Abstract
MicroRNAs (miRNAs) are a family of small non-coding RNA molecules that are made up of 18–25 nucleotides that function in post-transcriptional gene regulation. The expression of miRNAs is highly conserved and essential in regulating many cellular processes including formation, maintenance and the remodelling of the extracellular matrix (ECM). In this review, we examine different ECM molecules and the miRNAs involved in regulating their abundance and how these changes influence cell phenotype. For example, miRNAs and their target messenger RNAs (mRNAs) are involved in cell adhesion, by regulating the synthesis and turnover of key ECM adhesion molecules and their receptors including cadherins, integrins and other non-integrin ECM receptors. Other miRNAs regulate the abundance of cytokines and growth factors which in turn stimulate cells to synthesize and secrete specialized ECMs. For example, miR-125a/b and miR-146a and their downstream target mRNAs influence the production of the epidermal growth factor family which has a significant impact on the nature of the ECM formed. miRNAs affect structural ECM proteins important in the assembly, composition and organization of the ECM. Proteins such as collagen, fibronectin, versican, and nephronectin are targeted by several miRNAs. miRNAs can also control the expression of proteins such as matrix metalloproteinases (MMPs) and tissue inhibitor of metalloproteinases (TIMPs), which are involved in ECM remodelling and are important for tissue development, cell motility and wound healing. It has become clear that many different miRNAs control the balance in ECM composition that determines normal tissue function and alterations in the expression of these miRNAs can lead to pathological consequences.
Keywords: microRNA, 3′UTR, non-coding RNA, Extracellular matrix, Angiogenesis, Tumorigenesis
1. Introduction
The extracellular matrix (ECM) is a complex network of a number of different structural proteins, matricellular proteins, proteoglycans, hyaluronan and a variety of glycoproteins that interact by entanglement and cross linking to form a bioactive polymer that influences the mechanical properties of tissues and the phenotype of the cells that reside in those tissues. The ultimate composition of the ECM is determined by a combination of factors that influence new synthesis and turnover of individual ECM components. Changes in the individual components of the ECM and their organization have a significant impact on the cellular phenotype. MicroRNAs (miRNAs) provide one mechanism that controls the composition of the ECM and hence the phenotype of the cells that reside in that ECM. miRNAs are non-coding RNAs that are made up of 18–25 nucleotides and are able to modulate gene expression, by either inducing the degradation or blocking translation of the target messenger RNAs (mRNAs) (Bartel, 2009). miRNAs are highly conserved, and can be expressed in a tissue-specific manner and perform essential functions in regulating diverse cellular processes including those involved in development, differentiation, and disease (Johnston and Hobert, 2003; Chendrimada et al., 2005; Hatfield et al., 2005; Iorio et al., 2005; Lim et al., 2005; Dalmay and Edwards, 2006; Gauthier and Wollheim, 2006; Ruvkun, 2006; Chen and Stallings, 2007; Foekens et al., 2008; Huang et al., 2008b; Lowery et al., 2009; Wickramasinghe et al., 2009; Yang et al.). There are over 1000 human miRNAs that have been sequenced and reported, and it is estimated that one third of genes are regulated by miRNAs as one miRNA can regulate the expression of many genes (Lewis et al., 2005).
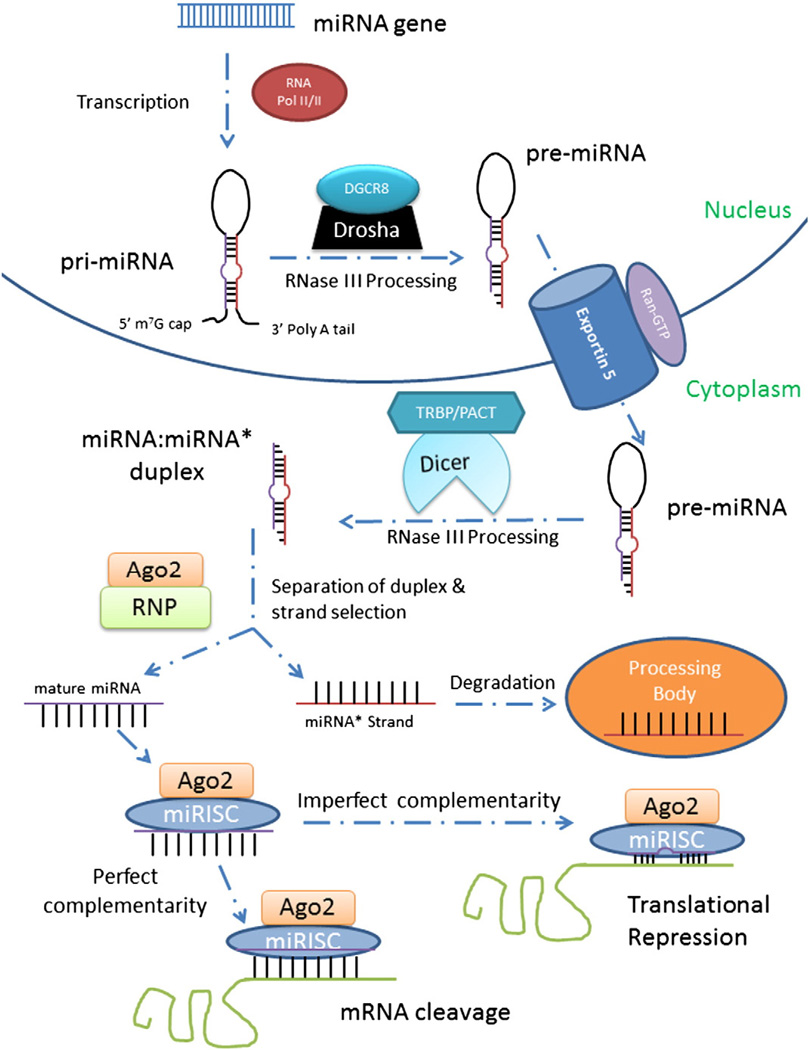
The biogenesis of miRNAs starts with transcription in the nucleus, similar to protein coding genes. Fig. 1 depicts the general biogenesis pathway of miRNAs. Typically, there is only one miRNA gene code for an individual miRNA; however, frequently some groups of miRNAs are transcribed as a single polycistronic transcript when they are clustered together (Baskerville and Bartel, 2005). miRNAs that are expressed in the same cluster usually do not share sequence similarities or target identical genes; however they do function together when they are co-expressed to control multiple target genes (Baskerville and Bartel, 2005). It is estimated that almost 70% of genes encoding for miRNAs overlap with defined transcriptional units and are transcriptionally linked to other genes, coding either for other non-coding RNAs (ncRNAs) or proteins (Rodriguez et al., 2004). miRNA genes have been reported between other genes, in the introns of protein-coding genes, and exons and introns of long non-coding RNA (lncRNA) transcripts (Rodriguez et al., 2004).
Fig. 1.
miRNA biogenesis pathway. miRNA genes are transcribed by RNA Pol II/III to make pri-miRNA. pri-miRNA is cleaved by Drosha complexed with DGCR8 to become pre-miRNA which is exported by Exportin-5 and RanGTP complex into the cytoplasm. Dicer and TRBP complex and process it to create the miRNA:miRNA* duplex. Ago2 and RNP separate the duplex to create the mature miRNA. The mature miRNA binds with miRISC and Ago2 to silence target mRNAs.
Many miRNA genes are also clustered together in the genome and can be transcribed as one transcript (Saini et al., 2007). In humans, over 247 miRNAs occur in clusters that have an inter-miRNA distance of less than 5000 bp (Griffiths-Jones et al., 2008). Also, the biological effect of these clusters can be strongly mediated by the fact that they have a high degree of conservation (Altuvia et al., 2005). For example the expression of the miR-23a–27a–24-2 cluster is upregulated in many disease conditions; heart failure, ulcerative colitis and cancers of the breast, pancreas, kidneys and bladder (van Rooij et al., 2006; Bloomston et al., 2007; Gottardo et al., 2007; Lee et al., 2007; Mertens-Talcott et al., 2007; Wu et al., 2008; Guttilla and White, 2009; Chow et al., 2010). However these miRNAs can also be independently transcribed as many of them have their own TATA box binding motifs which recruit transcriptional activators (Houbaviy et al., 2005). Furthermore, several transcription factors have been shown to be involved in regulating the expression of miRNAs by recruiting co-activators and other transcriptional elements (Shi et al., 2008). For example, Myc and the E2F family upregulate the transcription of the miR-17–92 cluster which has been shown to stimulate tumor growth (Aguda et al., 2008; Li et al., 2011c). In order to regulate translation, the association of the miRNA to the target 3′untranslated region (3′UTR) is believed to be driven through diffusion (Ameres et al., 2007).
miRNAs bind through base pairing to the 3′UTR of target mRNAs. The binding specificity and efficiency are believed to be determined by the 6–7 nucleotide sequence near the 5′ region of miRNA (Lewis et al., 2005). This sequence is called the “seed sequence” (Latronico et al., 2007), and is the initial binding site of the miRNA to the 3′UTR of the target mRNA (Lim et al., 2005). This seed sequence also determines whether the mRNA is degraded or translation repression occurs due to the degree of complementarity between the seed sequence of miRNA and the binding site in the target mRNA’s 3′UTR (Berezikov et al., 2005; Lim et al., 2005). It is also believed that mRNAs that are bound by miRNAs are targeted for degradation and are transported to the p-bodies leading to the translational repression of mRNAs (Liu et al., 2005). In this review, we give an overview of the current knowledge of the role of miRNAs in maintaining and remodelling the ECM structure.
2. Regulation of ECM composition and organization
Any given tissue has a preferred set of ECM components which are organized in such a way as to contribute to the mechanical properties of that tissue and to the organization and phenotype of the cells that reside in that tissue. The nature of the components and how they are organized determine the mechanical properties of the microenvironment surrounding the cells (i.e., stiff and rigid vs. soft and pliable) and these mechanical cues can have a major impact on regulating the differentiation and behavior of various cell types. Cells sense their surroundings by a set of receptors on their surface and this interaction results in signalling cascades that control the phenotype of the cells. Factors that control the composition of the ECM and the organization and availability of specific components of the ECM are critical for driving cellular events that form the basis of development and disease. miRNAs can regulate the composition of the ECM and impact the availability of certain ECMs in a number of different ways. Such a system that has an inherent redundancy generates complex overlapping pathways that can control cellular phenotypes. Some examples are discussed in the following sections.
2.1. Nephronectin
The composition of the ECM is critical in the organization and preservation of epithelial apical–basolateral polarity. Nephronectin is a secreted ECM protein that is expressed in a variety of tissues in the developing mouse embryo (Brandenberger et al., 2001). Exogenous overexpression of nephronectin promotes osteoblast differentiation and bone nodule formation, which appears to be regulated by miRNA (Kahai et al., 2009). While the exact mechanism responsible for the impact of nephronectin expression on osteoblast differentiation is not known, striking cell shape changes are seen in the nephronectin-expressing cells which could lead to altered gene expression and differentiation. Thus, cell shape change and differences in adhesion may result from altered ECM composition driving by miRNAs, resulting in a differentiated cellular phenotype.
The microRNA miR-378 can bind and target the 3′UTR of nephronectin which causes the modulation of osteoblast differentiation (Kahai et al., 2009). Interestingly, in later stages of MC3T3-E1 development, there are higher rates of cell differentiation and bone nodule formation due to the cells overexpressing the 3′UTR of nephronectin compared with earlier stages where there was a high amount of miR-378 that was present and active. This is the consequence of competition for miR-378 binding between nephronectin and UDP-N-acetylalpha-d-galactosamine:polypeptide N-acetylgalactosaminyltransferase 7 (GalNT7) (Kahai et al., 2009). In the late stages, there is an increased amount of GalNT7 activity due to the fact that endogenous miR-378 binds to the 3′UTR of nephronectin, which in turn leads to enhanced nephronectin glycosylation. Since glycosylation is an essential step in product synthesis and secretion, increased glycosylation from the upregulated GalNT7 activity facilitates nephronectin glycosylation and secretion. As a consequence, osteoblast differentiation is promoted (Kahai et al., 2009). This notion was further confirmed in a subsequent publication, in which over expression of the nephronectin 3′UTR in pre-osteoblast cells promoted osteoblast differentiation by modulating miRNA functions (Lee et al., 2011). Several microRNAs (miR-23a, miR-101a, miR-296-5p, miR-328, miR-340-3p, and miR-425) can bind and directly target the nephronectin 3′UTR, resulting in enhanced osteoblast differentiation (Lee et al., 2011). Identifying a precise mechanism by which altered expression of nephronectin by miRNAs regulates osteoblast differentiation awaits further studies (Table 1).
