Abstract
Oviposition dynamics and colonization of container habitats by the invasive species, Aedes (Finlaya) japonicus japonicus (Theobald) were examined through the use of ovistrips placed in buckets, and larval surveys of tree holes and tires at sites in central Michigan. In general, oviposition and colonization increased during the study periods, with several sites showing large increases from <10% Ae. j. japonicus initially to over 60% in the following years. Seasonally, higher proportions of Ae. j. japonicus were found in spring and fall collection periods. Ae. j. japonicus larvae co-occurred in the artificial containers with Ae. triseriatus, Ae. hendersoni, several Culex spp., and Anopheles spp. Recent surveys of tire and tree hole habitats at our study areas in mid-Michigan revealed that Ae. j. japonicus had colonized most of these habitats, but maintained relatively low populations in tree holes occupied by Ae. triseriatus. Trends seen in tires from 2008 to 2011, and from gravid trap and New Jersey light traps in 2005–2011, suggest that Ae. j. japonicus populations are stabilizing as they integrate into native Michigan mosquito communities.
Keywords: Aedes japonicus, Aedes triseriatus, tree hole
Spread of the invasive mosquito species, Aedes (Finlaya) japonicus japonicus (Theobald), part of a multi-subspecies Asian complex (Fonseca et al. 2001), across eastern North America has been rapid since it was first noticed in the United States in 1998 (Peyton et al. 1998). Its native range is Japan, China, and Korea (Tanaka et al. 1979), but it has quickly established in parts of the eastern and midwestern United States (reviewed by Andreadis and Wolfe 2010, Cameron et al. 2010), eastern Canada (Thielman and Hunter 2006), and central Europe (Schaffner et al. 2009) among other areas. Ae. j. japonicus has also invaded the northwest United States (Roppo et al. 2004, Irish and Pierce 2008) and has established in parts of Hawaii (Larish and Savage 2005). Ae. j. japonicus was first collected in Michigan in 2003 at the residence of the first author in southwest Eaton county as part of a casual survey of potential Culex larval habitats. Presumably, Ae. j. japonicus entered the United States and other regions via the same mechanism as Ae. albopictus, through the used tire trade (Tatem et al. 2006, Shaffner et al. 2009). However, this has not been firmly established for the United States. Evidence for multiple introductions of this single subspecies in the eastern United States may help explain its rapid range expansion in North America (Fonseca et al. 2001, 2005; Cameron et al. 2010). The species may play a role as a vector of current North American arboviral diseases (Sardelis et al. 2001, 2002a,b, 2003; Turell et al. 2001; Molaei et al. 2009) or in the event of future introduced diseases (Takashima and Rosen 1989, Schaffner et al. 2011), but currently is not thought to be a major vector of any human disease.
Ae. j. japonicus typically uses streamside rock pools and small containers as larval habitats, and seems to also prefer artificial containers in the United States (Andreadis et al. 2001, Scott et al. 2001a, Oliver et al. 2003, Joy and Sullivan 2005, Grim et al. 2007). Its larval habitat preference range overlaps with several other mosquito species, including Ae. atropalpus (Coquillett), Ae. triseriatus (Say), Culex pipiens (L.), and Cx. restuans (Theobald) (Bevins 2007, Armistead et al. 2008a, Andreadis and Wolfe 2010). Because it exploits many types of container habitats as larvae and has been implicated in the displacement of other species of larvae in some locales (Burger and Davis 2008, Andreadis and Wolfe 2010), its establishment may have indirect effects on disease transmission through interactions and competition with native species (Bevins 2007, Armistead et al. 2008b).
Our interests lie in how Ae. j. japonicus may interact with larval Ae. triseriatus, the primary vector of La Crosse encephalitis, and the subject of ongoing larval ecology research in our laboratory. Ae. triseriatus larvae develop primarily in tree holes, but will readily use tires and other artificial containers that contain decaying plant material. In tree-hole systems, it is by far the dominant mosquito species in our state and often the sole mosquito species found as larvae in tree holes near ground level. Ae. hendersoni is another common tree hole dwelling species, but tends to colonize tree holes that are elevated several meters or more above the tree base (Sinsko and Grimstadt 1977). Most previous studies have not examined if Ae. j. japonicus will successfully establish in tree-hole systems in the eastern United States. The species has been found in tree holes (Bevins 2007) but it more readily colonizes tires and other artificial containers that are also used as larval habitats by Ae. triseriatus (Joy and Sullivan 2005, Kaufman et al. 2005, Burger and Davis 2008, Andreas and Wolfe 2010).
In this study, we began investigation into the potential interactions between Ae. j. japonicus and Ae. triseriatus by quantifying oviposition of these species in artificial containers at sites expected to harbor Ae. triseriatus populations. We also surveyed co-occurring larvae in the artificial containers, and examined tree holes and tires at our long-term study sites for the presence and relative abundance of the invasive species.
Materials and Methods
The study was conducted at sites in Saginaw, Clinton, Eaton, and Ingham counties in central lower Michigan during the summer or early fall periods of 2006, 2007, and 2008, with additional larval surveys in tires and tree holes at two sites near the campus of Michigan State University in 2008–2011. Sites (Table 1; Fig. 1) were chosen based on previous collections of Ae. triseriatus in nearby areas and their low potential exposure to vandalism. Although 16 sites were monitored initially, only nine were consistently sampled over the entire study period described, and only the latter are included in the results.
Table 1.
Sites used for ovistrip collections from 2006 to 2008
| County | Classification | Site code | Description |
|---|---|---|---|
| Eaton | Rural | Ea-1 | Single family residence in farm land |
| Ingham | Rural | In-1 | Woodlot in farm land |
| Ingham | Rural | In-2 | Woodlot in farm land |
| Ingham | Suburban/urban | In-3 | Cemetery in suburban area |
| Clinton | Rural | Cl-1 | Single family residence in farm land |
| Saginaw | Suburban/urban | Sa-1 | Cemetery in suburban area |
| Saginaw | Suburban/urban | Sa-4 | Cemetery in urban area |
| Saginaw | Suburban/urban | Sa-5 | Cemetery in urban area |
| Saginaw | Rural | Sa-6 | Woodlot in wildlife refuge |
All sites are in lower Michigan.
Fig. 1.
County map of Michigan’s lower peninsula with expanded regions showing locations of ovistrip collection sites (see Table 1). Municipalities (gray squares) close to the sites (black circles) are indicated for reference.
We used dark green plastic buckets (19 liters) with 25 g decaying leaf material and 6 liters of distilled water as attractants for gravid females. The buckets had drain holes at the 6 liters mark to ensure a near-constant water level. Because the species of interest lay eggs on container walls at the water line, we attached “ovistrips” (a 4 × 6 inch strip of rough cloth material, off-white muslin in 2006 and light brown burlap in 2007, 2008) to the inside surface of the container, straddling the water line and weighted with fishing sinkers. We collected these on a weekly basis, replacing them with new strips at that time. Water and leaf material were not changed through the course of study each year, though distilled or rainwater was added as necessary, and additional leaf material was added to some if it appeared that quantities had diminished substantially because of decay or disturbance.
