Background: p40, a Lactobacillus rhamnosus GG-derived protein, ameliorates intestinal injury and inflammation through transactivation of the EGF receptor (EGFR).
Results: p40 up-regulates Muc2 gene expression and mucin production in LS174T cells and in mouse colonic epithelium in an EGFR-dependent manner.
Conclusion: p40 stimulates mucin production through transactivation of EGFR.
Significance: p40-stimulated mucin production may play a role in protecting the intestinal epithelium from injury.
Keywords: Apoptosis, Epidermal Growth Factor Receptor (EGFR), Intestine, Mucus, Probiotic, Goblet Cell, Intestine, Lactobacillus rhamnosus GG, Mucin, Probiotics
Abstract
The mucus layer coating the gastrointestinal tract serves as the first line of intestinal defense against infection and injury. Probiotics promote mucin production by goblet cells in the intestine. p40, a Lactobacillus rhamnosus GG-derived soluble protein, has been shown to transactivate the EGF receptor (EGFR) in intestinal epithelial cells, which is required for inhibition of apoptosis and preservation of barrier function in the colon, thereby ameliorating intestinal injury and colitis. Because activation of EGFR has been shown to up-regulate mucin production in goblet cells, the purpose of this study was to investigate the effects and mechanisms of p40 regulation of mucin production. p40 activated EGFR and its downstream target, Akt, in a concentration-dependent manner in LS174T cells. p40 stimulated Muc2 gene expression and mucin production in LS174T cells, which were abolished by inhibition of EGFR kinase activity, down-regulation of EGFR expression by EGFR siRNA transfection, or suppression of Akt activation. Treatment with p40 increased mucin production in the colonic epithelium, thus thickening the mucus layer in the colon of wild type, but not of Egfrwa5 mice, which have a dominant negative mutation in the EGFR kinase domain. Furthermore, inhibition of mucin-type O-linked glycosylation suppressed the effect of p40 on increasing mucin production and protecting intestinal epithelial cells from TNF-induced apoptosis in colon organ culture. Thus, these results suggest that p40-stimulated activation of EGFR mediates up-regulation of mucin production, which may contribute to the mechanisms by which p40 protects the intestinal epithelium from injury.
Introduction
Mucus plays an important role in protecting epithelial surfaces from bacterial and viral assault and mechanical damage in several systems, such as the gastrointestinal tract (1, 2), the trachea (3), and the conjunctiva (4). Mucin, a major component of mucus, is a heavily O-linked glycoprotein produced by goblet cells. There are two forms of mucins: secreted gel-forming mucin, the major component of the mucus system (1, 5), and transmembrane mucin, which may contribute to the formation of the protective glycocalyx in the intestines and the lungs (6). The colon and the stomach have a two-layered mucus system: an inner mucus layer attached to the epithelium and an outer, unattached, loose mucus layer with MUC2 in the colon and MUC5AC and MUC6 in the stomach. The small intestine has one layer of unattached mucus containing MUC2 (7, 8).
The mucus layer coating the gastrointestinal tract serves as the first line of the intestinal barrier. Qualitative and quantitative abnormalities of mucin gene expression have been found in several diseases. For example, a defect in the inner mucus layer that allows bacteria to reach the epithelium has been found in patients with ulcerative colitis and in mouse models of colitis (9). In necrotizing enterocolitis, the goblet cell number in the ileum is decreased (10). Animal studies have revealed that Muc2-deficient mice develop spontaneous colitis and are susceptible to experimental colitis because of increased intestinal permeability and bacterial adhesion to the mucosal cell surface (11). Thus, mucins play a role in protection of the intestinal epithelium and in maintaining mucosal homeostasis.
Probiotics have been reported to promote mucus production to enhance the host's defense against infection. Lactobacillus rhamnosus GG (LGG)3 has been shown to up-regulate Muc2 gene expression in intestinal epithelial cells (12). Furthermore, it has been demonstrated that LGG and Lactobacillus planetarium inhibit the adherence of enteropathogenic Escherichia coli to the intestinal epithelial cells through induction of MUC2 and MUC3 mucin production (13). However, information regarding the molecular mechanism of probiotic regulation of mucin production by goblet cells is limited.
EGFR is a member of the ErbB family that has an extracellular ligand-binding domain and an intracellular portion that contains a tyrosine kinase domain (14, 15). Binding of EGFR to its soluble ligands, EGF, heparin-binding (HB)-EGF, transforming growth factor (TGF)α, or amphiregulin, triggers autophosphorylation of cytoplasmic tyrosine residues (14, 15). These phosphorylated amino acids provide docking sites for a variety of signaling molecules that regulate intracellular signaling networks, such as MAPK and Akt. Activation of EGFR promotes cell proliferation, differentiation, migration, and survival (14, 15). EGFR signaling has also been reported to regulate mucin production (16).
LGG, a Gram-positive bacterium originally isolated from healthy human intestine (17), has been used in clinical trials for treating and/or preventing several diseases, including ulcerative colitis (18), diarrhea (19, 20), and atopic dermatitis (21). We have previously purified and cloned a LGG-derived soluble protein, p40, that prevents cytokine-induced epithelial damage and apoptosis, as well as hydrogen peroxide disruption of the epithelial barrier function through stimulation of EGFR ligand release, which activates EGFR in intestinal epithelial cells (22–24). Furthermore, specific delivery of p40 to the colon using hydrogel beads to protect it from degradation prevents and treats colonic epithelial cell injury and inflammation in mouse models of colitis in an EGFR-dependent manner (25).
The purpose of this study is to investigate the effects and mechanisms of p40 regulation of mucin production. These studies demonstrate that p40-stimulated mucin production in LS174T cells and in mouse colonic epithelium is mediated by EGFR transactivation. Our data define the mechanism by which p40 protects the intestinal epithelium from injury.
EXPERIMENTAL PROCEDURES
Cell Culture and Treatment
The LS174T cell line (ATCC CL-188TM) is a human colon cancer cell line that exhibits characteristics of normal colonic mucosal cells, including microvilli prominent in secretory cells and the presence of intracytoplasmic mucin vacuoles. LS174T cells were maintained in minimal essential medium supplemented with 10% heat-inactivated FBS, 1% penicillin and streptomycin at 37 °C in 5% carbon dioxide. Prior to all experiments, the cells were serum-starved for 16–18 h in minimal essential medium containing 0.5% FBS and 100 units/ml penicillin and streptomycin at 37 °C.
p40 was isolated from LGG culture supernatant, as previously described (23). Cells were treated with p40 or human EGF (Pepro Tech, Inc.) in the presence or absence of an EGFR tyrosine kinase inhibitor, AG1478 (Calbiochem), or a phosphoinositide 3-kinase inhibitor, wortmannin (Calbiochem).
Transient Transfection of siRNA
LS174T cells were transiently transfected with either 75 nm human EGFR siRNA (a set of four pooled siRNAs; Dharmacon) or 75 nm nontargeting siRNA using Lipofectamine RNAiMAX reagent (Invitrogen), according to the manufacturer's instructions. After a 48 h post-transfection, the cells were treated with p40 in serum-starved medium for up to 24 h for MUC2 immunostaining and RNA isolation for real time PCR assay. Cellular lysates were collected at the end of the experiment for Western blot analysis to determine levels of EGFR expression.
Preparation of Cellular Lysates for Western Blot Analysis
LS174T cells were solubilized in cell lysis buffer containing 1% Triton X-100, 10 mm Tris (pH 7.4), 1 mm EDTA, 1 mm EGTA, 150 mm NaCl, and a proteinase inhibitor mixture (Sigma-Aldrich) and incubated for 1 h on ice. The scraped suspensions were centrifuged at 14,000 rpm for 15 min at 4 °C, and the protein concentration was determined using a BCA protein assay kit (Pierce Thermo Scientific). The lysates were mixed with Laemmli sample buffer, and proteins were separated by SDS-PAGE for Western blot analysis using anti-total EGFR, anti-phospho-EGFR 1068, anti-total Akt (Cell Signaling Technology), anti-phospho-Ser473 Akt (Cell Signaling Technology), and anti-β-actin (Sigma-Aldrich) antibodies.
