Abstract
One of the central tenets of emotion theory is that emotions involve coordinated changes across experiential, behavioral, and physiological response domains. Surprisingly little is known, however, on how the strength of this emotion coherence is altered when people try to regulate their emotions. To address this issue, we recorded experiential, behavioral, and physiological responses while participants watched negative and positive pictures. Cross-correlations were used to quantify emotion coherence. Study 1 tested how two types of suppression (expressive and physiological) influence coherence. Results showed that both strategies decreased the response coherence measured in negative and positive contexts. Study 2 tested how multi-channel suppression (simultaneously targeting expressive and physiological responses) and acceptance influence emotion coherence. Results again showed that suppression decreased coherence. By contrast, acceptance was not significantly different from the unregulated condition. These findings help to clarify the nature of emotion response coherence by showing how different forms of emotion regulation may differentially affect it.
Keywords: Emotion, Synchronization, Coherence, Suppression, Acceptance
Emotions have been defined as patterned appraisals that lead to coordinated changes across experiential, behavioral, and physiological response systems (Ekman, 1992; Levenson 1994; Panksepp, 1994). Such coordination across response channels is referred to as “emotion coherence.” Despite the centrality of emotion coherence in emotion theorizing, surprisingly little is known about the strength of this response coherence, and even less is known about how it varies across contexts, for example when different forms of emotion regulation are attempted.
Emotion Coherence: Theory and Research
Many theorists see coherence among emotional response channels as the defining feature of an emotion episode (Ekman, 1972, 1992; Lazarus, 1991; Levenson, 1994; Tomkins, 1962). For example, Tomkins (1962) argued that emotions are composed of specific patterns of correlated responses. More recently, Scherer (1984, 2001) has argued that synchronization among components (or emotional responses) permits the emergence of the subjective experience of emotion (Grandjean, Sander, & Scherer, 2008), and is necessary to form a so-called full-blown emotion (see also Baumeister, Vohs, DeWall, & Zhang 2007), where all emotional components are engaged.
Most studies investigating emotion coherence have examined just two of the emotion response domains at a time. Coherence has been demonstrated between facial behaviors and self-report of experience (see for example Ekman, Davidson, & Friesen, 1990; Ekman, Friesen, & Ancoli, 1980; Larsen, Norris, & Cacioppo, 2003; Rosenberg & Ekman, 1994) and between facial behaviors and physiology (see for example Levenson, Ekman, & Friesen, 1990). In a study by Lang, Greenwald, Bradley, and Hamm (1993), bivariate associations between affective judgments, expressivity, and physiological responses were investigated. A factor analysis showed significant association between expressiveness, valence ratings, and heart rate. Other studies, however, have failed to show a reliable patterning of emotion responses (see Mauss, Levenson, McCarter, Wilhelm, & Gross, 2005 for a review). We are aware of only two major studies that have examined all three response domains at the same time with the specific aim of assessing the pattern of associations among them. In a study on the patterning of emotion responses in negative and positive contexts, Hubert and de Jong-Meyer (1990) found greater emotion coherence (measured by simple correlations) in high anxiety participants than in low anxiety participants. In a mixed emotional context (amusement/sad), Mauss et al. (2005) examined the coherence between emotional responses by taking online recordings of physiological responses, video coding of facial behavior, and obtaining continuous self-rating of experience. This study took into account the different dynamics of the diverse emotional responses by using cross-correlation. Results revealed that experience and behavior were highly associated but that physiological responses were only modestly associated with the two other responses.
Although promising, even these two more comprehensive studies of coherence have notable limitations. First, reactions were observed over a period of five to ten minutes, which potentially exceeds the time duration of an emotional response. Indeed, a period of five to ten minutes likely contains several phases of synchronization and desynchronization, which might lower the observed coherence. Second, interpretation of coherence was established on the basis of simple correlation comparisons (in Hubert & de Jong-Meyer, 1990) or with absolute strength of cross-correlations, interpreted on the basis of the differences observed with a correlation set to 0 (Mauss et al., 2005). These procedures, although indicative of the strength of the response link, either do not take into account the dynamic differences of the emotional responses (with simple correlations), or provide limited information regarding the factors that influence coherence.
Emotion Coherence: Impact of Emotion Regulation
One way to better understand emotion coherence is via an examination of factors that are hypothesized to influence emotion coherence. Of particular interest in this regard is emotion regulation, which refers to “the processes by which individuals influence which emotions they have, when they have them, and how they experience and express these emotions” (Gross, 1998b, p. 275). By attempting to regulate emotions, individuals alter the natural unfolding of emotional responses. For this reason, experimentally manipulating emotion regulation processes might provide a natural vehicle for probing emotion response coherence, as different forms of emotion regulation might be expected to differentially influence emotion coherence level.
In the process model of emotion regulation (Gross, 2001; Gross & John, 2003), one particularly relevant family of emotion regulation strategies is referred to as “response-focused” because these strategies involve changes to the emotion responses themselves (Gross, 2001). Response modulation is a common form of emotion regulation (Koole, 2009), and is predominantly used for down-regulation (suppression). In this case, the individual directly attempts to reduce one or several of his/her emotional responses. Suppression is therefore an excellent candidate for the observation of the modulation of emotion coherence, given that one, or several, of the elements that act in synchrony are directly targeted. In some ways, the opposite of emotion suppression, where individuals struggle to actively inhibit their emotion reactions, is acceptance. Acceptance is a strategy consisting in fully experiencing emotions, thoughts, and bodily sensations without trying to change, control, or avoid them. Despite the likely (divergent) effects suppression and acceptance might be expected to have on the unfolding emotional responses, no research has so far investigated how coherence is affected by such strategies.
Much of the research on response modulation has focused on suppression, i.e. the attempt to down-regulate the behavioral (mainly facial) manifestation of emotion. Past research has shown that participants instructed to suppress emotional expressions are indeed able to reduce their expressivity (Gross, 1998a; Gross & Levenson, 1993, 1997; Jackson, Malmstadt, Larson, & Davidson, 2000; Roberts, Levenson, & Gross, 2008), and also manifest a decrease in positive emotion (Gross & Levenson, 1993, 1997; John & Gross, 2004; Strack, Martin, & Stepper, 1988), as well as decreased heart rate, increased blood pressure, and increased sympathetic activation (Gross, 1998a; Gross & Levenson, 1993, 1997; Harris, 2001; Kunzmann, Kupperbusch, & Levenson, 2005; Roberts et al., 2008). The effects of emotion suppression strategies targeting other responses, like physiological activation, have also been investigated. Results of these studies show that voluntarily decreasing respiratory rate reduces both physiological activation (see review in Conde Pastor, Menéndez, Sanz, & Vila Abad, 2008) and anxious experience (McCaul, Solomon, & Holmes, 1979). A study systematically comparing these two regulation strategies (Dan-Glauser & Gross, 2011) showed that, in the context of picture viewing, these strategies differed in important ways. In fact, physiological suppression was more successful in decreasing positive emotion and had a greater impact on the level of blood pressure than expressive suppression. However, both suppression strategies had also surprisingly a lot of common effects. In fact, both strategies similarly decreased positive emotion and transiently increased negative emotion, while expressivity was strongly reduced in both contexts and for both strategies. Both suppression strategies also generally decreased cardiovascular activity and oxygenation, especially in the positive context. In the light of these results, and although targeting different responses, the two suppression strategies may thus also have very similar effects on other general parameters of emotional strength, including emotional response coherence.
