Abstract
Background: NY-ESO-1 is one of the most immunogenic members of the cancer/testis antigen family and its levels can be increased after exposure to demethylating and deacetylating agents. This cytoplasmic antigen can serve as a potent target for cancer immunotherapy and yet has not been well studied in differentiated thyroid cancer cells.
Methods: We studied the baseline expression of NY-ESO-1 messenger RNA and protein before and after exposure to 5-aza-2′-deoxycytidine (DAC) (72 hours) in a panel of thyroid cancer cell lines using quantitative polymerase chain reaction and Western blot. HLA-A2+, NY-ESO-1+ thyroid cell lines were then co-cultured with peripheral blood lymphocytes transduced with NY-ESO-1 specific T-cell receptor (TCR) and assayed for interferon-gamma and Granzyme-B release in the medium. SCID mice injected orthotopically with BCPAP cells were treated with DAC to evaluate for NY-ESO-1 gene expression in vivo.
Results: None of the thyroid cancer cell lines showed baseline expression of NY-ESO-1. Three cell lines, BCPAP, TPC-1, and 8505c, showed an increase in NY-ESO-1 gene expression with DAC treatment and were found to be HLA-A2 positive. DAC-treated target BCPAP and TPC-1 tumor cells with up-regulated NY-ESO-1 levels were able to mount an appropriate interferon-gamma and Granzyme-B response upon co-culture with the NY-ESO-1-TCR-transduced peripheral blood lymphocytes. In vivo DAC treatment was able to increase NY-ESO-1 expression in an orthotopic mouse model with BCPAP cells.
Conclusion: Our data suggest that many differentiated thyroid cancer cells can be pressed to express immune antigens, which can then be utilized in TCR-based immunotherapeutic interventions.
Introduction
The incidence of thyroid cancer is increasing in the United States (1). Papillary thyroid cancer (PTC), which accounts for a majority of thyroid cancer cases, is usually curative by surgery, radioiodine treatment, and thyroid-stimulating hormone suppression (2,3). Some patients with aggressive forms of PTC do poorly and there is a lack of effective therapies for these patients. Furthermore, the majority of patients with anaplastic thyroid cancer (ATC) fail to respond to current treatment regimens and show extremely poor prognosis (4). Targeted therapies against the BRAFV600E mutant oncoprotein with small molecule kinase inhibitors are currently in phase-2 trials in aggressive thyroid cancer (5,6). However, development of resistance to these inhibitors in melanoma has dampened enthusiasm, and there is concern that thyroid cancer patients will also develop resistance to these targeted therapies (7–9). Novel therapeutic strategies are needed for both of these groups of patients. There have been several advances in immunotherapeutic strategies over recent years especially in treating melanoma (10,11); however, immunotherapy as a treatment for thyroid cancer has not been well studied.
Effective strategies for immunotherapy in general are based on the generation of immunity against unique antigenic peptides exhibited on the surface or in the cytoplasm of tumor cells. Cancer/testis antigens (CTAs) have received attention as excellent therapeutic targets over the past decade. These antigens when presented by the major histocompatibility complex (MHC) class I molecules are recognized by cytotoxic T lymphocytes (CTLs). CTAs are expressed in various malignancies, but not in normal human tissues, with the exception of male germ line cells and placenta, which do not express MHC class 1 molecules, thus obviating any CTL response specific to these antigens (12,13). Among the CTAs, MAGE family genes and NY-ESO-1 have been used as potential targets for vaccine-based immunotherapy of cancer.
NY-ESO-1 is highly immunogenic and is expressed in melanoma, lung, esophageal, liver, gastric, prostate, ovarian, and bladder cancers, myxoid tumors, and a subset of liposarcomas (14,15). Its expression in the cytoplasm of malignant cells leads to a native strong cytotoxic T-cell immune response in many patients (16). The NY-ESO-1 gene is epigenetically regulated and low or no expression of this gene is sometimes a consequence of histone deacetylation or hypermethylation of its promoter (17). Strategies employing the treatment of cancer cells with demethylating agents as well as histone demethylase inhibitors to reactivate or increase the expression of the CTA genes including NY-ESO-1 have previously been published in glioma, myeloid leukemia, and melanoma (17–20). Clinical trials are now underway using NY-ESO-1 targeted immunotherapeutic strategies that include use of genetically engineered T-cells transduced with NY-ESO-1-T-cell receptors (TCRs) directly targeting melanoma cells (21–23).
Expression of CTAs and their utilization in immunotherapy is not extensively studied in thyroid cancer. Previous studies showed that NY-ESO-1 antibodies were expressed in 35.7% of screened medullary thyroid cancer patient samples (24). At the mRNA level, other CTA genes such as MAGE-A3 were expressed in 65% and MAGE-A1 in 30% of predominantly PTC patients (25–27).
Here, we studied the baseline expression levels of NY-ESO-1 in multiple thyroid cancer cell lines and analyzed the effect of demethylating agents on its expression levels. Furthermore, since some immune surface antigens in melanoma have been shown to be under the influence of oncoproteins such as BRAF cells (28–30), we analyzed the effectiveness of the BRAFV600E inhibitor PLX4720 in modulating NY-ESO-1 gene expression in the thyroid cancer cell lines. Our long-term goal is to study whether combining a cytotoxic immunotherapy strategy with BRAFV600E inhibition may result in better long-term results in the treatment of thyroid cancer.
Materials and Methods
Chemicals, antibodies, cell culture and peripheral blood lymphocyte culture
Demethylating agents
5′-Azacytidine (Aza) and 5′-Aza-3′-deoxycytidine (DAC) were purchased from Sigma Aldrich. PLX4720 was obtained from Plexxikon. Antibodies against β-actin, phospho-ERK1/2(p44/p42), and total ERK1/2(p44/p42) used in Western blots were purchased from Cell signaling technology. Antibodies against NY-ESO-1 (Santacruz Biotechnology), TTF-1 (Dako), and HLA-A2 (Lifespan Biosciences) were used for immunohistochemistry (IHC).
Thyroid cancer cell lines
PTC cell lines (BCPAP, TPC-1), ATC cell lines (8505c, HTh-7) and follicular thyroid cancer cell lines (FTC-133, FTC-236) were grown in RPMI medium supplemented with 10% fetal bovine serum and ampicillin/streptomycin (Gibco). The melanoma cell line A375 and the modified human embryonic kidney cell line GP2-293 were grown in Dulbecco's modified Eagle's medium supplemented with 10% fetal bovine serum and ampicillin/streptomycin (Gibco). All treatments were at 30% confluency for a total of 72 hours, changing media every 24 hours. Treatment concentrations for Aza and DAC were 0.1–10 μM, and PLX4720 concentrations were 1–10 μM. After completion of treatment, cells were collected for RNA or protein isolation.
