Abstract
Prior psychophysiological studies of cognitive reappraisal have generally focused on the down-regulation of negative affect, and have demonstrated either changes in self-reports of affective experience, or changes in facial EMG, but not both. Unfortunately, when taken separately, these measures are vulnerable to different sources of bias, and alternative explanations might account for changes in these indicators of negative affect. What is needed is a study that (1) obtains measures of self-reported affect together with facial EMG, and (2) examines the use of reappraisal to regulate externally and internally generated affective responses. In the present study, participants up- or down-regulated negative affect in the context of both negative and neutral pictures. Up-regulation led to greater self reports of negative affect, as well as greater corrugator and startle responses to both negative and neutral stimuli. Down-regulation led to lesser reports of negative affect, and lesser corrugator responses to negative and neutral stimuli. These results extend prior research by (1) showing simultaneous effects on multiple measures of affect, and (2) demonstrating that cognitive reappraisal may be used both to regulate responses to negative stimuli and to manufacture a negative response to neutral stimuli.
Keywords: Emotion, Affect, Regulation, Startle, EMG, Reappraisal
People report trying to regulate their emotions in many different ways, such as distracting themselves from emotional material, changing their environment so that it no longer elicits emotion, or altering their facial expression so that no one else knows that they are experiencing an emotion (Parkinson & Totterdell, 1999). These attempts at emotion regulation have been shown to impact both intra- and inter-personal functioning (Gross, 2002).
One type of emotion regulation that has received particular attention is cognitive reappraisal, which is a strategy that aims to change the trajectory of an emotional response by reinterpreting the meaning of the emotional stimulus (e.g., Gross, 1998; Ochsner et al., 2002; Ray, Wilhelm & Gross, 2008). Because appraisals are thought to be critical to emotion generation, it has been widely assumed that reappraisal – which presumably alters these appraisals – should simultaneously alter experiential, behavioral, and physiological aspects of the emotional response. However, evidence supporting this assumption is surprisingly scarce.
Early studies of reappraisal focused on decreased self-reports of negative affect but failed to find consistent physiological results (e.g., Koriat, Melkman, Averill, & Lazarus, 1972; Speisman, Lazarus, Mordkoff & Davison, 1964). By basing their evidence strictly on self-report, these studies have been vulnerable to the charge that their results are largely due to experimenter demand. Since emotion is thought to have both subjective and physiological aspects (Lang, 1995), these concerns were heightened by reports of decreased self-reports of negative affect with no concomitant changes in autonomic responding (Gross, 1998), as well as failures to find either self-report or physiological effects of cognitive regulation (Steptoe & Vogele, 1986).
To address the concern of demand characteristics, more recent studies have tested whether reappraisal can up- and down-regulate physiological correlates of negative affect, which are thought to be less subject to demand characteristics than verbal self reports. These studies have demonstrated that individuals can change the magnitude of startle eyeblink and corrugator EMG responses as directed during negative picture presentation (Dillon & LaBar, 2005; Eippert, Viet, Weiskopf, Birbaumer, & Anders, 2007; Jackson, Malmstadt, Larson, & Davidson, 2000).
One limitation of these recent studies is that with one notable exception (Eippert, Viet, Weiskopf, Birbaumer, & Anders, 2007), research on the cognitive regulation of emotion has measured physiological responses and excluded online measures of self-reported affective experience during cognitive regulation attempts. Physiological measures are also subject to bias, albeit from a different source than the biases that are a concern for self-report. For example, the startle response can be modulated by attention as well as affect (Bradley, Codispoti, & Lang, 2006). Therefore, a comprehensive multi-componential approach that operationalizes emotion in terms of experience, expression, and physiology is critical to assess the success of cognitive regulation of emotion. The conclusions about emotion regulation are far more reliable if the predicted patterns of responses are observed in multiple channels of emotion responding that are vulnerable to different sources of error. By collecting multiple measures of the affective response, we can investigate the degree to which reappraisal impacts individual response channels as well as coordinated affective responding across channels.
A second limitation of prior studies is that they generally have not addressed whether negative responses can only be modulated when they are induced by negative stimuli, or whether they can also be modulated in the context of neutral stimuli. The use of reappraisal to increase negative affect in response to neutral stimuli provides the opportunity to examine the voluntary up-regulation of negative affect even when no negative cues are physically present. This is important given that stimuli that are normatively rated as neutral can sometimes be perceived as negative when placed in the context of negative stimuli (Sommerville et al., 2004).