Acceptance involves being open to internal experiences, even if they are uncomfortable (Hayes, Strosahl, & Wilson, 1999). Originally, acceptance was elaborated as part of the acceptance and commitment therapy (ACT) and belonged to a set of core processes encouraging patients to increase psychological flexibility, which is the “ability to contact the present moment more fully as a conscious human being” (Hayes, Luoma, Bond, Masuda, & Lillis, 2006, p.7). Recently, researchers have started to consider acceptance as an emotion regulation strategy that can be used independently of an ACT program and whose effects can be compared to other regulation strategies. Campbell-Sills, Barlow, Brown, and Hofmann (2006), for example, tested individuals with anxiety and mood disorders and compared the effects of acceptance and suppression strategies on experience and physiological activation, before and after an emotional film viewing. Results showed that acceptance triggered decreased heart rate in response to the film, as well as less negative affect than suppression during the post-film period. More recently, Wolgast, Lundh, and Viborg (2011) compared the effects of reappraisal and acceptance on emotional responses, also during film-viewing. The authors showed that both strategies reduced affective experience, activity of a facial muscle (corrugator), as well as skin conductance. Both studies showed that acceptance can be consistently compared to other emotion regulation strategies and their differences reliably highlighted with classical emotional response measures. The results of the study by Wolgast and collaborators (2011) show that acceptance processes may indeed be an efficient tactic to reduce emotional negative effects, similarly to antecedent-focused emotion regulation strategies, such as reappraisal. The results of the study by Campbell-Sills and collaborators (2006), on the other hand, show that the processes underlying acceptance are different from those underlying suppression. It is possible, therefore, that these two strategies might differentially affect emotion coherence.
The Present Research
One particular challenge in assessing emotion coherence lies in the interpretation of any absolute measure of coherence. Without a scale to refer to, it is difficult to know whether any given emotion coherence value is low or high. One way to address this issue is to evaluate how emotion coherence varies across contexts. Using this relative approach, one can assess whether, and how much, the coherence observed in natural unfolding of emotional responses is affected by various kinds of perturbations of one or more of the emotion response channels. From this perspective, emotion regulation might serve as a tool for disrupting natural emotion unfolding. It would offer a vehicle for tackling the challenge of assessing emotion coherence by enabling the observation of how coherence varies across emotion regulation conditions.
In the present article, we report two studies designed to examine how emotion coherence is affected by emotion regulation. Despite the fact that coherence is assumed in negative as well as positive emotional states, emotional reactions to negative and positive contexts differ in important ways. For this reason, we separately examined coherence in negative and positive emotions that were elicited by showing participants well-standardized emotion-eliciting pictures.
In the first study, coherence in an unregulated context was compared to two forms of suppression (expressive and physiological). We selected this starting point because we wanted to show that cross-correlations were able to capture the hypothesized disruption in emotion coherence produced by suppression. We hypothesized that both types of suppression would sufficiently affect at least one emotion response channel to decrease emotion coherence. In fact, a drop in cross-correlation values between two time-series can be achieved in two major situations: either one of the time-series remains unchanged while the other increases or decreases, or the time-series start to develop in opposite directions, both situations reflecting previous results about the consequences of suppression. In the second study, we focused on a potential way to increase emotion coherence by asking participants to employ a global acceptance strategy. In fact, we supposed that unregulated emotions will still include some implicit suppressive processes, which can be diminished when an acceptance strategy is performed. Acceptance would thus allow responses to be better attuned to each other, thus showing higher coherence. This condition of acceptance was compared to a global suppressive condition, i.e. the attempt to simultaneously suppress physiological AND expressive responses. We hypothesized that, similar to results in the first study, global suppression should lower the coherence level observed in the unregulated condition, whereas acceptance should increase the coherence level.
Study 1: Effect of Different Suppression Strategies on Emotion Coherence
As part of a larger project on the psychophysiology of emotional responding, we previously investigated the effects of emotion suppression on emotion response magnitude. In particular, we examined the temporal dynamic of experiential, expressive, and physiological emotion responses to observe the time-locked response activity variations resulting from different suppression strategies (Dan-Glauser & Gross, 2011). To address our present question about the effects of emotional suppression on emotion coherence, we conducted new analyses that extend our prior report; with a focus on whether (and to what extent) two suppression strategies influence emotion coherence, as compared to a no-regulation condition. Coherence was calculated between each possible pairs of the three main emotional responses: expressive, experiential, and physiological reactions.
Method
Participants
Thirty-seven students participated in our study and were given course credit. Participation was restricted to right-handed females to eliminate laterality and gender variability. Their ages ranged between 18 and 40 years old, with a mean age of 20.2 years (SD=3.5). Participants identified as follows: 13 Asians, 10 Caucasians, two African Americans, and two Hispanics (10 participants indicated “other”, “more than one race”, or declined to answer).
Stimuli
One hundred and seventy-five pictures were selected from the International Affective Picture System (IAPS, Lang, Bradley, & Cuthbert, 1999) based on the affective norms for female participants (ratings from 1 to 9, middle point at 5). Of these pictures, 25 were of neutral valence (mean: 5.00, SD=0.07, range: 4.88-5.15), 75 were of negative valence (mean: 2.33, SD=0.37, range: 1.45-3.16), and 75 were of positive valence (mean: 7.44, SD=0.36, range: 6.91-8.34).
Measures
Measures were obtained in each of three response domains: emotion experience, expressive behavior, and autonomic responses. These measures were obtained continuously throughout the picture viewing period. All parameters were recorded and amplified with a 32-channel Bionex 8-slot chassis from Mindware Technologies (Gahanna, OH). Data were then converted (16 bit A/D) to a computer for viewing and storage. All acquired channels were sampled at 1000 Hz. Further details regarding the conversion of the data are provided in the Data reduction section (see below).
Emotion experience: Participants used a rating dial that provided a continuous recording of their degree of negative or positive feelings during picture presentation. Past research has shown that measuring emotion in this way does not seem to impact the emotion itself (Hutcherson et al., 2005) and is a reliable way to measure experience. The voltage output was linearly transformed to a scale from -100 (very negative) to +100 (very positive).
