CONSPECTUS
As a semi-permeable barrier that controls the flux of biomolecules in and out the cell, the plasma membrane is critical in cell function and survival. Many proteins interact with the plasma membrane and modulate its physiology. Within this large landscape of membrane-active molecules, researchers have focused significant attention on two specific classes of peptides, antimicrobial peptides (AMPs) and cell penetrating peptides (CPPs) because of their unique properties.
In this account, we describe our efforts over the last decade to build and understand synthetic mimics of antimicrobial peptides (SMAMPs). These endeavors represent one specific example of a much larger effort to understand how synthetic molecules interact with and manipulate the plasma membrane. Using both defined molecular weight oligomers and easier to produce, but heterogeneous, polymers, it has been possible to generate scaffolds with biological potency superior to the natural analogs. In one case, a compound has progressed through a phase II clinical trial for pan)staph infections. Modern biophysical assays highlighted the interplay between the synthetic scaffold and lipid composition leading to negative Gaussian curvature, a requirement for both pore formation and endosomal escape. The complexity of this interplay between lipids, bilayer components, and the scaffolds remains to be better resolved, but significant new insight has been provided. It is worthwhile to consider the various aspects of permeation and how these are related to ‘pore formation.’
More recently, our efforts have expanded toward protein transduction domains, or cell penetrating peptide, mimics. The combination of unique molecular scaffolds and guanidinium) rich side chains has produced an array of polymers with robust transduction (and delivery) activity. Being a new area, the fundamental interactions between these new scaffolds and the plasma membrane are just beginning to be understood. Negative Gaussian curvature is important but the detailed relationships between molecular structure, self)assembly with lipids, and translocation require more investigation. It has become clear that the combination of molecular design, biophysical models, and biological evaluation provide a robust approach to the generation and study of novel proteinomimetics.
INTRODUCTION
The plasma membrane constitutes a semi)permeable barrier which controls the flux of biomolecules in and out the cell. It has a fundamental role in cell function and survival. If its integrity is compromized, for example by the formation of large and permanent pores, it will result in cell death. Many proteins interact with the plasma membrane and modulate its physiology. Within this large landscape of membrane)active molecules, two specific classes of peptides, antimicrobial peptides (AMPs) and cell penetrating peptides (CPPs), have received significant attention due to their unique properties.1,2
Although AMPs and CPPs share structural and functional aspects, they have mainly existed as separate literatures until recently.3,4 Both consist of short sequences that are net cationic. Almost all AMPs have significant hydrophobic residues, or domains, while CPPs may not. Another difference has been the biological assays which evaluate most AMPs for their antibacterial and hemolytic activities while for CPPs tend to focus on mammalian cell translocation. Because the detailed mechanisms of membrane activity is complex and remain under investigation, it is difficult to say that any specific peptide, regardless of sequence, follows a mechanism consistent with an AMP or CPP. Future studies will surely provide important insight in this area. In this review we will describe structural and functional aspects of synthetic mimics of AMPs and CPPs developed in our group.
AMPs AND THEIR SYNTHETIC MIMICS
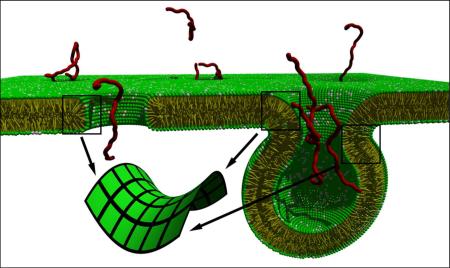
AMPs, isolated from organisms across the phylogenetic spectrum, are an important part of the innate immune system.5 Despite the diversity observed in AMP sequences, one hallmark is their facially amphiphilic (FA) topologies that appear crucial for membrane activity and antimicrobial properties (Figure 1). Although the exact mechanisms of membrane permeation are still not fully understood, it is thought that electrostatic interactions facilitate association with the anionic bacterial membrane and hydrophobic interactions promote pore formation and cell death.6 The differences in membrane composition between bacteria and eukaryotes have been used to justify AMPs selectivity. Bacterial membranes are rich in negative intrinsic curvature (NIC) lipids, such as phosphatidylethanolamine (PE) in Gram)negative and cardiolipin in Gram)positive, which play a critical role in pore)formation since they facilitate the negative)curvature circumferential barrels typical of transmembrane pores.7
Figure 1.
From AMPs to SMAMPs
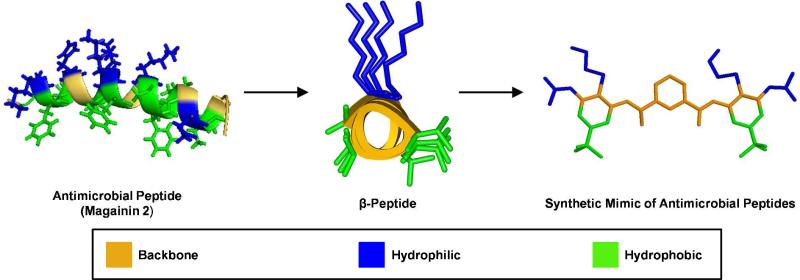
The ability to bind and control the integrity of phospholipid membranes is closely tied to the FA topology of AMPs. Over the past decade, their unique molecular architectures inspired the design of novel synthetic mimics of AMPs (SMAMPs) with tunable structural features.8)10 β) Peptides, a class of polyamides, have been shown to adopt a variety of secondary structures analogous to those of proteins. DeGrado and coworkers designed a series of amphiphilic, helical β)peptides to mimic natural membrane)active peptides.11 In particular, a series of β3) peptides showed reasonable antibacterial activity and selectivity (HC50/MIC) >100 for E. coli versus mammalian cells. Structure–function correlation studies provided important information about how the FA topology was related to their activities. The difference in vesicle leakage kinetics suggested that chain length might affect the bilayer disruption mechanisms. These initial studies, along with similar work by Gellman and Seebach, provided a useful guide for designing synthetic molecules and demonstrated that the α)helix was not essential for activity. However, these early designs still adopted overall FA secondary structures, with large surface areas of amphiphilic topology. Therefore, it remained unknown whether an inherent secondary structure was critical for activity until the first oligomers and polymers were prepared.12
Non-peptidic SMAMPs
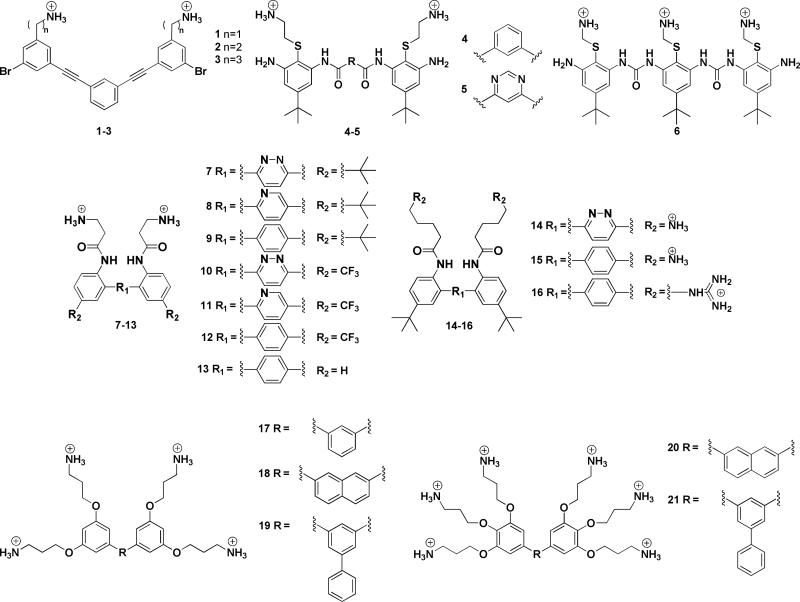
Motived by this question and by the desire to mimic the functions of biomolecules, our research group developed a series of novel FA synthetic polymers based on meta)phenylene ethynylene (mPE) backbone.13 This was one of the first attempts to produce synthetic mimics with a completely abiogenic backbone and no intramolecular H)bonding. These mPE polymers were found to be good mimics of AMPs, highlighting the importance of amphiphilicity, rather than peptide structure, on bioactivity.14,15 It was also possible to reduce the molecular weight (MW) leading to tri)aryl mPE SMAMPs (1-3) which were even more potent and selective than the polymeric counterparts (Figure 2).16 SMAMP 2 showed outstanding broad spectrum antibacterial activity and low toxicity, measured as minimum inhibitory concentration (MIC 0.1 Bg/mL against E. coli, 0.2 Bg/mL against S. aureus) and hemolytic concentration (HC50 75 μg/mL), respectively.16,17
Figure 2.
