Abstract
Objective
The goal of this study was to determine if we could establish a mesenchymal stromal line from zebrafish that would support hematopoietic cells. Such a co-culture system would be a great benefit to study the hematopoietic cell-stromal cell interaction in both the in vitro and in vivo environments.
Methods
Zebrafish stromal cells, ZStrC, were isolated from the “mesenchymal” tissue of the caudal tail and expanded in a specialized growth media. ZStrC were evaluated for phenotype, gene expression, and the ability to maintain zebrafish marrow cells in co-culture experiments.
Results
ZStrC showed mesenchymal and endothelial gene expression. Although ZStrC lacked the ability to differentiate into classic MSC lineages (osteocytes, adipocytes, chondrocytes), they did have the capacity for endotube formation on matrigel and LDL-uptake. ZStrC supported marrow cells for greater than 2 weeks in vitro. Importantly, the marrow cells were shown to retain homing ability in adoptive transfer experiments. ZStrC also were shown to improve hematopoietic recovery after sub-lethal irradiation after adoptive transfer.
Conclusion
As the zebrafish model grows in popularity and importance in the study of hematopoiesis, new tools to aid in our understanding of the hematopoietic cell-stromal cell interaction are required. ZStrC represent an additional tool in the study of hematopoiesis and will be useful to understand the factors that mediate the stromal cell-hematopoietic cell interaction that are important in hematopoietic maintenance.
Keywords: endothelial cells, mesenchymal stromal cell, zebrafish, stroma, hematopoiesis
Introduction
The ability to culture hematopoietic cells ex vivo is important as we try to understand the processes that control the hematopoietic program. Finding a suitable ex vivo culture environment has been sought for several decades, resulting in the production of a number of cell lines and various cocktails of growth factors in which hematopoietic cells are able to survive outside of their microenvironment. Several approaches have been taken in the past utilizing mesenchymal stromal cells (MSC), endothelial cells, and stroma-free conditions. Of these, MSC are probably the most well-characterized as a stromal support for the expansion of hematopoietic cells in vitro, mimicking the native stromahematopoietic cell interaction that takes place in the in vivo environment. By studying these interactions, the pathways that govern the ability of the marrow microenvironment to support hematopoiesis may be elucidated [1-3].
Endothelial cells have been shown to be a suitable stroma for hematopoietic cells as they can increase the number CD34+ cells from cord blood as well as increase SCID-repopulating frequencies (SRC) [4]. Comparable results also were obtained using both noncontact and contact co-culture of cord blood and endothelial cells giving rise to the hypothesis that soluble secreted factors from the endothelial cells play a role in hematopoietic cell support.
The advantages in using the zebrafish include: optical clarity for in vivo experiments, large offspring clutches to allow for higher throughput experiments, conservation of the critical genes involved in hematopoietic processes, and the ability to perform hematopoietic cell transplants [5-7].
We have been interested in the use of the zebrafish to model hematopoietic cell transplantation. Specifically, to better understand the stromal cell-hematopoietic cell niche interaction. Identifying a suitable stromal substrate has been difficult as there are very few suitable zebrafish cell lines, although Stachura et al recently has been successful in isolating a stromal cell line from the kidney of the zebrafish that can support hematopoietic cells in vitro [8]. In this report, we successfully developed several zebrafish cell lines from the stromal cells supporting the tail fine vasculature and have shown that these cells have similar properties to endothelial cells and are capable of supporting zebrafish hematopoietic cells that can be adoptively transferred and successfully home to the marrow space in myeloablated recipient fish.
Methods
Zebrafish
Fish were maintained by the University of Minnesota Zebrafish Core Facility according to standardized procedures with the approval of the International Animal Care and Use Committee, IACUC. Wild-type fish were obtained from Segrest Farms (Gibsonton, Florida) and bred in-house.
Cell isolation and culture
Adult zebrafish were anesthetized in 0.2% Tricaine until sedated. Tail fins were cut off with a sterile blade, and sterilized in 100% ethanol for 10 seconds before incubating in 0.01% bleach in phosphate-buffered saline (PBS), pH 7.4 for 10 minutes. Fins were then washed three times with PBS, pH 7.4 for 5 minutes to remove the bleach. Twenty microliters of Blendzyme in 1 mL of digest buffer (0.01M HEPES, 0.15M NaCl, 0.005M KCl, 0.001M MgCl2, 0.0018M CaCl2) was added to the fins. Fins were cut with sterile scissors, and triturated with P1000 pipette. Fins were then incubated at 32°C for 30 minutes, and triturated again with a P1000 pipette before another 30 minute incubation at 32°C. Fins were then spun down at 2000 × g for 6 minutes, washed with PBS, pH 7.4 before being plated in culture dishes with Zebrafish cell culture media (250 mL L-15, 175 mL DMEM, 75m L Ham's 12, 75mg sodium bicarbonate, 0.15M HEPES, 50 units penicillin/50 micrograms streptomycin, 2 mM L-Glutamine, 50 mL fetal bovine serum, 5 ng/ml selenium, 5 ng/ml human bFGF). Stromal cells would arise in about 2 - 3 weeks time. The resulting cellular outgrowths were maintained and split at 80 - 90% confluence once or twice per week. Incubator conditions were 4% CO2 and 5% O2 and a temperature of 28°C.
RT-PCR analysis
RNA was isolated from cells according to the manufacture's instructions using the Trizol® regent. Reverse transcription was performed using the SuperScript® III First-Strand Synthesis system (Invitrogen, Carlsbad, CA). Primers for PCR were designed for an annealing temperature of 60°C, used for an amplification of 40 cycles, and are listed in Table 1. For some experiments, endothelial cells were isolated from fli:EGFP transgenic fish. Tail cellular homogenates were prepared as above and the resulting cell suspension subject to fluorescence-activated cell sorting (FACS) based on enhanced green fluorescent protein (EGFP) positivity. The RNA was prepared from sorted cells as above and fgf20 expression evaluated using Taqman® primer/probe set Dr03084275_m1 and a universal 18S primer/probe set was used in normalization of qRT-PCR (Applied Biosystems, Foster City, CA).
