Abstract
Purpose.
We investigated relations between macular retinal ganglion cell plus inner plexiform layer (RGC+IPL) thickness and macular retinal function revealed by multifocal electroretinonography (mfERG) in a nonhuman primate model of experimental glaucoma.
Methods.
Retinal ganglion cell (RGC) structure and function were followed with spectral-domain optical coherence tomography (SD-OCT) and ERGs in five macaques with unilateral experimental glaucoma. Linear regression was used to study correlations in control (Con) and experimental (Exp) eyes between peripapillary retinal nerve fiber layer (RNFL) thickness, macular RGC+IPL thickness, multifocal photopic negative response (mfPhNR) and high-frequency multifocal oscillatory potentials (mfOP) in slow-sequence mfERG, and low-frequency component (mfLFC) in global-flash mfERG. We used ANOVA and paired t-tests to compare glaucoma-related mfERG changes between superior and inferior hemifields, foveal hexagon, inner three rings, and four quadrants of macula.
Results.
Average macular RGC+IPL and temporal RNFL thickness were strongly correlated (r2 = 0.90, P < 0.001). In hexagon-by-hexagon analysis, all three mfERG measures were correlated (P < 0.001) with RGC+IPL thickness for Con (r2, 0.33–0.51) and Exp eyes (r2, 0.17–0.35). The RGC structural and functional metrics decreased as eccentricity increased. The reduction in amplitude of mfERG measures in Exp eyes relative to Con eyes was proportionally greater, in general, than the relative thinning of RGC+IPL at the same location for eyes in which structural loss was not evident, or mild to moderate. Although not statistically significant, percent amplitude reduction of mfERG measures was greatest in the inferior temporal quadrant.
Conclusions.
Macular RGC+IPL thickness and mfERG measures of RGC function can be complementary tools in assessing glaucomatous neuropathy.
Keywords: multifocal ERG, experimental glaucoma, photopic negative response, retinal ganglion cell + inner plexiform layer complex
Relations were studied between macular retinal ganglion cell (RGC) plus inner plexiform layer thickness and macular RGC function assessed by mfERG in nonhuman primate model of experimental glaucoma. Function changed before structure in many loci, and local structure–function correlations were observed.
Introduction
The multifocal ERG (mfERG) technique developed by Sutter et al.1 is an objective and noninvasive method for simultaneous functional testing of multiple retinal locations. This technique can provide a sufficient spatial resolution and signal-to-noise (SNR) ratio to resolve focal defects with clinically acceptable time efficiency. The mfERG has been demonstrated to be capable of detecting retinal functional defects caused by glaucoma.2–9
While the standard fast flicker mfERG has been used in some studies of glaucoma,3,10,11 two other types of mfERG protocols, that is, slow-sequence protocols and global-flash protocols, have been used more extensively.4,5,7,8,12–14 In a slow-sequence protocol, the m-sequence is slowed down by inserting multiple dark frames into each m-step after the frame that contains a focal flash. It has been observed in several studies that high-frequency oscillating potentials (OPs) in slow-sequence mfERG rely on the integrity of retinal ganglion cells (RGCs) and are sensitive to glaucomatous injury.4,7,8,13,15
In global-flash protocols, at least one global flash frame is inserted into each m-step.4,5,9,16 Waveforms obtained with global flash protocols contain a direct component (DC), which is the response to the focal flash, and an induced component (IC), which is the response to the global flash(es) under the influence of preceding focal flash. The IC resembles higher order kernels in the fast flicker mfERG in terms of interflash interaction and is believed to be generated in inner retina.17,18 A characteristic nasotemporal asymmetry is augmented in certain global-flash protocols,5,6,16,19 due, at least partly, to an optic nerve head component (ONHC), which is a consequence of increased delays in temporal versus nasal retina in propagation of action potentials along the unmyelinated RGC axons to the optic nerve head.20,21
Recent work in a nonhuman primate model of experimental glaucoma has indicated that the low-frequency component isolated from a global-flash mfERG receives significant contribution from the ONHC, and correlates well with local visual sensitivity and peripapillary retinal nerve fiber layer (RNFL) thickness (RNFLT).5 However, a more direct assessment of relations between glaucoma-sensitive mfERG responses and local inner retinal structure is needed. With improvement in axial resolution and time efficiency in image acquisition with spectral-domain optical coherence tomography (SD-OCT) technology, accurate measurement of local thickness of the nerve fiber layer (NFL), RGC, and inner plexiform layer (IPL) has become possible. Among various inner retinal structural measures, the macular ganglion cell complex (mGCC), which is defined as the combination of the NFL, ganglion cell layer, and IPL, has shown repeatability better than that of RNFLT measures and promise for use in glaucoma diagnosis. The mGCC and peripapillary RNFL can detect RGC loss associated with preperimetric and early-stage perimetric glaucoma,22–24 and significant correlation between peripapillary RNFL and mGCC has been reported.25–28 However, mGCC thickness has similar or even higher power to detect glaucoma when compared to peripapillary RNFL.25,26,29–36 Correlations between mGCC and tests of visual function, such as visual fields29 and pattern ERG,37 also have been reported in glaucoma. Since the contribution from converging axons to mGCC thickness increases as the distance of the measured location from the optic nerve head (ONH) decreases, the macular RGC plus IPL (RGC+IPL) thickness, with RNFL excluded, should in theory correlate with local RGC function better than does mGCC. The RGC+IPL has been used in recent studies on relations between macular structure and function in glaucoma.38–41
The current study used SD-OCT and mfERG protocols known to elicit RGC responses to investigate relationships between local RGC+IPL thickness and RGC-related function in a model of experimental glaucoma in the macaque, whose retina is similar to that of humans. The findings could be useful for future translational studies, as well as in evaluating therapies objectively in a macaque model. Some of the results have been reported in abstract form (Luo X, et al. IOVS 2010;51:ARVO E-Abstract 1075).
Methods
Animals and Preparations
Five adult rhesus monkeys (Macaca mulatta, 7–8 years old, three males and two females) were included. These animals were subjects in other studies as well.42 They each received several unilateral blue-green argon laser procedures to the trabecular meshwork (TM) under general anesthesia to induce sustained ocular hypertension (OHT).43,44 The maximum (peak) IOP of each experimental (Exp) eye obtained with a Tono-pen Xl (Reichert, Inc., Depew, NY, USA) for the five animals, OHT 57 through OHT 61, was, in order, 48, 54, 47, 48, and 40 mm Hg. Most biweekly IOP tests (measured within 10 minutes of administering anesthesia, see below) were 10 to 20 mm Hg lower than the peak values; control eyes were between 8 and 17 mm Hg. The ERG recordings were done right after structural imaging sessions, which took one to two hours. The IOPs would have been reduced substantially by xylazine at that point.45,46 All experimental and animal care procedures adhered to the ARVO statement for the Use of Animals in Ophthalmic and Vision Research, and were approved by the Institutional Animal Care and Use Committee of the University of Houston.
For OCT and ERG experiments, animals were anesthetized intramuscularly with ketamine (2–25 mg/kg/h) and xylazine (0.8–0.9 mg/kg/h) and were treated with atropine sulfate (0.04 mg/kg injected subcutaneously), as previously described.8,13 Body temperature was maintained between 36.5°C and 38°C with a thermostatically controlled blanket (TC1000 temperature controller; CWE, Ardmore, PA, USA). Heart rate and blood oxygen were monitored with a pulse oximeter (model 9847V; Nonin Medical, Inc., Plymouth, MN, USA).
Pupils were fully dilated to approximately 8.5 mm in diameter with topical tropicamide (1%) and phenylephrine (2.5%). For mfERG recordings, the monkey's head was positioned carefully using a small bead-filled pillow under the chin to make the animal look straight ahead at the stimulus display. The eyes were refracted and fitted with appropriate contact lenses for the viewing distance, and the fovea of the stimulated eye was aligned with the center of the stimulus using a modified direct ophthalmoscope (American Optical Co., Buffalo, NY, USA). For OCT scans, done on the same day as the ERG experiments, a plano-power contact lens was placed on the cornea to maintain optical clarity. A bite bar and an occipital bar attached to a rotational mount were used to adjust the position and stabilize the head of the animal.42
ERG Experiments
Brief full-field photopic flash ERGs were recorded with an Espion system (Diagnosys, Lowell, MA, USA) to assess outer and inner retina function. The stimuli were brief red flashes (λmax = 650 nm, 0.04–2.84 cd s/m2) on a rod-saturating blue background (λmax = 462 nm, 100 scotopic cd/m2). The photopic negative response (PhNR) amplitude was measured from the ERG baseline 65 ms after the flash.47
A 103-element unstretched hexagon array generated by VERIS (Visual Evoked Response Imaging System; Electro-Diagnostic Imaging, Inc., Redwood City, CA, USA) 4.1 was used for mfERG recording. The hexagon array subtended an angle of approximately 35° × 34° on the retina at a viewing distance of 46 cm. Two mfERG protocols were used (Figs. 1A, 1B) to assess inner retinal function: a slow-sequence protocol with 14 dark frames in each m-step (F14), and a global-flash protocol that contained 2 global flash frames and 2 dark global frames in each m-step (MFOFO). The maximum luminance of m- and global-flash frames was 200 cd/m2, and the background and dark frames of both stimuli was 15 cd/m2.