Table 1.
miRNA–ECM relationships.
| ECM protein | microRNA | Disease process | References |
|---|---|---|---|
| Nephronectin | Mir-378 | Osteoblast differentiation | Kahai et al. (2009), Lee et al. (2011) |
| Collagen type I | miR-21 | Lung fibrogenesis | Li et al. (2011a) |
| Collagen type 1 alpha 1 | Let-7g | Hepatocellular carcinoma | Ji et al. (2010) |
| Collagen type 2 alpha 1 | Mir-301 | Breast cancer | Shi et al. (2011) |
| Collagen 1A1, 1A2, 3A1, 4A1, 4A2, 15A1 | miR-29c | Nasopharyngeal carcinomas | Sengupta et al. (2008) |
| Fibronectin, fibronectin type-III domain containing 3A | miR-17 | Growth retardation | Shan et al. (2009) |
| Fibronectin type III domain containing 3B | miR-143 | Metastasis of hepatitis B virus-related hepatocellular carcinoma | Zhang et al. (2009) |
| Fibronectin | miR-146a | Diabetes | Feng et al. (2011) |
| Fibronectin | miR-377 | Diabetic nephropathy | Wang et al. (2008b) |
| Fibronectin/versican | miR-199a* | Organ adhesion | Lee et al. (2009) |
| Versican | miR-138 | Disrupted cardiac morphogenesis | Morton et al. (2008) |
| Versican | miR-144 | Breast cancer | Lee et al. (2010) |
| Integrin β1 | miR-123 | Oral squamous carcinoma | Hunt et al. (2011) |
| Integrin β8 | miR-93 | Glioblastoma | Fang et al. (2011) |
| CD44 | miR-373, −520c, −216a, −330, −608, −328, −512-3p, −491 and −671 | Breast cancer | Jeyapalan et al. (2011), Rutnam and Yang (2012), Huang et al. (2008a) |
| CD44 | miR-34a | Pancreatic and prostate cancer cells | Liu et al. (2011), Pramanik et al. (2011) |
| E-cadherin | miR-495 | Breast cancer stem cells | Hwang-Verslues et al. (2011) |
| E-cadherin encoding mRNA, CDH1 | miR-92a | Esophageal squamous cell carcinoma | Chen et al. (2011) |
| EGFR | miR-128b | Non-small-cell lung cancer | Weiss et al. (2008) |
| ERBB2 and ERBB3 | miR-125a, −125b | Inhibition of cell motility and invasion | Scott et al. (2007) |
| MMP-1 | miR-203 | Synovial fibroblasts in rheumatoid arthritis | Stanczyk et al. (2011) |
| MMP-13 | miR-27b | Osteoarthritic chrondrocytes | Akhtar et al. (2010) |
| MMP-13, aggrecan, ADAMTS-5, collagen II | miR-146a | Human knee joint chondrocytes | Li et al. (2011b) |
| MMP-13 | miR-143 | Osteosarcoma | Osaki et al. (2011) |
| MMP-3 | miR-155 | Rheumatoid arthritic synovial fibroblasts | Stanczyk et al. (2008) |
| MMP-2 | miR-29b | Prostate cancer | Steele et al. (2010) |
| MMP-2 | miR-488 | Focal adhesion remodelling activity | Song et al. (2011) |
| MMP-2 and MMP-9 | miR-206, −340 | Breast cancer | Liu et al. (2010a) Wu et al. (2011) |
| MMP-9 | miR-340 | Glaucoma | Surgucheva et al. (2010) |
| MMP-9 | miR-212, −132 | Mammary gland development | Ucar et al. (2010) |
| MMP-9 | miR-218, −491-5p | Malignant glioma | Song et al. (2010), Yan et al. (2011) |
| MMP-9 | miR-338-3p | Hepatocellular carcinoma | Huang et al. (2011) |
| MMP-14 | Let-7 | Pancreatic ductal adenocarcinoma | Dangi-Garimella et al. (2011) |
| MMP-16 | miR-146b | Glioblastoma | Xia et al. (2009) |
| MMP-16 | miR-31 | Breast cancer | Valastyan et al. (2009) |
| TIMP3 | miR-21 | Breast cancer and cholangiocarcinomas | Gabriely et al. (2008), Selaru et al. (2009) |
| TIMP3 | miR-181, −181b | Heptaocellular carcinoma | Wang et al. (2010b) |
| TIMP3 | miR-221, −222 | Small cell lung cancer and hepatocellular carcinoma | Garofalo et al. (2009) |
The microRNA and extracellular matrix protein relationships involved in disease processes.
2.2. Collagen
The collagen family of proteins found in the ECM provides major structural integrity to tissues. There are approximately thirty distinct collagen genes in the human genome. These genes are transcribed and translated into proteins that join together in different ways to generate more than 20 distinct types of collagen fibrils. Collagen molecules are synthesized by many different cell types and there are a number of examples where collagen controls cell phenotypes. For example, the loss of epithelial polarity and increased tumor progression is promoted by increased ECM rigidity, which is caused by the increased deposition of fibrillar collagen, such as collagen type I (Col-1). There are numerous miRNAs involved in regulating collagen synthesis and accumulation. Col-1 has been reported to be upregulated by miR-21 in mammary gland and lung epithelial cell lines (Li et al., 2011a). Similarly, the upregulation of miR-21 is correlated with the increased accumulation of Col-1 in lung fibrogenesis (Li et al., 2011a). This upregulation of Col-1 causes a decrease in epithelial polarity as shown by the decreased formation of acinus structures, which are the in vitro equivalent of apical–basolateral polarity (Li et al., 2011a).
In breast cancer cells, the decrease of miR-301 results in decreased carcinogenesis, including diminished cell proliferation, migration, invasion, and tumor growth (Shi et al., 2011). Shi and colleagues reported that these functional changes were due to miR-301 targeting collagen type 2 alpha 1 (Col-2a1) and several other molecules including FOXF2, BBC3, and PTEN (Shi et al., 2011). This correlated with previous data showing that miR-301 overexpression is a negative clinical prognostic indicator in lymph node negative invasive ductal carcinoma of the breast (Shi et al., 2011).
Furthermore, the expression of multiple collagens is directly targeted by miRNAs let-7g, miR-29b and miR-29c, through an interaction with their 3′UTRs (Ji et al., 2010). In metastatic hepatocellular carcinomas (HCCs), let-7g expression is remarkably lower than that in normal cells. In addition, it was found to impede HCC cell motility by regulating the 3′UTR of Col-1α2 (COL1A2) (Ji et al., 2010). In nasopharyngeal carcinomas, miR-29c, which can target multiple collagens (collagen 1A1, 1A2, 3A1, 4A1, 4A2, 15A1) and laminin 1, is significantly decreased (Sengupta et al., 2008). In hepatic stellate cells, the expression of miR-29b is also decreased (Sekiya et al., 2011). The transfection of a miR-29b precursor significantly downregulates the expression of Col1A1 and Col1A2 mRNAs. miR-133a can directly target the 3′ UTR segment of Col1A1 (Castoldi et al., 2012). Furthermore, myocardial fibrosis was found to occur in Ang II-dependent hypertension which is regulated by the downregulation of miR-133a and miR-29b (Castoldi et al., 2012).
In mesangial cells, Kato and colleagues found that TGF-β1-induced expression of miR-192 and amplified the expression of Col 1A2 by downregulating ZEB2 (Kato et al., 2007). Furthermore, there is an amplified expression of miR-216a and miR-217 due to the downregulation of ZEB2. This also resulted in an augmentation of collagen production, downregulation of PTEN and the consequent activation of Akt and the development of diabetic nephropathy (Kato et al., 2009). Clearly these studies highlight not only the impact that collagen has on the structural integrity of the ECM surrounding cells but also illustrate that ECMs enriched in collagens can have a dramatic effect on the ability of cells to differentiate, proliferate, migrate and survive. Altered collagen accumulation around cells may impact cellular phenotype by promoting a rigid ECM which in turn could affect the proliferative and migratory activity of the cells. More experiments will be needed to decipher the actual mechanism(s) by which specific collagens, regulated by miRNA impact the behavior of cells.
2.3. Fibronectin
Fibronectin is a glycoprotein that is essential for wound healing and embryonic development. This ECM molecule binds to other ECM components such as integrins, collagens, fibrin and heparan sulfate proteoglycans to form complex ECM networks. These complexes serve as substrates for cell attachment and movement and impact the differentiation of certain cell types. The numerous isoforms of fibronectin are transcribed from a single gene, by alternative splicing at the pre-mRNA to create different isoforms. Fibronectin facilitates proliferation, adhesion, differentiation and motility. Altered fibronectin organization has been associated with a variety of diseases, including cancer, diabetes, and fibrosis. In the metastasis of hepatitis B virus-related hepatocellular carcinoma, miR-143 was significantly increased in transgenic mice and clinical specimens (Zhang et al., 2009). In addition, the upregulated expression of miR-143 favored liver tumor cell invasion and metastasis as local liver and distant lung metastases were dramatically decreased when miR-143 expression was inhibited (Zhang et al., 2009). These functions are directly due to the fact that the 3′UTR of fibronectin type III domain containing 3B, an important cell motility molecule, is directly suppressed by miR-143 (Zhang et al., 2009).
Recently, fibronectin levels in diabetic animals were found to be regulated by miR-146a (Feng et al., 2011). Feng and colleagues found that miR-146a binds to the 3′UTR of fibronectin and that miR-146a was concentrated in the retinal endothelial cells and was suppressed in animals with either type 1 and type 2 diabetes (Feng et al., 2011). Fibronectin was also found to be upregulated by miR-377 in diabetic nephropathy (Wang et al., 2008b). There was an increased amount of miR-377 found in mesangial cells cultured in high-glucose medium and diabetic glomeruli, which had decreased quantities of p21-activated kinase and superoxide dismutase proteins; which were due to miR-377 directly targeting their 3′UTRs (Wang et al., 2008b). Also, the downregulation of superoxide dismutase by miR-377 caused a greater oxidant production in mesangial cells (Wang et al., 2008b). These two proteins ultimately led to the enhancement in fibronectin protein production through a downstream signalling pathway caused by miR-377 (Wang et al., 2008b).
To study the role of miRNA in vitro and in vivo, we developed an expression vector that can stably overexpress a miRNA precursor (Lee et al., 2007). Using the vector, we expressed miR-17 in cells and in transgenic mice. The expression of miR-17 decreased endothelial cell adhesion, migration, and proliferation in vitro and overall growth retardation in transgenic mice (Shan et al., 2009). Further examination indicated that the expression of miR-17 can directly repress the expression of fibronectin and the fibronectin type-III domain containing 3A, both of which are targets of miR-17 (Shan et al., 2009). This represented the first report of a stably expressed miRNA precursor in both cells and mice. Since many miRNAs are expressed in clusters, it is difficult to dissect the precise function of an individual miRNA. This system allows the expression of a single miRNA precursor, providing an approach to study the function of a single miRNA. In addition, up to four copies of miR-17 are expressed, which can be used to increase the levels of ectopic miRNA expression.
2.4. Versican
Versican is a large chondroitin sulfate proteoglycan found in the ECM which controls numerous cellular events such as differentiation, migration, proliferation, and apoptosis (Wight, 2002; Kinsella et al., 2004; Wu et al., 2005; Xiang et al., 2006). Versican is prominent in the heart and vascular system and has a role in cardiomyocyte differentiation and heart development by influencing the migration and sorting of the cells that form the heart (Yao et al., 1994; Henderson and Copp, 1998; Chan et al., 2010). This ECM component increases in many pathological conditions including atherosclerosis and carcinogenesis (Wight and Merrilees, 2004; Ricciardelli et al., 2009).
The regulation of versican synthesis in development and disease can be controlled by miRNAs. For example, myocardin stimulates smooth muscle differentiation and induces the expression of miR-143, which attenuates versican expression by the smooth muscle cells (Wang et al., 2010c). The 3′-UTR of versican mRNA is a specific target of miR-143. Inhibiting versican expression by miR-143 inhibited the ability of the smooth muscle cells to migrate (Wang et al., 2010c). In zebrafish, miR-138 moderated the ventricular repression of the gene encoding versican and is required for establishing the correct chamber-specific gene expression patterns during cardiac morphogenesis (Morton et al., 2008). The inhibition of miR-138 resulted in the enhancement of gene expression in ventricular areas, which is naturally constrained to the atrio-ventricular valve region (Morton et al., 2008). This eventually led to the disrupted ventricular cardiomyocyte morphology and cardiac function (Morton et al., 2008).
