Abstract
Background: This pilot study was conducted to determine whether a 15-minute bout of moderate-intensity aerobic cycling exercise would affect symptoms (pain and fatigue) and function (Timed 25-Foot Walk test [T25FW] and Timed Up and Go test [TUG]) in people with multiple sclerosis (MS) or chronic fatigue syndrome (CFS), and to compare these results with those of a healthy control group.
Methods: Eight people with MS (Expanded Disability Status Scale score 5–6; Karnofsky score 50–80), eight people with CFS (Karnofsky score 50–80), and eight healthy volunteers participated in the study. Pain and fatigue levels and results of the T25FW and TUG were established at baseline as well as at 30 minutes, 2 hours, and 24 hours following a 15-minute stationary cycling aerobic exercise test. Repeated-measures analysis of variance (ANOVA) and covariance (ANCOVA) were used to analyze the findings over time.
Results: At baseline there were statistically significant differences between groups in fatigue (P = .039), T25FW (P = .034), and TUG (P = .010). A significant group/time interaction emerged for fatigue levels (P= .005). We found no significant group/time interaction for pain levels or function.
Conclusions: Undertaking 15 minutes of moderate-intensity aerobic cycling exercise had no significant adverse effects on pain or function in people with MS and CFS (with a Karnofsky score of 50–80) within a 24-hour time period. These initial results suggest that people with MS or CFS may undertake 15 minutes of cycling as moderate aerobic exercise with no expected negative impact on pain or function.
Multiple sclerosis (MS) is a progressive and disabling neurologic disease resulting from damage to the axons in the central nervous system. It differs between individuals in its clinical manifestations; however, common symptoms include increased pain, fatigue, and mobility problems.1 Chronic fatigue syndrome (CFS), by contrast, is a disorder that cannot be explained by any active medical condition2; however, people with CFS may also present with increased pain, fatigue, and mobility problems. The difference in fatigue between these clinical conditions has been acknowledged in the past3; however, it is unknown what the difference in other symptoms may be.
As MS differs from CFS in its etiology and presentation, it is not appropriate to compare symptoms in MS with symptoms in CFS directly, although both conditions may result in fatigue, pain, and mobility problems. Several weeks of aerobic exercise can be beneficial for people with CFS, including improving fatigue and physical functioning.4 Aerobic exercise over several weeks also has many benefits for people with MS, including improved exercise capacity and fatigue management.5
There is minimal evidence to suggest that short-term exercise has an effect on MS symptoms in the short term; Petruzzello et al.6 found a positive effect on anxiety levels, while Motl et al.7 found improvements in leg spasticity following a short bout of aerobic cycling exercise. In contrast, Mostert and Kesselring8 reported an immediate negative effect following cycling in people with MS, with increased muscle spasms. Qualitative evidence in studies of MS suggests that fatigue9–11 and muscle pain9 may result the day after exercise.
There is minimal evidence of the effect of a short bout of aerobic exercise in CFS. Paul et al.12 found no clear symptom change following 15 minutes of aerobic exercise on a stationary cycle, yet it is acknowledged that symptoms may increase following modest exercise, physical activity, or exercise testing.2 In addition, it has been shown that the physiological response (final heart rate and perceived exertion) to a short bout of exercise may differ between those with CFS and healthy individuals,12 yet results of such comparison between those with MS and healthy individuals have not been reported. This information may be important in prescribing exercise for people with MS. It is important to understand whether exercise has a positive or negative effect on symptoms immediately following exercise.
Participating in exercise over a prolonged period of time may have a positive impact on fatigue, pain, and mobility problems, while helping to prevent comorbidities associated with a lack of exercise.13 However, past evidence is unclear as to whether exercise may increase symptoms (ie, pain or fatigue) immediately following exercise in those with MS and CFS.2,9–11 Indeed, concern about a detrimental effect of exercise may deter people with these diseases from undertaking exercise, despite the absence of strong quantitative evidence that exercise may negatively affect symptoms over a longer period of time. In this study investigating the immediate effect of exercise on people with MS and CFS, we hypothesized that undertaking a 15-minute bout of aerobic exercise would increase pain and fatigue and decrease function for up to 24 hours following exercise.
The specific aim of this pilot study was to evaluate the effect of a 15-minute bout of moderate-intensity aerobic cycling exercise on heart rate and perceived exertion during the exercise and on levels of pain, fatigue, and function up to 24 hours following the exercise session in people with moderately disabling MS or CFS. Results were compared with those of a healthy, age-matched control group. The study was also intended to provide initial data for a future study investigating the immediate effect of a short bout of moderate-intensity aerobic cycling exercise on individuals with MS.
Methods
Design
This was a controlled pilot study with repeated measures over four time points. The study included eight people with MS, eight people with CFS, and eight healthy control (HC) participants. Ethical approval was provided by the West of Scotland Research Ethics Committee, and all participants gave written informed consent. Participants were involved in the study on 2 consecutive days. On day 1, they completed the first of four assessments of pain, fatigue, and function (described in the “Screening and Outcome Measures” section). They then undertook a 15-minute exercise test (described in the “Sub-Anaerobic Threshold Exercise Test” section). To monitor the physiological response to the exercise during the test, participants wore a Polar heart rate (HR) monitor (Polar Electro Oy, Kempele, Finland), allowing HR to be monitored every minute; at each minute, rating of perceived exertion (RPE 0–10)14 was also recorded. Heart rate and RPE scores on termination of the exercise test were analyzed.
Thirty minutes, 2 hours, and 24 hours after the exercise test, participants repeated the four assessments of pain, fatigue, and function.
