Abstract
Understanding mosquito dispersal is critically important for vector-borne disease control and prevention. Mark–release–recapture methods using various marking techniques have made substantial contributions to the study of mosquito biology. However, the ability to mark naturally breeding mosquitoes noninvasively and with life-long retention has remained problematic. Here, we describe a method to mark naturally breeding mosquitoes with stable isotopes. Culexpipiens f. molestus mosquitoes were provisioned as larvae in laboratory experiments with 15N-labeled potassium nitrate and 13C-labeled glucose. Larval enrichment was sufficient to differentiate marked adult mosquitoes from unmarked control mosquitoes and the natural source population from Chicago Illinois, using either δ15N or δ13C. Isotopic retention lasted for at least 55 d for adult male and females mosquitoes. There were no consistent effects of isotopic enrichment on immature mosquito survival or adult mosquito body size. We then applied this marking technique to naturally breeding Culex pipiens mosquitoes in suburban Chicago, IL, and for the first time, report successful isotopic enrichment of mosquitoes in the field. This stable isotope marking technique will facilitate studies of mosquito dispersal.
Keywords: Culex mosquito, mark–capture, mosquito dispersal, stable isotope
Mark–release–recapture studies have provided useful insights into adult mosquito dispersal, feeding behavior, duration of the gonotrophic cycle, survival rates, and population sizes (Silver 2008). Methods to mark mosquitoes have included dyes (Welch et al. 2006), paints (Trpis and Hausermann 1986), dusts (Reisen et al. 1992, Russell et al. 2005), trace elements (Wilkins et al. 2007), and radioactive isotopes (Lindquist et al. 1967). Hagler and Jackson (2001) reviewed marking techniques for insects and stated that the ideal marker should persist without inhibiting normal biology, should be environmentally safe, cost-effective, and easy to use. However, existing techniques tend to be labor intensive, as they require rearing mosquitoes, marking them in large quantities, and then inspecting large numbers of individuals to detect recaptures (Walker et al. 1987). In addition, the process of rearing adults, marking them, and releasing them may change behavior compared with natural populations (Reisen et al. 2003, Silver 2008).
Mark–release–recapture studies using fluorescent dust have been most widely adopted to date. Such methods typically recapture adult mosquitoes for <10 d (Lapointe 2008, Baber et al. 2010, Bellini et al. 2010) but occasionally up to 20 d (Pumpuni and Walker 1989, Harrington et al. 2005), and in one study up to 48-d post-release (Walker et al. 1987). Because of the constraints of the retention of the fluorescent marker, most studies primarily quantify dispersal of nulliparous female mosquitoes during the initial host-seeking event and occasionally a second host-seeking event. Although these studies have contributed valuable insights into mosquito behavior and biology, this short duration also is a shortcoming given that the vectorial capacity increases with longevity (Dye 1986, Milby and Reisen 1989). For example, the time from ingestion of an infectious bloodmeal until a mosquito is capable of delivering an infectious bite (i.e., extrinsic incubation period), is ≈8–30 d for West Nile virus (WNV) in Culex tarsalis and Cx. pipiens (Dohm et al. 2002, Reisen et al. 2006, Anderson et al. 2008). To characterize the dispersal of medically relevant infectious mosquitoes, markers ideally should be retained for life.
Stable isotopes have been adapted for biological and ecological studies on a wide variety of animals including insects (Hood-Nowotny and Knols 2007), mammals (Pauli et al. 2009), fish (Munro et al. 2008), and birds (Inger and Bearhop 2008). Stable isotopes occur naturally in the environment, are nontoxic and non-radioactive, and incorporate into living tissue, which make them safe and useful tracers. An isotope of an element has the same atomic number but different number of neutrons, thus a different atomic weight. For example, ≈0.4% of all nitrogen atoms in the environment are 15N and the rest are 14N, and ≈1% of all carbon atoms in the environment are 13C and the rest are 12C (Hood-Nowotny and Knols 2007). Studies have used variation in the natural abundance of stable isotopes to trace the origin and movement of animals (Rubenstein and Hobson 2004), or have relied on enrichment techniques by labeling animals with rare stable isotopes. For example, immature aquatic insects were enriched by the addition of 15N to streams and the emergent and marked adult insects were captured to estimate dispersal (Hershey et al. 1993, Briers et al. 2004, Macneale et al. 2005). Anopheles arabiensis Patton mosquitoes were enriched to address questions related to Sterile Insect Technique (SIT) programs (Hood-Nowotny et al. 2006, Helinski et al. 2007, Helinski et al. 2008a,b). In the context of SIT programs, the use of stable isotopes allowed investigators to determine the insemination success of irradiated versus unirradiated male mosquitoes. This is achieved by detecting isotopically enriched semen in unlabeled female spermathecae. These studies demonstrate the potential to use stable isotopes as a sensitive biological tracer in mosquitoes. Although the enrichment of naturally breeding mosquitoes by using stable isotopes in the context of a mark–capture study has been proposed (Hood-Nowotny and Knols 2007), more detailed development of the method is required before isotopic enrichment of naturally breeding mosquitoes can be used in the field.
We label Culex pipiens mosquitoes with stable isotopes in laboratory and field environments. We addressed the following objectives using Cx.pipiens mosquitoes reared under laboratory conditions: 1) determine the larval stable isotope enrichment level necessary to differentiate marked from unmarked adult mosquitoes, 2) determine the experimental conditions that allow the detection of one enriched mosquito in a pool of 5 or 10 individual mosquitoes, 3) identify the duration of enrichment at different days post emergence, 4) analyze positive or negative effects of isotopic enrichment on mosquito body size and survival, 5) quantify the impact of blood feeding or egg production on enrichment, and 6) determine if trans-generational marking occurs. Based on the results of the laboratory enrichment experiments, we applied the technique in the field to enrich naturally breeding mosquitoes with stable isotopes.
Materials and Methods
Mosquitoes
Culexpipiens f. molestus (Forskål) were used for all laboratory experiments. The colony was established from 2005 to 2009 from a natural population located in suburban Chicago, IL (Kothera et al. 2010). For the basic colony maintenance and experiments, larvae were reared in clear plastic trays (20 by 28 cm) in one liter of water (deionized water was used throughout the experiments). Larvae and adults were stored in a Percival Scientific incubator (Perry, IA) at 25°C and a photoperiod of 16:8 (L:D) h. Larvae were fed a 2% solution of desiccated and defatted liver powder (BioServ, Frenchtown, NJ) and brewer’s yeast hydrolysate (Bio-Serv, Frenchtown, NJ) at a ratio of 3:2. Larvae were transferred to fresh water by using a Calphalon stainless steel skimmer (Calphalon Corp., Toledo, OH) (third and fourth instars) or this same type of skimmer fitted with a finer mesh (210 μm) of fabric (first and second instars). The larvae in the skimmer were placed into a clean tray with one liter of water.
