Abstract
Background: Known factors related to distant metastases in follicular thyroid carcinoma (FTC) included age, primary tumor size, and invasiveness. Distant metastasis is a main cause of death in FTC patients. Several studies showed that the presence of RAS mutations is also associated with poor clinical outcomes. We analyzed RAS mutations in FTC with distant metastases, FTC without a distant metastasis, follicular adenoma (FA), and nodular hyperplasia (NH). Furthermore, we elucidated the relationship between RAS mutations and clinical outcomes in FTC patients.
Methods: We selected patients who underwent a thyroidectomy for FTC with distant metastases (n=28), size matched FTC specimens without a distant metastasis (n=28), FA (n=17), and NH (n=12). NRAS, HRAS, and KRAS mutations were assessed using direct sequencing.
Results: Among 85 patients, 39 patients (46%) had RAS mutations. The NRAS codon 61 mutation (n=21; 25%) was the most common point mutation. HRAS codon 61, KRAS codon 12/13, and KRAS codon 61 mutations were found in 7, 6, and 4 patients, respectively. A NRAS codon 12/13 mutation was found in only 1 patient, and a HRAS codon 12/13 mutation was not found. RAS mutations were significantly more common in the FTC than FA or NH groups. Especially, the NRAS codon 61 mutation was associated with distant metastasis in patients with FTC.
Conclusions: The presence of a RAS mutation, especially a NRAS codon 61 mutation, was significantly associated with the distant metastasis. The NRAS codon 61 mutation status might be a potential prognostic factor in FTC patients.
Introduction
RAS is a classical dual activator of the mitogen activated protein kinase and the phosphatidylinositol 3-kinase/AKT (PI3K/AKT) pathways. The RAS genes including NRAS, HRAS, and KRAS are known as proto-oncogenes. Many kinds of cancers, including thyroid, pancreas, biliary, colon, and melanoma, carry RAS mutations. The frequency of RAS mutations in thyroid cancer is reported to be about 14%, and many thyroid cancers carry mutations in all three isoforms (1). Several histologic types of malignant thyroid cancer (follicular thyroid carcinoma, encapsulated follicular variant papillary thyroid carcinoma, anaplastic thyroid cancer, and poorly differentiated thyroid cancer), as well as benign thyroid tumors carry RAS mutations with variable frequency (2,3). The higher rates of mutation in malignant thyroid tumors in comparison with benign tumors suggest that a RAS mutation is an early event in follicular thyroid carcinogenesis (4,5). Several studies reported that RAS mutations are also associated with aggressive thyroid cancer behavior, distant metastases, and poor clinical outcome (6–10). Garcia et al. have reported that RAS mutations are associated with loss of tumor differentiation, large tumor size, vascular invasion, distant metastasis, and survival. However, that study cohort included various histologic types of tumors other than follicular thyroid carcinomas (FTC) (10). A recent study retrospectively analyzed 58 FTC patients, and found that 14 of these cases had a distant metastasis, and 33 harbored a RAS mutation (9). That study reported that RAS mutations are associated with not only distant metastasis but also high mortality in FTC patients.
FTC is considered a more aggressive disease with poorer prognosis than papillary thyroid carcinoma (PTC). It is less likely to have lymph node metastases but more likely to hematogenously disseminate to distant organs in comparison with PTC (11). Some FTCs even initially present with a distant metastasis. According to previous reports, the percentage of patients who present with distant metastasis at the initial presentation varies from 3.1% to 21% of FTC patients (12–16). Distant metastasis is the most important prognostic factor, and patients with a distant metastasis demonstrate an approximately five-fold higher mortality than patients without distant metastasis (13). Known factors related to distant metastases included age, primary tumor size, and invasiveness (16,17).
In our present study, we analyze the frequency of RAS mutations in patients who present with FTC with or without distant metastasis, follicular adenomas (FA), and nodular hyperplasia (NH) to elucidate the roles of RAS mutations in follicular thyroid carcinogenesis and metastasis. In addition, we assessed the relationship between RAS mutations and clinical outcomes in FTC patients including survival and distribution of metastatic sites.
Material and Methods
Patients
We selected patients who underwent a thyroidectomy and were diagnosed with FTC and distant metastases (FTC M1) between 1998 and 2012 in the Asan Medical Center. We identified 28 patients with FTC M1 and 28 size matched FTC specimens without a distant metastasis (FTC M0). We also identified FA (n=17), and NH (n=12). For the mutational analysis, all tissue specimens were independently reviewed by two expert pathologists (DES and GG), who specialize in thyroid pathology. Pathological diagnoses were made according to the latest World Health Organization classifications for thyroid cancer. FTCs were further diagnosed as either minimally invasive or widely invasive according to the World Health Organization classification for capsular invasion and vascular invasion. We excluded the oncocytic subtype of FTCs and solid growth patterned tumors after reviewing the specimens. This study protocol was approved by the institutional review board of Asan Medical Center.