Emotion-expressive behavior: Facial behavior was assessed using bipolar surface electromyography (EMG). Electrodes were standard 4 mm Ag-AgCl sensors. Left Corrugator Supercilii and left Zygomaticus Major were the two targeted regions, as they have been associated with negative and positive emotions, respectively (Cacioppo, Petty, Losch, & Kim, 1986; Vrana, 1993). Skin was first cleaned with Kendall Webcol® skin cleansing alcohol pads (Tyco healthcare, Mansfield, MA) and gently rubbed with NuPrep® gel (Weaver and Company, Aurora, CA). Excess gel was then removed with alcohol pads. Electrodes were filled with Signagel® (Parker Laboratories, Inc, Fairfield, NJ).
Autonomic responses: Five measures were recorded. These measures were chosen to broadly sample response domains known to be sensitive to emotion, including: a) Electrocardiography: Two standard disposable pre-gelled Ag/AgCl electrodes were placed 5 cm below the lower rib on each side of the abdomen. A third electrode, which functioned as a ground, was placed at the level of the xiphoid process. b) Blood pressure: systolic and diastolic blood pressures were recorded by using a continuous inflatable finger cuff with a FINAPRES 2300 (Finger Arterial Pressure) system (Ohmeda, Madison, WI). Cuff size was determined for each participant, and was fitted to the third finger of the non-dominant hand. c) Finger pulse: Variation of amplitude of the blood volume at the finger site was recorded with a photoplethysmography transducer from Mindware Technologies (Gahanna, OH) clipped onto the extremity of the second finger of the non-dominant hand. d) Skin temperature: Finger temperature was recorded with a temperature probe from Mindware Technologies (Gahanna, OH) taped to the palmar surface of the extremity of the fourth finger of the non-dominant hand.1 e) Respiration: Thoracic and abdominal respirations were recorded with two respiration belts from Mindware Technologies (Gahanna, OH). The abdominal belt was placed around the waist just above the pants, whereas thoracic belt was placed high on the chest just below the armpits. A calibration procedure was conducted once belts were correctly attached and was used to later estimate respiration amplitude.
Procedure
Participants were run in individual sessions. After signing the consent form, they were prepared for the physiological recordings, allowed to observe the signals as they were tested, and to ask any questions they might have about the psychophysiological recordings. Participants first viewed a neutral 3-minute film clip as a resting baseline period. They then practiced using the rating dial on several negative and positive pictures. When they were ready to proceed, participants were acquainted with three types of instruction, presented on screen.
For the unregulated condition (cued by the word WATCH), the instruction was: “Observe the picture and let any emotion you may feel come and go naturally, the same way you did during the training. Continue monitoring yourself and use the dial to report your feelings.” For the expressive suppression trials (cued by the phrase DON’T SHOW), participants were instructed: “Observe the picture but don’t let any emotion you may feel on the inside show in your behavior. In other words, try to behave in such a way that a person watching you would not know that you are feeling an emotion. Remember that you don’t show, but you can feel. Continue monitoring yourself and use the dial to report your feelings.” This instruction was similar to the one used in previous studies on expressive suppression (Gross & Levenson, 1993, 1997). For the physiological suppression trials (cued by the phrase DON’T REACT), participants were instructed: “While watching the picture you may feel that your body is reacting to it. Observe the picture but don’t let any emotion you may feel affect your regular physiological responses. In other words, try to behave in such a way that a person watching your physiological reactions on a computer screen would not know that you are feeling an emotion. Try to calm down, breath normally, relax. Remember that you don’t react, but you can feel. Continue monitoring yourself and use the dial to report your feelings.”
After receiving these instructions, participants were given a training period and could then start the session. The session was composed of the 175 stimuli described in a previous section. The order of the picture presentation was randomly chosen for each participant and each time a semi-random assignment was made to one of the three conditions (unregulated, expressive suppression, physiological suppression). Each participant therefore viewed in a random order (no blocks): 25 neutral pictures under the unregulated instruction, 75 negative pictures (25 under each instruction), and 75 positive pictures (25 under each instruction).
On each trial, participants saw a black screen (1 s), a fixation cross (1.5 s), a black screen again (0.5 s), the instruction (WATCH, DON’T SHOW, or DON’T REACT, 1.5 s), the emotional picture (8 s), and then an instruction to reset the dial (1.5 s). Half way through the experiment, participants were given a rest break. After the viewing session, sensors were removed and participants were asked to summarize what they did when each of the instructions appeared. Responses to these questions were used as a control for instruction understanding. All participants having discriminated the three regulation conditions, all their data were therefore retained for analyses. Participants were then debriefed and thanked for their participation.
Data reduction
All recordings were exported to be analyzed with ANSLAB (Autonomic Nervous System Laboratory), a biosignal analysis program (shareware version available at the software repository of the Society for Psychophysiological Research; Wilhelm & Peyk, 2005). Channels were manually scanned for artifacts. The 8 second recordings were segmented into 16 epochs of 0.5 s each (see Bradley & Lang, 2000, for a similar segmentation). Rating dial data (measuring intensity of experience) were averaged for each epoch and transformed to represent the participant feeling on a scale from 0 to + 100 (no feeling to extreme feeling). EMG signals were rectified and smoothed before being averaged for each epoch. EMG was then expressed as the percentage of the baseline level (the 3.5 s preceding picture onset, calculated for each trial). Seven relevant parameters were extracted from the activity of the five recorded physiological channels. Heart rate was calculated from the ECG channel by transforming the interbeat interval obtained by the calculation of the duration between successive R waves. Pulse transit time (i.e., the time interval between the R wave of the ECG and the upstroke of the peripheral pulse at the finger site) and amplitude were exported as mean values for each epoch. Respiratory rate was obtained by converting the duration of the cycle intervals (in milliseconds) into a number of cycles per minute (c/min). Respiratory amplitude was interpolated by using the difference in volts between the point of maximum inspiration and the point of maximum expiration. Volumes in mL were then obtained using the calibration procedure values. Systolic and diastolic blood pressure values were first extracted as separate parameters and then averaged to create a measure of mean blood pressure for each epoch. All the autonomic response channels were calculated as change in activity with respect to trial baselines (the 3.5 s preceding picture onset).
Cross-correlation analyses
Rating dial data were used as a measure of experience. EMG data were used as a measure of expressivity (corrugator activity was taken into account for negative trials, and zygomatic activity for positive trials, an average of these two measures was taken into account for all neutral trials). To obtain a corresponding measure of overall physiological activation, six measures were aggregated: heart rate, blood pressure, respiratory rate, respiratory amplitude, pulse amplitude, and pulse transit time. After z-scoring all the variables, an averaged measure was created with a weight that reflects an increase in activation for an increasing value of the composite: + 1 for heart rate, blood pressure, and respiratory rate; −1 for pulse amplitude, pulse transit time, and respiratory amplitude.
To evaluate emotion coherence, cross-correlations were used. This approach makes possible to compare two time-series whose activities are shifted in time by expressing the simple correlation as a function of a time-shift operated on one of the two series (Hinton, 2002). For the present study, cross-correlation analyses were performed for each trial for each participant. For each trial, cross-correlations between time-series of 16 epochs were computed. Lags between −8 to + 8 were used in order to keep sufficient time-points for correlations, especially at the extreme bounds. Three comparisons were investigated. The first comparison addresses the correlation between experience and expressivity, the second comparison addresses the correlation between experience and physiological responding, while the third comparison addresses the correlation between expressivity and physiological responding.