For the colony maintenance only, pupae were transferred to a cup of water in an insect rearing cage (BugDorm; 299 cm3; BioQuip Products, Rancho Dominquez, CA) by using a pipette. Adults were fed a 10% sucrose solution ad libitum using 29.6-ml plastic containers (Bio-Serv, Frenchtown, NJ) with 5.1-cm dental rolls (Absorbal, Wheat Ridge, CO) inserted into the lid. Although some autogenous egg-laying occurred, we increased egg production by blood-feeding adults using defibrinated bovine blood (Hemostat Laboratories, Dixon, CA) provided in a glass blood-feeding apparatus heated to 36.5°C.
Cx. pipiens mosquitoes also were collected from suburban Chicago, IL in 2009 to obtain natural abundance levels of δ15N and δ13C. Adult male and female Cx. pipiens mosquitoes were collected from urban catch basins in suburban Chicago, IL in 2009 by using emergence traps (Hamer et al. 2011). Mosquitoes were stored in a −20°C freezer until they were prepared for stable isotope submission.
Experimental Labeling Protocols
We used 15N-labeled potassium nitrate (KNO3; 15N, 99 atom%; Cambridge Isotope Laboratories, Inc., Andover, MA) and 13C-labeled glucose (U-13C6, 99 atom%; Cambridge Isotope Laboratories, Inc., Andover, MA) for enrichment. In all three experiments, 15N-potassium nitrate or 13C-glucose was added based on the total amount of nitrogen and carbon present in the larval food. Values are expressed based on the final atom percentage of 15N or 13C added to the larval containers. Brewer’s yeast contains 13% nitrogen and 48% carbon, and liver powder contains 9% nitrogen and 39% carbon; thus 11% of the larval diet (liver powder and brewer’s yeast in a 3:2 ratio) consists of nitrogen and 43% consists of carbon. In experiment 3, for example, 0.32 g of larval food was added to each treatment, and because the percentage of nitrogen in potassium nitrate is 14%, 1.88 mg of 99 atom% 15N-potassium nitrate was added to reach 0.75 atom% 15N. The 13C-treatments in experiment 3 received the same amount of larval food, and because 40% of glucose consists of carbon, 1.88 mg of 99 atom% 13C-glucose was added to reach 0.55 atom% 13C. Quantities of 15N-potassium nitrate and 13C-glucose were measured using a balance with 0.1-mg sensitivity (Denver Instrument, Bohemia, NY). In all experiments, stable isotopes were prepared in a solution containing the larval food and were fed directly to trays beginning with first-instar larvae. Controls in all experiments were larvae reared with the same amount of particulate food, but without additions of any potassium nitrate or glucose.
The three experiments were designed to reach the objectives of the study described. For each experiment, we used separate larval trays, skimmers, transfer pipettes, emergence containers, spatula, and forceps to reduce the risk of cross-contamination of 15N and 13C among treatments. During all experiments, adult body size for individuals emerging from the different treatments was measured by obtaining a length of the wing by using a MZ16 F stereo microscope (Leica Microsystems, Wetzlar, Germany) fitted with a SPOT Pursuit charge-coupled device camera (Diagnostic Instruments, Sterling Heights, MI) and configured to a computer with image analysis software. Wing length, known to have a positive relationship with adult body size (Carron 2007), was measured from the alular notch to the distal margin (Bock and Milby 1981).
Experiment 1
The first experiment evaluated 15N-enrichment of larval mosquitoes under three concentrations in an initial attempt to identify the appropriate level of enrichment to distinguish marked from unmarked mosquitoes. The levels of enrichment were 0.04 atom% 15N (low), 0.08 atom% 15N (medium), and 0.12 atom% 15N (high). We ran two replicates for each dosage for six trays in total, containing 1 liter of water and 100 first-instar larvae each. In addition to the six trays receiving 15N additions, we had two trays of controls receiving the same amount of food but no 15N additions. The addition of food was constant among all treatments. The appropriate amount of 15N-potassium nitrate was added to each larval tray containing first-instar larvae to achieve the above atom% (0.05 mg, 0.09 mg, 0.14 mg for low, medium, and high, respectively). We added 0.09 g of liver powder and 0.06 g of brewer’s yeast into each tray per feeding. The water was changed and new larval food (same atom% 15N values but different mg of 15N-potassium nitrate per liter) was added at 3-d intervals until all larvae pupated. At days 9 and 12, the water was changed and we increased the amount of food (0.10 and 0.11 g of liver powder and 0.07 and 0.08 g of brewer’s yeast, respectively). Pupae were transferred to an emergence container (“mosquito breeder;” BioQuip Products, Rancho Dominquez, CA) and the newly emerged adults were transferred to 0.5-liter plastic cups (Bio-Serv) by using D-cell flashlight aspirators (BioQuip Products, Rancho Dominquez, CA). The adult holding cup lids had the centers cut out and replaced with a 1-mm mesh circle, 9 cm in diameter, glued to the outside of the lid. During adult rearing in the cups, adults were fed by placing sugar water soaked cotton balls on the top of the mesh lid, replaced daily. Individual adult male and female Cx. pipiens were collected at different days postemergence (0, 6, 15, 27) and stored in a −20°C freezer until samples were prepared for stable isotope analysis.
Experiment 2
The results of experiment 1 demonstrated that we needed to increase the concentrations of 15N, therefore, experiment 2 evaluated higher concentrations and also evaluated 13C enrichment. The concentrations for nitrogen were 0.33 atom% 15N (low) and 1.04 atom% 15N (high), and concentrations for carbon were 0.24 atom% 13C (low) and 0.76 atom% 13C (high). Two replicates were run for each dosage for a total of four 15N trays and four 13C trays containing 1 liter of water and 100 first-instar larvae each. In addition, two trays of controls received the same amount of food but no stable isotope additions. We added 0.2 g of liver powder, 0.14 g of brewer’s yeast, and 15N-potassium nitrate (0.9 mg and 2.7 mg for low and high, respectively) or 13C-glucose (0.9 mg and 2.7 mg for low and high, respectively) to each larval tray containing first-instar larvae to achieve the above atom percentage. No additional food or stable isotopes were added during larval development and water was not changed. Pupae were transferred to an emergence container and adults then were transferred to plastic cups, as described in experiment 1. Individual fourth-instar larvae were collected from the treatments and prepared for stable isotope analysis, as described in the Sample Preparation and Analysis section. Adult male and female Cx. pipiens were collected at 0-, 5-, and 25-d postemergence. In addition to sugar feeding described in experiment 1, we allowed adult females to blood feed through the mesh of the adult holding cups. Progeny from a 13C-enriched female that took a bloodmeal were reared and then submitted as newly-emerged adults for stable isotope analysis. To simulate pools of 5 and 10 individuals, we combined one adult from an enriched tray with four or nine natural abundance adult females, respectively, and prepared for stable isotope analysis.