Definitions
The diagnosis of distant metastasis was confirmed by pathological examination of metastatic site(s) of the FTC, assessment of 131I uptake in distant organs using 131I whole body scan following radioactive iodine (RAI) ablation, and/or assessment of nonradioactive iodine imaging including computed tomography, 18F-fluorodeoxyglucose positron emission tomography with computed tomography, or magnetic resonance imaging. FTC M1 was defined as both synchronous and metachronous metastasis. We classified FTC M1 according to the number of metastatic sites. Metastatic disease at one site was metastases in single organ (e.g., lung or bone only). Both lung and bone metastases were classified as metastatic disease at two sites and metastases at three or more sites were classified as metastatic disease at multiple sites (e.g., lung, bone, and brain). FTC M0 patients were defined as patients who did not demonstrate any evidence of metastasis at initial surgery or during the median follow up period (10.1 years; range, 2.0–14.8 years).
Mutational analysis of the RAS genes
We used DNA from primary thyroid tumors for polymerase chain reaction (PCR) to compare the RAS mutation status in all patients. Surgical removal of metastases was performed in only eight patients with FTC M1. However, we could not extract the DNA from the metastatic specimens because those samples were too old. Eighty-five paraffin-embedded thyroid specimens were microdissected and sectioned to 5–10 slices of 10 μm thickness. Thirty-five patients (41%) presented with multiple tumors, including 20 patients with FTC and NH, 7 patients with multiple FTCs, 5 patients with FTC and micro-PTC, 2 patients with FTC and FA, and 1 patient with multiple NHs. We extracted DNA from dominant tumors when the specimens contained multiple FTCs or NHs.
Genomic DNA from formalin-fixed paraffin-embedded (FFPE) tissues was extracted using the QIAamp DNA FFPE Tissue Kit (Qiagen, Hilden, Germany). Purified DNA was directly subjected to PCR with primers designed to detect each mutation site. NRAS, HRAS, and KRAS mutations were examined using PCR and amplified using appropriate primers (Supplementary Table S1; Supplementary Data are available online at www.liebertpub.com/thy).
DNA was used as template after quantification by NanoDrop (Thermo scientific, Wilmington, DE). PCR was performed using a BioMix kit (Bioline, Taunton, MA). Each PCR reaction (50 μL) contained 10 pmol/μL primer set with 1× PCR buffer, 2.5 mM MgCl2, and 1 μg genomic DNA. Most of the specimens used were old, so we used a large amount of template DNA to obtain an adequate PCR product. PCR reactions were performed using the following settings: 95°C initial denaturation for 5 minutes, denaturation at 94°C for 30 seconds, annealing of the primer to the template at 55–65°C for 30 seconds, and primer extension at 72°C for 30 seconds. Denaturation, annealing, and primer extension were repeated for 35 cycles followed by a final extension at 72°C for 5 minutes. The PCR reaction was then cooled to 4°C. The final PCR products were confirmed using electrophoresis. To determine any genetic mutation, DNA sequencing was performed using the antisense primers. The DNA sequences were then read using a DNA analyzer (Bioedit version 7.2.0, Carlsbad, CA), and RAS mutations were thereby identified. Each DNA sample was assayed at least twice in order to confirm the RAS mutation status.
Statistical analysis
R version 3.0 (R Foundation for Statistical Computing, Vienna, Austria, www.R-project.org) was used to analyze all data. Associations between the RAS mutation status and the four study groups (FTC M1, FTC M0, FA, and NH) were evaluated using the Kruskal-Wallis test. Correlations between RAS mutation, distant metastasis, and metastastic sites were analyzed using the chi-squared or Fisher's exact test. The predictive factors for distant metastases in FTC were assessed using univariate and multivariate logistic regression models. Overall survival was evaluated at the end of follow up using the Kaplan-Meier analysis. In this study, p-value<0.05 was considered to indicate statistical significance.
Results
Baseline characteristics of study subjects
Baseline characteristics of the 28 FTC M1 patients in our study cohort are listed in Supplementary Table S2. The mean age of patients in the FTC M1 group was 63.5 years (range; 42–82), and 22 patients were female (79%). All FTC M1 patients received a total thyroidectomy. The mean tumor size was 4.6±2.9 cm (range, 0.5–15.0 cm). Twenty-three (82%) and 22 of specimens (79%) of FTC M1 had capsular or vascular invasion, respectively. Lymph nodes dissection was performed in 15 patients (53%), and 3 of these patients (11%) had cervical lymph nodes metastasis. Twenty-seven patients received remnant RAI ablation after surgery, and one FTC patient with brain metastasis could not receive RAI ablation. Fifteen patients received external beam radiation therapy and/or systemic chemotherapy. Metachronous metastases were found in 7 patients (lung only in 3 patients, both lung and bone in 3 patients, and metastatic disease at multiple sites in 1 patient). The median time between initial surgery and detection of metastasis was 3.8 years in these patients.
