Abstract
Aedes (Finlaya) japonicus japonicus (Theobald) (Diptera: Culicidae) is recently invasive in North America and has expanded its range rapidly since 1998. Throughout its native and expanded range, Ae. j. japonicus larvae are commonly observed in many types of natural and artificial water-filled containers that vary in organic matter content and exposure to sunlight. Larvae are most often found in containers with decaying leaf material or algae, and we postulated that the added autocthonous primary production from algae could be both an important food source for larvae and an influential oviposition attractant to adult Ae. j. japonicus. We tested this hypothesis by placing plastic containers with varied levels of shading to manipulate algal density in the field, and then monitored oviposition by natural populations of Ae. j. japonicus. Over 99% of larvae hatching from eggs laid on the walls of our containers were Ae. j. japonicus, indicating that this species is a dominant colonizer of artificial containers in the study areas. Although full shading treatments effectively reduced algal biomass (significant reduction in chlorophyll a levels), at only one of three sites did this appear to affect Ae. j. japonicus oviposition. We conclude that algae in larval habitats are not a major factor in oviposition choices of adult Ae. j. japonicus females except when in situ primary production is high enough to substantially alter overall organic matter content cues.
Keywords: Aedes japonicus japonicus, algae, oviposition, container habitat
Aedes (Finlaya) japonicus japonicus (Theobald) (Diptera: Culicidae) is native to Japan, China, and Korea (Tanaka et al. 1979) and has recently invaded and expanded into parts of the United States, Canada, and Europe (Peyton et al. 1999, Thielman and Hunter 2006, Irish and Pierce 2008, Schaffner et al. 2009, Versteirt et al. 2009, Andreadis and Wolfe 2010, Cameron et al. 2010). Ae. j. japonicus is a species of medical importance because adults are competent vectors of a suite of encephalitis viruses, including Japanese encephalitis virus (Takashima and Rosen 1989), St. Louis encephalitis virus (Sardelis et al. 2003), Eastern Equine encephalitis virus (Sardelis et al. 2002), La-Crosse encephalitis virus (Sardelis et al. 2002), and West Nile virus (Sardelis and Turell 2001). This species is of special concern because it often occurs near human dwellings and has been implicated as a potential “bridge vector” of West Nile virus because of its tendency to blood feed on both mammals and birds (Williges et al. 2008, Molaei et al. 2009). Additionally, adult Ae. j. japonicus have been documented to occur in habitats with primary West Nile virus vectors Culex pipiens (L.) and Cx. restuans (Coquillett) (Chaves et al. 2011).
Larvae of Ae. j. japonicus are known to occur in a variety of natural and artificial water-filled containers spanning a wide range of compositions, sizes, and contents. Such diverse locations can include rock pools, tree holes, discarded tires, tarps, buckets, and cemetery vases, among others (Matuo 1961, Ebine 1969, Tanaka et al. 1979, Andreadis et al. 2001, Scott et al. 2001, Schaffner et al. 2009). Larval surveys in the United States as well as Japan indicate that Ae. j. japonicus colonizes containers spanning gradients of sunlight (Oliver et al. 2003, Joy and Sullivan 2005), elevation gradients (from 120 to 320 m; Iriarte et al. 1991), urban and rural settings (Iriarte et al. 1991, Joy and Sullivan 2005), and differing amounts and types of detrital content (Bevins 2007). The degree to which any specific habitat characters affect distribution of larvae is unclear, however, some authors have made note of Ae. j. japonicus larvae being particularly common in containers with algae or decaying leaves (Andreadis et al. 2001, Scott et al. 2001, Versteirt et al. 2009).
The availability of food resources is an important factor in determining the quality of larval habitats. Mosquito larvae are known to feed on heterotrophic microorganisms such as bacteria and fungi associated with decaying vegetation, as well as animal detritus, microcrustacea, rotifers, other larvae, and a wide variety of protists including algae (Merritt et al. 1992). Autocthonous primary production is generally considered secondary to allocthonous inputs of detritus in container habitats (Kitching 2001), but low levels of chlorophyll a indicative of algae have been measured in larval habitats such as tires and treeholes (Kaufman et al. 2001, Yee et al. 2010), and algae influence food web dynamics and are consumed by mosquito larvae in sun exposed tank-bromeliads (Brouard et al. 2011). Algae are commonly found in the dissected gut contents of mosquito larvae and are thought to be consumed in proportion to their abundance in the habitat (Howland 1930, Garros et al. 2008). Many algal taxa contain nutritious compounds that are required by mosquito larvae for growth, such as sterols and long-chain polyunsaturated fatty acids (Merritt et al. 1992). In addition, most algae are in the correct size range (1–50 μm) for ingestion by larval mosquitoes, and, as algae grow both in the water column and on submerged surfaces, are readily accessible through the different feeding modes employed by larvae (e.g., browsing, filtering, etc.; Merritt et al. 1992). Studies have noted associations between visible algae and increased mosquito larval density in several aquatic habitats (Bond et al. 2005, Barrera et al. 2006). We have observed algal blooms to be common in some containers used by Ae. j. japonicus larvae, thus algae may represent a primary or supplemental diet for this species.
The majority of studies of Ae. j. japonicus in its invasive range to date have addressed larval survival (i.e., presence and densities within surveyed habitats), with few examining oviposition habits (Scott 2003). Oviposition is primarily driven by female preferences based on cues of habitat suitability (Reiskind and Wilson 2004), and occurs in response to the physical and chemical stimuli that characterize a habitat (Bentley and Day 1989). Apart from perceiving the physical properties of the habitat (e.g., size, shape), certain mosquito species have been demonstrated as having the ability to detect signals from aquatic habitats that indicate the presence of conspecifics, predatory threats to larvae, and the availability of larval food resources, though responses to these signals are largely dependent upon the individual species in question (Edgerly et al. 1998, Reiskind and Wilson 2004, Silberbush et al. 2010, Warburg et al. 2011). In one study, response of Ae. triseriatus (Say) oviposition to conspecific larvae and eggs varied seasonally, with females generally avoiding laying eggs in habitats with conspecific larvae early in the season, and preferentially laying more eggs in habitats with conspecific larvae late in the season (Edgerly et al. 1998). Female Ae. aegypti (L.) tend to deposit more eggs in containers where conspecific larvae are present (Wong et al. 2011). An example of an effect of predator presence on mosquito oviposition is seen in Culiseta longiareolata (Macquart), which has been shown to respond negatively to hydrocarbons released by hemipteran predators (Silberbush et al. 2010). A similar response to the same hemipteran predator was seen in Anopheles gambiae (Giles) females, though this was apparently not mediated by hydrocarbons (Warburg et al. 2011).