Experiment 3
The isotopic enrichment obtained in experiment 2 was sufficient to differentiate marked from unmarked mosquitoes. In experiment 3, we evaluated 15N and 13C-enrichment of larval mosquitoes under one concentration for each stable isotope separately. In this experiment we focused on marker retention and kept adults alive for more days postemergence and encouraged blood-feeding of adult females. The concentration for nitrogen was 0.75 atom% 15N and the concentration for carbon was 0.55 atom% 13C. Three replicates were run for each stable isotope containing 1 liter of water and 100 first-instar larvae each. In addition, two trays of controls received the same amount of food but no stable isotope additions. We added 0.19 g of liver powder, 0.12 g of brewer’s yeast, and 15N-potassium nitrate (1.88 mg) or 13C-glucose (1.88 mg) was added to each larval tray containing first-instar larvae to achieve the above atom percentage. In experiment 2, the larval water was not changed and we did not add additional food or stable isotopes. Consequently, we observed high larval mortality. In experiment 3, the water was changed and new food enriched with 15N-potassium nitrate or 13C-glucose was added at the same dose as the initial feeding every 6 d. In addition to the sugar water, we also offered blood to adult females by using the same techniques described in experiment 2. Individual pupae were collected as described in experiment 1. Adult male and female Cx. pipiens were collected at 0-, 25-, 35-, 45-, and 55-d postemergence.
Field Labeling Protocols
From July to September 2010, we treated urban catch basins in Alsip, IL by using either 15N-potassium nitrate or 13C-glucose as part of a Culex mosquito mark–capture study. We obtained permission from the municipality, state, and county public health department, and the Illinois Environmental Protection Agency to amend catch basins with stable isotopes. We were unable to determine the total amount of nitrogen or carbon in the catch basins to calculate the necessary atom percentage to achieve the targeted levels of enrichment in the lab. Instead, we calculated the appropriate amount of stable isotope additions based on the volume of water in the catch basins. We measured the diameter of the sump in the catch basin and the depth of the water and sediment. We considered half of the sediment was water in our calculations to estimate the total volume of water. We treated nine catch basins with 15N-potassium nitrate and seven catch basins with 13C-glucose. All catch basins receiving either of the stable isotopes were connected by underground storm water drainage pipes that flowed directly to an outlet at the Stony Creek. For the initial treatment on 8 July, we added a solution of distilled water and stable isotopes targeting a treatment of 0.002 g of stable isotope per liter of water in the catch basin. This level of enrichment is similar to the amount of stable isotope added to 1 liter of water in experiment 3. We monitored enrichment by sampling larvae and pupae by using nets and emerging adults using emergence traps (Hamer et al. 2011). We sampled mosquitoes in untreated catch basins as controls to determine natural abundance levels of stable isotopes. Immature and adult mosquitoes were identified to species (Andreadis et al. 2005) and stored in 2-ml tubes in a −20°C freezer until submission for stable isotope analysis. Pools of mosquitoes were prepared by grouping one adult female Cx. pipiens collected from an enriched catch basin with four unenriched colony female Cx. pipiens mosquitoes. On 13 July and 15, we collected mosquitoes and submitted for the stable isotope analysis to Isotech Laboratories Inc. Results received 1 wk later demonstrated more enrichment than necessary, and we reduced the subsequent treatments to 0.001 g of stable isotopes per liter of water. We continued to monitor enrichment in mosquitoes and performed six subsequent treatments (16 July, 26 July, 6 August, 20 August, 3 September, and 19 September) after rain events that flushed the mosquitoes and stable isotope out of the catch basins. We collected invertebrates from the Stony Creek and found no evidence of downstream enrichment (data not shown). In total, we delivered 38.58 g of 15N-potassium nitrate and 5.18 g of 13C-glucose to the catch basins during the study.
Sample Preparation and Analysis
In preparation for stable isotope analysis, we removed the specimens from the −20°C freezer and arranged them in tin capsules in a 96-well tissue-culture microplate, round bottom (BD Falcon, Bedford, MA). For individual specimens, we used 5- by 9-mm tin capsules (Costech Analytical Technologies Inc., Valencia, CA) and for pools of 5–10 individuals, we used 9- by 10-mm tin capsules. The specimens were dried at 50°C for 24 h and the tubes then were crimped shut using forceps cleaned with ethanol between specimens. For the larger tin capsules, we initially used a 48-well cell culture plate to dry the specimens and then transferred the crimped tin capsules to a 96-well plates. Stable isotope analysis was performed by the University of California-Davis Stable Isotope Facility by using a PDZ Europa ANCA-GSL elemental analyzer interfaced to a PDZ Europa 20–20 isotope ratio mass spectrometer (IRMS; Sercon Ltd., Cheshire, United Kingdom). Additional analyses were performed by Isotech Laboratories Inc. by using a Carlo Erba CHNS-O EA1108 (CE Instruments, Milan, Italy) coupled to a ThermoFisher Delta V Plus IRMS (Thermo Fisher Scientific, Bremen, Germany) via a ThermoFinnigan ConFlo III interface (Thermo Electron Corp., Waltham, MA). Stable isotope values are reported as the ratio of the lighter to the heavier isotope referenced against international standards (nitrogen =AIR; carbon =Vienna Pee Dee Belemnite) and defined by δX = [(Rsample-Rstandard)/(Rstandard)] × 1,000.
Statistical Methods
We tested for differences in stable isotopes, larval survival, and wing length among groups by using one-way analysis of variance (ANOVA). Separate tests were used for each marker (15N or 13C), sex, and experiment. Once significant differences among groups were detected, we used a posthoc test (Tukey’s HSD) to compare means among groups (alpha = 0.05). All model residuals were assessed for normality by using diagnostic plots. All means are presented ± SE and all tests were performed using Program R (R Development Core Team 2011).
Results
Isotopic Enrichment
Adult Cx. pipiens were collected from seven different catch basins in 2009 by using emergence traps, and the natural abundance of δ15N and δ13C for females was 1.3 ± 0.81 (n = 5) and −25.4 ± 0.43 (n = 5), respectively, and natural abundance for males was 4.0 ± 0.92 (n = 19) and −28.1 ± 0.64 (n = 19), respectively (Table 1 and 2). For experiment 1, there were no significant differences for δ15N in adult males at different ages postemergence in the low, medium, and high treatment (F = 1.68, df = 9, P = 0.241; F = 0.98, df = 8, P = 0.449; F = 0.90, df = 7, P = 0.486, respectively) and no differences for δ15N in adult females at different ages postemergence for the low, medium, and high treatment (F = 2.22, df = 4, P = 0.229; F = 4.33, df = 6, P = 0.06; F = 3.28, df = 7, P = 0.089, respectively). Male and female mosquitoes from the 15N treatment had significantly higher δ15N than control and natural abundance mosquitoes (F = 66.84, df = 57, P < 0.001; F = 49.75, df = 36, P < 0.001; Table 1).
Table 1.