All of the peripheral blood lymphocytes (PBLs) used in this study were obtained from the blood of healthy male volunteer using BD Vacutainer CPT™ cell preparation tubes. The isolated PBLs were cultured in AIM-V medium (Invitrogen) supplemented with 5% human serum from donors of blood group AB (Sigma), 1 mM l-glutamine, 1 mM minimum essential medium nonessential amino acids, 20 μM 2-mercaptoethanol, 50 U/mL penicillin, 25 mM Hepes (pH 7.0), 50 mg/mL streptomycin (Invitrogen), and 300 IU/mL interleukin-2 (BD Biosciences) and maintained at 37°C with 5% CO2.
Patient samples
With Institutional Review Board approval, PTCs, benign adenomas and matched normal tissue samples collected from consenting patients were used for RNA isolation to perform quantitative polymerase chain reaction (qPCR) for NY-ESO-1 mRNA expression.
Quantitative real-time PCR
RNA was isolated from cells or mouse tissue using Trizol reagent (Invitrogen) according to manufacturer's instructions. Gene validation was performed by relative quantification using real-time PCR. Complementary DNA (cDNA) was synthesized from 1 μg total RNA with a Superscript VILO cDNA synthesis kit (Invitrogen). The cDNA was then used as the template for subsequent real-time PCR with primers for NY-ESO-1, HLA-A, and β-actin (TaqMan Gene Expression Assays; Applied Biosystems). For each sample, technical triplicates were performed. Controls included samples with or without Taq polymerase and RNA alone without reverse transcription. Gene expression levels in all the conditions were normalized to the β-actin gene. NY-ESO-1 and HLA-A expression levels showing more than 2.0-fold difference in relative mRNA expression and p-values <0.05 in unpaired two-tailed Student's t test were considered to be differentially expressed and statistically significant. All the NY-ESO-1 and HLA-A gene expression levels were compared to that of the melanoma cell line A375 (a positive control) at baseline.
Western blot
Cells treated with DAC and untreated cells were washed twice with phosphate-buffered saline and rapidly lysed in RIPA lysis buffer containing protease and phophatase inhibitor cocktails (Invitrogen). Total lysates were centrifuged at 15,000 g for 15 minutes at 4°C and supernatants collected and quantified using a Bradford protein assay kit (Pierce). Western blot analysis was performed using a standard procedure as described earlier (31), and the proteins were identified using specific antibodies.
HLA-A2 serotyping
HLA-A2 expression in cultured cancer cells was evaluated by flow cytometric techniques using the HLA-A2 antibody (BD Biosciences) that recognizes only functional HLA-A2 molecules. For analysis, the relative log fluorescence of live cells was measured using a FACScan flow cytometer.
Retroviral supernatant production and transduction of PBLs with NY-ESO-1 TCR
Retroviral supernatants were generated by transfecting a respective MSGV1-NY-ESO-1-TCR vector DNA (20) with a plasmid encoding RD114 envelope into GP2-293 cells using lipofectamine 2000 reagent (Invitrogen) in opti-MEM medium (Invitrogen) for 48 hours. A MSGV1 vector expressing green fluorescent protein (GFP) was used as a control in all experiments. Viral supernatants were loaded onto retronectin-coated (Takara Bio) non-tissue culture-treated six-well plates and transduction was carried out as described earlier (20). Briefly, PBLs were resuspended at a concentration of 106 cells/mL in stimulation media containing OKT3 (50 ng/mL) and recombinant human interleukin-2 (300 IU/mL) for 48 hours prior to transduction. Lymphocytes were then transduced with retroviral vectors by centrifugation (2,000 g) with retroviral supernatant and polybrene (8 μg/mL) in a retronectin-coated plate loaded with retroviral supernatant carrying NY-ESO-1 TCR gene or GFP.
Cytokine release and granzyme B release assays
In these assays, effector cells (1×105) were co-cultured in a 96-well plate with an equal number of target cells treated with DAC for 72 hours in AIM-V medium in a final volume of 0.2 mL, in triplicate. Culture supernatants were harvested 24 hours after the initiation of co-culture and assayed for interferon-gamma (IFN-γ) (BD Biosciences) and granzyme-B (Abcam) by enzyme-linked immunosorbent assay (ELISA) according to the manufacturer's instructions.
Orthotopic thyroid cancer mouse model
All animal work was done at the Massachusetts General Hospital (Harvard Medical School) in accordance with federal, local, and institutional guidelines. For the orthotopic tumor implantation, 10 (5 per group) SCID female mice, 10 weeks of age, were injected with BCPAP cells as previously described (32). One million BCPAP cells in 10 μL of serum-free RPMI medium were injected into the left thyroid; the unmanipulated right side served as an internal control. Three weeks after tumor implantation, the mice were given intraperitoneal injections of DAC (2.5 mg/kg body weight) or saline (controls) every other day for a week. At completion time, tumors were collected in 10% buffered formalin for histologic evaluation and RNAseLater for the RNA isolation. Histologic examination on paraffin embedded tumor tissue included: hematoxylin and eosin staining, TTF-1, HLA-A2, and NY-ESO-1.