The mean age of the FTC M0 group was 50.2 years (range, 34–71). Twenty-six patients in the FTC M0 group were female (93%). The mean tumor size was 4.1±2.2 cm (range, 1.0–12.0). Twenty- six (93%) and 3 specimens (11%) of the FTC M0 had capsular invasion or vascular invasion, respectively. Lymph node dissection was performed in 6 patients (21%), but no patient with lymph node metastasis was identified. Twenty-one (75%) patients in the FTC M0 group received remnant RAI ablation.
In the FA group, the mean age was 49.9 years (range, 38–64). All FA patients were female. The mean age of the NH group was 52.8 years (range, 34–74), and 10 patients were female (83%).
Frequency of RAS gene mutations in study patients
Among 85 patients, 39 patients (46%) had RAS mutations. The NRAS codon 61 mutation (n=21, 25%) was the most common point mutation (Table 1). HRAS codon 61, KRAS codon 12/13, and KRAS codon 61 mutations were found in 7, 6, and 4 patients, respectively. A NRAS codon 12/13 mutation was found in only 1 patient, and a HRAS codon 12/13 mutation was not found. The point mutations identified in our study are summarized in Supplementary Table S3.
Table 1.
The Frequency of RAS Mutations in Study Groups
| FTC | |||||
|---|---|---|---|---|---|
| Total | NH | FA | FTC M0 | FTC M1 | |
| Any RAS mutations | 39/85 (46)* | 1/12 (8) | 7/17 (41) | 14/28 (50) | 17/28 (61) |
| NRAS codon 61 | 21/85 (25) | 0/12 (0) | 3/17 (18) | 5/28 (18) | 13/28 (46) |
| HRAS codon 61 | 7/85 (8) | 1/12 (8) | 1/17 (6) | 3/28 (11) | 2/28 (7) |
| KRAS codon 12/13 | 6/85 (7) | 0/12 (0) | 2/17 (12) | 4/28 (14) | 0/28 (0) |
| KRAS codon 61 | 4/85 (5) | 0/12 (0) | 1/17 (6) | 1/28 (4) | 2/28 (7) |
| NRAS codon 12/13 | 1/85 (1) | 0/12 (0) | 0/17 (0) | 1/28 (4) | 0/28 (0) |
| HRAS codon 12/13 | 0/85 (0) | 0/12 (0) | 0/17 (0) | 0/28 (0) | 0/28 (0) |
RAS mutation positive patients /total patients (%).
NH, nodular hyperplasia; FA, follicular adenoma; FTC, follicular thyroid carcinoma; FTC M0, follicular thyroid carcinoma without distant metastasis; FTC M1, follicular thyroid carcinoma with distant metastases.
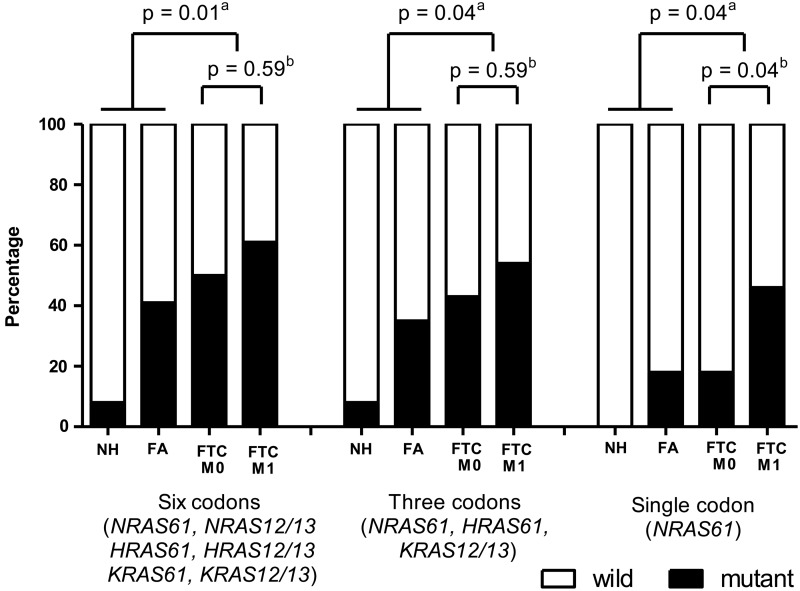
RAS mutations were detected more frequently in the FTC group than in the FA or NH groups (Fig. 1). We performed analyses of the presence of a mutation among 6 RAS codons (“six codons”; NRAS codon 61, NRAS codon 12/13, HRAS codon 61, HRAS codon 12/13, KRAS codon 61, and KRAS codon 12/13), presence of a mutation among 3 specific RAS codons (“three codons”; NRAS codon 61, HRAS codon 61, and KRAS codon 12/13), and presence of the NRAS codon 61 mutation (“single codon”). Three kinds of RAS mutation analyses demonstrated a significant association between RAS mutations and FTC. Especially, the NRAS codon 61 mutation was more frequently detected in the FTC M1 than in the FTC M0 group (46% vs. 18%; p=0.04, Fig. 1). However, analyses of “six codons” (p=0.59) and “three codons” (p=0.59) showed no statistically significant difference between the FTC M1 and FTC M0 groups (Fig. 1).