Mice and Treatment
Mice were kept on a 12-h light and 12-h dark cycle, and all animal experiments were performed according to a protocol approved by the Institutional Animal Care and Use Committee at Vanderbilt University (Nashville, TN). 6–8-week-old C57BL/6 WT and Egfrwa5 (EGFR dominant negative) (26) on C57BL/6J background were gavaged with p40-containing pectin/zein beads (10 μg of p40/day/mouse) or control pectin/zein beads without p40 for 4 h or 5 days. The preparation of p40-containing pectin/zein beads was described in our previous publication (25). Mice without any treatment were also used as no-treatment controls. After euthanasia, the colon was collected and opened, and the feces was removed. Then colon tissues were washed with PBS before preparation of paraffin-embedded formalin-fixed and Carnoy-fixed sections and for epithelial cell isolation.
Mouse Colon Organ Culture
Colon explants obtained from 6–8-week-old WT C57BL/6 mice were used for the organ culture, as previously described (23). Briefly, the colon explants were cultured on NetwellTM inserts in DMEM containing 0.5% FBS at 37 °C with 5% CO2 for 1 h before treatment with p40, murine TNF (Pepro Tech, Inc.), benzyl 2-acetamido-2-deoxy-α-d-galactopyranoside (benzyl-α-GalNAc; EMD Millipore), or various combinations of these three treatments for 8 h. At the end of the experiment, colon tissue was fixed in 4% paraformaldehyde in PBS at 4 °C overnight before preparing paraffin-embedded tissue sections. Mucosal lysates were prepared in PBS for a periodic acid-Schiff (PAS) assay.
Isolation of Colonic and Small Intestinal Epithelial Cells from Mice
Mouse colonic and small intestinal epithelial cells were isolated using a modified protocol (27). The colon and jejulium were incubated with 0.5 mm dithiothreitol and 3 mm EDTA at room temperature for 1.5 h without shaking. After removing the solution, PBS was added to the tissues. Villi and crypts were released from the tissues by vigorous shaking and then washed with PBS.
To determine the percentage of epithelial cells in the isolated cell population, the cells were incubated with a biotin-conjugated anti-mouse E-cadherin antibody (E-cadherin is a cell surface marker for epithelial cells) and PE-Cy5-labeled streptavidin (BD Biosciences). The cells were then fixed in 0.1% paraformaldyhyde in PBS and analyzed using multicolor flow cytometry to determine the percentage of positive cells using a BD LSRII system (BD Biosciences).
p40 ELISA
LS174T cells and colon and small intestinal epithelial cells isolated from mice were solubilized in 50 mm Tris, pH 7.5, containing 150 mm NaCl, 1% Triton X-100, and protease and phosphatase inhibitors. Proteins were coated on a 96-well dish with a flat bottom by incubating 1:10 dilution of proteins in 0.1 m sodium carbonate, pH 9.5, at 4 °C overnight. The plate was washed with wash buffer (0.05% Tween 20 in PBS) and blocked by 10% FBS in PBS for 1 h at room temperature. Proteins were incubated with a rabbit polyclonal anti-p40 antibody (generated by our group (23)) at 1:500 dilution in PBS for 2 h at room temperature, followed by a HRP-conjugated goat anti-rabbit IgG antibody (1:2000 dilution in PBS) for 1 h at room temperature. The substrate solution, teramethylbenzidine (BD Biosciences) was used to develop the reaction through incubation for 30 min. This reaction was stopped by 2 n H2SO4. The OD at 450-nm wavelength was measured. Purified p40 was used to generate a concentration curve.
PAS Assay
Colon epithelial cells isolated from mice, mucosal lysates from colon organ culture, and LS174T cells were disrupted in PBS using Tissuelyser to obtain soluble proteins. Protein concentration was determined using a BCA protein assay kit. Mucous glycoprotein in soluble fractions was measured as previously reported (28). Briefly, cellular soluble fractions were incubated with 0.1% periodic acid (Sigma-Aldrich) for 2 h at room temperature. Then the Schiff reagent (Sigma-Aldrich) was added and incubated for 30 min at room temperature. The OD of the resulting solution at 550 nm wavelength was taken as a measure of the amount of PAS-positive product present and compared with a known standard of porcine stomach mucin (Sigma-Aldrich).
Real Time PCR Assay
Total RNA was isolated from LS174T cells or colonic epithelial cells using an RNA isolation kit (Qiagen) and was treated with RNase-free DNase. Reverse transcription was performed using a high capacity cDNA reverse transcription kit (Applied Biosystems, Foster City, CA). Real time PCRs were set up by addition of 1.25 μl of the MUC2 (Mm 00439306) primer mix (containing 5 μm of reverse and forward primers; Applied Biosystems), 5 μl of diluted cDNA template, and 12.5 μl of TaqMan Gene Expression Master Mix. Real time PCR was performed using the 7300 real time PCR system (Applied Biosystems). The data were analyzed using the Sequence Detection System V1.4.0 software. The relative abundance of GAPDH mRNA was used to normalize levels of the mRNAs of MUC2. All of the cDNA samples were analyzed in triplicate.
MUC2 Immunostaining
LS174T cells were fixed in 4% paraformaldehyde for 15 min at room temperature. Cells were permeabilized using 2% Triton X-100 for 5 min in PBS at room temperature and blocked in 5% goat serum in PBS containing 1% Triton X-100 for 1 h at room temperature. Colonic tissue sections were deparaffinized and treated with an unmasking antigen solution (Jackson ImmunoResearch). Colonic sections were blocked using 5% goat serum in PBS for 1 h at room temperature. Tissue sections and cultured cell slides were incubated with an rabbit anti-MUC2 antibody (Santa Cruz Biotechnology, Inc.) for 48 h at 4 °C, followed by a FITC-conjugated (Jackson ImmunoResearch) or HRP-labeled goat anti-rabbit IgG antibodies at room temperature for 1 h. Slides using the horseradish peroxidase secondary antibody were developed using a 3, 3′-diaminobenzidine substrate kit (Vector Laboratories), counterstained with hematoxylin, and observed using light microscopy. The number of MUC2-positive cells in the distal colon tissues was determined by counting the absolute number of positive stained cells in at least 300 colonic crypts for each mouse. Slides using the FITC-labeled secondary antibody were mounted using mounting medium with DAPI and observed using fluorescence microscopy. FITC and DAPI images were taken from the same field. The thickness of the colonic attached mucus was determined by measuring the distance between the epithelial surface and the mucus surface in 20 fields for each mouse.
PAS Staining
LS174T cells were fixed in 4% paraformaldehyde at 4 °C overnight and stained using a PAS kit (Sigma-Aldrich), according to the manufacturer's instructions. The PAS-stained slides were counterstained with hematoxylin.
Apoptosis Assay
Apoptosis was detected in colon culture sections using the ApopTagTM in situ oligo ligation (ISOL) kit and T4 DNA ligase according to the manufacturer's guidelines (23, 25). The number of apoptotic cells was determined by counting the absolute number of positive stained cells in at least 100 colonic crypts/colonic explant.
Statistical Analysis
Statistical significance for multiple comparisons in each study was determined by one-way analysis of variance followed by a Newman-Keuls analysis using Prism 5.0 (GraphPad Software, Inc.). A p value < 0.05 was defined as statistically significant. The data are presented as means ± S.E.