We chose to use larval hatch numbers from collected eggs in lieu of identifying and counting individual eggs, even though egg surface features can allow discrimination between Ae. j. japonicus and Ae. triseriatus eggs (Haddow et al. 2009). We found the distinctions between the two species’ eggs to be somewhat subjective and much less obvious than readily observed larval characters, and hence used the following hatching procedures to estimate viable eggs laid and ensure accurate identification of the targeted species.
Collected ovistrips were incubated in closed plastic bags at 15°C, a photoperiod of 16:8 L:D h, for 4 wk (Shoyer and Craig 1983). They were then transferred to containers with dilute (1:10) Nutrient Broth (bacterial growth medium; BD Difco, Franklin Lake, NJ) and allowed to hatch over several days. Larvae were reared to third and fourth instar at low densities with food added ad libitum, preserved in ethanol, and later identified. In 2006, ovistrips were reincubated after the first hatch attempt for an additional 2–3 wk and then resubmerged in nutrient broth to induce a second hatch. The percentage of Ae. j. japonicus was consistent between the first and second hatches in 2006 (analysis of variance [ANOVA]; P = 0.8117; error df = 285), so we eliminated this practice in subsequent seasons. Additionally, in 2006, we recorded only the relative proportion of Ae. j. japonicus larvae for each strip, based on 2–4 ml subsamples of the entire preserved larval hatch (minimum of 10 larvae). In 2007 and 2008, we either counted the entire hatch from each strip or used a constant volume subsample (5 ml from 20 ml preserved larval sample) to determine total hatch numbers as well as the proportion of Ae. j. japonicus.
We also subsampled each container for larvae at the time of ovistrip collection in 2007 and 2008 with a mosquito dipper. These larvae were preserved in 70% ethanol and identified to the genus or species level where possible. Efforts were made to collect at least 10 larvae from each bucket at each collection time, but this was not always possible. Only collections with at least 10 identifiable larvae were used for calculations and presentations of mean relative abundance.
Monitoring of tires and tree holes for Ae. j. japonicus larvae at two of our ongoing Ae. triseriatus study sites near Michigan State University (MSUs) campus, Toumey and Hudson woodlots (Table 1) began in 2008. These sites contain over 60 tree holes, all beech tree pan types located at or near ground level, and over 20 tires that we use in field investigations of Ae. triseriatus larval ecology (Walker et al. 1991). All tires were located within Hudson woodlot, in close proximity (25–50 m) to some of the tree holes. We sampled larvae, when possible, on a monthly or bi-weekly basis during the spring through fall seasons using a turkey baster. At each larval habitat, we attempted to sub-sample at least 10 larvae on each sampling date. Because adequate larval yield because of hatching rates or habitat drying was inconsistent over the sampling period, all tree holes and tires were not necessarily included on each sampling date. Over the 4-yr period, we sampled between 17–23 tree holes and 15–25 tires at Hudson woodlot, and 34–36 tree holes at Toumey woodlot each year. Collected larvae that were third or fourth instar were preserved in ethanol immediately, and earlier stages were reared in low density conditions, with food provide ad libitum until they were large enough to be identified. Although mortality of early instars collected and reared in this fashion was not directly measured, we used general procedures in our laboratory developed for colony maintenance of Ae. triseriatus, Ae. japonicus, and Culex spp. that typically yield 90% or greater survival to the pupal stage.
Centers for Disease Control and Prevention (CDC) gravid traps and New Jersey light traps (NJLT) were set in Saginaw County as part of routine mosquito population and arbovirus surveillance (SCMAC, Saginaw County Mosquito Abatement Commission, www.scmac.org). Gravid traps targeted Culex spp., but also collected Ae. j. japonicus adult females from 2005 to 2011. Individual gravid traps were set at 18–20 locations throughout the county, including urban and rural areas, and were harvested two or three times weekly, 16–18 times per year, giving 263–317 trap nights per year. Gravid trap locations varied in response to reported or anticipated mosquito activity. Traps were baited with hay/yeast/lactalbumin infusions as described for Culex collections in the original description of the trap (Reiter 1983). NJLTs were set at 24–25 permanent locations and were collected three times per week for 16–18 wk, giving 1296–1350 trap nights per year. NJLTs are fairly nonspecific in mosquito species collected, but Ae. j. japonicus can be found in low numbers in these traps when present in an area.
We analyzed percent Ae. j. japonicus hatch from ovistrips, after arcsine-square-root transformation, using repeated measures multivariate ANOVA (MANOVA) and profile analysis. Because of missing values for some sites, particularly in early and late season periods in 2006, we determined percentages from combined hatch data during May through June, July, August, and September through October. Thus, four time periods were used as the repeated variable within a year. Because buckets were replaced at the start of each season and we were, therefore, not measuring the same individual units, we used year (2006/2007/2008) and site type (urban/rural) as main independent factors in the analysis. The interaction term between the repeated variable and main factors are used for hypothesis testing in this analysis (von Ende 2001). We also applied profile analysis to examine differences between the monthly periods (von Ende 2001) and adjusted P value significance levels using the sequential Bonferroni method (Rice 1989). Similarly, we applied MANOVA and profile analysis to total hatch data (log transformed) associated with the ovistrip studies, but because we did not measure total hatch numbers for most samples in 2006, only 2007 and 2008 data sets were analyzed. We also used repeated measures MANOVA to examine the percentage Ae. j. japonicus (arcsine-square root transformed) in larval collections from tree holes and tires in our study area. For these data, a single value was calculated for each container during each year because of missing value and inconsistent presence of adequate larvae for each tree hole during a year. Year was used as the repeated variable with container type (Hudson tree hole, Hudson tire, and Toumey tree hole) as the main factor in the analysis. We followed the MANOVA with individual year ANOVAs, adjusting P value significance levels with sequential Bonferroni methods as above, and using a-posteriori tests when ANOVA was significant. Lastly, we examined NJLT data collected by the Saginaw Mosquito Abatement Commission with a repeated measures MANOVA approach, using year as the repeated variable, to determine if numbers of Ae. j. japonicus (total per trap per year, log transformed) were influenced by urban/rural classifications. We could not analyze the gravid trap data based on urban/rural classifications because these traps were frequently moved throughout the year and therefore could not be assigned to a particular site or site type. Analytical and descriptive statistics were performed using SAS and JMP software (www.jmpin.com, SAS Institute, Inc., Cary, NC).
Results
The percentage of Ae. j. japonicus larvae hatched from ovistrips varied significantly between monthly period and year, with overall percentages increasing from 2006 to 2008 (Figs. 2 and 3; Table 2). Rural sites generally had higher percentages of Ae. j. japonicus than did the suburban/urban classification (grand means for urban and rural ovistrip hatches ranged between 43–81 and 68–95%, respectively, from 2006 to 2008), but site types were not significantly different (Table 2). However, it was clear that individual sites varied considerably and that all sites generally maintained similar levels or increased in Ae. j. japonicus oviposition between 2006 and 2008 (Fig. 4). The total number of larvae hatched varied significantly with monthly period, but this was not affected by year (2007–2008) or urban/rural classification (Figs. 2 and 3; Table 2). On a seasonal basis, the highest percentage of Ae. j. japonicus occurred in the May through June and September through October periods (Fig. 2). Profile analysis contrasts (Table 2) showed that the mean for the May through June period was significantly different from July, September through October was significantly different from August, but July and August did not differ. The differences between monthly periods were not affected by year or site (Table 2). Total larvae hatched increased significantly between May through June and July, but not between the other monthly intervals, and year and site type did not affect this difference (Fig. 2; Table 2).