In terms of resource detection, female Ae. triseriatus and Ae. albopictus (Skuse) seemingly are able to discriminate between different resource types (leaf species, leaf presence, or leaf absence) in larval habitats, which may then affect production of adult mosquitoes (Reiskind et al. 2009). Cx. quinquefaciatus (Say) oviposition is enhanced by increased nutrient content in larval habitats (Chaves et al. 2009). Although not a container mosquito, Anopheles pseudopunctipennis (Theobald) is able to distinguish between real algal filaments and analogs in the laboratory, and prefers real algae for oviposition (Torres–Estrada et al. 2007), suggesting an ability to detect viable larval resources. Oviposition responses of female Ae. j. japonicus to various habitat parameters including potential larval food items have thus far not been examined in detail, though Lee and Kokas (2004) demonstrated that a larger number of Ae. j. japonicus females were collected from Centers for Disease Control and Prevention gravid traps baited with lawn sod than with rabbit chow, indicating that this species may have the potential for selective oviposition based on available resources.
In this study we postulated that gravid Ae. j. japonicus females are attracted to algae and the associated changes of increased primary production when searching for sites in which to oviposit. We predicted that females would deposit greater numbers of eggs in habitats with higher algal biomass when offered a choice between containers with dense algal growth and containers with low or no algae. To test our hypothesis, we manipulated algal densities in oviposition containers in the field by altering light levels, and measured the ovipositional response of natural Ae. j. japonicus populations in the area.
Materials and Methods
Study Sites
The experiment was conducted at three field sites near the campus of Michigan State University (East Lansing, MI). The first site was located in a grassy field adjacent to Toumey Woodlot on Michigan State University (MSU) southern campus. The second site was located on the grounds of the Lower River Lab, part of the North Central Regional Aquaculture Center at MSU. The facility contains a large lawn with an experimental pond and small adjoining woodland, and is situated along the Red Cedar River. Our third field site was at Potter Park Zoo in Lansing, MI. The Potter Park Zoo is situated along the Red Cedar River ≈5 km to the west of MSU. All three sites featured a relatively open canopy and received direct sunlight during most of the day. Each site was found to have water-filled containers with Ae. j. japonicus larvae present within 30 m of the experimental plots. Experimental trials were conducted from 27 June through 4 July and repeated from 12 August through 19 August during the summer of 2010.
Experimental Design
Oviposition containers consisted of shallow plastic buckets measuring 31 cm long × 20 cm wide × 17 cm tall. The buckets were dark green in color, which prevented most light infiltration from the sides of the container and limited light to entering the container from above. To manipulate algal abundance we constructed canopies that consisted of a hardware cloth frame covered by a combination of plastic sheeting and shade cloth (Gempler’s s, Madison, WI). Each canopy measured 91 × 61 cm, with a 7-cm lip along each side, covering the containers completely and extending 20–30 cm beyond. Canopies were propped above each container and secured by a ground stake at each corner. Three shade treatments were used in the experiment: full sun (canopy consisted of a hardware cloth frame covered by clear plastic sheeting), partial shade (frame covered by Gempler’s 60% light reduction cloth and clear plastic sheeting), and full shade (frame covered by Gempler’s 80% light reduction cloth and two layers of black plastic). Photometer measurements taken before the June trial showed that the partial and full shade treatments reduced light penetration by 70 and 100%, respectively, compared with full sun treatments. Each light treatment was replicated three times at each of the three field sites, for a total of 27 containers. At each field site, experimental containers were situated into three groups of three containers, with one container of each treatment in each group. Each group was arranged in a line along an edge (border of the lawn against trees, shrubs, or a fence) to minimize human disturbance. The order of the treatments within the lines was randomized.
Each container included 4 liters of distilled water and 3 g of dried mixed oak and beech litter collected from the floor of Hudson Woodland, MSU Campus. In addition, 40 ml (1:100 concentration) of a solution of inorganic nutrients and glucose (5 ppm nitrate-N, 0.5 ppm phosphate-P, 5 ppm sulfate-S, and 20 ppm glucose; Kaufman et al. 2002), were added to encourage the development of biofilms. In the August trial, three unglazed ceramic tiles measuring 6.35 × 6.35 cm were also placed in each container for later quantification of benthic algal growth. Containers were inoculated with 4 ml of an algal inoculum collected from a water-filled scrap tire in Hudson Woodland. The algal inoculum was composed primarily of filamentous Chlorophytes and Scenedesmus. The containers were then covered with white tulle to prevent mosquito access and allowed to incubate under their respective shade treatments for a period of 10 d. After that time, the white tulle was removed and egg collections began. To determine the degree to which the shade treatments altered water temperature within the containers, the temperature was measured every 30 min in one full sun and one full shade container during each experimental trial using submerged temperature loggers (TidbiT v2 Temperature Logger, Onset, Bourne, MA).
Egg Collection and Processing
Ovipositional activity of Ae. j. japonicus females is greatest during the hours just after dusk and just before dawn (Scott 2003). To avoid any confounding effects of shading by the canopies themselves on oviposition behavior, canopies were removed at dusk and replaced at dawn during experimental trials. The inside of each container was lined with seed germination paper to facilitate egg collection, as Ae. j. japonicus oviposits on vertical surfaces just above the water line. At dawn on each day of each experimental trial, egg-laden papers from the previous night were collected and canopies were replaced. At dusk, canopies were removed and the sides of the containers were carefully checked for any eggs that had been laid during the day, before new oviposition papers were added to the containers. Any eggs that had been laid during the daytime were collected, placed onto damp paper towels in self-sealing plastic bags, and transported back to the laboratory. Upon collection at dawn, egg papers were placed into self-sealing plastic bags and returned to the laboratory. All eggs were counted under a dissecting microscope and stored for 10 d at 25 ± 1°C under a photoperiod of 16:8 (L:D) h regime before being hatched in broth composed of distilled water, yeast, and Proflo (Dulmage 1971). Larvae were reared to the third or fourth instar, then killed and preserved in a solution of 70% ethanol. All larvae were later identified to species according to Darsie and Ward (2005) and counted under a dissecting microscope. We hatched and identified larvae from our eggs to distinguish eggs of Ae. j. japonicus from other species.
Algal Sampling and Processing
Limnetic algae were sampled for biomass estimates (chlorophyll a analysis) on two dates during each experimental trial: once on the first day egg papers were collected, and once on the day after the last egg papers had been collected. However, the first day samples in the June trial were lost because of equipment failure, and only the final (posttrial) values for each trial are presented here. Limnetic algae were collected by submerging a 50-ml centrifuge tube in the center of each container an inch below the water’s surface. Limnetic samples were stored on ice under dark conditions until they could be processed in the laboratory. Each sample was poured into a large petri dish and gently mixed to homogenize. A small amount of sample (3–10 ml) was vacuum-filtered onto a glass fiber filter using a 12-well sampling manifold. The filter was immediately frozen at −20°C and stored under dark conditions for later analysis of chlorophyll a. Benthic samples were collected from the August trial only. One ceramic tile was randomly removed on the first (pretrial) and last (posttrial) day of egg collections from each container, and placed into individual self-sealing plastic bags. Tiles were stored on ice under dark conditions until they could be processed in the laboratory later that day. Biofilms were removed from each tile by scraping with a razor blade followed by a toothbrush, and brought up to a known volume with deionized water. The sample was filtered onto a glass fiber filter (Pall Life Sciences, Ann Arbor, MI) using a 60-ml syringe with a filter attached. The filter was immediately stored as above for limnetic samples. Frozen filters were submerged in 90% ethanol and extracted overnight at 4°C under dark conditions, before being quantified on a TD-700 fluorometer (Turner Designs, Sunnyvale, CA). Samples were acidified and fluorescence measured again to correct for pheophytin (protocol adapted from Arar and Collins 1997).