For each cross-correlation analysis, the maximum positive correlation was identified across all lags and retained. Fisher z-transformed correlations from trials of a same condition were then averaged for each participant and each comparison. The final matrix presents the data for 37 participants across two valence levels (positive or negative stimulation), three regulatory conditions (unregulated, expressive suppression, and physiological suppression), and the three comparisons between emotion responses. Analyses for this article were performed on these data, using repeated-measure ANOVAs with Greenhouse-Geisser corrections. As multivariate outlier analyses based on Mahalanobis’ distances showed no outliers for the relevant variables, the full dataset was included in the presented ANOVAs.
Results and Discussion
Regulation effects on the three response channels
To provide the context for our primary analyses, we examined the effects of condition on each of the three response channels. Data are reported in Table 1 for positive and negative contexts separately, together with the tests for main effects with repeated-measure ANOVAs. Detailed information regarding the temporal dynamics of expressive, physiological, and experiential responses have been reported elsewhere (Dan-Glauser & Gross 2011).
Table 1.
Regulation Effects on the Three Response Channels in Study 1
| Suppression |
||||
|---|---|---|---|---|
| Response | Unregulated | Expressive | Physiological | Main effect |
| Negative context | ||||
| Experience (/±100) | −45.51 (1.75) | −47.14 (1.85) | −47.58 (1.68) | F(2,58)=2.12, p=14 |
| Expressivity (%) | 114.80 (4.00) | 102.60 (2.24)** | 101.70 (2.49)** | F(1,44)=10.89, p<.01, η2=.23 |
| Physiological activation (Δz) | 0.012 (0.014) | −0.003 (0.026) | −0.025 (0.021) | F(2,65)=1.35, p=27 |
|
| ||||
| Positive context | ||||
| Experience (/±100) | 38.49 (2.24) | 35.65 (2.37)* | 33.92 (2.16)** | F(2,72)=7.95, p<.01, η2=18 |
| Expressivity (%) | 130.29 (8.39) | 94.85 (2.35)** | 96.34 (1.64)** | F(1,37)=15.67, p<.01, η2=.30 |
| Physiological activation (Δz) | 0.030 (0.017) | −0.004 (0.014) | −0.031 (0.018)** | F(2,71)=5.52, p<.01, η2=.13 |
Note: p<.05 and
p<.01 with the unregulated condition, evaluated by simple contrasts
Coherence variation in a negative context
To evaluate the variation in coherence due to suppression strategies in a negative context, a repeated-measure ANOVA was conducted using the maximum cross-correlation coefficients obtained between responses recorded during negative trials. Two within-factors were considered: the type of regulation performed during the trial (three levels: unregulated, expressive suppression, or physiological suppression) and the type of comparison between responses (three levels: experience-expressivity, experience-physiological activation, expressivity-physiological activation). Results show a main effect of the comparison type, F(2, 60) =34.77, p<.001, ηp 2 =.49.2 as well as a main effect of regulation, F(2,72)=3.76, p<.05, ηp 2=.10. The interaction was not significant, F(3,108)=0.86, p=ns. Details of the cross-correlation values obtained for each condition and each comparison are presented in Table 2, upper section.
Table 2.
Cross-correlation Values Obtained in Study 1
| Suppression |
|||
|---|---|---|---|
| Comparison | Unregulated | Expressive | Physiological |
| Negative context | |||
| Experience-Expressivity | 0.453 | 0.419 | 0.420 |
| Experience-Physiological activation | 0.527 | 0.520 | 0.528 |
| Expressivity-Physiological activation | 0.455 | 0.431 | 0.432 |
|
| |||
| Positive context | |||
| Experience-Expressivity | 0.403 | 0.390 | 0.408 |
| Experience-Physiological activation | 0.560 | 0.530 | 0.519 |
| Expressivity-Physiological activation | 0.490 | 0.453 | 0.466 |
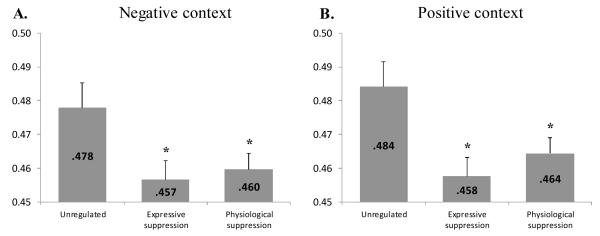
To answer our main research question, contrasts were computed to compare the level of coherence observed in unregulated trials with those of each of the two suppression strategies. Confirming our hypothesis, unregulated trials show a general association between responses (0.48) that is significantly higher than the association between emotional responses observed during each of the suppression conditions (0.46 during expressive suppression, and 0.46 during physiological suppression, p<.04). This result is depicted in Figure 1, panel A. Thus, in a negative context, performing either expressive or physiological suppression leads to a lower coherence level than is usually observed when emotion unfolding is left unregulated.
Figure 1.
Study 1 cross-correlations during negative emotion induction (Panel A) and positive emotion induction (Panel B) for unregulated trials and different suppression strategies. Results are the means of the maximum correlations observed for each trial in a given condition. Error bars are SEM. A-priori contrasts with the unregulated condition are reported above the suppression columns: * p<.05.
Coherence variation in a positive context
Using a similarly structured ANOVA in a positive picture context, results showed a main effect of the comparison type, F(2,60)=47.84, p<.001, ηp 2=.57, as well as a main effect of regulation, F(2,64)=4.86, p<.05, ηp 2=.12. The interaction was not significant, F(3,115)=1.69, p=ns. Details of the cross-correlation values obtained for each condition and each comparison are presented in Table 2, lower section.
As with the negative context, contrasts were computed to compare the level of coherence observed in unregulated trials with those of each of the two suppression strategies. Thus, in a positive context, unregulated trials show a general association between responses (0.48) that is significantly higher than the association between emotional responses observed during each of the suppression conditions (0.46 during expressive suppression, and 0.46 during physiological suppression, p<.04). This result is depicted in Figure 1, panel B. These results confirm our hypothesis that emotion regulation impacts negatively the level of coherence that is observed during unregulated emotional unfolding.
Study 2: Enhancing Emotion Coherence Using Acceptance
The results of Study 1 showed that cross-correlations are sensitive to coherence variation due to emotion regulation. The goal of Study 2 was to test whether emotion coherence could be augmented as well as diminished. To do this, we used the regulation strategy of acceptance, which is thought to enhance natural responding by limiting spontaneously occurring suppression. Acceptance condition should thus result in a greater coherence level, as it would allow responses to be better attuned to each other. As a contrast condition, we used a multi-channel suppression strategy (acting simultaneously on expressive and physiological responses). Study 2 thus compared a strategy of multi-channel suppression and a strategy of acceptance to unregulated emotion unfolding. For each condition, coherence was evaluated by cross-correlations between each possible pairs of the three main emotional responses: expressive, experiential, and physiological reactions.
