Abstract
Engraftment of human hematopoietic stem cells into immunodeficient mice that lack T cells, B cells and natural killer cells results in reconstitution of human blood lineage cells, especially B cells, in the recipient mice. However, these humanized mice do not make any significant level of IgG antibody in response to antigen stimulation. Here, we show that in humanized mice B cells are immature and there is a complete deficiency of CD209+ (DC-SIGN) human dendritic cells (DCs). These defects can be corrected by expression of human GM-CSF and IL-4 in humanized mice. As a result, these cytokine-treated humanized mice produced significant levels of antigen-specific IgG following immunization, including the production of neutralizing antibodies specific for H5N1 avian influenza virus. A significant level of antigen-specific CD4 T cell response was also induced. Thus, we have identified defects in humanized mice and devised approaches to correct these defects such that the platform can be used for studying antibody responses, and to generate novel human antibodies against virulent pathogens and other clinically relevant targets.
Keywords: humanized mice, dendritic cells, cytokines, antigen specific human IgG, neutralizing antibodies
Introduction
Adoptive transfer of human hematopoietic stem cells (HSCs) into immunodeficient mice that lack T cells, B cells and natural killer (NK) cells can lead to stable engraftment of human HSCs (1-5). Differentiation of the transferred HSCs gives rise to different lineages of human blood cells in the recipient mice, which are often referred to as humanized mice. Because humanized mice enable in vivo study of human hematopoiesis, infectious diseases, especially those caused by pathogens that only infect human blood lineage cells, and human blood cell diseases, such as leukemia and lymphoma, the platform has significant applications in both basic and translational biomedical research (1, 2, 6).
In humanized mice, human B cells and T cells are the most abundantly reconstituted cell types among all blood lineage cells. Despite this, humanized mice do not make a significant antibody response after immunization with different antigens in the presence of various adjuvants and through various routes (1, 3, 7). For example, SCID mice that were transplanted with human thymus, bone marrow and skin failed to generate any antigen-specific IgG following immunization with tetanus toxoid (TT) vaccine (7). Although additional transplantation of human lymph nodes into the recipient mice improved antibody response, the level of TT-specific human IgG was still very low (<0.2 μg/ml) (7). When BALB/c-Rag2-/- Il2rg-/- mice were used as recipients of human blood cells, the resulting mice had around 37 μg/ml and 191 μg/ml of circulating human IgM and IgG, respectively, which were, increased to 173 μg/ml and 459 μg/ml after TT immunization. However, the level of antigen-specific human IgM was barely detectable and no antigen-specific IgG was detected (8). When the same BALB/c-Rag2-/-Il2rg-/- recipient mice were treated with recombinant IL-15/IL-15Rα, agonist the total human IgG level increased to ~700 μg/ml. Following TT immunization, TT-specific human IgG was significantly elevated (0.5 IU/ml) as compared to the untreated but immunized humanized mice (0.1 IU/ml) (5). Furthermore, when NOD-scid, Il2rg-/- (NSG) mice were engrafted with human HSCs, no antigen-specific human IgG was elicited upon TT immunization, even after transplantation of human fetal liver and thymus (9). Similarly, humanized NOD-Rag1-/- Il2rg-/- mice had only a low level of human IgM (6 μg/ml) and failed to produce any antigen-specific IgG following TT immunization (10). The poor antibody response in humanized mice has significantly limited the potential of the platform for studying human B cell responses to pathogens and generation of antigen-specific human antibodies.
Induction of an IgG antibody response requires cognate interactions between antigen presentation cells (APCs) and CD4 T cells, and between CD4 T cells and B cells. Many factors are thought to contribute to the poor human antibody responses in humanized mice, including poor reconstitution of myeloid APCs, mismatch between human TCR and mouse MHC molecules, deficiency in human cytokines and block of T and B cell maturation in humanized mice (1, 3, 4, 11, 12). For example, myeloid cells, including dendritic cells (DCs), are poorly reconstituted due to a lack of appropriate human cytokines (11). As a result, CD209+ DCs, which are critical for antigen capture and priming of T cell immune responses (13-15), are completely absent in humanized mice. Similarly, development and maturation of human B cells is blocked in humanized mice and T cell function is impaired (12). Transgenic expression of human MHC class I or II molecules in the recipient mice have shown to enhance HLA-restricted cytotoxic T cell responses (6). However, antibody responses in these mice either remain very poor (9, 10) or have not been reported.
We have previously developed a simple and efficient method to enhance human immune cell reconstitution in humanized mice by expressing appropriate human cytokines via hydrodynamic injection of cytokine-encoding plasmids (11). In particular, we found that expression of human GM-CSF and IL-4 significantly increases reconstitution of human myeloid cells, including DCs. In the present study, we show that GM-CSF and IL-4 treatment promotes development of human CD209+ DCs and maturation of human B cells and T cells. Upon immunization, antigen-specific antibody responses are greatly improved in the cytokine-treated humanized mice, including induction of human neutralizing antibodies against H5N1 influenza viruses. These findings show that the poor antibody responses in humanized mice is partly due to a lack of human CD209+ DCs and a block in B and T cell maturation due to a lack of appropriate human cytokines. Provision of these human cytokines helps overcome these defects and improve antigen-specific antibody responses in humanized mice.
Materials and Methods
HSC isolation, construction of humanized mice and hydrodynamic gene delivery
Human fetal livers (n>8) were obtained from aborted fetuses at 15-23 weeks of gestation, in accordance with the institutional ethical guidelines of the National University Hospital of Singapore. All women gave written informed consent for the donation of their fetal tissue for research. Fetuses were collected within 2 h of the termination of pregnancy. Fetal liver tissue was initially cut into small pieces, and digested with 2 mg/ml collagenase VI (prepared in DMEM) for 15 min at 37°C with periodic mixing. A single cell suspension was prepared by passing the digested tissue through a 100 μm cell strainer (BD Biosciences). Umbilical cord blood was obtained from the National Disease Research Interchange (NDRI) or the Singapore Cord Blood Bank. CD34+ cells from both fetal liver and cord blood were purified with the use of a CD34 positive selection kit (Stem Cell Technologies, Vancouver, BC); the purity of CD34+ cells was 90 to 99%. Viable cells were counted by Trypan Blue exclusion of dead cells. All cells were isolated under sterile conditions. To construct humanized mice, 1-2×105 CD34+ fetal liver or cord blood cells were injected into pups within 48 hrs of birth intracardially. Ten to twelve weeks after engraftment, mice were analyzed for reconstitution by flow cytometry analysis of peripheral blood. Mice that had 40% or more human leukocyte reconstitution were used in subsequent experiments. Hydrodynamic injection of plasmid DNA was exactly as described (11). Briefly, human GM-CSF and IL-4 genes were cloned separately into pcDNA3.1(+) vector (Invitrogen, USA). Plasmid DNA was purified by Maxiprep Kit (Qiagen). For hydrodynamic gene delivery, 10 to 12 week-old humanized mice were injected with 50μg of each plasmid in a total of 1.8 ml saline within 7 seconds using a 27 gauge needle. All research with human samples and mice was performed in accordance of the institutional guidelines.