FIG. 1.
RAS mutation frequencies in study groups using three kinds of RAS mutation analyses (six codons, three codons, and single codon). aAnalysis of RAS mutation frequency between FTC group and FA or NH groups. FTC had significantly more RAS mutations than FA or NH groups in all three kinds of RAS mutation analyses (six codons, three codons, and single codon). bAnalysis of RAS mutation frequency between FTC M1 and FTC M0 groups. Only NRAS codon 61 mutation had a significant association with FTC M1 group. NH, nodular hyperplasia (n=12); FA, follicular adenoma (n=17); FTC, follicular thyroid carcinoma; FTC M0, FTC without a distant metastasis (n=28); FTC M1, FTC with distant metastases (n=28).
A comparison of the clinicopathological features in FTC patients according to NRAS codon 61 mutation status
The presence of a NRAS codon 61 mutation was significantly associated with tumor size and presence of distant metastases (Table 2). Although tumor size was smaller in NRAS codon 61 mutant tumors than in NRAS codon 61 wild type tumors, NRAS codon 61 mutant tumors were more likely to have distant metastases. The univariate model showed that age 45 years or older and the presence of a NRAS codon 61 mutation were significant factors associated with distant metastases. In the multivariate model, the presence of a NRAS codon 61 mutation was the only independent predictor of distant metastases (Table 3).
Table 2.
A Comparison of Clinicopathological Features in FTC Patients According to the NRAS Codon 61 Mutation Status
| Total | NRAS 61 wild type | NRAS 61 mutant | p-Value | |
|---|---|---|---|---|
| Patient, n/total (%) | 56/56 (100) | 38/56 (68) | 18/56 (32) | |
| Age, mean (range) | 56.8 (34–82) | 56.1 (34–80) | 58.4 (42–82) | 0.42 |
| Sex, female | 48/56 (86) | 32/38 (84) | 16/18 (89) | 0.99 |
| Tumor | ||||
| Mean size±SD, cm (range) | 4.4±2.5 (0.5–15.0) | 4.7±2.9 (0.5–15.0) | 3.6±1.2 (1.7–6.0) | 0.03 |
| Widely invasive FTC | 11/56 (20) | 7/38 (18) | 4/18 (22) | 0.73 |
| Lymph node metastasis | 3/56 (5) | 2/38 (5) | 1/18 (6) | 0.99 |
| Distant metastasis | 28/56 (50) | 15/38 (39) | 13/18 (72) | 0.04 |
| RAI treatment | 48/56 (86) | 32/38 (84) | 16/18 (89) | 0.99 |
| Mortality | 9/56 (16) | 6/38 (16) | 3/18 (17) | 0.99 |
NRAS 61, NRAS codon 61; SD, standard deviation; RAI, radioactive iodine.
Table 3.
The Predictive Factors for Distant Metastases in Follicular Thyroid Carcinomas
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| n (%) | OR [95% CI] | p-Value | OR [95% CI] | p-Value | |
| Age ≥45 yearsa | 26 (93) | 8.41 (1.66–42.76) | 0.01 | 4.43 (0.75–25.98) | 0.1 |
| Sex, female | 22 (79) | 0.28 (0.05–1.54) | 0.14 | ||
| Tumor sizeb | 1.08 (0.87–1.34) | 0.48 | 1.12 (0.82–1.53) | 0.47 | |
| Widely invasive FTCc | 11 (39) | NA | NA | NA | >0.99 |
| Lymph node metastasisc | 3 (11) | NA | NA | ||
| NRAS codon 61 mutationa | 13 (46) | 3.99 (1.18–13.5) | 0.026 | 5.75 (1.32–25.13) | 0.02 |
Covariates that were significant factors in the univariate analysis (age ≥45 years, NRAS codon 61 mutation), tumor size, and invasiveness were included in the multivariate model for distant metastases.
Tumor size was included in the model as a continuous variable.
Univariate analysis was not performed for widely invasive and lymph node metastasis due to low number of patients. All widely invasive follicular thyroid carcinomas included in FTC M1.
CI, 95% confidence interval; NA, not applicable; OR, odds ratio.
RAS mutation and FTC invasion
We subcategorized FTCs as minimally invasive FTC (n=45; 80%) or widely invasive FTC (n=11; 20%). All patients with widely invasive FTC demonstrated distant metastases, so there was a significant association between widely invasiveness and distant metastases (p<0.001). RAS mutation frequencies were similar in both groups (minimally invasive FTC group, 24 of 45 patients, 53%; widely invasive FTC group, 7 of 11 patients, 64%).