Statistical Analysis
Our experiment was a balanced design with one fixed effect (shade treatment) and two random effects (trial date and site). Because of the balanced design with no missing values, we fitted a simple linear mixed effects model via restricted maximum likelihood to estimate error and random effects variance, testing the significance of the main effects against an F distribution (Potvin 2001, Bolker et al. 2009, Chaves 2010). Our initial statistical and graphical analyses showed that site variation was considerable for both oviposition parameters and chlorophyll a, so we also used a mixed model to examine oviposition parameters at each site. We log-transformed our data on total number of eggs and total number of Ae. j. japonicus hatched, and used an arcsine-square-root transformation on the percentage (proportion) of eggs that hatched into Ae. j. japonicus larvae to achieve normality. Primarily because of zero values in the full shade treatment, limnetic chlorophyll a could not be transformed to yield a normal distribution and these data were analyzed using nonparametric methods (Wilcoxon/Kruskal–Wallis rank tests). Similarly, because of distributional issues with the full chlorophyll a dataset, we used a nonparametric correlation method (Spearman’s ρ), to examine overall relationships between oviposition variables and chlorophyll a. We applied sequential Bonferroni adjustments to significance levels of P values in table-wise comparisons (Rice 1989). Statistical analyses were performed with SAS and JMP software (www.sas.com, www.jmp.com, SAS Institute, Cary, NC).
Results
Shading treatment significantly affected limnetic and benthic chlorophyll a levels (Table 1), but the differences between partial shading and full sun were negligible, and site differences were also evident, particularly at Toumey where differences between fully shaded and full sun or partial shade were most pronounced (Fig. 1). Subsequent comparisons of treatments excluding full shade showed significant differences in limnetic chlorophyll a levels between sites (Wilcoxon/Kruskal–Wallis rank tests, P values of 0.0195 and 0.0292 for full sun and partial shade, respectively). Water temperatures in the full shade treatment averaged 16.4 (SD = ±5.3) and 21.6 (SD = ±2.5)°C in the June and August trials, respectively, while full sun water temperature averaged 19.5 (SD = ±8.0) and 23.3 (SD = ±4.6)°C, respectively. Higher temperatures associated with the full sun treatment were pronounced, as would be expected, only during daylight hours, and night period temperatures, when most oviposition likely occurred, were almost identical in the two treatments.
Table 1. Results of Wilcoxon/Kruskal–Wallis rank tests of shade treatment on limnetic and benthic chlorophyll a levels (log-transformed) in oviposition containers (site and trial effects are also listed).
| df | Limnetic chlorophyll a
|
Benthic chlorophyll a
|
|||
|---|---|---|---|---|---|
| χ2 | P value | χ2 | P value | ||
| Treatment | 2 | 35.6802 | <0.0001a | 17.4286 | 0.0002a |
| Site | 2 | 4.8837 | 0.0870 | 1.2734 | 0.5290 |
| Trial | 1 | 0.6226 | 0.4301 | — | — |
Significant with Bonferroni adjustment.
Fig. 1.
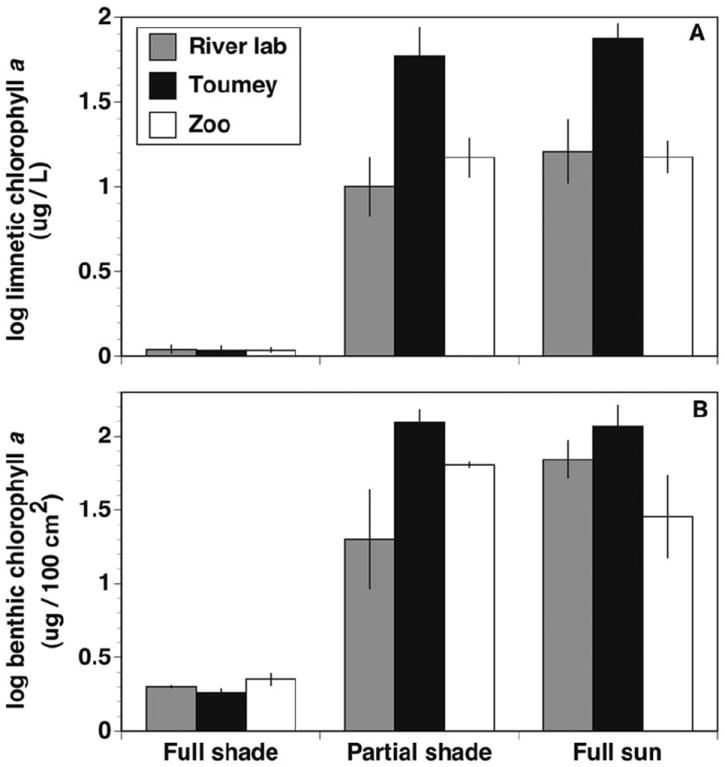
Mean (±1 SE) limnetic chlorophyll a (log μg/L) per container, measured posttrial (A), and benthic chlorophyll a (log μg/100 cm2) per container for trial 2, averaged pre- and posttrial (B); n = 6 for panel A, n = 3 for panel B.
We hatched a total of 58,062 larvae from 83,368 eggs, of which 57,697 were Ae. j. japonicus, 351 were Ae. triseriatus, 4 were Anopheles quadrimaculatus, and estimated fewer than 10 were Cx. restuans. Thus, at least 69% of the eggs we collected were Ae. j. japonicus, but this did not significantly vary with treatment, and site and trial random variance components were relatively low for this parameter compared with their contributions in the model for total eggs or total Ae. j. japonicus hatch (Table 2). The number of Ae. j. japonicus larvae hatched closely tracked the number of eggs collected (linear regression slope = 0.85; r2 = 0.94; P = <0.0001; data not illustrated).
Table 2. Mixed-model analysis results for shade treatment effect on total egg numbers, numbers of Ae. j. japonicus hatched (both variables log-transformed), and proportion Ae. j. japonicus hatched (arcsine-square root transformed).
| Fixed effect (shade treatment)
|
Random effects variance component estimates (% total)
|
||||||
|---|---|---|---|---|---|---|---|
| df | df den | F | P value | Site | Trial | Residual | |
| Total eggs | 2 | 48 | 2.3310 | 0.1079 | 62.7 | 9.1 | 28.2 |
| Total hatch | 2 | 48 | 3.4912 | 0.0384 | 65.2 | 6.6 | 28.2 |
| Proportion hatched | 2 | 48 | 1.8960 | 0.1692 | 13.9 | 6.7 | 79.5 |
Variance component estimates for random effects in each analysis are also listed (den, denominator). After sequential Bonferroni adjustment, no P value listed was considered significant.