While it has been well established that miRNA can regulate mRNA stability and translation, it is less clear whether miRNA function can be regulated. To explore this possibility, we initially used versican 3′UTR as a model by over expressing this sequence in a number of cell lines and in mice (Lee et al., 2009). In cells, the ectopic expression of the versican 3′UTR sequence increased cell adhesion. In transgenic mice expressing versican 3′UTR, organ adhesion was enhanced (Lee et al., 2009). The expressed versican 3′UTR could bind and modulate the functions of many, including miR-199a*, miR-136, miR-144, miR-133a, and miR-431 (Lee et al., 2009; Lee et al., 2010; Fang et al., 2012b). As a consequence, versican and fibronectin were upregulated in these tissues which functionally contributed to the adhesive phenotype (Lee et al., 2009; Fang et al., 2012b). The expression of versican 3′UTR can also lower the steady state expression of miR-199a-3p levels in the mouse breast carcinoma cell line, 4T1 (Lee et al., 2010). Other miRNAs such as miR-144 and miR-136 can also interact with the 3′UTR of versican as well as the Rb1 and PTEN 3′UTRs (Lee et al., 2010). The expression of versican, Rb1 and PTEN is promoted in the cells expressing versican 3′UTR, decreasing cell proliferation and tumor growth (Lee et al., 2010). In this way, the 3′ UTRs function as endogenous competing RNAs for binding and regulation of miRNA functions.
2.5. Small leucine-rich proteoglycans
The family of small leucine-rich proteoglycans (SLRPs) has a common multiple repeats of a leucine-rich structure motif that are flanks by cysteine residues. Proteins in this family include decorin, biglycan, lumican, fibromodulin, and keratocan. These proteins interact mostly with collagen by modifying their deposition and arrangement in the ECM. The research in the area of SLRPs and miRNAs is still expanding. Increased miR-21 expression in myometrial smooth muscle cells and leiomyoma smooth muscle cells results in reduced TGF-beta-induced expression of fibromodulin. This increases the growth rate of the cells while decreasing caspase-3/7 activity (Pan et al., 2010). Also the activity of decorin controls inflammation and tumor growth. This is because decorin can act as an endogenous ligand for Toll-like receptors 2 and 4. This leads to the production of proinflammatory molecules; PDCD4. Also, decorin decreases the activity of oncogenic miR-21, which is a translational inhibitor of PDCD4 (Merline et al., 2011). Such studies indicate that not only can miRNAs control the composition of the ECM but also that specific ECM components can influence the expression of specific miRNAs establishing a downstream relationship between miRNAs and their end point effects.
3. Regulation of cartilage differentiation
The interaction of the ECM with chondrocytes is important in the 3-dimensional organization of cartilage. Chondrogenesis of mesenchymal stem cells is accurately regulated by essential transcription factors and signalling cascades. During chondrogenic development and differentiation, miR-140 expression is increased and results in the inhibition of histone deacetylase 4 (Tuddenham et al., 2006; Miyaki et al., 2009). In addition, it is found that miR-145 appears to be a key negative regulator of chondrogenic differentiation since it decreases as a result of TGF-β3-induced chondrogenic differentiation of murine mesenchymal stem cells (Yang et al., 2011). miR-145 is reported to target the 3′UTR of SRY-related high mobility group-Box gene 9 (Sox9) gene which is a key transcription factor for chondrogenesis (Yang et al., 2011). In addition, overexpression of miR-145 decreased the expression of Sox9 only at protein levels and miR-145 inhibition significantly elevated Sox9 protein levels (Yang et al., 2011). Furthermore, overexpression of miR-145 decreased mRNA levels for chondrogenic marker genes, cartilage oligomeric matrix protein, type II collagen, aggrecan, type IX collagen and type XI collagen in C3H10T1/2 cells induced by TGF-β3, whereas anti-miR-145 inhibitor increased the expression of these chondrogenic marker genes (Yang et al., 2011). miR-221 regulates chondrogenic differentiation by inhibiting chondroprogenitor proliferation by targeting Mdm2 (Kim et al., 2010). In addition, chrondrogenic development is repressed by miR-199a, which regulates SMAD1 (Lin et al., 2009). Recently, Kim and colleagues reported the importance of miR-34 in the negative regulation of chondrocyte morphology (Kim et al. (2012)). It accomplishes this by regulating RhoA and RacI cross-talk and inhibits actin cytoskeleton reorganization (Kim et al., 2012). Thus many miRNAs are involved in the regulation of chondrogenesis by inhibiting important matrix molecules.
4. Regulation of ECM receptors
Integrins and non-integrin ECM receptors such as CD44 involved in cell–matrix interactions as well as junctional proteins such as cadherins involved in cell–cell interactions are under miRNA control. The expression and accumulation of these proteins in addition to their connection to the actin and intermediate cytoskeleton, control, in large part cell adhesion and migration.
4.1. Integrins
Integrins are another major group of proteins that are involved in cell-to-cell and cell-to-ECM adhesion and interaction. Integrins are composed of non-covalently associated α and β subunits. Integrins participate in a variety of events via their interaction with the ECM including intracellular signalling, regulating cytoskeletal formation and promoting cell adhesion and migration. Recently, miR-31 was shown to be a master modulator of integrins. Augoff and colleagues reported that miR-31 is involved in regulating multiple α subunit partners (α2, α5 and αV) of β1 integrins and also β3 integrins (Augoff et al. (2011)). Furthermore, overexpression of miR-31 in cancer cells resulted in a significant repression of mRNA and protein levels of these integrin subunits and affected cell spreading (Augoff et al., 2011). In addition, integrins also function in cell–cell adhesion indirectly by means of other intercellular ECM biomolecules. For example, α5β1 integrin binds to intercellular fibronectin and α6β1 integrin binds to intercellular laminin to facilitate cell adhesion. Another protein in the integrin family involved in cell migration is integrin β1. Hunt and colleagues reported that miRNA-123 can downregulate the expression of integrin β1 (Hunt et al. (2011)). This targeting resulted in reduced cell adhesion and motility of oral squamous carcinoma cells (Hunt et al., 2011). miR-183 was also shown to regulate the expression of integrin β1, which results in decreased adhesion to laminin, gelatin, and collagen type I in trabecular meshwork cells and human diploid fibroblasts (Li et al., 2010).
Integrin β8 was recently shown to be directly targeted by miR-93 (Fang et al., 2011). Overexpression of miR-93 promoted tumor growth, angiogenesis, and metastasis through enhanced endothelial cell spreading, migration, and tube-like structure formation (Fang et al., 2011; Fang et al., 2012a). These enhanced endothelial activities are important characteristics of angiogenesis. These phenotypic changes were due to miR-93 significantly decreasing integrin-β8 expression. The tumor cells with increased miR-93 and decreased integrin-β8 expression displayed an altered capacity for interaction with endothelial cells, contributing to tumor growth and angiogenesis.
4.2. CD44
CD44 is a cell-surface glycoprotein that plays a role in a variety of cell phenotypes, including cell migration, adhesion and cell-to-cell and cell-to-matrix interactions. CD44 can interact with hyaluronan and other ECM ligands such as collagens, osteopontin, and versican. In breast cancer, the standard isoforms of CD44 are involved in cell motility, invasion, and adhesion (Afify et al., 2009). Variant isoforms of CD44 also promote cell motility and invasion, to confer metastatic properties and to support tumor progression in diverse groups of cancers (Gunthert et al., 1991; Wielenga et al., 1993; Iida and Bourguignon, 1995; Ropponen et al., 1998; Marhaba and Zoller, 2004; Afify et al., 2009). While Huang and colleagues showed that miR-373 and miR-520c are able to target and downregulate CD44, which led to breast cancer cell invasion and changes in cell motility, it was unclear which isoforms of CD44 were the cause of this effect (Huang et al., 2008a). Recent work from our group has also found that miR-328 can bind and directly target the 3′UTR of CD44 (Wang et al., 2008a). This interaction causes a reduction in cell adhesion, aggregation and capillary formation (Wang et al., 2008a). MiR-328 modulated tissue morphogenesis could have occurred by regulating the binding interaction of CD44 with its ligand hyaluronan. Several other miRNAs, such as miR-34a, also target CD44 to decrease proliferation and increase apoptosis in pancreatic and prostate cancer cells (Liu et al., 2011; Pramanik et al., 2011). Furthermore, miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44 (Liu et al., 2011). CD117, also known as c-kit, performs an important function in endothelial progenitor cell motility and homing (Poliseno et al., 2006). miR-221 has been shown to regulate the expression of c-kit and inhibit human umbilical vein endothelial cell migration (Poliseno et al., 2006; Li et al., 2009). The suppression of migration was reversed by the downregulation of miR-221 (Li et al., 2009).
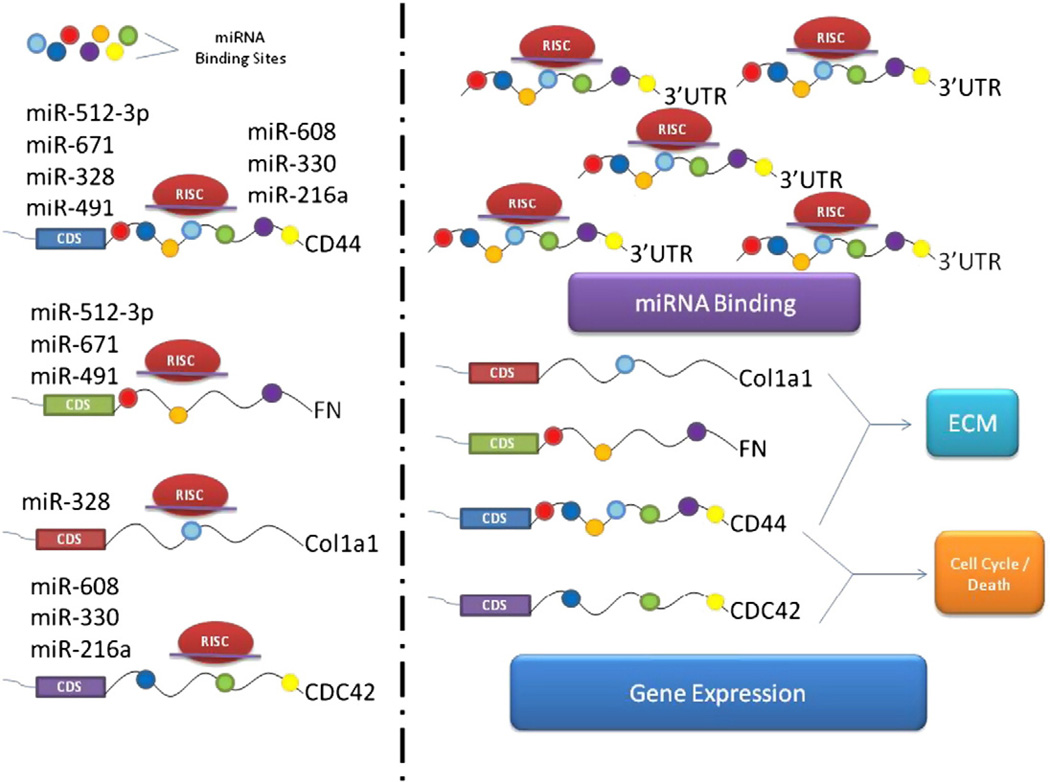
As indicated above, we used CD44 3′UTR as a model to confirm the role of a 3′UTR in regulating miRNA functions. Using a similar approach, we studied the modulation of multiple miRNAs by the CD44 3′UTR in modulating breast cancer cell activities (Jeyapalan et al., 2011; Rutnam and Yang, 2012). The 3′UTR can regulate miR-216a, miR-330 and miR-608, and thus increase CD44 and CDC42 protein levels and decrease cell proliferation and survival, with an increase in apoptosis and endothelial cell activities (Jeyapalan et al., 2011). The 3′UTR of CD44 could also be targeted by endogenous miR-328, miR-512-3p, miR-491 and miR-671, which resulted in inducing metastasis and regulating ECM functions by causing an increased expression of CD44, FN1, CDC42, and Col1a1 (Rutnam and Yang, 2012). A summary of the findings with the 3′UTR of CD44 is shown in Fig. 2. It was noted that the 3′UTRs of FN1, CDC42, and Col1a1 share common miRNA binding sites with the CD44 3′UTR. Overexpression of CD44 3′ UTR would arrest the functions of these miRNAs and promote the expression of the endogenous FN1, CDC42, and Col1a1. Since the expressed 3′ UTR is a fragment of existing mRNA, it is unlikely to cause potent side effects for the cells. This may serve as an approach for the future development of gene therapy.