RESULTS
p40 Activates EGFR and Up-regulates Mucin Production in LS174T Cells
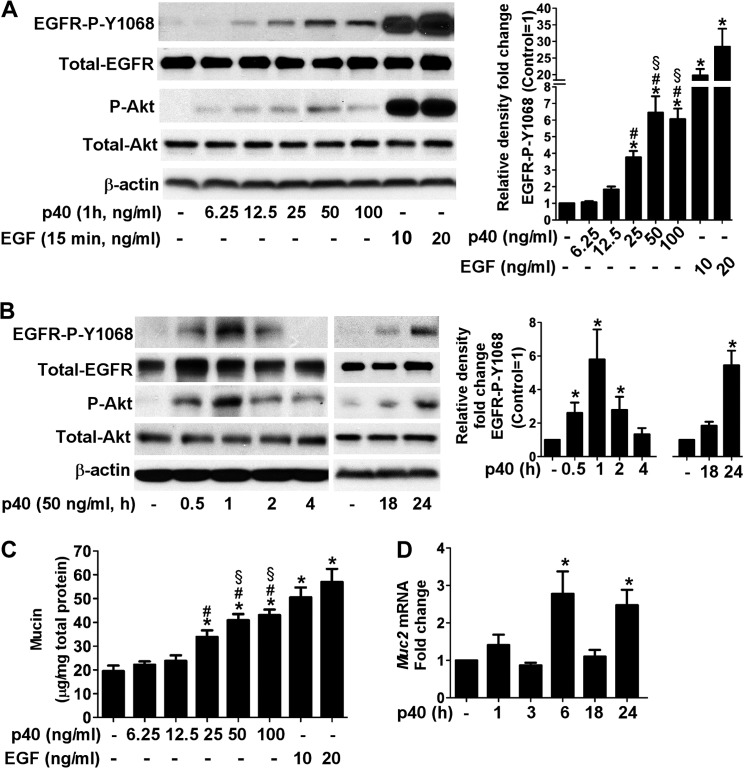
Our previous studies have demonstrated that p40 transactivates EGFR by stimulating HB-EGF release in intestinal epithelial cells (24), which is required for prevention and treatment of experimental colitis in mice (25). However, whether p40 transactivates EGFR in goblet cells and stimulates mucin production as a mucosal defense mechanism against injury and inflammatory assault in then intestinal tract is unknown. Thus, this work was focused on determining the role of p40 in activation of EGFR and mucin production in goblet cells. We treated LS174T cells, a human colon cancer cell line with characteristics of normal colonic mucosal cells with p40 at various concentrations and treatment times and analyzed the phosphorylation state of EGFR and Akt, a known target of EGFR by Western blot analysis. p40 at concentrations ranging from 6.25 to 50 ng/ml increased EGFR and Akt phosphorylation in LS174T cells in a concentration-dependent manner (Fig. 1A). No further increase was observed by p40 at 100 ng/ml, indicating that the effects of p40 on stimulation of EGFR activation in LS174T cells are saturated by the concentrations higher than 50 ng/ml (Fig. 1A). Furthermore, the time course of p40-stimulated EGFR and Akt phosphorylation was analyzed. p40 was able to stimulate EGFR and Akt phosphorylation at multiple time points. For example, two peaks of EGFR and Akt phosphorylation were observed: an early peak at 1 h and a later peak at 24 h after p40 at 50 ng/ml treatment (Fig. 1B). We chose to use p40 at a concentration of 50 ng/ml for the remainder of the cell culture studies, based on the dose-response analysis shown in Fig. 1A.
FIGURE 1.
p40 stimulates EGFR and Akt phosphorylation and up-regulates Muc2 gene expression and mucin production in LS174T cells. A, cells were treated with p40 for 1 h at indicated concentrations or EGF at 10 and 20 ng/ml for 15 min. B, cells were treated with p40 at 50 ng/ml for the indicated times. Cellular lysates were collected for Western blot analysis of total levels of EGFR and Akt and phosphorylation levels of EGFR (Tyr1068) and Akt (Ser473). The β-actin level was detected as a protein loading control. The relative densities of protein bands on Western blots were determined by comparing the densities of EGFR-Tyr1068 to total EGFR from the same sample. The relative density of bands from the untreated group was set as 1, and the relative densities of bands from the treatment groups were compared with those of the untreated group to obtain the fold changes. C, cells were treated with p40 at the indicated concentrations or EGF at 10 and 20 ng/ml for 24 h. Total cellular soluble proteins were prepared for a PAS assay. The data are presented as μg of mucin per mg of cellular protein. D, cells were treated with p40 at 50 ng/ml for the indicated times. RNA was isolated for real time PCR analysis of the Muc2 mRNA level. The Muc2 mRNA expression level in the control group was set as 1, and mRNA expression levels in treated groups were compared with the control group. The data are quantified from three to five separate experiments. *, p < 0.05 compared with the control group; #, p < 0.05 compared with the p40 at 12.5 ng/ml treated group; §, p < 0.05 compared with the p40 at 25 ng/ml treated group.
We next applied a PAS assay to examine the intracellular mucin level in LS174T cells. p40 treatment of 6.25 to 50 ng/ml for 24 h increased mucin production in LS174T cells in a concentration-dependent manner (Fig. 1C). p40 at 100 ng/ml did not further increase mucin production (Fig. 1C), which is consistent with the ability of p40 to activate EGFR, and Akt in LS174T cells is saturated by this concentration. LS174T cells were also treated with human EGF at 10 and 20 ng/ml for 15 min and 24 h as positive controls for EGFR and Akt activation (Fig. 1A) and stimulation of mucin production (Fig. 1C), respectively.
To test whether p40 up-regulates mucin gene expression, real time PCR analysis was performed to measure the Muc2 mRNA level in LS174T cells, which is a known mucin gene expressed in goblet cells in the colon (1, 5). p40 up-regulated Muc2 gene expression in LS174T cells (Fig. 1D). Interestingly, two peaks of increased Muc2 gene expression, an early peak at 6 h and a later peak at 24 h after p40 treatment, were found. This evidence is consistent with the two peaks of EGFR and Akt activation by p40 treatment (Fig. 1B). Thus, p40 up-regulates mucin gene expression and mucus production in LS174T cells, which is associated with p40 activation of EGFR.
Blocking EGFR Abolishes the Effects of p40 on Up-regulation of Mucin Production in LS174T Cells
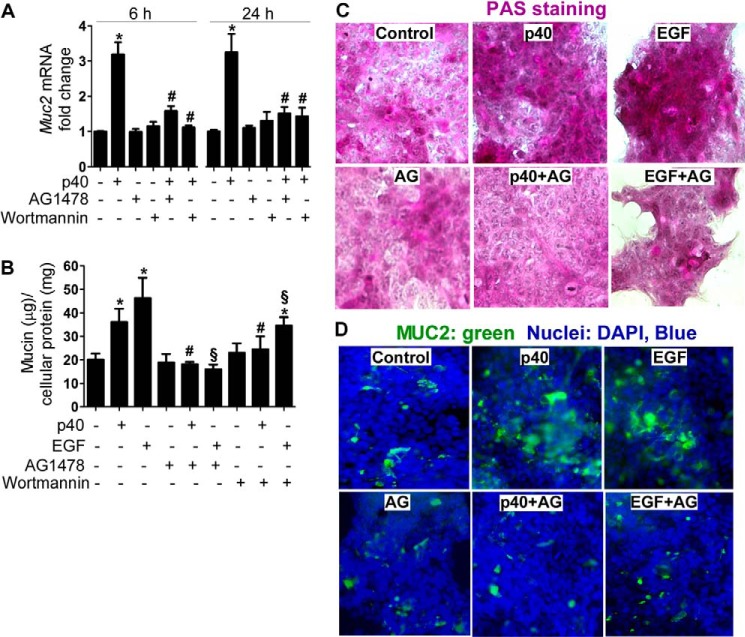
EGFR signaling has been linked to mucin production in airway cells (29). To determine the requirement of EGFR activation for the action of p40 in mucin production in LS174T cells, two approaches were used to block EGFR activity: LS174T cells were treated with a EGFR kinase inhibitor, AG1478, and knockdown EGFR expression in LS174T cells by siRNA transfection. In addition, wortmannin, a PI3K kinase inhibitor, was used to inhibit Akt activity. Inhibition of EGFR kinase activity using AG1478 or Akt activity using wortmannin significantly blocked p40-stimulated Muc2 gene expression at 6 and 24 h after treatment (Fig. 2A). These two inhibitors also blocked mucin production in LS174T cells (Fig. 2B). EGF-stimulated mucin production in LS174T cells was also suppressed by AG1478 and wortmannin (Fig. 2B). Furthermore, PAS staining and immunostaining using an anti-MUC2 antibody were performed. p40 treatment increased the mucous glycoprotein level detected by PAS staining (Fig. 2C) and the MUC2 level detected by immunostaining (Fig. 2D), both of which were blocked by AG1478 (Fig. 2, C and D).
