Abstract
We report a five-generation family with phenotypically diverse neurodegenerative disease including relentlessly progressive choreoathetoid movements, dysarthria, dysphagia, spastic paralysis, and behavioral dementia in descendants of a 67-year-old woman with amyotrophic lateral sclerosis. Disease onset varied with gender, occurring in male children and adult women. Exome sequence analyses revealed a novel mutation (c.1490C>T, p.P497L) in the ubiquilin-2 gene (UBQLN2) with X-linked inheritance in all studied affected individuals. As ubiquilin-2 positive inclusions were identified in brain we suggest that mutant peptide predisposes to protein misfolding and accumulation. Our findings expand the spectrum of neurodegenerative phenotypes caused by UBQLN2 mutations.
Introduction
Mutations in the gene UBQLN2 encoding ubiquilin-2 were recently described to cause X-linked dominant amyotrophic lateral sclerosis (ALS) and ALS/frontotemporal dementia (FTD) in children and adults.1 Subsequent analyses of UBQLN2 sequences have revealed mutations in less than 2.2% of affected patients,2-8 most often in sporadic ALS patients. As such the clinical spectrum of these mutations remains incompletely described.
We describe a progressive neurodegenerative disease in a five-generation family (Fig. 1) with symptom onset before 30 years of age in six affected members who developed spasticity, hyperkinesia, and dementia and after 60 years of age in a woman with prototypical ALS. Longitudinal evaluations, including clinical assessments, magnetic resonance imaging (MRI), and neuropathology in four autopsy brains failed to define a unified familial diagnosis. The absence of male-to-male transmission and earlier onset of more severe disease in males suggested an X-linked disorder; however, overt disease was also recognized in three of four women before age 30 years. To elucidate the cause of this disorder we performed exome sequencing in three family members. We report a novel UBQLN2 missense mutation (p.P497L) in all affected family members that were genetically studied. This study expands the phenotypic spectrum of neurodegenerative disease caused by UBQLN2 mutations and provides new insights into the nosology of ubiquitin-mediated disease.
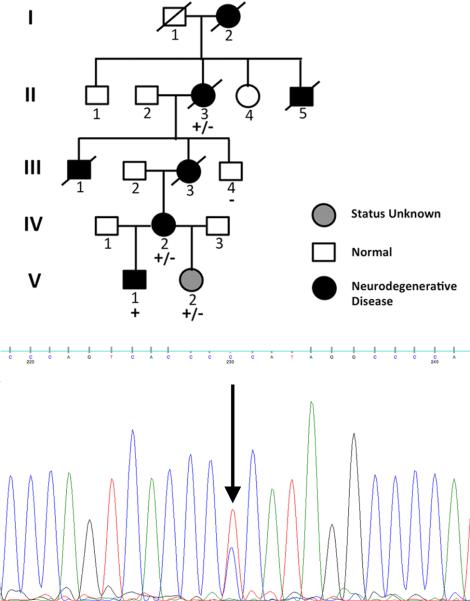
Figure 1.
X-linked dominant neurodegenerative disease in five generations of family 1T. Clinical status is denoted by fill of pedigree symbols: dark, affected; grey, unknown clinical status; clear, unaffected. Inset: Di-deoxy sequence trace of an affected female confirms the UBQLN2 c.1490C>T mutation identified by exome sequencing. Note the C (blue) and T (red) nucleotide residues, derived from the normal and mutant chromosome X UBQLN2 gene that encode respectively, proline and leucine at position 497. The genotype status for all family members is indicated: +/−, heterozygous mutation; +, hemizygous mutation; −, hemizygous normal allele.
Methods
Family Recruitment
Detailed clinical descriptions of three affected members of Family 1T (Fig.1; individuals I-2, II-5, and III-1) and an extended pathological description of individual II-5 were initially reported in 1964.9 Updated clinical assessment (including neurological evaluations, imaging, and pathology findings), genetic analyses, and return of genetic data were performed in accordance with protocols approved by the Partners Human Research Committee.