Table 1.
| Gene | Forward | Reverse | Product Size |
|---|---|---|---|
| nt5e | aggcccacatctgaagtttgagga | tgtcctccaatcacaacgtccact | 168 |
| thy-1 | gccgcaccagtgtcaatcattcaa | acaggaacgctactccactgtgtt | 97 |
| eng | tacacaggctccgtgttactgcat | tgatcttgtgcaggtgcaggtagt | 158 |
| cd45 | agttcctgaaatggaaaagc | gcacagaaaagtccagtacg | 188 |
| Ick | gacgcagccaggtagtttctcc | tggggcgctctggtctga | 219 |
| kitlga | aacgaaacggtttgcctgatgacc | ttcttctggactccggtcagtttg | 78 |
| kitlgb | cagaatgcaagccttcaagtgcca | tgccattctgctgtctgctttgac | 191 |
| il10 | atgaatccaacgatgacttg | tcttgcatttcaccatatcc | 222 |
| notch 1a | ccagcccaggaaacaacaacaaca | ttctgatgtccttcccgctgtctt | 93 |
| notch 1b | gaatgcatcttttcttcgtg | cagacacttgcattctcctc | 144 |
| notch2 | actgcagctctaatccatgccgta | agctgttcacgtagtctgtgcagt | 171 |
| notch3 | aatgcacaggataacacagg | gcttcaacgttattgactgc | 266 |
| jag 1a | agagtggcggataatagagcctca | gatcctccgttcctgacatgcaaa | 153 |
| jag 1b | tgtgtaatgtatacccgccgctga | catgcacgggtggagaacaacttt | 146 |
| jag2 | tgatcagaccgagggagagatcag | gatcctccgttcctgacatgcaaa | 81 |
| bmp1 | ggatggatattggaggaaag | ctttgttcggtctgtaatcg | 230 |
| ang1 | ctacaacagagcgccgtccac | aggtcctgctgtctctgaag | 419 |
| cd31 | gacgggcacgctgatgtttctcttc | ctgcacgctccctttctcggttttg | 427 |
| vegfr1 | atccgcagtgttatattcctccat | ttgcgctccttttgccgatacct | 410 |
| vegfr2 | ataagagcccgccaaaagaggat | gcgcggtgcagttgagtatgag | 435 |
| vegfr3 | ccccagggtatagagaaatca | caataaaggtggaagtgtt | 417 |
| vegfr4 | gcctcgggtcaatgctgttcc | gtccggttgccaagttcattcc | 561 |
| vwf | atgtggcctctgtgggaactacaa | aggtcctgctgtctctgaag | 165 |
| ve-cadherin | ccccgttttcgattctgacc | ctttgaggcttagcattccatctt | 515 |
| fgf20a | Taqman Dr03084275_m1 | 60 |
Lectin Staining
ZStrC were plated in chamber slides to achieve 70 - 80 % confluency. Cells were washed with PBS and fixed with acetone at room temperature for 5 minutes. Cells were then rehydrated in PBS containing 0.3 % bovine serum albumin (PBS/BSA). Incubation with FITC-conjugated Triticum vulgaris (wheat germ) from Sigma Chemical (St. Louis, MO) was performed at 1 microgram per milliliter in PBS/BSA for 2 hours at 4°C. Slides were then washed three times with PBS and nuclei stained with 4', 6-diamidino-2-phenylindole (DAPI) at a concentration of 1 microgram per milliliter in water for 10 minutes at room temperature. Slides were then washed once in PBS, mounted in glycerol, cover-slipped and viewed using a Leica DMI6000 microscope.
Scanning electron microscopy (SEM)
Cells were grown on pre-cleaned 12 mm round, #1.5 thickness coverslips and were fixed 4 hours in 2.5% glutaraldehyde, 2% formaldehyde in 0.1 M phosphate buffer adjusted to pH 7.4. The samples were washed in 0.1 M phosphate buffer and then post-fixed in 1% OsO4 for 2 hours at room temperature. The samples were rinsed in distilled water and stained enbloc in aqueous 2% unranyl acetate overnight followed by a water wash. Samples were then dehydrated in an ethanol series and embedded in Embed 812 resin (Electron Microscopy Sciences, Hatfield, Pennsylvania). Ultrathin sections 80 – 100 nm thick were cut on a Leica Ultracut UCT microtome using a diamond knife and post-stained with 3% uranyl acetate followed by Sato's triple-lead stain. Sections were examined with an FEI Phillips CM 12 transmission electron microscope operating at 60 kV. Images were recorded with a Maxim DL digital capture system.
LDL-uptake assay
The zebrafish stromal cells or rat blood outgrowth endothelial cells (BOECs) were [9] plated on an 8-well chamber slide at a density of 104 cells per well. Once the cells reached confluence, they were rinsed twice in pre warmed serum free medium and incubated with 20 mg/ml acetylated LDL conjugated to Alexa Fluor® 594 (Invitrogen) at 37°C for 4 hours in serum free medium. The cells were fixed with pre-warmed 4% paraformaldehyde, followed by fluorescent microscopy using the appropriate filter set.