Immunization and ELISA
Ten to twelve weeks after engraftment, mice were immunized with tetanus toxoid vaccine (Tetavax, Sanofi Pasteur, France). Specifically, 10 μl of the vaccine, representing 1/50 of the recommended vaccination dose for an adult human, was mixed with 90 μl aluminum hydroxide gel (Sigma) and then injected intraperitoneally. Mice were boosted twice with the same dose three weeks apart. After immunization, sera were analyzed by human TT specific IgG ELISA (MP Biomedicals Germany GmbH). Total human IgM and IgG levels were analyzed with human IgM and IgG kit (Bethyl Laboratories, Inc, TX). When NP-KLH (keyhole Limpet Hemocyanin) (Bioresearch Technologies, Novato, CA) was used as antigen, 100 μl of 1 mg/ml NP-KLH was mixed with 100 μl aluminum hydroxide gel and used for immunization as above. Blank ELISA plates (Bethyl Laboratories, INC, TX) were coated with NP-KLH and sera from immunized mice were added. HRP conjugated anti-human IgG antibody (Bethyl Laboratories, Inc, TX) was used as secondary antibody. To induce antibody response to H5N1 avian influenza viruses, humanized mice were immunized twice 2 weeks apart by intraperitoneal injection. Each immunization comprises 100 μl of inactivated H5N1 influenza virus (HA titer 28) with 100 μl of adjuvant (Seppic, France). Sera were collected 14 days after the second immunization by facial vein bleeding. H5N1 influenza viruses used for the immunization are inactivated A/Anhui/1/05, A/Indonesia/CDC669/06 and A/Hong Kong/213/03.
Single cell preparation, antibodies, and flow cytometry
Preparation of mononuclear cells (MNCs) from liver, lung, spleen and bone marrow of humanized mice was described previously (11). The following antibodies were used: CD20 (2H7), CD10 (HI10a), CD5 (UCHT2), CD27 (M-T271), IgM (G20-127), IgD (IA6-2), CD3 (HIT3a), CD4 (RPA-T4), CD40L (TRAP1), CD25 (2A3), CD19 (HIB19), HLA-DR (L243), CD209 (DCN46), CD45 (2D1), CD69 (L78), CD33(WM53) from BD Biosciences; CD40 (5C3), CD80 (2D10), CD86 (IT2.2), mouse CD45.1 (A20) from BioLegend. Cells were stained with appropriate antibodies in 100 μl PBS containing 0.2% BSA and 0.05% sodium azide for 30 min on ice. Flow cytometry was performed on a LSRII flow cytometer using the FACSDiva software (BD, Franklin Lakes, NJ). 10,000 to 1,000,000 events were collected per sample and analyzed using the Flowjo software.
ELISPOT assay
After the third immunization, single cell suspensions were prepared from spleens for ELISPOT assay (R&Dsystems, Minneapolis, MN). Splenocytes containing 1 × 105 CD3+ cells were plated per well in medium alone or in the presence of either 10 ng/ml of PMA or 0.5 μg/ml of a tetanus toxin peptide (Genscript, USA). Spots were counted using an ImmunoSpot S5 Versa Analyzer (Cellular Technology Ltd. Ohio) and analyzed with ImmunoCapture software (Analysis Software).
Immunofluorescence assay
MDCK cells cultured in 96-well plates were infected with H5N1 subtype viruses. The cells were rinsed with PBS and fixed with 4% paraformaldehyde 24h post-infection. Fixed cells were incubated with sera from humanized mice (1:50 in PBS) or H5 monoclonal antibody 2D9 at 37°C for 1 h, rinsed with phosphate buffered saline (PBS) and then incubated with a 1:500 dilution of fluorescein isothiocyanate (FITC)-conjugated rabbit anti-human or anti-mouse IgG (Dako, Denmark). Antibody binding was evaluated by wide-field epi-fluorescence microscopy (Olympus IX71).
Microneutralization assay
Monoclonal antibody (2D9) and polyclonal sera from humanized mice were serially diluted two-fold and incubated with 100 TCID50 of H5N1 virus isolates for 1 h at room temperature and plated in duplicate onto MDCK cells grown in a 96-well plate. The TCID50 of each H5 strain in MDCK cell culture was determined by the Reed and Muench method. The neutralizing titer was assessed as the highest antibody dilution in which no cytopathic effect was observed by light microscopy.
Statistical Analysis
Data are presented as mean and standard error of the mean (SEM). Differences between groups were analyzed via Student t test. A P value of < 0.05 was considered statistically significant. All calculations were performed using the Origin 8.0 software package.
Results
GM-CSF and IL-4 stimulate production of human CD209+ DCs and maturation of human B and T cells in mice
Humanized mice were constructed by adoptive transfer of CD34+ HSCs isolated from human cord blood or fetal liver into sublethally irradiated NSG newborn pups. Twelve weeks after HSC transfer, peripheral blood mononuclear cells (PBMCs) of recipient mice were stained for human and mouse CD45 to determine the reconstitution level of human leukocytes [% human CD45+ cells / (% human CD45+ cells + % mouse CD45+ cells)]. Mice with 40% or more human leukocyte reconstitution were used in this study. To stimulate human DC reconstitution, humanized mice between 12-16 weeks of age were hydrodynamically injected with vector plasmid (control) or plasmids encoding human GM-CSF and/or IL-4. Seven days after injection, splenocytes were stained for antibodies specific for human CD45, CD11c and CD209 and analyzed by flow cytometry. The frequency of CD11c+ cells within the human CD45+ leukocyte population was increased significantly in mice treated with GM-CSF (~17%) or GM-CSF plus IL-4 (~20%) as compared to control mice (~6%) or IL-4-treated mice (~7%) (Fig. 1A and 1B). A prominent population of human CD209+ DCs was detected only in GM-CSF plus IL-4-treated mice, reaching ~30% of CD45+CD11c+ human cells. To investigate whether the cytokine-induced CD209+ DCs are functional, 7 days post injection of GM-CSF and IL-4 plasmids, humanized mice were challenged with lipopolysaccharide (LPS). Twenty-four hrs later, splenocytes were stained for human CD45, CD209 plus CD40, CD80, CD86 or HLA-DR. Although CD40 expression did not change significantly following LPS stimulation, expression of CD80, CD86 and HLA-DR on CD209+ DCs was significantly up-regulated (Fig. 1C). When human CD209+ DCs were purified from peripheral blood and spleen by cell sorting and stimulated with LPS in vitro, a significant level of human IL-12p70 was detected in the culture supernatant (Fig. 1D). These results show that expression of GM-CSF and IL-4 in humanized mice stimulates reconstitution of functional human CD209+ DCs.
Fig. 1. GM-CSF and IL-4 stimulate production of human CD209+ DCs and maturation of human B and T cells in mice.