Experiment one results showing δ15N mean ± SE for adult male and female Cx. pipiens from natural abundance, control, and stable isotope enriched mosquitoes
| Mosquito |
δ15N
|
||
|---|---|---|---|
| n | Mean ± SE | Tukey’s HSDa | |
| Natural abundanceb | |||
| Male | 19 | 4.0 ± 0.9 | A |
| Female | 5 | 1.3 ± 0.8 | a |
| control | |||
| Male | 7 | 9.7 ± 0.5 | B |
| Female | 7 | 8.4 ± 0.5 | b |
| low treatmentc | |||
| Male | 13 | 15.7 ± 0.8 | C |
| Female | 8 | 13.9 ± 0.7 | c |
| medium treatment | |||
| Male | 12 | 21.5 ± 1.1 | D |
| Female | 10 | 15.9 ± 0.9 | c |
| high treatment | |||
| Male | 11 | 22.1 ± 1.2 | D |
| Female | 11 | 16.5 ± 0.8 | c |
Tukey’s HSD test for differences among means (P < 0.05) were run separately for males and females and results are provided as upper case letters and lower case letters, respectively.
Cx pipiens collected from seven different catch basins in 2009.
Stable isotope concentrations were 0.04, 0.08, and 0.12 atom% 15N for the low, medium, and high treatments, respectively.
Table 2.
Experiment 2 results showing δ15N and δ13C mean ± SE for adult male and female Cx. pipiens from the natural abundance, control, and stable isotope enriched mosquitoes at different days post-emergence
| Mosquito |
δ15N
|
δ13C
|
||||
|---|---|---|---|---|---|---|
| n | Mean ± SEa | Tukey’s HSDb | n | Mean ± SE | Tukey’s HSD | |
| Natural abundancec | ||||||
| Male | 19 | 4.0 ± 0.9 | A | 19 | −28.1 ± 0.6 | A |
| Female | 5 | 1.3 ± 0.8 | a | 5 | −25.4 ± 0.4 | a |
| Control | ||||||
| Male | 10 | 10.7 ± 0.2 | A | 10 | −22.5 ± 0.8 | A |
| Female | 2 | 9.5, 11.0 | a | 2 | −19.2, ±19.2 | a |
| Larvae | 3 | 10.9 ± 0.5 | A | 3 | −19.2 ± 0.3 | A |
| Low treatmentd | ||||||
| Zero days post-emergence | ||||||
| Male | 3 | 165.5 ± 9.2 | B | 4 | 108.3 ± 20.3 | C |
| Female | 3 | 164.8 ± 11.3 | b | 2 | 78.9, 99.9 | b |
| Larvae | 3 | 217.8 ± 2.9 | BC | 3 | 76.1 ± 3.6 | BC |
| Poole | 2 | 25.6, 47.6 | A | 2 | 0.6, 44.7 | AB |
| Five days post-emergence | ||||||
| Male | 4 | 190.0 ± 19.4 | B | 3 | 27.0 ± 1.9 | B |
| Female | 4 | 26.7 ± 12.3 | a | |||
| Twenty-five days post-emergence | ||||||
| Male | 4 | 169.5 ± 12.2 | B | 2 | 10.2, 41.8 | AB |
| Female | 4 | 1.5 ± 2.7 | a | |||
| High treatment | ||||||
| Zero days post-emergence | ||||||
| Male | 4 | 264.2 ± 37.8 | C | 2 | 283.1, 320.4 | D |
| Female | 2 | 169.0, 187.3 | b | |||
| Larvae | 3 | 185.3 ± 3.7 | B | 3 | 264.6 ± 12.5 | D |
| Five days post-emergence | ||||||
| Male | 3 | 125.2 ± 42.0 | C | |||
| Female | 2 | 84.3, 258.3 | b | |||
| Twenty-five days post-emergence | ||||||
| Male | 5 | 222.3 ± 23.1 | BC | 4 | 89.5 ± 17.8 | BC |
| Female | 1 | 71.6 | ab | |||
When sample size was less than three, actual values are presented.
Tukey’s HSD test for differences among means (P < 0.05) were run separately for males and females and results are provided as upper case letters and lower case letters, respectively. Larvae and pools were included with the males for the post-hoc tests.
Cx pipiens collected from seven different catch basins in 2009.
Stable isotope concentrations were 0.33 and 1.04 atom% 15N and 0.24 and 0.76 atom% 13C for the low and high treatment, respectively.
Pool contained one adult female from 15N treatment and four natural abundance adult females collected from the field.
For experiment 2, male and female mosquitoes in the 15N treatments had significantly higher δ15N than control and natural abundance mosquitoes (F=82.45, df = 49, P < 0.001; F = 228.35, df = 8, P < 0.001, respectively; Table 2). Male and female mosquitoes in the 13C treatments also had significantly higher δ13C than control and natural abundance mosquitoes (F = 99.28, df = 47, P < 0.001; F = 9.03, df = 13, P < 0.001, respectively), but the posthoc tests show not all means were different (Table 2). For experiment 3, male and female mosquitoes in the 15N treatments had significantly higher δ15N than control and natural abundance mosquitoes (F = 604.7, df = 30, P < 0.001; F = 293.3, df = 12, P < 0.001, respectively; Table 3). Male and female mosquitoes in the 13C treatments also had significantly higher δ13C than control and natural abundance mosquitoes (F=281.89, df =29, P<0.001; F = 13.21, df = 14, P < 0.001, respectively; Table 3). For experiments 2 and 3, we observed a slight decline of δ15N and δ13C as mosquitoes aged for both males and females. However, δ15N and δ13C for females were still significantly different from controls and natural abundance levels at 55 d postemergence. The δ15N for males at 55 d postemergence was significantly different from controls and natural abundance, but we did not have 55-d-old males from the 13C treatment to compare. Overall, females tended to have slightly lower δ15N and δ13C than did males.
Table 3.
Experiment 3 results showing δ15N and δ13C mean ± SE for adult male and female Cx. pipiens from the natural abundance, control, and stable isotope enriched mosquitoes at different days post-emergence
| Mosquito |
δ15N
|
δ13C
|
||||
|---|---|---|---|---|---|---|
| n | Mean ± SEa | Tukey’s HSDb | n | Mean ± SE | Tukey’s HSD | |
| Natural abundancec | ||||||
| Male | 19 | 4.0 ± 0.9 | A | 19 | −28.1 ± 0.6 | A |
| Female | 5 | 1.3 ± 0.8 | a | 5 | −25.4 ± 0.4 | a |
| Control | ||||||
| Male | 4 | 12.2 ± 0.7 | A | 4 | −23.2 ± 0.9 | A |
| Female | 3 | 13.2 ± 0.3 | a | 3 | −23.7 ± 0.5 | a |
| Treatmentd | ||||||
| Zero days post-emergence | ||||||
| Male | 2 | 795.3, 796.8 | C | 2 | 489.7, 603.1 | C |
| Female | ||||||
| Pupae | 2 | 727.6, 867.6 | C | 2 | 496.7, 592.5 | C |
| Twenty five days post-emergence | ||||||
| Male | 3 | 776.0 ± 39.8 | C | 3 | 163.6 ± 28.3 | B |
| Female | 3 | 541.9 ± 9.6 | b | 5 | 96.3 ± 13.1 | b |
| Thirty five days post-emergence | ||||||
| Male | 3 | 747.5 ± 12.1 | B | 2 | 115.0, 193.0 | B |
| Female | 4 | 754.0 ± 11.4 | c | 3 | 111.0 ± 16.8 | b |
| Forty five days post-emergence | ||||||
| Male | 2 | 735.7, 763.7 | C | 3 | 136.8 ± 14.4 | B |
| Female | 2 | 653.9, 824.2 | c | 2 | 80.9, 84.6 | ab |
| Fifty five days post-emergence | ||||||
| Male | 2 | 542.6, 711.2 | B | |||
| Female | 1 | 514.0 | b | 2 | 72.8, 241.3 | b |
When sample size was less than three, actual values are presented.
