Abstract
Aedes japonicus japonicus (Theobald) (Diptera: Culicidae) has recently expanded beyond its native range of Japan and Korea into large parts of North America and Central Europe. Population genetic studies begun immediately after the species was detected in North America revealed genetically distinct introductions that subsequently merged, likely contributing to the successful expansion. Interactions, particularly in the larval stage, with other known disease vectors give this invasive subspecies the potential to influence local disease dynamics. Its successful invasion likely does not involve superior direct competitive abilities, but it is associated with the use of diverse larval habitats and a cold tolerance that allows an expanded seasonal activity range in temperate climates. We predict a continued but slower expansion of Ae. j. japonicus in North America and a continued rapid expansion into other areas as this mosquito will eventually be considered a permanent resident of much of North America, Europe, Asia, and parts of Hawaii.
Keywords: Aedes japonicus or Ochlerotatus japonicus, invasive mosquitoes, disease vectors, container habitats, mosquito larvae interactions
INTRODUCTION
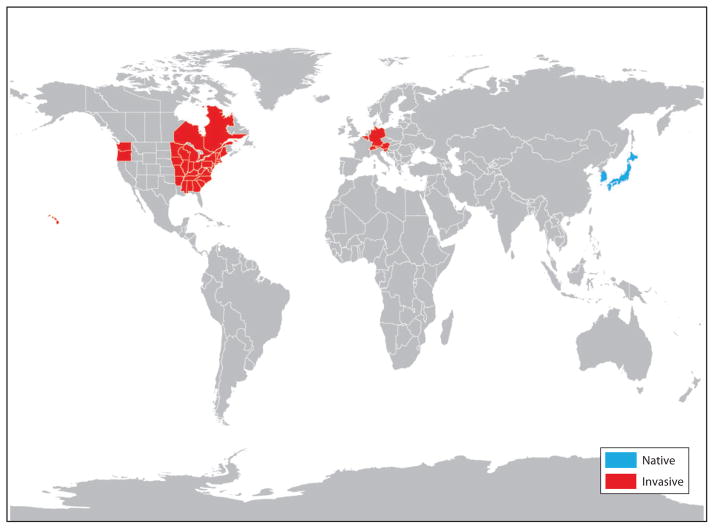
Aedes (Finlaya) japonicus japonicus (Theobald), the Asian rock pool or Asian bush mosquito, is part of a species group consisting of four subspecies and one very closely related sibling species (see below) whose native ranges encompass northeastern Russia to southern China and Taiwan, including Japan, Korea, and associated islands (102). Beginning in the mid- to late 1990s, Ae. j. japonicus, originally restricted to Japan north of the Ryukyu Islands and the Korean Peninsula, has spread throughout North America and later into Central Europe at a rate comparable to that of Ae. albopictus, the Asian tiger mosquito (69). In the approximately 15 years since it was recognized as established outside of its native range, Ae. j. japonicus has been found in all US states east of the Mississippi River (except Florida and Louisiana), in addition to Iowa, Missouri, Minnesota, Arkansas, Washington, Oregon, Hawaii, and the Canadian provinces of Quebec and Ontario (4, 11, 16, 21, 25, 29, 30, 32, 39, 42, 58, 70, 74, 77–79, 81, 83, 103, 104) (Figure 1). The subspecies has also become established Belgium, Germany, Switzerland, Austria, and Slovenia (38, 65, 89) (Figure 1). In addition, it has been intercepted and eradicated at entry points to France and New Zealand (57, 88).
Figure 1.
Current distribution map of Aedes japonicus japonicus at the state or province level in North America and the country level elsewhere. Note that this subspecies is established in Hawaii and has putatively been collected in parts of China and southeastern Russia.
Ae. j. japonicus is readily distinguished from similar mosquitoes in North America at both the larval and adult stages and has been included in the most recent comprehensive taxonomic keys (20). Adults have a distinctive bronze-colored, lyre-shaped pattern on the scutum, and larvae have a linear arrangement of branched frontal setae and a strongly spiculated anal saddle (20, 93). Ae. j. japonicus is distinctive compared to native species in Europe, where it is most likely to be confused with other invasive species such as Ae. albopictus and Ae. atropalpus (89) or, in particular, with Ae. koreicus (see below).
Although not considered a major vector of human pathogens, Ae. j. japonicus has been implicated in outbreaks of Japanese encephalitis (JE) in Asia (100). In the United States, Ae. j. japonicus has been found infected with West Nile virus (WNV) and La Crosse encephalitis (LAC) (93, 114) and has been found to be a highly competent vector of several other encephalitis viruses in laboratory studies (84–87, 90, 107, 108). Its role as a primary disease vector in North America or Europe is unclear; however, the impact of Ae. j. japonicus on local disease dynamics may result indirectly from its interactions with other established and critical vector mosquito species (e.g., 59). In this review, we discuss the characteristics of the subspecies in its expanded range that are likely involved in its invasion success and what appears to be permanent establishment among mosquito species that utilize container habitats in much of North America and Central Europe.
AEDES JAPONICUS SPECIES COMPLEX
Ae. japonicus is currently composed of four allopatric subspecies: Ae. j. japonicus, Ae. j. shintienensis Tsai & Lien, Ae. j. yaeyamensis Tanaka, Mizusawa & Saugstad, and Ae. j. amamiensis Tanaka, Mizusawa & Saugstad. The subspecies Ae. j. japonicus is a common mosquito in Palearctic Japan (Hokkaido, Honshu, Shikoku, and Kyushu) and shows little morphological variation in that region. Ae. j. japonicus is also found in Korea, where it overlaps with Ae. koreicus. There is some evidence that the subspecies is established in several areas in China (63, 113), although this awaits confirmation from molecular studies and determination of whether the populations may have recently expanded from a more restrictive native range. Ae. j. yaeyamensis is common on the Yaeyama Islands, the southernmost islands of the Ryukyu Archipelago, and is quite distinct from the other subspecies, although the diagnostic hind femur scale pattern clearly overlaps that of Ae. koreicus. Ae. j. shintienensis, which occurs in Taiwan, closely resembles its northern neighbor Ae. j. yaeyamensis in the aedeagus, implying a common ancestor, a possibility supported by DNA-based phylogenetic analyses (17; see below). Aedes j. amamiensis is found in Amami Ōshima, the northernmost islands of the Ryukyu Archipelago. Interestingly, although Ae. japonicus is considered absent from Okinawa, in the central Ryuku Archipelago, two specimens collected in Okinawa were morphologically similar to Ae. j. amamiensis (105, 106).
Close examination of the morphological evidence indicates there are insufficient diagnostic traits to reliably separate the adults of each subspecies in the Ae. japonicus complex from each other and from Ae. koreicus (102) (see sidebar, An Invasive Sibling Species). In contrast, analysis of two mitochondrial loci and a nuclear locus indicates the four subspecies are genetically quite distinct, averaging 8% nucleotide differences at the two mitochondrial loci (17). Furthermore, they form a monophyletic group that surprisingly includes Ae. koreicus as sibling to Ae. j. japonicus. These results suggest that a new taxonomic construct should be considered in which the subspecies of Ae. japonicus are raised to species (17).
AN INVASIVE SIBLING SPECIES.
Aedes koreicus, a very close relative of Ae. j. japonicus, has recently established in Belgium and Italy (19, 111). These taxa are difficult to separate morphologically because key features are not fixed in the two species and they also tend to be eroded during routine handling of specimens. However, a DNA-based rapid assay has been developed so that the species can be distinguished (17). Ae. koreicus is considered more peridomestic in its native range than Ae. j. japonicus and is also considered to be a vector of JE (65, 66, 90, 97). Because it is biologically similar to Ae. j. japonicus, it is quite likely that Ae. koreicus will spread throughout other temperate regions in the world and that it will eventually establish in North America. The spread of this species needs to be monitored closely because it adds another potential aspect to human disease transmission; however, it also represents a rare opportunity to examine the dynamics of multiple invasive mosquito species during the early stages of range expansion.
BACKGROUND AND BIOLOGY IN THE NATIVE RANGE
Although Ae. j. japonicus is relatively common, even in large cities (102), it is not particularly abundant in Japan and Korea. It has been implicated in JE transmission from pigs to humans in areas where Culex vectors were absent or too rare to be involved (100). It can be a nuisance biter in some locales but is not noted as an aggressive human biter (41, 56, 67, 102). Adults are active earlier in the spring and later in the fall compared with ecologically similar species (41, 67, 102). Larvae of Ae. j. japonicus, which also are found earlier in the year than other container-dwelling species (41), inhabit rock pools, tree holes, bamboo stumps, and artificial containers, particularly those formed from stone or concrete (67, 98). This subspecies tends to inhabit containers larger than those inhabited by similar species (98, 99). Other ecological information is somewhat sporadic and anecdotal (56, 98) and has been summarized by Scott (93). In general, this species tends to be inconspicuous in its native range because it is not an aggressive human biter, is not commonly collected with trapping methods typically used for adults (see below), and is not considered a primary vector of human disease, yet it appears to be found in a wider variety of habitats compared with ecologically similar species (41, 67, 98). Many of its native range characteristics—use of diverse larval habitats, host feeding preferences, propensity for forested areas, and cold tolerance—are important components of its success as an invader and potential role in disease transmission.