Genetic Studies
DNA was extracted from peripheral blood using standard protocols. PCR-amplified exons and flanking splice sequences for PANK2 and PLA2G6 were amplified and di-deoxy sequenced (ABI technology) from individuals II-3 and IV-2. Genomic libraries were also constructed from DNA from individuals II-3, IV-2, and unaffected male III-4. The exome was captured using SeqCap EZ Exome v2.0 kits from NimbleGen as per manufacturer's protocol, and 50 base paired-end sequencing was performed on the Illumina HiSeq. Exome sequences were aligned to hg19 using Novoalign, and variants were called using GATK. Sequences were subsequently filtered to define high quality rare (MAF< 1% in 1000Genomes and Exome Sequencing Project), nonsynonymous variants shared between II-3 and IV-2 but absent in III-4. The UBQLN2 variant was assessed in all family members using custom primers designed to amplify a 722bp region flanking UBQLN2 c.1490C>T mutation. Amplified fragments were analyzed by dideoxy sequencing (ABI technology).
Autopsy Specimens
Neuropathology reports for two affected individuals (II-5 and III-1) were reviewed as the autopsy specimens and slides were no longer stored. Brain and spinal cord specimens from individuals II-3 and III-3 were studied macroscopically (II-3) and microscopically (both) with Luxol fast blue-hematoxylin and eosin, histochemical, and immunohistochemical stains.
Results
Clinical Presentation
Three members of family 1T (Fig. 1, individuals I-2, II-5, and III-1) reported in 1964 had an atypical neurodegenerative disorder with clinical and neuropathological findings suggestive of, but indeterminant for neurodegeneration with brain iron accumulation (NBIA), formerly known as Hallervorden-Spatz disease.9
During five decades of follow-up, additional family members developed progressive neurodegeneration (Table 1). Disease began before age 10 years in three males (II-5, III-1, V-1). Three females (I-2, III-3, IV-2) developed symptoms between ages 20-30 years. Initial manifestations included dysarthria and diminished fine motor dexterity. Speech deficits progressively worsened, and drooling, dysphagia, and abnormal involuntary movements developed, followed by spastic paralysis in all limbs and behavioral dementia. Death occurred in affected males and females within 17 years after symptom onset. Brain images (computerized tomography or MRI) obtained at the time of presentation were unremarkable, but with disease progression atrophy of cerebral cortex, substantia nigra, caudate nucleus, and corticospinal tracts was reported. MRI findings in individual IV-2 suggested iron accumulation in the globus pallidus, red nucleus, and medial aspect of the pars reticulata of the substantia nigra bilaterally.
Table 1.
Phenotypes of 1T family members with UBQLN2 1490C>T
| ID | Age of onset (age at death† or current) | Presenting Manifestations | Clinical Features | Imaging & Neuropathology |
|---|---|---|---|---|
| I-2 | 30 (36†) | Awkward involuntary hand movements Dropped objects, slurred speech | Spastic paralysis, Dysarthria, Dysphagia, Involuntary movements, Loss of mental faculties | N/A |
| II-3 | 63 (67†) | Left lower extremity weakness | Dysarthria, Dysphagia, Diffuse muscle weakness without stiffness, involuntary movements or dementia. Clinically designated ALS | MRI: Mild diffuse atrophy consistent with age Moderate-severe atrophy frontotemporal lobes; Motor neuron degeneration (primary motor cortex, cranial nerve nuclei VII, X, XII, spinal cord) with corticospinal tract degeneration; Substantia nigra neuronal loss; Brain stem & limbic Lewy bodies; Ubiquilin-2 aggregates in hippocampus, brain stem |
| II-5 | 8 (19†) | Stumbling, falling, dropped objects, Slurred speech | Spastic paralysis, all extremities Dysarthria, Dysphagia, Dystonic athetoid somatic movements, Spastic gait, Behavioral dementia | Diffuse neuronal & Betz cell loss in cerebral cortex; Severe atrophy in caudate nucleus; Myelin pallor of cerebral hemispheric & deep cerebellar white matter; Corticospinal, corticopontine, & olivospinal tract degeneration; Brain stem atrophy |