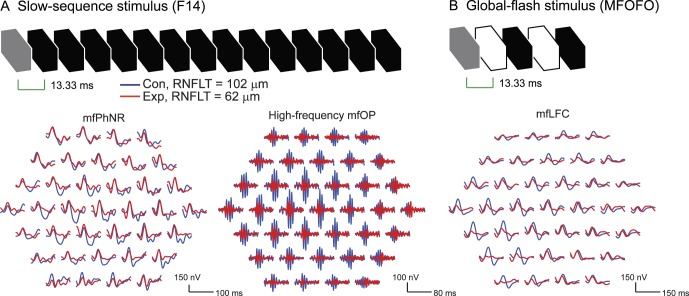
Figure 1.
Multifocal ERG stimuli and signal processing. (A) F14 stimulus and F14 trace arrays after the original responses were filtered by low-pass (50 Hz) and band-pass (110–224 Hz) filters. The low-pass filtered traces were used to quantify mfPhNR, whereas the band-passed traces were used to quantify mfOP. (B) The global-flash stimulus and the low-pass–filtered (25 Hz) trace array for LFC analysis. The gray frames were the m-frames. For the global-flash stimulus, the second and fourth frames contained the global flashes. All the other frames in both stimuli were dark frames. In this and subsequent figures, hexagon arrays are shown in retina view for the right eye. The arrays include approximately 11.5° of the central visual field with the fovea aligned with the central hexagon.
The mfERG recordings were amplified and filtered (1–300 Hz) by an amplifier (5A22N; Tektronix, Beaverton, OR, USA). Two recording epochs were obtained from each eye with each protocol and combined in VERIS. First order kernel responses were exported for off-line analysis.
The F14 recordings resembled the full-field flash ERG in two ways. First, there were multiple OPs that commenced along the ascending limb of P1.48,49 The high-frequency multifocal OPs (mfOPs) were isolated with a band-pass (110–224 Hz) filter and quantified in terms of RMS amplitude (signal window, 10–60 ms, Fig. 1A).7,8 Second, there was a slow and negative N2 component after P1, which resembled the PhNR in full-field flash ERG and, therefore, was referred to as mfPhNR in the present study. After signals had been filtered (low-pass, 50 Hz filter) to remove OPs, the mfPhNR amplitudes were measured from the baseline to the trough, rather than at a fixed time, because the position of the trough varied across the retina (Fig. 1A).
The low frequency components (mfLFC) in MFOFO recordings were isolated by filtering the signals with a low-pass (25 Hz) filter. As noted in the Introduction, this signal was observed previously to be altered in experimental glaucoma (Fig. 1B).5 The LFC RMS amplitude was calculated in the signal window from 10 to 70 ms.
OCT Experiments and Data Analysis
Spectralis HRA+OCT (Heidelberg Engineering, Jena, Germany) was used to acquire retinal images, always by the same investigator (NBP). The standard scanning protocols were a 12° peripapillary circular scan to measure RNFL thickness and a 31-section 30° × 25° horizontal raster scan to generate RGC+IPL thickness map. Each B-scan of the raster consisted of 1536 A-scans (51.2 scans/deg), and the distance between neighboring B-scans was 0.83° (Fig. 2B). A semiautomated algorithm was used to analyze intensity and slope of backscatter signal along each of the longitudinal reflectivity profiles (LRPs) in the acquired OCT images and to segment the RGC+IPL complex (Fig. 2A), which was a lamina between the hyper-reflective nerve fiber layer and the hyporeflective inner nuclear layer in the OCT images. The results then were interpolated linearly between neighboring B-scans to generate the RGC+IPL thickness map at the same lateral resolution of 51.2 scans/deg (Figs. 2B, 2C).
Figure 2.
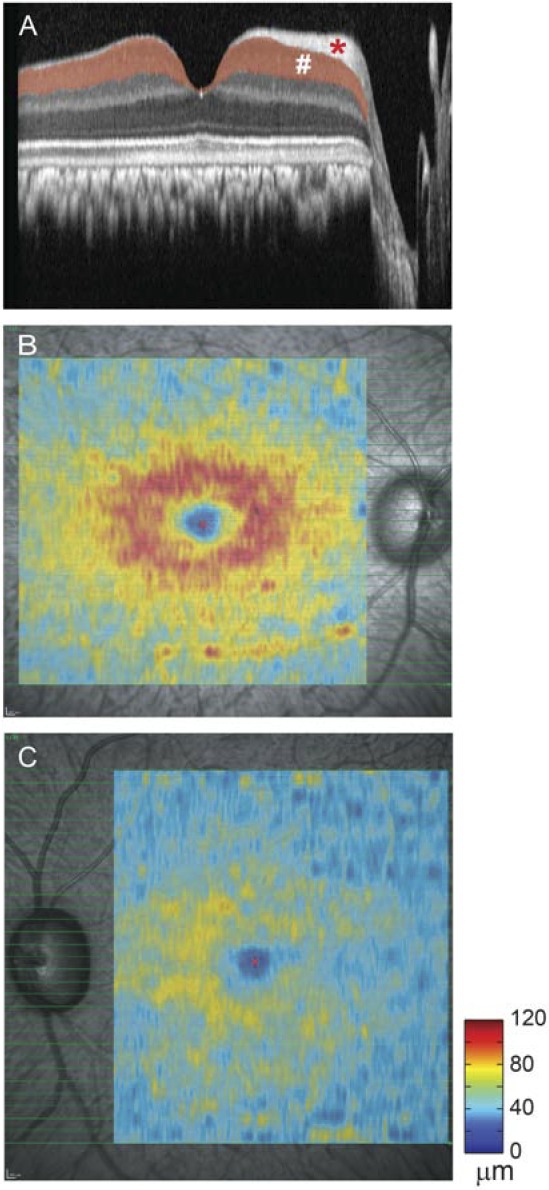
Optical coherence tomography image segmentation and RGC+IPL thickness maps from Con and Exp eyes of OHT-60 during the last imaging session. (A) A horizontal 30° OCT scan across the anatomical fovea of the Con eye. #RGC+IPL. *RNFL. (B) RGC+IPL thickness map for the Con eye (right eye) of OHT-60. (C) RGC+IPL thickness map for the Exp eye (left eye) of OHT-60.
A comparison between mfERG waves of RGC origin and RGC+IPL thickness averaged across retinal projections of mfERG hexagons is not directly available for central retina due to the RGC displacement in the foveal region. Previous studies, however, have modeled the relationship between RGC displacement and eccentricity along the principal hemimeridians in human retina,50 and have applied this model in structure–function studies in glaucoma.51,52 We assumed that the RGC displacement in macaques is similar to that in humans, when the scale of the smaller macaque eye is taken into account. To adapt the model for macaques, we used degrees of visual angle, rather than millimeters on the retina, as the metric for eccentricity in both species. Figure 3 shows projections of the mfERG hexagon array at the cone inner segment (IS) level (Figs. 3A, 3B) and the estimated projections at the RGC layer (Figs. 3A, 3C) with the model. The RGC displacement is largest in the center of the fovea, which causes significant distortion in RGC projections of central hexagons. The RGC+IPL thickness averaged by RGC projections (Fig. 3C) was used for further analysis.
Figure 3.
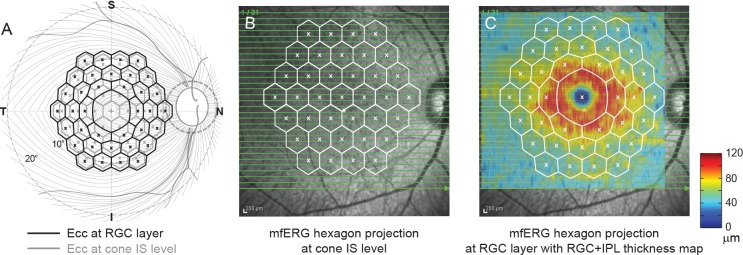
Correction of RGC displacement. (A) A schematic diagram of the relation between the projections of the mfERG hexagon array at cone IS and RGC layer levels. (B) Registration of the mfERG hexagon array projection at cone IS level on the 30° × 30° fundus image. (C) Registration of the mfERG hexagon array projection at RGC level on the fundus image and RGC+IPL thickness map.
In the present study, we analyzed data only from the fovea hexagon and central three rings of hexagons (37 hexagons in total, Fig. 1) extending to approximately 11.5° eccentricity on the horizontal and vertical meridians. Analyses were restricted to this region just slightly larger than the macula to ensure good overlap with the region represented in the OCT central raster scan and good signal-to-noise ratio in the mfERG from the region of retina where RGC density is the highest.
Statistical Analysis
Statistical analysis was performed using SPSS Statistics Version 20 (IBM Corporation, Somers, NY, USA) and MATLAB 7.4 (MathWorks, Natick, MA, USA). Most analyses were based on the final recording session for each of the five animals. Two-way ANOVA was used to compare structure and function grouped by retinal locations (hemifields, rings, and quadrants) in control (Con) eyes and eyes with experimental glaucoma (Exp). Paired t-test and 1-way ANOVA were used to compare glaucoma-related changes in these locations. Bonferroni correction was used in post hoc tests to adjust for multiple comparisons in the same eye. Data analyses, aside from those that already have been described, will appear in relevant sections of the results.
Results
Longitudinal ERG (full-field and multifocal) and RGC+IPL data were available for 4 of the 5 animals included in this study. The number of sessions per animal ranged from 1 to 12. The fifth subject, OHT-61, progressed rapidly to substantial RNFL thinning after unilateral TM lasering and mfERG data from only one session were available. Table 1 lists average RNFL thickness for the peripapillary circular scan and amplitude of full-field PhNR in response to a nearly saturating brief red full-field flash (2.84 cd.s/m2 flash) of five animals from their last ERG and imaging sessions. All of the animals showed losses in average RNFL thickness and full-field PhNR amplitude (but not a- or b-wave amplitude) in the eyes with laser-induced ocular hypertension. Previous work from the lab showed strong correlations between these measures longitudinally and cross-sectionally in macaques with unilateral experimental glaucoma.5
Table 1.