The goal of the present multi-method study was to assess the up- and down-regulation of negative affect using cognitive reappraisal during negative and neutral pictures. To this end, participants viewed negative and neutral pictures with instructions to increase, decrease, or to not change their negative response. Multiple channels of emotional responding, including physiology (startle eyeblink), expression (corrugator EMG), and experience (self-reported negative affect) were assessed for each picture presentation. Our expectation was that all three measures of emotion would be sensitive to instructions to up- and down-regulation emotion, and that these emotion modulatory effects would be largest in the context of negative pictures.
Method
Participants
Fifty-three women aged 18 – 29 years (M = 18.9, SD = 1.6) participated in the present study in return for two and a half hours of course credit. Only female participants were recruited due to sex differences in affective self-reports (Lang, Bradley, & Cuthbert, 2001) and physiological responses (Bradley, Codispoti, Sabatinelli, & Lang, 2001) to the International Affective Picture Set (IAPS) pictures.
Measures and Materials
Stimuli
Negative and neutral pictures were selected from the IAPS based on valence and arousal ratings for females (Lang, Bradley & Cuthbert, 2001). Eighty-one negative pictures were chosen on the basis of low (negative) valence (M=2.39±0.58) and high arousal scores (M = 6.08±1.18), and 81 neutral pictures were chosen for medium (neutral) valence (M=5.23±0.68) and low arousal scores (M=3.70±1.19). Pictures were presented in 3 quasi-random orders with no more than 3 of the same picture valence, instructions, or startle probe times presented in succession (see below). Across participants, all pictures were paired with all instruction types. All measures are within subjects.
Affect regulation instructions
Prior to the experimental task, each participant was familiarized with the instructions associated with the cue words. When participants saw the word INCREASE, they were instructed to reinterpret the meaning of the picture by “thinking about the picture in a way that increases your negative response to the picture.” When they saw the word AWARE, they were told to “be aware of your response and respond naturally.” It was emphasized that participants were to simply respond naturally in this condition. When participants saw the word DECREASE, they were instructed to “think about the picture in a way that decreases your negative response.”
During a practice session, participants were asked to report their reinterpretations aloud in response to several aversive and neutral images that were not used the experiment. Participants were guided by the experimenter to ensure that they did not use non-target regulation strategies (e.g., distraction, expressive suppression). The goal was to train participants to generate a narrative about the picture that either increased the negative content (e.g., a negative outcome is inevitable, people depicted are horribly injured, seemingly harmless objects can be used as weapons) or decreased the negative content (e.g., help is on the way, things will improve with time, it’s not as negative as it first seemed).
To decrease demand characteristics, the experimenter made it clear that up- and down-regulating emotions was difficult, and participants were encouraged to do their best and be honest in their verbal reports of emotion. Participants were told that after the task, they would be asked to review each of the images again and report how effortful it was to regulate their emotions as instructed. This created a context in which the demand to provide experience reports that were consistent with our instructions was minimized. Instead, it emphasized that the goal was to help us learn about the varying effects of our images by being honest both about how they felt when attempting to regulate and afterwards about how effortful it was to regulate.
Self-report
During the rating period of each trial, participants verbally reported the amount of negative affect that they were feeling at that moment according to a 0-7 scale where “0” was labeled “not negative at all” and “7” was labeled “strongly negative.” Ratings were later transcribed and averaged for each condition. After the slide viewing phase, participants retrospectively rated the effort required to follow the instructions for each picture on the same 0-7 scale where “0” was “not effortful at all” and “7” was “extremely effortful”.