Small molecule SMAMPs
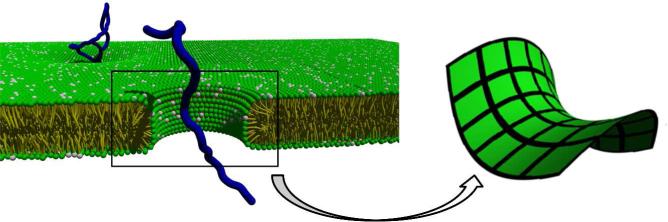
Extensive biophysical studies with this series of SMAMPs, such as small angle X)ray scattering (SAXS), dye release assays, solid)state NMR, and patch)clamp experiments were performed to evaluate the interaction between these SMAMPs and membranes. The ability of 2 to modulate the self)assembly and morphology of model membranes was studied in detail.7,17 SAXS data showed 2 restructured membranes, inducing an inverted hexagonal phase (HII) with 3nm water channels, in PE/PG (phosphatidylglycerol) model vesicles, but only when the PE lipid content in the membrane was above a minimum threshold of 64%.17 The HII phase is important for to the generation of negative Gaussian membrane curvature (or saddle)splay), topologically required for pore)formation (Figure 3).7 This indicates that the membrane activity of 2 critically depends on the concentration of the NIC PE lipid present in the membrane. This could also explain the selectivity of 2 because Gram)negative bacteria contain much higher volume fractions of NIC lipids than mammalian cells. In Gram)positive bacteria model membrane, cardiolipin (CL), in the presence of divalent metal cations (Ca2+ or Mg2+), acts as an NIC lipid and HII phases are induced by the presence of 2.18 An extensive dye leakage study supported SAXS findings indicating that these simpler biophysical assays can be used as a screening tool for more detailed and time)intensive experiments. 19,20
Figure 3.
Illustration of saddle)splay membrane curvature induced by SMAMPs
Another class of FA SMAMPs, with a conformationally stiff arylamide backbone, was also reported.12 For the arylamide oligomers, the conformational rigidity was derived from intramolecular H)bonding between the amide groups in the backbone and the thioether function in the side chains. Replacement of the central benzene ring (4) with pyrimidine (5), led to additional intramolecular H)bonding, resulting in a structure with higher rotational restriction, and enhanced antimicrobial activity (12 μg/mL and 0.8 μg/mL against E. coli, respectively).21 Within this arylamide series it was shown that increased conformational stiffness led to excellent antimicrobial activity (105 reduction in viable CFU of S. aureus) in a mouse model.22
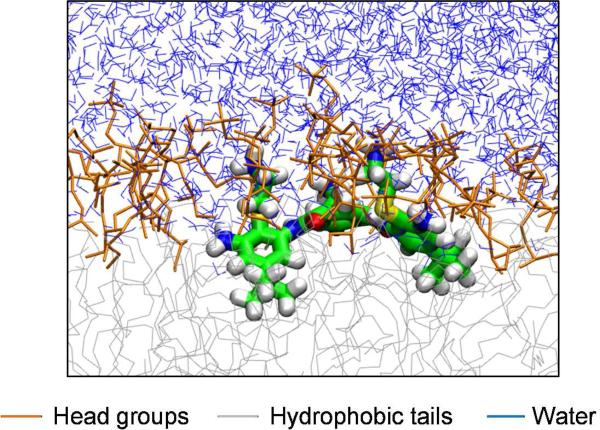
Molecular dynamic (MD) simulations revealed the preferential positions of the SMAMPs with respect to the bilayer. The simulations in n)octane/water and PC/water environments showed that arylamide oligomers based on 4 rapidly reached the interface with the charged side chains interacting with the charged phospholipid head groups and water; while the hydrophobic groups remained buried between the lipid tails (Figure 4).12,23 The primary driving force for insertion was hydrophobicity. MD simulations also showed that the preferential orientation of the arylamide oligomers was perpendicular to the bilayer normal, which was later supported experimentally.24 This particular orientation appears to maximize amphiphilic interactions. These computational studies have both verified and developed important SMAMP design principles. For instance, a semi)rigid backbone is apparently not an absolute requirement for optimal activity, provided that the SMAMPs can assemble into well) defined amphiphilic conformations in a heterogeneous lipid bilayer environment.
Figure 4.
Equilibrium conformation of a SMAMP in a hydrated PC lipid environment
To better investigate the role of backbone flexibility, several series of aromatic oligomers based on urea and tri)aryl scaffolds with intramolecular H)bonds between the rings were designed. Biological activity similar to 5 was observed for the urea)based oligomer 6, which had a totally locked conformation.25 However, when the same principle was applied to the tri) aryl series (7-16), the opposite trend was observed.26 This suggested that the potency of the compound was not the effect of one parameter alone but the result of a proper balance between the number of positive charges, amphiphilicity and hydrophobicity of the molecules. Without knowing the detailed mechanism of action, challenges arise in understanding structure)activity relationships. For example, within this series, the overall hydrophobicity had a higher impact than the conformational rigidity of the molecules. Modifications of the polar and non)polar side chains led to the same conclusion, since the molecules carrying the more hydrophobic tert) butyl (7-9) revealed to be more active.