| III-1 | 5 (18†) | Difficulty grasping objects; Slurred speech | Spastic paralysis, all extremities, Dysarthria, Dysphagia, Choreo-athetoid movements all extremities Spastic gait, Behavioral dementia | Diffuse atrophy of cerebral cortex with diffuse myelinated fiber loss in primary motor cortex; Severe atrophy of caudate nucleus with neuronal loss; Centrum semiovale atrophy & diffuse myelin pallor; Corticospinal, corticopontine, & olivospinal tract degeneration; Myelin absence in medullary pyramids |
| III-3 | 20 (37†) | Slurred speech | Spastic paralysis all extremities, Dysarthria, Dysphagia, Spastic gait, Behavioral dementia | CT: Frontal lobe atrophy Frontal cortex atrophy with neuronal loss & spongiosus; Neuronal loss & gliosis of caudate nucleus; Myelin pallor of frontal white matter with gliosis; Corticospinal tract degeneration with severe frontopontine fiber loss; Severe neuronal loss, gliosis of substantia nigra; Ubiquilin-2 aggregates in hippocampus, brain stem |
| IV-2 | 20 (24) | Dysarthria | Spastic paralysis all extremities, Dysarthria, Dysphagia, Spastic gait; Behavioral dementia | MRI: Symmetric T2 hypointensity in globus pallidus, red nucleus, & medial aspect of the pars reticulata of the substantia nigra bilaterally; Possible increased brain iron deposition |
| V-1 | 4 (5yrs 10mos) | Dysarthria, Decreased gross & fine motor dexterity, greatest in hands, right>left | Moderate dysarthric speech, Dystonia of upper & lower extremities bilaterally, Diffuse hypertonia, Marked decreased fine motor dexterity, Non-ambulatory | MRI: Normal |
| V-2 | (3yrs 11mos) | Normal | Normal | N/A |
All affected family members were close relatives of individual II-3, who remained well beyond the progressive debilitation and death of younger, affected family members (Table1). At the age of 63 years, she developed typical symptoms and signs of ALS, with initial unilateral weakness that progressed to worsening weakness, dysarthria, and dysphagia. An MRI was normal for age (65 years). She died at age 67 years.
Genetics
We initially sequenced PANK2 (which encodes pantothenate kinase 2) and PLA2G6 (which encodes phospholipase A2, group VI) in affected individuals as mutations in these genes cause NBIA. Neither novel deleterious variants nor previously identified NBIA mutations were identified.
Subsequently we performed exome sequencing in affected individuals II-3, IV-2, and unaffected individual III-4. High quality single nucleotide variants (SNVs) were filtered to identify variants (n= 21,015) predicted to alter protein structure or function, including deleterious missense, nonsense, or splice site variants. We restricted further analyses to 35 rare deleterious SNVs (maximum allele frequency <1% in the Exome Sequencing Project; ESP) that were present in affected individuals II-3 and IV-2 and were absent in unaffected individual III-4. Ten of these 35 deleterious rare variants were novel (absent ESP or 1000 Genomes databases), including one, a 1490C>T variant in the X-chromosome encoded gene UBQLN2. The 1490C>T variant encodes a leucine instead of the highly conserved proline at position 497 of the ubiquilin-2 protein.
We assessed the novel UBQLN2 missense variant in all family 1T members. A surviving affected male, two affected females, and a healthy 3-year-old girl (V-2) carried the rare 1490C>T variant. Based on the age-related penetrance of affected female family members, we suggest that age-related disease penetrance accounts for the normal clinical phenotype in individual V-2. Assuming an allele frequency of ≤0.1%, the probability of observing chance co-segregation of disease and the UBQLN2 Pro497Leu variant is 0.02. We conclude that UBQLN2 Pro497Leu is a pathogenic mutation that causes disease in family 1T.