Matrigel assays
ZStrC isolated from a Red Glofish® or rat BOECs were plated (8 × 104 cells) onto matrigel (BD Biosciences, Bedford, MA) precoated 24-well plates and incubated at 32°C for 24 hours in the presence of endothelial growth medium-2 (EGM-2) (Lonza, Basal, Switzerland) followed by fluorescent microscopy. Some images were done in a single plane and captured in RGB full color, others were done using confocal generation as a projection of 27 image planes acquired using an Olympus Fluo View FV 1000 Inverted microscope in gray scale mode. Human umbilical vein endothelial cells (HUVECs) were labeled with CellTracker™Violet prior according to the manufacture's instructions (Invitrogen) and placed on matrigel in a similar manner as above.
Hematopoietic cell support
To perform hematopoietic cell co-culture experiments, whole kidney marrow was harvested from adult either wild-type or h2afv:EGFP transgenic zebrafish provided by the Zebrafish International Resource Center (ZIRC, Eugene, OR). Isolated cells were passed through a 40-micron filter and washed with PBS. 50,000 cells were placed onto a ZStrC monolayer that was 90% confluent in a 24-well dish; another 50,000 cells were placed directly onto plastic. ZStrC growth media was changed twice per week. Cells were incubated for various time points up to 16 days prior to analysis by flow cytometry. In some experiments, hematopoietic cells were placed into a transwell basket atop of ZStrC.
Adoptive Transfer
For adoptive transfer experiments, whole kidney marrow cells from h2afv:EGFP transgenic fish were harvested and co-cultured with ZStrC for 16 days as described above. At the end of this period, the progeny were harvested from the media supernatant leaving behind the stromal cells and washed once in PBS. Cells were delivered by intracardiac injection into wild-type recipient zebrafish 2 days after receiving 20 Gy x-ray irradiation using a GE X-Rad 320 irradiator at a dose rate of 2.7 Gy/minute. Seven days after transplant, marrow was harvested and donor status was determined by flow cytometry for EGFP signal.
Radiation Recovery
Wild-type zebrafish were given 20 Gy irradiation and 500,000 ZStrC were delivered via the intraperitoneal route 2 days later. Seven days after injection, marrow was harvested and cells enumerated by hemocytometer counting.
Results
Our primary interest has been the characterization of zebrafish hematopoietic cells. Having a stromal cell line to support marrow cells in culture would be of benefit. A single report by Stachura et al. showed the isolation of a stromal cell line from the kidney, the hematopoietic organ of the zebrafish, capable or supporting zebrafish marrow cells in vitro. However, further characterization of the stromal cells was limited, and it is unclear whether that cell line represented a fibroblastic, mesenchymal or an endothelial progenitor lineage [8]. Because our initial attempts to establish kidney stromal lines were not successful, we decided to focus on other sources for stromal cells that included the cells surrounding the tail fin central vessels (Fig. 1A). These cells have been termed “mesenchymal” in a general sense, but have not been characterized to our knowledge [10, 11]. After optimizing the enzyme digestion conditions, we were able to establish cell lines with fibroblastoid morphology from the caudal tail tissue. We performed this isolation in wild-type as well as in fli:EGFP transgenic fish which have EGFP-labeled endothelial cells. Occasionally, small fragments of fin were still present after digestion, and we able to observe that the resulting stromal cells migrated from around the central vessel (EGFP labeled), while the endothelial cells making up the vessel remained and ultimately apoptosed after 48 hours (Supplemental Fig.1). This finding, combined with the fact that the cells were not EGFP positive, suggested that the stromal cells were not derived from the EGFP-endothelium, but from the surrounding mesenchyme.
Figure 1.
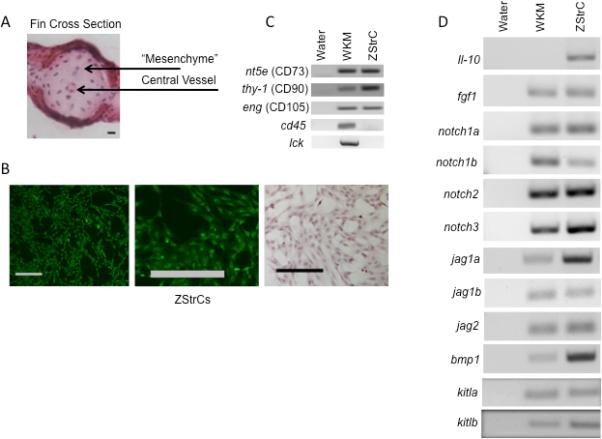
Isolation and initial characterization of ZStrCs from the mesenchymal tissue of the caudal tail. (A) A hematoxylin-eosin stained cross-section of the caudal tail prior to enzymatic digestion to release mesenchymal cells. Scale bar indicates 10 microns. (B) The resulting culture-derived stromal cells which were expanded from caudal tail mesenchyme and stained with Oregon Green® 488-conjugated wheat germ agglutinin to show their fibroblastoid morphology. Leftmost panel shows ZStrC after hematoxylin-eosin staining. Scale bars indicate 100 microns. (C) RT-PCR for typical positive and negative mesenchymal markers of whole kidney marrow (WKM) and ZStrCs. The human protein ortholog is given in parenthesis. (D) RT-PCR of factors important in hematopoietic cell support.
Such cells were plastic adherent and morphologically were very similar to fibroblasts, or mesenchymal stromal cells (Fig. 1B), therefore we have termed these cells zebrafish stromal cells (ZStrC). The cells were cultured at 32° C with 4% CO2 and 5% O2. ZStrC are very sensitive to the CO2 level as any increase above 4% caused the cultures to undergo apoptosis. We initially examined ZStrC growth kinetics and determined that their doubling time was ~ 44 hours (range 31 - 57 hours), and these cells have been maintained in culture for over 12 months and with no evidence of senescence.