Fig. 2.
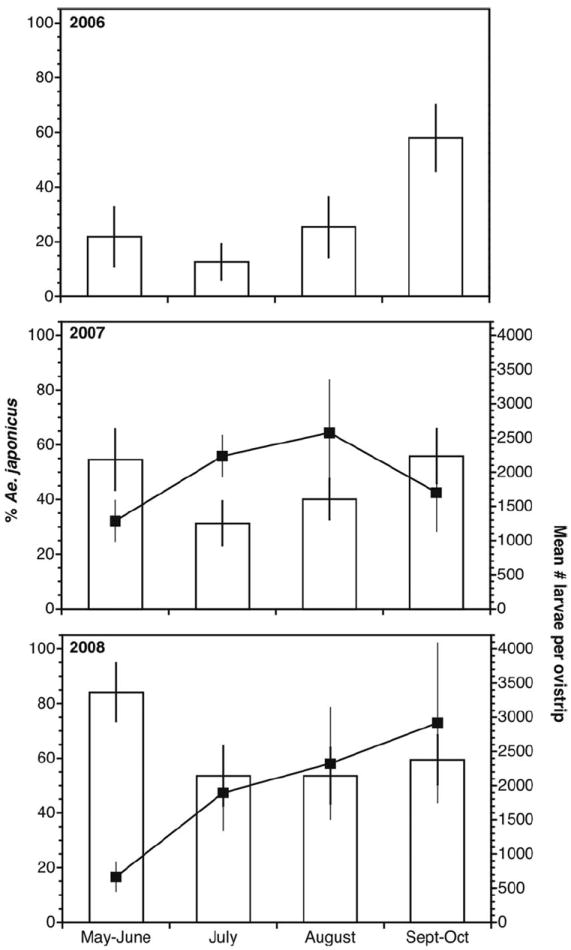
Seasonal trends of Ae. j. japonicus hatched from ovistrips in 2006 through 2008. Percent Ae. j. japonicus (bars) and total number of larvae hatched per ovistrip (black boxes connected by a line) are means ± 1 SE. Total hatch numbers were not measured in 2006.
Fig. 3.
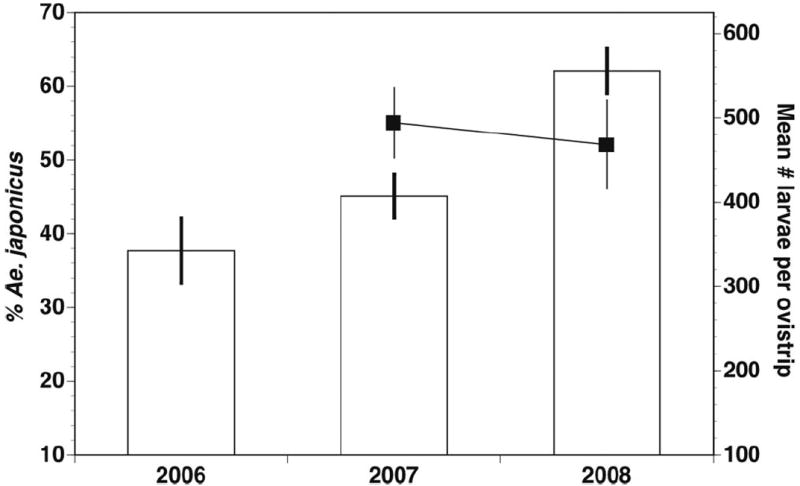
Overall trends by year for percent Ae. j. japonicus and total larval hatches from ovistrip collections in 2006–2008. Percent Ae. j. japonicus (bars) and total number of larvae hatched per ovistrip (black boxes connected by a line) are means ± 1 SE. Total hatch numbers were not measured in 2006.
Table 2.
Repeated measures profile analysis results for percentage Ae. j. japonicus and total larvae hatched from ovistrips
| MANOVA | % Ae. j. japonicus
|
Total larvae hatched
|
||
|---|---|---|---|---|
| F value | P value | F value | P value | |
| Month | 5.57 | 0.0065b | 4.77 | 0.0206b |
| Month*year | 8.78 | 0.0006b | 1.35 | 0.3036 |
| Month*site | 0.35 | 0.7911 | 0.75 | 0.5408 |
| Month*year*site | 0.78 | 0.5195 | 1.17 | 0.3619 |
| Contrastsa | ||||
| May/June–July | ||||
| Mean | 12.17 | 0.0022b | 13.81 | 0.0023b |
| Year | 2.15 | 0.1411 | 0.36 | 0.5557 |
| Site | 0.03 | 0.8595 | 0.96 | 0.3447 |
| July–Aug. | ||||
| Mean | 1.79 | 0.1951 | 0.14 | 0.7167 |
| Year | 1.04 | 0.3718 | 1.43 | 0.2518 |
| Site | 0.09 | 0.7667 | 0.3 | 0.5901 |
| Aug.–Sept./Oct. | ||||
| Mean | 9.73 | 0.0052b | 0.84 | 0.3741 |
| Year | 1.34 | 0.2841 | 1.2 | 0.292 |
| Site | 1.15 | 0.2966 | 0.42 | 0.5283 |
Site refers to rural or urban designation. Numerator and denominator df for MANOVA (Roy’s max root) were 3 and 19–20, respectively, for percent Ae. j. japonicus, and 3 and 12, respectively, for total larvae hatched. Error df for contrasts in profile analysis were 21.
Compares consecutive periods of repeated variable (monthly interval).
Significant with sequential Bonferroni adjustment.
Fig. 4.
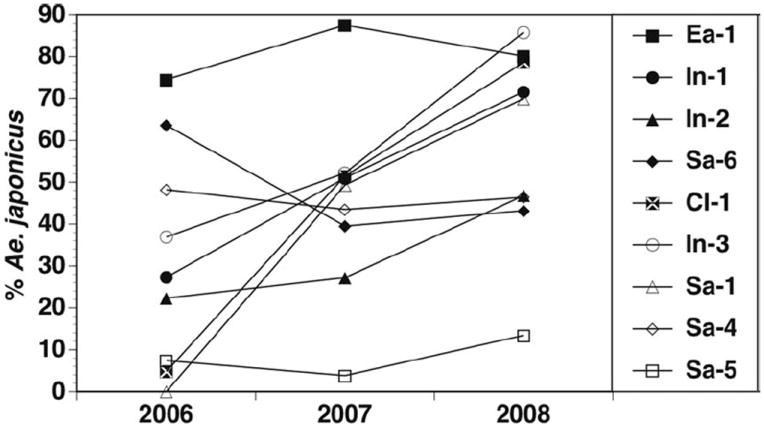
Mean percent Ae. j. japonicus hatched from ovistrips at each site from 2006 to 2007. See Table 1 for site codes and descriptions. Filled symbols are rural sites, open symbols are urban or suburban sites.