To further explore the influence of charge and hydrophobicity on SMAMP activity, a new series of molecules was synthesized containing four or six cationic charges and three different central rings: benzene (17), naphthalene (18, 20), and phenylbenzene (19, 21).27 By increasing the hydrophobicity and the number of cationic charges, the compounds became more potent and selective. 20 was one of the best candidates, with a selectivity >200 for both E. coli and S. aureus. These results confirmed the importance of fine)tuning the overall hydrophobicity and total number of cationic charge in order to improve the biological activities of SMAMPs.
Although not directly related to pore)formation, 20 also showed immunomodulatory activities similar to AMPs. Mounting evidence shows AMPs modulate the immune system. This SMAMP stimulated pro) and anti)inflammatory cytokines (TNF, IL)6 and IL)10) as well as induced murine chemokine production (CXCL1). The ability of 20 to further capture the immunomodulator properties of AMPs demonstrated the importance of developing these mimics. The potential to discover novel scaffold that control the immune system is tremendous.28 Thus, 20 is a promising model for the design and application of dual)functional SMAMPs.29
Polymers as SMAMPs
An important extension in the field of SMAMPs was the introduction of oligomeric and polymeric SMAMPs. The fact that biomimetic activity could be obtained with molecules of variable and less defined MWs, which deviate more from the original peptide sequences, greatly expanded our fundamental knowledge and design capabilities. The initial work on oligomeric systems as non)biological AMPs mimics was conducted with aromatic scaffolds.12) 14 Their activity against several bacterial strains demonstrated their broad spectrum; however, these polymers were also found to be hemolytic, likely due to the significant hydrophobicity of the aromatic groups.
Therefore, we developed aliphatic polymeric SMAMPs based on polynorbornene backbone obtained by ring opening metathesis polymerization (ROMP). The ease, efficiency and control of ROMP, as well as its high functional group tolerance made this synthetic platform advantageous for our purposes.
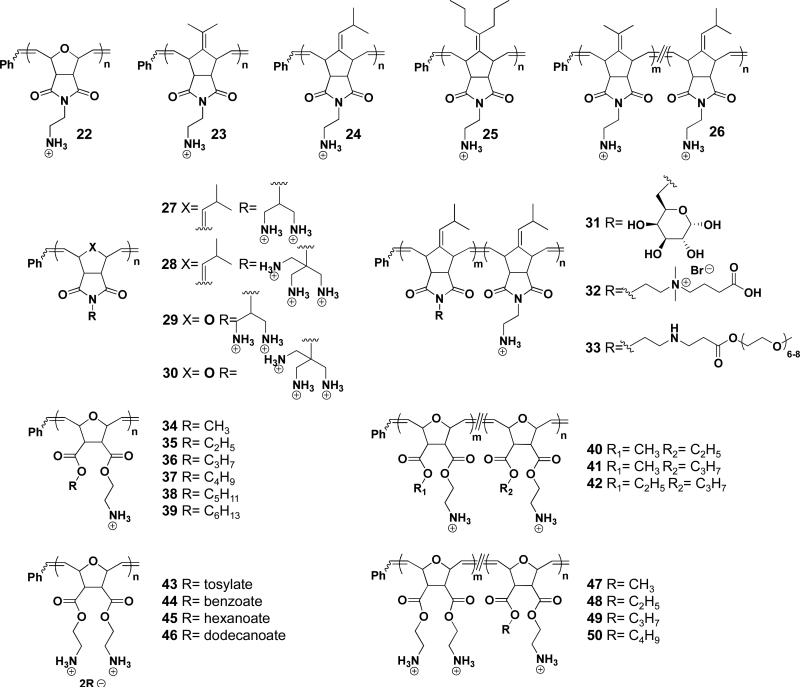
The first series of such antimicrobial ROMP polymers (22-25, Figure 5) included primary ammonium groups opposite a variety of hydrophobic side chains.30 The effects of hydrophobicity as well as the MW were studied in terms of MIC (E. coli, B. subtilis) and HC50. Four polymers with Mn=10,000 g/mol were first screened to determine antimicrobial activity. A general trend of increasing hemolytic and antimicrobial activity with increasing hydrophobicity was observed, with 22 and 25 performing poorly due to low activity and high hemolysis, respectively. Among these four antimicrobial polymers, no significant changes to MIC or HC50 values were observed as a function of MW, the only time this has been true for ROMP)synthesized SMAMPs.
Figure 5.
Antimicrobial polymers. Values for n and m are detailed in the text
Given the intermediate properties of 23 and 24, several random copolymers were synthesized using different monomer ratios in an effort to make a polymer that was both non)hemolytic and antimicrobial. A copolymer consisting primarily of 23 was found to have a selectivity of >100. This value demonstrates the versatility and tunability of this series of polymers.
An alternate approach to reducing the toxicity of 24 was to increase hydrophilicity. The ammonium density on 24 was increased to either two or three amines per residue (27-28).31 These SMAMPs maintained high antimicrobial activity (MIC>30 Bg/mL for E. coli, >50 Bg/mL for S. aureus) accompanied by a nearly 1000)fold decrease in hemolysis, yielding selectivities of nearly 100. In addition to changing the ammonium density, the monomer used in 24 was copolymerized with other hydrophilic monomers, yielding polymers 31-33.32 In order to reduce hemolysis, large ratios of the hydrophilic co)monomer were required, which also decreased the antimicrobial activity. This decrease was attributed to an overall decrease in positive charge, an important characteristic of active SMAMPs. One exception to this was 32 which had a 1:1 ratio of the two monomers. This polymer displayed low hemolysis (HC50=1500 Bg/mL) and moderate antimicrobial activity, resulting in selectivities of 10 for E. coli and 7.5 for S. aureus.
The second generation of antimicrobial oxanorbornene)based polymers (34-42) expanded the control of hydrophobicity and charge by introducing di)functionalized, diester monomers.33 It was found that homopolymers 34-39 displayed different biological activities at varying MWs, with lower MWs affording higher activity. 36 was selected due to its high activity, and a series of low MW oligomers (DP=2)7) was synthesized to determine effect of MW on biological activity. While selectivities towards E. coli remained similar across the series, those towards S. aureus dropped steadily from 280 (dimer) to >0.25 (Mn=10,000 g/mol). This trend was attributed to larger SMAMPs becoming trapped within the murein layer of gram)positive bacteria, decreasing their activity.
Copolymers were synthesized combining monomers used in 34 (non)active, non)hemolytic) with those used in 36 (active, hemolytic). Copolymers were also made using monomers in 35, as it displayed the highest selectivities among the homopolymers. Of these, 41 demonstrated the best selectivities towards S. aureus (>533) at all ratios tested (9:1, 1:1 and 1:9 of monomers in 34:36), while remaining relatively inactive (selectivity =10) towards E. coli. This difference was shown to be an effect of the double membrane present in Gram)negative bacteria rather than an issue of membrane composition.34
An alternative approach to controlling hydrophobicity examined the counterion. A series of diamine homopolymers coupled with counterions 43-46 were synthesized and it was determined that increasing the counterion size/hydrophobicity lowered antimicrobial activity due to strong polymer)counterion complexation, decreasing the membrane activity of the polymer.35
Similar to charge density studies performed on imide based SMAMPs (27-30), the diamine monomer was copolymerized with previously reported monomers to yield polymers 47-50. Of these copolymers, 47, with a ratio of 9:1 methyl/amine:diamine, showed the highest selectivity (650) towards S. aureus. On the contrary, 49 and 50 showed good activity against E. coli at high ratios of hydrophobic monomer due to the polymers’ increased abilities to disrupt the membrane via hydrophobic interactions. This study demonstrated the difference between Gram)positive and Gram)negative membrane disruption mechanisms as well as the ability to design selective SMAMPs by tuning the polymer compositions.