POPULATION GENETICS OF THE INVASION IN NORTH AMERICA
Fonseca et al. (26) examined the genetic signature at a mitochondrial DNA locus (ND4) and several random polymorphic amplified DNAs (RAPDs) of several stages of Ae. j. japonicus collected in 1999 in New Jersey, New York, Connecticut, Pennsylvania, and Ohio, and in Maryland in 2000. Included for comparison were specimens obtained from collaborators in six Japanese cities. Overall there were similar levels of genetic diversity in US and Japanese populations of Ae. j. japonicus and evidence of at least two separate introductions of the subspecies into the eastern United States. However, because mitochondrial DNA is sex-linked, and RAPD analyses do not differentiate between neutral loci and loci under selection, these results were considered inconclusive.
To test the hypothesis of a single introduction of Ae. j. japonicus into the United States, as well as to examine changes over time and interactions between distinct genetic signatures across a fine spatial gradient, Fonseca et al. (28) repeated and expanded the analyses using a panel of newly developed microsatellite loci (115). To obtain reliable microsatellite loci for Ae. j. japonicus, Widdel et al. (115) had to avoid the very abundant multi-copy DNA common in Aedes (24, 64) by using a protocol developed for Pinus (22). These techniques confirmed the existence of two abundant genetic forms in specimens originally collected in 1999–2000 that matched the disjunctive distribution of mitochondrial haplotypes (26). Additionally, a fine-scale genetic map of Ae. j. japonicus using nearly 500 specimens collected from 54 Pennsylvania counties in 2002–2003 enabled researchers to examine the distribution of the two genetic types across Pennsylvania. By making direct comparisons between specimens collected in 1999–2000 and new collections made in 2004–2005 obtained from the same areas in the northeastern United States, they observed that the strong association between mitochondrial DNA haplotype and microsatellite signature seen in 1999–2000 had weakened significantly by 2002 across Pennsylvania. This trend continued in 2004–2005 in Pennsylvania, New Jersey, and New York, indicating that distinct separate introductions were merging. These studies provide a high-resolution analysis of the spatial and temporal dynamics of the range expansion of Ae. j. japonicus and make this species an excellent case study to examine postintroduction patterns of evolution. The merger of multiple introductions partly hid the bottleneck subsequent to the introduction (28) and maintained high genetic diversity, a trait associated with invasiveness (55). If multiple introductions of mosquito species are a common occurrence, this phenomenon would explain the often high genetic diversity of invasive species that has previously been assumed to indicate introductions of large numbers of individuals (49, 110).
HOST RANGE AND IMPORTANCE AS A DISEASE VECTOR IN NORTH AMERICA AND EUROPE
Ae. j. japonicus adult females feed primarily on mammals but also on birds (6, 37, 68, 93, 116). Blood meal analyses of wild-caught Ae. j. japonicus have shown many instances of feeding on deer, but horse, opossum, chipmunk, and a high percentage of human blood meals (up to 63%) have been documented (6, 68, 93). The subspecies has been associated with nuisance biting in some locales (e.g., 91) and has been described as a day or crepuscular feeder in both its native and expanded ranges (41, 84, 93, 102). Direct evidence for feeding on birds and thus being able to serve as a viable bridge vector for several important viral diseases in North America is scant. Because WNV has been found in field-collected adults (93, 108), natural bird feeding is implied. However, other evidence of a willingness to take bird blood meals is based on maintenance of colonies using quail (116) and from the virus transmission studies cited below. These traits make Ae. j. japonicus a potential vector for several encephalitic diseases present in North America and Europe in addition to its demonstrated capacity to vector JE.
Laboratory studies show that Ae. j. japonicus is a highly competent vector for JE, WNV, and Saint Louis encephalitis (SLE) and a moderately competent vector for eastern equine encephalitis (EEE) and LAC (84, 86, 87, 90, 100, 107, 108). JE virus can be passed transovarially in this subspecies (100). Interestingly, Ae. j. japonicus had a higher estimated transmission rate for WNV compared with several Culex spp., the primary vectors in many areas of the world (107, 108). Scott (93) estimated the minimum field infection rate (MFIR, number per 1,000 tested) of Ae. j. japonicus with WNV to be 5.2—a value comparable to that for Culex spp. in areas of active WNV transmission, though the value for Ae. j. japonicus may be somewhat inflated because of small pool sizes. Ae. j. japonicus has also been shown to be a competent vector of dengue and chikungunya viruses (91). There is little field-collected evidence to implicate Ae. j. japonicus as a major vector for disease in its expanded range (90), but as a human blood feeder coupled with peridomestic habitat preferences, it must be considered a high risk in some areas. Recent rises in LAC cases in the Appalachian United States may be related to Ae. j. japonicus invasion dynamics, as it interacts with the native vector, Ae. triseriatus, and to the relatively recent arrival of Ae. albopictus in the area (59).
PHENOLOGY AND TEMPERATURE TOLERANCES
Ae. j. japonicus is a cold-tolerant species that overwinters primarily in the egg stage (for egg surface characteristics, see 34), but sometimes in the larval stage (4, 5, 41, 50, 93). Bartlett-Healy et al. (10) observed that Ae. j. japonicus larvae were more abundant in containers with lower mean water temperatures than co-occurring species and that the presence of this subspecies’ larvae was negatively correlated with temperature. Its range expansion is presumably limited in the southern latitudes of the Northern Hemisphere by temperatures regularly exceeding 30–35°C, and colonization of southern areas is usually associated with higher elevation sites and well-shaded areas (11, 32, 33). In northern areas, its cold tolerance and egg overwintering stage have likely allowed for rapid expansion into many areas. Scott (93) found that although the Ae. j. japonicus larval development rate was positively correlated with temperatures as high as ~30°C, higher temperatures were inhibitory and no emergence occurred at rearing temperatures of 34°C or 40°C. Some larvae reared at 10°C developed into adults, although the time to emergence was over 100 days (93). However, at 28°C, pupation success by fourth instars was poor, suggesting the developmental temperature limit was being approached. Eggs hatched at a range of temperatures, from 10°C to 40°C, with time to hatching from immersion to first instar varying inversely with temperature (93).
Ae. j. japonicus is multivoltine in most areas and early observations of the subspecies in Japan indicated that Ae. j. japonicus is active for a longer spring through fall period compared with similar container-dwelling mosquitoes (see above). This is also true in its expanded range (e.g., 4, 15, 27, 50). In some larval habitats, Ae. j. japonicus was the only species found in early spring and appeared to hatch in the field at temperatures typically associated with spring snow pool Aedes (15, 103). In more moderate winter climates, overwintering larvae could account for some of these early-season records (8). The laboratory studies on temperature and development, taken with the field observations of higher relative larval abundance in early spring and late fall, suggest an important key to the invasion success of Ae. j. japonicus: It gets a head start over many potential competitors and this phenology may allow both added generations and less direct overlap with similar larval stages of other species.
LARVAL HABITATS IN NORTH AMERICA AND EUROPE
Ae. j. japonicus utilizes a wide range of larval habitats. As the common name implies, it is often found associated with rock pools in its native range, but many natural and artificial containers are known oviposition sites within its current distribution. Within these general categories of habitats, larvae have been found repeatedly in tree holes, tires, and containers made of concrete, stone, plastic, or metal (4, 11, 33, 36, 44, 50, 51, 93, 98). The presence of Ae. j. japonicus larvae in New Jersey was found to be positively correlated with containers that were black/gray in color, primarily tires and large plastic buckets (10). Larvae of this subspecies have also been found in stormwater drainage systems, including catch basins (4, 31, 74), and sometimes in noncontainer habitats such as rain pools and depressions in soil, which are more commonly associated with floodwater mosquitoes or Culex spp. (4). Ae. j. japonicus larval abundances in the newly expanded range are often particularly high in rock pools and tires, with the invasive species dominating the mosquito fauna at many locations and/or within a habitat category (5, 8, 11, 15, 50, 89).
Within any particular geographic locale, Ae. j. japonicus larvae tend to be found more frequently in wooded/rural areas than in true urban sites (10) and this may be related to temperature tolerances (see above). Some researchers have observed more larvae in shaded habitats (8, 10), but others have found little evidence of discrimination between sunlit and shaded habitats (4). In addition, some researchers have observed a preference for open habitats or have found increased relative abundance of larvae in habitats located in sunlit areas (45, 98). Ae. j. japonicus readily oviposits in open areas (61), but it is not clear whether larval success is related to habitat microsite characters. Lorenz et al. (61) hypothesized that algae in sunlit habitats could play a role in the invasion success, but they found no overall preference for oviposition in containers that had been exposed to sun (high algal production) versus those that were shaded (low algal production). Sota et al. (98) suggested that Ae. j. japonicus egg resistance to desiccation plays a role in colonizing more open-area larval habitats, though desiccation-resistant eggs are not a characteristic of colonized strains in the United States (116).
It is assumed that introductions and subsequent expansion of Ae. j. japonicus into Europe and North America are associated with used tire imports and trade, as is the case for Ae. albopictus and many other container species (117). Although tires are not thought to be the means of introduction into Switzerland (89), the subspecies has been found in tires at ports and other entry sites (57, 78, 88) and its propensity for ovipositing in tires is well known (see above). Ae. j. japonicus is somewhat indiscriminate in its use of larval habitats, and the transport and trade of lawn ornaments, plant pots, and a wide variety of stone or concrete basins could serve as a means of spreading eggs and larvae. In addition, the consistent use of rock pools by the subspecies suggests that movement along river corridors could aid population dispersal (11).