Methods
Participants
Thirty-seven right-handed female students participated in this second study (these were different participants from Study 1). Their ages ranged between 18 and 27 years old, with a mean of 20.2 years (SD=2.4). Participants identified as follows: 12 Asians, 11 Caucasians, three Hispanics, three African Americans, and one American Indian (seven participants indicated “unknown”, “more than one race”, or declined to answer).
Stimuli
Stimuli were the same as in Study 1.
Measures
Measures were the same as in Study 1. Briefly, emotional experience was assessed using a rating dial, expressivity was assessed with EMG recordings on two facial sites, and autonomic responses were measured thanks to a composite of physiological activities including six parameters: heart rate, blood pressure, respiratory rate, respiratory amplitude, pulse amplitude, and pulse transit time.
Procedure
After signing the consent form, participants were prepared for the physiological recordings as in Study 1. Also for this study, participants were confronted with three types of instruction, presented on screen.
For the unregulated trials (cued by the word USUAL), the instruction was the same as in Study 1. Additionally, and to better stress the difference with the acceptance condition, the following sentence was added: “We want you to respond as you would if you encountered this picture during your daily life. Do whatever you would normally do.” For the suppression trials (cued by the phrase SUPPRESS), participants were instructed to suppress both expressive and physiological reactions: “When you see the instruction suppress, we would like you to try your best to strongly decrease your bodily and facial reactions. Try to calm down, try to breathe normally. Even if you feel emotion, try to act as if there is no emotion present, so that no-one watching your physiological responses or your face would know what you are feeling.” For the acceptance trials (cued by the phrase ACCEPT), participants were instructed: “When you see the instruction accept, we would like you to try your best to accept your emotions and experience them fully. We want you to allow yourself to stay with your emotions. Do not avoid them and don’t try to control or change your emotions in any way. Refrain from attempts to distract yourself or otherwise lessen or amplify your feelings, and instead allow yourself to feel your emotions and observe carefully the modifications that these emotions trigger within you.” This last instruction was adapted from Campbell-Sills and colleagues (2006). For each instruction, an additional sentence reminded participants to report their feeling with the dial.
Training period, stimuli, random assignment of pictures to conditions, and on-screen durations were the same as for Study 1. This time, however, two breaks (instead of one) were given to the participants, 13 and 26 minutes through the main task. After the computer session, participants were again asked to summarize what they did when each of the instructions appeared. As in Study 1, responses to these questions were used as a control for instruction understanding. Since all participants correctly discriminated the three instructions, all participant data were retained for analyses. Participants were then debriefed and thanked.
Data reduction and analyses
All data transformation and analyses were performed with the same software and procedure as in Study 1.
Cross-correlation analyses
Our focus was on the interrelation of three channels: experience, expressivity, and physiological activation (composed of six aggregated measures). Cross-correlation analyses were again performed for each trial for each participant, on 16 epoch series of 0.5 s each and with lags imposed from −8 to +8. As in Study 1, the maximum positive correlation across all lags was always retained. After the cross-correlation calculations, Fisher z-transformed maximum correlations from trials of a same condition were averaged for each participant and each comparison. The final matrix presents the data for 37 participants across two valence levels (positive or negative stimulation), three regulatory conditions (unregulated, suppression, acceptance), and the three comparisons between emotion responses. Analyses were mainly performed by using repeated-measure ANOVA with Greenhouse-Geisser corrections. As in Study 1, multivariate outlier analyses based on Mahalanobis’ distances showed no outliers for the relevant variables. The full dataset was thus included in the presented ANOVAs.
Results and Discussion
Regulation effects on the three response channels
As in Study 1, we provide the context for our primary analyses by examining the effects of condition on each of the three response channels. Data are reported in Table 3 for positive and negative contexts separately, together with the tests for main effects with repeated-measure ANOVAs.
Table 3.
Regulation Effects on the Three Response Channels in Study 2
| Comparison | Unregulated | Suppression | Acceptance | Main Effect |
|---|---|---|---|---|
| Negative context | ||||
| Experience (/±100) | −33.96 (2.51) | −35.27 (2.92) | −34.95 (2.35) | F(2,66)=0.65, p=51 |
| Expressivity (%) | 125.09 (3.98) | 106.50 (1.43)** | 128.39 (4.83) | F(1,48)=20.15, p<.001, η2=.36 |
| Physiological activation (Δz) | −0.003 (0.016) | −0.069 (0.019)** | −0.008 (0.017) | F(2,69)=7.43, p<.01, η2=17 |
|
| ||||
| Positive context | ||||
| Experience (/±100) | 24.68 (1.95) | 22.67 (2.03) | 26.74 (1.97)* | F(2,62)=5.93, p<.01, η2=14 |
| Expressivity (%) | 130.98 (7.20) | 97.72 (2.05)** | 156.49 (12.20)** | F(1,50)=18.20, p<.001, η2=.34 |
| Physiological activation (Δz) | 0.048 (0.020) | −0.025 (0.018)** | 0.037 (0.014) | F(2,63)=6.18, p<.01, η2=.15 |
Note: p<.05 and
p<.01 with the unregulated condition, evaluated by simple contrasts
Coherence variation in a negative context
To evaluate the variation in coherence due to suppression and acceptance strategies in a negative context, a repeated-measure ANOVA was conducted using the maximum cross-correlation coefficients obtained between responses recorded during negative trials. Two within-factors were considered: the type of regulation performed during the trial (three levels: unregulated, suppression, acceptance) and the type of comparison between responses (three levels: experience-expressivity, experience-physiological activation, expressivity-physiological activation). Results show a main effect of the comparison type, F(2,57)=37.37, p<.001, ηp 2=.51, as well as a main effect of regulation, F(2,69)=6.28, p<.01, ηp 2=.15. The interaction was not significant, F(3,124)=0.58, p=ns. Details of the cross-correlation values obtained for each condition and each comparison are presented in Table 4, upper section.
Table 4.
Cross-correlation Values Obtained in Study 2
| Comparison | Unregulated | Suppression | Acceptance |
|---|---|---|---|
| Negative context | |||
| Experience-Expressivity | 0.442 | 0.431 | 0.471 |
| Experience-Physiological activation | 0.561 | 0.546 | 0.573 |
| Expressivity-Physiological activation | 0.474 | 0.446 | 0.474 |
|
| |||
| Positive context | |||
| Experience-Expressivity | 0.237 | 0.233 | 0.233 |
| Experience-Physiological activation | 0.597 | 0.576 | 0.600 |
| Expressivity-Physiological activation | 0.333 | 0.293 | 0.337 |
To answer our main research question, contrasts were computed to compare the level of coherence observed in unregulated trials, in the suppression trials, and in the acceptance trials. Confirming our hypothesis and Study 1 results, unregulated trials showed a general association between responses (0.49) that was significantly greater than the association between emotional responses observed during the suppression condition (0.47, p<.05). Emotion coherence observed during acceptance (0.51) was also significantly higher than the emotion coherence observed during the suppression condition (0.47, p<.01). However, trials in which an acceptance strategy was used did not show a reliably greater level of coherence (0.51) than the one observed in unregulated trials (0.49, p=.10), although the difference in means was in the expected direction. These results are depicted in Figure 2, panel A.