Because ZStrC shared some physical characteristics with MSC, we next performed RT-PCR to determine expression of prototypical markers for human MSC (human CD73, CD90, CD105). These markers are not exclusive to MSC as CD73 is expressed on T and B cells, and CD90 or CD105 can be found on endothelial cells as well as other cell types. Nevertheless, their coexpression is one of the minimal criteria for an MSC [12]. Indeed, we did find that the zebrafish orthologs of the human antigens CD73, CD90, and CD105 (nt5e, thy-1, eng respectively) were expressed and the hematopoietic lineage genes, cd45 and lck, were not expressed (Fig. 1C). We were also interested to determine whether ZStrC expressed mRNAs encoding proteins known to be important in the support of hematopoietic cells. For this purpose, we focused on members of the notch-jagged family, kitl, and bmp1 (Fig. 1D). ZStrC expressed each of the aforementioned mRNAs and many were also expressed by whole kidney marrow (WKM), which we used as a reference being the hematopoietic organ of the zebrafish. These data indicated that ZStrC may be suitable for hematopoietic support.
The criteria for determining whether or not a cell type can be termed an MSC includes differentiation into the lineages of osteocytes, adipocytes, and chondrocytes [12]. Our cells failed to differentiate into these mesodermal lineages on multiple occasions (data not shown), and we concluded that either the conditions were not correct for differentiation (despite expressing MSC marker orthologs), or ZStrC had a different mesodermal capacity such as endothelial differentiation.
Further RT-PCR analysis showed certain endothelial markers to be expressed including pecam, ang-1, vegfr1-4 (Fig. 2A). These markers suggested that our stromal cells may be more related endothelial lineages or at least contain a population of cells with expression of endothelial markers [e.g. colony forming unit-Hill (CFU-Hill) or endothelial progenitor cells (EPCs)]. Functionally, CFU-Hill cells and EPCs have the ability to take up acetylated low density lipoprotein (LDL) which ZStrC were able to do similar to that of rat BOECs (Fig. 2B). We next explored the ultrastructure of the ZStrC using SEM and found typical cellular components of mitochondria, lysosomes, and golgi. In addition there were electron dense straining areas along the borders of cell-to-cell contact. Though not conclusive, these areas are similar to zonula occludens, which form tight junctions and are commonly found in several cellular structures including endothelial cells, kidney tubules, and some types of epithelia (Fig. 2C). These data show that ZStrcC have properties similar to cells of an endothelial lineage.
Figure 2.
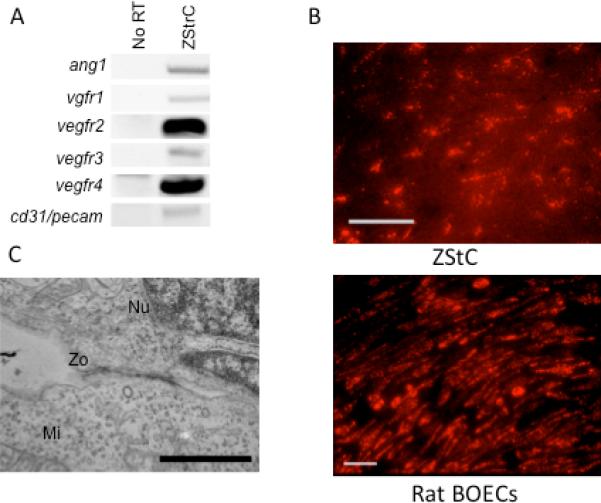
ZStrC have an endothelial-like phenotype. (A) RT-PCR of genes known to be expressed by endothelial cells. (B) Acetylated LDL uptake by ZStrCs and rat BOECs. Ten thousand cells per well of an 8-well chamber slide were cultured in growth media followed by exposure to acetylated LDL conjugated to Alexa Fluro® 594 for 4 hours prior to fixation and fluorescent imaging. Scale bar indicates 50 microns. (C) Electron microscopy of ZStrCs shows an electron dense region between two adjacent cells indicative of a possible zona occludens type cell junction (Zo). A nucleus (Nu) and mitochondrion (Mi) are also indicated for reference. Image taken at 11,500 × magnification and scale bar indicates 1 micron.
To determine if endothelial specific genes were upregulated when ZStrC were placed in endothelial growth media (EGM2), we performed RT-PCR for vecadherin, pecam, vwf, and ang1 (Fig. 3A) and found significant upregulation from 10-fold for ve-cadherin to 1000-fold for ang1. Additionally, ZStrC could undergo typical capillary network formation when placed onto Matrigel coated plates (Fig. 3B), which is a classic ability of cells with endothelial potential as exampled by the endothelial differentiation of the HUVECs (Fig. 3B) [13, 14]. While the capillary network is good evidence for an EPC or an endothelial-like cell, an actual lumen containing endotube is ideal evidence [15, 16], and we were able to visualize actual lumen containing endotubes in some of our matrigel experiments (Fig. 3C).
Figure 3.