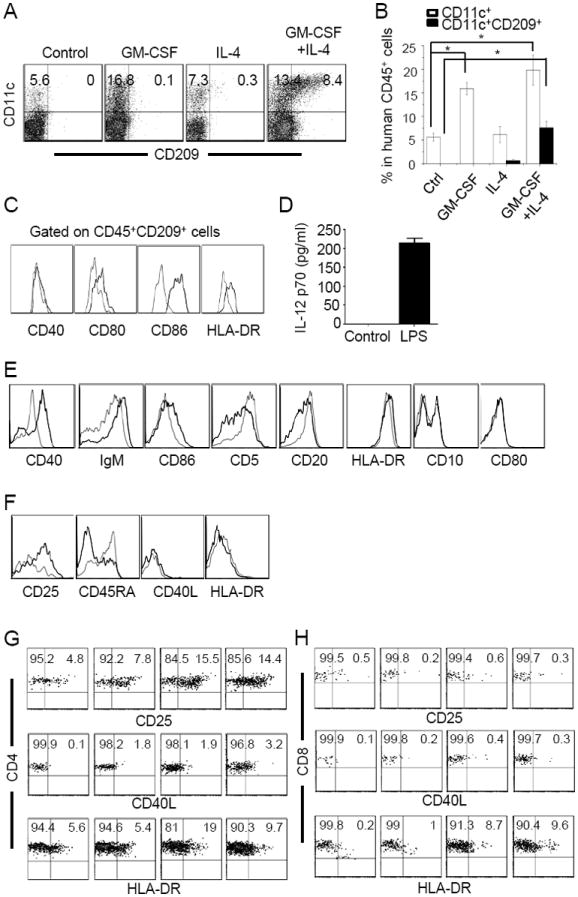
(A, B) Induction of human DCs by cytokine treatment. Humanized mice were hydrodynamically injected with empty pcDNA plasmid (Control) or pcDNA vectors expressing human GM-CSF and/or IL-4. Seven days later, splenocytes were stained for human CD45, CD11c and CD209. (A) representative CD11c versus CD209 plots gating on human CD45+ cells from one of three mice per group. (B) Comparison of frequencies of CD11c+ and CD11c+CD209+ cells within the human CD45+ cell population between non-treated and cytokine-treated mice. The values represent mean ± SEM of three mice per group. (C) Actiavtion of human CD209+ DCs by LPS. Seven days after injection of GM-CSF and IL-4 plasmids, humanized mice were injected with 10μg LPS. Twenty-four hrs later, splenocytes were stained for human CD45, CD209 plus CD40, CD80, CD86 or HLA-DR. Histograms show CD40, CD80, CD86 or HLA-DR expression by human CD45+CD209+ DCs without LPS (thin line) and with LPS treatment (thick line). Representative data from one of the three mice per group are shown.
(D) Induction of IL-12 expression by human CD209+ DCs by LPS in vitro. Seven days after injection of GM-CSF and IL-4 plasmids, CD45+CD33+CD209+ DCs were purified from PBMC and splenocytes by cell sorting. Purified DCs (2×105) were cultured alone (Control) or in the presence of LPS (1 μg/ml). Supernatants were analyzed 24 hrs later for human IL-12p70 by ELISA.
(E) Maturation of human B cells following GM-CSF and IL-4 treatment in humanized mice. Seven days after injection of empty plasmid or GM-CSF and IL-4 plasmids, spelnocytes were stained for human CD45, CD19 plus the indicated markers. Histograms compare CD40, IgM, CD86, CD5, CD20, HLA-DR, CD10 and CD80 expression by human CD45+CD19+ B cells in mice treated with empty plasmid (thin line) or GM-CSF and IL-4 plasmids (thick line). Representative data from one of the seven mice per group are shown.
(F) Effect of GM-CSF and IL-4 treatment on T cells in humanized mice. Seven days after injection of empty plasmid or GM-CSF and IL-4 plasmids, spelnocytes were stained for human CD45, CD3 plus CD25, C45RA, CD40L or HLA-DR. Histograms compare CD40L, CD25, CD45RA or HLA-DR expression by human CD45+CD3+ T cells in mice treated with empty plasmid (thin line) or GM-CSF and IL-4 plasmids (thick line). Representative data from one of the seven mice per group are shown.
(G, H) Staining profiles of CD4 and CD8 versus CD25, CD40L and HLA-DR gating on CD4+ (G) and CD8+ (H) human T cells, respectively. Ctrl: vector plasmid treated mice. Representative data from one of seven mice per group are shown. The numbers indicate percentages of cells in the gated quadrants.
Expression of human GM-CSF and IL-4 in humanized mice also has a significant effect on the maturation status of human T and B cells. Seven days post injection of vector plasmid or GM-CSF and IL-4 plasmids, splenocytes from humanized mice were stained for human CD45, CD19 plus CD5, CD40, CD20, HLA-DR, CD10, IgM, CD80 or CD86. Following IL-4 or GM-CSF plus IL-4 treatment, the levels of CD40, IgM and CD86 on CD45+CD19+ B cells were significantly elevated whereas the levels of CD5 and CD20 were reduced (Fig. 1E and Supplementary Fig. 1A and 1B). The expression of HLA-DR, CD10 and CD80 did not change. The frequency of CD19+CD27+ memory B cells were low (~1%) in humanized mice without cytokine treatment (Supplementary Fig. 1A). Following IL-4 or IL-4 plus GM-CSF treatment, the frequency was increased approximately ten-fold. Similarly, the proportion of human CD45+CD3+ T cells that expressed CD25 and the proportion of CD45RA-CD3+ T cells were significantly increased after IL-4 or GM-CSF plus IL-4 treatment (Fig. 1F and Supplementary Fig. 1C and 1D). Interestingly, most CD3+ T cells that expressed CD25 were CD4+ T cells but not CD8+ T cells (Fig. 1G and 1H), suggesting the development of CD4+CD25+ regulatory T cells in humanized mice. Thus, expression of Il-4 alone or GM-CSF plus IL-4 in humanized mice also stimulates T and B cell maturation.