Electromyography
Two electromyograph (EMG) measures were obtained using a SA Instruments 12-channel bioamplifier. Data were sampled at 1000 Hz and a 10 Hz high pass and a 500 Hz low pass filter were applied. The signal was rectified and smoothed using a 16 Hz filter. Integrated data were scored off-line using custom laboratory software written in MATLAB (Wilhelm, Grossman, & Roth, 1999). To measure corrugator EMG, which is associated with negative emotion experience (Lang et al., 1993), two Ag-AgCl mini-electrodes using standard electrolyte were applied to the abraded skin on the right corrugator. To measure startle blink EMG, which indexes a defensive blink reflex that is modulated by negative affect (Graham, 1979; Lang, 1995), two Ag-AgCl mini-electrodes using standard electrolyte were applied to the abraded skin of the left orbicularis oculi. The first sensor was placed 1 cm under the pupil and the second electrode was placed within 2 cm to the left. Only signals occurring within 20-120 ms of the startle probe were included, and startle magnitudes were calculated by subtracting the baseline 50 ms prior to startle probe from the maximum peak within the 120 ms following the probe. For both EMG channels, data were z-transformed and then averaged for each condition.
Procedure
Participants were individually trained and then tested in a chair facing a 20-inch television screen placed four feet away. After EMG sensors were attached, participants viewed 162 negative and neutral pictures selected from the International Affective Picture Set (IAPS; Center for the Study of Emotion and Attention [CSEA-NIMH], 1995). Pictures were presented in 9 blocks (18 trials per block), and in each trial the picture was preceded for 3 seconds by 1 of 3 instructions (Increase, Aware, or Decrease) presented in the center of the screen. The picture was presented for 8 seconds followed by a 6 second blank screen and then a 3 second rating period during which they rated how negative they felt on a 0 to 7 Likert scale. A 50 ms 95-db startle probe was delivered at one of two probe times during picture presentation (3 or 7 seconds after the start of the picture viewing period). Each startle probe time was represented equally in each block for each instruction type and valence for a total of 108 startle probes, 18 for each valence by instruction type1. After the task, participants viewed each picture and its associated instruction and rated the effort taken to regulate using a 0 to 7 Likert scale.
Results
Manipulation Check
To test whether participants responded differently to negative versus neutral slides viewed in the AWARE condition, paired t-tests were performed, comparing self-reported negative affect, corrugator EMG, and startle EMG responses for negative and neutral slides. Participants reported greater amounts of negative affect and had larger corrugator EMG and startle responses to negative than to neutral slides: self-reported negative affect, t(51)=21.92, p<.001; corrugator EMG, t(51)=8.18, p<.001; startle EMG, t(50)=4.78, p<.001.
A two-way ANOVA with Valence and Instruction as repeated factors was performed on subjective ratings of effort. The analysis produced a significant main effect of Valence (F(1,32)= 20.48, p<.001, η2 = .39, Negative M=2.84±0.92, Neutral M=2.44±0.82) showing that more effort was reported to regulate affect in response to a negative than neutral stimulus. It also produced a main effect of Instruction (F(2,64)= 34.18, p<.001, η2 = .52) and an interaction of Instruction × Valence, (F(2,64) = 71.04, p<.001, η2 = .69). Follow-up t-tests revealed that decreasing negative affect in response to negative pictures required more effort than either increasing or being aware of negative affect, which did not differ (ps <.001, Decrease M=3.67±0.95, Aware M=2.39±0.95, Increase M=2.46±0.81, t(32)=0.11, p=.596). Additionally, increasing negative affect to neutral pictures required more effort than either being aware or decreasing negative affect to neutral pictures, which did not differ (ps <.001, Decrease M=1.92±0.81, Aware M=1.84±0.81, Increase M=3.56±0.82, t(32)=0.81, p=.397).
Cognitive Regulation and Self-Reported Negative Affect
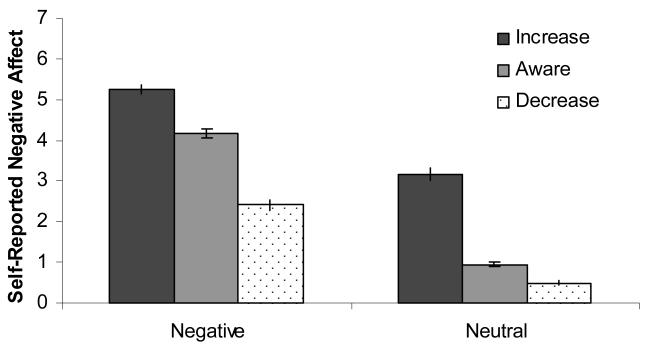
To examine the effects of instruction and slide type on self-reported negative affect reports, we conducted a 3 × 2 repeated measures ANOVA with Instruction (Decrease, Aware, Increase) and Valence (Neutral, Negative) as repeated factors. This analysis showed main effects of Instruction, F(1.26, 65.58) = 290.51, p<.001, η2 = .85, ε =.63, and Valence, F(1,52)= 635.83, p<.001, η2 = .92, as well as an interaction of Instruction × Valence, F(1.73,89.88) = 86.50, p<.001, η2 = .58, ε =.86. Repeated measures ANOVAs performed for negative and neutral pictures separately demonstrated significant effects of regulation on self-reports of negative affect in both contexts: negative F(1.44, 75.05)=275.95, p<.001, η2 = .84, ε =.72, and neutral F(1.15, 48.12)=198.99, p<.001, η2 = .83, ε =.57. All pairwise differences among these six means were significant after correcting for multiple comparisons (ps<.001, see Figure 1).