Average Peripapillary RNFL Thickness and Full-Field PhNR Amplitude of Five Animals From Their Last Recording and Imaging Sessions
|
Animal |
Average RNFL Thickness, μm |
PhNR Amplitude, μV |
||
|
Con |
Exp |
Con |
Exp |
|
| OHT-57 | 112.2 | 93.6 | 39.0 | 22.6 |
| OHT-58 | 115.3 | 61.3 | 31.2 | −7.3 |
| OHT-59 | 100.5 | 57.3 | 23.6 | −5.6 |
| OHT-60 | 102.4 | 61.8 | 71.0 | 16.4 |
| OHT-61 | 112.8 | 59.3 | 21.2 | −27.2 |
PhNR amplitude was measured in response to red full-field flashes on a rod-saturating blue background, from ERG baseline 65 ms after the stimulus flash.
The Relation Between Temporal RNFL Thickness and Central RGC+IPL Thickness
The present study was designed to use localized RGC+IPL thickness as the retinal structural measure to correlate with local ERG measures of retinal function. Previous studies in macaque have used RNFL thickness as the structural measure for local comparisons.5,15 An important question is whether the two structural measures from OCT are correlated in Con eyes, and are altered similarly by glaucoma in Exp eyes. As illustrated in Figure 3A, axons from RGCs residing in the central 37 hexagons converged to the temporal side of the ONH (Fig. 3A). Figure 4 shows the relation between the average RGC+IPL thickness for the central 37 hexagons and the average RNFL thickness measured over a 180° temporal portion of the 12° peripapillary circular scan. The distributions of Con and Exp data from the last session (open and filled black symbols) and all longitudinal sessions of the animals (gray symbols) suggested that they could be described with one continuous linear model. Linear regression analysis for data from last imaging sessions of the animals revealed a strong and statistically significant correlation between the two structural measures (r2 = 0.90, P < 0.001).
Figure 4.
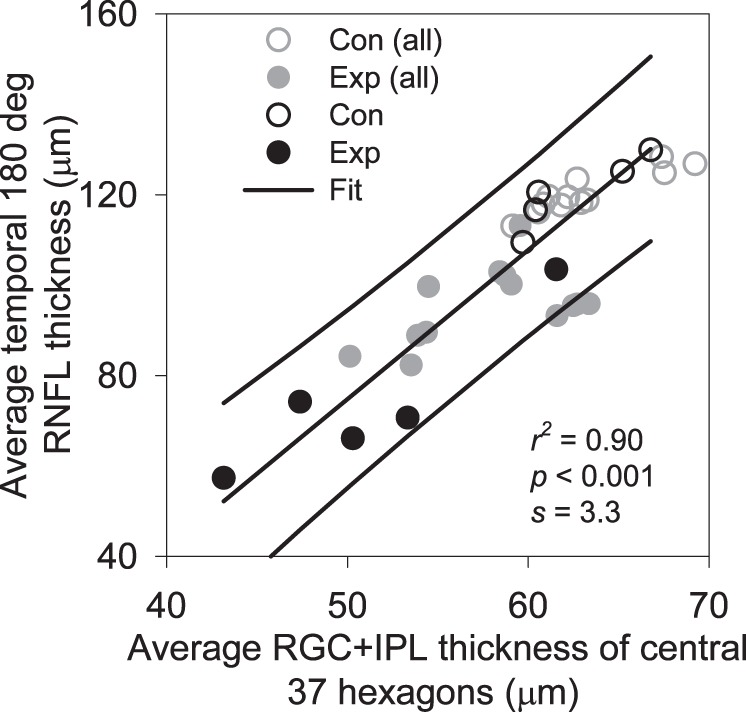
Relation between average macular RGC+IPL thickness and average temporal 180° RNFL thickness along the 12° peripapillary scan. Linear regression and 95% CI were fit to the data from the last recording sessions for Con (black open symbols) and Exp (filled dark symbols) of the five animals. Data from other recording sessions (gray symbols) almost all fell within the 95% CI.
Only data from the last session of each animal were used for linear regression for two reasons. First, since one of the animals had just one session of tests, and number of sessions varied from animal to animal, using one session from each animal balanced the contribution from each animal. Second, interocular differences should have been most prominent in the final session, thereby providing a large range of thickness values to determine the degree of correlation. However, the longitudinal data from four animals (gray symbols) clearly adhered to the trend and fell mostly within the 95% confidence interval (CI) determined by the regression analysis on the final session data. Therefore, the mean thickness of the temporal RNFL is well correlated with the RGC/IPL thickness in the macular region, but the macula scan also provides topographic representation that can be compared directly to the retinal locations of the stimulus elements of the mfERG.
Local RGC+IPL Thickness and mfERG Functional Losses in Experimental Glaucoma
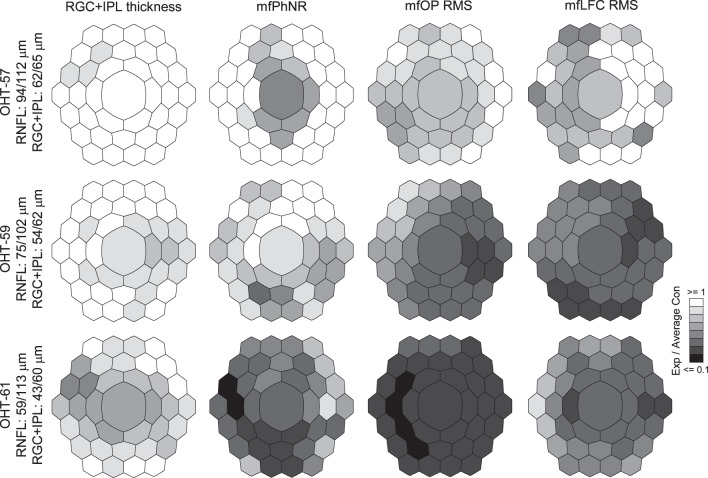
To determine whether local mfERG changes co-varied with RGC+IPL thickness changes caused by experimental glaucoma, normative data for hexagon-by-hexagon mfERG response and RGC+IPL thickness (adjusted for RGC displacement) were established for each animal, using longitudinal data from the Con eye. Con eye data from all sessions were averaged for each animal except OHT 61. Then, ratios of local RGC+IPL and mfERG measures in the Exp eye relative to the corresponding average for Con eye data for each animal were calculated. Figure 5 shows representative gray scale maps of RGC+IPL thickness and mfERG measures in this ratio format for one session each from three different animals. The sessions were selected, based on the severity of the relative structural loss (see notations on the left of the figure), to see animals at different stages of those losses.
Figure 5.
Ratio maps of RGC+IPL thickness and mfERG measures for three animals at different stages of structural loss. Top row: OHT-57 with mild structural loss. Middle row: OHT-59 with moderate structural loss. Bottom row: OHT-61 with the most severe structural loss. For OHT-57 and OHT-59, averages of all 4 recording and imaging sessions from the Con eyes were used to calculate the ratios of remaining structure and function in the Exp eyes. For OHT-61, only one recording and imaging session was available to calculate the ratios of remaining measures in the Exp eye.
For Figure 5, top row, structural losses were mild; average RGC+IPL thickness for the retinal area covered by the central 37 mfPhNR hexagons of OHT-57 was 62/65 μm (Exp/Con), and average RNFL thickness for the 12° peripapillary circular scan was 94/112 μm. The RGC+IPL thinning occurred mainly in the superior temporal region of the macula, and the central hexagon was spared. In contrast, all three mfERG measures captured functional loses that were more spatially extensive and profound than the structural losses. The proportional loss of retinal mfERG function extended into inferior regions, and the central hexagon RGC activity was dysfunctional by all three mfERG measures, although no RGC+IPL loss was detected there.
Average RGC+IPL and RNFL thickness for OCT session on OHT-59 illustrated in Figure 5 (middle row) were 54/62 and 75/102 μm, respectively. The RGC+IPL loss in this animal was more extensive at this stage than in OHT-57, illustrated above, and the central hexagon was involved. Both RGC+IPL and mfERG measures detected glaucomatous changes in the nasal side of the macula, but proportional losses comparing Exp to Con eyes were again greater and spatially more extensive for the mfERG measures than for the RGC+IPL thickness.
Data from the one session for OHT-61 are illustrated in Figure 5, bottom row, as an example of an eye that was more progressed in structural losses. Average RGC+IPL and RNFL thickness were 43/60 and 59/113 μm, respectively. In this animal, the proportional reduction of RGC+IPL thickness extended to most of the hexagons, and all hexagons were affected in all three mfERG analyses. Even at this stage the mfERG proportional losses were greater than the RGC+IPL proportional losses in most hexagons.
To further study the covariations observed in Figure 5, linear regression was used in the following session to analyze the correlations between these structural and functional measures of the inner retina.