Our primary Ae. j. japonicus oviposition measures, total eggs laid and total larval hatch, were also not significantly affected by treatment as indicated by mixed model analysis results (Table 2). However, as with chlorophyll a measures, there was considerable variation associated with site (Fig. 2; Table 2) and subsequent reanalysis of treatment effects within each site showed that the number of eggs collected was significantly affected by treatment (Table 3). Had we conducted the experiment only at Toumey, we would have concluded that shading significantly affected both the number of eggs collected and the number of Ae. j. japonicus larvae hatching. In all cases, trial contributed little to overall random variation in oviposition measures (Tables 2 and 3).
Fig. 2.
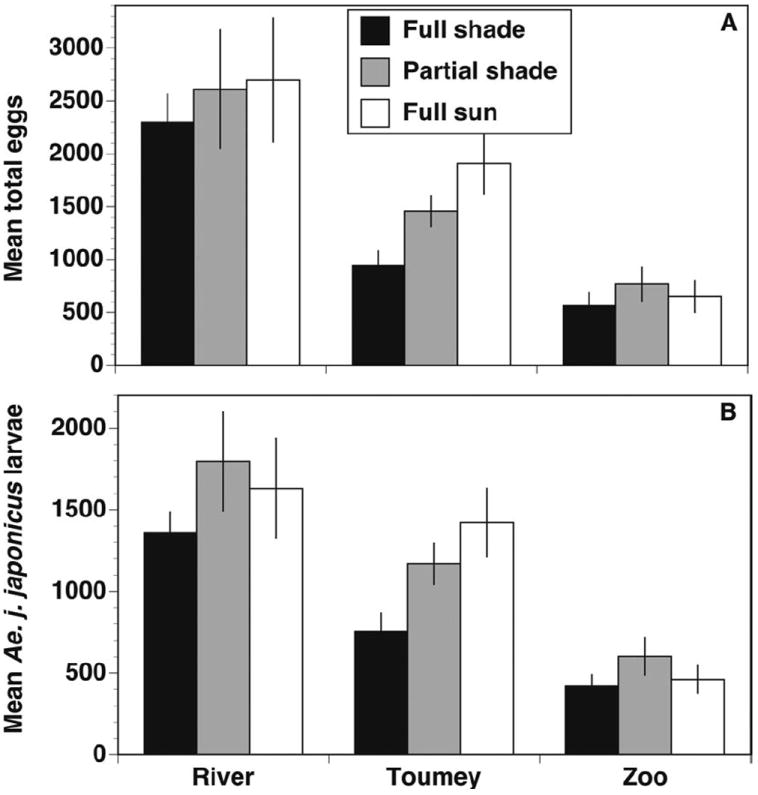
Mean (±1 SE) total number of eggs laid per container (A) and mean (±1 SE) total number of hatched Ae. j. japonicus individuals per container (B), with experimental trials one and two combined; n = 6 for all values.
Table 3. Mixed-model analysis results for shade treatment effects on total egg numbers and numbers of Ae. j. japonicus hatched (both variables log-transformed) within each site.
| Fixed effect (shade treatment)
|
Random effects variance component estimates (% total)
|
|||||
|---|---|---|---|---|---|---|
| df | df den | F | P value | Trial | Residual | |
| River lab | ||||||
| Total eggs | 2 | 14 | 0.0914 | 0.9132 | 35.1 | 64.9 |
| Total hatch | 2 | 14 | 0.6377 | 0.5432 | 36.6 | 63.4 |
| Toumey | ||||||
| Total eggs | 2 | 14 | 7.202 | 0.0071a | 12.2 | 87.8 |
| Total hatch | 2 | 14 | 6.2051 | 0.0118 | 26.5 | 73.5 |
| Zoo | ||||||
| Total eggs | 2 | 14 | 0.3110 | 0.7377 | 0.0 | 100 |
| Total hatch | 2 | 14 | 0.5281 | 0.6010 | 0.0 | 100 |
Variance component estimates for random effects in each analysis are also listed (den, denominator).
Significant with Bonferroni adjustment.
Overall associations of limnetic chlorophyll a with either total eggs or Ae. j. japonicus hatch numbers were only weakly positive and not significant using Spearman’s rank test, although Toumey site data more closely approached a nonzero slope (Table 4; Fig. 3).
Table 4. Results of Spearman’s ρ analysis of associations between limnetic chlorophyll a and total eggs laid or total Ae. j. japonicus hatch.
| Spearman’s ρ | P value | |
|---|---|---|
| Total eggs | 0.2239 | 0.1036 |
| Total hatch | 0.2486 | 0.0699 |
Fig. 3.
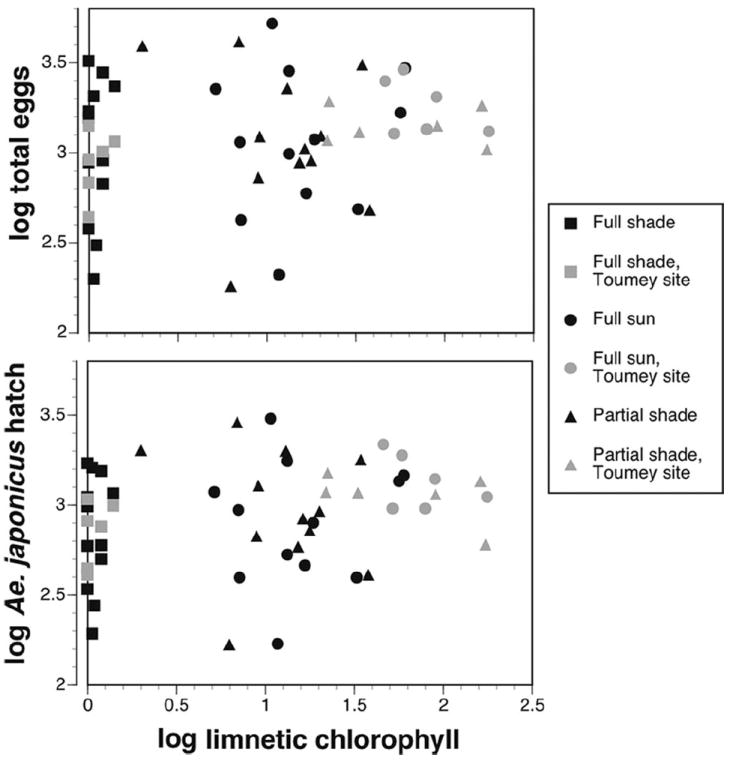
Scatter plots of total eggs collected per container (A) and total number of Ae. j. japonicus hatched (B) versus limnetic chlorophyll a levels for data from all sites and trials combined (Toumey site values are highlighted for emphasis).