Results
Baseline levels of NY-ESO-1 mRNA are low but can be boosted with the DNA methylation inhibitor deoxyazacytidine in thyroid cancer cell lines
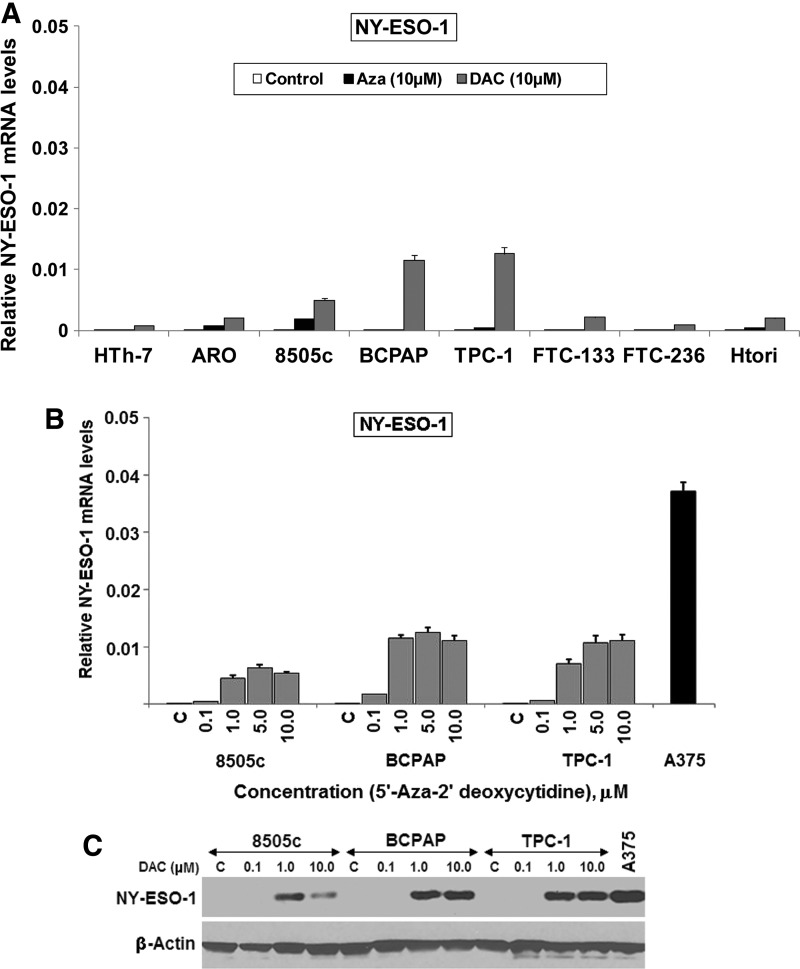
A panel of thyroid cancer cell lines including PTC (BCPAP, TPC-1), ATC (HTh-7, 8505c) and follicular thyroid cancer (FTC-133 and FTC-236) were tested for baseline expression of the NY-ESO-1 gene by real-time qPCR. None of these cell lines expressed NY-ESO mRNA at baseline. A panel of 28 patient's fresh frozen PTC tumors also showed no expression of NY-ESO-1 by qPCR (Table 1). However, treatment of the cell lines with the demethylating agents DAC and Aza for 72 hours resulted in a significant boost in expression of NY-ESO-1 mRNA in some of the tested cell lines, with DAC being significantly more effective than Aza across all cell lines. A normal thyroid cell line, HTori, did not express NY-ESO-1 mRNA at baseline, and the treatment with either of the demethylating agents did not show a significant increase in the expression level of this gene in this cell line (Fig. 1A). The PTC lines, BCPAP and TPC-1, showed high expression of NY-ESO-1 under DAC treatment along with the ATC cell line 8505c, which had lower levels of NY-ESO-1 mRNA than the other two cell lines (Fig. 1A). Maximum expression of NY-ESO-1 mRNA was achieved at 5 and 10 μM of DAC treatments—TPC-1 and BCPAP (∼0.35-fold) and 8505c (∼0.17-fold)—as compared with the baseline expression of NY-ESO-1 mRNA in A375 cells (Fig. 1B). Western blot of the DAC-treated cells also showed significant NY-ESO-1 protein expression at 1 and 10 μM in these cell lines (Fig. 1C). While the overall boost in NY-ESO-1 expression in thyroid cancer was less than the baseline expression in the tested melanoma cell line A375, it is thought that even these low expression levels can lead to cellular death when subsequently subjected to engineered T-cells expressing NY-ESO-1 peptide specific TCRs (20). Given the superiority of DAC treatment in increasing NY-ESO-1 levels, only DAC was used in all further experiments.
Table 1.
None of the Papillary Thyroid Cancer Patient Tumors Tested Showed Expression of NY-ESO-1 mRNA
| Pathology | Sex | No. of patients | No. of patients expressing NY-ESO-1 mRNA (qPCR) |
|---|---|---|---|
| PTC, classical type | F | 16 | 0 |
| M | 4 | 0 | |
| PTC, follicular variant | F | 6 | 0 |
| M | 1 | 0 | |
| PTC, tall cell variant | F | 1 | 0 |
qPCR, quantitative polymerase chain reaction; PTC, papillary thyroid cancer.
FIG. 1.
Treatment of thyroid cancer cell lines with demethylating agents induced the expression of NY-ESO-1 mRNA and protein. Thyroid cancer and normal cell lines were treated with 10 μM 5′-Azacytidine (Aza) or 0.1–10 μM 5′-Aza-3′-deoxycytidine (DAC) for 72 hours and analyzed for induction of NY-ESO-1 gene expression by quantitative real-time polymerase chain reaction (PCR) and Western blot. The melanoma cell line A375 was used as positive control. (A) None of the thyroid cancer cell lines showed NY-ESO-1 gene expression at baseline. DAC treatment induced significant NY-ESO-1 mRNA expression in 8505c, TPC-1, BCPAP cell lines. (B) Treatment of 8505c, BCPAP, and TPC-1 with increasing concentrations of DAC showed high NY-ESO-1 mRNA expression at 5 and 10 μM TPC, BCPAP (∼0.35-fold), and 8505c (∼0.17-fold), as compared with the baseline expression of NY-ESO-1 mRNA in A375 cells. (C) Western blot showed DAC (1 and 10μM) induced NY-ESO-1 protein expression in all cell lines.
Only three thyroid cancer cell lines are HLA-A2 serotype positive allowing recognition by NY-ESO-1 specific lymphocytes
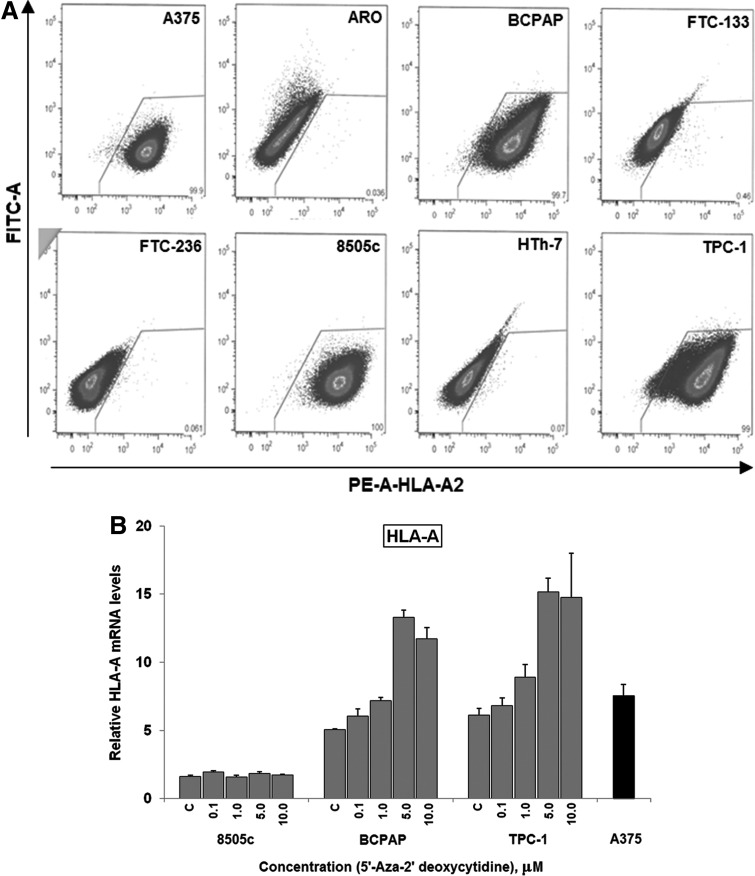
Since tumor cell recognition is bound initially by the ability of the immune system to recognize by HLA-A2 bound NY-ESO-1 derived peptide, we serotyped all the thyroid cancer cell lines with a HLA-A2 antibody. Only BCPAP, TPC-1, and 8505c were HLA-A2 positive (Fig. 2A), therefore limiting further study to these three cell lines. The melanoma cell line A375, which is also HLA-A2 positive and expresses NY-ESO-1 (20) at baseline was used as a positive control (C) throughout the study.