Correlations Between Local mfERG Measures and RGC+IPL Thickness
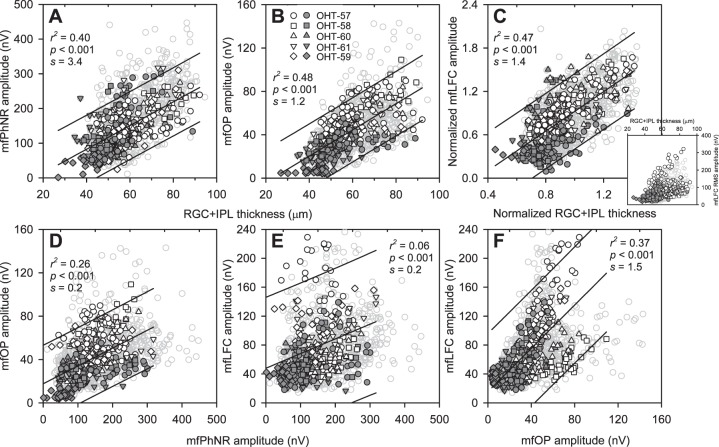
Analysis of the correlation of the three mfERG measures of inner retinal function with RGC+IPL thickness was approached first by assessing the hexagon-by-hexagon relations between the mfERG measures and corresponding RGC+IPL thickness from the last experimental session for each animal. These data are presented in Figures 6A through 6C for Con (open black symbols) and Exp (filled dark symbols), with data from all longitudinal sessions represented by gray symbols. Although interindividual variability in mfLFC data was the largest for the three mfERG measures (Fig. 6C, inset), previous work showed that this issue can be addressed by normalization, in this case of RGC+IPL thickness and mfLFC, by the averages across all the hexagons from the control sessions of the same animals (Fig. 6C, main panel).
Figure 6.
Relations between mfERG measures and RGC+IPL thickness, and between mfERG measures. Data for the central 37 hexagons were used for linear regression. (A) Relation between mfPhNR amplitude and RGC+IPL thickness. (B) Relation between mfOP RMS amplitude and RGC+IPL thickness. (C) Main panel: Relation between normalized mfLFC RMS amplitude (derived by normalizing Con and Exp data with the mean of Con from the same recording/imaging session) and normalized RGC+IPL thickness (derived by the same process). Inset: Scatterplot showing relation between mfLFC RMS amplitude and RGC+IPL thickness before normalization. (D) Relation between mfOP RMS and mfPhNR amplitude. (E) Relation between mfLFC RMS (without normalization) and mfPhNR amplitude. (F) Relation between mfLFC RMS (without normalization) and mfOP RMS amplitude. Symbol legends are listed in (B). s: slope of the fitted curves. Open black and filled dark symbols: Con and Exp data, respectively, from the last imaging/ERG session of all animals. Gray symbols: Con and Exp data from all sessions.
To study these structure–function relations within the same eyes, and between Con and Exp eyes, linear regression analysis was performed using data from the last session for each animal, and the results are listed in Table 2 with the animals sorted by ascending percent loss in RGC+IPL thickness in the Exp eye (100 − Exp/Con*100). The results showed significant structure–function correlations in Con and Exp eyes. Correlations generally were stronger in Con eyes than in Exp eyes and especially Exp eyes with the greatest RGC+IPL percent loss. This was consistent with the impression from Figures 6A through 6C that data points from Exp eyes were more likely to be packed close to the lower-left corner of the plots, reducing the range over which analyses could be done. The r2 values of the correlations for all animals combined ranged from 0.33 to 0.51 for Con eyes, and from 0.17 to 0.35 for Exp eyes (P < 0.001 in all cases).
Table 2.
Correlations Between RGC+IPL Thickness and mfERG Functional Measures by Animal and Eye (Last Session)
|
Animal |
% Loss of RGC+IPL in Exp |
mfPhNR |
mfOP |
mfLFC (Normalized Data) |
|||||||||
|
Con |
Exp |
Con |
Exp |
Con |
Exp |
||||||||
|
r2 |
P |
r2 |
P |
r2 |
P |
r2 |
P |
r2 |
P |
r2 |
P |
||
| OHT-57 | 5 | 0.51 | <0.001 | 0.00 | 0.711 | 0.38 | <0.001 | 0.02 | 0.362 | 0.48 | <0.001 | 0.19 | 0.007 |
| OHT-59 | 17 | 0.00 | 0.693 | 0.11 | 0.050 | 0.67 | <0.001 | 0.51 | <0.001 | 0.81 | <0.001 | 0.22 | 0.004 |
| OHT-58 | 20 | 0.64 | <0.001 | 0.22 | 0.005 | 0.58 | <0.001 | 0.68 | <0.001 | 0.74 | <0.001 | 0.22 | 0.005 |
| OHT-60 | 22 | 0.38 | <0.001 | 0.01 | 0.517 | 0.61 | <0.001 | 0.01 | 0.514 | 0.24 | 0.002 | 0.43 | <0.001 |
| OHT-61 | 28 | 0.45 | <0.001 | 0.22 | 0.004 | 0.22 | 0.003 | 0.06 | 0.156 | 0.42 | <0.001 | 0.01 | 0.532 |
| Average | 0.40 | 0.11 | 0.49 | 0.26 | 0.54 | 0.21 | |||||||
| All animals combined | 0.34 | <0.001 | 0.29 | <0.001 | 0.33 | <0.001 | 0.35 | <0.001 | 0.51 | <0.001 | 0.17 | <0.001 | |
When data from Con and Exp eyes were considered together, for each of the three mfERG measures, correlations with RGC+IPL thickness were similar to those of Con eyes alone: r2 = 0.40 for the correlation with mfPhNR, 0.48 for mfOP, and 0.47 for normalized mfLFC (P < 0.001 in all cases). Most of the data from all sessions (gray symbols) were within the 95% CI derived from the last session (Figs. 6A–C).
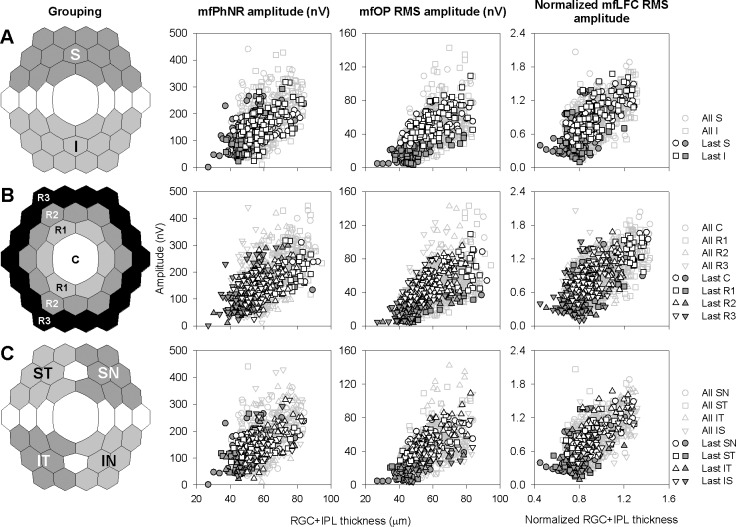
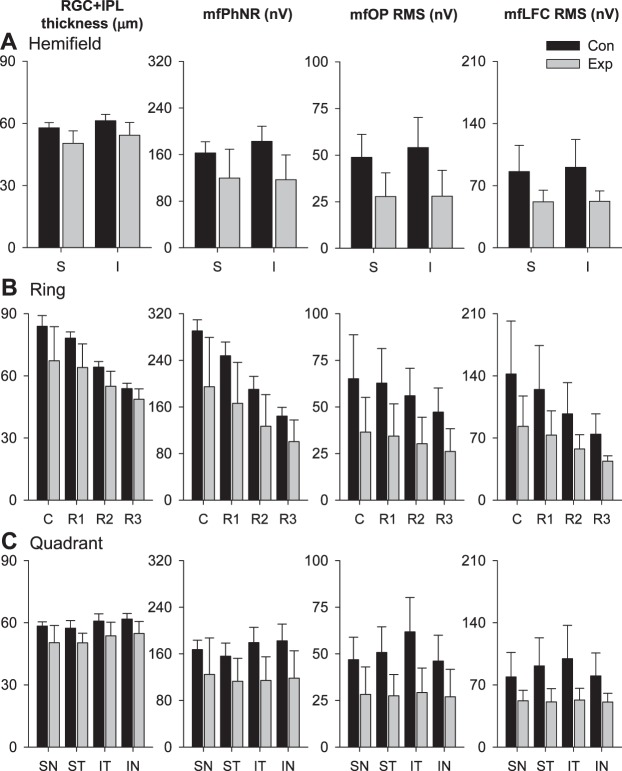
We then investigated the structure–function relations in the macular region, in different hemifields, eccentricities, and quadrants for each animal's last session. Figure 7A shows scatter plots for these relations for hexagons in superior and inferior hemifields. The scatter plots from the two hemifields followed the same trend and mostly overlapped with each other. Figure 8A shows average data separately for Con and Exp eyes for each hemifield, as mean ± SD for the five animals, with the percent losses of the RGC metrics (mean ± SE) reported in Table 3. Two-way ANOVA on last-session hemifield structural and functional measures from Con and Exp eyes found no statistically significant difference in these measures between the two hemifields (Table 4), and paired t-tests revealed no statistically significant difference in glaucoma-related percent losses between the two hemifields (data not shown). The strength of hexagon-by-hexagon correlations between last-session structure and functional metrics for Con and Exp eyes combined also was comparable between the two hemifields: r2 varied from 0.27 to 0.53, and all were significant (P < 0.001, Table 5).
Figure 7.
Grouping of central 37 hexagons and scatter plots showing relations between mfERG measures and RGC+IPL thickness with different grouping strategies. Black open and filled dark symbols: Con and Exp data, respectively, from last imaging/ERG session of all animals. Gray symbols: Con and Exp data from all sessions. (A) Grouping by hemifields and corresponding structure–function relations. S, superior retina; I, inferior retina. (B) Grouping by rings and corresponding structure–function relations. C, central hexagon; R1, Ring 1; R2, Ring 2; R3, Ring 3. (C) Grouping by quadrants and corresponding structure–function relations. SN,superonasal retina; ST, superotemporal retina; IT, inferotemporal retina; IN, inferonasal retina.