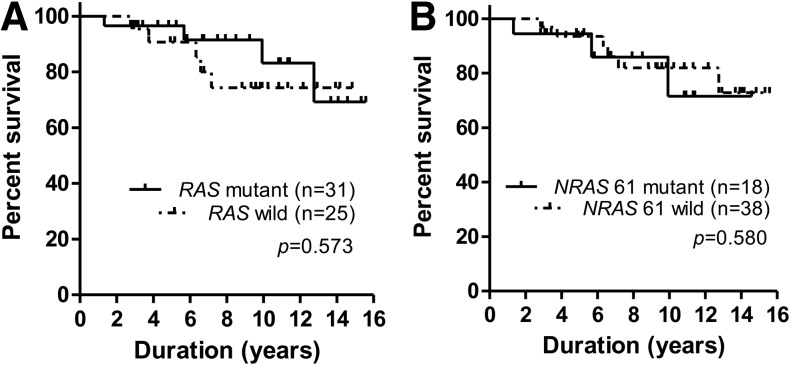
Survival in FTC patients according to RAS mutation status
The mean survival time in patients with FTC was 13.3±0.3 years. There was no significant difference in mean survival time between the RAS mutation positive FTC and RAS mutation negative FTC patients at the end of follow up (13.9±0.7 and 13.0±0.7 years respectively; Fig. 2A). NRAS codon 61 mutation positive FTC patients also demonstrated no difference in mean survival time compared with NRAS codon 61 mutation negative FTC patients (12.2±1.2 and 13.6±0.7 years respectively; Fig. 2B). RAS mutation positive FTC and RAS mutation negative FTC patients demonstrated similar clinicopathological features including age, sex, tumor size, FTC invasion, lymph node metastasis, and distant metastasis (Supplementary Table S4).
FIG. 2.
Survival analysis of FTC patients. (A) Overall survival in the FTC patients according to the RAS mutation status. There was no difference in the overall survival between RAS mutant FTC and wild-type FTC patients. (B) Overall survival in the FTC patients according to NRAS codon 61 mutation status demonstrated no difference between NRAS codon 61 mutant-FTC and NRAS codon 61 wild-type FTC patients.
Subgroup analysis in FTC patients with distant metastases
There was also no significant difference in mean survival time during the follow-up between RAS mutation positive FTC M1 and RAS mutation negative FTC M1 patients (12.8±2.9 years and 7.2±0.5 years respectively, p=0.067).
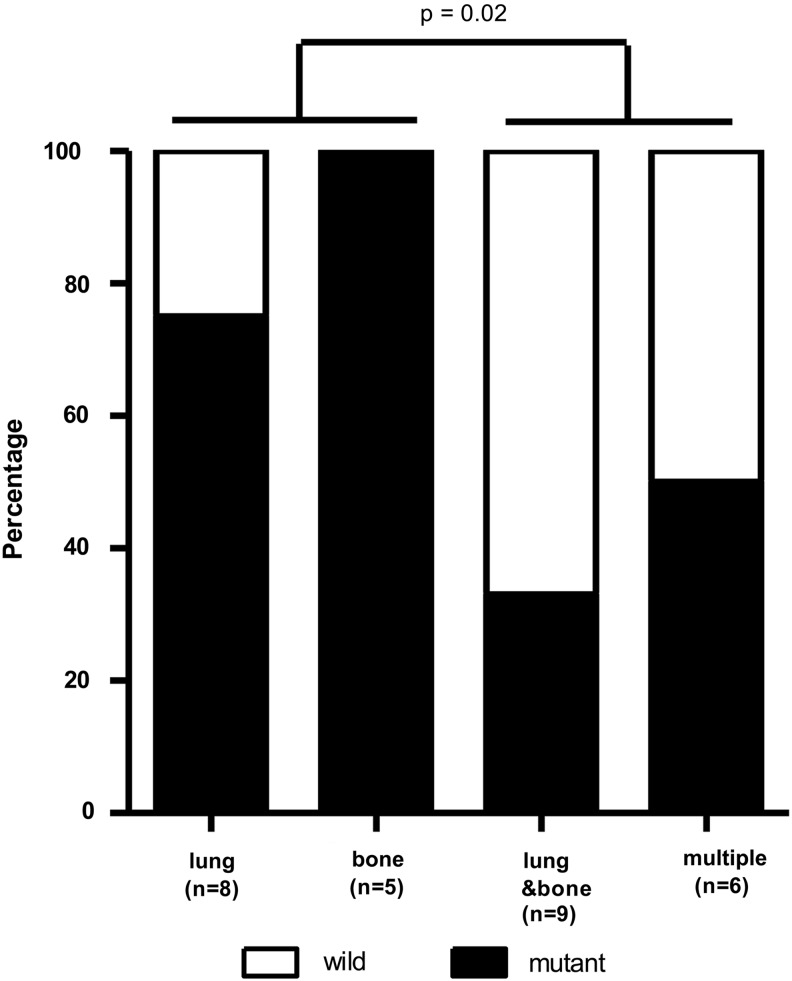
We analyzed the RAS mutation status according to the sites of metastases. Metastatic sites included lung only (n=8), bone only (n=5), both lung and bone (n=9), and multiple sites (n=6; Fig. 3). Metastases at multiple sites involved brain, muscle, liver, para-aortic lymph nodes, or kidney in addition to lung and bone. Patients with metastatic disease at one site (lung or bone only) were more likely to carry a RAS mutation than those with metastatic disease at two or more sites (p=0.02, odds ratio=8.25 [95% C.I. 1.33-51.26]; Fig, 3).