Discussion
Invasive mosquito species are unlike the majority of invasive species in that they have the potential to alter vector-borne disease cycles (Juliano and Lounibos 2005). They may do this either directly by vectoring the disease or indirectly through competition with native disease-carrying mosquitoes (Bevins 2008). In the United States many other disease vectors such as Ae. triseriatus, Cx. pipiens, and Cx. restuans occur in containers with Ae. j. japonicus. Competition for food and spatial resources in container habitats is often severe because of the discrete nature of the habitats and the limited amount of microbial food resources available. Autocthonous primary production and algal cells in particular are understudied components of the microbial food base in container habitats and this study addressed their potential role in influencing the local distribution of Ae. j. japonicus.
Within an area, the distribution of larvae of a given mosquito species is determined largely by oviposition habits of gravid females. These oviposition preferences are also important in determining larval developmental success (Blaustein and Kotler 1993). In container habitats that are exposed to sunlight, primary production in the form of algal biomass and associated increases in other microbial groups may enhance food levels and lessen the stresses of resource competition. Algae are known to be important food resources during the larval phase for some species (Merritt et al. 1992, Kaufman et al. 2006), but have received little attention in studies of container breeders. In both field and laboratory experiments, Ae. j. japonicus have been found to emerge sooner and in higher numbers from microcosms with abundant algal growth compared with microcosms with little algal growth (Lorenz 2012).
Although the results of our experiment showed no overall relationship between algal biomass and Ae. j. japonicus oviposition rates, at one of our field sites (Toumey) autocthonous primary production did appear to influence oviposition activity and it was the only site that appeared to show a graded response to shade treatments (Fig. 1). A prominent difference between the Toumey site and the other two sites was that limnetic and benthic chlorophyll a levels in both the full sun and partial shade treatments were at least twice as high as measured at the River Lab and Zoo field sites (Fig. 1). It is unclear why such a large difference in algal growth occurred between our sites, because all received the same inoculum at the beginning of the experiment. We did observe, however, that there seemed to be more invertebrate (mainly slug) mortalities within our containers at Toumey than at the other sites. This influx of invertebrate carcasses could have raised nutrient levels (e.g., via additions of phosphorous and nitrogen) within the containers at Toumey and subsequently enhanced primary production in treatments with sun exposure. Because the largest differences between chlorophyll a levels at any site were between the fully shaded and the other two treatments, it is likely that above a minimum threshold of light, inorganic nutrients quickly became limiting to algal growth, and Toumey container algal biomass increased with the added nutrient inputs observed there. The observed differences at Toumey also highlight the complex interactions between inputs and basal resources for mosquitoes in container habitats. It is unclear, for example, why oviposition at this site appeared to show a graded response to shading, while the others did not. It may be related to site- and/or shading-specific effects on microbial community structure, as particular assemblages of microorganisms have been shown to influence oviposition by Aedes spp. (Ponnusamy et al. 2010).
Because Toumey was the only location at which there was some evidence of shade treatment influencing oviposition, we suggest that algal biomass and associated heterotrophic microbial growth at high levels may influence oviposition of Ae. j. japonicus in ways that may be similar to those observed for other mosquito species. It is unclear whether females were responding to high algal levels specifically or were merely reacting to higher levels of organic matter and increased heterotrophic microbial activity in general, as seems to be the case for Culex. species (Reiskind et al. 2004, Chaves et al. 2009, Chavez and Kitron 2011). Our results also indicate that in most urban containers, where autocthonous primary production is unlikely to be as high as measured at Toumey, algae and associated microbes are probably not a consistent positive cue for oviposition by Ae. j. japonicus. This is supported by data from previous studies of artificial container habitats showing no association between Aedes mosquito abundance and algal growth (Beier et al. 1983, Kling et al. 2007, Yee et al. 2010). Studies that quantified chlorophyll a values in artificial containers found values that were lower than what we measured at Toumey, but similar to chlorophyll a measurements at our other sites (Kling et al. 2007, Yee et al. 2010).
One potentially confounding variable in our study was temperature. Containers exposed to full sun exhibited higher water temperatures than containers in partial or full shade and this may have directly or indirectly influenced Ae. j. japonicus oviposition. There is evidence that Ae. j. japonicus larvae are developmentally inhibited by water temperatures >30°C (Scott 2003, Andreadis and Wolfe 2010), and some avoidance of fully exposed containers might be expected. Our results suggest that there is little discrimination by ovipositing females in this regard and that any observed lowered Ae. j. japonicus densities in sun-exposed habitats (e.g., in rock pools; Andreadis and Wolfe 2010) may be a consequence of larval mortality at higher temperatures, rather than reduced initial oviposition and larval hatching. This may indicate a trade-off between higher primary productivity and temperature for Ae. j. japonicus such that containers exposed to high levels of sunlight and supporting higher primary production could be attractive to gravid females but are ultimately not suitable for larvae. Larvae may be optimally suited to containers with intermediate shade levels that still support some algal growth but do not reach lethal maximum temperatures. Regardless, the large numbers of eggs laid in our experimental containers that were all in sun-exposed areas demonstrate that Ae. j. japonicus females are clearly willing to deposit eggs in unshaded larval habitats and risk larval mortality.
Another potentially confounding factor comes from the algal community composition in our containers. We inoculated our containers at the beginning of the experiment with a culture of mixed algal taxa. This was augmented during the trials by natural algal colonization from the surrounding environment (i.e., airborne inocula). Freshwater algal communities are often highly diverse and reflect light and nutrient inputs, as well as physiochemical conditions such as pH and temperature (Wehr and Sheath 2003). In our containers, we documented the presence of Scenedesmus, Oedogonium, and many unidentifiable coccoid Chlorophytes, and some of these algae may not have been ideal foods for larvae. Marten (1986, 2007) documented the occurrence of certain algal taxa in mosquito habitats that are indigestible to larvae because of the presence of the carotenoid sporopollenin in their cell walls. In addition, certain taxa of algae, especially the Cyanobacteria, are known to produce toxins that are damaging or lethal to mosquito larvae (Dhillon et al. 1982, Kiviranta et al. 1993). Though we believe that our algal communities were representative of those occurring naturally in container habitats, some of the algae colonizing our containers may not have been good larval food resources and potentially less attractive as stimuli for oviposition. The effect of larval habitat microbial community composition on oviposition by container breeding mosquitoes is only beginning to be examined and algal communities may play an indirect role by influencing other microbial groups such as bacteria that are known to serve as attractants for mosquitoes (e.g., Espeland et al. 2001, Trexler et al. 2003, Ponnusamy et al. 2010). As such, increased primary production in container habitats is largely inseparable from other potential cues because it will have marked effects on nutrient and heterotrophic microbial dynamics in the system, as well as physiochemical parameters such as pH. Future studies that use axenic cultures of algae may be able to tease out any potential attractants associated with particular algal groups under laboratory conditions, but naturally colonized container systems will always contain algal and microbial assemblages that could obfuscate or enhance any oviposition cues provided by individual microbial species. Our results suggest that any specific algal-based cues are unimportant for ovipositing Ae. j. japonicus females.