GM-CSF and IL-4 treatment significantly enhances antigen specific IgG responses in humanized mice
To determine the effect of GM-CSF and IL-4 treatment on antigen-specific antibody responses, we immunized mice 7 days post injection of vector plasmid or GM-CSF and/or IL-4 plasmids with tetanus toxoid (TT) vaccine intraperitoneally three times every three weeks (Fig. 2A). Two weeks after the third immunization, mice were bled for measuring total human IgM and IgG and TT-specific human IgG in the sera by ELISA. Before immunization, the levels of human IgM were similar (~120 to ~170 μg/ml) in humanized mice injected with vector control plasmid and cytokine plasmids (Fig. 2B left panel). Although following injection of IL-4 or GM-CSF plasmid, the levels of human IgG were elevated 4-6 fold as compared to mice injected with vector plasmid, the absolute levels of IgG remained low (1 to 13 μg/ml, Fig. 2B, right panel). After immunization, the levels of the total IgM didn’t change much in mice injected with vector or GM-CSF or IL-4 plasmid alone, but were increased about 2.5 fold in mice injected with GM-CSF and IL-4 plasmids (Fig. 2C, left panel). Similarly, the levels of total IgG did not change significantly in mice injected with vector or GM-CSF plasmid. However, the levels of IgG were increased approximately 28 fold in IL-4 treated mice, reaching an average of ~120 μg/ml (Fig. 2C, middle panel). In GM-CSF and IL-4 treated mice, the levels of IgG were increased 55 fold, reaching ~700 μg/ml. Furthermore, TT-specific IgG (~0.14 IU/ml) was readily detected in GM-CSF and IL-4 plasmid injected mice following immunization but not in other groups of mice (Fig. 2C, right panel). Among the serum IgG isotypes, the predominant isotype was IgG1, followed by IgG2 and IgG4 (Supplementary Fig. 2A). These results show that GM-CSF and IL-4 treatment greatly stimulates antigen-specific IgG responses in humanized mice.
Fig. 2. GM-CSF and IL-4 treatment dramatically stimulates antigen specific IgG responses in humanized mice.
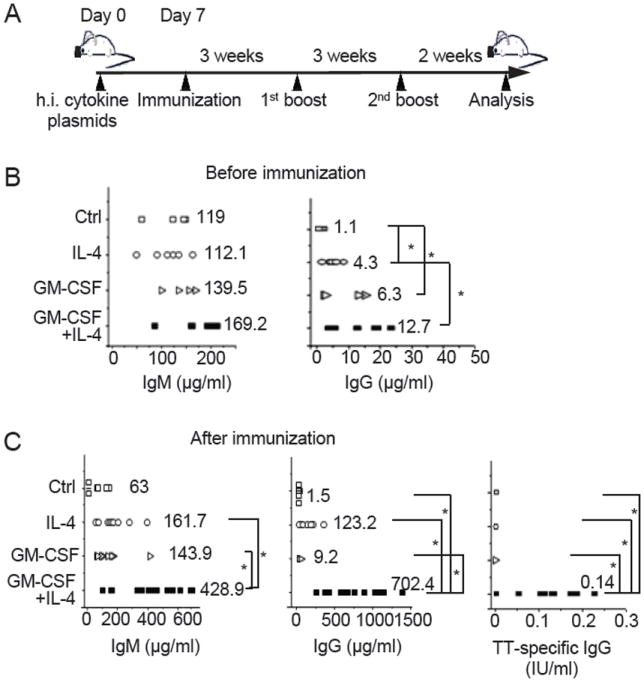
(A) Experimental scheme. Ten to twelve week old humanized mice were hydrodynamically injected (h.i.) with the vector plasmid or GM-CSF and/or IL-4 plasmids (50 μg each) on day 0. Seven days later, mice were immunized with TT vaccine and then boosted twice 3 weeks apart. Two weeks after the third immunization, mice were sacrificed and analyzed. (B and C) Total human IgG and IgM and TT-sepcific human IgG were analyzed in sera from mice that were injected with the vector plasmid (Ctrl), IL-4 plasmid, GM-CSF plasmid, or GM-CSF and IL-4 plasmids before (B) (n=6) and after immunization (C) (n=12). Each symbol represents one mouse. The numbers indicate the average level of IgM or IgG within the group. *p<0.05.
GM-CSF and IL-4 treatment promotes B and T cell responses following immunization
To investigate the cellular mechanisms of the enhanced antibody responses in GM-CSF and IL-4 treated mice, we analyzed T and B cell responses in the spleen two weeks after the third immunization. An immediate difference that was noticed was the enlarged spleens from immunized mice that have been injected with GM-CSF and IL-4 plasmids as compared to mice injected with vector, GM-CSF or IL-4 plasmids (Fig. 3A). Flow cytometry analysis revealed that nearly 90% of cells in the spleen of GM-CSF and IL-4 treated mice were human CD45+ (Supplementary Fig. 2B). The total numbers of human leukocytes (CD45+) in the spleen of GM-CSF and IL-4 treated mice were 52.7±12.5×106, representing ~10 fold increase over the numbers in the spleen of mice injected with vector plasmid (4.1±0.4×106), IL-4 plasmid (5.2±2.2×106) or GM-CSF plasmid (7±3.1×106) (Fig. 3B), correlating to the sizes of the spleens. Within the human CD45+ cells, ~30% was CD19+ B cells and ~60% was CD3+ T cells in GM-CSF and IL-4 treated mice (Supplementary Fig. 2C and 2D). In comparison, ~65% were CD19+ B cells and ~25% CD3+ T cells in control (vector plasmid) treated mice. As a result, the number of B cells was increased ~5 times in the spleen of mice injected with GM-CSF and IL-4 plasmids as compared to mice injected with vector plasmid, IL-4 plasmid or GM-CSF plasmid (Fig. 3B). Even more dramatically, the number of CD4+ T cells in the spleen was increased >12 times in mice injected with GM-CSF and IL-4 plasmids as compared to mice injected with vector plasmid, IL-4 plasmid or GM-CSF plasmid. Although the number of CD8+ T cells was also increased in the spleen of mice injected with GM-CSF and IL-4 plasmids, most splenic T cells were CD4+ T cells (Supplementary Fig. 2D).
Fig. 3. Enhanced B and T cell responses in GM-CSF and IL-4 treated mice following immunization.
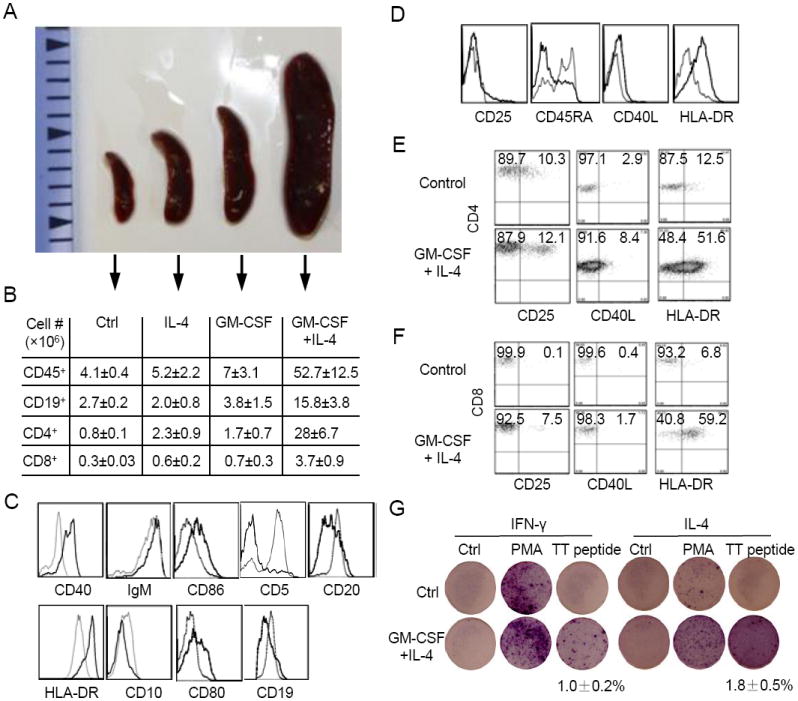
(A) Comparison of the sizes of spleens of humanized mice that were injected with vector (Ctrl), IL-4, GM-CSF, or GM-CSF plus IL-4 plasmids 2 weeks after the third immunization.