FIG. 3.
Distribution of metastatic sites in follicular thyroid carcinoma with distant metastases. Metastatic disease at one site (lung or bone only) was more significantly associated with RAS mutations than metastatic disease at two or more sites (both lung and bone, or multiple sites).
Discussion
In this study, we investigated the RAS mutation status according to the presence of a distant metastasis in a large number of FTC patients. This is the largest study addressing the role of RAS mutations in FTC patients with distant metastases. Our study shows that the presence of a RAS mutation is associated with FTC, and that a NRAS codon 61 mutation is significantly associated with distant metastases in FTC patients. This result suggests that, the presence of a NRAS codon 61 mutation could be a factor predicting distant metastases.
FTC frequently metastasizes to distant organs. Some FTCs initially present with distant metastasis, and the percentage of FTC patients with distant metastasis at initial diagnosis varies between 3.1% and 21% in the literature (12–16). Distant metastasis is the most important prognostic factor in FTC. Sugino et al. reported that 10-year cancer-specific survival in FTC patients with distant metastasis was 41% in comparison with 96% in patients without distant metastasis. Age 45 years old or more, tumor size over 4 cm, and widely invasive FTC are significant factors related to distant metastasis (16). A NRAS codon 61 mutation could also be an important factor to predict distant metastasis in FTC patients according to the result of our study. As our study cohort was larger than previous studies, we could demonstrate a clear association between the RAS mutation and distant metastases (9,10).
RAS mutations seem to be not only involved in early carcinogenesis but also in FTC progression according to the results of several studies (7–10). The neoplastic proliferation of thyroid follicular cells develops by altering multiple molecular pathways such as the PI3K/AKT pathway involving the RAS, RAF, RET, and NTRK1 genes (7,18). RAS, which is a dual activator of the mitogen activated protein kinase and PI3K/AKT pathways, predominantly activates the PI3K/AKT pathway in follicular thyroid carcinogenesis (19). Previous studies showed that the frequency of RAS gene mutations is higher in FTC (23.8–56.9%) than in FA patients (18.0–47.8%) (8,9,20–22). The higher rate of mutation in malignant thyroid tumors suggests that RAS mutations are an early event in follicular thyroid carcinogenesis (4,5). Furthermore, several in vivo and in vitro studies have reported that the introduction of mutant RAS to normal thyroid cells causes colonies to demonstrate well differentiated phenotypes and cell proliferation profiles that are consistent with FA (23–25). Other mutations, such as PTEN promoter hypomethylation, in addition to a RAS mutation could be needed to develop a FTC from a FA (26,27). More study is needed to find the genetic differences in FA and FTC patients.
Distant metastases of differentiated thyroid cancers usually show long-term stable or slowly progressive disease. Metastatic dormancy could explain the features of long-term stable distant metastases of thyroid cancer. Metastatic dormancy is defined as the ability of small clusters of cancer cells to migrate to distant sites and then survive in a quiescent state for long periods without significant growth (28,29). Although not significant, RAS mutation positive FTC M1 patients had longer survival time than RAS mutation negative FTC M1 patients. RAS mutation negative FTC with distant metastases demonstrated more extensive metastases than their RAS mutation positive counterparts, and this might have led to the offsetting of survival difference in FTC. RAS mutation positive FTC might gradually metastasize and in a stepwise manner unlike RAS mutation negative FTC. After metastasis develops, RAS mutations might not play a major role in cancer progression and dedifferentiation. Additional mutations might be needed to promote metastastatic progression in RAS mutation positive FTC with distant metastases. RAS mutation negative FTC with distant metastases might express undiscovered oncogenes that promote aggressive behavior. It is crucial to define the processes by which cancer metastasizes, and how metastatic dormancy is gained or lost in primary and metastatic settings in the future.