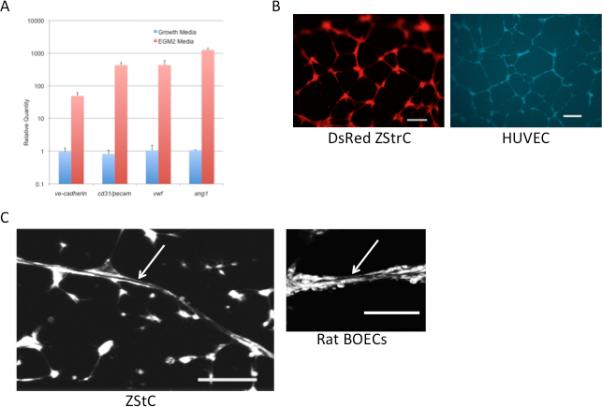
ZStrCs can be induced to express endothelial specific genes and are able to form capillary networks and endotubes on matrigel. (A) qRT-PCR of endothelial-specific genes expressed by ZStrCs in normal growth media versus EGM2 media. (B) Top panel shows zebrafish stromal cells isolated from a transgenic zebrafish constitutively expressing DsRed that were plated onto matrigel-precoated 96-well plates in EGM-2 media and incubated at 32°C for 24 hours followed by fluorescent microscopy. Shown is a typical capillary network. Scale bar indicates 100 microns. Bottom panel shows a similar endothelial differentiation using HUVECs labeled with CellTracker™Violet. Scale bar indicates 200 microns. (C) An example of a lumen-containing endotube (white arrow) formed by ZStrC or rat BOECs on matrigel. Confocal images were acquired using an Olympus Fluo View FV 1000 Inverted microscope in gray scale mode and final image generated as a projection of 27 image planes. Scale bar indicates 100 microns.
To determine whether ZStrC could be isolated in a clonal manner as has been performed with both MSC and endothelial cell cultures [17-19], we performed limiting dilution experiments, plating ZStrCs at 0.3 cells per well in 96-well plates. Over a period of 4 weeks, we observed that single-cell ZStrC had the ability to generate clonal cultures at a rate of about 15% (data not shown).
We next sought to determine if ZStrC could support hematopoietic cells, as might be predicted from our initial RT-PCR analysis for genes known to be important in the maintenance of hematopoietic cells (Fig. 1D), including members of the notch-jagged family, fgf and bmp1 [20-22]. Therefore, we placed fresh zebrafish whole kidney marrow cells in co-culture with ZStrC versus plastic alone. Although the input marrow cell number was not substantially increased over 16 days in culture, ZStrC were able to maintain starting cells significantly better than cells on plastic (Fig. 4A), resulting in a relative increase in cell yield between days 4 and 16 (2-fold to 7-fold, respectively). Furthermore, we found that the relative survival advantage given by ZStrC versus plastic was attenuated when whole kidney marrow cells were placed in a transwell, giving rise to the notion that contact factors are important in the maintenance of the marrow cells.
Figure 4.
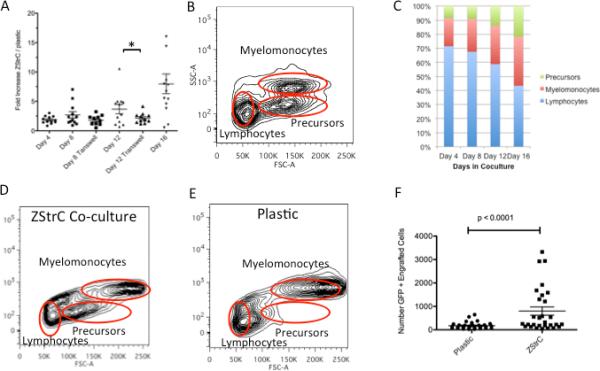
ZStrCs support hematopoietic cells in vitro that can be adoptively transferred. (A) WKM was harvested from h2afv:EGFP transgenic zebrafish and 50,000 cells were placed onto confluent ZStrC or plastic. The same donor was split between both conditions. At each time point, cells were harvested, counted, and analyzed by flow cytometry. The ratio of the number of cells on ZStrC versus plastic was calculated. In some experiments, marrow cells were placed into a transwell at the start of the experiment. Horizontal lines indicates the means, and the asterisk indicates a p-value < 0.05. (B) Forward and side scatter characteristics of zebrafish WKM previously perfused with saline to remove erythrocytes. Gating indicates where various cell populations are located. (C) Zebrafish WKM co-cultured with ZStrCs expands the precursor population. WKM was co-cultured with ZStrCs and the phenotype of the cells was determined at various time points over 16 days by flow cytometry forward and side scatter profiles of non-erythrocytes. Typical forward and side scatter profiles of marrow cells after 16 days of co-culture with ZStrC (D) or on plastic (E). (F) Marrow cells from h2afv:EGFP donor fish were cultured on ZStrCs or plastic for 16 days followed by adoptive transfer into myeloablated recipients. Flow cytometry was performed on recipient WKM 7 days after transplant and engraftment determined by gating on EGFP positive cells.
The lineages of zebrafish marrow cells can be classified to some extent based upon forward and side scatter properties [23]. Cell types include myelomonocytes (mature cells), precursors (immature cells), and lymphocytes (Fig. 4B). Over time, with ZStrC co-culture, we found there was a relative increase in the precursor population and a relative decrease in the lymphocyte population, suggesting that the environment may be suitable for immature cells to be maintained (Fig. 4C-E). To determine whether co-cultured marrow cells could home and engraft in the transplant setting, we next performed adoptive transfer experiments using co-cultured EGFP labeled marrow cells derived from h2afv:EGFP transgenic fish injected into myeloablated recipient wild-type fish. We analyzed the short term ability of the co-cultured marrow cells to engraft at day 7 post transplant and found they were significantly better than cells that were maintained on plastic alone (Fig. 4F).
Recently, Salter et al. showed the adoptive transfer of EPC can assist with autologous hematopoietic recovery after total body irradiation in mice, though the EPC did not engraft themselves [24]. In agreement with this previous finding, we also found that zebrafish given 500,000 ZStrCs following sub-lethal irradiation showed increased hematopoietic recovery 7 days after injection versus sham injection (Fig. 5A, p=0.03). Similarly, we also found that ZStrC did not engraft into the vasculature of the transplanted zebrafish when we performed such experiments with fluorescently labeled ZStrCs followed by dissection and visualization of various blood vessels (data not shown). Finally, FGF20 is a chemokine previously shown to have radioprotective capacity [25]. We compared fgf20a expression levels of ZStrC to that of flow sorted endothelial cells from the fli:EGFP transgenic zebrafish (which have EGFP-labeled endothelial cells) and found that fgf20a expression was greater than 100-fold higher in ZStrC than that of endothelial cells (Fig. 5B). We speculate that this relatively high level of fgf20a expression may be exerting a radio- or cyto-protective effect and explain some of the results in our autologous recovery experiments, but further definitive experiments blocking this pathway would be needed to be conclusive.