Culex spp. were the most common larvae found in the oviposition buckets, but their relative proportion declined seasonally as Ae. j. japonicus generally increased (Fig. 5). Ae. triseriatus tended to be more prominent in July and August periods, and in 2007 compared with 2008. In 2008, identifiable larvae (i.e., third and fourth instars) were dominated by Culex spp. and Ae. j. japonicus. The Culex species present in both years were primarily Cx. restuans and Cx. pipiens, with rare collections (<1%) of Cx. territans. Cx. restuans comprised over 70% of Culex collected in the first two seasonal periods (May through July), while Cx. pipiens was over 70% of identified Culex in the last two periods (August through October).
Fig. 5.
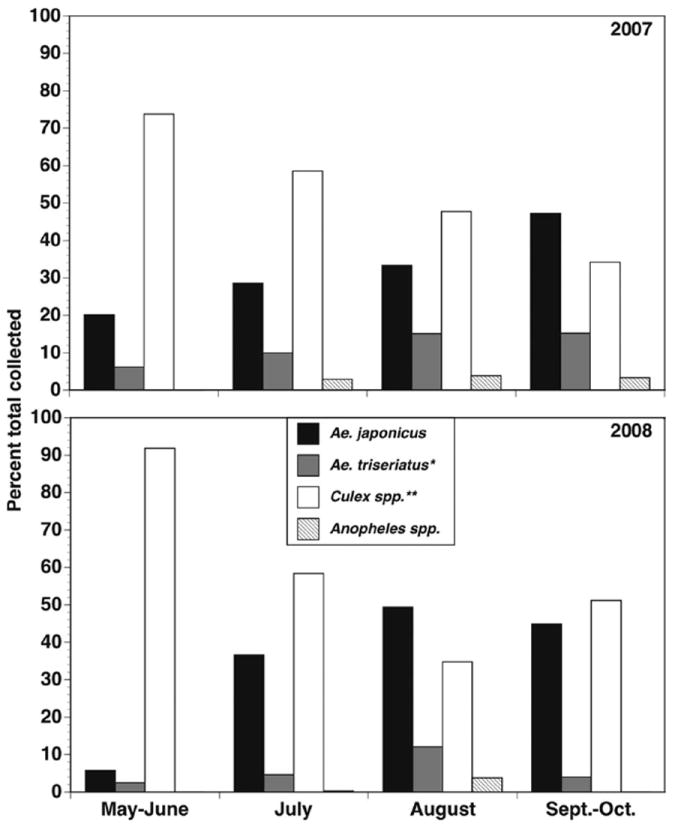
General larval groups and seasonal trends in collection buckets during 2007 and 2008. Values are mean percent for each larval group in each monthly category. *Primarily Ae. triseriatus but also includes Ae. hendersoni. **Primarily Cx. restuans and Cx. pipiens, and rarely Cx. territans.
Larval surveys of tree holes and tires at Hudson and Toumey woodlots indicated that Ae. j. japonicus maintained consistently low populations in natural tree holes, but was found in tires at significantly higher relative populations (Fig. 6; Table 3). Tree holes in Hudson woodlot, which also contained the tire array, had higher relative numbers of Ae. j. japonicus than Toumey woodlot. However, this difference was significant in 2010 only (Table 3). Ae. j. japonicus appeared to decline in Hudson woodlot during 2011 and this was primarily related to low numbers in both tree holes and tires in the early part of the season (April through June). During late season collections (August through September) in 2011, the percentage of Ae. j. japonicus larvae had returned to levels comparable to earlier years (>20 and 40% in tree holes and tires, respectively, data not shown).
Fig. 6.
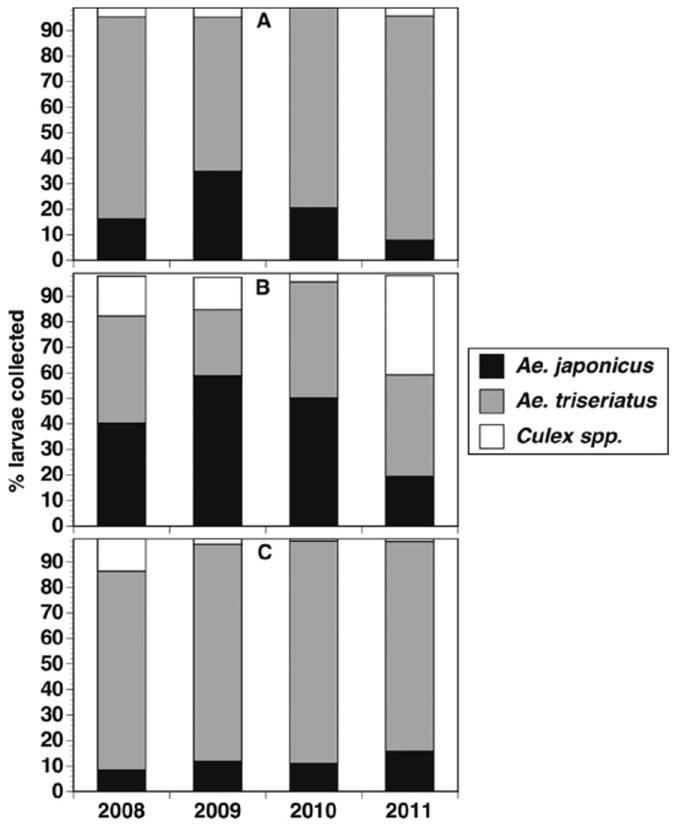
Mean percentages of main larval groups in tree holes and tires in two natural area woodlots near MSUs campus from 2008 to 2011. (A) Hudson woodlot tree holes. (B) Hudson woodlot tires. (C) Toumey woodlot tree holes. Toumey and Hudson woodlots correspond to In-1 and In-2, respectively, in Table 1.
Table 3.
Repeated measures MANOVA and individual ANOVAs by year results for container type effects on percent A. japonicus larvae collected from tree holes and tires in Hudson woodlot (H and HT, respectively), and tree holes in Toumey woodlot (T)
| MANOVA | df num, den | F value | P value |
|---|---|---|---|
| Year | 3,59 | 22.7 | <0.0001 |
| Year* type (H, HT, T) | 3,60 | 10.6 | <0.0001 |
| ANOVA | Error df | F value | P value | Tukey test means comparison |
|
| ||||
| 2008 type (H, HT, T) | 67 | 7.6 | 0.0010 | HT > H, T |
| 2009 type (H, HT, T) | 71 | 29.2 | <0.0001 | HT > H, T |
| 2010 type (H, HT, T) | 79 | 37.0 | <0.0001 | HT > H > T |
| 2011 type (H, HT, T) | 86 | 5.9 | 0.0105 | HT > H, T |
All P values are significant with Bonferroni adjustment. Tukey multiple means comparisons show significantly different (P > 0.05) container rankings for each year.
CDC gravid traps and NJLTs monitored in Saginaw Co. from 2005 through 2011 (Fig. 7) indicated that Ae. j. japonicus abundance has been roughly stable since 2006, with some evidence of decline in females attracted to gravid traps in 2011. We found no significant difference between total Ae. j. japonicus numbers collected from NJLTs in urban or rural locations (MANOVA: Year × Site type; F = 2.2951; P = 0.0832; df numerator/denominator = 6/17).
Fig. 7.