Figure 2.
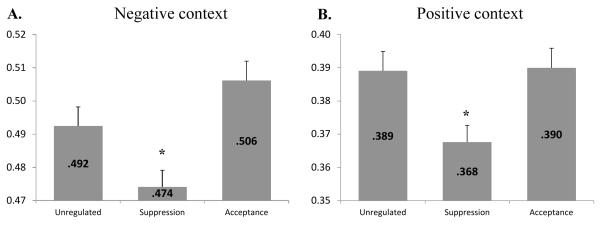
Study 2 cross-correlations during negative emotion induction (Panel A) and positive emotion induction (Panel B) for unregulated trials, a suppression strategy, and an acceptance strategy. Results are the means of the maximum correlations observed for each trial in a given condition. Error bars are SEM. Significant contrasts are reported above the suppression columns: * p<.05 both with the unregulated and with the acceptance condition.
Coherence variation in a positive context
Using a similarly structured ANOVA in the positive picture context, results showed a main effect of the comparison type, F(1,46)=336.65, p<.001, ηp 2=.90, as well as a main effect of regulation, F(2,71)=11.27, p<.001, ηp 2=.24. The interaction was not significant, F(3,101)=2.39, p=ns. Details of the cross-correlation values obtained for each condition and each comparison are presented in Table 4, lower section.
Similar to the analyses performed for the negative context, contrasts were computed to compare the level of coherence observed in the three conditions. In this context, unregulated trials showed a general association between responses (0.39) that was significantly higher than the association between emotional responses observed during the suppression condition (0.37, p<.01). This result is similar to what was found in Study 1 and in the negative context in Study 2, again showing how consistently suppression disrupts the coherence in emotional responses that is triggered by emotional stimulation. Coherence in acceptance trials did not differ from unregulated trials (both levels are equal to 0.39, p=ns), but did differ from the suppression trials (p<.01). These results are depicted in Figure 2, panel B.
General Discussion
It has long been hypothesized that emotion emerges when the various component responses implicated in emotional responding are coherently activated. Despite the centrality of this hypothesis, few studies have empirically investigated emotion coherence. In the present studies, we adopted a new approach to the problem, and focused on investigating whether and how emotion coherence varies as a function of different emotion regulation strategies.
Suppression and Emotion Coherence
Results of the present studies show that a disruption in the coherence between responses occurs when suppression is performed, and that this disruption is evident in both positive and negative contexts. This lessening of response coupling is evident across each pairwise comparison among experience, behavior, and physiology. Study 1 contrasted two regulation strategies, which differed by the response targeted by the suppression attempts. The similarity of the decrease in coherence for the two strategies may be due to the similar consequences of expressive and physiological suppression (Dan-Glauser & Gross, 2011).
This suppression effect on coherence may arise from the fact that suppression generally modifies one or two emotional response components, but generally does not have an effect on the three response components simultaneously (see Tables 1 and 3). This has been also seen in other contexts. For example, in the case of a confrontation with a negative stimulation, people generally succeed in reducing their emotional expressivity (Gross 1998a, Gross & Levenson, 1993, 1997; Jackson et al., 2000, Roberts et al., 2008), but the negative experience is often not affected (Gross & Levenson, 1993, 1997; Roberts et al., 2008).
It bears emphasizing that the reduction of coherence observed in both studies for the suppression conditions is very modest. In fact, it averages .02, which represents a decrease of 4.5% as compared to the unregulated coherence value. It is not yet known whether alterations of this magnitude have practical cognitive, affective, or social consequences in the immediate or longer term.
Nevertheless, the demonstration of this effect is of clear theoretical significance. Indeed, in a strikingly reproducible fashion, i.e. in both studies, and for both valence levels investigated, the suppression strategies sufficiently disrupted the natural unfolding of emotion to significantly reduce the coherence. This result indirectly sheds some light on the coherence attained when emotions are left unregulated. In fact, the fluctuations of emotion coherence caused by the suppression-mediated disruption of emotion unfolding tend to confirm that unregulated emotions require a significant coherence of emotional responses.
Acceptance and Emotion Coherence
Study 2 investigated whether acceptance would have the effect of increasing the coherence among emotion response components. Contrary to our hypothesis, results revealed that when acceptance is performed, emotion coherence is not reliably different from the coherence observed in an unregulated situation, although there was a trend toward higher coherence in a negative context (p=.10). This finding may be due to the tendency of participants to spontaneously engage in emotion regulation. It has been shown that, even when individuals are not asked to regulate their emotion, natural strategies are nevertheless employed to cope with the situation (Cole, Hall, & Radzioch, 2009; Drabant, McRae, Manuck, Hariri, & Gross, 2009; Egloff, Schmukle, Burns, & Schwerdtfeger, 2006; Tomkins, 1984). In our experiments, the natural disposition of our participants while confronted with emotional stimulations could well have been to accept their emotion, even when they were asked to refrain from modifying their emotion in any way (unregulated instruction). If so, this tendency would work against our ability to distinguish “unregulated” and “acceptance” conditions.
Limitations and Future Directions
Limiting our participants to one gender permitted us to avoid gender-related variations in emotional responding. However, this decision had the effect of constraining the generalizability of our findings. In future work, it will be important to examine male participants, other age groups, and a wider range of cultural groups. These steps will enable consideration of the broader consequences of differing patterns of emotion coherence. In this vein, one recent study on the dissociation (i.e. low coherence) between emotional responses showed that increased depressive symptoms and lower well-being were associated with lower coherence between expressive and experiential positive responses (Mauss et al., 2011). Thus, considering both antecedents and consequences of response synchronization in as many populations/contexts as possible will certainly expand our understanding of emotion coherence.
In keeping with prior work in this area, the instructions given in the present studies were face-valid and direct, but also transparent to the participants. This leaves open the possibility that demand characteristics may have played a role in the observed findings. Although it is difficult to see why participants’ expectations would have created effects of the precise magnitude and type as we observed, the possibility cannot completely be ruled out in the present studies. This therefore represents limitation of the present study that should not be neglected. In future research it will be important to consider employing less direct research approaches, perhaps involving experimental contexts that activate emotion regulation goals more indirectly. Having a full understanding of the effects of emotion regulation on emotion coherence will certainly require the use of converging methods, each of which with its own profile of strengths and weaknesses.