PGON: a non-membrane disruptive SMAMP
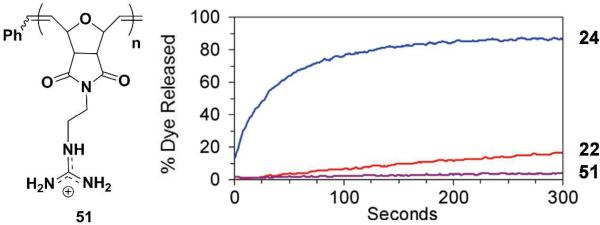
Although most AMPs and their synthetic mimics act on the membrane level, some have been shown to have intracellular targets, such as buforin;36 however, any intracellular target requires these molecules to transverse the lipid membrane. Polyguanidinium oxanorbornene (PGON, 51), a guanidine)functionalized version of 22, appears to fall within this category. The introduction of the guanidine functionality instead of amines led to totally new, unique membrane interactions. To the best of our knowledge, this was the first guanidine)containing, polymeric SMAMP. The presence of this group gave PGON significant activity against both Gram)positive and Gram)negative bacteria (MICs of 6 μg/mL for E. coli and 12 μg/mL for S. aureus) while remaining non)hemolytic.37 However, when tested in a dye release assay, PGON was clearly different from the many analogues previously studied. Even if highly bactericidal, it was found not to disrupt membranes of bacterial)like PE/PG vesicles (Figure 6). Cell staining confirmed a lack of membrane disruption, suggesting a different mechanism for killing bacteria.
Figure 6.
PGON structure and its membrane activity compared to its analogous 22 and 24 as measured in PE/PG vesicle dye release assay
The peculiar membrane activity of PGON and its chemical similarity with CPPs like polyarginine and TAT, suggested it may be an effective membrane transporter in addition to being antimicrobial. As anticipated, PGON indeed facilitated release of fluorescent dyes from PC vesicles without the help of an external activator, i.e. pyrenebutyrate.38 External activators are normally bulky, aromatic groups that provide the necessary hydrophobicity to aid in efficient membrane activity. None of the PGON derivatives studied (DP=5)41) required an activator, presumably due to the inherent hydrophobicity of the polyoxanorbornene backbone compared to peptides. Moreover, the membrane activity appeared to correlate in a non)linear manner to the degree of polymerization, with longer polymers performing better than short ones (EC50 = 2x10)8 M for DP= 41; EC50 = 3x10)6 M for DP = 5),38 in agreement with the behavior of well)known peptide systems like polyarginine. The DP)dependence of PGON membrane activity was also explored by SAXS.39 PGON derivatives were able to generate negative Gaussian curvature in PE)rich membranes, with a maximum induction at intermediate polymer length (DP = 14).
The unique membrane interaction properties of PGON are most likely due to the guanidine side chain. Many membrane)active peptides and proteins, such the TAT peptide or the amphipatic α) and θ)defensins, display a stronger cell)membrane interaction using arginine over lysine.40 As suggested by Wender and others, one of the advantages of arginine over lysine is its ability to form stable bidentate hydrogen bonds with phosphate and sulfate anions.41 More recently, quantum mechanical (QM) calculations showed that both guanidinium and amine groups are able to coordinate two phosphate groups together, with the same complexation energy (ca. 160 kcal/mol).39,42 However, because of the bidentate H) bonding ability and the planar Y)shape of the guanidinium groups, arginine side chains are induced to lay on the bilayer instead of staying perpendicular as in the case of lysine. Moreover, they are able to stack in a “face to face” conformation, in the case of polyarginine, and still coordinate two phosphate groups each even at a distance less than 5 Å. This creates a steric hindrance on the membrane and a lipid head crowding that generates a negative Gaussian curvature.43)46 As shown by QM calculations, curvature generation by PGON is also sensitive to the guanidinium group spacing, since an increase from 3.6 Å in polyarginine to 5.8 Å in PGON caused a 22% decrease in the maximum induced negative Gaussian curvature.
Introduction of the guanidine groups in oxanorbornene polymers resulted in a very unique membrane activity, making PGON both a good SMAMP with high antimicrobial activity and low cytotoxicity, and a good CPP)like membrane transporter. This unique membrane activity of guanidine)rich polyoxanorbornenes motivated our group to explore more carefully polymer designs that mimics arginine)rich CPPs.
CPPs AND THEIR SYNTHETIC MIMICS
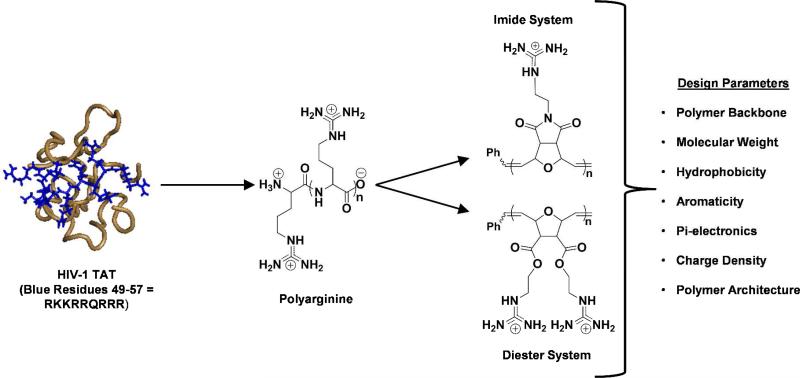
The field of CPPs started two decades ago when HIV)1 TAT, a small nuclear trans)activator of transcription protein, was shown to readily cross the cellular membrane and localize into the nucleus of many cell lines.47,48 It was then determined that this property was the result of a small, cation)rich domain between amino acids 49)57 (RKKRRQRRR).49,50 This sequence was referred to as PTD (Protein Transduction Domain). Synthetic variants, mostly peptides composed exclusively of arginine residues, such as polyarginine, outperform TAT and entered cells in a length)dependent fashion.40,51 The most active range was found to be between 5 and 17 arginine residues. Since then, β)peptides and several other molecular scaffolds (unique MWs) were richly decorated with guanidine functionality and showed similar internalization properties.41,52 In addition to TAT, in 1991, Antennapedia, a Drosophila homeoprotein widely studied since the 1980s, was found to be readily internalized by cells.53 Extensive studies demonstrated that the third helix, amino acids 43)58 (RQIKIWFQWRRMKWKK), were required for internalization, leading to the development of penetratin.54,55 Although this CPP contains arginine and lysine residues like Tat49)57, it also contains hydrophobic residues, which are critical for its cellular uptake. This expanded the design and suggested the importance of hydrophobic residues for efficient cellular uptake.