Another variable we did not address was the potential effect of conspecifics in a container on oviposition. In our experiment, adult females would have had to use cues associated with eggs rather than larvae, and these cues would have been minimal given that eggs were removed daily. Egg aggregation is a phenomenon that has been observed in other container-breeding mosquito species, and occurs when females of a species deposit eggs in the same habitat because of the presence of other conspecific eggs. Egg aggregation pheromones are cues for some Culex species (see Millar et al. 1994, Mboera et al. 1999). Reiskind and Wilson (2004) documented in their field oviposition experiment that Cx. restuans eggs tended to aggregate in different containers on different days, and postulate that egg pheromone is an oviposition attractant for this species. Additionally, eggs deposited on the container walls by congenerics such as Ae. triseriatus might have influenced overall oviposition by Ae. j. japonicus or larval hatching rates. Unfortunately, our study did not measure how many unhatched eggs were attributable to Ae. j. japonicus or other species, and hatch rates of Ae. triseriatus in this experiment were too low for meaningful comparisons. We are unaware of any previous studies that have examined oviposition inhibition or enhancement, or larval hatching rates related to conspecific or congeneric egg densities for these species.
We documented a small amount of outside oviposition from mosquito species other than Ae. j. japonicus occurring in our containers. The species with the largest contribution to this outside ovipositional activity based on larval hatching was Ae. triseriatus. These contributions were extremely minimal, representing <1% of the total number of larvae hatched. Some ovipositional activity by An. quadrimaculatus (Say) and Cx. restuans was also observed in our containers, but we estimate that the two species together made up <0.5% of the potential larval hatch in our containers during the study period. These hatch rates confirm that Ae. j. japonicus is a dominant artificial container-breeding mosquito in our area. Similar results were obtained by Andreadis and Wolfe (2010), who showed in larval surveys in Connecticut that Ae. triseriatus and Ae. atropalpus (Coquillett) were displaced to a significant percentage by Ae. j. japonicus in many container habitats.
From our study, we conclude that at low concentrations typical of shaded container habitats, algal biomass does not affect oviposition choices of Ae. j. japonicus. At higher concentrations, however, autocthonous primary production could have a positive impact on Ae. j. japonicus oviposition either directly or indirectly. The precise role of autotrophs in the ecology of water-filled container systems remains unresolved, and we believe the theme of autotrophy in detrital-based mosquito habitats necessitates further exploration. Because Ae. j. japonicus has become a dominant container mosquito in mid-Michigan and elsewhere, further studies into the ecology of this species and its impacts on native communities are clearly necessary. Ae. j. japonicus has the potential to alter disease cycles through interaction with local mosquito species, and its success is related to how well it exploits larval habitats.
Acknowledgments
We thank Ted Batterson and Tara Harrison for access to the River Lab and Potter Park Zoo field sites, respectively. For field and lab assistance, we thank Angeline Kosnik, Liu Yang, Blane Doyon, Marissa Cann, Catherine Lorenz, Sarah Willson Malakauskas, Brian Lovett, and Craig Bateman. We also thank Richard Merritt and R. Jan Stevenson for their valuable advice. Two anonymous reviewers provided helpful comments for which we are also grateful. Funding for this project was provided by National Institutes of Health grant R37 A121884 and by the Rhodes (Gene) Thompson Memorial Fellowship.
References Cited
- Andreadis TG, Anderson JF, Munstermann LE, Wolfe RJ, Florin DA. Discovery, distribution, and abundance of the newly introduced mosquito Ochlerotatus japonicus (Diptera : Culicidae) in Connecticut, USA. J Med Entomol. 2001;38:774–779. doi: 10.1603/0022-2585-38.6.774. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Wolfe BJ. Evidence for reduction of native mosquitoes with increased expansion of invasive Ochlerotatus japonicus japonicus (Diptera: Culicidae) in the Northeastern United States. J Med Entomol. 2010;47:43–52. doi: 10.1603/033.047.0106. [DOI] [PubMed] [Google Scholar]
- Arar EJ, Collins GB. EPA Method 445.0, Version 1.2. National Exposure Research Laboratory Office of Research and Development; Cincinnati, OH: 1997. In vitro determination of chlorophyll a and pheophytin a in marine and freshwater algae by fluorescence. [Google Scholar]
- Barrera R, Amador M, Clark GG. Ecological factors influencing Aedes aegypti (Diptera : Culicidae) productivity in artificial containers in Salinas, Puerto Rico. J Med Entomol. 2006;43:484–492. doi: 10.1603/0022-2585(2006)43[484:efiaad]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Beier JC, Patricoski C, Travis M, Kranzfelder J. Influence of water chemical and environmental parameters on larval mosquito dynamics in tires. Environ Entomol. 1983;12:434–438. [Google Scholar]
- Bentley MD, Day JF. Chemical ecology and behavioral aspects of mosquito oviposition. Annu Rev Entomol. 1989;34:401–421. doi: 10.1146/annurev.en.34.010189.002153. [DOI] [PubMed] [Google Scholar]
- Bevins SN. Establishment and abundance of a recently introduced mosquito species Ochlerotatus japonicus (Diptera : Culicidae) in the Southern Appalachians, USA. J Med Entomol. 2007;44:945–952. doi: 10.1603/0022-2585(2007)44[945:eaaoar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bevins SN. Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera : Culicidae) Biol Invasions. 2008;10:1109–1117. [Google Scholar]
- Blaustein L, Kotler BP. Oviposition habitat selection by the mosquito, Culiseta longiareolata: effects of conspecifics, food, and green toad tadpoles. Ecol Entomol. 1993;18:104–108. [Google Scholar]
- Bolker BM, Brooks ME, Clark CJ, Geange SW, Poulsen JR, Stevens MH, White JS. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol Evol. 2009;24:127–135. doi: 10.1016/j.tree.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Bond JG, Arredondo–Jimenez JI, Rodriguez MH, Quiroz–Martinez H, Williams T. Oviposition habitat selection for a predator refuge and food source in a mosquito. Ecol Entomol. 2005;30:255–263. [Google Scholar]