Fig. 2.
Modulation of miRNAs by the CD44 3′UTR. In the absence of the exogenous 3′UTR, miRNAs and RISC are able to bind to the 3′UTRs of CD44, FN, Col1a1 and CDC42 and inhibit mRNA translation (left side). The 3′UTR of CD44 is able to bind and antagonize endogenous miRNAs, effectively allowing for related mRNAs, FN, Col1a1 and CDC42, along with CD44 to be expressed (right side).
4.3. E-cadherin
E-cadherin is a calcium-dependent cell–cell adhesion glycoprotein that is often found in conjunction with actin-containing filaments in the cytoskeleton (Watabe et al., 1994). It is an important transmembrane molecule involved with the ECM because of its role in maintaining cell-to-cell adhesion junctions (Turner and Burridge, 1991). In cancer and morphogenesis, the loss of E-cadherin weakens cell junction adhesion and promotes cell migration, which is a feature of epithelial–mesenchymal transition (EMT) (Takeichi, 1993). In metastasis, this allows cancer cells to traverse the basement membrane and invade surrounding tissues. In breast cancer stem cells, miR-495 has been reported to directly suppress E-cadherin expression, which resulted in promotion of cell invasion (Hwang-Verslues et al., 2011). Ma and colleagues reported that miR-9 can inhibit cell adhesion by directly targeting an E-cadherin encoding mRNA, CDH1 (Ma et al. (2010)). Furthermore, increased cell motility and invasiveness was observed due to E-cadherin repression by miR-9 (Ma et al., 2010). In esophageal squamous cell carcinoma, the CDH1 3′UTR was directly targeted by miR-92a, which caused a repression in CDH1 expression (Chen et al., 2011). This resulted in increased cell motility and invasion in esophageal squamous carcinoma (Chen et al., 2011). Furthermore, many miRNAs have been reported to affect E-cadherin expression through the transforming growth factor-β (TGF-β) pathway. Gregory and colleagues have reported that the miRNA-200 family (miR-200a, miR-200b, miR-200c, miR-141 and miR-429) are able to repress the expression of ZEB1 and ZEB2, an E-box repressor (Gregory et al., 2008). ZEB1 and ZEB2 are transcriptional repressors of E-cadherin and are able to induce EMT and tumor metastasis (Gregory et al., 2008). These miRNAs decreased the levels of ZEB1 and ZEB2 and initiated mesenchymal-to-epithelial transition (Gregory et al., 2008). The inhibition of these miRNAs in MDCK cells showed an increase in a mesenchymal-like phenotype, such as an elongated cell morphology and rearrangement of the actin cytoskeleton (Gregory et al., 2008). Furthermore, E-cadherin has also been reported to be upregulated by miR-192/215, which directly binds to and represses the 3′UTR of ZEB2 mRNA (Wang et al., 2010a). This translational repression of ZEB2 caused an increase in E-cadherin mRNA. However, there was no other change detected in ECM protein expression in this pathway.
5. Regulation of cytokines and growth factors affecting ECM composition and assembly
ECM modulation requires a large number of growth factors and cytokines. These molecules are activated by ECM constituents and can be released rapidly in an active state when required. Transmembrane receptors which bind these molecules are essential for extracellular responses and the regulation of the ECM assembly. The epidermal growth factor receptor or ErbB family comprised four members that are structurally related receptor tyrosine kinases: EGFR (ErbB1, HER1), ErbB2 (HER2), ErbB3 (HER3), and ErbB4 (HER4). Of these members, ErbB2 is the only one that does not link with the known ligands at high affinity; however, it is the favored heterodimeric partner for the other EGFRs and operates as a shared co-receptor (Olayioye, 2001). These receptors are capable of coupling to extracellular growth factor ligands for intracellular signalling pathways affecting cell motility, proliferation, and differentiation. The expression of these receptors can be directly modulated by miRNAs. EGFR is negatively regulated by miR-145 in human lung adenocarcinoma cells (Cho et al., 2011). Furthermore, miR-128b directly regulates EGFR in non-small-cell lung cancer cell lines and miR-128b is lost frequently in clinical samples (Weiss et al., 2008). In breast cancer cells, the 3′UTR of breast cancer metastasis suppressor 1 (BRMS1) can be directly regulated by miR-146a (Lin et al., 2008). This repression caused an inhibition in the cell migration, invasion, and metastasis in these cells. This regulation was found to suppress the targeting of the expression of EGFR (Lin et al., 2008). In addition, miR-125a- or miR-125b overexpression in SKBR3 cells exhibited a significant reduction in cell motility and invasion (Scott et al., 2007). The overexpression of miR-125a and miR-125b was reported to reduce both the transcript and protein levels of ERBB2 and ERBB3 in these cells (Scott et al., 2007). This leads to a reduction in the downstream signalling of ERK1/2 and AKT (Scott et al., 2007). Interestingly, upon EGF stimulation, miR-125 was also shown to be extensively repressed (Wang et al., 2009). Therefore, a positive feedback loop for EGFR signalling may exist and may be due to the interaction between EGF, miR-125 and ERBB2/3. The involvement of miRNAs in regulating signalling pathways through effects on signalling receptors highlights the potential therapeutic value in the use of miRNAs in targeting specific pathways in disease progression.
6. Regulation of ECM degradation and turnover
Numerous miRNAs modulate the expression of genes that regulate ECM turnover and degradation such as matrix metalloproteinases and their inhibitors. Turnover or remodelling of the ECM is critical for influencing cell phenotypic changes such as proliferation, migration, differentiation and apoptosis.
6.1. Matrix metalloproteinases
Matrix metalloproteinases (MMPs) are zinc-dependent endopeptidases that perform an essential role in ECM modelling, cell migration, adhesion, dispersion, and differentiation (Zitka et al., 2010). They are capable of inducing ECM protein degradation and cleavage of cell surface receptors (Chambers and Matrisian, 1997). There are 25 members in the MMP family which contain a catalytic domain, a pro-domain, and an active site domain which includes a highly conserved HEXGHXXGXXH motif (Chambers and Matrisian, 1997; Malemud, 2006). The three histidine residues in the motif are essential for the binding of zinc ligands and MMP activity (Nagase and Woessner, 1999; Polette et al., 2004). The MMP family is subdivided depending on the protein’s functional and structural properties. The four subdivisions of the MMP family are collagenases, stromelysins, gelatinases, and membrane-type matrix metalloproteinase (MT-MMPs) (Malemud, 2006; Zitka et al., 2010).
Collagenases (MMP-1, MMP-8, and MMP-13) instigate the breakdown of several native fibrillar collagens (Johansson et al., 1997; Nielsen et al., 2001). An increasing number of reports have shown that miRNAs modulate collagenase activities in rheumatoid arthritis and osteoarthritis. For example, an increased expression of miR-203 led to a considerable increase in MMP-1 levels through the NF-κB pathway in rheumatoid arthritic synovial fibroblasts. This elevation in levels contributes to synovial fibroblasts in rheumatoid arthritis (RA) having an activated phenotype (Stanczyk et al., 2011). Recently, Akhtar and colleagues reported that miR-27b downregulated MMP-13 in both normal and osteoarthritic chrondrocytes (Akhtar et al., 2010). miR-146a is also involved in osteoarthritis and cartilage degeneration. Overexpression of exogenous miR-146a significantly repressed MMP-13 and other ECM associated proteins, such as aggrecan, a disintegrin and metalloproteinase with thrombospondin motifs-5 (ADAMTS-5), and collagen II in human knee joint chondrocytes (Li et al., 2011b). In the osteosarcoma cells, the exogenous expression of miR-143 resulted in decreased cell invasion and downregulation of MMP-13 protein levels. This correlated with clinical samples, which showed that MMP-13 was present in lung metastasis-positive cases that had low miR-143 expression; however, MMP-13 was absent in cases with no metastasis and higher miR-143 expression (Osaki et al., 2011).
Stromelysins (MMP-3, MMP-10, and MMP-11) are capable of degrading a variety of substrates, such as collagen types IV, V, IX and X, elastin, fibronectin, gelatine, laminin, and proteoglycan core proteins (Polette et al., 2004). In rheumatoid arthritic synovial fibro-blasts, MMP-3 was directly repressed by the exogenous expression of miR-155. Additionally, miR-155 was involved in the induction of Toll-like receptor ligands and cytokines; these ligands and cytokines were able to further reduce the stimulation of MMPs-1 and 3 (Stanczyk et al., 2008). Additional investigation is needed on the effect of miRNAs on MMP-11 as it is not expressed in the normal tissues, but only in tumors. Gelatinases (MMP-2 and MMP-9), also called type IV collagenases, are involved in degrading denatured collagen, laminins, gelatine, and basement membrane components (Polette et al., 2004). In prostate cancer cells, MMP-2 was identified and confirmed to be a miR-29b target (Steele et al., 2010). Overexpression of miR-29b regulated cell growth by regulating pro-metastatic and anti-apoptotic matrix molecules (Steele et al., 2010). In human breast cancer, miR-206 was found to downregulate Cdc42, MMP-2 and MMP-9 protein levels (Liu et al., 2010a). This regulation of protein levels resulted in the suppression of cell invasion and migration of MDA-MB-231 cells due to the regulation of actin cytoskeleton remodelling such as filopodia formation (Liu et al., 2010a). Furthermore, aggressive behavior in specific breast cancer cell lines has been linked to the downregulation of miR-340 (Wu et al., 2011). The induction of miR-340 expression caused the suppression of breast tumor cell motility and invasion due to its direct targeting of c-Met, which resulted in the modulation of the expression of MMP-2 and MMP-9 (Wu et al., 2011).
In chrondrocytes, miR-468 was reported to repress cell motility and migration by regulating cell to ECM interactions (Song et al., 2011). This is a result of focal adhesion remodelling activity by MMP-2 (Song et al., 2011). This was further confirmed as shown that MMP-2 activity increased when anti-miRNA-488 oligonucleotides were introduced into the cells (Song et al., 2011).
MMP-9 is instrumental in various normal biological activities, and the expression of MMP-9 is regulated by many miRNAs. Alterations to normal MMP-9 protein levels are known to cause pathological processes. The abnormal expression of MMP-9 in retinal ganglion cells is associated with the pathology of glaucoma (Surgucheva et al., 2010). miR-340 controls the expression of MMP-9. Apoptosis of retinal ganglion cells is dependent on MMP-9-mediated changes of the ECM (Surgucheva et al., 2010). In mammary gland development, the miR-212/132 family is an important modulator of epithelial–stromal interactions (Ucar et al., 2010). miR-212 and miR-132 are expressed only in mammary stroma where they regulate the outgrowth of the epithelial ducts (Ucar et al., 2010). Both miR-212 and miR-132 directly target MMP-9 and in glands where they are absent, MMP-9 is overexpressed and accumulates around the ducts (Ucar et al., 2010). This overexpression is believed to obstruct the deposition of collagen and result in the hyperactivation of the TGF-β signalling pathway (Ucar et al., 2010).
In various types of cancers, MMP-9 has been shown to be regulated by numerous miRNAs. MMP-9 plays a major function in regulating the invasiveness of malignant glioma cells (Song et al., 2010; Yan et al., 2011). The increased expression of miR-218 decreases the cell motility and invasion of glioma cells (Song et al., 2010). This is a result of MMP-9 being directly downregulated by miR-218, which was reversed when miR-218 was inhibited (Song et al., 2010). IKK-β is also an important protein in the invasive function of glioma cells and its 3′UTR was directly targeted by miR-218 (Song et al., 2010). Furthermore, miR-218 was able to inactivate the NF-κB pathway through the downregulation of the IKK-β expression (Song et al., 2010). miR-885-5p and miR-491-5p overexpression was reported to repress MMP-9 expression and hinders cellular invasion in U87 and U251 glioma cells (Yan et al., 2011). This was correlated with the observation that the 3′UTR of MMP-9 was directly targeted by miR-491-5p to suppress glioma cell invasion (Yan et al., 2011). In hepatocellular carcinoma, miR-338-3p stimulates cell invasion and metastasis by downregulating MMP-9 expression (Huang et al., 2011). miR-338-3p was also found to directly target the 3′UTR of smoothened, transmembrane proteins involved in embryogenesis and the maintenance of tissue homeostasis.