Since then the study of peptide sequences has expanded tremendously in the search for PTDs. These include Transportan (GWTLNS-AGYLLGKINLKALAALAKKIL-NH2), which is a fusion between the neuropeptide galanin)1)13 and wasp venom peptide mastoparan first reported in 1998, and Pep)1 (KETWWETWWTEWSQPKKKRKV-cya), which is the fusion of the lysine)rich NLS from Simian Virus 40 large T antigen and a tryptophan)rich sequence linked by the SQP sequence, first reported in 2001.56,57 It is worth noting that few CPPs have FA topologies but tend toward more blocking arrangements (Pep)1, Transportan, MPG..).
Inspired by the abilities of these native and chimeric proteins to translocate membranes, our group aimed to develop a series of non)peptidic, synthetic CPP mimics (CPPMs) that captured these unique features (Figure 7). Kiessling and Wender/Hedrick/Waymouth have independently reported polymeric CPPMs.50,58)60 Much like our antimicrobial polymers, we utilized ROMP with Grubbs 3rd generation catalyst, since it is fast, efficient, and yields polymers with low PDIs. In addition to homopolymers and random copolymers, the living nature of this synthetic platform has allowed for the synthesis of block copolymers. Moreover, a wider range of synthetic variations became available compared to native proteins or peptides, which is important for easy structural and physicochemical optimizations.
Figure 7.
From CPPs to CPPMs
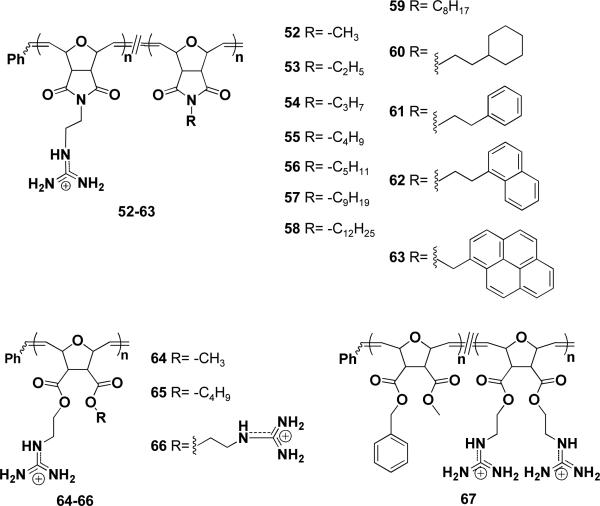
Based on previous work, which indicated that supramolecular hydrophobic activators enhance membrane activity of polyarginine, we aimed to directly incorporate these activators into CPPMs. Guanidine)containing monomers were copolymerized with a series of increasingly hydrophobic monomers ranging from methyl to dodecyl alkyl chains (52-58, Figure 8).61 The activity of this series, compared to PGON, was significantly improved. The butyl)containing molecule 55 showed the best activity (EC50 = 0.003 μM compared to 6.4 μM of 52); while polymers containing longer alkyl chains showed lower activities, due to poor solubility. This structure)activity study additionally demonstrated that “neutralization” of the guanidinium cationic charge by hydrophobic counteranions is not required for activity.
Figure 8.
Polymeric CPPMs
Knowing that aromatic amino acids are present in both membrane proteins and many CPP sequences, like penetratin and Pep-1, and that the best activators are also aromatic,62 we further investigated the role of aromaticity in CPPMs. A series of CPPMs (61-65) was designed to compare linear and cyclic aliphatic side chains versus aromatic side chains.63 Among the series, the aromatic 63 was the most active (EC50 = 4.3 nM) while also being the least hydrophobic of the series according to HPLC RTs. This suggested that aromaticity may indeed play a role in CPP activity.
Besides the imide)based series, we also developed the di)ester synthetic system (Fig. 3), which easily allowed doubling of the functional group density and offered the opportunity to vary two side chains independently. Homopolymers containing one guanidine functionality and one hydrophobic group (methyl or butyl alky chains) per repeat units (64-65) were synthesized and tested. Vesicle studies showed that the polymers behaved similar to the imide system (52-58). In addition, polymers 64 and 66 of various MWs were evaluated in vitro for cellular uptake with HEK293T, CHO, and Jurkat T cells.64 The two series of polymers studied were different based on the presence of a methyl group (64) and the density of guanidine groups (double for 66). Both polymers were able to function as CPPMs, and 64 with 9 guanidine groups even outperformed the control peptide R9. The fact that 64 and 66, with 12 and 18 guanidinium groups, respectively, showed similar internalization efficiencies in HEK293T suggests that, not only the number, but also the guanidine density has an effect in this cell line.
Expanding these preliminary reports, a block copolymer (67) with a total DP=10 and hydrophobic to hydrophilic ratio of 1:1 was synthesized to mimic the best features of Tat49)57 and Pep)1. This polymer was compared to 66 (DP=9) for siRNA delivery against hNOTCH1 into Jurkat and human peripheral blood mononuclear cells (PBMCs).65 67 outperformed 66 and reduced hNOTCH1 expression by 50 %. This is a substantial knockdown considering NOTCH1 is a highly regulated gene and not a reporter gene.
OUTLOOK
Using AMPs and CPPs as case studies, it has been possible to build synthetic mimics of the natural systems using simple, synthetic building blocks. This is essential for numerous reasons including the fact that it provides new model systems for understanding fundamental mechanisms. The larger toolbox of synthetic chemistry also enabled us to eliminate detrimental features of peptides leading to clinical development of a novel antibiotic. It would be reasonable to expect similar results in the CPPM field. Finally, these mimics are leading to new insight on polymer)membrane assemblies which are expected to have important implications in manipulating cell biology.
In this Account, we describe our efforts over the last decade to build and understand synthetic mimics of antimicrobial peptides (SMAMPs). These endeavors represent one specific example of a much larger effort to understand how synthetic molecules interact with and manipulate the plasma membrane.
Using both defined molecular weight oligomers and easier to produce, but heterogeneous, polymers, we have generated scaffolds with biological potency exceeding that of the natural analogs. One of these compounds has progressed through a phase II clinical trial for pan-staph infections. Modern biophysical assays have highlighted the interplay between the synthetic scaffold and lipid composition: a negative Gaussian curvature is required both for pore formation and for the initiation of endosome creation. Although work remains to better resolve the complexity of this interplay between lipids, other bilayer components, and the scaffolds, significant new insights have been discovered. These results point to the importance of considering the various aspects of permeation and how these are related to ‘pore formation.’