FIGURE 2.
Inhibition of EGFR kinase activity blocks p40-stimulated Muc2 gene expression and mucin production in LS174T cells. The cells were treated with p40 at 50 ng/ml or EGF (20 ng/ml) for 6 and 24 h in the presence or absence of AG1478 (200 nm) or wortmannin (10 μm). A, RNA was isolated for real time PCR analysis of the Muc2 mRNA level as described in Fig. 1D. B, total cellular soluble proteins were prepared for a PAS assay as described in Fig. 1C. *, p < 0.05 compared with the control group; #, p < 0.05 compared with the p40-treated group; §, p < 0.05 compared with the EGF-treated group. The data are quantified from five separate experiments. C, cells were treated as shown above for 24 h and stained with PAS. D, cells were treated as shown above for 24 h for immunostaining using an anti-MUC2 antibody and a FITC-conjugated secondary antibody and observed under fluorescence microscopy. Green staining represents MUC2-positive staining. The nuclei were stained by DAPI. In C and D, data are representative of three separate experiments.
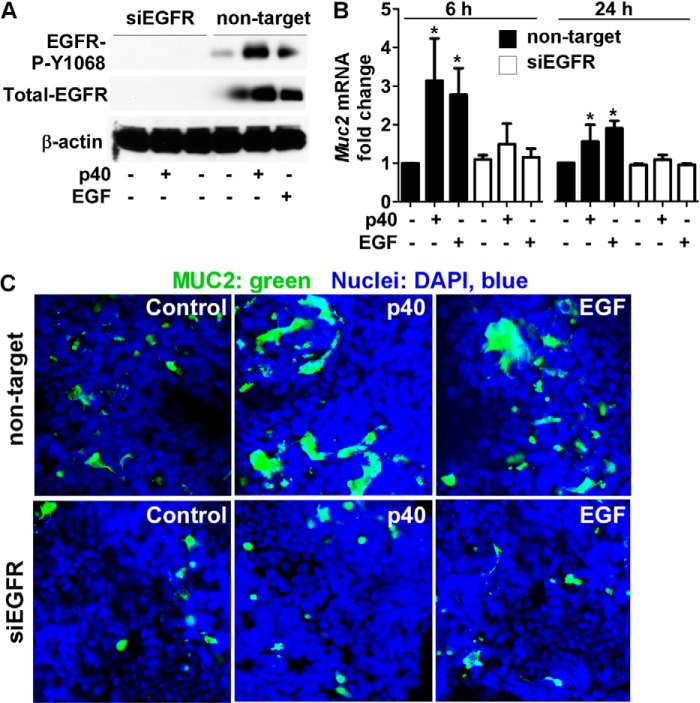
We next transfected LS174T cells with EGFR siRNA to suppress EGFR expression. EGFR expression, detected as a protein band with molecular mass of 180 kDa by Western blot, was suppressed in cells transduced with EGFR siRNA, but not in cells treated with nontargeting siRNA (Fig. 3A). As expected, p40 activated EGFR in nontargeting siRNA-transfected cells (Fig. 3A). p40 failed to stimulate Muc2 gene expression after 6- and 24-h treatment in cells with knockdown of EGFR expression (Fig. 3B). EGF treatment was used as a control for EGFR inhibition. EGF-stimulated Muc2 gen expression was blocked in cells transfected with EGFR siRNA (Fig. 3B). Next, the effects of p40 and EGF on mucin production were examined. p40 and EGF stimulated mucin production in LS174T cells transduced with nontargeting siRNA, but not EGFR siRNA (Fig. 3C). These data indicate a requirement of EGFR for the stimulatory effects of p40 on mucin production.
FIGURE 3.
p40 up-regulation of mucin production in LS174T cells requires EGFR. A, cells transduced with siRNA against EGFR or nontargeting siRNA were treated with p40 (50 ng/ml) for 1 h or EGF (20 ng/ml) for 15 min. Cellular lysates were collected for Western blot analysis of total and phosphorylation levels of EGFR. The β-actin level was detected as a protein loading control. B, cells were treated with p40 (50 ng/ml) or EGF (20 ng/ml) for the indicated times. RNA was isolated for real time PCR analysis of the Muc2 mRNA level. The Muc2 mRNA expression level in the control group with nontargeting siRNA transfection was set as 1, and mRNA expression levels in treated groups were compared with this group. *, p < 0.05 compared with the control group with nontargeting siRNA transfection. C, cells treated with p40 (50 ng/ml) for 24 h were immunostained using an anti-MUC2 antibody and a FITC-conjugated secondary antibody. The nuclei were stained by DAPI. The images were observed under fluorescence microscopy. The images shown are representative of three separate experiments.
p40 Promotes Mucin Production in Mice, Which Requires EGFR Kinase Activity
Data from in vitro experiments indicate that p40 stimulates mucin production, which requires activation of EGFR. Next, we examined whether p40 exerts the same function in vivo. We generated pectin/zein beads, a hydrogel formulation that protects associated drugs from abundant intestinal enzymes (30, 31), to specifically deliver p40 to the colon. By using this delivery system, p40 was recovered in the colon, with a limited amount in the small intestine in mice by immunostaining 4 h after the administration of 10 μg of p40 in pectin/zein beads. This treatment has also been shown to activate EGFR in colon epithelial cells (25). In this experiment, WT mice were treated with p40 in pectin/zein beads as before (10 μg of p40 for 4 h). Colonic and jejunum epithelial cells were isolated. To determine the percentage of epithelial cells in isolated cells, phenotypic characterization of expression of E-cadherin (an epithelial cell marker) in these isolated cells was performed using flow cytometry analysis. The percentage of E-cadherin-positive staining cells was 91.33 ± 4.045% (Fig. 4A), suggesting that the majority of isolated cell population is epithelial cells.
FIGURE 4.
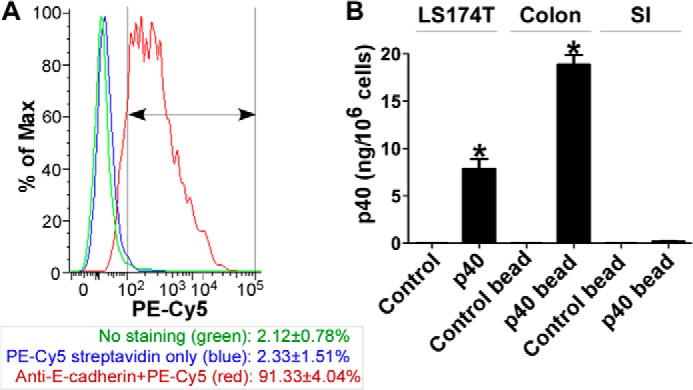
Detecting the p40 content in intestinal epithelial cells isolated from mice and in LS174T cells. WT mice were gavaged with 10 μg of p40 in two pectin/zein beads (each bead contains 5 μg of p40) or two control pectin/zein beads without p40 for 4 h. Colonic and small intestinal tissues were prepared for isolation of epithelial cells. A, phenotypic characterization of the isolated cell population from the colon was performed by staining cells using biotin-labeled anti-mouse E-cadherin and streptavidin-PE-Cy5 and analyzed by flow cytometry. The gate is shown as the line with double arrowheads. The percentage of E-cadherin-positive staining cells is shown (n = 5 mice/group). B, LS174T cells treated with p40 at 50 ng/ml for 1 h and small intestinal (SI), and colonic epithelial cells isolated from mice were solubilized for an ELISA assay to detect the intracellular p40 level. Purified p40 protein was used to generate a concentration curve. The data are presented as ng of p40/106 cells. *, p < 0.05 compared with the control group (for LS174T cells) or control bead-treated mice (n = 5 mice/group). The p40 level in LS174T cells is representative of three separate experiments.