Figure 1.
Self-reported negative affect on a 0-7 scale, where 0 = “not negative at all” and “7” = “strongly negative.”
Negative affect in response to negative pictures was highest after the “Increase” cue, intermediate after the “Aware” cue, and lowest after the “Decrease” cue. Similarly, in response to neutral pictures, self-reported negative affect was highest after the “Increase” cue, intermediate after the “Aware” cue, and lowest after the “Decrease” cue. Furthermore, self-reported negative affect was higher in the exogenously negative condition “Aware” negative, than in the endogenously generated negative condition “Increase” neutral.
Cognitive Regulation and Corrugator EMG
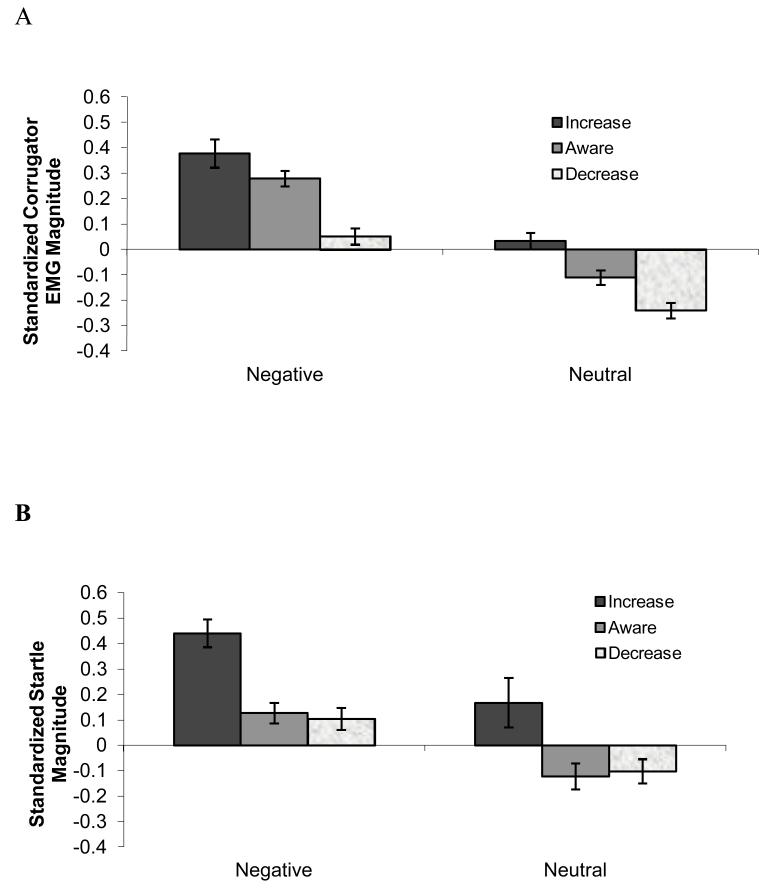
To examine the effects of instruction and slide type on corrugator EMG, we conducted a 3 × 2 repeated measures ANOVA with Instruction and Valence as repeated factors. This analysis revealed main effects of Instruction, F(1.40,71.59)=24.62, p<.001, η2 = .33, ε =.72 and Valence, F(1,51)=77.30, p<.001, η2 = .60, with negative pictures producing larger corrugator EMG responses (M=.24±0.29) than neutral pictures (M=−.11±0.22). Follow-up paired t-tests demonstrated that across negative and neutral contexts, the cue to increase one’s negative response produced larger corrugator EMG responses (M= 0.21±0.32) than the cue to be aware (M = 0.08±0.21; t(71.59)=2.95, p<.014). The cue to “Decrease” produced smaller corrugator EMG magnitudes (M =−0.09±0.23) than the cue to be “Aware” of one’s responses (t(71.59)=5.93, p<.001) (See Figure 2.) However, there was no interaction between Instruction type and Valence, F(2,102)=1.49, p=.23, η2 = .03.