Tukey’s HSD test for differences among means (P < 0.05) were run separately for males and females and results are provided as upper case letters and lower case letters, respectively. Pupae were included with the adult males for the post-hoc tests.
Cx pipiens collected from seven different catch basins in 2009.
Stable isotope concentrations were 0.75 atom% 15N and 0.55 atom% 13C.
Isotopic Enrichment in Mosquito Pools, Larvae, and Pupae
Two pools, each containing one enriched Cx. pipiens (low 15N and 13C treatment in experiment 2) and 4 natural abundance Cx.pipiens had higher, but not significantly higher, δ15N and δ13C compared with control and natural abundance values. Pools containing 10 individuals (1 enriched and 9 natural abundance) contained too much nitrogen and carbon for the IRMS to provide reliable results. Fourth-instar larvae and pupae had similar δ15N and δ13C compared with newly emerged adults from the same treatment (Tables 2, 3).
Isotopic Enrichment After Blood-feeding and in Progeny
Three females from the 13C treatment in experiment 3 took a bloodmeal, laid an egg raft, and then were harvested at 25 d postemergence for stable isotope analysis. These females with a previous blood-meal had a mean δ13C of 95.9 ± 16.1, and two females that were harvested from the same treatment at the same age but did not take a bloodmeal had a mean δ13C of 96.8 ± 16.1, showing no reduction in enrichment because of blood feeding. None of the 15N-labeled females took a bloodmeal. A 14-d-old female from the 13C low treatment in experiment 2 took a bloodmeal, laid an egg raft, and was collected at 27-d postemergence with a δ13C of −1.33. We were able to rear four of the progeny and the four males had a mean δ13C of −21.7 ±0.18. Unenriched control males from the same experiment had a δ13C of −22.5 ± 0.85 (n = 10), showing no evidence of transgenerational marking.
Influence of Enrichment on Larval Survival, Development, and Wing Length
There was no significant difference in immature mosquito survival among treatments for experiment 1 (F = 1.18, df = 3, P = 0.423) or experiment 3 (F = 0.10, df = 2, P = 0.906) but there was for experiment 2 (F = 21.6, df = 4, P = 0.002; Table 4). Experiment 2 showed that the 13C-enriched mosquitoes had higher survival compared with the control and the 15N-enriched mosquitoes, but this pattern was not consistent in experiment 3, and the rearing protocol in experiment 2 resulted in high larval mortality and smaller adults relative to the other experiments. Mosquito wing lengths were not significantly different among isotopic-enriched treatments and controls for males but there were significant differences for females in experiment 1 and 3 (F = 2.93, df = 48, P = 0.043; F = 2.93, df = 48, P = 0.043; Table 5).
Table 4.
Larval survival (percent of adults emerging) and number of days for immature development from each treatment in each experiment
| Experiment | Treatment | Survival
|
Days until emergence (Mean ± SE) | |
|---|---|---|---|---|
| Mean ± SE | Tukey’s HSDa | |||
| 1 | Control | 73.0 ± 4.0 | 16.5 ± 0.1 | |
| 15N-Low | 75.5 ± 3.5 | 17.0 ± 0.2 | ||
| 15N-Medium | 76.5 ± 4.5 | 17.1 ± 0.2 | ||
| 15N-High | 87.0 ± 9.0 | 17.8 ± 0.2 | ||
| 2 | Control | 21.0 ± 3.0 | A | 14.3 ± 0.3 |
| 13C-Low | 35.0 ± 2.0 | B | 14.2 ± 0.2 | |
| 13C-High | 29.0 ± 0.0 | AB | 14.1 ± 0.2 | |
| 15N-Low | 20.0 ± 1.0 | A | 13.5 ± 0.2 | |
| 15N-High | 13.5 ± 1.5 | A | 13.7 ± 0.2 | |
| 3 | Control | 50.5 ± 0.5 | 17.0 ± 0.3 | |
| 13C | 46.0 ± 15.6 | 15.6 ± 0.2 | ||
| 15N | 43.3 ± 3.3 | 14.9 ± 0.2 | ||
Post-hoc tests show differences among means. Blank values indicate no difference among groups.
Table 5.
Adult Culex pipiens wing length (mm) from the different treatments in each experiment
| Experiment | Treatment | Male wing length (mm)
|
Female wing length (mm)
|
||||
|---|---|---|---|---|---|---|---|
| n | Mean ± SE | Tukey’s HSDa | n | Mean ± SEb | Tukey’s HSD | ||
| 1 | Control | 10 | 3.0 ± 0.1 | 13 | 3.6 ± 0.1 | AB | |
| 15N Low | 14 | 3.1 ± 0.0 | 12 | 3.7 ± 0.0 | B | ||
| 15N Med | 12 | 3.0 ± 0.0 | 12 | 3.6 ± 0.1 | AB | ||
| 15NHigh | 13 | 3.0 ± 0.0 | 15 | 3.5 ± 0.0 | A | ||
| 2 | Control | 5 | 2.6 ± 0.1 | 2 | 2.9, 3.6 | ||
| 15N Low | 8 | 2.7 ± 0.1 | 1 | 4.0 | |||
| 15NHigh | 3 | 2.8 ± 0.1 | 1 | 3.0 | |||
| 13C Low | 9 | 2.8 ± 0.1 | 8 | 3.0 ± 0.1 | |||
| 13CHigh | 5 | 2.8 ± 0.0 | 5 | 3.2 ± 0.2 | |||
| 3 | Control | 10 | 3.4 ± 0.0 | 10 | 3.7 ± 0.0 | a | |
| 15N | 17 | 3.2 ± 0.0 | 10 | 4.0 ± 0.0 | b | ||
| 13C | 13 | 3.3 ± 0.1 | 9 | 3.7 ± 0.3 | ab | ||
Post-hoc tests show differences among means. Blank values indicate no difference among groups.
When sample size was less than three, actual values are presented.