Fine needle aspiration cytology (FNAC) is the standard tool for detecting thyroid cancer (30). Recently, Gupta et al. reported that 83% of nodules with indeterminate and preoperative RAS mutation positive FNAC were diagnosed as cancer after surgery (31). If the thyroid nodules are diagnosed as a follicular neoplasm, and have a RAS mutation in a FNAC specimen, these tumors are expected to show more aggressive behavior (such as distant metastases) than those without a mutation based on our data. Therefore, preoperative RAS mutation analysis on FNAC samples diagnosed as follicular neoplasm will be useful for clinical decision making including the determination of the extent of surgical intervention. However, we need further studies based on long-term follow up data for elucidation of clinical usefulness of RAS mutation examination on FNAC.
In conclusion, the presence of a RAS mutation was significantly associated with FTC and a NRAS codon 61 mutation was especially associated with distant metastases in FTC patients. Our findings in this study suggest that NRAS codon 61 mutation status might be a potential prognostic factor in FTC patients.
Supplementary Material
Acknowledgment
This study was supported by the National Research Foundation (NRF) of Korea Research Grant (NRF-2012R1A1A2038383).
Author Disclosure Statement
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- 1.Prior IA, Lewis PD, Mattos C.2012A comprehensive survey of Ras mutations in cancer. Cancer Res 72:2457–2467 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nikiforov YE.2008Thyroid carcinoma: molecular pathways and therapeutic targets. Mod Pathol 21:S37–S43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ricarte-Filho JC, Ryder M, Chitale DA, Rivera M, Heguy A, Ladanyi M, Janakiraman M, Solit D, Knauf JA, Tuttle RM, Ghossein RA, Fagin JA.2009Mutational profile of advanced primary and metastatic radioactive iodine-refractory thyroid cancers reveals distinct pathogenetic roles for BRAF, PIK3CA, and AKT1. Cancer Res 69:4885–4893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lemoine NR, Mayall ES, Wyllie FS, Williams ED, Goyns M, Stringer B, Wynford-Thomas D.1989High frequency of ras oncogene activation in all stages of human thyroid tumorigenesis. Oncogene 4:159–164 [PubMed] [Google Scholar]
- 5.Namba H, Rubin SA, Fagin JA.1990Point mutations of ras oncogenes are an early event in thyroid tumorigenesis. Mol Endocrinol 4:1474–1479 [DOI] [PubMed] [Google Scholar]
- 6.Hou P, Liu D, Shan Y, Hu S, Studeman K, Condouris S, Wang Y, Trink A, El-Naggar AK, Tallini G, Vasko V, Xing M.2007Genetic alterations and their relationship in the phosphatidylinositol 3-kinase/Akt pathway in thyroid cancer. Clin Cancer Res 13:1161–1170 [DOI] [PubMed] [Google Scholar]
- 7.Xing M.2013Molecular pathogenesis and mechanisms of thyroid cancer. Nat Rev Cancer 13:184–199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manenti G, Pilotti S, Re FC, Della Porta G, Pierotti MA.1994Selective activation of ras oncogenes in follicular and undifferentiated thyroid carcinomas. Eur J Cancer 30A:987–993 [DOI] [PubMed] [Google Scholar]
- 9.Fukahori M, Yoshida A, Hayashi H, Yoshihara M, Matsukuma S, Sakuma Y, Koizume S, Okamoto N, Kondo T, Masuda M, Miyagi Y.2012The associations between RAS mutations and clinical characteristics in follicular thyroid tumors: new insights from a single center and a large patient cohort. Thyroid 22:683–689 [DOI] [PubMed] [Google Scholar]
- 10.Garcia-Rostan G, Zhao H, Camp RL, Pollan M, Herrero A, Pardo J, Wu R, Carcangiu ML, Costa J, Tallini G.2003ras mutations are associated with aggressive tumor phenotypes and poor prognosis in thyroid cancer. J Clin Oncol 21:3226–3235 [DOI] [PubMed] [Google Scholar]
- 11.Grebe SK, Hay ID.1995Follicular thyroid cancer. Endocrinol Metab Clin North Am 24:761–801 [PubMed] [Google Scholar]
- 12.DeGroot LJ, Kaplan EL, Shukla MS, Salti G, Straus FH.1995Morbidity and mortality in follicular thyroid cancer. J Clin Endocrinol Metab 80:2946–2953 [DOI] [PubMed] [Google Scholar]
- 13.Passler C, Scheuba C, Prager G, Kaczirek K, Kaserer K, Zettinig G, Niederle B.2004Prognostic factors of papillary and follicular thyroid cancer: differences in an iodine-replete endemic goiter region. Endocr-Relat Cancer 11:131–139 [DOI] [PubMed] [Google Scholar]