(B) Comparison of the total human CD45+ leukocytes, CD19+ B cells, CD4+ T cells, and CD8+ T cells in the spleens of immunized humanized mice that were injected with vector (Ctrl), IL-4, GM-CSF, or GM-CSF plus IL-4 plasmids. Single cell suspensions of spleens were prepared, counted and analyzed by flow cytometry for human CD45, CD19, CD3, CD4 and CD8. The numbers of human cells were calculated by multiplying the total cell numbers with the frequency the specific cell type. Shown are mean ± SEM (n=3-5).
(C) Flow cytometry analysis of human B cells. Splenocytes were analyzed for human CD45, CD19 plus the indicated markers. Histograms compare CD40, IgM, CD86, CD5, CD20, HLA-DR, CD10, CD80 and CD19 expression gating on CD45+CD19+ cells between mice injected with vector plasmid (thin line) and GM-CSF and IL-4 plasmids (thick line).
(D) Flow cytometry analysis of human T cells. Splenocytes were analyzed for human CD45, CD3 plus the indicated markers. Histograms compare CD25, CD45RA, CD40L and HLA-DR expression gating on CD45+CD3+ cells between mice injected with vector plasmid (thin line) and GM-CSF and IL-4 plasmids (thick line).
(E and F) Splenocytes were analyzed for human CD45, CD4 or CD8 plus the indicated markers. Shown are plots of CD4 (E) and CD8 (F) versus CD25, CD40L and HLA-DR gating on CD4+ and CD8+ human T cells, respectively. The numbers indicate percentages of cells in the gated quadrants.
(G) ELISpot analysis of TT-specific human T cell responses. Purified CD3+ T cells (1×105 cells/well) were incubated with medium, PMA or TT peptide for 24 hrs and then analyzed by ELISpot assay. The numbers indicate frequencies of T cells that secreted human IFN-γ or IL-4. Shown are mean ± SEM (n=4).
We further analyzed B cells in immunized mice by staining splenocytes for human CD45, CD19 plus CD40, IgM, CD86, CD5, CD20, HLA-DR, CD10, or CD80. In mice injected with vector plasmid, the phenotype of CD45+CD19+ human B cells did not change significantly following immunization; 80% of CD19+ human B cells expressed CD5 in humanized mice (Fig. 1E) and vector plasmid-injected humanized mice (Fig. 3C). Following immunization, 80% of CD19+ human B cells were CD5-negative in GM-CSF and IL-4 treated mice (Fig. 3C), indicating an induction of conventional (CD5-) B cells. Furthermore, most B cells in the spleen of GM-CSF and IL-4 treated mice exhibited an elevated expression of CD40, IgM, HLA-DR, CD80 and CD86, but reduced expression of CD19, CD20 and CD10 (Fig. 3C and Supplementary Fig. 2B), suggesting maturation of human B cells. Thus, the enhanced antigen-specific IgG response in GM-CSF and IL-4 treated mice is associated with induction of mature conventional B cells.
We also analyzed T cells in immunized mice by staining splenocytes for human CD45, CD3 plus CD25, CD45RA, CD40L or HLA-DR. Compared to the vector plasmid-injected humanized mice, CD3 T cells upregulated CD40L and HLA-DR and a small fraction of the T cells were also positive for CD25 in humanized mice injected with GM-CSF and IL-4 plasmids (Fig. 3D and Supplementary Fig. 2D), suggesting that T cells were further activated after immunization. The proportion of CD45RA- T cells also increased significantly in cytokine-treated mice. HLA-DR was upregulated in both CD4+ and CD8+ T cells (Fig. 3E and 3F). When splenocytes were stimulated with a dominant CD4 peptide (QYIKANSKFIGITE) from tetanus toxin, no IFN-γ or IL-4 secretion was detected by ELISpot in vector plasmid-injected (control) mice. In contrast, TT-specific IFN-γ and IL-4 secreting T cells were readily detected in the splenocytes of GM-CSF and IL-4 treated mice, reaching 1.0±0.2% and 1.8±0.5%, respectively (Fig. 3G). As controls, no IFN-γ or IL-4-secretion was detected without TT peptide stimulation and much higher frequencies of IFN-γ or IL-4-secreting T cells were detected following PAM stimulation. These results show that GM-CSF and IL-4 stimulate T cell maturation and antigen specific responses in humanized mice.
To distinguish the effect of cytokines versus immunization on T and B cell numbers, we analyzed humanized mice without immunization 9 weeks after injection with control plasmid, GM-CSF and/or IL-4 plasmids. The numbers of human CD45+ leukocytes in the spleens were increased slightly in IL-4 (5.7±0.7×106) and GM-CSF plus IL-4 (6.5±0.8×106) treated mice as compared to control mice (4.5±0.4×106) (Supplementary Fig. 3). The numbers of CD19+ B cells in the spleens did not change significantly following cytokine treatment. In contrast, the number of CD3+ T cells were increased ~2 fold following IL-4 or GM-CSF plus IL-4 treatment. Most of the increase in total T cell numbers was due to increase in CD4+ T cells (~2.5 times). Because 9 weeks following immunization the numbers of CD4+ T cells increased 28 fold in GM-CSF plus IL-4 treated mice as compared to control mice (Fig. 3B), the observed effects are primarily due to the combination of cytokine treatment and immunization.