Further limitations to these studies represent additional opportunities for future research. For example, we focused here on one particularly well-defined context, namely picture viewing, and we exerted stringent control over the different emotion regulation strategies participants were asked to employ when cued. It will be particularly important in future research to consider other emotion-eliciting contexts and other emotion regulation strategies, particularly those that have less systematic identified variations on emotion responses than the well described expressive suppression strategy. Additionally, limiting the analyses to only the first eight seconds of the reaction certainly limits the generalizability of the findings. On this matter, future research will need to investigate whether the coherence results reported here are also present in an observation window taken later in the emotional process or over a longer time frame.
With respect to variation across participants, we should note that different people have different patterns of emotion regulation, and that these may vary by emotion-eliciting context. Future studies could thus compare the coherence among emotional response components in participants who have different preferred regulation strategies. Conversely, coherence may also be differentially affected according to the position of the stimulation in the valence-arousal space. In the present research, we have focused on stimulations of similar arousal, both in the negative and positive affect space. Future studies could focus on different levels of arousal and also investigate how coherence is differentiated between different emotional categories. Finally, this research cannot determine how the magnitude of emotion response variations influences the degree of coherence deviation. Systematic analyses of the effects of emotion response magnitude will be an important complement to research in which the type of emotion is varied.
In conclusion, our studies demonstrate that cross-correlations are sensitive enough to spot the coherence differences between emotional responses and between settings, and may therefore be used for subsequent research on emotion coherence. More importantly, our studies show the viability of documenting emotional response coherence, not by judging an absolute level of coherence, but by using a relative approach examining how disruption of natural emotion processes through regulation attempts can impact emotion coherence.
Acknowledgments
E.D-G. was financed by a prospective researcher fellowship PBGE11-119321, granted by the Swiss National Science Foundation. Other sources of financing include MH66957 to J.G.
Footnotes
Temperature was later omitted from the study analyses due to poor reactivity of this measure in this context.
Corrected degrees of freedom are reported for all analyses.
References
- Baumeister RF, Vohs KD, DeWall CN, Zhang L. How Emotion Shapes Behavior: Feedback, Anticipation, and Reflection, Rather Than Direct Causation. Personality and Social Psychology Review. 2007;11(2):167–203. doi: 10.1177/1088868307301033. doi: 10.1177/1088868307301033. [DOI] [PubMed] [Google Scholar]
- Bradley MM, Lang PJ. Measuring emotion: Behavior, feeling, and physiology. In: Lane RD, Nadel L, editors. Cognitive Neuroscience of Emotion. Oxford University Press; New York, NY: 2000. pp. 242–276. [Google Scholar]
- Cacioppo JT, Petty RE, Losch ME, Kim HS. Electromyographic activity over facial muscle regions can differentiate the valence and intensity of affective reactions. Journal of Personality and Social Psychology. 1986;50(2):260–268. doi: 10.1037//0022-3514.50.2.260. [DOI] [PubMed] [Google Scholar]
- Campbell-Sills L, Barlow DH, Brown TA, Hofmann SG. Effects of suppression and acceptance on emotional responses of individuals with anxiety and mood disorders. Behaviour Research and Therapy. 2006;44(9):1251–1263. doi: 10.1016/j.brat.2005.10.001. [DOI] [PubMed] [Google Scholar]
- Cole PM, Hall SE, Radzioch AM. Emotional Dysregulation and the Development of Serious Misconduct. In: Olson SL, Sameroff AJ, editors. Biopsychosocial Regulatory Processes in the Development of Childhood Behavioral Problems. Cambridge University Press; New York, NY: 2009. pp. 186–211. [Google Scholar]
- Conde Pastor M, Menéndez FJ, Sanz M, Vila Abad E. The influence of respiration on biofeedback techniques. Applied Psychophysiology and Biofeedback. 2008;33(1):49–54. doi: 10.1007/s10484-007-9048-4. [DOI] [PubMed] [Google Scholar]
- Dan-Glauser ES, Gross JJ. The temporal dynamics of two response-focused forms of emotion regulation: Experiential, expressive, and autonomic consequences. Psychophysiology. 2011;48(9):1309–1322. doi: 10.1111/j.1469-8986.2011.01191.x. doi: 10.1111/j.1469-8986.2011.01191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drabant EM, McRae K, Manuck SB, Hariri AR, Gross JJ. Individual differences in typical reappraisal use predict amygdala and prefrontal responses. Biological Psychiatry. 2009;65(5):367–373. doi: 10.1016/j.biopsych.2008.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egloff B, Schmukle SC, Burns LR, Schwerdtfeger A. Spontaneous emotion regulation during evaluated speaking tasks: Associations with negative affect, anxiety expression, memory, and physiological responding. Emotion. 2006;6(3):356–366. doi: 10.1037/1528-3542.6.3.356. doi: 10.1037/1528-3542.6.3.356. [DOI] [PubMed] [Google Scholar]
- Ekman P. Universals and cultural differences in facial expressions of emotion. In: Cole J, editor. Nebraska Symposium on Motivation. University of Nebraska Press; Lincoln, NE: 1972. pp. 207–282. [Google Scholar]
- Ekman P. An argument for basic emotions. Cognition and emotion. 1992;6(3/4):169–200. [Google Scholar]
- Ekman P, Davidson RJ, Friesen WV. The Duchenne smile: Emotional expression and brain physiology II. Journal of Personality and Social Psychology. 1990;58:342–353. [PubMed] [Google Scholar]
- Ekman P, Friesen WV, Ancoli S. Facial signs of emotional experience. Journal of Personality and Social Psychology. 1980;39:1125–1134. [Google Scholar]
- Grandjean D, Sander D, Scherer KR. Conscious emotional experience emerges as a function of multilevel, appraisal-driven response synchronization. Consciousness and Cognition. 2008;17(2):484–495. doi: 10.1016/j.concog.2008.03.019. [DOI] [PubMed] [Google Scholar]
- Gross JJ. Antecedent- and response-focused emotion regulation: divergent consequences for experience, expression, and physiology. Journal of Personality and Social Psychology. 1998a;74(1):224–237. doi: 10.1037//0022-3514.74.1.224. [DOI] [PubMed] [Google Scholar]
- Gross JJ. The emerging field of emotion regulation: An integrative review. Review of General Psychology. 1998b;2(3):271–299. doi: 10.1037/1089-2680.2.3.271. [Google Scholar]