More recently, our efforts have expanded toward protein transduction domains, or mimics of cell penetrating peptides. Using a combination of unique molecular scaffolds and guanidinium-rich side chains, we have produced an array of polymers with robust transduction (and delivery) activity. In this new area, researchers are just beginning to understand the fundamental interactions between these new scaffolds and the plasma membrane. Negative Gaussian curvature is also important in these systems, but the detailed relationships between molecular structure, self-assembly with lipids, and translocation will require more investigation. It has become clear that the combination of molecular design, biophysical models, and biological evaluation provide a robust approach to the generation and study of novel proteinomimetics.
ACKNOLEDGMENTS
This work was supported by grants from NSF (CHE)0910963) and NIH (AI)074866, AI) 082192). Authors also would like to thank Dr. Morris Slutsky for his graphical contribution to the manuscript.
Biography
Federica Sgolastra is a Post)Doc in PSE having received her Ph.D. in Biomolecular Sciences from the Università Politecnica delle Marche, Italy, in 2011. Her current research interests include the development of synthetic delivery agents.
Brittany M. deRonde received a Bachelor's degree in Chemistry from Rutgers University in 2009, and is a graduate student in PSE who received an NIH training fellowship (T32 GMO8515).
Joel M. Sarapas received a Bachelor's degree in Chemistry from the University of Minnesota Twin Cities in 2011 and is a graduate student in PSE.
Abhigyan Som received his Ph.D. in Chemistry from the University of Geneva, Switzerland, in 2004. In 2005 he joined Professor Tew's group at UMASS Amherst as a Post)Doc. Currently, he is a research scientist at Metrex Research, Anaheim, CA.
Gregory N. Tew was trained in Chemistry, Materials Science, and Biophysics before joining the Faculty at PSE in 2001, since he has received a number of awards including the PECASE and currently serves as Chair of the ACS Polymer Chemistry Division. It has been a pleasure to work with a diverse group of talented students interested in complex and challenging scientific questions.
REFERENCES
- 1.Zasloff M. Antimicrobial peptides of multicellular organisms. Nature. 2002;415:389–395. doi: 10.1038/415389a. [DOI] [PubMed] [Google Scholar]
- 2.Wender PA, Cooley CB, Geihe EI. Beyond Cell Penetrating Peptides: Designed Molecular Transporters. Drug Discov Today Technol. 2012;9:e49–e55. doi: 10.1016/j.ddtec.2011.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Takeshima K, Chikushi A, Lee KK, Yonehara S, Matsuzaki K. Translocation of analogues of the antimicrobial peptides magainin and buforin across human cell membranes. J. Biol. Chem. 2003;278:1310–1315. doi: 10.1074/jbc.M208762200. [DOI] [PubMed] [Google Scholar]
- 4.Henriques ST, Melo MN, Castanho MARB. Cell-penetrating peptides and antimicrobial peptides: how different are they? Biochem J. 2006;399:1–7. doi: 10.1042/BJ20061100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brogden KA. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nature Reviews Microbiology. 2005;3:238–250. doi: 10.1038/nrmicro1098. [DOI] [PubMed] [Google Scholar]
- 6.Yeaman MR, Yount NY. Mechanisms of antimicrobial peptide action and resistance. Pharmacological Reviews. 2003;55:27–55. doi: 10.1124/pr.55.1.2. [DOI] [PubMed] [Google Scholar]
- 7.Yang LH, Gordon VD, Trinkle DR, Schmidt NW, Davis MA, DeVries C, Som A, Cronan JE, Tew GN, Wong GCL. Mechanism of a prototypical synthetic membrane-active antimicrobial: Efficient hole-punching via interaction with negative intrinsic curvature lipids. Proc. Natl. Acad. Sci. U. S. A. 2008;105:20595–20600. doi: 10.1073/pnas.0806456105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gabriel GJ, Som A, Madkour AE, Eren T, Tew GN. Infectious disease: Connecting innate immunity to biocidal polymers. Materials Science & Engineering R-Reports. 2007;57:28–64. doi: 10.1016/j.mser.2007.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Som A, Vemparala S, Ivanov I, Tew GN. Synthetic mimics of antimicrobial peptides. Biopolymers. 2008;90:83–93. doi: 10.1002/bip.20970. [DOI] [PubMed] [Google Scholar]
- 10.Lienkamp K, Tew GN. Synthetic Mimics of Antimicrobial Peptides-A Versatile Ring-Opening Metathesis Polymerization Based Platform for the Synthesis of Selective Antibacterial and Cell-Penetrating Polymers. Chem. Eur. J. 2009;15:11784–11800. doi: 10.1002/chem.200900049. [DOI] [PubMed] [Google Scholar]
- 11.Hamuro Y, Schneider JP, DeGrado WF. De novo design of antibacterial beta-peptides. J. Am. Chem. Soc. 1999;121:12200–12201. [Google Scholar]
- 12.Tew GN, Liu DH, Chen B, Doerksen RJ, Kaplan J, Carroll PJ, Klein ML, DeGrado WF. De novo design of biomimetic antimicrobial polymers. Proc. Natl. Acad. Sci. U. S. A. 2002;99:5110–5114. doi: 10.1073/pnas.082046199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Arnt L, Tew GN. New poly(phenyleneethynylene)s with cationic, facially amphiphilic structures. J. Am. Chem. Soc. 2002;124:7664–7665. doi: 10.1021/ja026607s. [DOI] [PubMed] [Google Scholar]
- 14.Arnt L, Nusslein K, Tew GN. Nonhemolytic abiogenic polymers as antimicrobial peptide mimics. J. Polym. Sci. Pol. Chem. 2004;42:3860–3864. [Google Scholar]
- 15.Ishitsuka Y, Arnt L, Majewski J, Frey S, Ratajczek M, Kjaer K, Tew GN, Lee KYC. Amphiphilic poly(phenyleneethynylene)s can mimic antimicrobial peptide membrane disordering effect by membrane insertion. J. Am. Chem. Soc. 2006;128:13123–13129. doi: 10.1021/ja061186q. [DOI] [PubMed] [Google Scholar]
- 16.Som A, Tew GN. Influence of lipid composition on membrane activity of antimicrobial phenylene ethynylene oligomers. J. Phys. Chem. B. 2008;112:3495–3502. doi: 10.1021/jp077487j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yang LH, Gordon VD, Mishra A, Sorn A, Purdy KR, Davis MA, Tew GN, Wong GCL. Synthetic antimicrobial, oligomers induce a composition-dependent topological transition in membranes. J. Am. Chem. Soc. 2007;129:12141–12147. doi: 10.1021/ja072310o. [DOI] [PubMed] [Google Scholar]