INTERACTIONS WITH NATIVE SPECIES IN ROCK POOLS AND CONTAINER HABITATS
Ae. j. japonicus co-occurs with many other mosquito species in the larval habitat (Table 1). For many of these species it is unclear whether there is any significant interaction in terms of resource competition or potential displacement. However, observations of larval abundances in a variety of habitats throughout the invasive range have suggested that Ae. j. japonicus is displacing at least two native species, Ae. triseriatus and Ae. atropalpus, from some of their larval habitats and that interactions with another invasive species, Ae. albopictus, may be limiting the extent of the Ae. j. japonicus invasion.
Table 1.
List of North American species that have been reported to co-occur with Aedes j. japonicus in larval habitats and estimates of potential for interaction with the invasive species
| Co-occurring species | Potential for interactiona | Evidence of negative effects on native species or larval competition? | Reference(s) |
|---|---|---|---|
| Aedes albopictus (Skuse) | +++ | Some habitat segregation between the two species; Aedes japonicus inferior competitor in lab and field studies | 7, 8, 10, 52 |
| Aedes atropalpus (Coquillett) | +++ | Reduction or elimination in rock pools and tires; Aedes japonicus marginally better competitor in lab studies? | 4, 5, 8–11, 15, 35, 50, 94 |
| Aedes hendersoni (Cockerell) | + | N/A | N/A |
| Aedes triseriatus (Say) | +++ | Reduction in tires; little evidence of asymmetric competition in lab studies | 1, 5, 11, 15, 35, 40, 45, 50 |
| Anopheles barberi (Coquillett) | + | N/A | N/A |
| Anopheles punctipennis (Say) | ++ | Displacement in tires? (Unexpectedly absent in surveys) | 103 |
| Anopheles quadrimaculatus (Say) | + | N/A | N/A |
| Culex pipiens (Linnaeus) | +++ | Reduction in some containers; little evidence of asymmetric competition in lab studies | 35, 60 |
| Culex restuans (Theobald) | +++ | Reduction in tires and other artificial containers | 5, 71 |
| Culex salinarius (Coquillett) | + | N/A | N/A |
| Culex territans (Coquillett) | ++ | N/A | N/A |
| Culiseta melanura (Coquillett) | + | N/A | N/A |
| Orthopodomyia signifera (Coquillett) | ++ | N/A | N/A |
| Toxorhynchites rutilus (Dyar & Knab) | ++ | Predator on Aedes j. japonicus; evidence of negative impact on Aedes j. japonicus in lab and field studies | 53, 71 |
Low (+) to high (+++) potential based on overlapping use of container types and high relative abundance of each species.
N/A, not available.
Interactions with Aedes atropalpus
Among the native species that appear to overlap strongly with Ae. j. japonicus in larval habitats, Ae. atropalpus is most likely to be displaced. Ae. atropalpus is the native rock pool mosquito in North America and thus could be considered the ecological equivalent to Ae. j. japonicus. Interestingly, Ae. atropalpus also appears to have recently adapted to tires as larval habitats (73), which likely facilitated its introduction into Europe. Ae. atropalpus is a worrisome invasive species in Europe (65) but ironically could eventually be limited in its distribution by the successful invasion of Ae. j. japonicus. Ae. atropalpus is generally not considered to be a strong larval competitor with co-occurring species because it is primarily autogenous and requires a longer larval development time (see below).
Several research groups in North America have observed lower relative abundance of Ae. atropalpus in rock pools and tires after the introduction of Ae. j. japonicus (4, 5, 8, 11, 15, 94). In some cases, an absence of Ae. atropalpus in rock pools formerly known to consistently harbor the native species suggests complete displacement by Ae. j. japonicus (10). Though not typically a dominant member of larval mosquito communities in tires, Ae. atropalpus has suffered a similar decline in relative abundance or has disappeared entirely from these habitats in some locales as Ae. j. japonicus has established (5, 10, 50).
Laboratory competition studies between larval Ae. j. japonicus and Ae. atropalpus have been few and somewhat equivocal. Using simulated rock pools and leaf detritus, Armistead et al. (9) concluded that Ae. j. japonicus slightly outperformed Ae. atropalpus in terms of reproductive potential, and attributed most of the advantage to the latter’s autogenous adult stages that presumably required more nutritional reserves acquired in the larval stage. Using a lab diet of yeast and lactal-bumin added every 2 days, Hardstone & Andreadis (35) found only weak evidence for competition, though Ae. j. japonicus generally had lower adult mortality and achieved higher adult female size across a range of inter- and intraspecific larval densities.
Interactions with Aedes triseriatus
Ae. j. japonicus and Ae. triseriatus larvae co-occur in a number of natural and artificial containers, including tree holes and tires. Research groups have often reported that Ae. j. japonicus is numerically dominant in tires and has increased its abundance in these habitats in many parts of North America at the expense of Ae. triseriatus (5, 11, 15, 45, 50). Although the invasive subspecies colonizes tree holes, sometimes in relatively high abundance (11), there is little evidence to indicate that it will displace the native species in these ancestral habitats. In a multiyear survey of over 60 tree holes in two different woodlots in Michigan, Kaufman et al. (50) found consistently low abundances of Ae. j. japonicus compared with Ae. triseriatus, with little evidence of an increasing trend over time. In that study, Ae. j. japonicus larval abundance was maintained at nearly 50% of overall larval abundance in tires found at the same location. Additionally, Bartlett-Healy et al. (10) found no Ae. j. japonicus larvae in tree holes, but substantial populations in tires, as part of a survey of container habitats in New Jersey.
Several studies that have examined larval competition between Ae. triseriatus and Ae. j. japonicus conclude that only weak competition occurs and that the strongest constraints on larval development are related to overall larval density regardless of whether the other larvae are conspecifics or congenerics (1, 35, 40, 60). In a study using leaf detritus as the basal resource, Alto (1) showed that, although Ae. j. japonicus may have an advantage in development time when in competition with Ae. triseriatus, survival was reduced, particularly under low resource levels, and potential rates of population increase were less than those for Ae. triseriatus. Ingrassia (40) also found poor survival of Ae. j. japonicus relative to Ae. triseriatus under conditions of low food resources (ground leaf material). Similarly, Hardstone & Andreadis (35) used a lab diet and found no evidence of strong competition between the two species. In that study, Ae. j. japonicus generally had faster developmental rates and larger adult females, and adult mortality was not significantly different from that of Ae. triseriatus, but larval survival was reduced when in direct competition with the native species. Similar to the above-mentioned studies, Lorenz (60) used decaying leaves and algae as basal resources in a field microcosm experiment and found that Ae. j. japonicus developed faster than Ae. triseriatus regardless of inter- or intraspecific species assemblages, and that both species showed enhanced adult production when algal productivity was high.
Interactions with Culex restuans and Culex pipiens
Perhaps more than any other container-dwelling species listed here, Ae. j. japonicus consistently co-occurs with Culex spp. Its ability to colonize stormwater drainage systems including catch basins (4, 43, 74) and its use of sunlit container habitats (e.g., 45) and habitats with higher organic matter content (112) mirror some of the oviposition selection habits of Cx. restuans and Cx. pipiens. Andreadis & Wolfe (5) noted a significant decline in the relative abundance of Cx. restuans in containers in Connecticut after Ae. j. japonicus became established in the state, and Murrell & Juliano (71) observed negative effects of Ae. j. japonicus on Culex (primarily Cx. restuans) larval populations in artificial containers in Missouri. However, Armistead et al. (8) observed no significant positive or negative associations between Ae. j. japonicus larval relative abundance and either Cx. pipiens or Cx. restuans in a survey of containers in Virginia. Kaufman et al. (50) observed an increase in Culex spp. larval abundance in tires when Ae. j. japonicus larvae declined during one year of a multiyear study in Michigan. Overall, there is little evidence to suggest that Culex larval populations are as negatively affected by the Ae. j. japonicus range expansion in the United States, in contrast to what has been seen in larval populations of Ae. atropalpus and Ae. triseriatus.
Direct larval competition studies between Ae. j. japonicus and Culex spp. are limited. In a study using a lab diet, Hardstone & Andreadis (35) found that Cx. pipiens larval survival was higher than that of Ae. j. japonicus when in interspecific competition, but other indications of asymmetrical competition were lacking. Also in that study, Ae. j. japonicus generally had faster pupation times and reached larger female sizes than Cx. pipiens across the range of treatment densities in both intra- and interspecific competition. Lorenz (60) found that Ae. j. japonicus generally outperformed Cx. pipiens in microcosms with leaves and algae as basal resources. Although low emergence rates for both species obscured conclusions, only Ae. j. japonicus emerged as adults from the interspecific competition treatment. To our knowledge, no studies have examined direct larval competition between Ae. j. japonicus and Cx. restuans or Culex spp. other than Cx. pipiens. Because there seems to be evidence of displacement in larval habitat interactions between Ae. j. japonicus and Cx. restuans (see above), competition studies with those two species in tires or microcosms are warranted.