Neuropathology
The neuropathological findings from four deceased family members who underwent autopsy are listed in Table 1. Ubiquilin-2 positive inclusions were identified in the brain stems and hippocampal formations from individuals II-3 and III-3 (Figs. 2A-D). TDP-43 immunoreactive inclusions were present in both brains. Glial fibrillary acid protein (GFAP) immunohistochemical stains confirmed gliosis within the corticospinal tracts in both brains. Immunohistochemical stains for beta-amyloid and phospho-tau, and histochemical staining with thioflavin S were performed on multiple sections from both brains. No positive staining for these proteins was detected in subject III-3. In subject II-3, the cerebral cortex contained sparse to moderate beta-amyloid immunoreactive diffuse plaques with sparse thioflavin S fluorescence. There was limited and sparse phospho-tau in the brain of subject II-3.
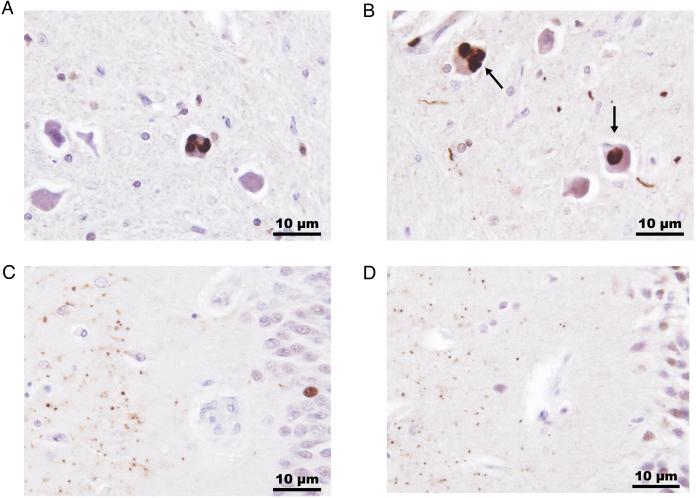
Figure 2.
Immunopositive ubiquilin-2 inclusions in brain stem and hippocampus from two affected members of family 1T. Ubiquilin-2 positive intracytoplasmic aggregates (arrows) were identified in neurons in the inferior olivary nucleus along with sparse immunopositive neurites (A, individual II-3; B, individual III-3). Hippocampal aggregates of ubiquilin-2 were detected in the molecular layer (left side of section) of the fascia dentata (C, individual II-3; D, individual III-3). Ubiquilin-2 immunohistochemistry was performed with an ubiquilin-2 polyclonal antibody (Sigma Life Sciences) using diamobenzidine and a hematoxylin counterstain, x400 original magnification.
Discussion
Ubiquilin-2 functions to deliver ubiquitinated proteins targeted for degradation to the proteasome. Neuronal cells that express mutated UBQLN2 are incapable of ubiquitin-mediated proteasomal degradation and as a consequence, develop inclusions composed of damaged and misfolded proteins that are molecular markers for and linked to neurodegenerative disease.1 The novel UBQLN2 Pro497Leu mutation reported here, like previously described familial pathogenic UBQLN2 mutations, encodes missense residues that alter a proline residue within or upstream to a 12 PXX repeat region of the ubiquilin-2 protein, a finding that implies critical roles for this domain in ubiquilin-2 biology and disease-related pathophysiology. Whether these UBQLN2 missense mutations cause neurotoxicity by dominant gain of function or loss of normal protein function remains unclear. The age of disease onset in male and most female mutation carriers is compatible with a loss of ubiquilin-2 function. However the early manifestations in female IV-2 could indicate dominant toxic effects by the mutation or unfortunate X-inactivation of the normal UBQLN2 allele.