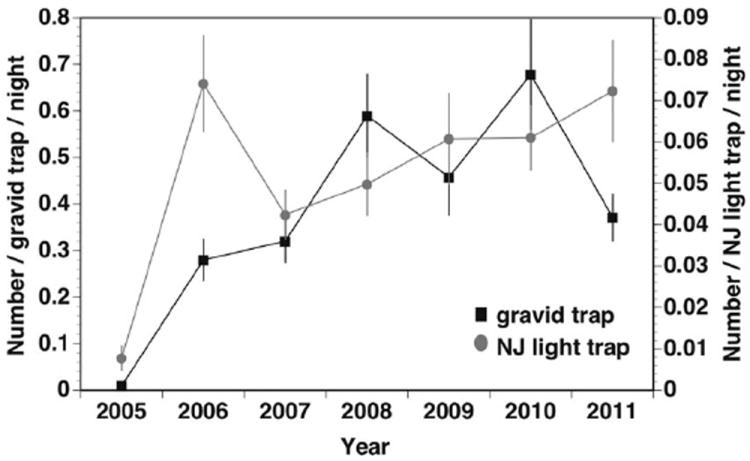
Ae. j. japonicus collected from CDC gravid traps and NJLT in Saginaw County, MI.
Discussion
Ae. j. japonicus appears to be well-established in areas of Michigan and showed high or increasing levels of relative abundance at most of our study sites from 2006 through 2008. Notably, the site of our initial discovery in Eaton County in 2003 continued to yield larvae in high numbers. We have sampled many other locations in Michigan and have always found larvae or collected eggs of this invasive species, usually associated with artificial containers. In its native range and in the eastern United States, Ae. j. japonicus is associated with rock pools along rivers and streams (Tanaka et al. 1979, Andreadis et al. 2001, Scott et al. 2001a), and we have also collected larvae from such habitats in Michigan’s upper peninsula, showing that it has established well north of our current study areas.
Although we did not find a significant positive association with Ae. j. japonicus and rural sites, previous studies have reported that the species tends to be more abundant in rural versus urban areas (e.g., Falco et al. 2002). Bevins (2007) has postulated that this species, because of its adaptability to rock pools, may use river corridors as well as normal transportation routes (e.g., commercial transport of tires and other containers) to expand. The rapid establishment of the species in artificial containers in rural, agricultural areas (Kaufman et al. 2005) indicates that transport and storage of used tires in rural areas is also important. Because Ae. j. japonicus seems particularly sensitive to high temperatures, as reflected in the limited extent of its southern range both in Asia and the United States (Tanaka et al. 1979, Andreadis and Wolfe 2010), it’s possible that less overall shaded areas and urban heat islands also play a role in limiting the species’ abundance in urban areas. We found no difference for urban and rural site designations in the Saginaw NJLT data, but these traps have very low capture rates (average of <1 individual for every 10 trap nights) for Ae. j. japonicus and are not the best measures of local abundance for this species.
Ae. j. japonicus is a reported colonizer of tree hole habitats in its native range (Tanaka et al. 1979, Sota et al. 1994), but this has not often been observed in previous studies in the United States or in Europe (Bevins 2007, Verstreit et al. 2009, Andreadis and Wolf 2010). Bevins (2007) found a high relative abundance of Ae. j. japonicus in a small number of tree holes in the southeastern United States, but this is the only study that suggests the invading species may be very successful in these habitats. Our results suggest that it will not displace Ae. triseriatus from tree holes in Michigan and similar northern areas. However, we consistently find Ae. j. japonicus larvae in natural tree holes in our study area and it remains to be seen if some level of permanent integration into these habitats will occur. Ae. j. japonicus is a very competent competitor with Ae. triseriatus and other tree hole colonizers (Armistead et al. 2008b, Alto 2011, Hardstone and Andreadis 2012).
In contrast, our study and several previous investigations (Andreadis et al. 2001, Joy and Sullivan 2005, Burger and Davis 2008) do show that the species must now be considered a regular component of tire mosquito communities in most parts of the eastern United States and Canada. Proportions of Ae. j. japonicus in tires from Hudson woodlot (Fig. 6) show that this species was routinely dominant in these containers over the study period. Others have found even higher percentages of Ae. j. japonicus larvae in tires over a much wider geographic range and sample size than examined here (e.g., Joy and Sullivan 2005, Burger and Davis 2008, Andreadis and Wolfe 2010). Comparable to our results that examined tires in a rural woodlot, Joy and Sullivan (2005) found that Ae. j. japonicus larvae were relatively less abundant than Ae. triseriatus in nonperidomestic areas. Presumably this reflects higher overall populations of Ae. triseriatus originating from tree holes in these areas. We suggest that this interplay between larval habitat suitability (tree holes supporting more Ae. triseriatus and tires more Ae. j. japonicus) partially explains the trend toward higher tree hole populations of the invader in Hudson woodlot compared with Toumey. However, in a study that examined tires placed in a wooded area in New Hampshire (Burger and Davis 2008) showed that Ae. j. japonicus appeared to be displacing Ae. triseriatus even though tree holes would likely have been a source for Ae. triseriatus in that location. It is of interest to note that ovistrip collections from Toumey woodlot generally showed higher percentages of Ae. j. japonicus than those at Hudson (Fig. 4). This may reflect a lack of alternative preferred oviposition sites because Toumey had no tires and fewer potential larval habitats than Hudson except tree holes.
Many factors could be involved in the low rates of Ae. j. japonicus larvae abundance in natural tree holes, and aside from studies that show it is a competent competitor against Ae. triseriatus in laboratory settings, these factors are uninvestigated. Predators, parasites, pathogens, and partitioning of larval resources are all possible mechanisms that might explain an invading mosquito species success and propensity for displacement or co-existence (Juliano and Lounibos 2005) With the exception of the rarely found Anopheles barberi, larval predators similar to Toxorhynchites are not found in Michigan tree hole systems, so differential predation susceptibility is unlikely. Even An. barberi is not thought to be an important predator in these systems and it is also found in tires where Ae. j. japonicus predominates (Nannini and Juliano 1998). Parasites and pathogens that differentially affect Ae. j. japonicus and are either exclusive to tree holes or are more abundant in those habitats have not yet been studied. Some of our own work (M.G.K., unpublished data) suggests that Ae. j. japonicus and Ae. triseriatus feed essentially on the same groups of microbial resources, with any partitioning favoring Ae. j. japonicus. However, there is little available information on how these resources might be exploited by either species in tree holes compared with tires. It has been suggested that Ae. albopictus’ displacement of Ae. triseriatus from tires, but not tree holes, in the southern United States is related to food resource partitioning (Livdahl and Willey 1991). Another obvious mechanism that needs further study is whether Ae. j. japonicus females generally avoid ovipositing in tree holes. We have collected larvae from tree holes early in the season and the first hatches are almost always dominated by Ae. triseriatus, in contrast to what we have observed in other habitats where Ae. j. japonicus is often the first to hatch. Therefore, there’s little evidence that oviposition rates in tree holes are similar for the two species or that Ae. j. japonicus’ lower relative abundance is the result of attrition in the larval stage assuming initial hatch rates comparable to Ae. triseriatus.