Although MT1-MMPs (MMP-14, MMP-15, MMP-16, MMP-24, and MMP-25) are known to be the main physiological activators of pro-MMP-2, they can also break down types I–III collagens, fibronectin, fibrin, gelatin, laminins, nidogen, and cartilage proteoglycan core proteins (Hernandez-Barrantes et al., 2002). The overexpression of MMP-14 in pancreatic ductal adenocarcinoma cells resulted in decreased let-7 levels (Dangi-Garimella et al., 2011). The fibrosis that contributes to this cancer progression can be regulated due to MMP-14 acting in synergy with its substrate type I collagen to repress let-7 miRNA (Dangi-Garimella et al., 2011). Furthermore, miR-10b has an increased expression in glioma samples and this is directly correlated to the glioma’s pathological grade and malignancy (Sun et al., 2011). miR-10b induced glioma cell invasion by regulating tumor invasion factors MMP-14 and uPAR expression via the targeting of the 3′UTR of HOXD10 (Sun et al., 2011). Furthermore, invasive ability was lost when treated with specific miR-10b inhibitors (Sun et al., 2011). The miR-10b/HOXD10/MMP-14/uPAR signalling pathway was shown to influence the invasion of glioma cells (Sun et al., 2011).
The inhibition of MMP-16 by miR-146b transfection significantly suppresses invasion and cell motility, while not affecting proliferation, in human glioblastoma U373 cells (Xia et al., 2009). Furthermore, miR-31 also suppresses cell motility, metastasis, and invasion in breast cancer cells, MB-231 (Valastyan et al., 2009). This was shown to be a direct result of miR-31 targeting the 3′UTR of MMP-16, along with the 3′UTRs of frizzled3, integrin α5, myosin phosphatase-Rho interacting protein, radixin, and RhoA (Valastyan et al., 2009). In correlation with human samples, miR-31 was found to be decreased in breast carcinomas with metastasis (Valastyan et al., 2009).
6.2. Regulation of tissue inhibitor of metalloproteinases
Tissue inhibitor of matrix metalloproteinases (TIMPs) are molecules with two domains that control ECM remodelling, which are specific endogenous MMP inhibitors (Woessner, 1991). TIMPs are produced by many different types of cells and exist in most body fluids, cells, and tissues (Lambert et al., 2004). TIMPs are also able to inhibit adamalysins (ADAMs, and ADAMTS), which are also a class of metalloproteinases (Murphy et al., 2003). The TIMP family consists of four members (TIMP-1–4) that have a high structural homology (Gomez et al., 1997; Murphy et al., 2003). Of the four members, TIMP3 has been extensively investigated. In myogenesis, miR-206 mediated the suppression of TIMP3 expression (Liu et al., 2010b). In addition, TIMP3 downregulation caused the release of TNF-α, the activation of p38 MAPK, the expression of myogenic genes and the formation of myotubes (Liu et al., 2010b).
miR-21 regulates TIMP3 levels in breast cancer and controls breast cancer invasion. miR-21 can also control multiple genes associated with glioma cell apoptosis, migration, and invasiveness, such as reversion-inducing-cysteine-rich protein with kazal motifs protein (Gabriely et al., 2008). Furthermore, miR-21 also represses protein levels of TIMP3, along with programmed cell death 4, in cholangiocarcinomas (Selaru et al., 2009).
In hepatocellular carcinoma, TIMP3 is a target of miR-181 and miR-181b (Wang et al., 2010b). Wang and colleagues show that miR-181b enhanced MMP-2 and MMP-9 activities and promoted cell growth, survival, migration, and invasion that could be reversed by increasing TIMP3 protein levels (Wang et al., 2010b). miR-221 and miR-222 are overexpressed in invasive non-small cell lung cancer and hepatocellular carcinomas, compared to lung and liver cells that have lower invasiveness and a normal phenotype. As a result of miR-221 and miR-222 targeting PTEN and TIMP3, there is an enhanced cellular motility due to the activation of metallopeptidases and the AKT pathway (Garofalo et al., 2009).
7. Conclusion
miRNAs play important functional roles in the targeting of many proteins that are part of the ECM, since many of these proteins interact with cells and influence their phenotype. miRNAs have evolved as one of a number of different mechanisms that regulate cell activity and phenotype. The composition and organization of the ECM in large part control numerous biological events, such as cell proliferation, movement, and differentiation which ultimately affect development and wound healing. In addition, indirect targeting, through complex signalling pathways, has been shown to significantly affect ECM gene expression. For example, miR-377 upregulates fibronectin by directly targeting p21-activated kinase and superoxide dismutase proteins. Furthermore, numerous miRNAs are able to regulate multiple genes involved in the ECM and in various tissue types. For example, miR-221 has been reported to target c-kit, PTEN, and TIMP3. Equally, a single gene can be regulated by several or many miRNAs. Versican, fibronectin, collagens, MMP-2, MMP-9, and TIMP3, and numerous others mentioned in this review, have all been found to be regulated by more than one miRNA. These facts highlight how the integrity and structure of the ECM which are critical to development and disease can be controlled onmultiple levels. While there are a number of examples of specific miRNAs affecting cellular events through altering the composition and organization of the microenenvironment, the mechanism(s) by which these ECM changes regulate cellular phenotype have not been thoroughly investigated and more work is needed to sort this out. The discovery of miRNAs and their related ECM targets reveals important information about the complex molecular mechanisms involved in the structure, maintenance, and remodelling of the ECM. Utilizing this knowledge will be extremely important in developing strategies to target specific components of the ECM that contribute to the development of a variety of diseases.
Acknowledgments
The authors thank Dr. Virginia M. Green for her careful reading and manuscript preparation.
Abbreviations
- miRNA
microRNA
- ECM
extracellular matrix
- mRNA
messenger RNA
- MMP
matrix metalloproteinase
- TIMP
tissue inhibitor of metalloproteinases
- ncRNA
non-coding RNAs
- lncRNA
long non-coding RNA
- 3′UTR
3’-untranslated region
- Col-1
collagen type I
- HCC
hepatocellular carcinoma
- SLRP
small leucine-rich proteoglycans
- Sox9
SRY-related high mobility group-Box gene 9
- EMT
epithelial-mesenchymal transition
- BRMS1
breast cancer metastasis suppressor 1
- RA
rheumatoid arthritis
- ADAMTS-5
A disintegrin and metalloproteinase with thrombospondin motifs-5
Footnotes
This work was supported by grants from the Canadian Institutes of Health Research (MOP-102635 andMOP-111171) to Burton B. Yang who is the recipient of a Career Investigator Award (CI 7418) from the Heart and Stroke Foundation of Ontario and by NIH grants HL18645 and HL098067 to Thomas N. Wight
References
- Afify A, Purnell P, Nguyen L. Role of CD44s and CD44v6 on human breast cancer cell adhesion, migration, and invasion. Exp. Mol. Pathol. 2009;86:95–100. doi: 10.1016/j.yexmp.2008.12.003. [DOI] [PubMed] [Google Scholar]
- Aguda BD, Kim Y, Piper-Hunter MG, Friedman A, Marsh CB. MicroRNA regulation of a cancer network: consequences of the feedback loops involving miR-17-92, E2F, and Myc. Proc. Natl. Acad. Sci. U. S. A. 2008;105:19678–19683. doi: 10.1073/pnas.0811166106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akhtar N, Rasheed Z, Ramamurthy S, Anbazhagan AN, Voss FR, Haqqi TM. MicroRNA-27b regulates the expression of matrix metalloproteinase 13 in human osteoarthritis chondrocytes. Arthritis Rheum. 2010;62:1361–1371. doi: 10.1002/art.27329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altuvia Y, Landgraf P, Lithwick G, Elefant N, Pfeffer S, Aravin A, Brownstein MJ, Tuschl T, Margalit H. Clustering and conservation patterns of human microRNAs. Nucleic Acids Res. 2005;33:2697–2706. doi: 10.1093/nar/gki567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ameres SL, Martinez J, Schroeder R. Molecular basis for target RNA recognition and cleavage by human RISC. Cell. 2007;130:101–112. doi: 10.1016/j.cell.2007.04.037. [DOI] [PubMed] [Google Scholar]
- Augoff K, Das M, Bialkowska K, McCue B, Plow EF, Sossey-Alaoui K. miR-31 is a broad regulator of beta1-integrin expression and function in cancer cells. Mol. Cancer Res. 2011;9:1500–1508. doi: 10.1158/1541-7786.MCR-11-0311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baskerville S, Bartel DP. Microarray profiling of microRNAs reveals frequent coexpression with neighboring miRNAs and host genes. RNA. 2005;11:241–247. doi: 10.1261/rna.7240905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berezikov E, Guryev V, van de Belt J, Wienholds E, Plasterk RH, Cuppen E. Phylogenetic shadowing and computational identification of human microRNA genes. Cell. 2005;120:21–24. doi: 10.1016/j.cell.2004.12.031. [DOI] [PubMed] [Google Scholar]
- Bloomston M, Frankel WL, Petrocca F, Volinia S, Alder H, Hagan JP, Liu CG, Bhatt D, Taccioli C, Croce CM. MicroRNA expression patterns to differentiate pancreatic adenocarcinoma from normal pancreas and chronic pancreatitis. JAMA. 2007;297:1901–1908. doi: 10.1001/jama.297.17.1901. [DOI] [PubMed] [Google Scholar]
- Brandenberger R, Schmidt A, Linton J, Wang D, Backus C, Denda S, Muller U, Reichardt LF. Identification and characterization of a novel extracellular matrix protein nephronectin that is associated with integrin alpha8beta1 in the embryonic kidney. J. Cell Biol. 2001;154:447–458. doi: 10.1083/jcb.200103069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castoldi G, Di Gioia CR, Bombardi C, Catalucci D, Corradi B, Gualazzi MG, Leopizzi M, Mancini M, Zerbini G, Condorelli G, Stella A. MiR-133a regulates collagen 1A1: potential role of miR-133a in myocardial fibrosis in angiotensin II-dependent hypertension. J. Cell. Physiol. 2012;227:850–856. doi: 10.1002/jcp.22939. [DOI] [PubMed] [Google Scholar]
- Chambers AF, Matrisian LM. Changing views of the role of matrix metalloproteinases in metastasis. J. Natl. Cancer Inst. 1997;89:1260–1270. doi: 10.1093/jnci/89.17.1260. [DOI] [PubMed] [Google Scholar]
- Chan CK, Rolle MW, Potter-Perigo S, Braun KR, Van Biber BP, Laflamme MA, Murry CE, Wight TN. Differentiation of cardiomyocytes from human embryonic stem cells is accompanied by changes in the extracellular matrix production of versican and hyaluronan. J. Cell. Biochem. 2010;111:585–596. doi: 10.1002/jcb.22744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Stallings RL. Differential patterns of microRNA expression in neuroblastoma are correlated with prognosis, differentiation, and apoptosis. Cancer Res. 2007;67:976–983. doi: 10.1158/0008-5472.CAN-06-3667. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Zhao XH, Wang JW, Li BZ, Wang Z, Sun J, Tan FW, Ding DP, Xu XH, Zhou F, Tan XG, Hang J, Shi SS, Feng XL, He J. microRNA-92a promotes lymph node metastasis of human esophageal squamous cell carcinoma via E-cadherin. J. Biol. Chem. 2011;286:10725–10734. doi: 10.1074/jbc.M110.165654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chendrimada TP, Gregory RI, Kumaraswamy E, Norman J, Cooch N, Nishikura K, Shiekhattar R. TRBP recruits the Dicer complex to Ago2 for microRNA processing and gene silencing. Nature. 2005;436:740–744. doi: 10.1038/nature03868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho WC, Chow AS, Au JS. MiR-145 inhibits cell proliferation of human lung adenocarcinoma by targeting EGFR and NUDT1. RNA Biol. 2011;8:125–131. doi: 10.4161/rna.8.1.14259. [DOI] [PubMed] [Google Scholar]