- Brouard O, LeJeune AH, Leroy C, Cereghino R, Roux O, Pelozuelo L, Dejean A, Corbara B, Carrias JF. Are algae relevant to the detritus-based food web in tank-bromeliads? PLoS ONE. 2011;6:10. doi: 10.1371/journal.pone.0020129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron EC, Wilkerson RC, Mogi M, Miyagi I, Toma T, Kim HC, Fonseca DM. Molecular phylogenetics of Aedes japonicus, a disease vector that recently invaded Western Europe, North America, and the Hawaiian Islands. J Med Entomol. 2010;47:527–535. doi: 10.1093/jmedent/47.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaves LF. An entomologist’s guide to demystify pseudoreplication: data analysis of field studies with design constraints. J Med Entomol. 2010;47:291–298. doi: 10.1603/me09250. [DOI] [PubMed] [Google Scholar]
- Chaves LF, Keogh CL, Vazquez–Prokopec GM, Kitron UD. Combined sewage overflow enhances oviposition of Culex quinquefasciatus (Diptera: Culicidae) in urban areas. J Med Entomol. 2009;46:220–226. doi: 10.1603/033.046.0206. [DOI] [PubMed] [Google Scholar]
- Chaves LF, Hamer GL, Walker ED, Brown WM, Ruiz MO, Kitron UD. Climatic variability and landscape heterogeneity impact urban mosquito diversity and vector abundance and infection. Ecosphere. 2011;2 art70. [Google Scholar]
- Chaves LF, Kitron UD. Weather variability impacts on oviposition dynamics of the southern house mosquito at intermediate time scales. Bull Entomol Res. 2011;101:633–641. doi: 10.1017/S0007485310000519. [DOI] [PubMed] [Google Scholar]
- Darsie RF, Ward RA. Identification and geographical distribution of the mosquitos of North America, north of Mexico. University Press of Florida; Gainesville, FL: 2005. [Google Scholar]
- Dhillon MS, Mulla MS, Hwang YS. Biocidal activity of algal toxins against immature mosquitoes. J Chem Ecol. 1982;8:557–566. doi: 10.1007/BF00987803. [DOI] [PubMed] [Google Scholar]
- Dulmage HT. Production of δ-endotoxin by eighteen isolates of Bacillus thuringiensis, serotype 3, in 3 fermentation media. J Invertebr Pathol. 1971;18:353–358. doi: 10.1016/0022-2011(71)90037-1. [DOI] [PubMed] [Google Scholar]
- Ebine I. Studies on the ecology of mosquitoes in Saitama Prefecture: part 2. Seasonal distribution of larvae which living in rock pools in the river bed of Nagatoro. Jpn J Sanit Zool. 1969;20:27–31. [Google Scholar]
- Edgerly JS, McFarland M, Morgan P, Livdahl T. A seasonal shift in egg-laying behaviour in response to cues of future competition in a treehole mosquito. J Anim Ecol. 1998;67:805–818. [Google Scholar]
- Espeland EM, Francoeur SN, Wetzel RG. Influence of algal photosynthesis on biofilm bacterial production and associated glucosidase and xylosidase activities. Microb Ecol. 2001;42:524–530. doi: 10.1007/s00248-001-1022-8. [DOI] [PubMed] [Google Scholar]
- Garros C, Ngugi N, Githeko AE, Tuno N, Yan G. Gut content identification of larvae of the Anopheles gambiae complex in western Kenya using a barcoding approach. Mol Ecol Resour. 2008;8:512–518. doi: 10.1111/j.1471-8286.2007.02013.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howland LJ. Bionomical investigation of English mosquito larvae with special reference to their algal food. J Ecol. 1930;18:81–125. [Google Scholar]
- Iriarte WLZ, Tsuda Y, Wada Y, Takagi M. Distribution of mosquitoes on a hill of Nagasaki city, with emphasis to the distance from human dwellings. Trop Med. 1991;33:55–60. [Google Scholar]
- Irish SR, Pierce CS. Update on the distribution of Ochlerotatus japonicus in Oregon and Washington. J Am Mosq Control Assoc. 2008;24:110–111. doi: 10.2987/5572.1. [DOI] [PubMed] [Google Scholar]
- Joy JE, Sullivan SN. Occurrence of tire inhabiting mosquito larvae in different geographic regions of West Virginia. J Am Mosq Cont Assoc. 2005;21:380–386. doi: 10.2987/8756-971X(2006)21[380:OOTIML]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman MG, Bland SN, Worthen ME, Walker ED, Klug MJ. Bacterial and fungal biomass responses to feeding by larval Aedes triseriatus (Diptera: Culicidae) J Med Entomol. 2001;38:711–719. doi: 10.1603/0022-2585-38.5.711. [DOI] [PubMed] [Google Scholar]
- Kaufman MG, Goodfriend W, Kohler–Garrigan A, Walker ED, Klug MJ. Soluble nutrient effects on microbial communities and mosquito production in Ochlerotatus triseriatus habitats. Aqua Microb Ecol. 2002;29:73–88. [Google Scholar]
- Kaufman MG, Wanja E, Maknojia S, Bayoh MN, Vulule JM, Walker ED. Importance of algal biomass to growth and development of Anopheles gambiae larvae. J Med Entomol. 2006;43:669–676. doi: 10.1603/0022-2585(2006)43[669:ioabtg]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Kitching RL. Food webs and container habitats: the natural history and ecology of phytotelmata. Cambridge University Press; Cambridge, MA: 2001. [Google Scholar]
- Kiviranta J, Abdelhameed A, Sivonen K, Niemela SI, Carlberg G. Toxicity of cyanobacteria to mosquito larvae: screening of active compounds. Environ Toxicol Water Qual. 1993;8:63–71. [Google Scholar]
- Kling LJ, Juliano SA, Yee DA. Larval mosquito communities in discarded vehicle tires in a forested and unforested site: detritus type, amount, and water nutrient differences. J Vect Ecol. 2007;32:207–217. doi: 10.3376/1081-1710(2007)32[207:lmcidv]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kokas JE. Field evaluation of CDC gravid trap attractants to primary West Nile virus vectors, Culex mosquitoes in New York State. J Am Cont Assoc. 2004;20:248–253. [PubMed] [Google Scholar]
- Lorenz AR. M S Thesis. Michigan State University; East Lansing: 2012. The role of algae in the invasion ecology of the mosquito species Aedes japonicus japonicus (Diptera: Culicidae) [Google Scholar]
- Marten GG. Mosquito control by plankton management: the potential of indigestible green algae. J Trop Med Hyg. 1986;89:213–222. [PubMed] [Google Scholar]
- Marten GG. Larvicidal algae. J Am Mosq Cont Assoc Bull No 7. 2007;23(Supp. to No. 2):177–183. doi: 10.2987/8756-971X(2007)23[177:LA]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Matuo K. On the successions of mosquito larvae breeding in basins for holy water hollowed on gravestones surrounded by bamboo groves in Kyoto. Jpn J Sanit Zool. 1961;12:257–261. [Google Scholar]
- Mboera LEG, Mdira KY, Salum FM, Takken W, Pickett JA. Influence of synthetic oviposition pheromone and volatiles from soakage pits and grass infusions upon oviposition site: selection of Culex mosquitoes in Tanzania. J Chem Ecol. 1999;25:1855–1865. [Google Scholar]
- Merritt RW, Dadd RH, Walker ED. Feeding behavior, natural food, and nutritional relationships of larval mosquitoes. Annu Rev Entomol. 1992;37:349–376. doi: 10.1146/annurev.en.37.010192.002025. [DOI] [PubMed] [Google Scholar]