Burger and Davis (2008) point out a key competitive advantage that Ae. j. japonicus has over Ae. triseriatus and other species. They note, and we have observed the same here in Michigan, that the invasive species hatches earlier and is active (as larvae and as ovipositing females) later into the fall than Ae. triseriatus. This translates into at least one more generation per year for Ae. j. japonicus. Our data show that the seasonal range of both ovipositional activity and larval presence of Ae. j. japonicus in artificial containers exceeds that of Ae. triseriatus. This phenology may provide Ae. j. japonicus an advantage in these habitats through avoidance or reduction of intense larval competition during some times of the year, and contribute to its successful integration into the new mosquito community.
It is unclear how Ae. j. japonicus interactions with Ae. triseriatus might affect La Crosse encephalitis transmission in Michigan. Although human cases, including a fatality in the Saginaw area, have been attributed to the disease in the past decade, the incidence of disease in our state is relatively low, even compared with neighboring states (ArboNET/CDC/USGS, http://diseasemaps.usgs.gov). Our laboratory routinely tests Ae. triseriatus and Ae. j. japonicus adults collected by SCMAC and our mosquito surveillance activities near MSU for the virus, however, we have only found it in Ae. triseriatus pools collected from a historically active area south of Saginaw. Bevins (2008) has suggested that larval competition between Ae. triseriatus and Ae. albopictus could result in production of fewer in number, but larger in individual size Ae. triseriatus females that are more capable of transmitting the virus. La Crosse encephalitis dynamics in Tennessee are thought to be linked to the establishment of Ae. albopictus (Erwin et al. 2002), but it is unknown if this is related to vector competency of the invader species or the indirect effects suggested by Bevins (2008). Given that Ae. j. japonicus is reducing relative proportions Ae. triseriatus in some habitats in Michigan, and that it is a very competent vector of La Crosse encephalitis virus (Sardelis et al. 2002b), it might be expected that human cases could change in terms of distribution or frequency. However, because activity has remained relatively low because Ae. j. japonicus was first observed in Michigan almost 10 yr ago, it will be difficult to attribute future changes in La Crosse dynamics solely to influx of the invasive species.
Because Ae. j. japonicus colonizes a wide range of natural and artificial container habitats, it co-occurs with a number of other mosquito species other than Ae. triseriatus. There is some evidence to suggest that it may displace native species (Andreadis and Wolfe 2010), and this is particularly true in the case of Ae. atropalpus in some rock pool habitats. A competitive advantage for Ae. j. japonicus when co-occurring with Ae. atropalpus is that the latter species is autogenous and requires a longer larval stage to meet nutrient demands (Armistead et al. 2008b). Although Ae. atropalpus was reported as widespread in artificial containers in Michigan including tires (Nawrocki and Craig 1989), it was not observed in the current study, suggesting that its population has declined along with Ae. japonicus establishment. Other species likely to co-occur with Ae. j. japonicus larvae in Michigan, including Ae. triseriatus, are not constrained by autogeny and we found little evidence to indicate complete displacement of native species here. Among co-occurring species other than Ae. triseriatus and Ae. atropalpus, Culex spp. appear likely to suffer production losses from some container habitats if Ae. j. japonicus larvae are present. We make this statement based on a general negative association of Culex relative abundance to that of Ae. j. japonicus larvae in the ovistrip buckets (Fig. 5) and woodlot tires (Fig. 6). For the buckets, we are likely underestimating Ae. j. japonicus abundance because we removed many potential larvae when ovistrips were collected on a weekly basis. Because Culex spp. does not oviposit on container walls, their abundance reflected total inputs. Additionally, we have found Ae. j. japonicus to be a superior competitor to Cx. pipiens when larvae are grown in microcosms with decaying leaves and algae as basal food resources (Lorenz 2012). These observations contrast somewhat with studies that indicate Cx. pipiens maintained similar larval populations in tires before and after the introduction of Ae. j. japonicus in Connecticut (Andreadis and Wolfe 2010), and with studies of larval competition between Ae. j. japonicus and Cx. pipiens reared on laboratory diets that indicate the two species are equivalent competitors (Hardstone and Andreadis 2012). Cx. restuans and Cx. pipiens are broadly cosmopolitan in Michigan, and colonize a wider range of larval habitats than even Ae. j. japonicus, so overall populations in a region will probably not be impacted greatly by the invasive species.
From our larval surveys in tree holes and tires in our study area, it might appear that Ae. j. japonicus is experiencing a decline. Because this is only one site, any conclusions are tenuous; however, our general observations of larvae in early 2011 at other sites that normally produce large numbers of the species were that the spring brood was far below what we had seen in the past 5 yr. Gravid trap data from Saginaw Co. also indicate a decline in 2011 (Fig. 7) relative to previous years. However, NJLTs at permanent locations in Saginaw Co. suggest populations between 2006 and 2011 were similar. Whether these observations collectively indicate an overall trend toward stabilization at lower than initial densities as Ae. j. japonicus permanently integrates into the areas is unknown, but similar patterns have been seen for other invasive organisms (Simberloff and Gibbons 2004).
We found that the use of plastic buckets with leaf material is an excellent means for determining the presence of this species because these monitoring devices are inexpensive, convenient, low maintenance, and colonized rapidly by Ae. j. japonicus if present locally. Occasional addition of water of rain or distilled water, and leaf material if necessary, is usually all that is required for season-long monitoring. Ae. j. japonicus eggs hatch readily from May through October in our area, and regular larval collections, as opposed to the use of cloth ovistrips, can determine if the species is present in any number of locations. These observations are consistent with previous studies that have found gravid traps to be the most effective way to collect adults (Scott et al. 2001b, Falco et al. 2002, Lee and Kokas 2004). Although Ae. j. japonicus females will oviposit in sunlit areas (Joy and Sullivan 2005), placement of the buckets in shaded or partially shaded areas is preferable. The proximity to human activity appears to be a nonfactor (Joy 2004) as containers at some of our sites were placed directly adjacent to occupied human dwellings and high numbers of eggs and larvae were still collected.
The use of ovistrips to monitor the relative abundance of Ae. j. japonicus in an area has more limited utility, but we believe it represents an accurate means to compare its abundance to that of Ae. triseriatus and other species that lay eggs on container walls. There are a number of caveats attached to the use and interpretation of ovistrip data. First, there may be some biases related to the strip itself. Not all eggs laid on the container walls were laid on the ovistrip and it is possible that Ae. j. japonicus females were more attracted to the cloth than Ae. triseriatus females. Ae. j. japonicus females are attracted to light colored expanded polystyrene foam, for example (Scott and Crans 2003). However, if it is assumed that larval surveys from the buckets (Fig. 5) were an indicator of eggs not deposited on the cloth strips, then estimates of Ae. j. japonicus relative to Ae. triseriatus would still be high. Secondly, the treatment of collected eggs for hatching was based upon our experience with rearing Ae. triseriatus and breaking diapause of field collected eggs. These conditions (e.g., storage time and temperature) should have been adequate to break any diapause in Ae. triseriatus eggs (Shoyer and Craig 1983), but may not have been optimal for Ae. j. japonicus (Williges et al. 2008). Recent studies in our lab (Lorenz 2012) show that similar storage conditions for eggs collected on seed germination paper from plastic containers at Toumey woodlot yielded hatch rates of over 75%. Taken together, these caveats suggest we may have actually underestimated the proportions of Ae. j. japonicus at our study sites.