The amount of p40 delivered to epithelial cells was then detected using an ELISA method. The levels of p40 in colon and jejunum epithelial cells isolated from p40-treated mice were 18.87 ± 1.48 and 0.19 ± 0.04 ng/106 cells, respectively. No p40 was detected in the colon or jejunum epithelial cells isolated from mice gavaged with control lectin/zein beads (Fig. 4B). The p40 level in LST174T cells treated with 50 ng/ml p40 for 1 h was 7.89 ± 1.46 ng/106, but no p40 was detected in control LS174T cells (Fig. 4B).
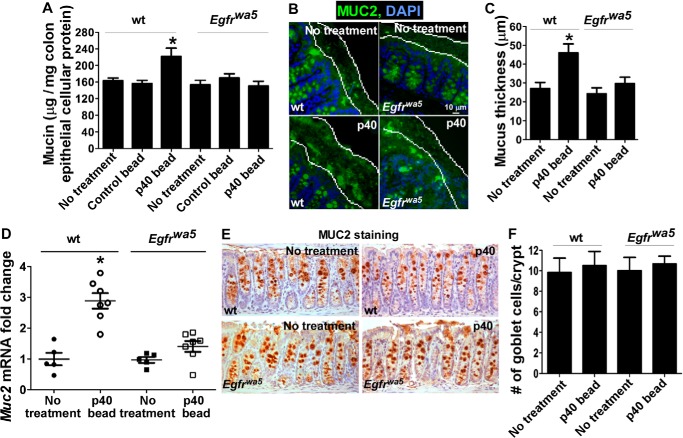
To test the effects of p40 on mucin production in vivo, WT mice were gavaged with p40-containing pectin/zein beads (10 μg p40/day) or control beads for 5 days. We first examined the mucin level in colon epithelial cells and found that the mucin level was significantly increased in colon epithelial cells isolated from p40-containing bead treated mice, compared with that from mice with no treatment or treated with control beads (Fig. 5A). Mucin production in the colon was further examined by measuring the thickness of the attached inner mucus layer by immunostaining using an anti-MUC2 antibody. It was determined that p40 treatment significantly increased the thickness of the inner mucus layer in the WT colon (Fig. 5, B and C).
FIGURE 5.
p40 promotes mucin production in the colonic epithelium of WT, but not Egfrwa5 mice. WT and Egfrwa5 mice were gavaged with 10 μg of p40 in two pectin/zein beads or two control pectin/zein beads without p40 for 5 days. A, colonic epithelial cells were isolated, and cellular soluble proteins were prepared for a PAS assay. The data are presented as μg of mucin per mg of cellular protein. *, p < 0.05 compared with the no-treatment group and control bead-treated group. B and C, Carnoy-fixed colon sections were immunostained using an anti-MUC2 antibody and a FITC-conjugated secondary antibody. The slides were mounted using mounting medium containing DAPI to stain nuclei and observed using fluorescence microscopy. FITC and DAPI images were taken from the same field. The thickness of the attached mucus layer in the proximal colon was determined by measuring the distance between the epithelial surface and the mucus surface in at least 20 fields for each mouse. D, RNA was prepared from colonic epithelial cells for real time PCR analysis of the Muc2 mRNA level. The Muc2 mRNA expression level in the no-treatment group of WT mice was set as 1, and mRNA expression levels in other groups were compared with that in this group. E and F, formalin-fixed colon sections were immunostained using an anti-MUC2 antibody and a HRP-conjugated secondary antibody. The slides were developed using 3, 3′-diaminobenzidine counterstained with hematoxylin, and observed using light microscopy. The number of MUC2-positive cells in the distal colonic tissues was determined by counting the absolute number of positive stained cells in at least 300 crypts for each mouse. *, p < 0.05 compared with the no-treatment group of WT mice (n = 5/group).
To determine whether EGFR mediates p40 regulation of mucin production in vivo, Egfrwa5 mice with a dominant negative mutation in the EGFR kinase domain (26) were utilized. The mucus content in colon epithelial cells (Fig. 5A) and the thickness of the mucus layer in the colon (Fig. 5, B and C), of Egfrwa5 mice were similar to those in WT mice. Our previous studies have shown that p40 activated EGFR in colonic epithelial cells of WT, but not Egfrwa5 mice (25). In the present study, p40 treatment failed to stimulate mucin production in colon epithelial cells (Fig. 5A) and increased the thickness of the inner mucus layer in the colon of Egfrwa5 mice (Fig. 5, B and C), compared with those from untreated Egfrwa5 mice.
Next, we examined whether p40 regulates Muc2 gene expression in vivo. There is no difference in the basal level of Muc2 gene expression in the colonic epithelial cells in WT mice, compared with that in Egfrwa5 mice. p40 stimulated Muc2 gene expression in the colonic epithelial cells of WT, but not Egfrwa5 mice (Fig. 5D).
Because the number of mucin-producing cells in the colon was not affected by p40 treatment in either WT or Egfrwa5 mice (Fig. 5, E and F), these data suggest that p40 transactivation of EGFR may play a role in promoting mucin production through stimulation of mucin synthesis in existing cells, but not in increasing the number of mucin producing cells in vivo.
p40-stimulated Mucin Production Mediates Inhibition of TNF-induced Epithelial Cell Apoptosis
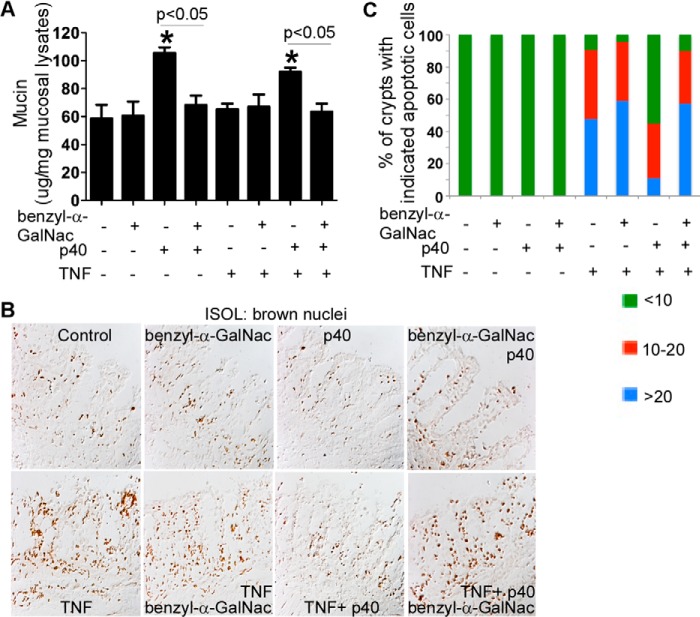
We have reported that p40 inhibits TNF-induced apoptosis in intestinal epithelial cells in vitro and in the colon organ culture in an EGFR-dependent manner (25). This study further investigated the role of p40-stimulated mucin production in protection of cytokine-induced apoptosis in the colonic explant culture. The colonic explants were cultured for 8 h with the treatment of benzyl-α-GalNAc. Benzyl-α-GalNAc, a specific inhibitor of the glycosylation of O-glycosidically linked glycoproteins, has been shown to inhibit mucin production in LS174T cells (32) and in HT-29 cells, another colon tumor cell line (33). p40 stimulated mucin production in the presence and absence of TNF, which was inhibited by benzyl-α-GalNAc (Fig. 6A). Consistent with our previous data (25), p40 inhibited TNF-induced epithelial apoptosis, which was suppressed by benzyl-α-GalNAc (Fig. 6, B and C). These results indicate that p40-up-regulation of mucin production mediates its protective effects on the intestinal epithelium against injury and inflammation.