Figure 8.
Macular RGC+IPL and mfERG measures grouped and averaged by hemifields, rings and quadrants. Bar heights are means (N = 5 animals) for averaged measures, and error bars are 1 SD (N = 5 animals) for averaged measures. Dark bars: Con data. Light gray bars: Exp data. RGC+IPL thickness and mfERG measures in Con and Exp eyes by hemifields, rings, and quadrants for the last imaging and mfERG sessions are plotted in (A), (B), and (C), respectively.
Table 3.
Percent Loss in RGC+IPL Thickness and mfERG Functional Measures (Mean ± SE)
|
Group |
RGC+IPL Thickness |
mfPhNR |
mfOP RMS |
mfLFC RMS |
|
| Hemifield | S | 13.0 ± 3.6 | 28.1 ± 10.9 | 43.5 ± 10.8 | 35.3 ± 9.4 |
| I | 11.6 ± 2.8 | 34.1 ± 12.1 | 47.8 ± 10.8 | 38.2 ± 8.5 | |
| Ring | C | 20.2 ± 7.6 | 32.6 ± 13.0 | 44.8 ± 10.8 | 37.2 ± 10.6 |
| R1 | 18.3 ± 5.5 | 32.5 ± 12.5 | 46.1 ± 10.3 | 37.0 ± 10.3 | |
| R2 | 14.6 ± 3.8 | 33.8 ± 11.4 | 45.7 ± 10.6 | 36.6 ± 9.5 | |
| R3 | 9.6 ± 2.6 | 31.2 ± 10.0 | 43.9 ± 11.4 | 36.8 ± 8.5 | |
| Quadrant | SN | 13.7 ± 6.3 | 27.0 ± 14.7 | 40.4 ± 12.3 | 28.7 ± 11.5 |
| ST | 12.3 ± 2.6 | 29.3 ± 7.5 | 45.0 ± 10.6 | 40.7 ± 8.5 | |
| IT | 12.0 ± 3.1 | 35.0 ± 11.7 | 52.3 ± 8.6 | 41.6 ± 10.2 | |
| IN | 11.4 ± 2.7 | 32.8 ± 12.9 | 40.9 ± 13.8 | 32.9 ± 8.0 | |
Descriptive statistics listed in this table were derived from last-session data plotted in Figure 8 for different grouping strategies.
Table 4.
Two-Way ANOVA Based on Group Averages of Structure and Function Measures (N = 5 Animals)
|
Group |
RGC+IPL Thickness |
mfPhNR |
mfOP |
mfLFC |
|||||
|
F |
P |
F |
P |
F |
P |
F |
P |
||
| Hemifields | Con vs. Exp | 11.70 | 0.004 | 11.17 | 0.004 | 14.49 | 0.002 | 12.01 | 0.003 |
| Between hemifields | 3.09 | 0.098 | 0.28 | 0.606 | 0.19 | 0.666 | 0.06 | 0.804 | |
| Post hoc | |||||||||
| Rings | Con vs. Exp | 19.24 | <0.001 | 22.47 | <0.001 | 23.64 | <0.001 | 16.29 | <0.001 |
| Between rings | 18.58 | <0.001 | 12.42 | <0.001 | 1.38 | 0.266 | 4.46 | 0.010 | |
| Post hoc | C, R1 – R2, R3 | C – R2, R3 | C – R3 | ||||||
| R1 – R3 | |||||||||
| Quadrants | Con vs. Exp | 20.84 | <0.001 | 19.90 | <0.001 | 27.37 | <0.001 | 22.98 | <0.001 |
| Between quadrants | 1.81 | 0.165 | 0.33 | 0.801 | 0.81 | 0.496 | 0.48 | 0.696 | |
| Post hoc | |||||||||
--, statistically significant difference between groups it separates.
Table 5.
Coefficient of Determination (r2) From Linear Regression Analysis of RGC+IPL Versus the Three mfERG Measures of RGC Function
|
mfPhNR |
mfOP RMS |
Normalized mfLFC RMS |
||
| Hemifield | Superior | 0.27 | 0.53 | 0.48 |
| Inferior | 0.44 | 0.48 | 0.44 | |
| Ring | Center | 0.460.03 | 0.400.05 | 0.510.02 |
| Ring 1 | 0.59 | 0.42 | 0.44 | |
| Ring 2 | 0.47 | 0.52 | 0.35 | |
| Ring 3 | 0.19 | 0.45 | 0.21 | |
| Quadrant | SN | 0.32 | 0.51 | 0.55 |
| ST | 0.20 | 0.56 | 0.45 | |
| IT | 0.48 | 0.59 | 0.48 | |
| IN | 0.41 | 0.38 | 0.48 |
< 0.01 except for central hexagon analyses (P values marked as superscript).
Both RGC+IPL thickness and mfERG measures decreased as eccentricity increased (Fig. 7B, data for individual hexagons and Fig. 8B, average data for Con and Exp eyes for each ring). Using 2-way ANOVA, a statistically significant difference between rings was found in all structural and functional metrics except for mfOP (Table 4). Although glaucoma-related average structural and mfERG functional losses tended to be larger for the central hexagon and more central rings (Fig. 8B), the difference in percent loss across rings were not statistically significant for any of the metrics. Hexagon-by-hexagon correlations between RGC+IPL thickness and mfERG metrics also tended to be stronger for the central hexagon and more central rings (Table 5).
Scatter plots for hexagons in the quadrants (Fig. 7C) looked very similar to those for hemifields: they all followed the same trend and overlapped with each other. Structural and functional measures tended to be largest in inferotemporal quadrant (Fig. 8C), although this trend was not statistically significant (Table 4). Glaucoma-related percent loss also tended to be greater in the inferotemporal quadrant (Fig. 8C). However 1-way ANOVA did not find differences in any of these changes to be statistically significant across the four quadrants. The RGC+IPL thickness–mfERG correlations were consistently high or highest in the inferotemporal quadrant for all three mfERG measures (Table 5).
Relations Between the Three mfERG Measures
The present study also provided an opportunity to investigate correlations across glaucoma-sensitive mfERG measures. When the last-session data were analyzed, hexagon-by-hexagon correlation, whether with data grouped for all animals for Con and Exp eyes (Figs. 6D–F) or with data from individual animals and eyes (Tables 2, 3), was the highest between mfOP and mfLFC (r2 = 0.37, P < 0.001, Fig. 6F; average r2 was 0.57 for Con and 0.20 for Exp, Table 6), with the correlation between mfPhNR and mfOP (r2 = 0.26, P < 0.001, Fig. 6D; average r2 was 0.24 for Con and 0.10 for Exp, Table 3) lower, and correlation between mfPhNR and mfLFC (r2 = 0.06, P < 0.001, Fig. 6E; average r2 was 0.18 for Con and 0.13 for Exp, Table 6) the lowest. Correlations were again stronger for data from individual Con eyes than Exp eyes, and correlations tended to be stronger in Exp eyes with less RGC+IPL loss, as noted above for Figures 6A through 6C.
Table 6.
Correlations Between mfERG Functional Measures by Animal and Eye (Last Session)
|
Animal |
% Loss of RGC+IPL in Exp |
mfPhNR vs. mfOP |
mfPhNR vs. mfLFC |
mfOP vs. mfLFC |
|||||||||
|
Con |
Exp |
Con |
Exp |
Con |
Exp |
||||||||
|
r2 |
P |
r2 |
P |
r2 |
P |
r2 |
P |
r2 |
P |
r2 |
P |
||
| OHT-57 | 5 | 0.28 | <0.001 | 0.02 | 0.363 | 0.15 | 0.016 | 0.00 | 0.958 | 0.61 | <0.001 | 0.11 | 0.042 |
| OHT-59 | 17 | 0.01 | 0.560 | 0.07 | 0.102 | 0.00 | 0.829 | 0.42 | <0.001 | 0.70 | <0.001 | 0.42 | <0.001 |
| OHT-58 | 20 | 0.66 | <0.001 | 0.26 | 0.002 | 0.55 | <0.001 | 0.07 | 0.131 | 0.52 | <0.001 | 0.36 | <0.001 |
| OHT-60 | 22 | 0.32 | <0.001 | 0.05 | 0.175 | 0.02 | 0.408 | 0.07 | 0.104 | 0.44 | <0.001 | 0.03 | 0.276 |
| OHT-61 | 28 | 0.04 | 0.266 | 0.07 | 0.131 | 0.26 | 0.001 | 0.09 | 0.077 | 0.55 | <0.001 | 0.00 | 0.907 |
| Average | 0.26 | 0.10 | 0.20 | 0.13 | 0.56 | 0.19 | |||||||
| All animals combined | 0.07 | <0.001 | 0.22 | <0.001 | 0.00 | 0.814 | 0.13 | <0.001 | 0.20 | <0.001 | 0.30 | <0.001 | |
Discussion
In the present study, we examined the local relationship between RGC+IPL thickness and RGC function assessed using mfERG measures that are known to be affected in experimental glaucoma within a central retinal region slightly larger than the macula. The RGC+IPL became thinner and amplitudes of mfERG measures were reduced in Exp eyes. Proportional losses (Figs. 5, 8; Exp eye relative to Con eye) in the functional measures generally were larger than corresponding losses in the structural measures. There were significant correlations between local RGC+IPL thickness and RGC function, and between mfERG measures, with the strongest between mfOPs and mfLFC. Correlations between these structural and functional metrics tended to be best in central retina and to become weaker as eccentricity increased (Table 5). This was true in Con and Exp eyes. In Con eyes, center to periphery decline in RGC density provided a nearly linear relationship between the measures (Fig. 6, open black symbols), and in Exp eyes as glaucoma progressed loss of RGCs and their function extended the relationship to lower values (Fig. 6, filled dark symbols). Correlations were not found to differ much between hemifields, but tended to be strongest in inferior temporal retina (Table 5). The diffuse nature of damage in the nonhuman primate experimental glaucoma model has been a common observation.