Isotopic Enrichment of Mosquitoes in the Field
We treated catch basins in the field for three months beginning 8 July, 2011. Mosquitoes were collected from catch basins receiving stable isotopes and untreated controls on 18 different days between 13 July and 8 October 2011.
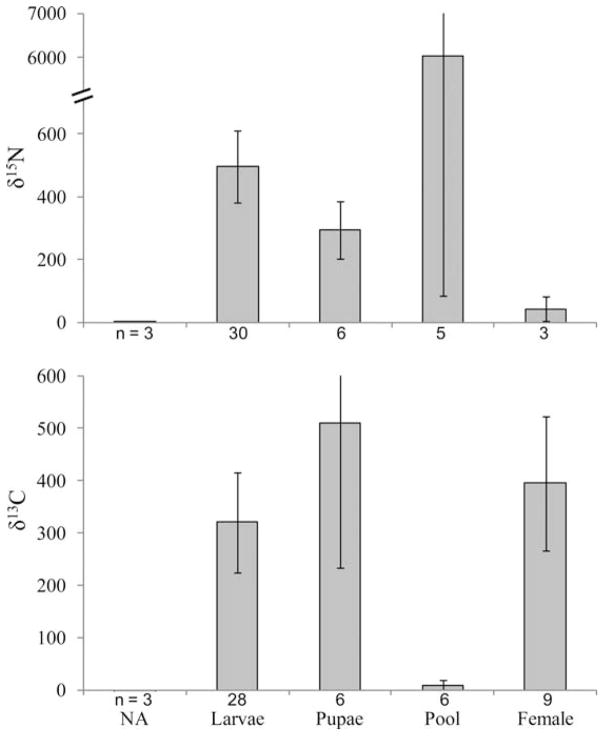
The mean δ15N for all mosquitoes (larvae, pupae, and adults combined) collected from catch basins receiving 15N-potassium nitrate was 1,067.7 ± 673.8 and the mean δ15N for mosquitoes collected from untreated catch basins was 2.6 ± 0.69 (Fig. 1). The mean δ13C for all mosquitoes (larvae, pupae, and adults combined) collected from catch basins receiving 13C-glucose was 319.2 ±69.3 and the mean δ13C for mosquitoes collected from untreated catch basins was −24.4 ± 1.04.
Fig. 1.
Results of isotopic enrichment in the field showing δ15N or δ13C (mean±SE) for natural abundance (NA) Cx. pipiens collected from untreated catch basins and larvae, pupae, pools, and adult female Cx. pipiens mosquitoes collected directly from catch basins receiving stable isotope enrichment. Treatment of the catch basins began 8 July and mosquitoes were collected on 18 different days between 13 July and 8 October, 2011. Each pool includes one adult female mosquito collected from a treated catch basin and four untreated colony control adult females. Sample sizes are indicated below the x-axis.
Discussion
These results demonstrate that sufficient isotopic enrichment can be achieved in the laboratory and field setting through larval enrichment to mark adult Cx. pipiens mosquitoes. Through addition of 0.75 atom% 15N and 0.55 atom% 13C, we sufficiently enriched mosquitoes beyond the recommended 2–3 standard deviations above the isotopic abundance of the natural population of Cx. pipiens (IAEA 2009). We also show that one enriched mosquito can be detected in a pool of five individuals. However, we also found that a pool of 10 individuals contained too much nitrogen and carbon for the IRMS instruments to provide reliable results. We found no evidence that blood feeding reduces 13C enrichment, although small sample sizes warrant additional investigations to the influence of blood feeding on isotopic enrichment. We were unable to judge the influence of blood feeding on 15N enrichment because of the limited numbers of females and limited blood feeding. We found no evidence of transgenerational marking; the progeny of a 13C-enriched female were similar to δ13C levels from unenriched controls. Given that transgenerational marking has been observed in other studies (Williamson et al. 2009, Pecl et al. 2010), and given the limited sample size in this study, additional studies to confirm our results in Cx. pipiens mosquitoes are warranted. Finally, our study also demonstrated that fourth-instar larvae and pupae had similar levels of enrichment as newly emerged adults. This observation is important for monitoring enrichment levels during field studies.
Importantly, we did not observe consistent positive or negative consequences of isotopic enrichment on mosquito morphology or survival. Wing lengths, used as a proxy for adult body size, were very similar among treatments. Also, survival rates of immature mosquitoes in the 15N and 13C treatments showed no positive or negative trends compared with the controls. Positive effects of enrichment might be expected if the nutrient added is limiting and negative effects of enrichment might be expected if toxic levels of nitrogen or carbon are reached, neither of which likely would occur in a field application in a natural container. We assume that bioaccumulation of the enriched nitrogen and carbon in the microbial community (Hamilton et al. 2004), which is a food source for larval mosquitoes, was the primary mechanism for the enrichment observed in this study. The loss of δ13C occurred at a faster rate than δ15N, indicating that the 15N was incorporated into structural body tissues (e.g., chitin), whereas 13C was subject to higher biochemical turnover (e.g., respiration).
Utilizing stable isotopes as a mosquito marking technique offers several advantages. First, a “mark–capture” design can be implemented where naturally breeding mosquitoes can be isotopically enriched without removing them from the environment (Hagler and Jackson 2001). The enrichment of container breeding species such as most Culex spp. mosquitoes offers the advantage that the larval environment is confined, and thus can be easily treated with stable isotopes during larval growth. Other container-breeding mosquito species such as Aedes aegypti and Ae. albopictus might be enriched in the same way, whereas mosquitoes such as An. gambiae or Ae. sollicitans, which use larger bodies of water for larval habitat, might be enriched through broadcast application of stable isotopes. Our breeding-site labeling technique also offers the advantage of being able to mark thousands of mosquitoes with relative ease. For example, a single highly productive septic tank produced 1,663 adult Ae. aegypti and 6,670 adult Cx. quinquefasciatus per day in a small community in southern Puerto Rico (Mackay et al. 2009). Finally, stable isotopes also provide long-lasting retention. The current study demonstrates enrichment for Cx. pipiens up to 55 d old, which were the oldest individuals we tested. Depending on the initial level of enrichment, the rate of loss, and the number of individuals in a pool, mosquitoes marked with stable isotopes essentially can be marked for life.
The negative aspects of the use of stable isotopes are mainly the cost for the isotopically enriched material and the cost for the analysis. The 15N-labeled potassium nitrate and 13C-labeled glucose used in this study cost $62.70 and $176.00 for 1 g, respectively. In all the experiments, we used 0.024 g of 15N-labeled potassium nitrate (costing $1.50) and 0.021 g of 13C-labeled glucose (costing $3.69). When implementing a field study enriching naturally breeding mosquitoes, the cost of the material would depend on the atom percentage necessary to sufficiently enrich individuals and can be estimated based on the total volume of water. The catch basins used in this study were either 60 cm or 120 cm in diameter and the mean volume of water in the sump was 318.6 liters. We delivered a total of $2,418 worth of 15N-potassium nitrate and $912 worth of 13C-glucose, however, when purchased in bulk, the price per gram is reduced considerably. In this study, we used 99 atom% 15N potassium nitrate and glucose but a lower grade compound containing a lower atom % could also be used for a cost savings. The cost for stable isotope analysis is very high, but improvements in technology and market demand continue to decrease the cost per sample (Hood-Nowotny and Knols 2007). For example, the cost of the dual analysis for 15N and 13C in this study was $7 per sample. In the context of a field mark–capture study, mosquitoes can be pooled with up to five individuals to increase efficiency.