- Chow TF, Youssef YM, Lianidou E, Romaschin AD, Honey RJ, Stewart R, Pace KT, Yousef GM. Differential expression profiling of microRNAs and their potential involvement in renal cell carcinoma pathogenesis. Clin. Biochem. 2010;43:150–158. doi: 10.1016/j.clinbiochem.2009.07.020. [DOI] [PubMed] [Google Scholar]
- Dalmay T, Edwards DR. MicroRNAs and the hallmarks of cancer. Oncogene. 2006;25:6170–6175. doi: 10.1038/sj.onc.1209911. [DOI] [PubMed] [Google Scholar]
- Dangi-Garimella S, Strouch MJ, Grippo PJ, Bentrem DJ, Munshi HG. Collagen regulation of let-7 in pancreatic cancer involves TGF-beta1-mediated membrane type 1-matrix metalloproteinase expression. Oncogene. 2011;30:1002–1008. doi: 10.1038/onc.2010.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Deng Z, Shatseva T, Yang J, Peng C, Du WW, Yee AJ, Ang LC, He C, Shan SW, Yang BB. MicroRNA miR-93 promotes tumor growth and angiogenesis by targeting integrin-beta8. Oncogene. 2011;30:806–821. doi: 10.1038/onc.2010.465. [DOI] [PubMed] [Google Scholar]
- Fang L, Du WW, Yang W, Rutnam ZJ, Peng C, Li H, O’Malley YQ, Askeland RW, Sugg S, Liu M, Mehta T, Deng Z, Yang BB. MiR-93 Enhances Breast Cancer Tumorigenesis by Targeting LATS2. Cell Cycle. 2012a;11(23) doi: 10.4161/cc.22670. (Electronic publication ahead of print, PMID: 23111389, 2012 Oct 30) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang L, Du WW, Yang X, Chen K, Ghanekar A, Levy G, Yang W, Lu WY, Xuan JW, Gao ZL, Xie F, He C, Deng Z, Yang BB. Versican 3’-Untranslated Region (3′UTR) functions as a ceRNA in inducing the development of hepatocellular carcinoma by regulating miRNA activity. FASEB J. 2012b doi: 10.1096/fj.12-220905. (Electronic publication ahead of print, PMID: 23180826, 2012 Dec 4) [DOI] [PubMed] [Google Scholar]
- Feng B, Chen S, McArthur K, Wu Y, Sen S, Ding Q, Feldman RD, Chakrabarti S. miR-146a–mediated extracellular matrix protein production in chronic diabetes complications. Diabetes. 2011;60:2975–2984. doi: 10.2337/db11-0478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foekens JA, Sieuwerts AM, Smid M, Look MP, de Weerd V, Boersma AW, Klijn JG, Wiemer EA, Martens JW. Four miRNAs associated with aggressiveness of lymph node-negative, estrogen receptor-positive human breast cancer. Proc. Natl. Acad. Sci. U. S. A. 2008;105:13021–13026. doi: 10.1073/pnas.0803304105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gabriely G, Wurdinger T, Kesari S, Esau CC, Burchard J, Linsley PS, Krichevsky AM. MicroRNA 21 promotes glioma invasion by targeting matrix metalloproteinase regulators. Mol. Cell. Biol. 2008;28:5369–5380. doi: 10.1128/MCB.00479-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garofalo M, Di Leva G, Romano G, Nuovo G, Suh SS, Ngankeu A, Taccioli C, Pichiorri F, Alder H, Secchiero P, Gasparini P, Gonelli A, Costinean S, Acunzo M, Condorelli G, Croce CM. miR-221&222 regulate TRAIL resistance and enhance tumorigenicity through PTEN and TIMP3 downregulation. Cancer Cell. 2009;16:498–509. doi: 10.1016/j.ccr.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Gauthier BR, Wollheim CB. MicroRNAs: ‘ribo-regulators’ of glucose homeostasis. Nat. Med. 2006;12:36–38. doi: 10.1038/nm0106-36. [DOI] [PubMed] [Google Scholar]
- Gomez DE, Alonso DF, Yoshiji H, Thorgeirsson UP. Tissue inhibitors of metalloproteinases: structure, regulation and biological functions. Eur. J. Cell Biol. 1997;74:111–122. [PubMed] [Google Scholar]
- Gottardo F, Liu CG, Ferracin M, Calin GA, Fassan M, Bassi P, Sevignani C, Byrne D, Negrini M, Pagano F, Gomella LG, Croce CM, Baffa R. Micro-RNA profiling in kidney and bladder cancers. Urol. Oncol. 2007;25:387–392. doi: 10.1016/j.urolonc.2007.01.019. [DOI] [PubMed] [Google Scholar]
- Gregory PA, Bert AG, Paterson EL, Barry SC, Tsykin A, Farshid G, Vadas MA, Khew-Goodall Y, Goodall GJ. The miR-200 family and miR-205 regulate epithelial to mesenchymal transition by targeting ZEB1 and SIP1. Nat. Cell Biol. 2008;10:593–601. doi: 10.1038/ncb1722. [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S, Saini HK, van Dongen S, Enright AJ. miRBase: tools for microRNA genomics. Nucleic Acids Res. 2008;36:D154–D158. doi: 10.1093/nar/gkm952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gunthert U, Hofmann M, Rudy W, Reber S, Zoller M, Haussmann I, Matzku S, Wenzel A, Ponta H, Herrlich P. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell. 1991;65:13–24. doi: 10.1016/0092-8674(91)90403-l. [DOI] [PubMed] [Google Scholar]
- Guttilla IK, White BA. Coordinate regulation of FOXO1 by miR-27a, miR-96, and miR-182 in breast cancer cells. J. Biol. Chem. 2009;284:23204–23216. doi: 10.1074/jbc.M109.031427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatfield SD, Shcherbata HR, Fischer KA, Nakahara K, Carthew RW, Ruohola-Baker H. Stem cell division is regulated by the microRNA pathway. Nature. 2005;435:974–978. doi: 10.1038/nature03816. [DOI] [PubMed] [Google Scholar]
- Henderson DJ, Copp AJ. Versican expression is associated with chamber specification, septation, and valvulogenesis in the developing mouse heart. Circ. Res. 1998;83:523–532. doi: 10.1161/01.res.83.5.523. [DOI] [PubMed] [Google Scholar]
- Hernandez-Barrantes S, Bernardo M, Toth M, Fridman R. Regulation of membrane type-matrix metalloproteinases. Semin. Cancer Biol. 2002;12:131–138. doi: 10.1006/scbi.2001.0421. [DOI] [PubMed] [Google Scholar]
- Houbaviy HB, Dennis L, Jaenisch R, Sharp PA. Characterization of a highly variable eutherian microRNA gene. RNA. 2005;11:1245–1257. doi: 10.1261/rna.2890305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang Q, Gumireddy K, Schrier M, le Sage C, Nagel R, Nair S, Egan DA, Li A, Huang G, Klein-Szanto AJ, Gimotty PA, Katsaros D, Coukos G, Zhang L, Pure E, Agami R. The microRNAs miR-373 and miR-520c promote tumour invasion and metastasis. Nat. Cell Biol. 2008a;10:202–210. doi: 10.1038/ncb1681. [DOI] [PubMed] [Google Scholar]
- Huang TH, Zhu MJ, Li XY, Zhao SH. Discovery of porcine microRNAs and profiling from skeletal muscle tissues during development. PLoS One. 2008b;3:e3225. doi: 10.1371/journal.pone.0003225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang XH, Chen JS, Wang Q, Chen XL, Wen L, Chen LZ, Bi J, Zhang LJ, Su Q, Zeng WT. miR-338-3p suppresses invasion of liver cancer cell by targeting smoothened. J. Pathol. 2011;225:463–472. doi: 10.1002/path.2877. [DOI] [PubMed] [Google Scholar]
- Hunt S, Jones AV, Hinsley EE, Whawell SA, Lambert DW. MicroRNA-124 suppresses oral squamous cell carcinoma motility by targeting ITGB1. FEBS Lett. 2011;585:187–192. doi: 10.1016/j.febslet.2010.11.038. [DOI] [PubMed] [Google Scholar]
- Hwang-Verslues WW, Chang PH, Wei PC, Yang CY, Huang CK, Kuo WH, Shew JY, Chang KJ, Lee EY, Lee WH. miR-495 is upregulated by E12/E47 in breast cancer stem cells, and promotes oncogenesis and hypoxia resistance via downregulation of E-cadherin and REDD1. Oncogene. 2011;30:2463–2474. doi: 10.1038/onc.2010.618. [DOI] [PubMed] [Google Scholar]
- Iida N, Bourguignon LY. New CD44 splice variants associated with human breast cancers. J. Cell. Physiol. 1995;162:127–133. doi: 10.1002/jcp.1041620115. [DOI] [PubMed] [Google Scholar]
- Iorio MV, Ferracin M, Liu CG, Veronese A, Spizzo R, Sabbioni S, Magri E, Pedriali M, Fabbri M, Campiglio M, Menard S, Palazzo JP, Rosenberg A, Musiani P, Volinia S, Nenci I, Calin GA, Querzoli P, Negrini M, Croce CM. MicroRNA gene expression deregulation in human breast cancer. Cancer Res. 2005;65:7065–7070. doi: 10.1158/0008-5472.CAN-05-1783. [DOI] [PubMed] [Google Scholar]
- Jeyapalan Z, Deng Z, Shatseva T, Fang L, He C, Yang BB. Expression of CD44 3′-untranslated region regulates endogenous microRNA functions in tumorigenesis and angiogenesis. Nucleic Acids Res. 2011;39:3026–3041. doi: 10.1093/nar/gkq1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji J, Zhao L, Budhu A, Forgues M, Jia HL, Qin LX, Ye QH, Yu J, Shi X, Tang ZY, Wang XW. Let-7g targets collagen type I alpha2 and inhibits cell migration in hepatocellular carcinoma. J. Hepatol. 2010;52:690–697. doi: 10.1016/j.jhep.2009.12.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansson N, Airola K, Grenman R, Kariniemi AL, Saarialho-Kere U, Kahari VM. Expression of collagenase-3 (matrix metalloproteinase-13) in squamous cell carcinomas of the head and neck. Am. J. Pathol. 1997;151:499–508. [PMC free article] [PubMed] [Google Scholar]
- Johnston RJ, Hobert O. A microRNA controlling left/right neuronal asymmetry in Caenorhabditis elegans . Nature. 2003;426:845–849. doi: 10.1038/nature02255. [DOI] [PubMed] [Google Scholar]
- Kahai S, Lee SC, Lee DY, Yang J, Li M, Wang CH, Jiang Z, Zhang Y, Peng C, Yang BB. MicroRNA miR-378 regulates nephronectin expression modulating osteoblast differentiation by targeting GalNT-7. PLoS One. 2009;4:e7535. doi: 10.1371/journal.pone.0007535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Zhang J, Wang M, Lanting L, Yuan H, Rossi JJ, Natarajan R. MicroRNA-192 in diabetic kidney glomeruli and its function in TGF-beta-induced collagen expression via inhibition of E-box repressors. Proc. Natl. Acad. Sci. U. S. A. 2007;104:3432–3437. doi: 10.1073/pnas.0611192104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato M, Putta S, Wang M, Yuan H, Lanting L, Nair I, Gunn A, Nakagawa Y, Shimano H, Todorov I, Rossi JJ, Natarajan R. TGF-beta activates Akt kinase through a microRNA-dependent amplifying circuit targeting PTEN. Nat. Cell Biol. 2009;11:881–889. doi: 10.1038/ncb1897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim D, Song J, Jin EJ. MicroRNA-221 regulates chondrogenic differentiation through promoting proteosomal degradation of slug by targeting Mdm2. J. Biol. Chem. 2010;285:26900–26907. doi: 10.1074/jbc.M110.115105. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kim D, Song J, Kim S, Park HM, Chun CH, Sonn J, Jin EJ. MicroRNA-34a modulates cytoskeletal dynamics through regulating RhoA/Rac1 cross-talk in chondroblasts. J. Biol. Chem. 2012;287:12501–12509. doi: 10.1074/jbc.M111.264382. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Kinsella MG, Bressler SL, Wight TN. The regulated synthesis of versican, decorin, and biglycan: extracellular matrix proteoglycans that influence cellular phenotype. Crit. Rev. Eukaryot. Gene Expr. 2004;14:203–234. doi: 10.1615/critreveukaryotgeneexpr.v14.i3.40. [DOI] [PubMed] [Google Scholar]
- Lambert E, Dasse E, Haye B, Petitfrere E. TIMPs as multifacial proteins. Crit. Rev. Oncol. Hematol. 2004;49:187–198. doi: 10.1016/j.critrevonc.2003.09.008. [DOI] [PubMed] [Google Scholar]