Several factors may, potentially, have contributed to the finding in the present study that proportional losses in the ERG functional measures generally were larger and more extensive than corresponding losses in the OCT structural measures (Figs. 5, 8). First, this discrepancy is consistent with neuronal (and/or glial) dysfunction before structural loss, which has been reported previously in human ERG and OCT studies on glaucoma suspects53 and patients with early diabetes.54 Second, the glaucoma-unrelated residuals in RGC+IPL thickness and mfERG functional measures, which predetermined the maximal proportional losses that could be observed with these measures, apparently were quite different (Figs. 6A–C). Since the maximal proportional losses that these structural and functional measures could approach were different, visualizing the losses with the same grayscale also could have contributed the mismatch observed in Figure 5. A third consideration is the effect of IOP of the Exp eyes in the five animals. Maximum IOP (see Methods) was similarly high for most of the animals, which could have affected function. However, IOP would have been lowered by Xylazine during recording sessions,45,46 making it difficult to relate any mismatch of structure and function in a given session to IOP.
Involvement of Macular Region in Early Glaucoma
The present study found that macular structure and function were affected in glaucoma. Macular involvement in early glaucoma has been reported previously.38,51,55–57 One phenomenon frequently noted in these glaucomatous changes and correlations between structure and function measures is that the changes often are more prominent in the inferior retina (usually the inferotemporal quadrant, as illustrated in Fig. 8C), and structure–function and function–function correlations usually also are stronger in the same region (Table 5). Since axons from inferior macular region project to the inferior temporal sector of the ONH,58–62 the preferential structure–function loss in the inferior macular region observed in the present and previous studies translates to axon damage and/or rim change of the inferior temporal sector of the ONH, which have been described in humans63 and nonhuman primates with laser-induced ocular hypertension.64 Previous studies on monkeys and humans have shown that OCT-derived neuroretinal rim parameters detect glaucomatous lesion earlier than does RNFL thickness.65,66 It would be interesting to see if changes in these OCT-derived neuroretinal rim parameters also are more profound in the inferior temporal sector of the ONH. Profound structure and function loss due to experimental glaucoma in inferior quadrants helped to extend dynamic ranges and better define relations of these measures.
Central RGC+IPL Thickness Loss Versus Peripapillary RNFL Thickness Change in Glaucoma
Previous studies, such as those cited in the introduction, have shown that OCT quantification of macular inner retinal structures is useful in diagnosis of glaucoma, and can be as good as or superior to peripapillary RNFL in detecting glaucomatous changes. Recent work on a macaque model with experimental glaucoma has shown that loss of ONH neural and connective tissues precedes loss of peripapillary RNFL thickness. The onset of macular RGC structure compared to the onset of ONH changes, however, was not compared in that study.66 The strong and statistically significant correlation between the average temporal peripapillary RNFL thickness and the average RGC+IPL thickness of central 37 hexagons revealed in the present study (Fig. 4) suggests that the two structural measures follow a similar course in the Exp eyes, and it is possible that macular RGC structural changes occur later than ONH neural and connective tissue changes as well, which requires further study.
PhNR Recorded Locally With Multifocal Technique
The PhNR was described initially in nonhuman primate and human full-field flash photopic ERG as a negative component that reaches its maximum amplitude after the b-wave.67,68 This slow negative wave is reduced in optic neuropathy due to experimental and clinical glaucoma as well as in other diseases that affect inner retina, such as diabetic retinopathy, optic nerve atrophy, central retinal artery occlusion, multiple sclerosis, and retinal vein occlusion.69–71 The PhNR in macaques also can be removed by intravitreal tetrodotoxin (TTX), an agent that prevents the generation of sodium-dependent spikes in retinal neurons.72,73 These observations suggested that the neuronal origin of the PhNR is from spike-generating inner retinal neuron (mainly RGCs in primate, but perhaps more from amacrine cells in rodents).68,74,75 Evidence also points to a Müller-glial cell contribution to PhNR in rat76 and in humans.77
A fundus camera-based stimulus delivery technique also has been used to study focal macular PhNR in nonhuman primates with RGC lesions.78–82 A study in glaucoma patients using this technique showed that the focal macular PhNR has high sensitivity and specificity in detecting functional loss due to early glaucomatous damage.80 Consistent with this finding, even the full field PhNR in macaque has been observed to show significant reduction in amplitude even when visual sensitivity losses due to experimental glaucoma are minor.68 These studies encourage further use of focal stimuli, simultaneously in a multifocal presentation for detecting glaucoma, as was done in the present study using a slow-sequence (F14) mfERG protocol and isolated with a low-pass filter (cutoff at 50 Hz). Although compared to full-field and focal-flash PhNR, mfPhNR recorded with the slow-sequence protocol was more oscillatory (Fig. 1A), the resemblance of the two in waveform and reduction of amplitude by experimental glaucoma on their amplitude suggests that they share origin(s). We found that mfPhNR correlated well with local RGC+IPL thickness detected by SD-OCT (Fig. 6A) and was significantly smaller in Exp eyes than in Con eyes (Table 4).
Each m-step of the F14 protocol used in the present study was twice as long as an m-step in the more commonly used F7 protocol. The advantages of a shorter recording time include better patient cooperation and performance during the test, more reliable recording, and fewer artifact rejections. However, previous studies failed to identify significant change in N2 in the low-frequency component in F7 mfERG (equivalent to mfPhNR measured from P1 peak in the present study) in experimental glaucoma,12,15,83 which may be partially because the F7 protocol is not long enough for the full development of the mfPhNR.
A Comparison of Three mfERG Measures
The naso-temporal asymmetry in mfOPs and mfLFC seen in Figure 8C for inferior quadrants has been noted previously for these measures.5,7,8,13 Both measures are manifestations of the ONHC of Sutter and Bearse,21 whose nasotemporal asymmetry is due to longer delays in propagation of action potentials to the ONH in temporal retina. This asymmetry is lost in experimental glaucoma when signals from RGCs and their axons are reduced or eliminated.
The mfPhNR did not show the nasotemporal asymmetry seen for the other two mfERG RGC functional measures. A previous focal PhNR study in macaques reported that focal PhNR was largest in superior and nasal macula, and suggested that this was due to higher RGC cell densities in those regions.79 This asymmetry is not consistent with a dominant input from the ONHC for the mfPhNR, making it different in this respect from the mfOPs and mfLFC.
Of all the three mfERG measures, mfPhNR had the largest dynamic range, which was followed by mfLFC RMS and high-frequency mfOP RMS (Fig. 6). The mfLFC RMS amplitude measure had large interindividual variability, as has been reported previously.5 However, after this interindividual variability had been minimized by self-normalization (Fig. 6C, major panel), all three mfERG measures showed similar moderate-to-strong correlations with local RGC+IPL thickness (Fig. 6; Tables 2, 6).
Glaucoma-related percentage losses in mfOP and mfLFC were similar and both were larger than those seen with mfPhNR, and mfOP and mfLFC were largest in the inferotemporal quadrant and showed most profound loss in that region as noted above.
Conclusions
Results of this study suggested that in nonhuman primate experimental glaucoma, structure–function correlation can be observed not only with global RGC measures, but also with focal RGC measures in the macular region. These local retinal structure and function tests may be useful complementary tools for clinical assessment of glaucoma, as well as for studying effectiveness of therapies in an animal model whose retina is similar to that of humans.
Acknowledgments
Supported by National Eye Institute Grants R01-EY01139, K23 EY021761, and P30-EY07751.