FIGURE 6.
p40-stimulated mucin production mediates inhibition of TNF-induced epithelial cell apoptosis in colon organ culture. Colon explants derived from 6–8-week-old WT mice were cultured in DMEM containing 0.5% FBS and treated with p40 (50 ng/ml) in the presence or absence of TNF (100 ng/ml), benzyl-α-GalNAc (2 mm), or both for 8 h. A, total mucosal lysates were collected for a PAS assay. The data are presented as μg of mucin per mg protein in mucosal lysates. *, p < 0.05 compared with the control group. B and C, paraffin-embedded tissue sections were prepared for detecting apoptosis using ISOL staining. Apoptotic nuclei labeled with peroxidase and developed by 3, 3′-diaminobenzidine (brown nuclei) were visualized using differential interference contrast microscopy (B). The percentage of crypts with indicated apoptotic cells is shown (C) (n = 5 mice/group).
DISCUSSION
One of the mechanisms by which probiotics protect the host from pathogenic bacterial invasion is through stimulating mucin gene expression, synthesis, and secretion (34). LGG has been reported to induce mucin gene expression and production in cell cultures, including LS174T, Caco2, and HT-29 cells (12, 13, 35). However, how LGG regulates mucin production remains unclear. To elucidate the mechanisms of probiotic action, we purified and cloned p40 from LGG culture supernatants. p40 has beneficial effects on colon epithelial homeostasis through activation of EGFR and Akt in intestinal epithelial cells (23–25). In the present study, we demonstrate that p40 promotes Muc2 gene expression and MUC2 production in LS174T cells, which requires EGFR activation. We also show that p40 treatment increases Muc2 gene expression, the amount of mucin in the colon epithelial cells, and the thickness of the mucus layer in the colon of mice in an EGFR-dependent manner. These results suggest that production of soluble proteins may serve as a mechanism underlying LGG up-regulation of mucin production.
EGF was used to treat LS174T cells in this study as a positive control to study the involvement of EGFR activation in mucin production. We have reported that EGFR transactivation by p40 occurs through the release of an EGFR ligand, HB-EGF. Thus, p40 stimulates a delayed (30 min) and longer period of EGFR activation (up to 2 h), compared with HB-EGF (15–30 min) (24). This study further showed that p40 stimulated several peaks of EGFR activation, which are correlated with increased Muc2 gene expression at these time points in LS174T cells. This evidence may result from the accumulation of HB-EGF released by p40 in the medium at these time points for activation of EGFR.
The levels of EGFR phosphorylation stimulated by EGF at 10 and 20 ng/ml for 15 min are about 3- and 4-fold, respectively, compared with those induced by p40 at 50 ng/ml for 1 h in LS174T cells. However, EGF treatment (10 and 20 ng/ml for 24 h) stimulated mucin production in LS174 cells at a level less than 2-fold compared with that by p40 at 50 ng/ml for 24 h. These results may be explained by EGFR kinetics. Following ligand binding to EGFR, the Cbl ubiquitin ligase, a family of E3 ubiquitin ligases, is tyrosine-phosphorylated by the EGFR receptor (36) or indirectly through interaction with the adaptor protein growth factor receptor-bound protein 2 (GRB2). Phosphorylated Cbl promotes ubiquitinylation of EGFR at the plasma membrane (37), which leads to translocation of EGFR to the endosomal compartment (38). EGFR is subsequently sorted by incompletely understood mechanisms through distinct vesicles for either destruction or recycling to the plasma membrane with the loss of its activity (39, 40). Therefore, EGFR phosphorylation and activation of its downstream targets by direct ligand binding is a transient event. p40-stimulated EGFR phosphorylation is sustained longer than that by EGF and can stimulate more than one peak of EGFR activation. Thus, the cumulative effects of EGFR activation by p40 on stimulating its downstream targets and cellular responses cannot be reproduced by the level of EGFR phosphorylation at a specific time point.
Mucin production is regulated by a variety of stimuli, including growth factors, inflammatory cytokines, microbes, microbial products, toxins, and hormones (2). Studies have shown that EGFR ligands, EGF, and TGF-α up-regulate Muc2 and Muc5AC through Ras/MAPK/ERK in a lung cancer cell line (41). In addition to the Ras/MAPK/ERK pathway, PI3K has been shown to be involved in interleukin-1β-stimulated expression of the MUC2 gene in NCI-H292 human airway cells (42). Our results show that p40 stimulates Muc2 gene expression that is inhibited by down-regulation of EGFR expression and kinase activity or by inhibition of Akt activation. We have reported previously that p40 does not have effects on ERK1/2 activation (23). Although inhibition of ERK1/2 activation down-regulates the basal levels of mucin production in LS174T cells, p40 still stimulates higher level of mucin production, compared with that in untreated cells (data not shown). Thus, these results suggest that the EGFR/Akt pathway mediates the effects of p40 on the stimulation of mucin production.
Increased synthesis of mucin is frequently coupled with mucin secretion, but chronic secretion may result in the depletion of mucin in goblet cells (43). Our study suggests that p40 treatment does not deplete mucin from goblet cells because p40 increases the intracellular mucus level in LS174T cells and in colon epithelial cells in mice, and the number of goblet cells in mice identified by MUC2 immunostaining is not affected by p40 treatment. In addition to increasing mucin synthesis (16), EGF has been shown to regulate mucin secretion (44), indicating that p40 plays a possible role in mucin secretion.
p40 promotes mucin production but does not affect the number of goblet cells in the colon in mice, suggesting that p40 may increase mucin synthesis in the existing goblet cells but does not have significant effects on goblet cell differentiation and proliferation under the conditions presented in this study. Notch signaling is required for differentiation and maturation of intestinal stem cells into goblet cells (45). The SAM pointed domain Ets factor (SPDEF) transcription factor has been shown to be downstream of Math1 to promote goblet cell differentiation and hyperplasia and is a major regulator of secretory gene products such as MUC2 (46, 47). We did not find any significant effects of p40 on SPDEF gene expression in LS174T cells and in colonic epithelial cells isolated from mice (data not shown). However, we cannot rule out the role of p40 in the regulation of notch signaling for promoting goblet cell differentiation during development and under disease status.
Clinical studies have revealed evidence regarding the defect of mucin production in disease development. An association with an alteration of goblet cell response and mucin production has been found in intestinal infections caused by bacteria, viruses, and parasites (48). Quantitative and qualitative alterations in mucins are observed in intestinal inflammation in humans (49). Loss of intestinal core 1-derived O-glycans causes decreased mucin production and spontaneous colitis in mice (50). This study further shows that inhibition of p40-stimulted mucin production by blocking O-linked glycosylation abolishes the protective effects of p40 on TNF-induced apoptosis. Thus, regulation of mucin production by probiotics may play a role in maintaining intestinal mucosal homeostasis.
In addition to the protective roles in the epithelium, mucus provides binding sites and an energy source for intestinal commensal microbiota. Both commensal and pathogenic bacteria benefit from their ability to regulate mucin synthesis and/or secretion in host goblet cells (5, 51). Our finding that p40 stimulates MUC2 production suggests a beneficial effect of p40 on the intestinal microbial microenvironment. Further investigations are clearly needed to verify this possibility.
In summary, results from our previous studies show that p40 fulfills protective roles in mouse models of experimental colitis through transactivation of the EGFR (25). We have further demonstrated that p40 transactivation of EGFR mediates up-regulation of mucin production in mice and in human mucus-producing colon cancer cells. Thus, this finding provides insights into the mechanisms of the action of p40 in regulation of intestinal epithelial homeostasis and prevention of intestinal inflammatory diseases.