FIG. 2.
DAC treatment increased the HLA-A mRNA in BCPAP and TPC-1 cell lines. All thyroid cancer cell lines were serotyped using HLA-A2 antibody and the HLA-A2+ cell lines were treated with 0.1–10 μM DAC for 72 hours and analyzed for the HLA-A mRNA expression using quantitative reverse transcription PCR. (A) 8505c, BCPAP, and TPC-1 cells are HLA-A2 positive. (B) DAC (5 and 10 μM) treatment resulted in a ∼2.5-fold increase of HLA-A mRNA in BCPAP and TPC-1 cell lines; 8505c cells, on the other hand, did not show any significant changes in the HLA-A expression with DAC treatment. FITC, fluorescein isothiocyanate; PE, phycoerythrin dyes.
HLA-A levels are not decreased by the DNA methylation inhibitor deoxyazacytidine in thyroid cancer cell lines
Since demethylating agents can also alter HLA-A gene products (18,33,34) and the ability of the antigen presenting cells to detect cancer cells, we wanted to see whether DAC treatment increased the expression of the HLA-A mRNA as measured by qPCR. DAC treatment at 5 and 10 μM showed increases in HLA-A expression in BCPAP (∼2.3-fold increase) and TPC-1 (∼2.4-fold increase) as compared with the baseline expression of HLA-A mRNA in these cells. However, 8505c cells did not show any change in the expression of the HLA-A gene in the DAC treatment (Fig. 2B). When the surface expression of HLA-A2 in these cell lines was tested by flow cytometry using a specific antibody, none of them showed a significant increase (result not shown). It appears that DAC treatment does not reduce the ability of tumor cells to be detected by the antigen presenting cells.
Inhibition of BRAFV600E in BRAF mutant thyroid cancer cell lines results in a decrease in deoxyazacytidine-induced NY-ESO-1 protein expression
There is exciting new literature looking at the role of BRAF in cloaking the immunogenic antigens in melanoma cells and also influencing the immune responses of the host. In fact, BRAF inhibition in melanoma overcomes this BRAF-induced repression of the immunogenic antigens such as melanoma differentiation antigen (MDAs) (30), and also results in increased T-cell infiltration into treated tumors (29). While BRAF inhibition does not appear to directly influence the expression of MHC class-1 proteins on melanoma cells, it can alter MHC-1 expression in the presence of cytokines such as IFN-γ (35). We therefore, wanted to see whether treatment with the BRAF inhibitor, PLX4720 altered NY-ESO-1 expression levels in the thyroid tumor cells.
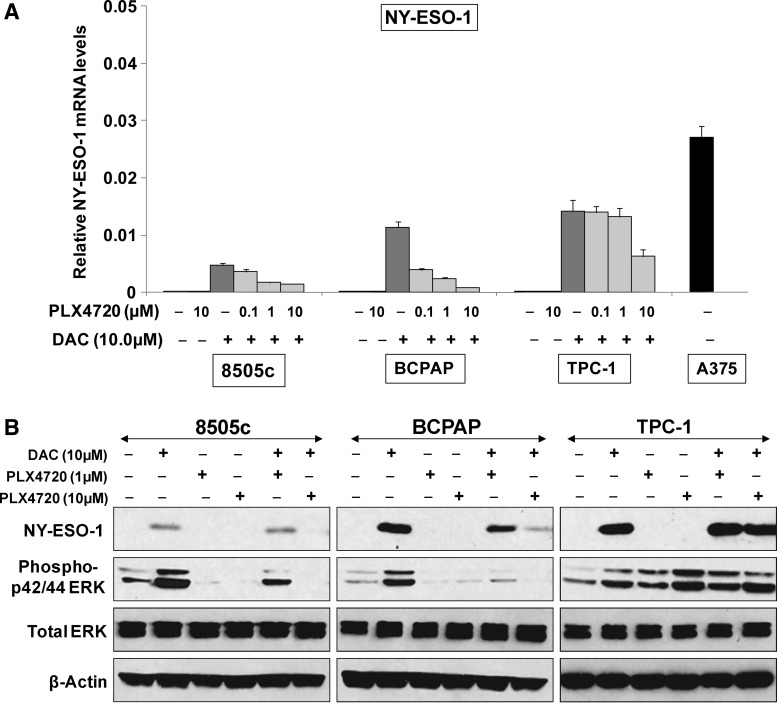
First, we tested the role of MAP kinase/BRAF inhibition on the NY-ESO-1 expression in the thyroid cancer cell lines. Treatment of all HLA-A2 positive cell lines with PLX4720 (0.1–10 μM) appropriately decreased phospho-ERK44/42 protein (Fig. 3B) but did not result in the induction of NY-ESO-1 gene expression from its low baseline level (Fig. 3A). In fact, even though DAC normally increases NY-ESO-1 expression, here we found that adding PLX4720 to DAC-treated BRAFV600E mutant cell lines BCPAP and 8505c resulted in a decrease in mRNA and protein expression of NY-ESO-1 (Fig. 3A, B), ranging from ∼3-fold to 14-fold. On the other hand, in TPC-1 cells (BRAFwt), DAC-induced increases in NY-ESO-1 were maintained with BRAF inhibition (Fig. 3A, B).
FIG. 3.
PLX4720 decreased the NY-ESO-1 mRNA and protein expression in BRAFV600E mutation harboring thyroid cancer cell lines. 8505c, BCPAP and TPC-1 cells were treated with increasing concentrations of PLX4720 (0.1–10 μM), either alone or in combination with DAC (10 μM). (A) Addition of 10 μM PLX4720 to the DAC treated cells decreased the DAC-induced NY-ESO-1 mRNA expression in 8505c and BCPAP cell lines by 3- and 14-fold respectively. (B) Western blot demonstrating the decrease in DAC-induced NY-ESO-1 protein expression in 8505c and BCPAP cell lines when treated in combination with (1 and 10 μM) PLX4720. TPC-1, which has BRAFwt, did not show a decrease in either NY-ESO-1 mRNA or protein when treated with this combination of DAC and PLX4720. PLX4720 treatment appropriately decreased the phospho-p42/44 ERK protein in 8505c and BCPAP cells by inhibiting the BRAFV600E oncoprotein.