GM-CSF and IL-4 treated mice are capable of generating H5N1 virus-neutralizing antibodies
To test whether GM-CSF and IL-4 treated mice produce human antibody responses to other antigens, we used NP-KLH, a commonly used model antigen, and inactivated avian influenza virus H5N1. Cytokine-treated humanized mice were immunized three times at three week intervals with NP-KLH and sera were measured for total and NP-specific human IgM and/or IgG. Immunization of GM-CSF and IL-4 treated humanized mice with NP-KLH also led to elevated levels of total human IgM (~445 μg/ml), IgG (~770 μg/ml) and NP-specific IgG (~1350 ng/ml) (Supplementary Fig. 4). Similarly, cytokine-treated humanized mice were immunized twice at two weeks internal with one of the three inactivated H5N1 virus isolates: A/Anhui/1/05 (Anhui), A/Indonesia/CDC669/06 (Idn) and A/Hong Kong/213/03 (HK). Seven days after the second immunization, sera were collected and assayed for the presence of anti-viral antibodies using two methods. In the immunofluorescent assay, MDCK cells were infected with Anhui, Idn or HK viruses, fixed, incubated with sera from the respective virus-immunized mice, and then developed with labeled antibodies specific to human IgG or mouse IgG. As shown in Fig. 4A, no positive staining was detected when sera from non-immunized GM-CSF and IL-4 treated (Ctrl) mice were used. As a positive control, when a murine anti-H5N1 monoclonal antibody 2D9 (16) was used, positive signal was detected only with labeled secondary antibody specific for the mouse, but not human, IgG. In contrast, sera from all six H5N1-immunized GM-CSF and IL-4 treated mice (two for each viral isolate) reacted with infected MDCK cells when labeled secondary antibody was specific to human, but not mouse, IgG. In the neutralization assay, 100 TCID50 of viruses were incubated with different dilutions of sera or 2D9 monoclonal antibody and then analyzed for cytopathic effect on MDCK cells. While control serum did not show any neutralizing activity, 2D9 neutralized viruses at a maximum dilution factor of 128 (starting with 100 μg/ml of antibody) (Fig. 4B). Sera from GM-CSF and IL-4-treated and immunized mice also exhibited significant neutralizing activity against respective strains of H5N1 virus isolates with maximum dilution factors of 18 for the Anhui isolate, 14 for the Indonesia isolate and 22 for the Hong Kong isolate. These results show that GM-CSF and IL-4 treatment stimulates human antibody responses to a diverse set of antigens, including avian H5N1 influenza virus.
Fig. 4. Induction of neutralizing antibodies against H5N1 in GM-CSF and IL-4 treated mice.
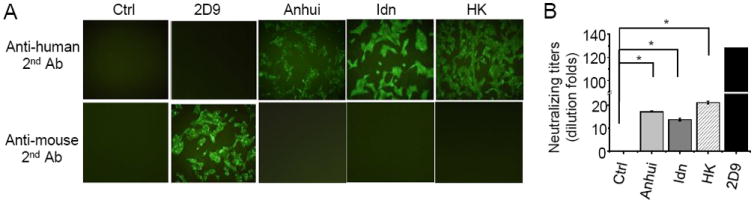
(A) MDCK cells were infected with H5N1 isolates A/Anhui/1/05, A/Indonesia/CDC669/06 (Idn) or A/Hong Kong/213/03 (HK), fixed, incubated with sera from the respective virus isolate-immunized mice, or control (Ctrl) sera from non-immunized GM-CSF and IL-4 treated humanized mice, or 2D9, a mouse monoclonal antibody specific for H5N1. Binding of primary antibody was detected using FITC-conjugated anti-human or anti-mouse secondary antibodies. Two humanized mice were immunized with each H5N1 virus isolate and representative data from one mouse are shown.
(B) H5N1 virus isolates (100 TCID50) were incubated with different dilution of sera from corresponding virus isolate-immunized mice, or non-immunized mice, or 2D9 monoclonal antibody. The mixtured was then added to MDCK cells and cytopathic effect was observed by light microscopy. Shown are the neutralizing titers at the highest serum or antibody dilution at which no cytopathic effect was observed. *p<0.05.
Discussion
Following engraftment of human HSCs into immunodeficient recipient mice, B cells are the most abundantly reconstituted human blood cell type. However, they do not make any significant IgG antibody response following immunization with specific antigen. Studies have suggested multiple defects contribute to the poor human antibody responses, including poor reconstitution of myeloid APCs, mismatch between human TCR and mouse MHC molecules, block of T and B cell maturation and deficiency in human cytokines in humanized mice (1, 3, 4, 11, 12). In this study, we show that in humanized mice majority of B cells express CD5 and are blocked at the transition stage, and there is a profound deficiency of human CD209+ DCs. More importantly, we show that these defects can be partially corrected by expression of human GM-CSF and IL-4 in mice. As a result, the cytokine-treated humanized mice make significant levels of antigen-specific IgG following immunization, including neutralizing antibodies specific for H5N1 avian influenza viruses.
The vast majority of human B cells in humanized mice are IgM+ (8). Consistently, we found that over 85% of human CD19+ B cells are IgM+ in the spleens of normal humanized mice (Figure 1E). We noticed that most of these B cells also express CD5, consistent with a previous report (17), whereas CD5+IgM+ B cells are only a minor fraction of the B cell population in human (18, 19). Based on CD5 expression, human B cells can be divided into CD5+ B1 cells and CD5- conventional B2 cells. Serum IgM is thought to be secreted primarily by CD5+ B1 cells whereas IgG antibody responses are mediated by CD5- conventional B2 cells. The relatively high levels of serum IgM but little IgG in humanized mice is consistent with this notion and raises the possibility that the observed defect in IgG antibody response is due to the composition of B cells in humanized mice.
CD5 is also expressed on transitional human pre-naive B cells which also express CD10 and a low level of CD40 (20). We found that the majority of human B cells (>50%) in humanized mice exhibited a phenotype of CD19+CD10+CD40lowIgM+IgD+ (Figure 1E), indicating that the development of human B cells in humanized mice is blocked at the transitional stage. Our finding is consistent with a previous study showing accumulation of CD24int/hiCD38hi immature B cells which comprised of 60% of the splenic CD19+ cells in humanized mice using the NOG mice as recipients (12). Our study extends the previous study by defining the stage at which B cell development is blocked. Furthermore, we show that maturation of B cells can be stimulated by the expression of GM-CSF and IL-4 in humanized mice, as indicated by the increased expression of IgM, CD40, CD86, and decreased expression of CD20 (Supplementary Figure 1). Interestingly, cytokine treatment alone also results in a significant increase in the proportion of CD5- conventional B cells. The maturation of human B cells and development of CD5- B cells are further stimulated by immunization of cytokine-treated humanized mice. Besides further upregulation of CD40 and downregulation of CD20, HLA-DR and CD80 are upregulated and CD10 and CD19 are down regulated on human B cells in immunized mice (Figure 3C), consistent with observation that expression of CD19 and CD20 decreases during B cell antibody responses in human (21, 22). Most profoundly, the majority of B cells become CD5- following immunization. This is even more remarkable considering that total numbers of B cells increased around 6 fold in immunized GM-CSF and IL-4-treated mice as compared to mice injected with control vector. As CD10 disappears upon B cell maturation (21, 23), these results suggest that B cell development in humanized mice is likely blocked at the pre-naïve B cell stage. Thus, the deficiency in B cell maturation as well as preferential development of CD5+ B1 cells are likely contributing factors to the poor IgG antibody response in humanized mice.