Deng et al. reported two mutations (Pro497Ser and Pro497His) in patients with ALS or ALS/dementia with a median age of onset of 33 years for males and 44.5 years for females.1 Notably, these mutations alter the same proline residue that is mutated in the 1T family, who had earlier ages of onset (males, age 4 years; females, age 25 years) and clinical features that led to a diagnosis of ALS in only one individual, II-3. Although in retrospect, other affected members of family 1T exhibited phenotypes that are consistent with a very rare and severe form of ALS with bulbar onset (dysarthria and dysphagia) followed by severe spastic paralysis, involuntary choreo-athetoid movements, which were particularly overt in individuals I-2, II-5, and III-1, are not usually seen in ALS. The combination of early onset and rapid progression from dysarthria and spasticity to dysphagia and spastic paralysis is also atypical for ALS.
The disease in family 1T was initially considered to be a variant of NBIA, a disease that can sometimes mimic bulbar ALS.10 NBIA is characterized neuropathologically by globus pallidus and pars reticulata degeneration, pigment accumulation, including iron in affected areas, and widely distributed axonal spheroids. The brains from individuals II-5 and III-1 were described to contain9 “numerous neuroplasmic swellings” (II-5) and “variable amounts and types of pigment in the globus pallidus and adjacent brain areas” (III-1). However, these particular neuropathological findings were not observed in the brains from individuals II-3 and III-3.
MRI in NBIA can reveal iron deposition in the basal ganglia, usually seen as hypointense lesions on T2 weighted images. PANK2 mutations cause central hyperintensity in the globus pallidus surrounded by hypointensity, commonly known as “eye of the tiger,” a pathognomonic sign of NBIA. While individual IV-2 had symmetric T2 hypointensity in the globus pallidus, red nucleus, and medial aspect of the pars reticulata of the substantia nigra bilaterally, the “eye of the tiger” was not reported in this individual or in MRI studies of two other affected individuals. Absence of PANK2 or PLA2G6 mutations further excluded NBIA as the etiology of this neurodegenerative disease.
UBQLN2 mutations in some ALS/FTD patients cause dementia.1 Early recognition of dementia in family 1T members was difficult due to very young age at presentation. In retrospect, individuals II-5, III-1, III-3, and IV-2 had suggestive findings but the rapid progression of their brain degenerative processes made accurate and consistent formal assessment of their mental status difficult.
Identification of a UBQLN2 mutation ended the diagnostic odyssey in family 1T and provided definitive risk assessment for family members. The normal genotype and current age of individual III-4 provides strong evidence that he is unaffected. In contrast, V-2, who is phenotypically normal at the age of 3 years, carries the pathogenic genotype. However, as her mother and grandmother had disease onset and relentless progression from the age of 20 years while her great-grandmother developed classic ALS only at age 63, her genetic status cannot predict her clinical history. Markedly delayed or incomplete penetrance of UBQLN2 mutations that alter the PXX repeat domain is sometimes observed in mutation carriers from other families.1
This study expands the phenotypic spectrum of neurodegenerative disease caused by UBQLN2 mutations. These defects impair proteasomal degradation, resulting in a pathological endpoint of protein aggregate accumulations in the central nervous system. As protein aggregates are common in many neurological diseases,1 and ubiquitin-positive inclusions in the spinal cord are a hallmark of ALS, genetic strategies both improve the precision of clinical and pathologic diagnosis and implicate a common pathway of neuronal damage due to accumulation of misfolded proteins in multiple neurodegenerative disorders. Genetic analyses of UBQLN2 in more families with mutation-negative ALS, ALS/FTD, NBIA, or other atypical neurodegenerative disease may further expand the spectrum of disease caused by mutations in this gene. Understanding the mechanism through which mutations in the PXX repeat region of UBQLN2 cause neurodegenerative disease and elucidation of the genetic or environmental factors that modify the effect of the abnormal proteins direct therapeutic strategies for many neurodegenerative disorders.