Short-term deoxyazacytidine treatment in vivo results in expression of NY-ESO-1 on thyroid cancer cells in an orthotopic thyroid cancer model
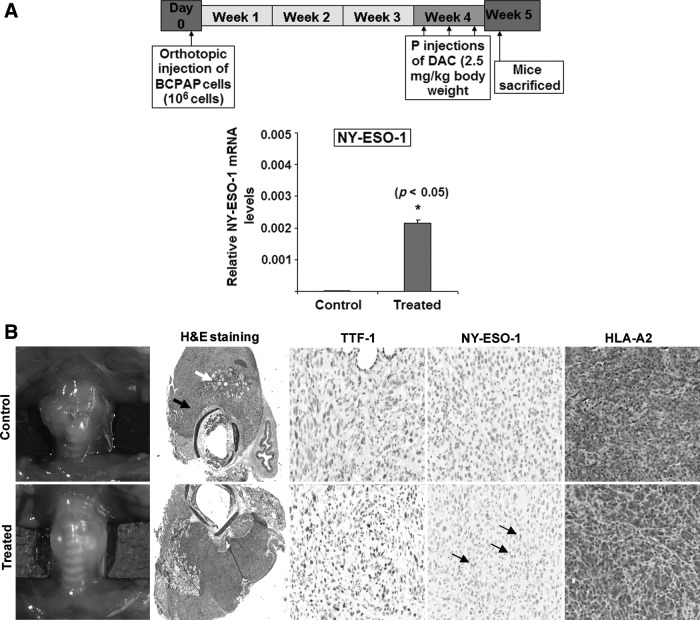
To evaluate whether the boosted NY-ESO-1 gene expressed in the thyroid cancer cells observed in vitro after DAC treatments could be reproduced in vivo, we examined the efficacy of short-term DAC treatment in SCID mice bearing orthotopically implanted BCPAP and 8505c thyroid tumors (Fig. 4A). TPC-1 cells also had high boost of NY-ESO-1 protein with DAC treatment in vitro but these cells do not form orthotopic tumors (data not shown). The BCPAP cell line implanted orthotopically into mice produces large tumors reproducibly by 8 weeks with no evidence of metastases. The 8505c cell line implanted orthotopically results in large, aggressive tumors and distant metastases by 5 weeks. Mice were implanted with one million BCPAP or 8505c cells into the left thyroid and DAC (intraperitoneal, 2.5 mg/kg body weight) was started 3 weeks after tumor implantation. DAC treatment resulted in detectable expression of NY-ESO-1 mRNA in the BCPAP tumors as evaluated by qPCR (Fig. 4A) and protein expression using IHC staining using an NY-ESO-1 antibody (Fig. 4B). DAC treatment in the orthotopically implanted 8505c ATC cell line also resulted in very mild increased expression of in vivo NY-ESO-1 (data not shown). Both the BCPAP (Fig. 4B) and 8505c (data not shown) cell lines retained the expression of HLA-A2 protein in vivo in both the untreated and the DAC-treated conditions.
FIG. 4.
Short-term DAC treatment in vivo in mice orthotopically injected with BCPAP cells resulted in expression of NY-ESO-1 protein. (A) BCPAP cells were implanted into the left thyroid of SCID mice. Three weeks post implantation, mice (n=5) were injected thrice intraperitoneally (IP) with DAC (2.5 mg/kg body weight) or saline every other day for a week. DAC treatment did not reduce tumor volume but did result in expression of NY-ESO-1 mRNA in the BCPAP tumors as evaluated by quantitative PCR and immunohistochemistry (IHC). (B) BCPAP orthotopic tumors are shown at 5 weeks post implantation along with hematoxylin and eosin (H&E) staining, which shows the tumor (black arrow) and entrapped normal thyroid follicles (white arrow), both of which show strong TTF-1 and HLA-A2 IHC staining. DAC treatment in these mice induced a moderate expression of NY-ESO-1 protein in the cytoplasm of the tumor cells (arrow) but not the normal thyroid cells.
NY-ESO-1 expression leads to increase in interferon gamma and Granzyme-B production in the co-culture experiments
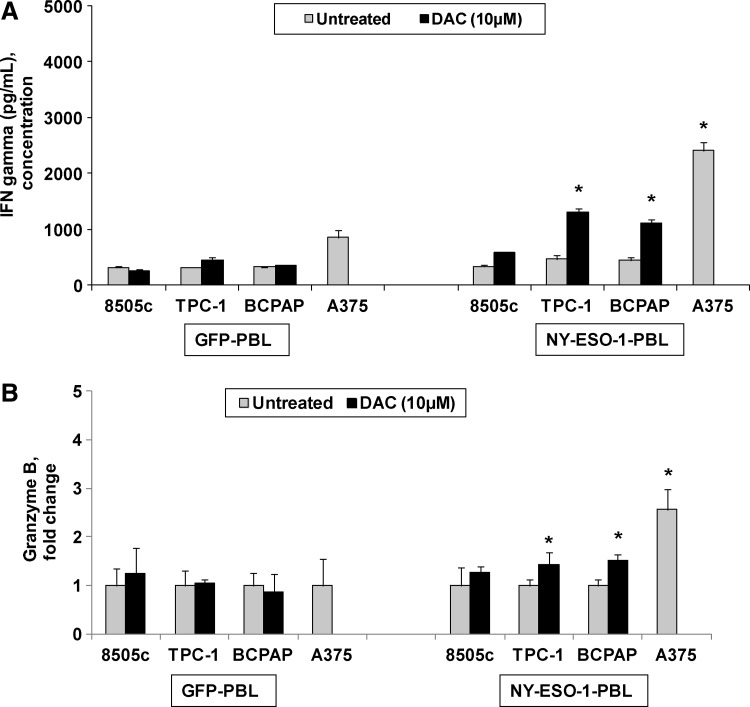
In the next step, we wanted to study whether the increased NY-ESO-1 protein expression obtained in DAC-treated cells is able to activate an immune response through recognition of NY-ESO-1 specific lymphocytes. PBLs were transduced with retroviral vectors encoding a TCR specific for NY-ESO-1 antigen (MSGV1-NY-ESO-1-TCR) and the control GFP-vector. Both these vectors and the PBLs transduction were elaborately described in our previous study (20) HLA A2+ thyroid cell lines, BCPAP, TPC-1, and 8505c, were treated with the demethylating agent DAC and co-cultured with PBLs transduced with NY-ESO-1-TCR and control-GFP for 24 hours. ELISA was then performed to assess IFN-γ release as a surrogate for T-cell recognition of tumor cells. There was significant ∼2.4-fold (BCPAP) to ∼2.8-fold (TPC-1) increase in IFN-γ (Fig. 5A) and ∼1.4-fold (TPC-1) to ∼1.5-fold (BCPAP) increase in Granzyme-B (Fig. 5B) (p<0.05) when NY-ESO-1-T lymphocytes were co-cultured with the thyroid cancer cell lines treated with DAC compared with untreated cells. There was no significant IFN-γ and granzyme-B release from PBLs transduced with NY-ESO-1-TCR in 8505c co-cultures experiments. This data suggests that, in thyroid tumor cells, increased NY-ESO-1 expression through treatment with demethylating agents is able to cause activation of NY-ESO-1-TCR transduced peripheral blood T-cells.
FIG. 5.