- Latronico MV, Catalucci D, Condorelli G. Emerging role of microRNAs in cardiovascular biology. Circ. Res. 2007;101:1225–1236. doi: 10.1161/CIRCRESAHA.107.163147. [DOI] [PubMed] [Google Scholar]
- Lee DY, Deng Z, Wang C, Yang BB. MicroRNA mir-378 promotes cell survival, tumor growth, and angiogenesis by targeting Sufu and Fus-1 expression. Proc. Natl. Acad. Sci. U. S. A. 2007;104:20350–20355. doi: 10.1073/pnas.0706901104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Shatseva T, Jeyapalan Z, Du WW, Deng Z, Yang BB. A 3′-untranslated region (3′UTR) induces organ adhesion by regulating miR-199a* functions. PLoS One. 2009;4:e4527. doi: 10.1371/journal.pone.0004527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee DY, Jeyapalan Z, Fang L, Yang J, Zhang Y, Yee AY, Li M, Du WW, Shatseva T, Yang BB. Expression of versican 3′-untranslated region modulates endogenous microRNA functions. PLoS One. 2010;5:e13599. doi: 10.1371/journal.pone.0013599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SC, Fang L, Wang CH, Kahai S, Deng Z, Yang BB. A non-coding transcript of nephronectin promotes osteoblast differentiation by modulating microRNA functions. FEBS Lett. 2011;585:2610–2616. doi: 10.1016/j.febslet.2011.07.016. [DOI] [PubMed] [Google Scholar]
- Lewis BP, Burge CB, Bartel DP. Conserved seed pairing, often flanked by adenosines, indicates that thousands of human genes are microRNA targets. Cell. 2005;120:15–20. doi: 10.1016/j.cell.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Li Y, Song YH, Li F, Yang T, Lu YW, Geng YJ. MicroRNA-221 regulates high glucose-induced endothelial dysfunction. Biochem. Biophys. Res. Commun. 2009;381:81–83. doi: 10.1016/j.bbrc.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Luna C, Qiu J, Epstein DL, Gonzalez P. Targeting of integrin beta1 and kinesin 2alpha by microRNA 183. J. Biol. Chem. 2010;285:5461–5471. doi: 10.1074/jbc.M109.037127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li C, Nguyen HT, Zhuang Y, Lin Y, Flemington EK, Guo W, Guenther J, Burow ME, Morris GF, Sullivan D, Shan B. Post-transcriptional up-regulation of miR-21 by type I collagen. Mol. Carcinog. 2011a;50:563–570. doi: 10.1002/mc.20742. [DOI] [PubMed] [Google Scholar]
- Li X, Gibson G, Kim JS, Kroin J, Xu S, van Wijnen AJ, Im HJ. MicroRNA-146a is linked to pain-related pathophysiology of osteoarthritis. Gene. 2011b;480:34–41. doi: 10.1016/j.gene.2011.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Zhang H, Chen Y. MicroRNA-mediated positive feedback loop and optimized bistable switch in a cancer network Involving miR-17-92. PLoS One. 2011c;6:e26302. doi: 10.1371/journal.pone.0026302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim LP, Lau NC, Garrett-Engele P, Grimson A, Schelter JM, Castle J, Bartel DP, Linsley PS, Johnson JM. Microarray analysis shows that some microRNAs downregulate large numbers of target mRNAs. Nature. 2005;433:769–773. doi: 10.1038/nature03315. [DOI] [PubMed] [Google Scholar]
- Lin SL, Chiang A, Chang D, Ying SY. Loss of mir-146a function in hormone-refractory prostate cancer. RNA. 2008;14:417–424. doi: 10.1261/rna.874808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin EA, Kong L, Bai XH, Luan Y, Liu CJ. miR-199a, a bone morphogenic protein 2-responsive MicroRNA, regulates chondrogenesis via direct targeting to Smad1. J. Biol. Chem. 2009;284:11326–11335. doi: 10.1074/jbc.M807709200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J, Valencia-Sanchez MA, Hannon GJ, Parker R. MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell Biol. 2005;7:719–723. doi: 10.1038/ncb1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H, Cao YD, Ye WX, Sun YY. Effect of microRNA-206 on cytoskeleton remodelling by downregulating Cdc42 in MDA-MB-231 cells. Tumori. 2010a;96:751–755. doi: 10.1177/030089161009600518. [DOI] [PubMed] [Google Scholar]
- Liu H, Chen SE, Jin B, Carson JA, Niu A, Durham W, Lai JY, Li YP. TIMP3: a physiological regulator of adult myogenesis. J. Cell Sci. 2010b;123:2914–2921. doi: 10.1242/jcs.057620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat. Med. 2011;17:211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowery AJ, Miller N, Devaney A, McNeill RE, Davoren PA, Lemetre C, Benes V, Schmidt S, Blake J, Ball G, Kerin MJ. MicroRNA signatures predict oestrogen receptor, progesterone receptor and HER2/neu receptor status in breast cancer. Breast Cancer Res. 2009;11:R27. doi: 10.1186/bcr2257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma L, Young J, Prabhala H, Pan E, Mestdagh P, Muth D, Teruya-Feldstein J, Reinhardt F, Onder TT, Valastyan S, Westermann F, Speleman F, Vandesompele J, Weinberg RA. miR-9, a MYC/MYCN-activated microRNA, regulates E-cadherin and cancer metastasis. Nat. Cell Biol. 2010;12:247–256. doi: 10.1038/ncb2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malemud CJ. Matrix metalloproteinases (MMPs) in health and disease: an overview. Front. Biosci. 2006;11:1696–1701. doi: 10.2741/1915. [DOI] [PubMed] [Google Scholar]
- Marhaba R, Zoller M. CD44 in cancer progression: adhesion, migration and growth regulation. J. Mol. Histol. 2004;35:211–231. doi: 10.1023/b:hijo.0000032354.94213.69. [DOI] [PubMed] [Google Scholar]
- Merline R, Moreth K, Beckmann J, Nastase MV, Zeng-Brouwers J, Tralhao JG, Lemarchand P, Pfeilschifter J, Schaefer RM, Iozzo RV, Schaefer L. Signaling by the matrix proteoglycan decorin controls inflammation and cancer through PDCD4 and microRNA-21. Sci. Signal. 2011;4:ra75. doi: 10.1126/scisignal.2001868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens-Talcott SU, Chintharlapalli S, Li X, Safe S. The oncogenic microRNA-27a targets genes that regulate specificity protein transcription factors and the G2-M checkpoint in MDA-MB-231 breast cancer cells. Cancer Res. 2007;67:11001–11011. doi: 10.1158/0008-5472.CAN-07-2416. [DOI] [PubMed] [Google Scholar]
- Miyaki S, Nakasa T, Otsuki S, Grogan SP, Higashiyama R, Inoue A, Kato Y, Sato T, Lotz MK, Asahara H. MicroRNA-140 is expressed in differentiated human articular chondrocytes and modulates interleukin-1 responses. Arthritis Rheum. 2009;60:2723–2730. doi: 10.1002/art.24745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morton SU, Scherz PJ, Cordes KR, Ivey KN, Stainier DY, Srivastava D. microRNA-138 modulates cardiac patterning during embryonic development. Proc. Natl. Acad. Sci. U. S. A. 2008;105:17830–17835. doi: 10.1073/pnas.0804673105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy G, Knauper V, Lee MH, Amour A, Worley JR, Hutton M, Atkinson S, Rapti M, Williamson R. Role of TIMPs (tissue inhibitors of metalloproteinases) in pericellular proteolysis: the specificity is in the detail. Biochem. Soc. Symp. 2003:65–80. doi: 10.1042/bss0700065. [DOI] [PubMed] [Google Scholar]
- Nagase H, Woessner JF., Jr. Matrix metalloproteinases. J. Biol. Chem. 1999;274:21491–21494. doi: 10.1074/jbc.274.31.21491. [DOI] [PubMed] [Google Scholar]
- Nielsen BS, Rank F, Lopez JM, Balbin M, Vizoso F, Lund LR, Dano K, Lopez-Otin C. Collagenase-3 expression in breast myofibroblasts as a molecular marker of transition of ductal carcinoma in situ lesions to invasive ductal carcinomas. Cancer Res. 2001;61:7091–7100. [PubMed] [Google Scholar]
- Olayioye MA. Update on HER-2 as a target for cancer therapy: intracellular signaling pathways of ErbB2/HER-2 and family members. Breast Cancer Res. 2001;3:385–389. doi: 10.1186/bcr327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osaki M, Takeshita F, Sugimoto Y, Kosaka N, Yamamoto Y, Yoshioka Y, Kobayashi E, Yamada T, Kawai A, Inoue T, Ito H, Oshimura M, Ochiya T. MicroRNA-143 regulates human osteosarcoma metastasis by regulating matrix metalloprotease-13 expression. Mol. Ther. 2011;19:1123–1130. doi: 10.1038/mt.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Q, Luo X, Chegini N. microRNA 21: response to hormonal therapies and regulatory function in leiomyoma, transformed leiomyoma and leiomyosarcoma cells. Mol. Hum. Reprod. 2010;16:215–227. doi: 10.1093/molehr/gap093. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Polette M, Nawrocki-Raby B, Gilles C, Clavel C, Birembaut P. Tumour invasion and matrix metalloproteinases. Crit. Rev. Oncol. Hematol. 2004;49:179–186. doi: 10.1016/j.critrevonc.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Poliseno L, Tuccoli A, Mariani L, Evangelista M, Citti L, Woods K, Mercatanti A, Hammond S, Rainaldi G. MicroRNAs modulate the angiogenic properties of HUVECs. Blood. 2006;108:3068–3071. doi: 10.1182/blood-2006-01-012369. [DOI] [PubMed] [Google Scholar]
- Pramanik D, Campbell NR, Karikari C, Chivukula R, Kent OA, Mendell JT, Maitra A. Restitution of tumor suppressor microRNAs using a systemic nanovector inhibits pancreatic cancer growth in mice. Mol. Cancer Ther. 2011;10:1470–1480. doi: 10.1158/1535-7163.MCT-11-0152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricciardelli C, Sakko AJ, Ween MP, Russell DL, Horsfall DJ. The biological role and regulation of versican levels in cancer. Cancer Metastasis Rev. 2009;28:233–245. doi: 10.1007/s10555-009-9182-y. [DOI] [PubMed] [Google Scholar]
- Rodriguez A, Griffiths-Jones S, Ashurst JL, Bradley A. Identification of mammalian microRNA host genes and transcription units. Genome Res. 2004;14:1902–1910. doi: 10.1101/gr.2722704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ropponen KM, Eskelinen MJ, Lipponen PK, Alhava E, Kosma VM. Expression of CD44 and variant proteins in human colorectal cancer and its relevance for prognosis. Scand. J. Gastroenterol. 1998;33:301–309. doi: 10.1080/00365529850170900. [DOI] [PubMed] [Google Scholar]
- Rutnam ZJ, Yang BB. The non-coding 3′UTR of CD44 induces metastasis by regulating extracellular matrix functions. J. Cell Sci. 2012;125:2075–2085. doi: 10.1242/jcs100818. [DOI] [PubMed] [Google Scholar]
- Ruvkun G. Clarifications on miRNA and cancer. Science. 2006;311:36–37. doi: 10.1126/science.311.5757.36d. [DOI] [PubMed] [Google Scholar]
- Saini HK, Griffiths-Jones S, Enright AJ. Genomic analysis of human microRNA transcripts. Proc. Natl. Acad. Sci. U. S. A. 2007;104:17719–17724. doi: 10.1073/pnas.0703890104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott GK, Goga A, Bhaumik D, Berger CE, Sullivan CS, Benz CC. Coordinate suppression of ERBB2 and ERBB3 by enforced expression of micro-RNA miR-125a or miR-125b. J. Biol. Chem. 2007;282:1479–1486. doi: 10.1074/jbc.M609383200. [DOI] [PubMed] [Google Scholar]
- Sekiya Y, Ogawa T, Yoshizato K, Ikeda K, Kawada N. Suppression of hepatic stellate cell activation by microRNA-29b. Biochem. Biophys. Res. Commun. 2011;412:74–79. doi: 10.1016/j.bbrc.2011.07.041. [DOI] [PubMed] [Google Scholar]