- 18.Som A, Yang LH, Wong GCL, Tew GN. Divalent Metal Ion Triggered Activity of a Synthetic Antimicrobial in Cardiolipin Membranes. J. Am. Chem. Soc. 2009;131:15102. doi: 10.1021/ja9067063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen JM, Hessler JA, Putchakayala K, Panama BK, Khan DP, Hong S, Mullen DG, DiMaggio SC, Som A, Tew GN, Lopatin AN, Baker JR, Holl MMB, Orr BG. Cationic Nanoparticles Induce Nanoscale Disruption in Living Cell Plasma Membranes. J. Phys. Chem. B. 2009;113:11179–11185. doi: 10.1021/jp9033936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu WG, Som A, Tew GN. Interaction between Lipids and Antimicrobial Oligomers Studied by Solid-State NMR. J Phys Chem B. 2011;115:8474–8480. doi: 10.1021/jp202414m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tang H, Doerksen RJ, Jones TV, Klein ML, Tew GN. Biomimetic facially amphiphilic antibacterial oligomers with conformationally stiff backbones. Chem Biol. 2006;13:427–435. doi: 10.1016/j.chembiol.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Choi S, Isaacs A, Clements D, Liu DH, Kim H, Scott RW, Winkler JD, DeGrado WF. De novo design and in vivo activity of conformationally restrained antimicrobial arylamide foldamers. P Natl Acad Sci USA. 2009;106:6968–6973. doi: 10.1073/pnas.0811818106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu DH, Choi S, Chen B, Doerksen RJ, Clements DJ, Winkler JD, Klein ML, DeGrado WF. Nontoxic membrane-active antimicrobial arylamide oligomers. Angew. Chem., Int. Ed. 2004;43:1158–1162. doi: 10.1002/anie.200352791. [DOI] [PubMed] [Google Scholar]
- 24.Chen XY, Tang HZ, Even MA, Wang J, Tew GN, Chen Z. Observing a molecular knife at work. J. Am. Chem. Soc. 2006;128:2711–2714. doi: 10.1021/ja057029t. [DOI] [PubMed] [Google Scholar]
- 25.Tang HZ, Doerksen RJ, Tew GN. Synthesis of urea oligomers and their antibacterial activity. Chemical Communications. 2005:1537–1539. doi: 10.1039/b413679a. [DOI] [PubMed] [Google Scholar]
- 26.Thaker HD, Sgolastra F, Clements D, Scott RW, Tew GN. Synthetic Mimics of Antimicrobial Peptides from Triaryl Scaffolds. J. Med. Chem. 2011;54:2241–2254. doi: 10.1021/jm101410t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Thaker HD, Som A, Ayaz F, Lui DH, Pan WX, Scott RW, Anguita J, Tew GN. Synthetic Mimics of Antimicrobial Peptides with Immunomodulatory Responses. J. Am. Chem. Soc. 2012;134:11088–11091. doi: 10.1021/ja303304j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hancock REW, Sahl HG. Antimicrobial and host-defense peptides as new anti-infective therapeutic strategies. Nat. Biotech. 2006;24:1551–1557. doi: 10.1038/nbt1267. [DOI] [PubMed] [Google Scholar]
- 29.Som A, Navasa N, Percher A, Scott RW, Tew GN, Anguita J. Identification of Synthetic Host Defense Peptide Mimics That Exert Dual Antimicrobial and Anti-Inflammatory Activities. Clinical and Vaccine Immunology. 2012;19:1784–1791. doi: 10.1128/CVI.00291-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ilker MF, Nusslein K, Tew GN, Coughlin EB. Tuning the hemolytic and antibacterial activities of amphiphilic polynorbornene derivatives. J. Am. Chem. Soc. 2004;126:15870–15875. doi: 10.1021/ja045664d. [DOI] [PubMed] [Google Scholar]
- 31.Al-Badri ZM, Som A, Lyon S, Nelson CF, Nusslein K, Tew GN. Investigating the Effect of Increasing Charge Density on the Hemolytic Activity of Synthetic Antimicrobial Polymers. Biomacromolecules. 2008;9:2805–2810. doi: 10.1021/bm800569x. [DOI] [PubMed] [Google Scholar]
- 32.Colak S, Nelson CF, Nusslein K, Tew GN. Hydrophilic Modifications of an Amphiphilic Polynorbornene and the Effects on its Hemolytic and Antibacterial Activity. Biomacromolecules. 2009;10:353–359. doi: 10.1021/bm801129y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lienkamp K, Madkour AE, Musante A, Nelson CF, Nusslein K, Tew GN. Antimicrobial polymers prepared by ROMP with unprecedented selectivity: A molecular construction kit approach. J. Am. Chem. Soc. 2008;130:9836–9843. doi: 10.1021/ja801662y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lienkamp K, Kumar KN, Som A, Nusslein K, Tew GN. “Doubly Selective” Antimicrobial Polymers: How Do They Differentiate between Bacteria? Chem. Eur. J. 2009;15:11710–11714. doi: 10.1002/chem.200802558. [DOI] [PubMed] [Google Scholar]
- 35.Lienkamp K, Madkour AE, Kumar KN, Nusslein K, Tew GN. Antimicrobial Polymers Prepared by Ring-Opening Metathesis Polymerization: Manipulating Antimicrobial Properties by Organic Counterion and Charge Density Variation. Chem. Eur. J. 2009;15:11715–11722. doi: 10.1002/chem.200900606. [DOI] [PubMed] [Google Scholar]
- 36.Pavia KE, Spinella SA, Elmore DE. Novel histone-derived antimicrobial peptides use different antimicrobial mechanisms. Biochimica Et Biophysica Acta-Biomembranes. 2012;1818:869–876. doi: 10.1016/j.bbamem.2011.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gabriel GJ, Madkour AE, Dabkowski JM, Nelson CF, Nusslein K, Tew GN. Synthetic Mimic of Antimicrobial Peptide with Nonmembrane-Disrupting Antibacterial Properties. Biomacromolecules. 2008;9:2980–2983. doi: 10.1021/bm800855t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hennig A, Gabriel GJ, Tew GN, Matile S. Stimuli-responsive polyguanidino-oxanorbornene membrane transporters as multicomponent sensors in complex matrices. J Am Chem Soc. 2008;130:10338–10344. doi: 10.1021/ja802587j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schmidt NW, Lis M, Zhao K, Lai GH, Alexandrova AN, Tew GN, Wong GCL. Molecular Basis for Nanoscopic Membrane Curvature Generation from Quantum Mechanical Models and Synthetic Transporter Sequences. J. Am. Chem. Soc. 2012;134:19207–19216. doi: 10.1021/ja308459j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mitchell DJ, Kim DT, Steinman L, Fathman CG, Rothbard JB. Polyarginine enters cells more efficiently than other polycationic homopolymers. Journal of Peptide Research. 2000;56:318–325. doi: 10.1034/j.1399-3011.2000.00723.x. [DOI] [PubMed] [Google Scholar]
