with magic-angle spinning and dynamic nuclear polarization below 25 K. J. Magn. Reson. 2013;226:100–106. doi: 10.1016/j.jmr.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Potapov A, Thurber KR, Yau WM, Tycko R. Dynamic nuclear polarization-enhanced H-1-C-13 double resonance NMR in static samples below 20 K. J. Magn. Reson. 2012;221:32–40. doi: 10.1016/j.jmr.2012.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Thurber KR, Yau WM, Tycko R. Low-temperature dynamic nuclear polarization at 9.4 T with a 30 mW microwave source. J. Magn. Reson. 2010;204:303–313. doi: 10.1016/j.jmr.2010.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thurber KR, Tycko R. Prospects for sub-micron solid state nuclear magnetic resonance imaging with low-temperature dynamic nuclear polarization. Phys. Chem. Chem. Phys. 2010;12:5779–5785. doi: 10.1039/c0cp00157k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Procopio A, Dalpozzo R, De Nino A, Nardi M, Sindona G, Tagarelli A. Erbium(III) triflate: a valuable catalyst for the rearrangement of epoxides to aldehydes and ketones. Synlett. 2004;14:2633–2635. [Google Scholar]
- 32.Paleos CM, Dais P. Ready reduction of some nitroxide free-radicals with ascorbic-acid. J. Chem. Soc. Chem. Commun. 1977:345–346. [Google Scholar]
- 33.Dempsey CE. The actions of melittin on membranes. Biochim. Biophys. Acta. 1990;1031:143–161. doi: 10.1016/0304-4157(90)90006-x. [DOI] [PubMed] [Google Scholar]
- 34.Terwilliger TC, Eisenberg D. The structure of melittin. 2. Interpretation of the structure. J. Biol. Chem. 1982;257:6016–6022. [PubMed] [Google Scholar]
- 35.Terwilliger TC, Eisenberg D. The structure of melittin. 1. Structure determination and partial refinement. J. Biol. Chem. 1982;257:6010–6015. doi: 10.2210/pdb1mlt/pdb. [DOI] [PubMed] [Google Scholar]
- 36.Bello J, Bello HR, Granados E. Conformation and aggregation of melittin - dependence on ph and concentration. Biochemistry. 1982;21:461–465. doi: 10.1021/bi00532a007. [DOI] [PubMed] [Google Scholar]
- 37.Thurber KR, Tycko R. Biomolecular solid state NMR with magic-angle spinning at 25 K. J. Magn. Reson. 2008;195:179–186. doi: 10.1016/j.jmr.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Thurber KR, Tycko R. Measurement of sample temperatures under magic-angle spinning from the chemical shift and spin-lattice relaxation rate of Br-79 in KBr powder. J. Magn. Reson. 2009;196:84–87. doi: 10.1016/j.jmr.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bennett AE, Rienstra CM, Auger M, Lakshmi KV, Griffin RG. Heteronuclear decoupling in rotating solids. J. Chem. Phys. 1995;103:6951–6958. [Google Scholar]
- 40.Lange S, Linden AH, Akbey U, Franks WT, Loening NM, van Rossum BJ, Oschkinat H. The effect of biradical concentration on the performance of DNP-MAS-NMR. J. Magn. Reson. 2012;216:209–212. doi: 10.1016/j.jmr.2012.01.002. [DOI] [PubMed] [Google Scholar]
- 41.Rossini AJ, Zagdoun A, Lelli M, Gajan D, Rascon F, Rosay M, Maas WE, Coperet C, Lesage A, Emsley L. One hundred fold overall sensitivity enhancements for Silicon-29 NMR spectroscopy of surfaces by dynamic nuclear polarization with CPMG acquisition. Chem. Sci. 2012;3:108–115. [Google Scholar]
- 42.Corzilius B, Andreas LB, Smith AA, Ni QZ, Griffin RG. Paramagnet induced signal quenching in MAS-DNP experiments in frozen homogeneous solutions. J. Magn. Reson. 2014;240:113–123. doi: 10.1016/j.jmr.2013.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thurber KR, Tycko R. Theory for cross effect dynamic nuclear polarization under magic-angle spinning in solid state nuclear magnetic resonance: the importance of level crossings. J. Chem. Phys. 2012;137:084508. doi: 10.1063/1.4747449. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.