Figure 2.
Standardized (A) corrugator EMG and,(B) startle magnitude (averaged over Times 1 and 2).
Cognitive Regulation and Startle EMG
To examine the effects of instruction and slide type on startle EMG, a repeated ANOVA was performed with Instruction and Valence as the repeated factors and the startle magnitude as the dependent measure. This analysis showed main effects of Instruction, F(1.56,77.81)=12.67, p<.001, η2 = .20, ε =.78 and Valence, F(1,50)=27.56, p<.001, η2 = .36, with negative pictures corresponding to larger startle magnitudes (M =0.23±0.33) than neutral pictures (M=−.02±0.47). However, there was no interaction F(1.75,87.42)=0.21, p=.779, η2 = .004, ε =.87). Follow-up paired t-tests demonstrated that across negative and neutral contexts, the cue to increase produced greater startle magnitudes (M= 0.31±0.54) than the cue to be aware of negative responses (M= 0.01±0.33; t(77.81)=2.88, p<.002. The cue to decrease, however, did not produce significantly lower startle responses (M=0.00±0.33) than the cue to be aware of ones responses (t(77.81)=0.01, p=.497) (See Figure 2.)2, 3
Discussion
Growing evidence suggests that cognitive reappraisal may be used to up- and down-regulate negative emotion. Findings from this study replicate prior findings by demonstrating instructed modulation of corrugator EMG response and startle eyeblink. This study also extends prior work in several important ways. First, it shows that online reports of negative affect can be modulated in parallel with more objective corrugator and eyeblink startle responses. Second, the present study reveals the effectiveness of cognitive reappraisal to generate a negative affective response as measured by several channels of emotional responding to a neutral stimuli. This response is compared to unregulated and regulated responses to exogenously negative stimuli.
Similar to previous studies, the current study demonstrated that cognitive reappraisal can be used to modulate expressive and physiological behavior (Dillon & LaBar, 2005; Eippert, Viet, Weiskopf, Birbaumer, & Anders, 2007; Jackson, Malmstadt, Larson, & Davidson, 2000). However, unlike previous studies, online ratings of negative affect were taken. By measuring different channels of on-line affective responding (expression, experience, and physiology) that are prone to different sources of bias, a clearer picture forms of the effects of cognition on affective responding. Furthermore, affective responding when in accord with physiology is less easily dismissed as due to demand effects. While there may be times when awareness of one’s internal states is either obscured or not veridically reported, it is instructive to understand under what conditions self-reported experience, expression and physiology do and do not correspond.
Our demonstration of the modulation of negative responding in the context of neutral stimuli warrants particular comment. The previous study that attempted to do so asked individuals to increase or decrease whatever affect the stimuli naturally invoked in them (Dillon & LaBar, 2005). Therefore, it is not surprising that the authors reported no greater or lesser affective responding when the stimuli were neutral, as no affect was present to be modulated. The present study adopted a different goal —individuals were asked to increase or decrease the amount of negative affect they experienced, regardless of the valence of the presented stimuli. When trained with this instruction, individuals were able to successfully modulate the different channels of their affective responding in accordance with instructions. In the case of increasing negative affect when looking at a neutral image, this demonstrates the internal generation of images and thoughts to produce several aspects of emotional responding. This internal generation of negative affect is an important phenomenon common in affective disorders, and so it’s important to note that emotions generated in this way share several experiential and physiological characteristics with those generated by normatively negative stimuli.
One puzzle is that in the current study, startle responses were increased in accordance with regulation instructions in both negative and neutral contexts by 246% and 237% over of that when instructed to be aware, respectively, but we did not observe a significant decrease in startle response to the decrease instruction. This asymmetrical startle result was also found by Eippert et al (2007). While it is not clear exactly why we were not able to see differences in the startle between the Aware and Decrease negative condition, we have several hypotheses.