Correlating with the maturation of B cells, GM-CSF and IL-4 treatment stimulates a significant increase in serum IgG as compared to non-treated humanized mice (Figure 2B). Following immunization, the levels of total IgM and especially total IgG are further increased, reaching a much significant level of 400 to 700 μg/ml, respectively. Importantly, a significant level of antigen-specific IgG is also induced to immunizing antigens tetanus toxoid and NP-KLH (Figure 2C and Supplementary Fig. 4). The level of TT-specific human IgG is at the minimum protective level in humans. As in humans, the most abundant immunoglobulin isotypes are IgG1, followed by IgG2, and then IgM and IgG4. In contrast to humanized mice that were not treated with GM-CSF and IL-4, the predominant immunoglobulin isotype is IgM. In addition, GM-CSF and IL-4 treated humanized mice were able to produce neutralizing antibodies against three different isolates of H5N1 avian influenza virus. IL-4 is known to stimulate B cell antibody responses and immunoglobulin class switching. It is notable that expression of IL-4 alone is not sufficient to elevate serum IgG levels and antigen-specific IgG response, suggesting that combined GM-CSF and IL-4 treatment contribute to the improved antibody responses through additional mechanisms beyond stimulating B cell maturation.
Another major contributing factor for the improved antibody responses in GM-CSF and IL-4 treated humanized mice is the induction of CD209+ myeloid DCs and the enhanced priming of CD4 T cells. The functions of human T cells, particularly CD4+ T cells, are known to be impaired in humanized mice as they only developed very weak responses to stimulations and immunizations (1, 3, 5, 12). Specifically, it has been shown that human T cells in humanized mice are unable to produce detectable amount of IFN-γ and IL-4 upon antigen restimulation (12). We have shown previously that GM-CSF and IL-4 treatment induces the generation of CD11c+CD209+ cells in humanized mice (11). In this study, we further demonstrated that these CD209+ DCs are functional as they up-regulated expression of co-stimulatory molecules CD80, CD86 and HLA-DR as well as secretion of IL-12 upon stimulation with LPS. Previous reports have suggested that CD209+ DCs are critical for the initiation of T cell immune responses (15, 24), and their induction in cytokine-treated mice likely contributes to the elevated antibody responses in humanized mice through induction of CD4 T cell responses. Expression of GM-CSF and IL-4 also increased the proportion of human T cells that were CD45RA- (Supplementary Fig. 1C and 1D). As human CD45RA- T cells preferentially undergo homeostatic expansion and acquisition of memory-like phenotype in mice (25), the result suggests that expression of GM-CSF and IL-4 stimulates T cell maturation in humanized mice. Upon immunization, T cells further upregulated CD40L and HLA-DR, with a small fraction of T cells still expressing the activation marker CD25 (Figure 3D). Furthermore, a significant fraction of human CD4 T cells responded to restimulation with tetanus toxoid peptide by secreting IFN-γ and IL-4 as shown by ELISPOT assay (Figure 3E). It is worth to note that in our ELISPOT assay, total splenocytes were stimulated with tetanus toxoid peptide QYIKANSKFIGITE. This peptide is known to be a dominant CD4 epitope in human (26), but also can stimulate CD4+ T cell response in mice (27). Although it is not known whether the peptide was presented by human and/or mouse MHC in the ELISPOT assay, our findings clearly show that GM-CSF and IL-4 treatment stimulates induction of CD209+ DCs and effective CD4 T cell priming in humanized mice.
In summary, the poor antigen-specific IgG antibody response in humanized mice is correlated with a block of B cell development at the pre-naïve B cell stage, preferential development of CD5+ B cells and a lack of CD209+ DCs. The expression of GM-CSF and IL-4 in humanized mice can correct these defects by stimulating B cell maturation, generation of CD5- conventional B cells and induction of CD209+ DCs. As a result, these cytokine-treated mice can make a significant antigen-specific IgG antibody response following immunization. We have now identified the specific defects in humanized mice with regards to the generation of a physiological antibody response, and defined a novel approach to correct them such that this platform can be used to study antibody responses and generate novel human antibodies against virulent pathogens and other clinically relevant targets.
Supplementary Material
Acknowledgments
We thank Dr. William Hwang of Singapore Cord Blood Bank for providing cord blood, Lan Hiong Wong and Hooi Linn Loo for technical assistance, Drs. Maroun Khoury, Adam Drake, Bettina Iliopoulou for discussion and critical review of the manuscript, and Dr. Farzad Olfat for general support.
This work was supported in part by funds from Singapore-MIT Alliance for Research and Technology. Jerry Chan received salary support from the National Medical Research Council, Singapore (NMRC/CSA/012/2009).
Abbreviations
- NSG
NOD-scid Il2r-/- mice
- MNCs
mononuclear cells
- DCs
dendritic cells
- IL
interleukin
- GM-CSF
granulocyte macrophage colony stimulating factor
- APC
antigen presenting cells
- TT
tetanus toxoid
- NP-KLH
4-hydroxy-3-nitrophenyl acetyl-keyhole limpet hemocyanin
Footnotes
Disclosures
The authors have no financial conflict of interest.
References
- 1.Shultz LD, Ishikawa F, Greiner DL. Humanized mice in translational biomedical research. Nat Rev Immunol. 2007;7:118–130. doi: 10.1038/nri2017. [DOI] [PubMed] [Google Scholar]
- 2.Ishikawa F, Yasukawa M, Lyons B, Yoshida S, Miyamoto T, Yoshimoto G, Watanabe T, Akashi K, Shultz LD, Harada M. Development of functional human blood and immune systems in NOD/SCID/IL2 receptor {gamma} chain(null) mice. Blood. 2005;106:1565–1573. doi: 10.1182/blood-2005-02-0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Legrand N, Weijer K, Spits H. Experimental models to study development and function of the human immune system in vivo. J Immunol. 2006;176:2053–2058. doi: 10.4049/jimmunol.176.4.2053. [DOI] [PubMed] [Google Scholar]
- 4.Traggiai E, Chicha L, Mazzucchelli L, Bronz L, Piffaretti JC, Lanzavecchia A, Manz MG. Development of a human adaptive immune system in cord blood cell-transplanted mice. Science. 2004;304:104–107. doi: 10.1126/science.1093933. [DOI] [PubMed] [Google Scholar]