- 41.Wender PA, Galliher WC, Goun EA, Jones LR, Pillow TH. The design of guanidinium-rich transporters and their internalization mechanisms. Adv. Drug Deliv. Rev. 2008;60:452–472. doi: 10.1016/j.addr.2007.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kawamoto S, Takasu M, Miyakawa T, Morikawa R, Oda T, Futaki S, Nagao H. Binding of Tat peptides on DOPC and DOPG lipid bilayer membrane studied by molecular dynamics simulations. Mol Simulat. 2012;38:366–368. [Google Scholar]
- 43.Kawamoto S, Miyakawa T, Takasu M, Morikawa R, Oda T, Saito H, Futaki S, Nagao H. Cell-Penetrating Peptide Induces Various Deformations of Lipid Bilayer Membrane: Inverted Micelle, Double Bilayer, and Transmembrane. Int. J. Quantum Chem. 2012;112:178–183. [Google Scholar]
- 44.Hirose H, Takeuchi T, Osakada H, Pujals S, Katayama S, Nakase I, Kobayashi S, Haraguchi T, Futaki S. Transient Focal Membrane Deformation Induced by Arginine-rich Peptides Leads to Their Direct Penetration into Cells. Mol Ther. 2012;20:984–993. doi: 10.1038/mt.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Marschall ALJ, Frenzel A, Schirrmann T, Schungel M, Dubel S. Targeting antibodies to the cytoplasm. Mabs-Austin. 2011;3:3–16. doi: 10.4161/mabs.3.1.14110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kawamoto S, Takasu M, Miyakawa T, Morikawa R, Oda T, Futaki S, Nagao H. Inverted micelle formation of cell-penetrating peptide studied by coarse-grained simulation: Importance of attractive force between cell-penetrating peptides and lipid head group. J. Chem. Phys. 2011:134. doi: 10.1063/1.3555531. [DOI] [PubMed] [Google Scholar]
- 47.Green M, Loewenstein PM. Autonomous Functional Domains of Chemically Synthesized Human Immunodeficiency Virus Tat Trans-Activator Protein. Cell. 1988;55:1179–1188. doi: 10.1016/0092-8674(88)90262-0. [DOI] [PubMed] [Google Scholar]
- 48.Frankel AD, Pabo CO. Cellular Uptake of the Tat Protein from Human Immunodeficiency Virus. Cell. 1988;55:1189–1193. doi: 10.1016/0092-8674(88)90263-2. [DOI] [PubMed] [Google Scholar]
- 49.Vives E, Brodin P, Lebleu B. A truncated HIV-1 Tat protein basic domain rapidly translocates through the plasma membrane and accumulates in the cell nucleus. J Biol Chem. 1997;272:16010–16017. doi: 10.1074/jbc.272.25.16010. [DOI] [PubMed] [Google Scholar]
- 50.Wender PA, Mitchell DJ, Pattabiraman K, Pelkey ET, Steinman L, Rothbard JB. The design, synthesis, and evaluation of molecules that enable or enhance cellular uptake: Peptoid molecular transporters. Proc. Natl. Acad. Sci. U. S. A. 2000;97:13003–13008. doi: 10.1073/pnas.97.24.13003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Goun EA, Pillow TH, Jones LR, Rothbard JB, Wender PA. Molecular transporters: Synthesis of oligoguanidinium transporters and their application to drug delivery and real-time imaging. Chembiochem. 2006;7:1497–1515. doi: 10.1002/cbic.200600171. [DOI] [PubMed] [Google Scholar]
- 52.Potocky TB, Silvius J, Menon AK, Gellman SH. HeLa cell entry by guanidinium-rich beta-peptides: Importance of specific cation-cell surface interactions. Chembiochem. 2007;8:917–926. doi: 10.1002/cbic.200600563. [DOI] [PubMed] [Google Scholar]
- 53.Joliot A, Pernelle C, Deagostinibazin H, Prochiantz A. Antennapedia Homeobox Peptide Regulates Neural Morphogenesis. P Natl Acad Sci USA. 1991;88:1864–1868. doi: 10.1073/pnas.88.5.1864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Derossi D, Joliot AH, Chassaing G, Prochiantz A. The 3rd Helix of the Antennapedia Homeodomain Translocates through Biological-Membranes. J. Biol. Chem. 1994;269:10444–10450. [PubMed] [Google Scholar]
- 55.Derossi D, Chassaing G, Prochiantz A. Trojan peptides: the penetratin system for intracellular delivery. Trends in Cell Biology. 1998;8:84–87. [PubMed] [Google Scholar]
- 56.Pooga M, Hallbrink M, Zorko M, Langel U. Cell penetration by transportan. FASEB J. 1998;12:67–77. doi: 10.1096/fasebj.12.1.67. [DOI] [PubMed] [Google Scholar]
- 57.Morris MC, Depollier J, Mery J, Heitz F, Divita G. A peptide carrier for the delivery of biologically active proteins into mammalian cells. Nat Biotechnol. 2001;19:1173–1176. doi: 10.1038/nbt1201-1173. [DOI] [PubMed] [Google Scholar]
- 58.Kolonko EM, Kiessling LL. A polymeric domain that promotes cellular internalization. J. Am. Chem. Soc. 2008;130:5626. doi: 10.1021/ja8001716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cooley CB, Trantow BM, Nederberg F, Kiesewetter MK, Hedrick JL, Waymouth RM, Wender PA. Oligocarbonate Molecular Transporters: Oligomerization-Based Syntheses and Cell-Penetrating Studies. J. Am. Chem. Soc. 2009;131:16401. doi: 10.1021/ja907363k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Geihe EI, Cooley CB, Simon JR, Kiesewetter MK, Edward JA, Hickerson RP, Kaspar RL, Hedrick JL, Waymouth RM, Wender PA. Designed guanidinium-rich amphipathic oligocarbonate molecular transporters complex, deliver and release siRNA in cells. Proc. Natl. Acad. Sci. U. S. A. 2012;109:13171–13176. doi: 10.1073/pnas.1211361109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Som A, Tezgel AO, Gabriel GJ, Tew GN. Self Activation in De Novo Designed Mimics of Cell Penetrating Peptides. Angew. Chem., Int. Ed. 2011;50:6147–6150. doi: 10.1002/anie.201101535. [DOI] [PubMed] [Google Scholar]
- 62.Nishihara M, Perret F, Takeuchi T, Futaki S, Lazar AN, Coleman AW, Sakai N, Matile S. Arginine magic with new counterions up the sleeve. Organic & Biomolecular Chemistry. 2005;3:1659–1669. doi: 10.1039/b501472g. [DOI] [PubMed] [Google Scholar]
- 63.Som A, Reuter A, Tew GN. Protein Transduction Domain Mimics: The Role of Aromatic Functionality. Angew Chem Int Edit. 2012;51:980–983. doi: 10.1002/anie.201104624. [DOI] [PubMed] [Google Scholar]
- 64.Tezgel AO, Telfer JC, Tew GN. De Novo Designed Protein Transduction Domain Mimics from Simple Synthetic Polymers. Biomacromolecules. 2011;12:3078–3083. doi: 10.1021/bm200694u. [DOI] [PubMed] [Google Scholar]
- 65.Tezgel AO, Gonzalez-Perez G, Telfer JC, Osborne BA, Minter LM, Tew GN. Novel Protein Transduction Domain Mimics as Nonviral Delivery Vectors for siRNA Targeting NOTCH1 in Primary Human T cells. Mol Ther. 2013;21:201–209. doi: 10.1038/mt.2012.209. [DOI] [PMC free article] [PubMed] [Google Scholar]