- Selaru FM, Olaru AV, Kan T, David S, Cheng Y, Mori Y, Yang J, Paun B, Jin Z, Agarwal R, Hamilton JP, Abraham J, Georgiades C, Alvarez H, Vivekanandan P, Yu W, Maitra A, Torbenson M, Thuluvath PJ, Gores GJ, LaRusso NF, Hruban R, Meltzer SJ. MicroRNA-21 is overexpressed in human cholangiocarcinoma and regulates programmed cell death 4 and tissue inhibitor of metalloproteinase 3. Hepatology. 2009;49:1595–1601. doi: 10.1002/hep.22838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sengupta S, den Boon JA, Chen IH, Newton MA, Stanhope SA, Cheng YJ, Chen CJ, Hildesheim A, Sugden B, Ahlquist P. MicroRNA 29c is downregulated in nasopharyngeal carcinomas, up-regulating mRNAs encoding extracellular matrix proteins. Proc. Natl. Acad. Sci. U. S. A. 2008;105:5874–5878. doi: 10.1073/pnas.0801130105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan SW, Lee DY, Deng Z, Shatseva T, Jeyapalan Z, Du WW, Zhang Y, Xuan JW, Yee SP, Siragam V, Yang BB. MicroRNA MiR-17 retards tissue growth and represses fibronectin expression. Nat. Cell Biol. 2009;11:1031–1038. doi: 10.1038/ncb1917. [DOI] [PubMed] [Google Scholar]
- Shi XB, Tepper CG, deVere White RW. Cancerous miRNAs and their regulation. Cell Cycle. 2008;7:1529–1538. doi: 10.4161/cc.7.11.5977. [DOI] [PubMed] [Google Scholar]
- Shi W, Gerster K, Alajez NM, Tsang J, Waldron L, Pintilie M, Hui AB, Sykes J, P’ng C, Miller N, McCready D, Fyles A, Liu FF. MicroRNA-301 mediates proliferation and invasion in human breast cancer. Cancer Res. 2011;71:2926–2937. doi: 10.1158/0008-5472.CAN-10-3369. [DOI] [PubMed] [Google Scholar]
- Song L, Huang Q, Chen K, Liu L, Lin C, Dai T, Yu C, Wu Z, Li J. miR-218 inhibits the invasive ability of glioma cells by direct downregulation of IKK-beta. Biochem. Biophys. Res. Commun. 2010;402:135–140. doi: 10.1016/j.bbrc.2010.10.003. [DOI] [PubMed] [Google Scholar]
- Song J, Kim D, Jin EJ. MicroRNA-488 suppresses cell migration through modulation of the focal adhesion activity during chondrogenic differentiation of chick limb mesenchymal cells. Cell Biol. Int. 2011;35:179–185. doi: 10.1042/CBI20100204. [DOI] [PubMed] [Google Scholar]
- Stanczyk J, Pedrioli DM, Brentano F, Sanchez-Pernaute O, Kolling C, Gay RE, Detmar M, Gay S, Kyburz D. Altered expression of MicroRNA in synovial fibroblasts and synovial tissue in rheumatoid arthritis. Arthritis Rheum. 2008;58:1001–1009. doi: 10.1002/art.23386. [DOI] [PubMed] [Google Scholar]
- Stanczyk J, Ospelt C, Karouzakis E, Filer A, Raza K, Kolling C, Gay R, Buckley CD, Tak PP, Gay S, Kyburz D. Altered expression of microRNA-203 in rheumatoid arthritis synovial fibroblasts and its role in fibroblast activation. Arthritis Rheum. 2011;63:373–381. doi: 10.1002/art.30115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steele R, Mott JL, Ray RB. MBP-1 upregulates miR-29b that represses Mcl-1, collagens, and matrix-metalloproteinase-2 in prostate cancer cells. Genes Cancer. 2010;1:381–387. doi: 10.1177/1947601910371978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L, Yan W, Wang Y, Sun G, Luo H, Zhang J, Wang X, You Y, Yang Z, Liu N. MicroRNA-10b induces glioma cell invasion by modulating MMP-14 and uPAR expression via HOXD10. Brain Res. 2011;1389:9–18. doi: 10.1016/j.brainres.2011.03.013. [DOI] [PubMed] [Google Scholar]
- Surgucheva I, Chidambaram K, Willoughby DA, Surguchov A. Matrix metalloproteinase 9 expression: new regulatory elements. J. Ocul. Biol. Dis. Inform. 2010;3:41–52. doi: 10.1007/s12177-010-9054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherins in cancer: implications for invasion and metastasis. Curr. Opin. Cell Biol. 1993;5:806–811. doi: 10.1016/0955-0674(93)90029-p. [DOI] [PubMed] [Google Scholar]
- Tuddenham L, Wheeler G, Ntounia-Fousara S, Waters J, Hajihosseini MK, Clark I, Dalmay T. The cartilage specific microRNA-140 targets histone deacetylase 4 in mouse cells. FEBS Lett. 2006;580:4214–4217. doi: 10.1016/j.febslet.2006.06.080. [DOI] [PubMed] [Google Scholar]
- Turner CE, Burridge K. Transmembrane molecular assemblies in cell-extracellular matrix interactions. Curr. Opin. Cell Biol. 1991;3:849–853. doi: 10.1016/0955-0674(91)90059-8. [DOI] [PubMed] [Google Scholar]
- Ucar A, Vafaizadeh V, Jarry H, Fiedler J, Klemmt PA, Thum T, Groner B, Chowdhury K. miR-212 and miR-132 are required for epithelial stromal interactions necessary for mouse mammary gland development. Nat. Genet. 2010;42:1101–1108. doi: 10.1038/ng.709. [DOI] [PubMed] [Google Scholar]
- Valastyan S, Reinhardt F, Benaich N, Calogrias D, Szasz AM, Wang ZC, Brock JE, Richardson AL, Weinberg RA. A pleiotropically acting microRNA, miR-31, inhibits breast cancer metastasis. Cell. 2009;137:1032–1046. doi: 10.1016/j.cell.2009.03.047. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- van Rooij E, Sutherland LB, Liu N, Williams AH, McAnally J, Gerard RD, Richardson JA, Olson EN. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. U. S. A. 2006;103:18255–18260. doi: 10.1073/pnas.0608791103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang CH, Lee DY, Deng Z, Jeyapalan Z, Lee SC, Kahai S, Lu WY, Zhang Y, Yang BB. MicroRNA miR-328 regulates zonation morphogenesis by targeting CD44 expression. PLoS One. 2008a;3:e2420. doi: 10.1371/journal.pone.0002420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Q, Wang Y, Minto AW, Wang J, Shi Q, Li X, Quigg RJ. MicroRNA-377 is up-regulated and can lead to increased fibronectin production in diabetic nephropathy. FASEB J. 2008b;22:4126–4135. doi: 10.1096/fj.08-112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang G, Mao W, Zheng S, Ye J. Epidermal growth factor receptor-regulated miR-125a-5p–a metastatic inhibitor of lung cancer. FEBS J. 2009;276:5571–5578. doi: 10.1111/j.1742-4658.2009.07238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Herman-Edelstein M, Koh P, Burns W, Jandeleit-Dahm K, Watson A, Saleem M, Goodall GJ, Twigg SM, Cooper ME, Kantharidis P. E-cadherin expression is regulated by miR-192/215 by a mechanism that is independent of the profibrotic effects of transforming growth factor-beta. Diabetes. 2010a;59:1794–1802. doi: 10.2337/db09-1736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang B, Hsu SH, Majumder S, Kutay H, Huang W, Jacob ST, Ghoshal K. TGFbeta-mediated upregulation of hepatic miR-181b promotes hepatocarcinogenesis by targeting TIMP3. Oncogene. 2010b;29:1787–1797. doi: 10.1038/onc.2009.468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Hu G, Zhou J. Repression of versican expression by microRNA-143. J. Biol. Chem. 2010c;285:23241–23250. doi: 10.1074/jbc.M109.084673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watabe M, Nagafuchi A, Tsukita S, Takeichi M. Induction of polarized cell-cell association and retardation of growth by activation of the E-cadherin-catenin adhesion system in a dispersed carcinoma line. J. Cell Biol. 1994;127:247–256. doi: 10.1083/jcb.127.1.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss GJ, Bemis LT, Nakajima E, Sugita M, Birks DK, Robinson WA, Varella-Garcia M, Bunn PA, Jr., Haney J, Helfrich BA, Kato H, Hirsch FR, Franklin WA. EGFR regulation by microRNA in lung cancer: correlation with clinical response and survival to gefitinib and EGFR expression in cell lines. Ann. Oncol. 2008;19:1053–1059. doi: 10.1093/annonc/mdn006. [DOI] [PubMed] [Google Scholar]
- Wickramasinghe NS, Manavalan TT, Dougherty SM, Riggs KA, Li Y, Klinge CM. Estradiol downregulates miR-21 expression and increases miR-21 target gene expression in MCF-7 breast cancer cells. Nucleic Acids Res. 2009;37:2584–2595. doi: 10.1093/nar/gkp117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wielenga VJ, Heider KH, Offerhaus GJ, Adolf GR, van den Berg FM, Ponta H, Herrlich P, Pals ST. Expression of CD44 variant proteins in human colorectal cancer is related to tumor progression. Cancer Res. 1993;53:4754–4756. [PubMed] [Google Scholar]
- Wight TN. Versican: a versatile extracellular matrix proteoglycan in cell biology. Curr. Opin. Cell Biol. 2002;14:617–623. doi: 10.1016/s0955-0674(02)00375-7. [DOI] [PubMed] [Google Scholar]
- Wight TN, Merrilees MJ. Proteoglycans in atherosclerosis and restenosis: key roles for versican. Circ. Res. 2004;94:1158–1167. doi: 10.1161/01.RES.0000126921.29919.51. [DOI] [PubMed] [Google Scholar]
- Woessner JF., Jr. Matrix metalloproteinases and their inhibitors in connective tissue remodeling. FASEB J. 1991;5:2145–2154. [PubMed] [Google Scholar]
- Wu Y, Wu J, Lee DY, Yee A, Cao L, Zhang Y, Kiani C, Yang BB. Versican protects cells from oxidative stress-induced apoptosis. Matrix Biol. 2005;24:3–13. doi: 10.1016/j.matbio.2004.11.007. [DOI] [PubMed] [Google Scholar]
- Wu F, Zikusoka M, Trindade A, Dassopoulos T, Harris ML, Bayless TM, Brant SR, Chakravarti S, Kwon JH. MicroRNAs are differentially expressed in ulcerative colitis and alter expression of macrophage inflammatory peptide-2 alpha. Gastroenterology. 2008;135(1624–1635):e1624. doi: 10.1053/j.gastro.2008.07.068. [DOI] [PubMed] [Google Scholar]
- Wu ZS, Wu Q, Wang CQ, Wang XN, Huang J, Zhao JJ, Mao SS, Zhang GH, Xu XC, Zhang N. miR-340 inhibition of breast cancer cell migration and invasion through targeting of oncoprotein c-Met. Cancer. 2011;117:2842–2852. doi: 10.1002/cncr.25860. [DOI] [PubMed] [Google Scholar]
- Xia H, Qi Y, Ng SS, Chen X, Li D, Chen S, Ge R, Jiang S, Li G, Chen Y, He ML, Kung HF, Lai L, Lin MC. microRNA-146b inhibits glioma cell migration and invasion by targeting MMPs. Brain Res. 2009;1269:158–165. doi: 10.1016/j.brainres.2009.02.037. [DOI] [PubMed] [Google Scholar]
- Xiang YY, Dong H, Wan Y, Li J, Yee A, Yang BB, Lu WY. Versican G3 domain regulates neurite growth and synaptic transmission of hippocampal neurons by activation of epidermal growth factor receptor. J. Biol. Chem. 2006;281:19358–19368. doi: 10.1074/jbc.M512980200. [DOI] [PubMed] [Google Scholar]
- Yan W, Zhang W, Sun L, Liu Y, You G, Wang Y, Kang C, You Y, Jiang T. Identification of MMP-9 specific microRNA expression profile as potential targets of anti-invasion therapy in glioblastoma multiforme. Brain Res. 2011;1411:108–115. doi: 10.1016/j.brainres.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Yang B, Guo H, Zhang Y, Chen L, Ying D, Dong S. MicroRNA-145 regulates chondrogenic differentiation of mesenchymal stem cells by targeting Sox9. PLoS One. 2011;6:e21679. doi: 10.1371/journal.pone.0021679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W, Lee DY, Ben-David Y. The roles of microRNAs in tumorigenesis and angiogenesis. Int. J. Physiol. Pathophysiol. Pharmacol. 2011;3:140–155. [PMC free article] [PubMed] [Google Scholar]
- Yao LY, Moody C, Schonherr E, Wight TN, Sandell LJ. Identification of the proteoglycan versican in aorta and smooth muscle cells by DNA sequence analysis, in situ hybridization and immunohistochemistry. Matrix Biol. 1994;14:213–225. doi: 10.1016/0945-053x(94)90185-6. [DOI] [PubMed] [Google Scholar]
- Zhang X, Liu S, Hu T, He Y, Sun S. Up-regulated microRNA-143 transcribed by nuclear factor kappa B enhances hepatocarcinoma metastasis by repressing fibronectin expression. Hepatology. 2009;50:490–499. doi: 10.1002/hep.23008. [DOI] [PubMed] [Google Scholar]
- Zitka O, Kukacka J, Krizkova S, Huska D, Adam V, Masarik M, Prusa R, Kizek R. Matrix metalloproteinases. Curr. Med. Chem. 2010;17:3751–3768. doi: 10.2174/092986710793213724. [DOI] [PubMed] [Google Scholar]