- Gross JJ. Emotion regulation in adulthood: Timing is everything. Current Directions in Psychological Science. 2001;10(6):214. [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: implication for affect, relationships, and well-being. Journal of Personality and Social Psychology. 2003;85(2):348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Emotional suppression: Physiology, self-report, and expressive behavior. Journal of Personality and Social Psychology. 1993;64:970–986. doi: 10.1037//0022-3514.64.6.970. [DOI] [PubMed] [Google Scholar]
- Gross JJ, Levenson RW. Hiding Feelings: The acute effects of inhibiting negative and positive emotion. Journal of Abnormal Psychology. 1997;106(1):95–103. doi: 10.1037//0021-843x.106.1.95. [DOI] [PubMed] [Google Scholar]
- Harris C. Cardiovascular responses of embarrassment and effects of emotional suppression in a social setting. Journal of Personality and Social Psychology. 2001;81(5):886–897. doi: 10.1037//0022-3514.81.5.886. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Luoma JB, Bond FW, Masuda A, Lillis J. Acceptance and commitment therapy: Model, processes and outcomes. Behaviour Research and Therapy. 2006;44(1):1–25. doi: 10.1016/j.brat.2005.06.006. doi: 10.1016/j.brat.2005.06.006. [DOI] [PubMed] [Google Scholar]
- Hayes SC, Strosahl KD, Wilson KG. Acceptance and commitment therapy: An experiential approach to behavior change. Guilford Press; New York, NY: 1999. [Google Scholar]
- Hinton O. Chapter 6: Describing Random Sequences. University of Newcastle upon Tyne; UK: [Retrieved November 7th, 2011]. 2002. pp. 6.1–6.8. [Online ressources for course EEE305 - Digital Signal Processing] from http://www.staff.ncl.ac.uk/oliver.hinton/eee305. [Google Scholar]
- Hubert W, de Jong-Meyer R. Psychophysiological response patterns to positive and negative film stimuli. Biological psychology. 1990;31(1):73–93. doi: 10.1016/0301-0511(90)90079-c. [DOI] [PubMed] [Google Scholar]
- Hutcherson CA, Goldin PR, Ochsner KN, Gabrieli JD, Barrett LF, Gross JJ. Attention and emotion: Does rating emotion alter neural responses to amusing and sad films? NeuroImage. 2005;27(3):656–668. doi: 10.1016/j.neuroimage.2005.04.028. doi: 10.1016/j.neuroimage.2005.04.028. [DOI] [PubMed] [Google Scholar]
- Jackson D, Malmstadt J, Larson C, Davidson R. Suppression and enhancement of emotional responses to unpleasant pictures. Psychophysiology. 2000;37(4):515–522. [PubMed] [Google Scholar]
- John OP, Gross JJ. Healthy and unhealthy emotion regulation: personality processes, individual differences, and life span development. Journal of Personality. 2004;72(6):1301–1333. doi: 10.1111/j.1467-6494.2004.00298.x. [DOI] [PubMed] [Google Scholar]
- Koole S. The psychology of emotion regulation: An integrative review. Cognition and Emotion. 2009;23(1):4–41. [Google Scholar]
- Kunzmann U, Kupperbusch CS, Levenson RW. Behavioral inhibition and amplification during emotional arousal: A comparison of two age groups. Psychology and Aging. 2005;20(1):144–158. doi: 10.1037/0882-7974.20.1.144. doi: 10.1037/0882-7974.20.1.144. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. International Affective Picture System (IAPS): Instruction manual and affective ratings. Center for Research in Psychophysiology: University of Florida; 1999. Technical Report A-4. [Google Scholar]
- Lang P, Greenwald M, Bradley M, Hamm A. Looking at pictures: Affective, facial, visceral, and behavioral reactions. Psychophysiology. 1993;30:261–261. doi: 10.1111/j.1469-8986.1993.tb03352.x. [DOI] [PubMed] [Google Scholar]
- Lazarus RS. Emotion and adaptation. Oxford University Press; London, UK: 1991. [Google Scholar]
- Levenson RW. Human emotions: A functional view. In: Ekman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. Oxford University Press; New York, NY: 1994. pp. 123–126. [Google Scholar]
- Levenson RW, Ekman P, Friesen WV. Voluntary facial action generates emotion-specific autonomic nervous system activity. Psychophysiology. 1990;27:363–384. doi: 10.1111/j.1469-8986.1990.tb02330.x. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Levenson RW, McCarter L, Wilhelm FH, Gross J. The tie that binds? Coherence among emotion experience, behavior, and physiology. Emotion. 2005;5(2):175–190. doi: 10.1037/1528-3542.5.2.175. [DOI] [PubMed] [Google Scholar]
- Mauss IB, Shallcross AJ, Troy AS, John OP, Ferrer E, Wilhelm FH, Gross JJ. Don’t hide your happiness! Positive emotion dissociation, social connectedness, and psychological functioning. Journal of personality and social psychology. 2011;100(4):738–748. doi: 10.1037/a0022410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCaul K, Solomon S, Holmes D. Effects of paced respiration and expectations on physiological and psychological responses to threat. Journal of Personality and Social Psychology. 1979;37(4):564–571. doi: 10.1037//0022-3514.37.4.564. [DOI] [PubMed] [Google Scholar]
- Panksepp J. The basics of basic emotion. In: Ekman P, Davidson RJ, editors. The nature of emotion: Fundamental questions. Oxford University Press; New York, NY: 1994. pp. 237–242. [Google Scholar]
- Roberts N, Levenson RW, Gross JJ. Cardiovascular costs of emotion suppression cross ethnic lines. International Journal of Psychophysiology. 2008;70(1):82–87. doi: 10.1016/j.ijpsycho.2008.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenberg EL, Ekman P. Coherence between expressive and experiential systems in emotion. Cognition & Emotion. 1994;8(3):201–229. doi: 10.1080/02699939408408938. [Google Scholar]
- Scherer KR. On the nature and function of emotion: A component process approach. In: Scherer KR, Ekman P, editors. Approaches to emotion. Erlbaum; Hillsdale, NJ: 1984. pp. 293–317. [Google Scholar]
- Scherer KR. Appraisal considered as a process of multi-level sequential checking. In: Scherer KR, Schorr A, Johnstone T, editors. Appraisal processes in emotion: Theory, Methods, Research. Oxford University Press; New York, NY: 2001. pp. 92–120. [Google Scholar]
- Strack F, Martin LL, Stepper S. Inhibiting and facilitating conditions of the human smile: A nonobstructive test of the facial feedback hypothesis. Journal of Personality and Social Psychology. 1988;54:768–777. doi: 10.1037//0022-3514.54.5.768. [DOI] [PubMed] [Google Scholar]
- Tomkins S. Affect, imagery, consciousness (Vol. 1 The positive affects) Springer; New York, NY: 1962. [Google Scholar]
- Tomkins S. Affect theory. In: Scherer K, Ekman P, editors. Approaches to emotion. Erlbaum; Hillsdale, NJ: 1984. pp. 353–400. [Google Scholar]
- Vrana SR. The psychophysiology of disgust: Differentiating negative emotional contexts with facial EMG. Psychophysiology. 1993;30(3):279–286. doi: 10.1111/j.1469-8986.1993.tb03354.x. [DOI] [PubMed] [Google Scholar]
- Wilhelm FH, Peyk P. ANSLAB: Autonomic Nervous System Laboratory (Version 2.5) 2005 Freeware available at the SPR Software Respository: http://www.sprweb.org.
- Wolgast M, Lundh L-G, Viborg G. Cognitive reappraisal and acceptance: An experimental comparison of two emotion regulation strategies. Behaviour Research and Therapy. 2011;49(12):858–866. doi: 10.1016/j.brat.2011.09.011. [DOI] [PubMed] [Google Scholar]