Disclosure: X. Luo, None; N.B. Patel, None; L.P. Rajagopalan, None; R.S. Harwerth, None; L.J. Frishman, None
References
- 1. Sutter EE, Tran D. The field topography of ERG components in man—I. The photopic luminance response. Vision Res. 1992; 32: 433–446 [DOI] [PubMed] [Google Scholar]
- 2. Chan HH. Detection of glaucomatous damage using multifocal ERG. Clin Exp Optom. 2005; 88: 410–414 [DOI] [PubMed] [Google Scholar]
- 3. Chan HL, Brown B. Multifocal ERG changes in glaucoma. Ophthalmic Physiol Opt. 1999; 19: 306–316 [DOI] [PubMed] [Google Scholar]
- 4. Fortune B, Bearse MA Jr, Cioffi GA, Johnson CA. Selective loss of an oscillatory component from temporal retinal multifocal ERG responses in glaucoma. Invest Ophthalmol Vis Sci. 2002; 43: 2638–2647 [PubMed] [Google Scholar]
- 5. Luo X, Patel NB, Harwerth RS, Frishman LJ. Loss of the low-frequency component of the global-flash multifocal electroretinogram in primate eyes with experimental glaucoma. Invest Ophthalmol Vis Sci. 2011; 52: 3792–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Miguel-Jimenez JM, Boquete L, Ortega S, Rodriguez-Ascariz JM, Blanco R. Glaucoma detection by wavelet-based analysis of the global flash multifocal electroretinogram. Med Eng Phys. 2012; 36: 103–111 [DOI] [PubMed] [Google Scholar]
- 7. Rangaswamy NV, Hood DC, Frishman LJ. Regional variations in local contributions to the primate photopic flash ERG: revealed using the slow-sequence mfERG. Invest Ophthalmol Vis Sci. 2003; 44: 3233–3247 [DOI] [PubMed] [Google Scholar]
- 8. Rangaswamy NV, Zhou W, Harwerth RS, Frishman LJ. Effect of experimental glaucoma in primates on oscillatory potentials of the slow-sequence mfERG. Invest Ophthalmol Vis Sci. 2006; 47: 753–767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chu PH, Chan HH, Brown B. Glaucoma detection is facilitated by luminance modulation of the global flash multifocal electroretinogram. Invest Ophthalmol Vis Sci. 2006; 47: 929–937 [DOI] [PubMed] [Google Scholar]
- 10. Frishman LJ, Saszik S, Harwerth RS, et al. Effects of experimental glaucoma in macaques on the multifocal ERG. Multifocal ERG in laser-induced glaucoma. Doc Ophthalmol. 2000; 100: 231–251 [DOI] [PubMed] [Google Scholar]
- 11. Hood DC, Greenstein V, Frishman L, et al. Identifying inner retinal contributions to the human multifocal ERG. Vision Res. 1999; 39: 2285–2291 [DOI] [PubMed] [Google Scholar]
- 12. Fortune B, Wang L, Bui BV, Cull G, Dong J, Cioffi GA. Local ganglion cell contributions to the macaque electroretinogram revealed by experimental nerve fiber layer bundle defect. Invest Ophthalmol Vis Sci. 2003; 44: 4567–4579 [DOI] [PubMed] [Google Scholar]
- 13. Zhou W, Rangaswamy N, Ktonas P, Frishman LJ. Oscillatory potentials of the slow-sequence multifocal ERG in primates extracted using the Matching Pursuit method. Vision Res. 2007; 47: 2021–2036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Palmowski-Wolfe AM, Todorova MG, Orguel S, Flammer J, Brigell M. The ‘two global flash' mfERG in high and normal tension primary open-angle glaucoma. Doc Ophthalmol. 2007; 114: 9–19 [DOI] [PubMed] [Google Scholar]
- 15. Fortune B, Burgoyne CF, Cull GA, Reynaud J, Structural Wang L. and functional abnormalities of retinal ganglion cells measured in vivo at the onset of optic nerve head surface change in experimental glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 3939–3950 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Sutter EE, Bearse MA. The retinal topography of local and lateral gain control mechanisms. Vision Science and Its Applications, OSA Technical Digest Series. 1998; 1: 20–23 [Google Scholar]
- 17. Chu PH, Chan HH, Ng YF, et al. Porcine global flash multifocal electroretinogram: possible mechanisms for the glaucomatous changes in contrast response function. Vision Res. 2008; 48: 1726–1734 [DOI] [PubMed] [Google Scholar]
- 18. Shimada Y, Li Y, Bearse MA Jr, Sutter EE, Fung W. Assessment of early retinal changes in diabetes using a new multifocal ERG protocol. Br J Ophthalmol. 2001; 85: 414–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Sutter EE, Shimada Y, Li Y, Bearse MA. Mapping inner retinal function through enhancement of adaptive components in the m-ERG. Vision Science and Its Applications, OSA Technical Digest Series. 1999; 1: 52–55 [Google Scholar]
- 20. Hood DC, Bearse MA Jr, Sutter EE, Viswanathan S, Frishman LJ. The optic nerve head component of the monkey's (Macaca mulatta) multifocal electroretinogram (mERG). Vision Res. 2001; 41: 2029–2041 [DOI] [PubMed] [Google Scholar]
- 21. Sutter EE, Bearse MA Jr. The optic nerve head component of the human ERG. Vision Res. 1999; 39: 419–436 [DOI] [PubMed] [Google Scholar]
- 22. Arintawati P, Sone T, Akita T, Tanaka J, Kiuchi Y. The applicability of ganglion cell complex parameters determined from SD-OCT images to detect glaucomatous eyes. J Glaucoma. 2013; 22: 713–718 [DOI] [PubMed] [Google Scholar]
- 23. Na JH, Kook MS, Lee Y, Baek S. Structure–function relationship of the macular visual field sensitivity and the ganglion cell complex thickness in glaucoma. Invest Ophthalmol Vis Sci. 2012; 53: 5044–5051 [DOI] [PubMed] [Google Scholar]
- 24. Takagi ST, Kita Y, Takeyama A, Tomita G. Macular retinal ganglion cell complex thickness and its relationship to the optic nerve head topography in glaucomatous eyes with hemifield defects. J Ophthalmol. 2011; 2011: 914250 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chen J, Huang H, Wang M, Sun X, Qian S. Fourier domain OCT measurement of macular, macular ganglion cell complex, and peripapillary RNFL thickness in glaucomatous Chinese eyes. Eur J Ophthalmol. 2012; 22: 972–979 [DOI] [PubMed] [Google Scholar]
- 26. Firat PG, Doganay S, Demirel EE, Colak C. Comparison of ganglion cell and retinal nerve fiber layer thickness in primary open-angle glaucoma and normal tension glaucoma with spectral-domain OCT. Graefes Arch Clin Exp Ophthalmol. 2013; 251: 831–838 [DOI] [PubMed] [Google Scholar]
- 27. Kita Y, Kita R, Nitta A, Nishimura C, Tomita G. Glaucomatous eye macular ganglion cell complex thickness and its relation to temporal circumpapillary retinal nerve fiber layer thickness. Jpn J Ophthalmol. 2011; 55: 228–234 [DOI] [PubMed] [Google Scholar]
- 28. Takagi ST, Kita Y, Yagi F, Tomita G. Macular retinal ganglion cell complex damage in the apparently normal visual field of glaucomatous eyes with hemifield defects. J Glaucoma. 2012; 21: 318–325 [DOI] [PubMed] [Google Scholar]
- 29. Cho JW, Sung KR, Lee S, et al. Relationship between visual field sensitivity and macular ganglion cell complex thickness as measured by spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2010; 51: 6401–6407 [DOI] [PubMed] [Google Scholar]
- 30. Kim NR, Hong S, Kim JH, Rho SS, Seong GJ, Kim CY. Comparison of macular ganglion cell complex thickness by Fourier-domain OCT in normal tension glaucoma and primary open-angle glaucoma. J Glaucoma. 2013; 22: 133–139 [DOI] [PubMed] [Google Scholar]
- 31. Kim NR, Lee ES, Seong GJ, Kim JH, An HG, Kim CY. Structure–function relationship and diagnostic value of macular ganglion cell complex measurement using Fourier-domain OCT in glaucoma. Invest Ophthalmol Vis Sci. 2010; 51: 4646–4651 [DOI] [PubMed] [Google Scholar]
- 32. Kita Y, Kita R, Takeyama A, Takagi S, Nishimura C, Tomita G. Ability of optical coherence tomography-determined ganglion cell complex thickness to total retinal thickness ratio to diagnose glaucoma. J Glaucoma. 2013; 22: 757–762 [DOI] [PubMed] [Google Scholar]
- 33. Kotowski J, Folio LS, Wollstein G, et al. Glaucoma discrimination of segmented cirrus spectral domain optical coherence tomography (SD-OCT) macular scans. Br J Ophthalmol. 2012; 96: 1420–1425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Moreno PA, Konno B, Lima VC, et al. Spectral-domain optical coherence tomography for early glaucoma assessment: analysis of macular ganglion cell complex versus peripapillary retinal nerve fiber layer. Can J Ophthalmol. 2011; 46: 543–547 [DOI] [PubMed] [Google Scholar]
- 35. Rolle T, Briamonte C, Curto D, Grignolo FM. Ganglion cell complex and retinal nerve fiber layer measured by fourier-domain optical coherence tomography for early detection of structural damage in patients with preperimetric glaucoma. Clin Ophthalmol. 2011; 5: 961–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Schulze A, Lamparter J, Pfeiffer N, Berisha F, Schmidtmann I, Hoffmann EM. Diagnostic ability of retinal ganglion cell complex, retinal nerve fiber layer, and optic nerve head measurements by Fourier-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2011; 249: 1039–1045 [DOI] [PubMed] [Google Scholar]
- 37. Bowd C, Tafreshi A, Zangwill LM, Medeiros FA, Sample PA, Weinreb RN. Pattern electroretinogram association with spectral domain-OCT structural measurements in glaucoma. Eye (Lond). 2011; 25: 224–232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Hood DC, Raza AS, de Moraes CG, Liebmann JM, Ritch R. Glaucomatous damage of the macula. Prog Retin Eye Res. 2013; 32: 1–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Raza AS, Cho J, de Moraes CG, et al. Retinal ganglion cell layer thickness and local visual field sensitivity in glaucoma. Arch Ophthalmol. 2011; 129: 1529–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang M, Hood DC, Cho JS, et al. Measurement of local retinal ganglion cell layer thickness in patients with glaucoma using frequency-domain optical coherence tomography. Arch Ophthalmol. 2009; 127: 875–881 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Moura AL, Raza AS, Lazow MA, De Moraes CG, Hood DC. Retinal ganglion cell and inner plexiform layer thickness measurements in regions of severe visual field sensitivity loss in patients with glaucoma. Eye (Lond). 2012; 26: 1188–1193 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Patel NB, Luo X, Wheat JL, Harwerth RS. Retinal nerve fiber layer assessment: area versus thickness measurements from elliptical scans centered on the optic nerve. Invest Ophthalmol Vis Sci. 2011; 52: 2477–2489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Harwerth RS, Smith EL III, DeSantis L. Experimental glaucoma: perimetric field defects and intraocular pressure. J Glaucoma. 1997; 6: 390–401 [PubMed] [Google Scholar]