It is possible that this asymmetric finding was due to a difference in our non-regulation instruction. Previous reports have used the cue “maintain” to instruct the individual to leave their natural response unaltered. However, because the cue is delivered after the stimulus has been viewed naturally for 3 seconds, the word “maintain” might encourage participants to artificially prolong the duration of their natural response to the stimulus, which in itself is a type of affect regulation (Gross 1998). Therefore, our non-regulation instruction of “aware” may result in lesser affective responding than previous studies, driving responding in the non-regulated condition closer to that in the “decrease” condition. Also, we had more than one startle time to decrease predictability and a large number of trails; however, we may have increased both variability in the strength of the responses and habituation enough to suppress the difference between the Aware and the Decrease trials. These findings are a reminder of the importance of the exact nature of the instructions given in the “unregulated” comparison condition and the timing of response measurement.
Another possible reason for the lack of a decrease in the startle response may be the increased difficulty involved in decreasing one’s response to a negative stimulus. Effort ratings collected after the task demonstrated that decreasing one’s negative affect to negative pictures was more effortful than either attempting to increase or be aware of one’s negative affect. Increased effort may have run counter to the physiological effects of decreasing negative affect (Silvestrini & Gendolla, 2007). However, it should be noted that there are several other conditions for which relative effort does not align with relative startle magnitude. Therefore, future research should investigate the effects of cognitive efforts to decrease negative affect in response to stimuli that are difficult to reinterpret versus those that are easier to reinterpret.
Several limitations of the present study should be noted. First, to increase the homogeneity of affective responding, we limited our sample to female participants. Because sex differences in affective responding have been documented (Bradley, Codispoti, Sabatinelli & Lang, 2001; Kring & Gordon, 1998), it is unknown whether we would have observed similar results in a male sample. Second, in addition to investigating the role of valence, future studies might more tightly control and manipulate arousal levels of the stimuli. The present images, while chosen for valence, do not allow for valence versus arousal inferences. Manipulation of arousal within valence would more fully disentangle valence from arousal in the context of emotion regulation. Also, online measurement of effort ratings would allow us to more closely account for the effects of effort on self-reported experience, expression and physiology.
Cognitive reappraisal has now been shown in several studies to flexibly modulate self-reported experience, expression and physiology. In the current study, we extended prior work by demonstrating the affects of cognitive reappraisal across multiple channels of affective responding that include self-reported experience. Second, the current study shows that cognitive reappraisal can be deployed to internally generate a negatively valenced affective response to neutral stimuli, providing a small demonstration of how flexibly cognitive mechanisms can be employed to create distress when there is no observable cause.
Acknowledgments
Preparation of this manuscript was supported by NIH grants MH66957, MH58147, and MH76074.
Footnotes
Probes were also presented after the picture offset on some trials (data not reported here).
Comparisons between instruction types revealed that the correlation between self-report and corrugator EMG measures was higher during the Aware condition (M=.17 ± .30) than in the Increase condition (M=.05 ± .31, t(47)=3.14, p=.003, but neither differed from the Decrease condition, (M=.12 ± .31, ps>.05). Also, correlations between self-report and startle EMG measures were higher during the Decrease condition (M=.12 ± .32) than in the Increase condition (M=.00 ± .30, t(41)=2.36, p<.026), but neither differed from the Aware condition, (M=.05 ± .30, ps>.05).
The startle magnitude in response to the Increase and Decrease cues to negative pictures was negatively correlated (r(50)= −.45, p=.001). The same is true for the corrugator EMG (r(49)=−.497, p<.001), and there was a trend for self-report (r(49)=−.25, p=.08).