Increased NY-ESO-1 expression in thyroid cell lines with the DAC treatment allows recognition by NY-ESO-1-specific T-cell lymphocytes. One hundred thousand thyroid cancer cells were treated with DAC and then co-cultured with an equal number of NY-ESO-1 specific T-cell receptor (TCR)-transduced T lymphocytes for 24 hours. DAC treatment of TPC-1 and BCPAP resulted in a significant increase in (A) interferon-gamma (IFN-γ) (2.4- to 2.8-fold) and (B) granzyme-B levels (∼1.4- to 1.5-fold) in the co-culture media when compared with untreated controls. Although 8505c cells show a mild increase in NY-ESO-1 mRNA and protein, it appears not to be significant enough to result in T-cell activation. *P<0.05 versus untreated control. GFP, green fluorescent protein; PBL, peripheral blood lymphocyte.
Discussion
Using immune-based therapeutics to treat tumors resistant to other interventions is being intensely studied. Anaplastic and recurrent aggressive papillary thyroid cancers currently lack effective treatment. Early studies in thyroid cancer are showing promise using information about specific derangement in a variety of signaling pathways such as the PTEN, MAP kinase, and RAS pathways to target these aggressive cancers. Tumors in general co-opt and then become dependent upon very specific and often small number of altered signaling pathways which then lead to cell cycle progression, angiogenesis, prevention of apoptosis and alterations in the host defense processes (36,37). Initial efforts in thyroid cancer predominantly involved use of agents that are now considered nonspecific inhibitors of BRAF, such as sorafenib and lower potency MAP-ERK kinase (MEK) inhibitors such as selumetinib (5,38). Multiple, small phase-2 clinical trials are currently underway in metastatic radioiodine resistant thyroid cancer using more refined targeted approaches; for example, using vemurafenib, a specific inhibitor of the oncogenic mutant BRAFV600E (39,40). Studies in thyroid cancer patients are not yet mature, making conclusions about efficacy of targeting specific signaling pathways at this early time period is difficult. Based on previous studies in melanoma, it is expected that there might be rapid early responders, but that they will quickly develop resistance over a short period of time due to selective pressure on the tumor cell population.
Interestingly, parallel efforts in melanoma with small molecular BRAF inhibitors and MEK inhibitors have shown that there is interaction of these inhibitors with the immune system, and understanding these interactions is important to uncovering ways to overcome resistance and develop sensible combinatorial strategies. In melanoma studies, targeting the BRAF oncoprotein with PLX4720 resulted in the increase of cell surface expression of melanoma differentiation antigens (MDA) and also enhanced the T-cell recognition of melanoma tumors (30). The opportunity is ripe to transform the care of patients with these aggressive thyroid cancers by exploring rational combinations of molecularly targeted therapies and immunotherapies.
Here we focused on the potential role of immunotherapy as an alternative therapeutic strategy, one that has been used with some success in melanoma (11,41). Next to nothing is known about the effects of the signaling pathway alterations seen in differentiated thyroid cancers on the immune antigens of the thyroid cancer cells. Immune targets can include surface or cytoplasmic antigens, which are different between tumor cells and normal cells. Expression of these immune antigens is low at baseline largely controlled epigenetically through methylation of their promoters. Treatment of the cancer cells with demethylating agents can trigger a significant increase in the expression of these antigens, which then leads to recognition and destruction of the cancer cell by the patient's native immune system. While the native T-cells of any given patient might not recognize these tumor antigens, newer technologies such as transduced TCRs and chimeric antigen receptors are rapidly being developed providing clinicians with tools to attack tumor cells. These immune-mediated reagents are becoming increasingly available, as they have already undergone clinical testing in a number of malignancies with some success (42–44).
NY-ESO-1, one such molecule, is a cytoplasmic highly immunogenic molecule present in many malignant cells and has been the subject of intense translational research in patients with melanoma. Adoptive immunotherapy clinical trials using both tumor specific vaccines and NY-ESO-1 specific TCR transduced T-cells in patients with metastatic melanoma and synovial cell sarcoma showed promising tumor regression without any significant toxicity (21). The NY-ESO-1 antigen has not been well studied in thyroid cancer; only one previous study showed that about one-third of patients with medullary carcinoma have antibodies to NY-ESO-1, implying exposure from their tumors, while only 7% actually expressed the antigen in their tumors (24). We set out to assess whether NY-ESO-1 could be used as a potential immune target in thyroid cancer. We studied a wide variety of differentiated thyroid cancer cells including PTCs, ATCs, and FTCs with known mutations in a variety of signaling pathways. We found that at baseline neither patient PTC samples nor any of the tested thyroid cancer cell lines show NY-ESO-1 gene expression. However, treatment with the DNA demethylating agent, DAC, effectively increased NY-ESO-1 levels in vitro and led to effective recognition and activation by NY-ESO-1 specific T-cells. Furthermore, in vivo treatment with DAC, also leads to detectable tissue expression of NY-ESO-1 mRNA in an orthotopic murine model of PTC. Unfortunately, while the most aggressive ATC cell line studied, had some boost in NY-ESO-1 gene expression with use of demethylating agents in vitro, there was minimal increased in expression in vivo with one week of treatment. Longer or earlier treatment with demethylating agents or alternative ways to increase the expression may be necessary in these ATC cells.
It has been suggested that the BRAFV600E mutation, apart from contributing to tumor aggressiveness, may also promote tumor cell immune escape by decreasing both the expression of immune antigens and the expression of MHC class I molecules on the surface of the cancer cells. In fact, in melanoma BRAF inhibition has resulted in the re-expression of both melanoma differentiation antigens (MDA) and MHC molecules which results in antigen recognition by T-cells (30,35). In our studies, some of the thyroid cancer cell lines (BCPAP, TPC-1, and 8505c) had expression of HLA-A2 protein, though levels were quite dampened in the ATC cell line, which is hemizygous for the BRAFV600E mutation. However, in thyroid cancer cells, BRAF inhibition did not result in any significant increase in the expression of either HLA-A or the target antigen NY-ESO-1 itself indicating that mechanisms may be quite different between melanoma and thyroid cancer. While the induction of peptide-MHC expression is a crucial step in T-cell-mediated immunity, there is the possibility that the expression of inhibitory molecules (e.g. CTLA4, PD-1, PD-L1 and others) will attenuate an antitumor immune response. Investigation into the use of antibodies that target such inhibitory molecules, both as monotherapies and in combination with other therapeutic approaches, is of interest to the field and is actively being pursued.
In conclusion, the results in this study demonstrate that thyroid cancer cells express negligible or no NY-ESO-1 at baseline and that treatment with DAC can increase its expression both in vitro and in vivo. This study demonstrates a potentially new alternative treatment pathway for aggressive thyroid cancers that has not been previously well studied. Further detailed study on NY-ESO-1 expression and utilization in developing immunotherapy, will likely prove valuable, especially in treating certain aggressive radioiodine resistant cancers for which no alternative therapies are currently available.