Interactions with Aedes albopictus
The potential interaction between two invasive container-dwelling mosquito species, one moving into the United States from the south and the other from the northeast, is intriguing from many perspectives. Leisnham & Juliano (59) have reviewed this within the context of LAC emergence in Appalachia but repeatedly note that relatively little is known about the Ae. j. japonicus side of the interaction. In areas of overlap, the two species seem to coexist without much evidence of displacement. This is partly because prior baseline abundance levels have not often been established while both species are still expanding their ranges. In Virginia, Armistead et al. (8) found a significant negative association between Ae. j. japonicus and Ae. albopictus larval abundances and suggested that interspecific repulsion via crowding accounted for the result. Ae. j. japonicus larvae generally encountered higher numbers of Ae. albopictus larvae than conspecifics across a range of containers but probably circumvented direct competition owing to faster larval development early in the year. Bartlett-Healy et al. (10) found that the two species in New Jersey were segregated on the basis of container type and container characteristics to a great extent, with Ae. japonicus being more rural and found in containers with lower mean water temperatures.
The few studies that have examined direct competition show that Ae. albopictus is the superior larval competitor, as it is in most studies of competition with other larval species (48, but see 47 for exceptions). In a field study using leaf material in microcosms and natural populations of Ae. j. japonicus and Ae. albopictus, Armistead et al. (7) found that Ae. albopictus had a higher potential population growth rate overall and in interspecific competition with Ae. j. japonicus. Kesavaraju et al. (52) found similar results in a laboratory study and further showed that Ae. j. japonicus was more susceptible to the pesticide malathion. Under conditions of interspecific competition between the two species in the presence of malathion, the developmental advantages of Ae. albopictus larvae were magnified. Larval competition between Ae. j. japonicus and Ae. albopictus may also be altered by the presence of predators and parasites (see below).
Taken together, studies of interactions and larval competition between Ae. j. japonicus and established species in North America suggest that the success of the invasive species is not related to superior competitive ability in the larval stage, as is thought to be a component of the invasion success of Ae. albopictus (48, 69). Weak or no competitive advantages for Ae. j. japonicus larvae have been detected thus far, and with the exception of Ae. albopictus, Ae. j. japonicus larvae fail to distinguish between conspecifics and co-occurring larval species such that intra- and interspecific larval competition outcomes are comparable and density dependent. In several studies, however, Ae. j. japonicus appeared to have a developmental speed advantage over the native species regardless of competition type (intra- or interspecific) being examined, but this finding needs to be verified with more experiments under field conditions and over a wider range of temperatures. The results from laboratory competition studies varied with larval food resources, and this also should be explored in future experiments. Additionally, almost all competition experiments have utilized a single strain of Ae. j. japonicus originating in the Rutgers Center for Vector Ecology lab [a notable exception is the study conducted by Armistead et al. (7)] because of the relative difficulty in starting and maintaining viable colonies from field sources (see below). It remains to be seen whether the strain is representative of the diversity in established field populations. Coexistence with or displacement of native species and Ae. albopictus may include some aspects of superior larval competitiveness but more likely relate to the use of diverse containers, the tolerance of low temperatures, interactions with predators and parasites (see below), and the accelerated activity of Ae. j. japonicus earlier in the year, which may allow the subspecies to avoid intense larval competition entirely. Invasion success of a mosquito species is clearly related to a multitude of factors (48, 62), among which direct larval competitive ability may play a minor role or be important only under particular circumstances (46). Ae. j. japonicus alters the relative abundances of existing species in larval habitats, which may have implications for disease transmission regardless of any complete displacement in larval habitats. Bevins (12, 13), for example, has presented evidence that Ae. triseriatus may become a more efficient vector of LAC after emerging from larval habitats that contain competing Ae. albopictus. Other studies have shown that stresses in the larval environment, in the form of either competitors or abiotic influences, can greatly affect the vectorial capacity of emerging adults, often leading to an increased ability for virus transmission (2, 72).
Even if Ae. j. japonicus appears to be displacing species from larval habitats, there is little indication that a related decline in adult populations of these mosquitoes is occurring. Rochlin et al. (80) present evidence based on light trap data that Ae. triseriatus adult numbers are declining in an area where both Ae. j. japonicus and Ae. albopictus are also present. However, differences in trap efficiency for collecting adult Ae. j. japonicus versus other species raise questions about accurate relative adult population estimates. More studies are needed to determine, for example, whether Ae. atropalpus adult populations have suffered severe declines in areas now colonized by Ae. j. japonicus in view of the observed severe declines in larval populations of the former.
PREDATORS AND PARASITES
Very little is known about specific parasites and predators of Ae. j. japonicus. In its native range an unclassified microsporidian and a gregarine parasite, Ascogregarina japonicus, appear to be associated primarily with this species complex (23, 82, 101). However, they have not yet been found with the subspecies in its expanded range. Ae. j. japonicus can be infected by the same ascogregarine that infects Ae. albopictus and that parasite has been found in a few field-collected Ae. j. japonicus larvae. Because the Ae. albopictus ascogregarine Ascogregarina taiwanensis can complete its life cycle in Ae. j. japonicus, its presence might influence interactions between the two species, with Ae. albopictus potentially benefitting from host dilution (23). Ae. j. japonicus larvae are susceptible to general predators in the environment, and two studies have examined the foraging behavior of larvae that might influence susceptibility to Toxorhynchites. One study (75) was designed to investigate foraging behavior under different food conditions but concluded that Ae. j. japonicus was more susceptible to Toxorhynchites predation because of greater browsing activity below the water surface. A more recent study that examined larval foraging behavior in the presence and absence of predator cues concluded that Ae. albopictus was more susceptible to Toxorhynchites rutilus (53). Additionally, Ae. j. japonicus is less susceptible to T. rutilus predation than Culex spp., which have different foraging behaviors than Aedes spp. in general (71). Therefore, it is not yet clear whether Ae. j. japonicus has any behavioral advantage over co-occurring larval species in the presence of a common predator. Toxorhynchites, however, is generally found in more southern locations and does not overlap as much with the current expanded range of Ae. j. japonicus in North America as it does with Ae. albopictus or Culex spp., although Tx. rutilus appears to be moving northward in the United States (54).
ESTABLISHMENT OF LABORATORY COLONIES AND OTHER PRACTICAL CONSIDERATIONS
Several Ae. j. japonicus laboratory colonies have been successfully established, but this species appears to require special care compared with several other co-occurring species (e.g., Ae. triseriatus, Ae. albopictus, Ae. atropalpus, Cx. pipiens) in the United States (37, 116). Only a few colonies exist, although the Rutgers Center for Vector Biology currently maintains colonies started from specimens from the two original strains established in the northeastern United States (New Jersey and Pennsylvania) and from specimens collected in Hawaii. These colonies were started from field collections, sometimes with few individuals, but have always required forced mating often followed by free mating in enclosures with high densities of adults (37, 116). Even though blood meal analyses show the species to be predominantly mammophilic, they have been maintained on quail, partly for economic reasons (116). Fecundity from field-collected adults has been estimated at 114 eggs per female (76) and lab-reared females have similar values (37). Eggs are readily deposited in small, dark containers with a rough substrate such as seed germination paper or Styrofoam (96, 116). Ae. j. japonicus egg storage requires higher humidity than other Aedes spp., suggesting a low tolerance to desiccation (116), though this is in contrast to field observations by Sota et al. (98) in its native range. Eggs are deposited almost exclusively in the hours just after sunset and before dawn (93), and egg surface characteristics that distinguish Ae. j. japonicus from other Aedes spp. have been described (34).
Although Ae. j. japonicus is often very abundant in some locations according to larval sampling studies, adults are not readily trapped with conventional methods. Therefore, common surveillance methods that utilize CDC (Centers for Disease Control and Prevention) or New Jersey light traps, or even the BG-Sentinel trap developed for day-biting mosquitoes, likely underestimate populations of Ae. j. japonicus (e.g., 93, 103). Many researchers consider gravid traps or oviposition traps better methods to collect adults and monitor for presence of the subspecies (50, 95, 96); however, some standard oviposition traps are ineffective for surveillance of this species (4). The use of polystyrene floats or pieces of rough cloth for oviposition substrates aids in egg collection (50, 96). Recently, a modification to CDC, CO2-baited light traps, namely an additional octenol-based lure, has shown great promise in increasing catch rates of Ae. j. japonicus adults by as much as 100 times over normal CDC light traps (3). One of the best methods for detecting the subspecies in an area, however, is the surveillance of larval habitats. Placing a dark plastic bucket with some decaying leaf material and water in an appropriately protected area is a cost-effective and simple means of detection (50).
CONCLUSIONS
The range expansion of Ae. j. japonicus has been rapid and extensive. In contrast to Ae. albopictus, which expanded in the United States at a similar rate, Ae. j. japonicus does not appear to have a remarkable intrinsic capacity for population growth and it is not a clearly superior larval competitor when compared with many co-occurring native species. Instead, our review of the ecology and phylogenetics of this subspecies indicates the main reasons for its success:
Multiple introductions maintaining high genetic diversity, a feature often associated with invasiveness.
A wide-ranging use of potential larval habitats that include typical natural and artificial containers as well as stormwater drainage system components. This ability not only allows colonization of diverse habitats, but also may help the range expansion of the species because of cross-regional transport of domestic containers, tires, and perhaps even construction supplies and equipment.