This study shows that Ae. j. japonicus has established in many areas of Michigan and continues to integrate itself into container habitat communities. We suggest that the species is reaching more stable populations, but additional monitoring will be needed to determine what will eventually be considered typical levels in our area. There is little evidence to indicate Ae. j. japonicus will totally displace native species in Michigan such as Ae. triseriatus, Cx. pipiens, or Cx. restuans, even at a local level, but studies of its interactions with Ae. atropalpus in Michigan are lacking. However, it is very likely affecting local population levels of vector species and the effect on arbovirus transmission remains to be seen.
Acknowledgments
We gratefully acknowledge Renee Bloome, Amanda Lorenz, Craig Bateman, Blane Doyon, and Angeline Kosnik for assistance with collections and identifications. We also thank S. A. Juliano and two anonymous reviewers for very helpful comments that improved the manuscript. This work was supported by National Institutes of Health MERIT award AI21884.
References Cited
- Alto BW. Interspecific Larval competition between invasive Aedes japonicus and native Aedes triseriatus (Diptera: Culicidae) and adult longevity. J Med Entomol. 2011;48:232–242. doi: 10.1603/me09252. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Anderson JF, Munstermann LE, Wolfe RJ, Florin DA. Discovery, distribution, and abundance of the newly introduced mosquito Ochlerotatus japonicus (Diptera : Culicidae) in Connecticut, USA. J Med Entomol. 2001;38:774–779. doi: 10.1603/0022-2585-38.6.774. [DOI] [PubMed] [Google Scholar]
- Andreadis TG, Wolfe BJ. Evidence for reduction of native mosquitoes with increased expansion of invasive Ochlerotatus japonicus japonicus (Diptera: Culicidae) in the Northeastern United States. J Med Entomol. 2010;47:43–52. doi: 10.1603/033.047.0106. [DOI] [PubMed] [Google Scholar]
- Armistead JS, Nishimura N, Escher RL, Lounibos LP. Larval competition between Aedes japonicus and Aedes atropalpus (Diptera: Culicidae) in simulated rock pools. J Vector Ecol. 2008a;33:238–246. doi: 10.3376/1081-1710-33.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armistead JS, Arias JR, Nishimura N, Lounibos LP. Interspecific larval competition between Aedes albopictus and Aedes japonicus (Diptera : Culicidae) in northern Virginia. J Med Entomol. 2008b;45:629–637. doi: 10.1603/0022-2585(2008)45[629:ilcbaa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevins SN. Establishment and abundance of a recently introduced mosquito species Ochlerotatus japonicus (Diptera : Culicidae) in the Southern Appalachians, USA. J Med Entomol. 2007;44:945–952. doi: 10.1603/0022-2585(2007)44[945:eaaoar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Bevins SN. Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species. Biol Invasions. 2008;10:1109–1117. [Google Scholar]
- Burger JF, Davis H. Discovery of Ochlerotatus japonicus japonicus (Theobald) (Diptera: Culicidae) in southern New Hampshire, USA and its subsequent increase in abundance in used tire casings. Entomol News. 2008;119:439–444. [Google Scholar]
- Cameron EC, Wilkerson RC, Mogi M, Miyagi I, Toma T, Kim HC, Fonseca DM. Molecular phylogenetics of Aedes japonicus, a disease vector that recently invaded Western Europe, North America, and the Hawaiian Islands. J Med Entomol. 2010;47:527–535. doi: 10.1093/jmedent/47.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erwin PC, Jones TF, Gerhardt RR, Halford SK, Smith AB, Patterson LER, Gottfried KL, Burkhalter KL, Nasci RS, Schaffner W. La Crosse encephalitis in eastern Tennessee: clinical, environmental, and entomological characteristics from a blinded cohort study. Am J Epidemiol. 2002;1555:1060–1065. doi: 10.1093/aje/155.11.1060. [DOI] [PubMed] [Google Scholar]
- Falco RC, Daniels TJ, Slamecka MC. Prevalence and distribution of Ochlerotatus japonicus (Dip-tera : Culicidae) in two counties in southern New York State. J Med Entomol. 2002;39:920–925. doi: 10.1603/0022-2585-39.6.920. [DOI] [PubMed] [Google Scholar]
- Fonseca DM, Campbell S, Crans WJ, Mogi M, Miyagi I, Toma T, Bullians M, Andreadis TG, Berry RL, Pagac B, Sardelis MR, Wilkerson RC. Aedes (Finlaya) japonicus (Diptera : Culicidae), a newly recognized mosquito in the United States: analyses of genetic variation in the United States and putative source populations. J Med Entomol. 2001;38:135–146. doi: 10.1603/0022-2585-38.2.135. [DOI] [PubMed] [Google Scholar]
- Fonseca DM, Widdel AK, Spilchiger SE, Hutchinson M, Kramer LD. Fine-scale spatial and temporal population genetics of Aedes japonicus (Diptera : Culicidae), a US newcomer. Am J Trop Med Hyg. 2005;73:304–304. [Google Scholar]
- Grim DC, Jackson BT, Paulson SL. Abundance and bionomics of Ochlerotatus j-japonicus in two counties in Southwestern Virginia. J Am Mosq Control Assoc. 2007;23:259–263. doi: 10.2987/8756-971X(2007)23[259:AABOOJ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Haddow AD, Moulton JK, Gerhardt RR, Mc-Cuiston LJ, Jones CJ. Description of the egg of Ochlerotatus japonicus japonicus (Diptera: Culicidae) using variable pressure scanning electron microscopy. J Med Entomol. 2009;46:9–14. doi: 10.1603/033.046.0102. [DOI] [PubMed] [Google Scholar]
- Hardstone MC, Andreadis TG. Weak larval competition between the invasive mosquito Aedes japonicus japonicus (Diptera: Culicidae) and three resident container-inhabiting mosquitoes in the laboratory. J Med Entomol. 2012;49:277–285. doi: 10.1603/me11050. [DOI] [PubMed] [Google Scholar]
- Irish SR, Pierce CS. Update on the distribution of Ochlerotatus japonicus in Oregon and Washington. J Am Mosq Control Assoc. 2008;24:110–111. doi: 10.2987/5572.1. [DOI] [PubMed] [Google Scholar]
- Joy JE. Larval mosquitoes in abandoned tire pile sites from West Virginia. J Am Mosq Control Assoc. 2004;20:12–17. [PubMed] [Google Scholar]
- Joy JE, Sullivan SN. Occurrence of tire inhabiting mosquito larvae in different geographic regions of West Virginia. J Am Mosq Control Assoc. 2005;21:380–386. doi: 10.2987/8756-971X(2006)21[380:OOTIML]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–574. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PE, Harrington LC, Waldron JK, Rutz DA. The importance of agricultural tire habitats for mosquitoes of public health importance in New York State. J Am Mosq Control Assoc. 2005;21:171–176. doi: 10.2987/8756-971X(2005)21[171:TIOATH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Larish LB, Savage HM. Introduction and establishment of Aedes (Finlaya) japonicus japonicus (Theobald) on the island of Hawaii: implications for arbovirus transmission. J Am Mosq Control Assoc. 2005;21:318–321. doi: 10.2987/8756-971X(2005)21[318:IAEOAF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Livdahl TP, Willey MS. Prospects for invasion: competition between Aedes albopictus and native Aedes triseriatus. Science. 1991;253:189–191. doi: 10.1126/science.1853204. [DOI] [PubMed] [Google Scholar]