Figure 5.
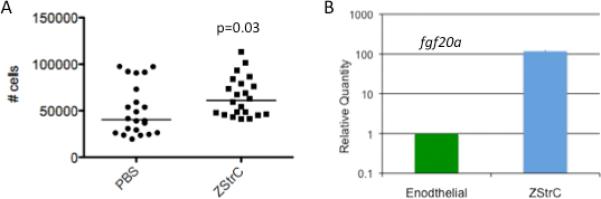
ZStrCs have a cytoprotective effect and express fgf-20a. (A) Wild-type zebrafish were irradiated with 20 Gy and 500,000 ZStrC were delivered via intraperitoneal injection 2 days later. Seven days after transplant, WKM was harvested and the number of non-erythrocyte cells was enumerated by hemocytometer. The graph shows the median cell count obtained, and the p-value calculated using Student's t-test. (B) RNA was prepared from endothelial cells isolated from Tg(fli:EGFP) zebrafish by FACS and from cultured ZStrC followed by qRT-PCR (figure represents 3 biologic replicates, p < 0.05).
Discussion
Our initial goal of this study was to determine a stromal line that could support hematopoietic cells from the zebrafish so that future manipulations and experiments involving hematopoietic cell homing could be accomplished. The resultant stromal cell line, termed ZStrC, had several characteristics of endothelial cells including vegf expression, LDL uptake, and capillary network/endotube formation on matrigel. ZStrC had the ability to maintain marrow cells for greater than 2 weeks, and such cells were engraftable short term (evaluated at 7 days post transplant). ZStrC also augmented autologous hematopoietic recovery after irradiation when delivered intraperitoneally.
In recent years, many studies have focused on the MSC (typically derived from bone marrow) to mimic the in vivo microenvironment and allow for hematopoietic cell maintenance or expansion. Although the bone marrow is a common source, other tissues can give rise to MSC, which support hematopoietic cells [26]. Our initial theory was that ZStrC were, in fact, MSC. However, despite the fact that ZStrC expressed similar markers as MSC, we were unable to differentiate them into the mesodermal lineages of osteocytes, adipocytes, or chondrocytes, a major criterion for categorization of cells as MSC [12]. It is possible that we did not use the proper media formulation or serum base for cells derived from fish, however our cell lines and other zebrafish cell lines do utilize fetal calf serum for growth and survival; therefore not all components need to be of fish origin. The cell line derived by Stachura et al. was also grown in fetal calf serum containing media and has a fibroblastoid morphologic appearance, but whether those cells represent a MSCs is not known [8].
MSC have previously been shown to differentiate into endothelial cells, although it is possible that such MSC cultures may have contained EPCs [27]. It is probably true that MSC and other stromal-derived cultures including ZStrC represent a heterogeneous mixture of cells with different potentials. This may be resolved by future work using established clones from the stromal populations being studied – whether human, mouse, or zebrafish. Even though we found expression of the zebrafish orthologs of classical MSC markers CD73, CD90, and CD105, these proteins have also been shown to be expressed by endothelial cells or cells with endothelial potential which only adds to the confusion as to what kind of cells are present in a bulk-derived culture [28-30]. Finally, there is some evidence that MSC and endothelial cells share a common mesodermal progenitor, as Vodyanik et al. was able to isolate MSC from embryonic stem cells after being differentiated toward mesoderm, but not after MSC differentiation giving an evidence of a common progenitor [31].
Further characterization of our stromal cells showed that they had a phenotype closer to that of endothelial progenitor cell type. The specific type of progenitor that existed in our cultures is unknown, and there is some debate in the literature about the differences between isolated endothelial progenitors, specifically regarding the description of the CFU-Hill cell and the EPC [17]. Given the characteristics examined in this work: endothelial antigens, LDL-uptake, matrigel endotube formation, and lack of vascular formation in vivo (data not shown), our cells seem more closely related to CFU-Hill, although they do not necessarily form colonies in culture nor do they express cd45. This may be because their maintenance is not in an endothelial type media, but a more generic serum-containing media similar to that of MSC.
The ability of endothelial cells to support hematopoietic cells has been described. Human brain-derived endothelial cells (HUBEC) have been shown to support and expand cord blood derived CD34+ cells which had improved SCID repopulating ability in mice [4]. While we could not show true high-fold expansion of marrow cells, we did find a definite contact mediated survival advantage offered by ZStrCs. The fact that contact was important leads us to speculate that this is perhaps due to engagement of the Notch-Jagged pathway, and we did find expression of various notch-jagged family members (Fig. 1D) [22]. Of course, there are probably other ligand-receptor pathways yet to be elucidated. We were also able to show that the co-cultured marrow cells could engraft short term in adoptive transfer experiments indicating that co-cultured marrow cells maintained their functional ability to home to the marrow microenvironment after irradiation. This is an important finding for future studies evaluating homing after genetic manipulation of either hematopoietic cells or of stromal cells.