Finally, an additional drawback of our method is the difficulty of releasing cohorts of marked adults at defined intervals and quantifying the number of marked adults being released. The number of marked adults being released at a distinct time is a necessary parameter when using existing equations to estimate dispersal, population sizes (e.g., Lincoln Index), and daily survival rates (Silver 2008). Clever field designs might allow the estimation of these parameters but new methodological and quantitative techniques also might need to be developed. For example, estimating Culex mosquito production might require the deployment of emergence traps (Hamer et al. 2011). Analytically, it might be necessary to incorporate uncertainty in detection probabilities at different distances from the release point (e.g., occupancy modeling; Mackenzie et al. 2006).
In summary, this study demonstrates that stable isotopes can be used to enrich naturally breeding larval mosquitoes for adult mosquito dispersal studies. We demonstrate proper enrichment levels to segregate marked mosquitoes from natural populations and isotopic marker retention for the life of the mosquito. Utilizing stable isotopes as a marking technique offers advantages that will complement mark–release–recapture studies using traditional markers. Advancements in measuring mosquito movement and dispersal will greatly improve estimates of parameters that constrain and facilitate disease transmission in nature.
Acknowledgments
This project was supported by NSF-EF-0429124, NIH grant AI21884, and the Michigan Agricultural Experiment Station and the Agricultural Research Service, multi-state project NE-507. The mosquito colony was started with eggs provided by Harry Savage and the Cx. pipiens f. molestus colony protocol was provided by Theodore Andreadis. The Todd Ciche Laboratory provided access to equipment for measuring wing lengths. We thank Garrett Berry, Cherie Bryant, Milica Dimic, Patrick Kelly, Angeline Kosnik, and Jennifer Sidge for assistance with the laboratory experiments. Two anonymous reviewers provided constructive comments on an earlier draft of this manuscript.
References Cited
- Anderson JF, Main AJ, Delroux K, Fikrig E. Extrinsic incubation periods for horizontal and vertical transmission of West Nile virus by Culex pipiens pipiens (Diptera: Culicidae) J Med Entomol. 2008;45:445–451. doi: 10.1603/0022-2585(2008)45[445:eipfha]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Thomas MC, Shepard JJ. Identification guide to the mosquitoes of Connecticut. The Connecticut Agricultural Experiment Station; New Haven, CT: 2005. [Google Scholar]
- Baber I, Keita M, Sogoba N, Konate M, Diallo M, Doumbia S, Traore SF, Ribeiro JMC, Manoukis NC. Population size and migration of Anopheles gambiae in the Bancoumana region of Mali and their significance for efficient vector control. PLoS ONE. 2010:5. doi: 10.1371/journal.pone.0010270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellini R, Albieri A, Balestrino F, Carrieri M, Porretta D, Urbanelli S, Calvitti M, Moretti R, Maini S. Dispersal and survival of Aedes albopictus (Diptera: Culicidae) males in Italian urban areas and significance for sterile insect technique application. J Med Entomol. 2010;47:1082–1091. doi: 10.1603/me09154. [DOI] [PubMed] [Google Scholar]
- Bock ME, Milby MM. Seasonal variation of wing length and egg raft size in Culex tarsalis. Proc Calif Mosq Vector Control Assoc. 1981;49:64–66. [Google Scholar]
- Briers RA, Gee JHR, Cariss HM, Geoghegan R. Inter-population dispersal by adult stoneflies detected by stable isotope enrichment. Freshw Biol. 2004;49:425–431. [Google Scholar]
- Carron A. Correlation between wing measurements and dry body weight in male and female Ochlerotatus (Ochlerotatus) caspius (Pallas, 1771) (Diptera: Culicidae) Eur Mosq Bull. 2007:4–8. [Google Scholar]
- Dohm DJ, Sardelis MR, Turell MJ. Experimental vertical transmission of West Nile virus by Culex pipiens (Diptera : Culicidae) J Med Entomol. 2002;39:640–644. doi: 10.1603/0022-2585-39.4.640. [DOI] [PubMed] [Google Scholar]
- Dye C. Vectorial capacity: must we measure all its components? Parasitol Today. 1986;2:203–209. doi: 10.1016/0169-4758(86)90082-7. [DOI] [PubMed] [Google Scholar]
- Hagler JR, Jackson CG. Methods for marking insects: current techniques and future prospects. Annu Rev Entomol. 2001;46:511–543. doi: 10.1146/annurev.ento.46.1.511. [DOI] [PubMed] [Google Scholar]
- Hamer GL, Kelly PH, Focks DA, Goldberg TL, Walker ED. Evaluation of a novel emergence trap to study Culex mosquitoes in urban catch basins. J Am Mosq Control Assoc. 2011;27:142–147. doi: 10.2987/10-6090.1. [DOI] [PubMed] [Google Scholar]
- Hamilton SK, Tank JL, Raikow DF, Siler ER, Dorn NJ, Leonard NE. The role of instream vs allochthonous N in stream food webs: modeling the results of an isotope addition experiment. J N Am Benthol Soc. 2004;23:429–448. [Google Scholar]
- Harrington LC, Scott TW, Lerdthusnee K, Coleman RC, Costero A, Clark GG, Jones JJ, Kitthawee S, Kittayapong P, Sithiprasasna R, et al. Dispersal of the dengue vector Aedesaegypti within and between rural communities. Am J Trop Med Hyg. 2005;72:209–220. [PubMed] [Google Scholar]
- Helinski MEH, Hood-Nowotny R, Mayr L, Knols BGJ. Stable isotope-mass spectrometric determination of semen transfer in malaria mosquitoes. J Exp Biol. 2007;210:1266–1274. doi: 10.1242/jeb.002642. [DOI] [PubMed] [Google Scholar]
- Helinski MEH, Hood RC, Knols BGJ. A stable isotope dual-labelling approach to detect multiple insemination in un-irradiated and irradiated Anopheles arabiensis mosquitoes. Parasit Vectors. 2008a;1 doi: 10.1186/1756-3305-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helinski MEH, Hood RC, Gludovacz D, Mayr L, Knols BGJ. A N-15 stable isotope semen label to detect mating in the malaria mosquito Anopheles arabiensis. Patton Parasit Vectors. 2008b;1 doi: 10.1186/1756-3305-1-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hershey AE, Pastor J, Peterson BJ, Kling GW. Stable isotopes resolve the drift paradox for baetis mayflies in an arctic river. Ecology. 1993;74:2315–2325. [Google Scholar]