Acknowledgments
We thank Dr. Dawn A. Israel and Dr. Richard M. Peek, Jr., from Vanderbilt University Medical Center for technical support and assistance in preparation of this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grants R01 DK081134 (to F. Y.), and P01 DK033506 and R01 HD059126 (to W. A. W.), and P30 DK058404 (to Vanderbilt University Medical Center's Digestive Disease Research Center). This work was also supported by Research Fund for the Doctoral Program of Higher Education of China Grant 20121202110018 (to B. M. W.), and National Natural Science Foundation of China Grant 81300272 (to H. L. C.) and Tianjin Research Program of Application Foundation and Advanced Technology of China Grant 13JCQNJC10600 (to H. L. C.).
- LGG
- L. rhamnosus GG
- benzyl-α-GalNAc
- benzyl 2-acetamido-2-deoxy-α-d-galactopyranoside
- EGFR
- EGF receptor
- HB
- heparin-binding
- PAS
- periodic acid-Schiff.
REFERENCES
- 1. Johansson M. E., Sjövall H., Hansson G. C. (2013) The gastrointestinal mucus system in health and disease. Nat. Rev. Gastroenterol. Hepatol. 10, 352–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Kim Y. S., Ho S. B. (2010) Intestinal goblet cells and mucins in health and disease: recent insights and progress. Curr. Gastroenterol. Rep. 12, 319–330 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Rubin B. K. (2002) Physiology of airway mucus clearance. Respir. Care 47, 761–768 [PubMed] [Google Scholar]
- 4. Dartt D. A., Rios J. D., Kanno H., Rawe I. M., Zieske J. D., Ralda N., Hodges R. R., Zoukhri D. (2000) Regulation of conjunctival goblet cell secretion by Ca2+ and protein kinase C. Exp. Eye Res. 71, 619–628 [DOI] [PubMed] [Google Scholar]
- 5. Dharmani P., Srivastava V., Kissoon-Singh V., Chadee K. (2009) Role of intestinal mucins in innate host defense mechanisms against pathogens. J. Innate Immun. 1, 123–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Button B., Cai L. H., Ehre C., Kesimer M., Hill D. B., Sheehan J. K., Boucher R. C., Rubinstein M. (2012) A periciliary brush promotes the lung health by separating the mucus layer from airway epithelia. Science 337, 937–941 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ermund A., Schütte A., Johansson M. E., Gustafsson J. K., Hansson G. C. (2013) Studies of mucus in mouse stomach, small intestine, and colon: I. gastrointestinal mucus layers have different properties depending on location as well as over the Peyer's patches. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G341–G347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Rodríguez-Piñeiro A. M., Bergström J. H., Ermund A., Gustafsson J. K., Schütte A., Johansson M. E., Hansson G. C. (2013) Studies of mucus in mouse stomach, small intestine, and colon: II. gastrointestinal mucus proteome reveals Muc2 and Muc5ac accompanied by a set of core proteins. Am. J. Physiol. Gastrointest. Liver Physiol. 305, G348–G356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Johansson M. E., Gustafsson J. K., Holmén-Larsson J., Jabbar K. S., Xia L., Xu H., Ghishan F. K., Carvalho F. A., Gewirtz A. T., Sjövall H., Hansson G. C. (2014) Bacteria penetrate the normally impenetrable inner colon mucus layer in both murine colitis models and patients with ulcerative colitis. Gut 63, 281–291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. McElroy S. J., Prince L. S., Weitkamp J. H., Reese J., Slaughter J. C., Polk D. B. (2011) Tumor necrosis factor receptor 1-dependent depletion of mucus in immature small intestine: a potential role in neonatal necrotizing enterocolitis. Am. J. Physiol. Gastrointest. Liver Physiol. 301, G656–G666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Van der Sluis M., De Koning B. A., De Bruijn A. C., Velcich A., Meijerink J. P., Van Goudoever J. B., Büller H. A., Dekker J., Van Seuningen I., Renes I. B., Einerhand A. W. (2006) Muc2-deficient mice spontaneously develop colitis, indicating that MUC2 is critical for colonic protection. Gastroenterology 131, 117–129 [DOI] [PubMed] [Google Scholar]
- 12. Mattar A. F., Teitelbaum D. H., Drongowski R. A., Yongyi F., Harmon C. M., Coran A. G. (2002) Probiotics up-regulate MUC-2 mucin gene expression in a Caco-2 cell-culture model. Pediatr. Surg. Int. 18, 586–590 [DOI] [PubMed] [Google Scholar]
- 13. Mack D. R., Michail S., Wei S., McDougall L., Hollingsworth M. A. (1999) Probiotics inhibit enteropathogenic E. coli adherence in vitro by inducing intestinal mucin gene expression. Am. J. Physiol. 276, G941–G950 [DOI] [PubMed] [Google Scholar]
- 14. Yarden Y. (2001) The EGFR family and its ligands in human cancer: signalling mechanisms and therapeutic opportunities. Eur. J. Cancer 37, (Suppl. 4) S3–S8 [DOI] [PubMed] [Google Scholar]
- 15. Yarden Y., Sliwkowski M. X. (2001) Untangling the ErbB signalling network. Nat. Rev. Mol. Cell Biol. 2, 127–137 [DOI] [PubMed] [Google Scholar]
- 16. Nadel J. A. (2001) Role of epidermal growth factor receptor activation in regulating mucin synthesis. Respir. Res. 2, 85–89 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Gorbach S. L. (1996) The discovery of Lactobacillus GG. Nutr. Today 31, 2S–4S [Google Scholar]
- 18. Zocco M. A., dal Verme L. Z., Cremonini F., Piscaglia A. C., Nista E. C., Candelli M., Novi M., Rigante D., Cazzato I. A., Ojetti V., Armuzzi A., Gasbarrini G., Gasbarrini A. (2006) Efficacy of Lactobacillus GG in maintaining remission of ulcerative colitis. Aliment. Pharmacol. Ther. 23, 1567–1574 [DOI] [PubMed] [Google Scholar]
- 19. Basu S., Paul D. K., Ganguly S., Chatterjee M., Chandra P. K. (2009) Efficacy of high-dose Lactobacillus rhamnosus GG in controlling acute watery diarrhea in Indian children: a randomized controlled trial. J. Clin. Gastroenterol. 43, 208–213 [DOI] [PubMed] [Google Scholar]
- 20. Szajewska H., Wanke M., Patro B. (2011) Meta-analysis: the effects of Lactobacillus rhamnosus GG supplementation for the prevention of healthcare-associated diarrhoea in children. Aliment. Pharmacol. Ther. 34, 1079–1087 [DOI] [PubMed] [Google Scholar]
- 21. Doron S., Snydman D. R., Gorbach S. L. (2005) Lactobacillus GG: bacteriology and clinical applications. Gastroenterol. Clin. North Am. 34, 483–498 [DOI] [PubMed] [Google Scholar]
- 22. Yan F., Cao H., Cover T. L., Whitehead R., Washington M. K., Polk D. B. (2007) Soluble proteins produced by probiotic bacteria regulate intestinal epithelial cell survival and growth. Gastroenterology 132, 562–575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Seth A., Yan F., Polk D. B., Rao R. K. (2008) Probiotics ameliorate the hydrogen peroxide-induced epithelial barrier disruption by a PKC- and MAP kinase-dependent mechanism. Am. J. Physiol. Gastrointest. Liver Physiol. 294, G1060–G1069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Yan F., Liu L., Dempsey P. J., Tsai Y. H., Raines E. W., Wilson C. L., Cao H., Cao Z., Liu L., Polk D. B. (2013) A Lactobacillus rhamnosus GG-derived soluble protein, p40, stimulates ligand release from intestinal epithelial cells to transactivate epidermal growth factor receptor. J. Biol. Chem. 288, 30742–30751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Yan F., Cao H., Cover T. L., Washington M. K., Shi Y., Liu L., Chaturvedi R., Peek R. M., Jr., Wilson K. T., Polk D. B. (2011) Colon-specific delivery of a probiotic-derived soluble protein ameliorates intestinal inflammation in mice through an EGFR-dependent mechanism. J. Clin. Invest. 121, 2242–2253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lee D., Cross S. H., Strunk K. E., Morgan J. E., Bailey C. L., Jackson I. J., Threadgill D. W. (2004) Wa5 is a novel ENU-induced antimorphic allele of the epidermal growth factor receptor. Mamm. Genome 15, 525–536 [DOI] [PubMed] [Google Scholar]