A tolerance for cold temperatures by both larvae and adults. In temperate climates, Ae. j. japonicus extends the range of seasonal activity beyond that of native container dwellers. In addition, the subspecies can overwinter as eggs or as larvae in milder climates.
The ability to establish early in the season and remain active longer, allowing Ae. j. japonicus to circumvent intense larval competition or stagger its exposure to co-occurring species in the often highly density-dependent, resource-limited environment of the larval habitat.
Ae. j. japonicus should continue to expand its worldwide range, albeit at a decelerated rate, in North America. It is likely that Ae. j. japonicus will eventually overlap considerably with the current and potential ranges of Ae. atropalpus, since these two species are so similar in their ecological characteristics. The potential for Ae. atropalpus expansion is discussed by Scholte et al. (92). Expansion across Europe appears more possible because of the many favorable climates and habitats in nearby Eastern Europe, Scandinavia, and Russia (109). However, transport of suitable containers between European countries is more restricted than interstate and US–Canadian transport (14). This may limit the rate of expansion in Europe to something more similar to those of Ae. albopictus, a species that arrived in Europe in 1979 but is still well short of its potential range in the region (18). In addition, increasing temperatures in the Northern Hemisphere may begin to reduce the range of Ae. j. japonicus in this century. Climate change models indicate that Ae. j. japonicus could eventually be restricted to the northern US states and Canada and that part of the range restriction will involve the northward spread of Ae. albopictus as temperatures rise (80). However, these predictions assume that Ae. j. japonicus will be unable to adapt to higher temperatures, an assumption that is being tested, especially in surprising new locations for this species such as Hawaii, where Ae. j. japonicus is now also established (58). Regardless of the eventual final range, we should now consider Ae. j. japonicus as part of the normal mosquito fauna in much of North America and Europe.
SUMMARY POINTS.
Ae. j. japonicus has greatly expanded its range to include large parts of North America, Europe, and Hawaii. The rate of its expansion rivals that of Ae. albopictus. Importantly, their co-occurrence in North America hinges on the ecology and temperature tolerances of Ae. j. japonicus relative to those of Ae. albopictus. Comparisons of the two invasive species as they expand and adapt to their new ranges may uncover critical processes that drive mosquito populations, exotic or native.
Population genetic studies of Ae. j. japonicus in North America, which began very shortly after the species was identified outside its native range, found clear evidence of the occurrence of more than one introduction into the northeast, an area where the species has become widespread. Comparisons across time demonstrated that initially isolated introductions have merged, a process that may be related to increased invasiveness.
Ae. j. japonicus is a competent vector of several arboviral diseases, is associated with peridomestic habitats, is known to feed on humans, and should be considered a risk for transmission of pathogens, particularly arboviruses. Also, because its presence can affect populations of other disease vectors in larval habitats, it may be affecting disease system dynamics indirectly.
The rapid spread of Ae. j. japonicus is likely related to its cold hardiness and expansive use of container habitats for oviposition and larval development. Transport via used tires is clearly involved, but a wide variety of artificial containers moved through commercial or domestic means would also serve in dispersal. An additional propensity to utilize rock pool habitats along river corridors may play a role.
Although Ae. j. japonicus appears to be displacing or reducing the abundance of native mosquito species, particularly Ae. atropalpus and Ae. triseriatus, in many larval habitats, initial studies of interspecific competition do not indicate a superior competitive advantage. Instead, this invasive subspecies may be increasing in relative abundance because of an expanded seasonal activity range in temperate climates that allows an early start in spring in larval habitats and a longer window for oviposition activity in the fall.
The recent worldwide spread of Ae. j. japonicus and its sibling species Ae. koreicus presents a unique opportunity to examine the impacts of multiple introductions of exotic vectors on disease systems and on the populations of native mosquito species. Their spread also indicates an increase in the expansion rate of new exotic mosquitoes and potential disease vectors, and the need to develop strategies to curb their spread while reducing or eliminating established populations.
Acknowledgments
We thank Marc Klowden for the invitation to submit the article and subsequent help throughout. We also thank Claire Luckachina for constructing the distribution map. This work was supported by NIH award AI21884.
Footnotes
DISCLOSURE STATEMENT
The authors are not aware of any affiliations, memberships, funding, or financial holdings that might be perceived as affecting the objectivity of this review.
Contributor Information
Michael G. Kaufman, Email: kaufma15@msu.edu.
Dina M. Fonseca, Email: dinafons@rci.rutgers.edu.
LITERATURE CITED
- 1.Alto BW. Interspecific larval competition between invasive Aedes japonicus and native Aedes triseriatus (Diptera: Culicidae) and adult longevity. J Med Entomol. 2011;48:232–42. doi: 10.1603/me09252. [DOI] [PubMed] [Google Scholar]
- 2.Alto BW, Lounibos LP, Higgs S, Juliano SA. Larval competition differentially affects arbovirus infection in Aedes mosquitoes. Ecology. 2005;86:3279–88. doi: 10.1890/05-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anderson JF, McKnight S, Ferrandino FJ. Aedes japonicus japonicus and associated woodland species attracted to Centers for Disease Control and Prevention miniature light traps baited with carbon dioxide and the Traptech® Mosquito Lure. J Am Mosq Control Assoc. 2012;28:184–91. doi: 10.2987/12-6260R.1. [DOI] [PubMed] [Google Scholar]
- 4.Andreadis TG, Anderson JF, Munstermann LE, Wolfe RJ, Florin DA. Discovery, distribution, and abundance of the newly introduced mosquito Ochlerotatus japonicus (Diptera: Culicidae) in Connecticut, USA. J Med Entomol. 2001;38:774–79. doi: 10.1603/0022-2585-38.6.774. [DOI] [PubMed] [Google Scholar]
- 5.Andreadis TG, Wolfe RJ. Evidence for reduction of native mosquitoes with increased expansion of invasive Ochlerotatus japonicus japonicus (Diptera: Culicidae) in the northeastern United States. J Med Entomol. 2010;47:43–52. doi: 10.1603/033.047.0106. [DOI] [PubMed] [Google Scholar]
- 6.Apperson CS, Hassan HK, Harrison BA, Savage HM, Aspen SE, et al. Host feeding patterns of established and potential mosquito vectors of West Nile virus in the eastern United States. Vector Borne Zoonotic Dis. 2004;4:71–82. doi: 10.1089/153036604773083013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Armistead JS, Arias JR, Nishimura N, Lounibos LP. Interspecific larval competition between Aedes albopictus and Aedes japonicus (Diptera: Culicidae) in northern Virginia. J Med Entomol. 2008;45:629–37. doi: 10.1603/0022-2585(2008)45[629:ilcbaa]2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Armistead JS, Nishimura N, Arias JR, Lounibos LP. Community ecology of container mosquitoes (Diptera: Culicidae) in Virginia following invasion by Aedes japonicus. J Med Entomol. 2012;49:1318–27. doi: 10.1603/me11261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Armistead JS, Nishimura N, Escher RL, Lounibos LP. Larval competition between Aedes japonicus and Aedes atropalpus (Diptera: Culicidae) in simulated rock pools. J Vector Ecol. 2008;33:238–46. doi: 10.3376/1081-1710-33.2.238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bartlett-Healy K, Unlu I, Obenauer P, Hughes T, Healy S, et al. Larval mosquito habitat utilization and community dynamics of Aedes albopictus and Aedes japonicus (Diptera: Culicidae) J Med Entomol. 2012;49:813–24. doi: 10.1603/me11031. [DOI] [PubMed] [Google Scholar]