- 14.Ito Y, Hirokawa M, Higashiyama T, Takamura Y, Miya A, Kobayashi K, Matsuzuka F, Kuma K, Miyauchi A.2007Prognosis and prognostic factors of follicular carcinoma in Japan: importance of postoperative pathological examination. World J Surg 31:1417–1424 [DOI] [PubMed] [Google Scholar]
- 15.Asari R, Koperek O, Scheuba C, Riss P, Kaserer K, Hoffmann M, Niederle B.2009Follicular thyroid carcinoma in an iodine-replete endemic goiter region: a prospectively collected, retrospectively analyzed clinical trial. Ann Surg 249:1023–1031 [DOI] [PubMed] [Google Scholar]
- 16.Sugino K, Ito K, Nagahama M, Kitagawa W, Shibuya H, Ohkuwa K, Yano Y, Uruno T, Akaishi J, Kameyama K, Ito K.2011Prognosis and prognostic factors for distant metastases and tumor mortality in follicular thyroid carcinoma. Thyroid 21:751–757 [DOI] [PubMed] [Google Scholar]
- 17.Lang BH, Wong KP, Cheung CY, Wan KY, Lo CY.2013Evaluating the prognostic factors associated with cancer-specific survival of differentiated thyroid carcinoma presenting with distant metastasis. Ann Surg Oncol 20:1329–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kondo T, Ezzat S, Asa SL.2006Pathogenetic mechanisms in thyroid follicular-cell neoplasia. Nate Rev Cancer 6:292–306 [DOI] [PubMed] [Google Scholar]
- 19.Liu Z, Hou P, Ji M, Guan H, Studeman K, Jensen K, Vasko V, El-Naggar AK, Xing M.2008Highly prevalent genetic alterations in receptor tyrosine kinases and phosphatidylinositol 3-kinase/akt and mitogen-activated protein kinase pathways in anaplastic and follicular thyroid cancers. J Clin Endocrinol Metab 93:3106–3116 [DOI] [PubMed] [Google Scholar]
- 20.Esapa CT, Johnson SJ, Kendall-Taylor P, Lennard TW, Harris PE.1999Prevalence of Ras mutations in thyroid neoplasia. Clin Endocrinol 50:529–535 [DOI] [PubMed] [Google Scholar]
- 21.Vasko V, Ferrand M, Di Cristofaro J, Carayon P, Henry JF, de Micco C.2003Specific pattern of RAS oncogene mutations in follicular thyroid tumors. J Clin Endocrinol Metab 88:2745–2752 [DOI] [PubMed] [Google Scholar]
- 22.Schulten HJ, Salama S, Al-Ahmadi A, Al-Mansouri Z, Mirza Z, Al-Ghamdi K, Al-Hamour OA, Huwait E, Gari M, Al-Qahtani MH, Al-Maghrabi J.2013Comprehensive survey of HRAS, KRAS, and NRAS mutations in proliferative thyroid lesions from an ethnically diverse population. Anticancer Res 33:4779–4784 [PubMed] [Google Scholar]
- 23.Rochefort P, Caillou B, Michiels FM, Ledent C, Talbot M, Schlumberger M, Lavelle F, Monier R, Feunteun J.1996Thyroid pathologies in transgenic mice expressing a human activated Ras gene driven by a thyroglobulin promoter. Oncogene 12:111–118 [PubMed] [Google Scholar]
- 24.Bond JA, Wyllie FS, Rowson J, Radulescu A, Wynford-Thomas D.1994In vitro reconstruction of tumour initiation in a human epithelium. Oncogene 9:281–290 [PubMed] [Google Scholar]
- 25.Gire V, Wynford-Thomas D.2000RAS oncogene activation induces proliferation in normal human thyroid epithelial cells without loss of differentiation. Oncogene 19:737–744 [DOI] [PubMed] [Google Scholar]
- 26.Alvarez-Nunez F, Bussaglia E, Mauricio D, Ybarra J, Vilar M, Lerma E, de Leiva A, Matias-Guiu X, Thyroid Neoplasia Study G 2006PTEN promoter methylation in sporadic thyroid carcinomas. Thyroid 16:17–23 [DOI] [PubMed] [Google Scholar]
- 27.Miller KA, Yeager N, Baker K, Liao XH, Refetoff S, Di Cristofano A.2009Oncogenic Kras requires simultaneous PI3K signaling to induce ERK activation and transform thyroid epithelial cells in vivo. Cancer Res 69:3689–3694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aguirre-Ghiso JA.2007Models, mechanisms and clinical evidence for cancer dormancy. Nat Rev Cancer 7:834–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Phay JE, Ringel MD.2013Metastatic mechanisms in follicular cell-derived thyroid cancer. Endocr-Relat Cancer 20:R307–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kwak JY.2013Indications for fine needle aspiration in thyroid nodules. Endocrinol Metab 28:81–85 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gupta N, Dasyam AK, Carty SE, Nikiforova MN, Ohori NP, Armstrong M, Yip L, Lebeau SO, McCoy KL, Coyne C, Stang MT, Johnson J, Ferris RL, Seethala R, Nikiforov YE, Hodak SP.2013RAS mutations in thyroid FNA specimens are highly predictive of predominantly low-risk follicular-pattern cancers. J Clin Endocrinol Metab 98:E914–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.