The ability of the endothelial cells to support hematopoietic cells in vitro and in vivo is not entirely surprising as we learn more about the hematopoietic niche microenvironment. There are at least two niches in the hematopoietic microenvironment: an endosteal bone niche and a vascular niche [32, 33]. The factors that are responsible for the growth and maintenance of hematopoietic cells, recently termed angiocrine factors, include members of the Notch-Jagged pathway, FGF2, BMP4, and others, secreted both by endothelial cells and bone “niche” cells [20, 22, 34-36]. ZStrC do produce some of these angiocrine factors. ZStrC will be useful as an in vitro environment in which we can modulate these growth-signaling pathways to determine their specific effects on hematopoietic cell maintenance.
Previously, it was shown that EPC could help autologous HSC recovery as well as sinusoidal vessel recovery after total body irradiation in a mouse model [24]. Our data are in agreement with this phenomenon as we showed hastened autorecovery of marrow cells in zebrafish receiving ZStrCs post sublethal irradiation. Whether this is an effect of the endothelial cells, or due to other cell types within the line, such as MSC or even fibroblasts, is not known. Furthermore, the recovery observed by ZStrC does not seem to require them to engraft into the vasculature as we did not observe this, which is similar to what was found with EPC [24]. Finally, prior studies have demonstrated a radioprotective effect of FGF-20 [25], and we were able to show ZStrC expressed significantly more fgf20a than endothelial cells. We speculate on a mechanism that involves ZStrC secreting high levels of Fgf20 which serves to upregulate oxygen radical scavenging enzyme pathways such as MnSOD and activated signal transduction pathways (such as ERK), although these latter downstream events remain to be investigated in our system.
In conclusion, we have established stromal cell lines from mesenchymal tissue surrounding the tail fin vasculature of adult zebrafish. These stromal cells, ZStrC, may be a heterogeneous cell culture, but contain a population with endothelial markers and endothelial potential. ZStrC are able to support the maintenance of zebrafish marrow cells for greater than 2 weeks which still retain the ability for homing and short-term rescue of sublethally irradiated recipients following adoptive. ZStrC will prove to be a valuable tool for studying the cell-cell interactions that take place between hematopoietic cells and the microenvironment.
Supplemental Fig1. Zebrafish stromal cells come from cells exclusive of the central vessel. Fins from fli:EFGP transgenic fish were prepared as described in the methods section. The resulting “fin-digest” preparation was placed in a glass-bottom dish and imaged with fluorescent and phase-contrast microscopy and images were merged as shown. The white arrows indicate some of the nearly translucent stromal cells visible with phase-contrast. Clearly shown are the remaining EGFP positive endothelial cells making up the central vessel in the fin ray which disintegrated over 48 hours. This figure represents one frame from a 12-hour time-lapse video.
Supplementary Material
Acknowledgements
This work was supported by the Children's Cancer Research Fund.
References
- 1.Zhang Y, Chai C, Jiang XS, Teoh SH, Leong KW. Co-culture of umbilical cord blood CD34+ cells with human mesenchymal stem cells. Tissue Eng. 2006;12:2161–2170. doi: 10.1089/ten.2006.12.2161. [DOI] [PubMed] [Google Scholar]
- 2.Xie CG, Wang JF, Xiang Y, et al. Cocultivation of umbilical cord blood CD34(+) cells with retro-transduced hMSCs leads to effective amplification of long-term culture-initiating cells. World J Gastroenterol. 2006;12:393–402. doi: 10.3748/wjg.v12.i3.393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Robinson SN, Ng J, Niu T, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. 2006 doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chute JP, Muramoto G, Fung J, Oxford C. Quantitative analysis demonstrates expansion of SCID-repopulating cells and increased engraftment capacity in human cord blood following ex vivo culture with human brain endothelial cells. Stem Cells. 2004;22:202–215. doi: 10.1634/stemcells.22-2-202. [DOI] [PubMed] [Google Scholar]
- 5.Major RJ, Poss KD. Zebrafish Heart Regeneration as a Model for Cardiac Tissue Repair. Drug Discov Today Dis Models. 2007;4:219–225. doi: 10.1016/j.ddmod.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Davidson AJ, Zon LI. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene. 2004;23:7233–7246. doi: 10.1038/sj.onc.1207943. [DOI] [PubMed] [Google Scholar]
- 7.Bahary N, Zon LI. Use of the zebrafish (Danio rerio) to define hematopoiesis. Stem Cells. 1998;16:89–98. doi: 10.1002/stem.160089. [DOI] [PubMed] [Google Scholar]
- 8.Stachura DL, Reyes JR, Bartunek P, Paw BH, Zon LI, Traver D. Zebrafish kidney stromal cell lines support multilineage hematopoiesis. Blood. 2009;114:279–289. doi: 10.1182/blood-2009-02-203638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Somani A, Nguyen J, Milbauer L, Solovey A, Sajja S, Hebbel R. The establishment of murine blood outgrowth endothelial cells and observations relevant to gene therapy. Translational Research. 2007;150:30–39. doi: 10.1016/j.trsl.2007.02.002. [DOI] [PubMed] [Google Scholar]