- 11.Bevins SN. Establishment and abundance of a recently introduced mosquito species Ochlerotatus japonicus (Diptera: Culicidae) in the southern Appalachians, USA. J Med Entomol. 2007;44:945–52. doi: 10.1603/0022-2585(2007)44[945:eaaoar]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 12.Bevins SN. Effects of expanded mosquito range. Science. 2008;321:1634. doi: 10.1126/science.321.5896.1634. [DOI] [PubMed] [Google Scholar]
- 13.Bevins SN. Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae) Biol Invas. 2008;10:1109–17. [Google Scholar]
- 14.Buehler R. Transport policies, automobile use, and sustainable transport: a comparison of Germany and the United States. J Plan Educ Res. 2010;30:76–93. [Google Scholar]
- 15.Burger JF, Davis H. Discovery of Ochlerotatus japonicus japonicus (Theobald) (Diptera: Culicidae) in southern New Hampshire, USA and its subsequent increase in abundance in used tire casings. Entomol News. 2008;119:439–44. [Google Scholar]
- 16.Caldwell ND, Gerhardt RR, Jones CJ. First collection of Ochlerotatus japonicus japonicus in the state of Tennessee. J Am Mosq Control Assoc. 2005;21:322–24. doi: 10.2987/8756-971X(2005)21[322:FCOOJJ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 17.Cameron EC, Wilkerson RC, Mogi M, Miyagi I, Toma T, et al. Molecular phylogenetics of Aedes japonicus, a disease vector that recently invaded Western Europe, North America, and the Hawaiian Islands. J Med Entomol. 2010;47:527–35. doi: 10.1093/jmedent/47.4.527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Caminade C, Medlock JM, Ducheyne E, McIntyre KM, Leach S, et al. Suitability of European climate for the Asian tiger mosquito Aedes albopictus: recent trends and future scenarios. J R Soc Interface. 2012;9:2708–17. doi: 10.1098/rsif.2012.0138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Capelli G, Drago A, Martini S, Montarsi F, Soppelsa M, et al. First report in Italy of the exotic mosquito species Aedes (Finlaya) koreicus, a potential vector of arboviruses and filariae. Parasites Vectors. 2011;4:188. doi: 10.1186/1756-3305-4-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Darsie RF., Jr Revision of Darsie and Ward 1981 to include Ochlerotatus japonicus Theobald and a checklist of species referred to the genus Ochlerotatus in the Nearctic region. J Am Mosq Control Assoc. 2002;18:237–40. [PubMed] [Google Scholar]
- 21.Dunphy BM, Tucker BJ, Petersen MJ, Blitvich BJ, Bartholomay LC. Arrival and establishment of Aedes japonicus japonicus (Diptera: Culicidae) in Iowa. J Med Entomol. 2009;46:1282–89. doi: 10.1603/033.046.0605. [DOI] [PubMed] [Google Scholar]
- 22.Elsik CG, Williams CG. Low-copy microsatellite recovery from a conifer genome. Theor Appl Genet. 2001;103:1189–95. [Google Scholar]
- 23.Erthal JA, Soghigian JS, Livdahl T. Life cycle completion of parasite Ascogregarina taiwanensis (Api-complexa: Lecudinidae) in non-native host Ochlerotatus japonicus (Diptera: Culicidae) J Med Entomol. 2012;49:1109–17. doi: 10.1603/me12018. [DOI] [PubMed] [Google Scholar]
- 24.Fagerberg AJ, Fulton RE, Black WC. Microsatellite loci are not abundant in all arthropod genomes: analyses in the hard tick, Ixodes scapularis and the yellow fever mosquito, Aedes aegypti. Insect Mol Biol. 2001;10:225–36. doi: 10.1046/j.1365-2583.2001.00260.x. [DOI] [PubMed] [Google Scholar]
- 25.Falco RC, Daniels TJ, Slameck MC. Prevalence and distribution of Ochlerotatus japonicus (Diptera: Culicidae) in two counties in southern New York State. J Med Entomol. 2002;39:920–25. doi: 10.1603/0022-2585-39.6.920. [DOI] [PubMed] [Google Scholar]
- 26.Fonseca DM, Campbell S, Crans WJ, Mogi M, Miyagi I, et al. Aedes (Finlaya) japonicus (Diptera: Culicidae), a newly recognized mosquito in the United States: analyses of genetic variation in the United States and putative source populations. J Med Entomol. 2001;38:135–46. doi: 10.1603/0022-2585-38.2.135. [DOI] [PubMed] [Google Scholar]
- 27.Fonseca DM, Unlu I, Crepeau T, Farajollahi A, Healy S, et al. Area-wide management of Aedes albopictus Part 2: Gauging the efficacy of traditional integrated pest control measures against urban container mosquitoes. Pest Manag Sci. 2013;69:1351–61. doi: 10.1002/ps.3511. [DOI] [PubMed] [Google Scholar]
- 28.Fonseca DM, Widdel AK, Hutchinson M, Spichiger SE, Kramer LD. Fine-scale spatial and temporal population genetics of Aedes japonicus, a new US mosquito, reveal multiple introductions. Mol Ecol. 2010;19:1559–72. doi: 10.1111/j.1365-294X.2010.04576.x. [DOI] [PubMed] [Google Scholar]
- 29.Gallitano S, Blaustein L, Vonesh J. First occurrence of Ochlerotatus japonicus in Missouri. J Vector Ecol. 2005;30:347–48. [PubMed] [Google Scholar]
- 30.Gaspar JP, McKay T, Huss MJ. First report of Aedes japonicus in natural and artificial habitats in northeastern Arkansas. J Am Mosq Control Assoc. 2012;28:38–42. doi: 10.2987/11-6196.1. [DOI] [PubMed] [Google Scholar]
- 31.Gingrich JB, Anderson RD, Williams GM, O’Connor L, Harkins K. Stormwater ponds, constructed wetlands, and other best management practices as potential breeding sites for West Nile virus vectors in Delaware during 2004. J Am Mosq Control Assoc. 2006;22:282–91. doi: 10.2987/8756-971X(2006)22[282:SPCWAO]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 32.Gray EW, Harrison BA, Womack ML, Kerce J, Neely CJ, Noblet R. Ochlerotatus japonicus japonicus (Theobald) in Georgia and North Carolina. J Am Mosq Control Assoc. 2005;21:144–46. doi: 10.2987/8756-971X(2005)21[144:OJJTIG]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 33.Grim DC, Jackson BT, Paulson SL. Abundance and bionomics of Ochlerotatus j. japonicus in two counties in southwestern Virginia. J Am Mosq Control Assoc. 2007;23:259–63. doi: 10.2987/8756-971X(2007)23[259:AABOOJ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 34.Haddow AD, Moulton JK, Gerhardt RR, McCuiston LJ, Jones CJ. Description of the egg of Ochlerotatus japonicus japonicus (Diptera: Culicidae) using variable pressure scanning electron microscopy. J Med Entomol. 2009;46:9–14. doi: 10.1603/033.046.0102. [DOI] [PubMed] [Google Scholar]
- 35.Hardstone MC, Andreadis TG. Weak larval competition between the invasive mosquito Aedes japonicus japonicus (Diptera: Culicidae) and three resident container-inhabiting mosquitoes in the laboratory. J Med Entomol. 2012;49:277–85. doi: 10.1603/me11050. [DOI] [PubMed] [Google Scholar]
- 36.Harrison BA, Whitt PB, Cope SE, Payne GR, Rankin SE, et al. Mosquitoes (Diptera: Culicidae) collected near the Great Dismal Swamp: new state records, notes on certain species, and a revised checklist for Virginia. Proc Entomol Soc Wash. 2002;104:655–62. [Google Scholar]
- 37.Hoshino K, Isawa H, Tsuda Y, Kobayashi M. Laboratory colonization of Aedes japonicus japonicus (Diptera: Culicidae) collected in Narita, Japan and the biological properties of the established colony. Jpn J Infect Dis. 2010;63:401–4. [PubMed] [Google Scholar]
- 38.Huber K, Pluskota B, Jost A, Hoffmann K, Becker N. Status of the invasive species Aedes japonicus japonicus (Diptera: Culicidae) in southwest Germany in 2011. J Vector Ecol. 2012;37:462–65. doi: 10.1111/j.1948-7134.2012.00252.x. [DOI] [PubMed] [Google Scholar]
- 39.Hughes TH, Irwin PM, Kaufman A, Sage H, Pagac BB, Jr, Paskewitz SM. First records of Aedes japonicus japonicus in Wisconsin. J Am Mosq Control Assoc. 2008;24:583–84. doi: 10.2987/5735.1. [DOI] [PubMed] [Google Scholar]
- 40.Ingrassia A. MSc thesis. SUNY; Binghamton, NY: 2006. Larval competition between the native treehole mosquito, Ochlerotatus triseriatus, and the invasive mosquito, Ochlerotatus japonicus, using natural diets; p. 104. [Google Scholar]
- 41.Iriate WLZ, Tsuda Y, Wada Y, Takagi M. Distribution of mosquitoes on a hill of Nagasaki City, with emphasis to the distance from human dwellings. Trop Med. 1991;33:55–60. [Google Scholar]
- 42.Irish SR, Pierce CS. Update on the distribution of Ochlerotatus japonicus in Oregon and Washington. J Am Mosq Control Assoc. 2008;24:110–11. doi: 10.2987/5572.1. [DOI] [PubMed] [Google Scholar]
- 43.Johnson KA, Brogren SJ, Crane DM, Lamere CA. Status of Aedes japonicus in the Metropolitan Mosquito Control District, Minnesota. J Am Mosq Control Assoc. 2010;26:328–31. doi: 10.2987/10-6018.1. [DOI] [PubMed] [Google Scholar]
- 44.Joy JE. Larval mosquitoes in abandoned tire pile sites from West Virginia. J Am Mosq Control Assoc. 2004;20:12–17. [PubMed] [Google Scholar]