- Hood-Nowotny R, Knols BGJ. Stable isotope methods in biological and ecological studies of arthropods. Entomol Exp Appl. 2007;124:3–16. [Google Scholar]
- Hood-Nowotny R, Mayr L, Knols BGJ. Use of carbon-13 as a population marker for Anophelesarabiensis in a sterile insect technique (SIT) context. Malar J. 2006;5 doi: 10.1186/1475-2875-5-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inger R, Bearhop S. Applications of stable isotope analyses to avian ecology. Ibis. 2008;150:447–461. [Google Scholar]
- [IAEA] International Atomic Energy Agency. Manual for the use of stable isotopes in entomology. Vienna, Austria: 2009. p. 69. [Google Scholar]
- Kothera L, Godsey M, Mutebi JP, Savage HM. A comparison of aboveground and belowground populations of Culex pipiens (Diptera: Culicidae) mosquitoes in Chicago, Illinois, and New York City, New York, using microsatellites. J Med Entomol. 2010;47:805–813. doi: 10.1603/me10031. [DOI] [PubMed] [Google Scholar]
- Lapointe DA. Dispersal of Culex quinquefasciatus (Diptera : Culicidae) in a Hawaiian rain forest. J Med Entomol. 2008;45:600–609. doi: 10.1603/0022-2585(2008)45[600:docqdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Lindquist AW, Ikeshoji T, Grab B, Demeillo B, Khan ZH. Dispersion studies of Culex pipiens fatigans tagged with 32P in Kemmendine area of Rangoon, Burma. Bull World Health Org. 1967;36:21–37. [PMC free article] [PubMed] [Google Scholar]
- Mackay AJ, Amador M, Diaz A, Smith J, Barrera R. Dynamics of Aedes aegypti and Culex quinquefasciatus in septic tanks. J Am Mosq Control Assoc. 2009;25:409–416. doi: 10.2987/09-5888.1. [DOI] [PubMed] [Google Scholar]
- Mackenzie DI, Nichols JD, Royle JD, Pollock KH, Bailey LL, Hines JE. Occupancy estimation and modeling: inferring patterns and dynamics of species occurrence. Elsevier Inc; London, United Kingdom: 2006. [Google Scholar]
- Macneale KH, Peckarsky BL, Likens GE. Stable isotopes identify dispersal patterns of stonefly populations living along stream corridors. Freshw Biol. 2005;50:1117–1130. [Google Scholar]
- Milby MM, Reisen WK. Estimation of vectorial capacity: vector survivorship. Bull Soc Vector Ecol. 1989;14:47–54. [Google Scholar]
- Munro AR, Gillanders BM, Elsdon TS, Crook DA, Sanger AC. Enriched stable isotope marking of juvenile golden perch (Macquaria ambigua) otoliths. Can J Fish Aquat Sci. 2008;65:276–285. [Google Scholar]
- Pauli JN, Ben-David M, Buskirk SW, DePue JE, Smith WP. An isotopic technique to mark mid-sized vertebrates non-invasively. J Zool. 2009;278:141–148. [Google Scholar]
- Pecl GT, Doubleday ZA, Danyushevsky L, Gilbert S, Moltschaniwskyj NA. Transgenerational marking of cephalopods with an enriched barium isotope: a promising tool for empirically estimating post-hatching movement and population connectivity. ICES J Mar Sci. 2010;67:1372–1380. [Google Scholar]
- Pumpuni CB, Walker ED. Population-size and survivorship of adult Aedes triseriatus in a scrap tire-yard in Northern Indiana. J Am Mosq Control Assoc. 1989;5:166–172. [PubMed] [Google Scholar]
- R Development Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing. Version 2.13; Vienna, Austria: 2011. [Google Scholar]
- Reisen WK, Milby MM, Meyer RP. Population-dynamics of adult Culex mosquitoes (Diptera, culicidae) along the Kern Kern River, Kern-County, California, in 1990. J Med Entomol. 1992;29:531–543. doi: 10.1093/jmedent/29.3.531. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Lothrop HD, Lothrop B. Factors influencing the outcome of mark–release–recapture studies with Culex tarsalis (Diptera : Culicidae) J Med Entomol. 2003;40:820–829. doi: 10.1603/0022-2585-40.6.820. [DOI] [PubMed] [Google Scholar]
- Reisen WK, Fang Y, Martinez VM. Effects of temperature on the transmission of West Nile virus by Culex tarsalis (Diptera : Culicidae) J Med Entomol. 2006;43:309–317. doi: 10.1603/0022-2585(2006)043[0309:EOTOTT]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Rubenstein DR, Hobson KA. From birds to butterflies: animal movement patterns and stable isotopes. Trends Ecol Evol. 2004;19:256–263. doi: 10.1016/j.tree.2004.03.017. [DOI] [PubMed] [Google Scholar]
- Russell RC, Webb CE, Williams CR, Ritchie SA. Mark–release–recapture study to measure dispersal of the mosquito Aedes aegypti in Cairns, Queensland, Australia. Med Vet Entomol. 2005;19:451–457. doi: 10.1111/j.1365-2915.2005.00589.x. [DOI] [PubMed] [Google Scholar]
- Silver JB. Mosquito ecology: field sampling methods. Springer; New York: 2008. [Google Scholar]
- Trpis M, Hausermann W. Dispersal and other population parameters of Aedes aegypti in an African village and their possible significance in epidemiology of vector-borne diseases. Am J Trop Med Hyg. 1986;35:1263–1279. doi: 10.4269/ajtmh.1986.35.1263. [DOI] [PubMed] [Google Scholar]
- Walker ED, Copeland RS, Paulson SL, Munstermann LE. Adult survivorship, population-density, and body size in sympatric populations of Aedes triseriatus and Aedes hendersoni (Diptera, Culicidae) J Med Entomol. 1987;24:485–493. doi: 10.1093/jmedent/24.4.485. [DOI] [PubMed] [Google Scholar]
- Welch CH, Kline DL, Allan SA, Barnard DR. Laboratory evaluation of a dyed food marking technique for Culex quinquefasciatus (Diptera : Culicidae) J Am Mosq Control Assoc. 2006;22:626–628. doi: 10.2987/8756-971X(2006)22[626:LEOADF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Wilkins EE, Smith SC, Roberts JM, Benedict M. Rubidium marking of Anopheles mosquitoes detectable by field-capable X-ray spectrometry. Med Vet Entomol. 2007;21:196–203. doi: 10.1111/j.1365-2915.2007.00683.x. [DOI] [PubMed] [Google Scholar]
- Williamson DH, Jones GP, Thorrold SR, Frisch AJ. Transgenerational marking of marine fish larvae: stable-isotope retention, physiological effects and health issues. J Fish Biol. 2009;74:891–905. doi: 10.1111/j.1095-8649.2008.02176.x. [DOI] [PubMed] [Google Scholar]