- 27. Whitehead R. H., Demmler K., Rockman S. P., Watson N. K. (1999) Clonogenic growth of epithelial cells from normal colonic mucosa from both mice and humans. Gastroenterology 117, 858–865 [DOI] [PubMed] [Google Scholar]
- 28. Garcia M. A., Yang N., Quinton P. M. (2009) Normal mouse intestinal mucus release requires cystic fibrosis transmembrane regulator-dependent bicarbonate secretion. J. Clin. Invest. 119, 2613–2622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Takeyama K., Dabbagh K., Lee H. M., Agustí C., Lausier J. A., Ueki I. F., Grattan K. M., Nadel J. A. (1999) Epidermal growth factor system regulates mucin production in airways. Proc. Natl. Acad. Sci. U.S.A. 96, 3081–3086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liu L., Fishman M. L., Hicks K. B., Kende M., Ruthel G. (2006) Pectin/zein beads for potential colon-specific drug delivery: synthesis and in vitro evaluation. Drug Deliv. 13, 417–423 [DOI] [PubMed] [Google Scholar]
- 31. Liu L., Fishman M. L., Kost J., Hicks K. B. (2003) Pectin-based systems for colon-specific drug delivery via oral route. Biomaterials 24, 3333–3343 [DOI] [PubMed] [Google Scholar]
- 32. Kuan S. F., Byrd J. C., Basbaum C., Kim Y. S. (1989) Inhibition of mucin glycosylation by aryl-N-acetyl-α-galactosaminides in human colon cancer cells. J. Biol. Chem. 264, 19271–19277 [PubMed] [Google Scholar]
- 33. Truant S., Bruyneel E., Gouyer V., De Wever O., Pruvot F. R., Mareel M., Huet G. (2003) Requirement of both mucins and proteoglycans in cell-cell dissociation and invasiveness of colon carcinoma HT-29 cells. Int. J. Cancer 104, 683–694 [DOI] [PubMed] [Google Scholar]
- 34. Vanderpool C., Yan F., Polk D. B. (2008) Mechanisms of probiotic action: implications for therapeutic applications in inflammatory bowel diseases. Inflamm. Bowel Dis. 14, 1585–1596 [DOI] [PubMed] [Google Scholar]
- 35. Mack D. R., Ahrne S., Hyde L., Wei S., Hollingsworth M. A. (2003) Extracellular MUC3 mucin secretion follows adherence of Lactobacillus strains to intestinal epithelial cells in vitro. Gut 52, 827–833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Oksvold M. P., Thien C. B., Widerberg J., Chantry A., Huitfeldt H. S., Langdon W. Y. (2003) Serine mutations that abrogate ligand-induced ubiquitination and internalization of the EGF receptor do not affect c-Cbl association with the receptor. Oncogene 22, 8509–8518 [DOI] [PubMed] [Google Scholar]
- 37. de Melker A. A., van der Horst G., Borst J. (2004) c-Cbl directs EGF receptors into an endocytic pathway that involves the ubiquitin-interacting motif of Eps15. J. Cell Sci. 117, 5001–5012 [DOI] [PubMed] [Google Scholar]
- 38. Dikic I., Giordano S. (2003) Negative receptor signalling. Curr. Opin. Cell Biol. 15, 128–135 [DOI] [PubMed] [Google Scholar]
- 39. Bucci M., Roviezzo F., Cicala C., Sessa W. C., Cirino G. (2000) Geldanamycin, an inhibitor of heat shock protein 90 (Hsp90) mediated signal transduction has anti-inflammatory effects and interacts with glucocorticoid receptor in vivo. Br J. Pharmacol. 131, 13–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. de Renzis S., Sönnichsen B., Zerial M. (2002) Divalent Rab effectors regulate the sub-compartmental organization and sorting of early endosomes. Nat. Cell Biol. 4, 124–133 [DOI] [PubMed] [Google Scholar]
- 41. Perrais M., Pigny P., Copin M. C., Aubert J. P., Van Seuningen I. (2002) Induction of MUC2 and MUC5AC mucins by factors of the epidermal growth factor (EGF) family is mediated by EGF receptor/Ras/Raf/extracellular signal-regulated kinase cascade and Sp1. J. Biol. Chem. 277, 32258–32267 [DOI] [PubMed] [Google Scholar]
- 42. Kim Y. D., Jeon J. Y., Woo H. J., Lee J. C., Chung J. H., Song S. Y., Yoon S. K., Baek S. H. (2002) Interleukin-1β induces MUC2 gene expression and mucin secretion via activation of PKC-MEK/ERK, and PI3K in human airway epithelial cells. J. Korean Med. Sci. 17, 765–771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Davis C. W., Dickey B. F. (2008) Regulated airway goblet cell mucin secretion. Annu. Rev. Physiol. 70, 487–512 [DOI] [PubMed] [Google Scholar]
- 44. Hodges R. R., Bair J. A., Carozza R. B., Li D., Shatos M. A., Dartt D. A. (2012) Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Exp. Eye Res. 103, 99–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Okamoto R., Tsuchiya K., Nemoto Y., Akiyama J., Nakamura T., Kanai T., Watanabe M. (2009) Requirement of Notch activation during regeneration of the intestinal epithelia. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G23–G35 [DOI] [PubMed] [Google Scholar]
- 46. Engelman J. A., Zejnullahu K., Mitsudomi T., Song Y., Hyland C., Park J. O., Lindeman N., Gale C. M., Zhao X., Christensen J., Kosaka T., Holmes A. J., Rogers A. M., Cappuzzo F., Mok T., Lee C., Johnson B. E., Cantley L. C., Jänne P. A. (2007) MET amplification leads to gefitinib resistance in lung cancer by activating ERBB3 signaling. Science 316, 1039–1043 [DOI] [PubMed] [Google Scholar]
- 47. Noah T. K., Kazanjian A., Whitsett J., Shroyer N. F. (2010) SAM pointed domain ETS factor (SPDEF) regulates terminal differentiation and maturation of intestinal goblet cells. Exp. Cell Res. 316, 452–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Hansson G. C. (2012) Role of mucus layers in gut infection and inflammation. Curr. Opin. Microbiol. 15, 57–62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Swidsinski A., Loening-Baucke V., Theissig F., Engelhardt H., Bengmark S., Koch S., Lochs H., Dörffel Y. (2007) Comparative study of the intestinal mucus barrier in normal and inflamed colon. Gut 56, 343–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Fu J., Wei B., Wen T., Johansson M. E., Liu X., Bradford E., Thomsson K. A., McGee S., Mansour L., Tong M., McDaniel J. M., Sferra T. J., Turner J. R., Chen H., Hansson G. C., Braun J., Xia L. (2011) Loss of intestinal core 1-derived O-glycans causes spontaneous colitis in mice. J. Clin. Invest. 121, 1657–1666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Martens E. C., Roth R., Heuser J. E., Gordon J. I. (2009) Coordinate regulation of glycan degradation and polysaccharide capsule biosynthesis by a prominent human gut symbiont. J. Biol. Chem. 284, 18445–18457 [DOI] [PMC free article] [PubMed] [Google Scholar]