- 45.Joy JE, Sullivan SN. Occurrence of tire inhabiting mosquito larvae in different geographic regions of West Virginia. J Am Mosq Control Assoc. 2005;21:380–86. doi: 10.2987/8756-971X(2006)21[380:OOTIML]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 46.Juliano SA. Species interactions among larval mosquitoes: context dependence across habitat gradients. Annu Rev Entomol. 2009;54:37–56. doi: 10.1146/annurev.ento.54.110807.090611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Juliano SA. Coexistence, exclusion, or neutrality? A meta-analysis of competition between Aedes albopictus and resident mosquitoes. Isr J Ecol Evol. 2010;56:325–51. doi: 10.1560/IJEE.55.3-4.325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Juliano SA, Lounibos LP. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol Lett. 2005;8:558–74. doi: 10.1111/j.1461-0248.2005.00755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kambhampati S, Black WC, Rai KS, Sprenger D. Temporal variation in genetic structure of a colonizing species: Aedes albopictus in the United States. Heredity. 1990;64:281–87. doi: 10.1038/hdy.1990.34. [DOI] [PubMed] [Google Scholar]
- 50.Kaufman MG, Stanuszek WW, Brouhard EA, Knepper RG, Walker ED. Establishment of Aedes japonicus japonicus and its colonization of container habitats in Michigan. J Med Entomol. 2012;49:1307–17. doi: 10.1603/me12061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kaufman PE, Harrington LC, Waldron JK, Rutz DA. The importance of agricultural tire habitats for mosquitoes of public health importance in New York State. J Am Mosq Control Assoc. 2005;21:171–76. doi: 10.2987/8756-971X(2005)21[171:TIOATH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 52.Kesavaraju B, Alto B, Afify A, Gaugler R. Malathion influences competition between Aedes albopictus and Aedes japonicus. J Med Entomol. 2010;47:1011–18. doi: 10.1603/me10011. [DOI] [PubMed] [Google Scholar]
- 53.Kesavaraju B, Khan DF, Gaugler R. Behavioral differences of invasive container-dwelling mosquitoes to a native predator. J Med Entomol. 2011;48:526–32. doi: 10.1603/me10200. [DOI] [PubMed] [Google Scholar]
- 54.Kokas J, Lee JH. Toxorhynchites rutilus septentrionalis: new occurrences in upstate New York. J Am Mosq Control Assoc. 2004;20:199–200. [PubMed] [Google Scholar]
- 55.Kolbe JJ, Glor RE, Rodríguez Schettino L, Lara AC, Larson A, Losos JB. Genetic variation increases during biological invasion by a Cuban lizard. Nature. 2004;43:177–81. doi: 10.1038/nature02807. [DOI] [PubMed] [Google Scholar]
- 56.LaCasse WJ, Yamaguti S. Mosquito fauna of Japan and Korea. Parts I & II. Mosquito survey data on Japan and their application in the control of mosquito-borne diseases. Off Surg, HQ 1 Corps APO 301 (Japan) 1950 [Google Scholar]
- 57.Laird M, Calder L, Thornton RC, Syme R, Holder PW, Mogi M. Japanese Aedes albopictus among four mosquito species reaching New Zealand in used tires. J Am Mosq Control Assoc. 1994;10:14–23. [PubMed] [Google Scholar]
- 58.Larish LB, Savage HM. Introduction and establishment of Aedes (Finlaya) japonicus japonicus (Theobald) on the island of Hawaii: implications for arbovirus transmission. J Am Mosq Control Assoc. 2005;21:318–21. doi: 10.2987/8756-971X(2005)21[318:IAEOAF]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 59.Leisnham PT, Juliano SA. Impacts of climate, land use, and biological invasion on the ecology of immature Aedes mosquitoes: implications for La Crosse emergence. Ecohealth. 2012;9:217–28. doi: 10.1007/s10393-012-0773-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lorenz A. MSc thesis. Michigan State Univ; 2012. The role of algae in the invasion ecology of the mosquito species Aedes japonicus japonicus (Diptera: Culicidae) p. 115. [Google Scholar]
- 61.Lorenz AR, Walker ED, Kaufman MG. Does autochthonous primary production influence oviposition by Aedes japonicus japonicus (Diptera: Culicidae) in container habitats? J Med Entomol. 2013;50:69–78. doi: 10.1603/me12083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lounibos LP. Invasions by insect vectors of human disease. Annu Rev Entomol. 2002;47:233–66. doi: 10.1146/annurev.ento.47.091201.145206. [DOI] [PubMed] [Google Scholar]
- 63.Lu B, Li B, Ji S, Chen H, Meng Q, et al. Fauna Sinica, Insecta Vol. 8, Diptera: Culicidae 1. Beijing: Science Press; 1997. pp. 125–35.pp. 593 [Google Scholar]
- 64.McLain DK, Rai KS, Fraser MJ. Intraspecific and interspecific variation in the sequence and abundance of highly repeated DNA among mosquitoes of the Aedes albopictus subgroup. Heredity. 1987;58(Pt. 3):373–81. doi: 10.1038/hdy.1987.65. [DOI] [PubMed] [Google Scholar]
- 65.Medlock JM, Hansford KM, Schaffner F, Versteirt V, Hendrickx G, et al. A review of the invasive mosquitoes in Europe: Ecology, public health risks, and control options. Vector Borne Zoonotic Dis. 2012;12:435–47. doi: 10.1089/vbz.2011.0814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Miles JA. Some ecological aspects of the problem of arthropod-borne animal viruses in the Western Pacific and South-East Asia regions. Bull World Health Organ. 1964;30:197–210. [PMC free article] [PubMed] [Google Scholar]
- 67.Miyagi Notes on the Aedes (Finlaya) chrysolineatus subgroup in Japan and Korea (Diptera: Culicidae) Trop Med. 1971;13:141–51. [Google Scholar]
- 68.Molaei G, Farajollahi A, Scott JJ, Gaugler R, Andreadis TG. Human bloodfeeding by the recently introduced mosquito, Aedes japonicus japonicus, and public health implications. J Am Mosq Control Assoc. 2009;25:210–14. doi: 10.2987/09-0012.1. [DOI] [PubMed] [Google Scholar]
- 69.Moore CG. Aedes albopictus in the United States: current status and prospects for further spread. J Am Mosq Control Assoc. 1999;15:221–27. [PubMed] [Google Scholar]
- 70.Morris JA, Lampman RL, Ballmes G, Funes J, Halvorsen J, Novak RJ. First record of Aedes japonicus japonicus in Illinois: defining its spatial distribution and associated mosquito species. J Am Mosq Control Assoc. 2007;23:243–51. doi: 10.2987/8756-971X(2007)23[243:FROAJJ]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 71.Murrell EG, Juliano SA. Predation resistance does not trade off with competitive ability in early-colonizing mosquitoes. Oecologia. 2013;173:1033–42. doi: 10.1007/s00442-013-2674-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Muturi EJ, Kim CH, Alto BW, Berenbaum MR, Schuler MA. Larval environmental stress alters Aedes aegypti competence for Sindbis virus. Trop Med Int Health. 2011;16:955–64. doi: 10.1111/j.1365-3156.2011.02796.x. [DOI] [PubMed] [Google Scholar]
- 73.Narwrocki SJ, Craig GB. Further extension of the range of the rock pool mosquito, Aedes atropalpus, via tire breeding. J Am Mosq Control Assoc. 1989;5:110–14. [PubMed] [Google Scholar]
- 74.Neitzel DF, Johnson KA, Brogren S, Kemperman MM. First collection records of Aedes japonicus in Minnesota. J Am Mosq Control Assoc. 2009;25:367–69. doi: 10.2987/09-0015.1. [DOI] [PubMed] [Google Scholar]
- 75.O’Donnell DL, Armbruster P. Comparison of larval foraging behavior of Aedes albopictus and Aedes japonicus (Diptera: Culicidae) J Med Entomol. 2007;44:984–89. doi: 10.1603/0022-2585(2007)44[984:colfbo]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 76.Oliver J, Howard JJ. Fecundity of naturally blood-fed Ochlerotatus japonicus. J Med Entomol. 2005;42:254–59. doi: 10.1093/jmedent/42.3.254. [DOI] [PubMed] [Google Scholar]
- 77.Oliver J, Means RG, Howard JJ. Geographic distribution of Ochlerotatus japonicus in New York State. J Am Mosq Control Assoc. 2003;19:121–24. [PubMed] [Google Scholar]
- 78.Peyton EL, Campbell SR, Candeletti TM, Romanowski M, Crans WJ. Aedes (Finlaya) japonicus japonicus (Theobald), a new introduction into the United States. J Am Mosq Control Assoc. 1999;15:238–41. [PubMed] [Google Scholar]
- 79.Qualls WA, Mullen GR. Larval survey of tire-breeding mosquitoes in Alabama. J Am Mosq Control Assoc. 2006;22:601–8. doi: 10.2987/8756-971X(2006)22[601:LSOTMI]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 80.Rochlin I, Gaugler R, Williges E, Farajollahi A. The rise of the invasives and decline of the natives: insights revealed from adult populations of container-inhabiting Aedes mosquitoes (Diptera: Culicidae) in temperate North America. Biol Invas. 2013;15:991–1003. [Google Scholar]