- Lee JH, Kokas JE. Field evaluation of CDC gravid trap attractants to primary West Nile virus vectors, Culex mosquitoes in New York State. J Am Mosq Control Assoc. 2004;20:248–253. [PubMed] [Google Scholar]
- Lorenz AR. M S thesis. Michigan State University; East Lansing, MI: 2012. The role of algae in the invasion ecology the mosquito species Aedes japonicus japonicus (Diptera: Culicidae) [Google Scholar]
- Molaei G, Farajollahi A, Scott JJ, Gaugler R, Andreadis TG. Human bloodfeeding by the recently introduced mosquito, Aedes japonicus japonicus, and public health implications. J Am Mosq Control Assoc. 2009;25:210–214. doi: 10.2987/09-0012.1. [DOI] [PubMed] [Google Scholar]
- Nannini MA, Juliano SA. Effects of the facultative predator Anopheles barberi on population performance of its prey Aedes triseriatus (Diptera: Culicidae) Ann Entomol Soc Am. 1998;91:33–42. [Google Scholar]
- Narwrocki SJ, Craig GB., Jr Further extension of the range of the rock pool mosquito, Aedes atropalpus, via tire breeding. J Am Mosq Control Assoc. 1989;5:110–114. [PubMed] [Google Scholar]
- Oliver J, Means RG, Howard JJ. Geographic distribution of Ochlerotatus japonicus in New York State. J Am Mosq Control Assoc. 2003;19:121–124. [PubMed] [Google Scholar]
- Peyton EL, Campbell SR, Candeletti TM, Romanowski M, Crans WJ. Aedes (Finlaya) japonicus japonicus (Theobald), a new introduction into the United States. J Am Mosq Control Assoc. 1999;15:238–241. [PubMed] [Google Scholar]
- Reiter P. A portable, battery-powered trap for collecting gravid Culex mosquitoes. Mosq News. 1983;43:496–498. [Google Scholar]
- Rice WR. Analyzing tables of statistical tests. Evolution. 1989;43:223–225. doi: 10.1111/j.1558-5646.1989.tb04220.x. [DOI] [PubMed] [Google Scholar]
- Roppo MR, Lilja JL, Maloney FA, Sames WJ. First occurrence of Ochlerotatus japonicus in the State of Washington. J Am Mosq Control Assoc. 2004;20:83–84. [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ. Ochlerotatus j. japonicus in Frederick County, Maryland: discovery, distribution, and vector competence for West Nile virus. J Am Mosq Control Assoc. 2001;17:137–141. [PubMed] [Google Scholar]
- Sardelis MR, Dohm DJ, Pagac B, Andre RG, Turell MJ. Experimental transmission of eastern equine encephalitis virus by Ochlerotatus j. japonicus (Diptera : Culicidae) J Med Entomol. 2002a;39:480–484. doi: 10.1603/0022-2585-39.3.480. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Andre ARG. Laboratory transmission of La Crosse virus by Ochlerotatus j. japonicus (Diptera : Culicidae) J Med Entomol. 2002b;39:635–639. doi: 10.1603/0022-2585-39.4.635. [DOI] [PubMed] [Google Scholar]
- Sardelis MR, Turell MJ, Andre RG. Experimental transmission of St. Louis encephalitis virus by Ochlerotatus j. japonicus. J Am Mosq Control Assoc. 2003;19:159–162. [PubMed] [Google Scholar]
- Schaffner F, Kaufmann C, Hegglin D, Mathis A. The invasive mosquito Aedes japonicus in Central Europe. Med Vet Entomol. 2009;23:448–451. doi: 10.1111/j.1365-2915.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- Schaffner F, Vazeille M, Kaufmann C, Failloux A, Mathis A. Vector competence of Aedes japonicus for chikungunya and dengue viruses. Eur Mosquito Bull. 2011;29:141–142. [Google Scholar]
- Scott JJ, Carle FL, Crans WJ. Ochlerotatus japonicus collected from natural rockpools in New Jersey. J Am Mosq Control Assoc. 2001a;17:91–92. [PubMed] [Google Scholar]
- Scott JJ, Crans SC, Crans WJ. Use of an infusion-baited gravid trap to collect adult Ochlerotatus japonicus. J Am Mosq Control Assoc. 2001b;17:142–143. [PubMed] [Google Scholar]
- Scott JJ, Crans WJ. Expanded polystyrene (EPS) floats for surveillance of Ochlerotatus japonicus. J Am Mosq Control Assoc. 2003;19:376–381. [PubMed] [Google Scholar]
- Shroyer DA, Craig GB., Jr Egg diapause in Aedes triseriatus (Diptera: Culicidae): geographic variation in photoperiodic response and factors influencing diapause termination. J Med Entomol. 1983;20:601–607. doi: 10.1093/jmedent/20.6.601. [DOI] [PubMed] [Google Scholar]
- Simberloff D, Gibbons L. Now you see them, now you don’t! Population crashes of established introduced species. Biol Invasions. 2004;6:161–172. [Google Scholar]
- Sinsko MU, Grimstad PR. Habitat separation by differential vertical oviposition of two treehole Aedes in Indiana. Environ Entomol. 1977;6:485–487. [Google Scholar]
- Sota T, Mogi M, Hayamizu E. Habitat stability and the larval mosquito community in treeholes and other containers on a temperate island. Res Popul Ecol. 1994;36:93–104. [Google Scholar]
- Takashima I, Rosen L. Horizontal and vertical transmission of Japanese encephalitis-virus by Aedes japonicus (Diptera, Culicidae) J Med Entomol. 1989;26:454–458. doi: 10.1093/jmedent/26.5.454. [DOI] [PubMed] [Google Scholar]
- Tanaka KK, Mizusawa K, Saugstad ES. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasawara Islands) and Korea (Diptera: Culicidae) Contrib Am Entomol Inst. 1979;16:1–987. [Google Scholar]
- Tatem AJ, Rogers DJ, Hay SI. Global transport networks and infectious disease spread. Adv Parasit. 2006;62:293–343. doi: 10.1016/S0065-308X(05)62009-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thielman A, Hunter FF. Establishment of Ochlerotatus japonicus (Diptera : culicidae) in Ontario, Canada. J Med Entomol. 2006;43:138–142. doi: 10.1603/0022-2585(2006)043[0138:eoojdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera : Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–134. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- Versteirt V, Schaffner F, Garros C, Dekoninck W, Coosemans M, Van Bortel W. Introduction and establishment of the exotic mosquito species Aedes japonicus japonicus (Diptera: Culicidae) in Belgium. J Med Entomol. 2009;46:1464–1467. doi: 10.1603/033.046.0632. [DOI] [PubMed] [Google Scholar]
- Walker ED, Lawson DL, Morgan WT, Klug MJ. Nutrient dynamics bacterial populations and mosquito productivity in tree hole ecosystems. Ecology. 1991;72:1529–1546. [Google Scholar]
- Williges E, Farajollahi A, Scott JJ, McCuiston LJ, Crans WJ, Gaugler R. Laboratory colonizationof Aedes japonicus japonicus. J Am Mosq Control Assoc. 2008;24:591–593. doi: 10.2987/5714.1. [DOI] [PubMed] [Google Scholar]