- Millar JG, Chaney J, Beehler JW, Mulla MS. Interaction of the Culex quinquefasciatus egg raft pheromone with a natural chemical associated with oviposition sites. J Am Mosq Control Assoc. 1994;10:374–379. [PubMed] [Google Scholar]
- Molaei G, Farajollahi A, Scott JJ, Gaugler R, Andreadis TG. Human bloodfeeding by the recently introduced mosquito, Aedes japonicus japonicus, and public health implications. J Am Mosq Cont Assoc. 2009;25:210–214. doi: 10.2987/09-0012.1. [DOI] [PubMed] [Google Scholar]
- Oliver J, Means RG, Howard JJ. Geographic distribution of Ochlerotatus japonicus in New York State. J Am Mosq Cont Assoc. 2003;19:121–124. [PubMed] [Google Scholar]
- Peyton EL, Campbell SR, Candeletti TM, Romanowski M, Crans WJ. Aedes (Finlaya) japonicus japonicus (Theobald), a new introduction into the United States. J Am Mosq Cont Assoc. 1999;15:238–241. [PubMed] [Google Scholar]
- Ponnusamy L, Wesson DM, Arellano C, Schal C, Apperson CS. Species composition of bacterial communities influences attraction of mosquitoes to experimental plant infusions. Microb Ecol. 2010;59:158–173. doi: 10.1007/s00248-009-9565-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potvin C. ANOVA: experimental layout and analysis. In: Scheiner SM, Gurevitch J, editors. Design and Analysis of Ecological Experiments. 2. Oxford University Press; New York, NY: 2001. pp. 63–76. [Google Scholar]
- Reiskind MH, Wilson ML. Culex restuans (Diptera : Culicidae) oviposition behavior determined by larval habitat quality and quantity in southeastern Michigan. J Med Entomol. 2004;41:179–186. doi: 10.1603/0022-2585-41.2.179. [DOI] [PubMed] [Google Scholar]
- Reiskind MH, Greene KL, Lounibos LP. Leaf species identity and combination affect performance and oviposition choice of two container mosquito species. Ecol Entomol. 2009;34:447–456. doi: 10.1111/j.1365-2311.2008.01067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Dohm DJ, Pagac B, Andre RG, Turell MJ. Experimental transmission of eastern equine encephalitis virus by Ochlerotatus j. japonicus (Diptera : Culicidae) J Med Entomol. 2002;39:480–484. doi: 10.1603/0022-2585-39.3.480. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ. Ochlerotatus j. japonicus in Frederick County, Maryland: discovery, distribution, and vector competence for West Nile virus. J Am Mosq Cont Assoc. 2001;17:137–141. [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Andre ARG. Laboratory transmission of La Crosse virus by Ochlerotatus j. japonicus (Diptera : Culicidae) J Med Entomol. 2002;39:635–639. doi: 10.1603/0022-2585-39.4.635. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Andre RG. Experimental transmission of St. Louis encephalitis virus by Ochlerotatus j. japonicus. J Am Mosq Cont Assoc. 2003;19:159–162. [PubMed] [Google Scholar]
- Schaffner F, Kaufmann C, Hegglin D, Mathis A. The invasive mosquito Aedes japonicus in Central Europe. Med Vet Entomol. 2009;23:448–451. doi: 10.1111/j.1365-2915.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- Scott JJ. Ph D dissertation. The State University of New Jersey; New Brunswick, NJ: 2003. The ecology of the exotic mosquito Ochlerotatus (Finlaya) japonicus japonicus (Theobald 1901) (Diptera: Culicidae) and an examination of its role in the West Nile virus cycle in New Jersey. [Google Scholar]
- Scott JJ, Carle FL, Crans WJ. Ochlerotatus japonicus collected from natural rockpools in New Jersey. J Am Mosq Cont Assoc. 2001;17:91–92. [PubMed] [Google Scholar]
- Silberbush A, Markman S, Lewinsohn E, Bar E, Cohen JE, Blaustein L. Predator-released hydrocarbons repel oviposition by a mosquito. Ecol Lett. 2010;13:1129–1138. doi: 10.1111/j.1461-0248.2010.01501.x. [DOI] [PubMed] [Google Scholar]
- Takashima I, Rosen L. Horizontal and vertical transmission of Japanese encephalitis virus by Aedes japonicus (Diptera, Culicidae) J Med Entomol. 1989;26:454–458. doi: 10.1093/jmedent/26.5.454. [DOI] [PubMed] [Google Scholar]
- Tanaka K, Mizusawa K, Saugstad ES. A revision of the adult and larval mosquitoes of Japan including the Ryukyu Archipelago and the Ogasawara Islands and Korea (Diptera: Culicidae) Contrib Am Entomol Inst. 1979;16:1–979. [Google Scholar]
- Thielman A, Hunter FF. Establishment of Ochlerotatus japonicus (Diptera : Culicidae) in Ontario, Canada. J Med Entomol. 2006;43:138–142. doi: 10.1603/0022-2585(2006)043[0138:eoojdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Torres–Estrada JL, Meza–Alvarez RA, Cruz–Lopez L, Rodriguez MH, Arredondo–Jimenez JI. Attraction of gravid Anopheles pseudopunctipennis females to oviposition substrates by Spirogyra majuscula (Zygnematales : Zygnmataceae) algae under laboratory conditions. J Am Mosq Cont Assoc. 2007;23:18–23. doi: 10.2987/8756-971X(2007)23[18:AOGAPF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Trexler JD, Apperson CS, Zurek L, Gemeno C, Schal C, Kaufman MG, Walker ED, Watson DW, Wallace L. Role of bacteria in mediating the oviposition responses of Aedes albopictus (Diptera: Culicidae) J Med Entomol. 2003;40:841–848. doi: 10.1603/0022-2585-40.6.841. [DOI] [PubMed] [Google Scholar]
- Versteirt V, Schaffner F, Garros C, Dekoninck W, Coosemans M, Van Bortel W. Introduction and establishment of the exotic mosquito species Aedes japonicus japonicus (Diptera: Culicidae) in Belgium. J Med Entomol. 2009;46:1464–1467. doi: 10.1603/033.046.0632. [DOI] [PubMed] [Google Scholar]
- Warburg A, Faiman R, Shtern A, Silberbush A, Markman S, Cohen JE, Blaustein L. Oviposition habitat selection by Anopheles gambiae in response to chemical cues by Notonecta maculata. J Vect Ecol. 2011;36:421–425. doi: 10.1111/j.1948-7134.2011.00183.x. [DOI] [PubMed] [Google Scholar]
- Wehr JD, Sheath RG, editors. Freshwater algae of North America: ecology and classification. Academic; San Diego, CA: 2003. [Google Scholar]
- Williges E, Farajollahi A, Scott JJ, McCuiston LJ, Crans WJ, Gaugler R. Laboratory colonization of Aedes japonicus japonicus. J Am Mosq Cont Assoc. 2008;24:591–593. doi: 10.2987/5714.1. [DOI] [PubMed] [Google Scholar]
- Wong J, Stoddard ST, Astete H, Morrison AC, Scott TW. Oviposition site selection by the dengue vector Aedes aegypti and its implications for dengue control. PLoS Negl Trop Dis. 2011;5:12. doi: 10.1371/journal.pntd.0001015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yee DA, Kneitel JM, Juliano SA. Environmental correlates of abundances of mosquito species and stages in discarded vehicle tires. J Med Entomol. 2010;47:53–62. doi: 10.1603/033.047.0107. [DOI] [PMC free article] [PubMed] [Google Scholar]