- 81.Roppo MR, Lilja JL, Maloney FA, Sames WJ. First occurrence of Ochlerotatus japonicus in the state of Washington. J Am Mosq Control Assoc. 2004;20:83–84. [PubMed] [Google Scholar]
- 82.Roychoudhury S, Isawa H, Hoshino K, Sasaki T, Saito N, et al. Comparison of the morphology of oocysts and the phylogenetic analysis of four Ascogregarina species (Eugregarinidae: Lecudinidae) as inferred from small subunit ribosomal DNA sequences. Parasitol Int. 2007;56:113–18. doi: 10.1016/j.parint.2006.12.006. [DOI] [PubMed] [Google Scholar]
- 83.Saenz VL, Townsend LH, Vanderpool RM, Schardein MJ, Trout RT, Brown GC. Ochlerotatus japonicus japonicus in the state of Kentucky. J Am Mosq Control Assoc. 2006;22:754–55. doi: 10.2987/8756-971X(2006)22[754:OJJITS]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 84.Sardelis MR, Dohm DJ, Pagac B, Andre RG, Turell MJ. Experimental transmission of eastern equine encephalitis virus by Ochlerotatus j. japonicus (Diptera: Culicidae) J Med Entomol. 2002;39:480–84. doi: 10.1603/0022-2585-39.3.480. [DOI] [PubMed] [Google Scholar]
- 85.Sardelis MR, Turell MJ. Ochlerotatus j. japonicus in Frederick County, Maryland: discovery, distribution, and vector competence for West Nile virus. J Am Mosq Control Assoc. 2001;17:137–41. [PubMed] [Google Scholar]
- 86.Sardelis MR, Turell MJ, Andre RG. Laboratory transmission of La Crosse virus by Ochlerotatus j. japonicus (Diptera: Culicidae) J Med Entomol. 2002;39:635–39. doi: 10.1603/0022-2585-39.4.635. [DOI] [PubMed] [Google Scholar]
- 87.Sardelis MR, Turell MJ, Andre RG. Experimental transmission of St. Louis encephalitis virus by Ochlerotatus j japonicus. J Am Mosq Control Assoc. 2003;19:159–62. [PubMed] [Google Scholar]
- 88.Schaffner F, Chouin S, Guilloteau J. First record of Ochlerotatus (Finlaya) japonicus japonicus (Theobald, 1901) in metropolitan France. J Am Mosq Control Assoc. 2003;19:1–5. [PubMed] [Google Scholar]
- 89.Schaffner F, Kaufmann C, Hegglin D, Mathis A. The invasive mosquito Aedes japonicus in Central Europe. Med Vet Entomol. 2009;23:448–51. doi: 10.1111/j.1365-2915.2009.00825.x. [DOI] [PubMed] [Google Scholar]
- 90.Schaffner F, Medlock JM, Van Bortel W. Public health significance of invasive mosquitoes in Europe. Clin Microbiol Infect. 2013;19:685–92. doi: 10.1111/1469-0691.12189. [DOI] [PubMed] [Google Scholar]
- 91.Schaffner F, Vazeille M, Kaufmann C, Failloux A, Mathis A. Vector competence of Aedes japonicus for chikungunya and dengue viruses. Eur Mosq Bull. 2011;29:141–42. [Google Scholar]
- 92.Scholte JE, Den Hartog W, Braks M, Ruesken C, Dik M, Hessels A. First report of a North American invasive species Ochlerotatus atropalpus (Coquillett) in the Netherlands, 2009. Euro Surveill. 2009;14 doi: 10.2807/ese.14.45.19400-en. pii. [DOI] [PubMed] [Google Scholar]
- 93.Scott J. PhD thesis. Rutgers Univ; New Jersey: 2003. The ecology of the exotic mosquito Ochlerotatus (Finlaya) japonicus japonicus (Theobald 1901) (Diptera: Culicidae) and an examination of its role in the West Nile virus cycle in New Jersey; p. 179. [Google Scholar]
- 94.Scott JJ, Carle FL, Crans WJ. Ochlerotatus japonicus collected from natural rockpools in New Jersey. J Am Mosq Control Assoc. 2001;17:91–92. [PubMed] [Google Scholar]
- 95.Scott JJ, Crans SC, Crans WJ. Use of an infusion-baited gravid trap to collect adult Ochlerotatus japonicus. J Am Mosq Control Assoc. 2001;17:142–43. [PubMed] [Google Scholar]
- 96.Scott JJ, Crans WJ. Expanded polystyrene (EPS) floats for surveillance of Ochlerotatus japonicus. J Am Mosq Control Assoc. 2003;19:376–81. [PubMed] [Google Scholar]
- 97.Shestakov VI, Mikheeva AI. Study of vectors of Japanese encephalitis in the Maritime Territory. Med Parazitol Parazit Bolezn. 1966;35:545–50. [PubMed] [Google Scholar]
- 98.Sota T, Mogi M, Hayamizu E. Habitat stability and the larval mosquito community in treeholes and other containers on a temperate island. Res Popul Ecol. 1994;36:93–104. [Google Scholar]
- 99.Sunahara T, Ishizaka K, Mogi M. Habitat size: a factor determining the opportunity for encounters between mosquito larvae and aquatic predators. J Vector Ecol. 2002;27:8–20. [PubMed] [Google Scholar]
- 100.Takashimi I, Rosen L. Horizontal and vertical transmission of Japanese encephalitis virus by Aedes japonicus (Diptera: Culicidae) J Med Entomol. 1989;26:454–58. doi: 10.1093/jmedent/26.5.454. [DOI] [PubMed] [Google Scholar]
- 101.Takoaka H. Microsporidian infection of Aedes japonicus larvae in Japan. Jpn J Sanit Zool. 1982;33:71–72. [Google Scholar]
- 102.Tanaka K, Mizusawa K, Saugstad ES. A revision of the adult and larval mosquitoes of Japan (including the Ryukyu Archipelago and the Ogasaware Islands) and Korea (Diptera: Culicidae) Contrib Am Entomol Inst. 1979;16:1–987. [Google Scholar]
- 103.Thielman A, Hunter FF. Establishment of Ochlerotatus japonicus (Diptera: Culicidae) in Ontario, Canada. J Med Entomol. 2006;43:138–42. doi: 10.1603/0022-2585(2006)043[0138:eoojdc]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 104.Thorn M, Varnado WC, Goddard J. First record of Aedes japonicus japonicus in Mississippi. J Am Mosq Control Assoc. 2012;28:43–44. doi: 10.2987/11-6204.1. [DOI] [PubMed] [Google Scholar]
- 105.Toma T, Miyagi I. Notes on the mosquitoes collected at forest areas in the northern part of Okinawajima, Ryukyu Islands, Japan. Jpn J Sanit Zool. 1981;32:271–79. [Google Scholar]
- 106.Toma T, Miyagi I. The mosquito fauna of the Ryukyu Archipelago with identification keys, pupal descriptions and notes on the biology, medical importance and distribution. Mosq Syst. 1986;18:1–109. [Google Scholar]
- 107.Turell MJ, O’Guinn ML, Dohm DJ, Jones JW. Vector competence of North American mosquitoes (Diptera: Culicidae) for West Nile virus. J Med Entomol. 2001;38:130–34. doi: 10.1603/0022-2585-38.2.130. [DOI] [PubMed] [Google Scholar]
- 108.Turell MJ, Sardelis MR, Dohm DJ, O’Guinn ML. Potential North American vectors of West Nile virus. Ann N Y Acad Sci. 2001;951:317–24. doi: 10.1111/j.1749-6632.2001.tb02707.x. [DOI] [PubMed] [Google Scholar]
- 109.Unlu I, Farajollahi A. Vectors without borders: imminent arrival, establishment, and public health implications of the Asian bush (Aedes japonicus) and Asian tiger (Aedes albopictus) mosquitoes in Turkey. Hacet J Biol Chem. 2012;40:23–36. [Google Scholar]
- 110.Urbanelli S, Bellini R, Carrieri M, Sallicandro P, Celli G. Population structure of Aedes albopictus (Skuse): the mosquito which is colonizing Mediterranean countries. Heredity. 2000;84:331–37. doi: 10.1046/j.1365-2540.2000.00676.x. [DOI] [PubMed] [Google Scholar]
- 111.Versteirt V, De Clercq EM, Fonseca DM, Pecor J, Schaffner F, et al. Bionomics of the established exotic mosquito species Aedes koreicus in Belgium, Europe. J Med Entomol. 2012;49:1226–32. doi: 10.1603/me11170. [DOI] [PubMed] [Google Scholar]
- 112.Versteirt V, Schaffner F, Garros C, Dekoninck W, Coosemans M, Van Bortel W. Introduction and establishment of the exotic mosquito species Aedes japonicus japonicus (Diptera: Culicidae) in Belgium. J Med Entomol. 2009;46:1464–67. doi: 10.1603/033.046.0632. [DOI] [PubMed] [Google Scholar]
- 113.Wang G, Li C, Guo X, Xing D, Dong Y, et al. Identifying the main mosquito species in China based on DNA barcoding. PLoS One. 2012;7:e47051. doi: 10.1371/journal.pone.0047051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Westby K, Fritzen C, Huang J, Jaske E, Paulsen D, et al. La Crosse encephalitis in Eastern Tennessee: evidence of invasive mosquito (Aedes albopictus and Ochlerotatus japonicus) involvement in the transmission of an indigenous disease. Am J Trop Med Hyg. 2011;85(Suppl):374. [Google Scholar]
- 115.Widdel AK, McCuiston LJ, Crans WJ, Kramer LD, Fonseca DM. Finding needles in the haystack: single copy microsatellite loci for Aedes japonicus (Diptera: Culicidae) Am J Trop Med Hyg. 2005;73:744–48. [PubMed] [Google Scholar]
- 116.Williges E, Farajollahi A, Scott JJ, McCuiston LJ, Crans WJ, Gaugler R. Laboratory colonization of Aedes japonicus japonicus. J Am Mosq Control Assoc. 2008;24:591–93. doi: 10.2987/5714.1. [DOI] [PubMed] [Google Scholar]
- 117.Yee DA. Tires as habitats for mosquitoes: a review of studies within the eastern United States. J Med Entomol. 2008;45:581–93. doi: 10.1603/0022-2585(2008)45[581:tahfma]2.0.co;2. [DOI] [PubMed] [Google Scholar]