- 5.Huntington ND, Alves NL, Legrand N, Lim A, Strick-Marchand H, Mention JJ, Plet A, Weijer K, Jacques Y, Becker PD, Guzman C, Soussan P, Kremsdorf D, Spits H, Di Santo JP. IL-15 transpresentation promotes both human T-cell reconstitution and T-cell-dependent antibody responses in vivo. Proc Natl Acad Sci U S A. 108:6217–6222. doi: 10.1073/pnas.1019167108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shultz LD, Saito Y, Najima Y, Tanaka S, Ochi T, Tomizawa M, Doi T, Sone A, Suzuki N, Fujiwara H, Yasukawa M, Ishikawa F. Generation of functional human T-cell subsets with HLA-restricted immune responses in HLA class I expressing NOD/SCID/IL2r gamma(null) humanized mice. Proc Natl Acad Sci U S A. 107:13022–13027. doi: 10.1073/pnas.1000475107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carballido JM, Namikawa R, Carballido-Perrig N, Antonenko S, Roncarolo MG, de Vries JE. Generation of primary antigen-specific human T- and B-cell responses in immunocompetent SCID-hu mice. Nat Med. 2000;6:103–106. doi: 10.1038/71434. [DOI] [PubMed] [Google Scholar]
- 8.Becker PD, Legrand N, van Geelen CM, Noerder M, Huntington ND, Lim A, Yasuda E, Diehl SA, Scheeren FA, Ott M, Weijer K, Wedemeyer H, Di Santo JP, Beaumont T, Guzman CA, Spits H. Generation of human antigen-specific monoclonal IgM antibodies using vaccinated “human immune system” mice. PLoS One. 5 doi: 10.1371/journal.pone.0013137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rajesh D, Zhou Y, Jankowska-Gan E, Roenneburg DA, Dart ML, Torrealba J, Burlingham WJ. Th1 and Th17 immunocompetence in humanized NOD/SCID/IL2rgammanull mice. Hum Immunol. 71:551–559. doi: 10.1016/j.humimm.2010.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Danner R, Chaudhari SN, Rosenberger J, Surls J, Richie TL, Brumeanu TD, Casares S. Expression of HLA class II molecules in humanized NOD.Rag1KO.IL2RgcKO mice is critical for development and function of human T and B cells. PLoS One. 6:e19826. doi: 10.1371/journal.pone.0019826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen Q, Khoury M, Chen J. Expression of human cytokines dramatically improves reconstitution of specific human-blood lineage cells in humanized mice. Proc Natl Acad Sci U S A. 2009;106:21783–21788. doi: 10.1073/pnas.0912274106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Watanabe Y, Takahashi T, Okajima A, Shiokawa M, Ishii N, Katano I, Ito R, Ito M, Minegishi M, Minegishi N, Tsuchiya S, Sugamura K. The analysis of the functions of human B and T cells in humanized NOD/shi-scid/gammac(null) (NOG) mice (hu-HSC NOG mice) Int Immunol. 2009;21:843–858. doi: 10.1093/intimm/dxp050. [DOI] [PubMed] [Google Scholar]
- 13.Gijzen K, Tacken PJ, Zimmerman A, Joosten B, de Vries IJ, Figdor CG, Torensma R. Relevance of DC-SIGN in DC-induced T cell proliferation. J Leukoc Biol. 2007;81:729–740. doi: 10.1189/jlb.0606414. [DOI] [PubMed] [Google Scholar]
- 14.Engering A, Geijtenbeek TB, van Vliet SJ, Wijers M, van Liempt E, Demaurex N, Lanzavecchia A, Fransen J, Figdor CG, Piguet V, van Kooyk Y. The dendritic cell-specific adhesion receptor DC-SIGN internalizes antigen for presentation to T cells. J Immunol. 2002;168:2118–2126. doi: 10.4049/jimmunol.168.5.2118. [DOI] [PubMed] [Google Scholar]
- 15.Geijtenbeek TB, Engering A, Van Kooyk Y. DC-SIGN, a C-type lectin on dendritic cells that unveils many aspects of dendritic cell biology. J Leukoc Biol. 2002;71:921–931. [PubMed] [Google Scholar]
- 16.Ho HT, Qian HL, He F, Meng T, Szyporta M, Prabhu N, Prabakaran M, Chan KP, Kwang J. Rapid detection of H5N1 subtype influenza viruses by antigen capture enzyme-linked immunosorbent assay using H5- and N1-specific monoclonal antibodies. Clin Vaccine Immunol. 2009;16:726–732. doi: 10.1128/CVI.00465-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Matsumura T, Kametani Y, Ando K, Hirano Y, Katano I, Ito R, Shiina M, Tsukamoto H, Saito Y, Tokuda Y, Kato S, Ito M, Motoyoshi K, Habu S. Functional CD5+ B cells develop predominantly in the spleen of NOD/SCID/gammac(null) (NOG) mice transplanted either with human umbilical cord blood, bone marrow, or mobilized peripheral blood CD34+ cells. Exp Hematol. 2003;31:789–797. doi: 10.1016/s0301-472x(03)00193-0. [DOI] [PubMed] [Google Scholar]
- 18.Hayakawa K, Hardy RR, Herzenberg LA, Herzenberg LA. Progenitors for Ly-1 B cells are distinct from progenitors for other B cells. J Exp Med. 1985;161:1554–1568. doi: 10.1084/jem.161.6.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yuling H, Ruijing X, Xiang J, Yanping J, Lang C, Li L, Dingping Y, Xinti T, Jingyi L, Zhiqing T, Yongyi B, Bing X, Xinxing W, Youxin J, Fox DA, Lundy SK, Guohua D, Jinquan T. CD19+CD5+ B cells in primary IgA nephropathy. J Am Soc Nephrol. 2008;19:2130–2139. doi: 10.1681/ASN.2007121303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee J, Kuchen S, Fischer R, Chang S, Lipsky PE. Identification and characterization of a human CD5+ pre-naive B cell population. J Immunol. 2009;182:4116–4126. doi: 10.4049/jimmunol.0803391. [DOI] [PubMed] [Google Scholar]
- 21.Loken MR, Shah VO, Dattilio KL, Civin CI. Flow cytometric analysis of human bone marrow. II. Normal B lymphocyte development. Blood. 1987;70:1316–1324. [PubMed] [Google Scholar]
- 22.Nadler LM, Anderson KC, Marti G, Bates M, Park E, Daley JF, Schlossman SF. B4, a human B lymphocyte-associated antigen expressed on normal, mitogen-activated, and malignant B lymphocytes. J Immunol. 1983;131:244–250. [PubMed] [Google Scholar]
- 23.Stashenko P, Nadler LM, Hardy R, Schlossman SF. Characterization of a human B lymphocyte-specific antigen. J Immunol. 1980;125:1678–1685. [PubMed] [Google Scholar]
- 24.van Kooyk Y, Geijtenbeek TB. A novel adhesion pathway that regulates dendritic cell trafficking and T cell interactions. Immunol Rev. 2002;186:47–56. doi: 10.1034/j.1600-065x.2002.18605.x. [DOI] [PubMed] [Google Scholar]
- 25.Onoe T, Kalscheuer H, Chittenden M, Zhao G, Yang YG, Sykes M. Homeostatic expansion and phenotypic conversion of human T cells depend on peripheral interactions with APCs. J Immunol. 184:6756–6765. doi: 10.4049/jimmunol.0901711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valmori D, Pessi A, Bianchi E, Corradin G. Use of human universally antigenic tetanus toxin T cell epitopes as carriers for human vaccination. J Immunol. 1992;149:717–721. [PubMed] [Google Scholar]
- 27.Beignon AS, Briand JP, Rappuoli R, Muller S, Partidos CD. The LTR72 mutant of heat-labile enterotoxin of Escherichia coli enhances the ability of peptide antigens to elicit CD4(+) T cells and secrete gamma interferon after coapplication onto bare skin. Infect Immun. 2002;70:3012–3019. doi: 10.1128/IAI.70.6.3012-3019.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