Acknowledgments
We thank Paul Lin and Gideon Bollag of Plexxikon for providing PLX4720 and sharing their expertise. This work was supported by The National Institute of Health grant to S. Parangi (NIH-NCI R01 1R01CA149738-01A1).
Author Disclosure Statement
All authors certify that they have no competing financial interests pertaining to any of the data or statements given in this article.
References
- 1.Simard EP, Ward EM, Siegel R, Jemal A.2012Cancers with increasing incidence trends in the United States: 1999 through 2008. CA Cancer J Clin 62:118–128 [DOI] [PubMed] [Google Scholar]
- 2.Brown RL.2008Standard and emerging therapeutic approaches for thyroid malignancies. Semin Oncol 35:298–308 [DOI] [PubMed] [Google Scholar]
- 3.Harris PJ, Bible KC.2011Emerging therapeutics for advanced thyroid malignancies: rationale and targeted approaches. Expert Opin Investig Drugs 20:1357–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sugitani I, Miyauchi A, Sugino K, Okamoto T, Yoshida A, Suzuki S.2012Prognostic factors and treatment outcomes for anaplastic thyroid carcinoma: ATC Research Consortium of Japan cohort study of 677 patients. World J Surg 36:1247–1254 [DOI] [PubMed] [Google Scholar]
- 5.Gupta-Abramson V, Troxel AB, Nellore A, Puttaswamy K, Redlinger M, Ransone K, Mandel SJ, Flaherty KT, Loevner LA, O'Dwyer PJ, Brose MS.2008Phase II trial of sorafenib in advanced thyroid cancer. J Clin Oncol 26:4714–4719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wong KK.2009Recent developments in anti-cancer agents targeting the Ras/Raf/MEK/ERK pathway. Recent Pat Anticancer Drug Discov 4:28–35 [DOI] [PubMed] [Google Scholar]
- 7.Mandala M, Voit C.2013Targeting BRAF in melanoma: Biological and clinical challenges. Crit Rev Oncol Hematol 87:239–255 [DOI] [PubMed] [Google Scholar]
- 8.Fedorenko IV, Paraiso KH, Smalley KS.2011Acquired and intrinsic BRAF inhibitor resistance in BRAF V600E mutant melanoma. Biochem Pharmacol 82:201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Romano E, Pradervand S, Paillusson A, Weber J, Harshman K, Muehlethaler K, Speiser D, Peters S, Rimoldi D, Michielin O.2013Identification of multiple mechanisms of resistance to vemurafenib in a patient with BRAFV600E-mutated cutaneous melanoma successfully rechallenged after progression. Clin Cancer Res 19:5749–5757 [DOI] [PubMed] [Google Scholar]
- 10.Callahan MK, Wolchok JD.2013At the bedside: CTLA-4- and PD-1-blocking antibodies in cancer immunotherapy. J Leukoc Biol 94:41–53 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Menzies AM, Long GV.2013New combinations and immunotherapies for melanoma: latest evidence and clinical utility. Ther Adv Med Oncol 5:278–285 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Scanlan MJ, Gure AO, Jungbluth AA, Old LJ, Chen YT.2002Cancer/testis antigens: an expanding family of targets for cancer immunotherapy. Immunol Rev 188:22–32 [DOI] [PubMed] [Google Scholar]
- 13.Caballero OL, Chen YT.2009Cancer/testis (CT) antigens: potential targets for immunotherapy. Cancer Scie 100:2014–2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gnjatic S, Nishikawa H, Jungbluth AA, Gure AO, Ritter G, Jager E, Knuth A, Chen YT, Old LJ.2006NY-ESO-1: review of an immunogenic tumor antigen. Adv Cancer Res 95:1–30 [DOI] [PubMed] [Google Scholar]
- 15.Hemminger JA, Ewart Toland A, Scharschmidt TJ, Mayerson JL, Kraybill WG, Guttridge DC, Iwenofu OH.2013The cancer-testis antigen NY-ESO-1 is highly expressed in myxoid and round cell subset of liposarcomas. Mod Pathol 26:282–288 [DOI] [PubMed] [Google Scholar]
- 16.Weide B, Zelba H, Derhovanessian E, Pflugfelder A, Eigentler TK, Di Giacomo AM, Maio M, Aarntzen EH, de Vries IJ, Sucker A, Schadendorf D, Buttner P, Garbe C, Pawelec G.2012Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J Clinl Oncol 30:1835–1841 [DOI] [PubMed] [Google Scholar]
- 17.Almstedt M, Blagitko-Dorfs N, Duque-Afonso J, Karbach J, Pfeifer D, Jager E, Lubbert M.2010The DNA demethylating agent 5-aza-2′-deoxycytidine induces expression of NY-ESO-1 and other cancer/testis antigens in myeloid leukemia cells. Leuk Res 34:899–905 [DOI] [PubMed] [Google Scholar]
- 18.Natsume A, Wakabayashi T, Tsujimura K, Shimato S, Ito M, Kuzushima K, Kondo Y, Sekido Y, Kawatsura H, Narita Y, Yoshida J.2008The DNA demethylating agent 5-aza-2′-deoxycytidine activates NY-ESO-1 antigenicity in orthotopic human glioma. Int J Cancer 122:2542–2553 [DOI] [PubMed] [Google Scholar]
- 19.Weiser TS, Guo ZS, Ohnmacht GA, Parkhurst ML, Tong-On P, Marincola FM, Fischette MR, Yu X, Chen GA, Hong JA, Stewart JH, Nguyen DM, Rosenberg SA, Schrump DS.2001Sequential 5-Aza-2 deoxycytidine-depsipeptide FR901228 treatment induces apoptosis preferentially in cancer cells and facilitates their recognition by cytolytic T lymphocytes specific for NY-ESO-1. J Immunother 24:151–161 [DOI] [PubMed] [Google Scholar]
- 20.Wargo JA, Robbins PF, Li Y, Zhao Y, El-Gamil M, Caragacianu D, Zheng Z, Hong JA, Downey S, Schrump DS, Rosenberg SA, Morgan RA.2009Recognition of NY-ESO-1+ tumor cells by engineered lymphocytes is enhanced by improved vector design and epigenetic modulation of tumor antigen expression. Cancer Immunol Immunother 58:383–394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Robbins PF, Morgan RA, Feldman SA, Yang JC, Sherry RM, Dudley ME, Wunderlich JR, Nahvi AV, Helman LJ, Mackall CL, Kammula US, Hughes MS, Restifo NP, Raffeld M, Lee CC, Levy CL, Li YF, El-Gamil M, Schwarz SL, Laurencot C, Rosenberg SA.2011Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J Clin Oncol 29:917–924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ebert LM, MacRaild SE, Zanker D, Davis ID, Cebon J, Chen W.2012. A cancer vaccine induces expansion of NY-ESO-1-specific regulatory T cells in patients with advanced melanoma. PloS One 7:e48424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Karbach J, Neumann A, Brand K, Wahle C, Siegel E, Maeurer M, Ritter E, Tsuji T, Gnjatic S, Old LJ, Ritter G, Jager E.2012Phase I clinical trial of mixed bacterial vaccine (Coley's toxins) in patients with NY-ESO-1 expressing cancers: immunological effects and clinical activity. Clin Cancer Res 18:5449–5459 [DOI] [PubMed] [Google Scholar]