References
- Bradley MM, Codispoti M, Lang PJ. A multi-process account of startle modulation during affective perception. Psychophysiology. 2006;43:486–497. doi: 10.1111/j.1469-8986.2006.00412.x. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Codispoti M, Sabatinelli D, Lang PJ. Emotion and motivation II: Sex differences in picture processing. Emotion. 2001;1:300–319. [PubMed] [Google Scholar]
- Demaree HA, Schmeichel BJ, Robinson JL, Pu J, Everhart DE, Berntson GG. Up- and down-regulating facial disgust: Affective, vagal, sympathetic, and respiratory consequences. Biological Psychology. 2006;71:90–99. doi: 10.1016/j.biopsycho.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Dillon DG, LaBar KS. Startle-modulation during conscious emotion regulation is arousal-dependent. Behavioral Neuroscience. 2005;119:1118–1124. doi: 10.1037/0735-7044.119.4.1118. [DOI] [PubMed] [Google Scholar]
- Eippert F, Viet R, Weiskopf N, Birbaumer N, Anders S. Regulation of emotional responses elicited by threat-related stimuli. Human Brain Mapping. 2007;28:409–423. doi: 10.1002/hbm.20291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham FK. Distinguishing among orienting, defense, and startle reflexes. In: Kimmel HD, van Olst EH, Orlebeke JF, editors. The Orienting Reflex in Humans (Conference sponsored by the Scientific Affairs Division of NATO) Erlbaum; Hillsdale, NJ: 1979. pp. 137–67. [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: Divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998;74:224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation: Affective, cognitive, and social consequences. Psychophysiology. 2002;39:281–291. doi: 10.1017/s0048577201393198. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Emotion regulation. In: Lewis M, Haviland-Jones JM, Barrett LF, editors. Handbook of emotions. 3rd ed Guilford; New York, NY: 2008. pp. 497–512. [Google Scholar]
- Jackson DC, Malmstadt JR, Larson CL, Davidson RJ. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37:515–522. [PubMed] [Google Scholar]
- Koriat A, Melkman R, Averill JR, Lazarus R,S. The self-control of emotional reactions to a stressful film. Journal of Personality. 1972;40:601–619. doi: 10.1111/j.1467-6494.1972.tb00083.x. [DOI] [PubMed] [Google Scholar]
- Kring AM, Gordon AH. Sex differences in emotion: Expression, experience, and physiology. Journal of Social and Personality Psychology. 1998;74:686–703. doi: 10.1037//0022-3514.74.3.686. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Greenwald MK, Bradley MM, Hamm AO. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–273. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lang PJ. The emotion probe: Studies of motivation and attention. American Psychologist. 1995;50:372–385. doi: 10.1037//0003-066x.50.5.372. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International affective picture system (IAPS): Instruction manual and affective ratings. University of Florida, Center for Research in Psychophysiology; Gainsville: 2001. [Google Scholar]
- Ochsner KN, Bunge SA, Gross JJ, Gabrieli JD. Rethinking feelings: An fMRI study of the cognitive regulation of emotion. Journal of Cognitive Neuroscience. 2002;14:1215–1229. doi: 10.1162/089892902760807212. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends in Cognitive Sciences. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Parkinson B, Totterdell P. Classifying affect-regulation strategies. Cognition and Emotion. 1999;13:277–303. [Google Scholar]
- Ray R, Wilhelm FH, Gross JJ. All in the mind’s eye: Anger rumination and reappraisal. Journal of Personality and Social Psychology. 2008;94 doi: 10.1037/0022-3514.94.1.133. [DOI] [PubMed] [Google Scholar]
- Silvestrini N, Gendolla GHE. Mood state effects on autonomic activity in mood regulation. Psychophysiology. 2007;44:650–659. doi: 10.1111/j.1469-8986.2007.00532.x. [DOI] [PubMed] [Google Scholar]
- Sommerville LH, Kim H, Johnstone T, Alexander AL, Whalen PJ. Human amygdala responses during presentation of happy and neutral faces: Correlations with state anxiety. Biological Psychiatry. 2004;55:897–903. doi: 10.1016/j.biopsych.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Speisman JC, Lazarus RS, Mordkoff A, Davison L. Experimental reduction of stress based on ego-defense theory. Journal of Abnormal and Social Psychology. 1964;68:367–380. doi: 10.1037/h0048936. [DOI] [PubMed] [Google Scholar]
- Steptoe A, Vogele C. Are stress responses influenced by cognitive appraisal? An experimental comparison of coping strategies. British Journal of Psychology. 1986;77:243–255. doi: 10.1111/j.2044-8295.1986.tb01999.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Grossman P, Roth WT. Analysis of cardiovascular regulation. Biomed Sci Intrum. 1999;35:135–140. [PubMed] [Google Scholar]