- 44. Quigley HA, Hohman RM. Laser energy levels for trabecular meshwork damage in the primate eye. Invest Ophthalmol Vis Sci. 1983; 24: 1305–1307 [PubMed] [Google Scholar]
- 45. Burke JA, Potter DE. The ocular effects of xylazine in rabbits, cats, and monkeys. J Ocul Pharmacol. 1986; 2: 9–21 [DOI] [PubMed] [Google Scholar]
- 46. Frishman LJ, Shen FF, Du L, et al. The scotopic electroretinogram of macaque after retinal ganglion cell loss from experimental glaucoma. Invest Ophthalmol Vis Sci. 1996; 37: 125–141 [PubMed] [Google Scholar]
- 47. Rangaswamy NV, Shirato S, Kaneko M, Digby BI, Robson JG, Frishman LJ. Effects of spectral characteristics of Ganzfeld stimuli on the photopic negative response (PhNR) of the ERG. Invest Ophthalmol Vis Sci. 2007; 48: 4818–4828 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hood DC. Assessing retinal function with the multifocal technique. Prog Retin Eye Res. 2000; 19: 607–646 [DOI] [PubMed] [Google Scholar]
- 49. Hood DC, Seiple W, Holopigian K, Greenstein V. A comparison of the components of the multifocal and full-field ERGs. Vis Neurosci. 1997; 14: 533–544 [DOI] [PubMed] [Google Scholar]
- 50. Drasdo N, Millican CL, Katholi CR, Curcio CA. The length of Henle fibers in the human retina and a model of ganglion receptive field density in the visual field. Vision Res. 2007; 47: 2901–2911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Hood DC, Raza AS. Method for comparing visual field defects to local RNFL and RGC damage seen on frequency domain OCT in patients with glaucoma. Biomed Opt Express. 2011; 2: 1097–1105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Hood DC, Raza AS, de Moraes CG, et al. Initial arcuate defects within the central 10 degrees in glaucoma. Invest Ophthalmol Vis Sci. 2011; 52: 940–946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Banitt MR, Ventura LM, Feuer WJ, et al. Progressive loss of retinal ganglion cell function precedes structural loss by several years in glaucoma suspects. Invest Ophthalmol Vis Sci. 2013; 54: 2346–2352 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Dhamdhere KP, Bearse MA Jr, Harrison W, Barez S, Schneck ME, Adams AJ. Associations between local retinal thickness and function in early diabetes. Invest Ophthalmol Vis Sci. 2012; 53: 6122–6128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Anctil JL, Anderson DR. Early foveal involvement and generalized depression of the visual field in glaucoma. Arch Ophthalmol. 1984; 102: 363–370 [DOI] [PubMed] [Google Scholar]
- 56. Langerhorst CT, Carenini LL, Bakker D, De Bie-Raakman MAC. Measurements for description of very early glaucomatous field defects. In: Wall M, Heiji A. eds Perimetry Update 1996/1997. New York, NY: Kugler Publications; 1997: 67–73 [Google Scholar]
- 57. Tan O, Li G, Lu AT, Varma R, Huang D. Mapping of macular substructures with optical coherence tomography for glaucoma diagnosis. Ophthalmology. 2008; 115: 949–956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Garvin MK, Abramoff MD, Lee K, Niemeijer M, Sonka M, Kwon YH. 2-D pattern of nerve fiber bundles in glaucoma emerging from spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012; 53: 483–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Garway-Heath DF, Poinoosawmy D, Fitzke FW, Hitchings RA. Mapping the visual field to the optic disc in normal tension glaucoma eyes. Ophthalmology. 2000; 107: 1809–1815 [DOI] [PubMed] [Google Scholar]
- 60. Harwerth RS, Vilupuru AS, Rangaswamy NV, Smith EL III. The relationship between nerve fiber layer and perimetry measurements. Invest Ophthalmol Vis Sci. 2007; 48: 763–773 [DOI] [PubMed] [Google Scholar]
- 61. Jansonius NM, Nevalainen J, Selig B, et al. A mathematical description of nerve fiber bundle trajectories and their variability in the human retina. Vision Res. 2009; 49: 2157–2163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Shields M. The Optic Nerve Head. Textbook of Glaucoma. Philadelphia, PA: Williams & Wilkins; 1987: 71–104 [Google Scholar]
- 63. Jonas JB, Schiro D. Localised wedge shaped defects of the retinal nerve fibre layer in glaucoma. Br J Ophthalmol. 1994; 78: 285–290 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Jonas JB, Hayreh SS. Localised retinal nerve fibre layer defects in chronic experimental high pressure glaucoma in rhesus monkeys. Br J Ophthalmol. 1999; 83: 1291–1295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Chauhan BC, O'Leary N, Almobarak FA, et al. Enhanced detection of open-angle glaucoma with an anatomically accurate optical coherence tomography-derived neuroretinal rim parameter. Ophthalmology. 2013; 120: 535–543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. He L, Yang H, Gardiner SK, et al. Longitudinal detection of optic nerve head changes by spectral domain optical coherence tomography in early experimental glaucoma. Invest Ophthalmol Vis Sci. 2013; 55: 574–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Colotto A, Falsini B, Salgarello T, Iarossi G, Galan ME, Scullica L. Photopic negative response of the human ERG: losses associated with glaucomatous damage. Invest Ophthalmol Vis Sci. 2000; 41: 2205–2211 [PubMed] [Google Scholar]
- 68. Viswanathan S, Frishman LJ, Robson JG, Harwerth RS, Smith EL III. The photopic negative response of the macaque electroretinogram: reduction by experimental glaucoma. Invest Ophthalmol Vis Sci. 1999; 40: 1124–1136 [PubMed] [Google Scholar]
- 69. Fortune B, Wang L, Bui BV, Burgoyne CF, Cioffi GA. Idiopathic bilateral optic atrophy in the rhesus macaque. Invest Ophthalmol Vis Sci. 2005; 46: 3943–3956 [DOI] [PubMed] [Google Scholar]
- 70. Machida S. Clinical applications of the photopic negative response to optic nerve and retinal diseases. J Ophthalmol. 2012; 2012: 397178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Wang J, Cheng H, Hu YS, Tang RA, Frishman LJ. The photopic negative response of the flash electroretinogram in multiple sclerosis. Invest Ophthalmol Vis Sci. 2012; 53: 1315–1323 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Bloomfield SA. Effect of spike blockade on the receptive-field size of amacrine and ganglion cells in the rabbit retina. J Neurophysiol. 1996; 75: 1878–1893 [DOI] [PubMed] [Google Scholar]
- 73. Stafford DK, Dacey DM. Physiology of the A1 amacrine: a spiking, axon-bearing interneuron of the macaque monkey retina. Vis Neurosci. 1997; 14: 507–522 [DOI] [PubMed] [Google Scholar]
- 74. Machida S, Raz-Prag D, Fariss RN, Sieving PA, Bush RA. Photopic ERG negative response from amacrine cell signaling in RCS rat retinal degeneration. Invest Ophthalmol Vis Sci. 2008; 49: 442–452 [DOI] [PubMed] [Google Scholar]
- 75. Viswanathan S, Frishman LJ, Robson JG. The uniform field and pattern ERG in macaques with experimental glaucoma: removal of spiking activity. Invest Ophthalmol Vis Sci. 2000; 41: 2797–2810 [PubMed] [Google Scholar]
- 76. Raz-Prag D, Grimes WN, Fariss RN, et al. Probing potassium channel function in vivo by intracellular delivery of antibodies in a rat model of retinal neurodegeneration. Proc Natl Acad Sci U S A. 2010; 107: 12710–12715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Thompson DA, Feather S, Stanescu HC, et al. Altered electroretinograms in patients with KCNJ10 mutations and EAST syndrome. J Physiol. 2011; 589: 1681–1689 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Kondo M, Kurimoto Y, Sakai T, et al. Recording focal macular photopic negative response (PhNR) from monkeys. Invest Ophthalmol Vis Sci. 2008; 49: 3544–3550 [DOI] [PubMed] [Google Scholar]
- 79. Kurimoto Y, Kondo M, Ueno S, Sakai T, Machida S, Terasaki H. Asymmetry of focal macular photopic negative responses (PhNRs) in monkeys. Exp Eye Res. 2009; 88: 92–98 [DOI] [PubMed] [Google Scholar]
- 80. Machida S, Tamada K, Oikawa T, Yokoyama D, Kaneko M, Kurosaka D. Sensitivity and specificity of photopic negative response of focal electoretinogram to detect glaucomatous eyes. Br J Ophthalmol. 2010; 94: 202–208 [DOI] [PubMed] [Google Scholar]
- 81. Tamada K, Machida S, Yokoyama D, Kurosaka D. Photopic negative response of full-field and focal macular electroretinograms in patients with optic nerve atrophy. Jpn J Ophthalmol. 2009; 53: 608–614 [DOI] [PubMed] [Google Scholar]
- 82. Machida S, Toba Y, Ohtaki A, Gotoh Y, Kaneko M, Kurosaka D. Photopic negative response of focal electroretinograms in glaucomatous eyes. Invest Ophthalmol Vis Sci. 2008; 49: 5636–5644 [DOI] [PubMed] [Google Scholar]
- 83. Fortune B, Cull GA, Burgoyne CF. Relative course of retinal nerve fiber layer birefringence and thickness and retinal function changes after optic nerve transection. Invest Ophthalmol Vis Sci. 2008; 49: 4444–4452 [DOI] [PMC free article] [PubMed] [Google Scholar]