- 24.Maio M, Coral S, Sigalotti L, Elisei R, Romei C, Rossi G, Cortini E, Colizzi F, Fenzi G, Altomonte M, Pinchera A, Vitale M.2003Analysis of cancer/testis antigens in sporadic medullary thyroid carcinoma: expression and humoral response to NY-ESO-1. J Clin Endocrinol Metab 88:748–754 [DOI] [PubMed] [Google Scholar]
- 25.Milkovic M, Sarcevic B, Glavan E.2006Expression of MAGE tumor-associated antigen in thyroid carcinomas. Endocr Pathol 17:45–52 [DOI] [PubMed] [Google Scholar]
- 26.Ruschenburg I, Kubitz A, Schlott T, Korabiowska M, Droese M.1999MAGE-1, GAGE-1/-2 gene expression in FNAB of classic variant of papillary thyroid carcinoma and papillary hyperplasia in nodular goiter. Int J Mol Med 4:445–448 [DOI] [PubMed] [Google Scholar]
- 27.Gunda V, Cogdill AP, Bernasconi MJ, Wargo JA, Parangi S.2013Potential role of 5-Aza-2′-deoxycytidine induced MAGE-A4 expression in immunotherapy for anaplastic thyroid cancer. Surgery 154:1456–1462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blank CU, Hooijkaas AI, Haanen JB, Schumacher TN.2011Combination of targeted therapy and immunotherapy in melanoma. Cancer Immunol Immunother 60:1359–1371 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wilmott JS, Long GV, Howle JR, Haydu LE, Sharma RN, Thompson JF, Kefford RF, Hersey P, Scolyer RA.2012Selective BRAF inhibitors induce marked T-cell infiltration into human metastatic melanoma. Clin Cancer Res 18:1386–1394 [DOI] [PubMed] [Google Scholar]
- 30.Boni A, Cogdill AP, Dang P, Udayakumar D, Njauw CN, Sloss CM, Ferrone CR, Flaherty KT, Lawrence DP, Fisher DE, Tsao H, Wargo JA.2010Selective BRAFV600E inhibition enhances T-cell recognition of melanoma without affecting lymphocyte function. Cancer Res 70:5213–5219 [DOI] [PubMed] [Google Scholar]
- 31.Nucera C, Nehs MA, Nagarkatti SS, Sadow PM, Mekel M, Fischer AH, Lin PS, Bollag GE, Lawler J, Hodin RA, Parangi S.2011Targeting BRAFV600E with PLX4720 displays potent antimigratory and anti-invasive activity in preclinical models of human thyroid cancer. Oncologist 16:296–309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Nucera C, Nehs MA, Mekel M, Zhang X, Hodin R, Lawler J, Nose V, Parangi S.2009. A novel orthotopic mouse model of human anaplastic thyroid carcinoma. Thyroid 19:1077–1084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Serrano A, Tanzarella S, Lionello I, Mendez R, Traversari C, Ruiz-Cabello F, Garrido F.2001Rexpression of HLA class I antigens and restoration of antigen-specific CTL response in melanoma cells following 5-aza-2′-deoxycytidine treatment. Int J Cancer 94:243–251 [DOI] [PubMed] [Google Scholar]
- 34.Mora-Garcia Mde L, Duenas-Gonzalez A, Hernandez-Montes J, De la Cruz-Hernandez E, Perez-Cardenas E, Weiss-Steider B, Santiago-Osorio E, Ortiz-Navarrete VF, Rosales VH, Cantu D, Lizano-Soberon M, Rojo-Aguilar MP, Monroy-Garcia A.2006Up-regulation of HLA class-I antigen expression and antigen-specific CTL response in cervical cancer cells by the demethylating agent hydralazine and the histone deacetylase inhibitor valproic acid. J Transl Med 4:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sapkota B, Hill CE, Pollack BP.2013Vemurafenib enhances MHC induction in BRAF homozygous melanoma cells. Oncoimmunology 2:e22890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gottesman MM.2002Mechanisms of cancer drug resistance. Annu Rev Med 53:615–627 [DOI] [PubMed] [Google Scholar]
- 37.Ganss R, Arnold B, Hammerling GJ.2004Mini-review: overcoming tumor-intrinsic resistance to immune effector function. Eur J Immunol 34:2635–2641 [DOI] [PubMed] [Google Scholar]
- 38.Hayes DN, Lucas AS, Tanvetyanon T, Krzyzanowska MK, Chung CH, Murphy BA, Gilbert J, Mehra R, Moore DT, Sheikh A, Hoskins J, Hayward MC, Zhao N, O'Connor W, Weck KE, Cohen RB, Cohen EE.2012Phase II efficacy and pharmacogenomic study of Selumetinib (AZD6244; ARRY-142886) in iodine-131 refractory papillary thyroid carcinoma with or without follicular elements. Clin Cancer Res 18:2056–2065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gild ML, Bullock M, Robinson BG, Clifton-Bligh R.2011Multikinase inhibitors: a new option for the treatment of thyroid cancer. Nat Rev Endocrinol 7:617–624 [DOI] [PubMed] [Google Scholar]
- 40.Chapman PB, Hauschild A, Robert C, Haanen JB, Ascierto P, Larkin J, Dummer R, Garbe C, Testori A, Maio M, Hogg D, Lorigan P, Lebbe C, Jouary T, Schadendorf D, Ribas A, O'Day SJ, Sosman JA, Kirkwood JM, Eggermont AM, Dreno B, Nolop K, Li J, Nelson B, Hou J, Lee RJ, Flaherty KT, McArthur GA.2011Improved survival with vemurafenib in melanoma with BRAF V600E mutation. New Engl J Med 364:2507–2516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kyi C, Postow MA.2013Checkpoint blocking antibodies in cancer immunotherapy. FEBS Letters 588:368–376 [DOI] [PubMed] [Google Scholar]
- 42.Rosenberg SA, Restifo NP, Yang JC, Morgan RA, Dudley ME.2008Adoptive cell transfer: a clinical path to effective cancer immunotherapy. Nat Rev Cancer 8:299–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R.2006Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol 24:5060–5069 [DOI] [PubMed] [Google Scholar]
- 44.Goedegebuure PS, Douville LM, Li H, Richmond GC, Schoof DD, Scavone M, Eberlein TJ.1995Adoptive immunotherapy with tumor-infiltrating lymphocytes and interleukin-2 in patients with metastatic malignant melanoma and renal cell carcinoma: a pilot study. J Clin Oncol 13:1939–1949 [DOI] [PubMed] [Google Scholar]