- 10.Huang CC, Lawson ND, Weinstein BM, Johnson SL. reg6 is required for branching morphogenesis during blood vessel regeneration in zebrafish caudal fins. Dev Biol. 2003;264:263–274. doi: 10.1016/j.ydbio.2003.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang CC, Wang TC, Lin BH, Wang YW, Johnson SL, Yu J. Collagen IX is required for the integrity of collagen II fibrils and the regulation of vascular plexus formation in zebrafish caudal fins. Dev Biol. 2009;332:360–370. doi: 10.1016/j.ydbio.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 13.Arnaoutova I, George J, Kleinman HK, Benton G. The endothelial cell tube formation assay on basement membrane turns 20: state of the science and the art. Angiogenesis. 2009;12:267–274. doi: 10.1007/s10456-009-9146-4. [DOI] [PubMed] [Google Scholar]
- 14.Kubota Y, Kleinman HK, Martin GR, Lawley TJ. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988;107:1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Koh W, Stratman AN, Sacharidou A, Davis GE. In vitro three dimensional collagen matrix models of endothelial lumen formation during vasculogenesis and angiogenesis. Methods Enzymol. 2008;443:83–101. doi: 10.1016/S0076-6879(08)02005-3. [DOI] [PubMed] [Google Scholar]
- 16.Kamei M, Brian Saunders W, Bayless KJ, Dye L, Davis GE, Weinstein BM. Endothelial tubes assemble from intracellular vacuoles in vivo. Nature. 2006;442:453–456. doi: 10.1038/nature04923. [DOI] [PubMed] [Google Scholar]
- 17.Yoder MC, Mead LE, Prater D, et al. Redefining endothelial progenitor cells via clonal analysis and hematopoietic stem/progenitor cell principals. Blood. 2007;109:1801–1809. doi: 10.1182/blood-2006-08-043471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De Bari C, Dell'accio F, Vanlauwe J, et al. Mesenchymal multipotency of adult human periosteal cells demonstrated by single-cell lineage analysis. Arthritis Rheum. 2006;54:1209–1221. doi: 10.1002/art.21753. [DOI] [PubMed] [Google Scholar]
- 19.Russell KC, Phinney DG, Lacey MR, Barrilleaux BL, Meyertholen KE, O'Connor KC. In vitro high-capacity assay to quantify the clonal heterogeneity in trilineage potential of mesenchymal stem cells reveals a complex hierarchy of lineage commitment. Stem Cells. 2010;28:788–798. doi: 10.1002/stem.312. [DOI] [PubMed] [Google Scholar]
- 20.Varnum-Finney B, Purton LE, Yu M, et al. The Notch ligand, Jagged-1, influences the development of primitive hematopoietic precursor cells. Blood. 1998;91:4084–4091. [PubMed] [Google Scholar]
- 21.Fujita S, Toguchida J, Morita Y, Iwata H. Clonal analysis of hematopoiesis-supporting activity of human mesenchymal stem cells in association with Jagged1 expression and osteogenic potential. Cell Transplant. 2008;17:1169–1179. doi: 10.3727/096368908787236611. [DOI] [PubMed] [Google Scholar]
- 22.Weber JM, Calvi LM. Notch signaling and the bone marrow hematopoietic stem cell niche. Bone. 2010;46:281–285. doi: 10.1016/j.bone.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Traver D, Paw BH, Poss KD, Penberthy WT, Lin S, Zon LI. Transplantation and in vivo imaging of multilineage engraftment in zebrafish bloodless mutants. Nat Immunol. 2003;4:1238–1246. doi: 10.1038/ni1007. [DOI] [PubMed] [Google Scholar]
- 24.Salter AB, Meadows SK, Muramoto GG, et al. Endothelial progenitor cell infusion induces hematopoietic stem cell reconstitution in vivo. Blood. 2009;113:2104–2107. doi: 10.1182/blood-2008-06-162941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Maclachlan T, Narayanan B, Gerlach VL, et al. Human fibroblast growth factor 20 (FGF-20; CG53135-05): a novel cytoprotectant with radioprotective potential. Int J Radiat Biol. 2005;81:567–579. doi: 10.1080/09553000500211091. [DOI] [PubMed] [Google Scholar]
- 26.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 27.Oswald J, Boxberger S, Jorgensen B, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22:377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 28.Strijbos MH, Verhoef C, Gratama JW, Sleijfer S. On the origin of (CD105+) circulating endothelial cells. Thromb Haemost. 2009;102:347–351. doi: 10.1160/TH08-11-0762. [DOI] [PubMed] [Google Scholar]
- 29.De Francesco F, Tirino V, Desiderio V, et al. Human CD34/CD90 ASCs are capable of growing as sphere clusters, producing high levels of VEGF and forming capillaries. PLoS One. 2009;4:e6537. doi: 10.1371/journal.pone.0006537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Airas L, Niemela J, Salmi M, Puurunen T, Smith DJ, Jalkanen S. Differential regulation and function of CD73, a glycosyl-phosphatidylinositol-linked 70-kD adhesion molecule, on lymphocytes and endothelial cells. J Cell Biol. 1997;136:421–431. doi: 10.1083/jcb.136.2.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vodyanik MA, Yu J, Zhang X, et al. A mesoderm-derived precursor for mesenchymal stem and endothelial cells. Cell Stem Cell. 2010;7:718–729. doi: 10.1016/j.stem.2010.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bianco P. Bone and the hematopoietic niche: a tale of two stem cells. Blood. 2011 doi: 10.1182/blood-2011-01-315069. [DOI] [PubMed] [Google Scholar]
- 33.Boyerinas B, Sipkins DA. HSPCs in the balance: The vascular niche. Cell Stem Cell. 2010;7:645–646. doi: 10.1016/j.stem.2010.11.021. [DOI] [PubMed] [Google Scholar]
- 34.Kobayashi H, Butler JM, O'Donnell R, et al. Angiocrine factors from Akt-activated endothelial cells balance self-renewal and differentiation of haematopoietic stem cells. Nat Cell Biol. 2010;12:1046–1056. doi: 10.1038/ncb2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ratajczak MZ. Fibroblast growth factors and early hemopoietic cell development. Leuk Lymphoma. 1997;27:221–229. doi: 10.3109/10428199709059678. [DOI] [PubMed] [Google Scholar]
- 36.McReynolds LJ, Tucker J, Mullins MC, Evans T. Regulation of hematopoiesis by the BMP signaling pathway in adult zebrafish. Exp Hematol. 2008;36:1604–1615. doi: 10.1016/j.exphem.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