Acknowledgements
We would like to thank the family for their help and perseverance for many years. This work was supported in part by grants from the Dubai Harvard Foundation for Medical Research (A.C.F., J.G.S., C.E.S.), the Howard Hughes Medical Institute (C.E.S.), and the National Institutes of Health (NIH (National Institute on Aging P30AG35982; C.M.G.; National Heart, Blood and Lung 5U01HL098166 and 5R01HL080494; J.G.S., C.E.S).
Footnotes
Author Contributions
B.M., L.S.D., M.B., and D.H.H. performed clinical evaluations and medical record reviews. C.M.G. performed immunohistochemical stains. K.L.N. provided neuropathological evaluations and reviewed historical neuropathology records. A.C.F., A.G.B., J.G.S., and C.E.S. performed genetic and genomic analyses. All authors contributed to data interpretation and manuscript preparation.
References
- 1.Deng HX, Chen W, Hong ST, et al. Mutations in UBQLN2 cause dominant X-linked juvenile and adult-onset ALS and ALS/dementia. Nature. 2011 Sep 8;477(7363):211–5. doi: 10.1038/nature10353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Daoud H, Suhail H, Szuto A, et al. UBQLN2 mutations are rare in French and French-Canadian amyotrophic lateral sclerosis. Neurobiology of Aging. 2012 Sep;33(9):2230, e1–e5. doi: 10.1016/j.neurobiolaging.2012.03.015. [DOI] [PubMed] [Google Scholar]
- 3.Dillen L, Van Langenhove T, Engelborghs S, et al. Explorative genetic study of UBQLN2 and PFN1 in an extended Flanders-Belgian cohort of frontotemporal lobar degeneration patients. Neurobiology of Aging. 2013 Jan 8; doi: 10.1016/j.neurobiolaging.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 4.Gellera C, Tiloca C, Del Bo R, et al. Ubiquilin 2 mutations in Italian patients with amyotrophic lateral sclerosis and frontotemporal dementia. Journal of Neurology, neurosurgery, and psychiatry. 2013 Feb;84(2):183–7. doi: 10.1136/jnnp-2012-303433. [DOI] [PubMed] [Google Scholar]
- 5.Millecamps S, Corcia P, Cazeneuve C, et al. Mutations in UBQLN2 are rare in French amyotrophic lateral sclerosis. Neurobiology of Aging. 2012 Apr;33(4):839, e1–3. doi: 10.1016/j.neurobiolaging.2011.11.010. [DOI] [PubMed] [Google Scholar]
- 6.Synofzik M, Maetzler W, Grehl T, et al. Screening in ALS and FTD patients reveals 3 novel UBQLN2 mutations outside the PXX domain and a pure FTD phenotype. Neurobiology of Aging. 2012 Dec;33(12):2949, e13–17. doi: 10.1016/j.neurobiolaging.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 7.van Doormaal PT, van Rheenen W, van Blitterswijk M, et al. UBQLN2 in familial amyotrophic lateral sclerosis in The Netherlands. Neurobiology of Aging. 2012 Sep;33(9):2233, e7–8. doi: 10.1016/j.neurobiolaging.2012.02.032. [DOI] [PubMed] [Google Scholar]
- 8.Williams KL, Warraich ST, Yang S, et al. UBQLN2/ubiquilin 2 mutation and pathology in familial amyotrophic lateral sclerosis. Neurobiology of Aging. 2012 Oct;33(10):2527, e3–10. doi: 10.1016/j.neurobiolaging.2012.05.008. [DOI] [PubMed] [Google Scholar]
- 9.DeMyer W, Harter D, Zeman W. Familial spasticity, hyperkinesia and dementia. Acta Neuropathologica. 1964;4(1):28–45. 1964/01/01. [Google Scholar]
- 10.Vasconcelos OM, Harter DH, Duffy C, et al. Adult Hallervorden-Spatz syndrome simulating amyotrophic lateral sclerosis. Muscle & Nerve. 2003 Jul;28(1):118–22. doi: 10.1002/mus.10389. [DOI] [PubMed] [Google Scholar]