Participants
Participants with MS and CFS were recruited through the local National Health Service rehabilitation service. People with MS were eligible to take part in the study if they had clinically confirmed MS and an Expanded Disability Status Scale (EDSS) score of 5 to 6 (ie, “ambulatory without aid or rest for about 200 meters; disability severe enough to impair full daily activities” to “intermittent or unilateral constant assistance [cane, crutch, brace] required to walk about 100 meters with or without resting”). People with CFS were eligible if they had been diagnosed with CFS and met both the Centers for Disease Control and Prevention research guidelines for CFS15 and the Canadian clinical guidelines for CFS.16 Healthy control (HC) participants were a convenience sample of volunteers.
To determine a similar level of disability in those with MS and those with CFS, all participants were required to have a Karnofsky performance score of 50 to 80 (ie, “requires occasional assistance, but is able to care for most of his personal needs” to “normal activity with effort; some signs or symptoms of disease”),17 indicative of moderate disability. Participants were eligible if they had an International Physical Activity Questionnaire (IPAQ)18 score indicating low or moderate physical activity. The IPAQ comprises seven questions to ascertain length of time being physically active; the total score indicates low, moderate, or high levels of physical activity.
To reduce some potential confounding factors, people with MS or CFS were excluded if they had experienced exacerbation of their symptoms in the 3 months prior to the study or if they had taken anticonvulsant therapy, disease-modifying drugs, or immune suppressants in the 4 weeks prior to the study. Potential participants were excluded if they were known to be pregnant, were under the age of 18 years, had taken β-blockers in the 4 weeks prior to the study, or had a history of cardiovascular, respiratory, or another neurologic or metabolic disease, any other medical condition, or signs of cognitive difficulties that might affect ability to participate in the study. All participants were clinically stable with no change in drug therapy for 30 days prior to the study.
Screening and Outcome Measures
Demographic data were collected on participants' age, sex, height, weight, and physical activity level using the IPAQ. Psychological status was assessed using the Hospital Anxiety and Depression Scale (HADS),19 which yields a total score of 0 to 42 and individual scores for anxiety (0–21) and depression (0–21); fatigue was assessed using the Fatigue Severity Scale (FSS),20 which ranges in score from 0 to 7. In both scales, higher scores are indicative of higher levels of anxiety and depression and fatigue, respectively. The HADS and FSS have been previously validated in MS and CFS studies.20–24
The effect of the exercise specifically on fatigue and pain was monitored using two separate visual analogue scales (VASs). The scales were 10 cm in length anchored between 0 (no pain/fatigue) and 10 (most severe pain/fatigue imaginable).25,26 Participants were asked to mark a line through the scale to indicate current pain and fatigue levels. The distance (in millimeters) of this mark from the 0 anchor was then used for analysis.
The Timed 25-Foot Walk test (T25FW)27 and the Timed Up and Go test (TUG)28 were performed twice at each time point, with mean scores analyzed. Participants were given standardized instructions. The T25FW was performed by participants walking across a flat marked 25-foot course, while the TUG involved participants standing up from a standard chair, walking around a cone placed 3 m ahead of them, and returning to the chair as per previous protocol.29
Sub-Anaerobic Threshold Exercise Test
A simple, clinically applicable exercise field test that does not require the intricate equipment of a maximal oxygen consumption exercise test was performed, the Sub-Anaerobic Threshold Exercise Test (SATET).30 The SATET is based on normative data from healthy populations and was performed on an exercise cycle, with participants maintaining a wattage of 90% of their predicted work rate. The SATET protocol has been safely used in a clinical study involving people with CFS12 and was considered a clinically usable submaximal exercise test to standardize prescription of effort level during the test across all the participants. Participants' weight, gender, and age were used to determine work rate for the SATET, following the standardized protocol.30 Participants cycled for 15 minutes at 90% of their calculated anaerobic threshold to achieve the required work rate. They were encouraged to keep within 5 watts of the required power output to maintain a constant work rate. Heart rate response was recorded each minute to ensure safety—that is, that they worked at a level lower than their age-predicted maximum HR (ie, 220−age).31 Final HR response for each group was reported, as was a mean age-predicted maximum final HR.
Statistical Analysis
Data were analyzed using SPSS version 18 (SPSS Inc, Chicago, IL). Descriptive statistics were established across all groups; an analysis of variance (ANOVA) was employed to determine any differences at baseline and for resting and final HR and RPE response. Where appropriate, the Tukey multiple comparison post hoc test was performed between groups. As pretreatment scores for fatigue, T25FW, and TUG varied widely across the three groups, a repeated-measures analysis of covariance (ANCOVA), with pretreatment scores as the covariate,32,33 was performed for fatigue, T25FW, and TUG; pretreatment pain scores were not significantly different, so a repeated-measures ANOVA was performed for pain. This allowed for comparisons to be made between groups and over time (30-minute, 2-hour, and 24-hour assessments). The ANCOVA was performed with a Greenhouse-Geisser correction, and statistical significance is reported inclusive of a Bonferroni correction. For variables where significant group/time results emerged, effect size (ηp2) and power were calculated. To provide data to power a future study, we noted the variation in T25FW results for the MS group and assumed a desired power of 80% at the 5% level of significance.
Results
Baseline Comparison Between Groups
Eight MS participants, eight CFS participants, and eight HC participants were involved in the study. The groups were matched for sex and age. At baseline there were statistically significant differences between the three groups. For IPAQ scores, the HC group reported higher activity levels than both the MS and CFS groups. The total HADS score and HADS Depression score showed statistically significant differences, with both the MS group and the CFS group reporting higher scores than the HC group for this self-reported scale (Table 1).
Table 1.
Demographic details, baseline characteristics, and physiological response to exercise stimulus
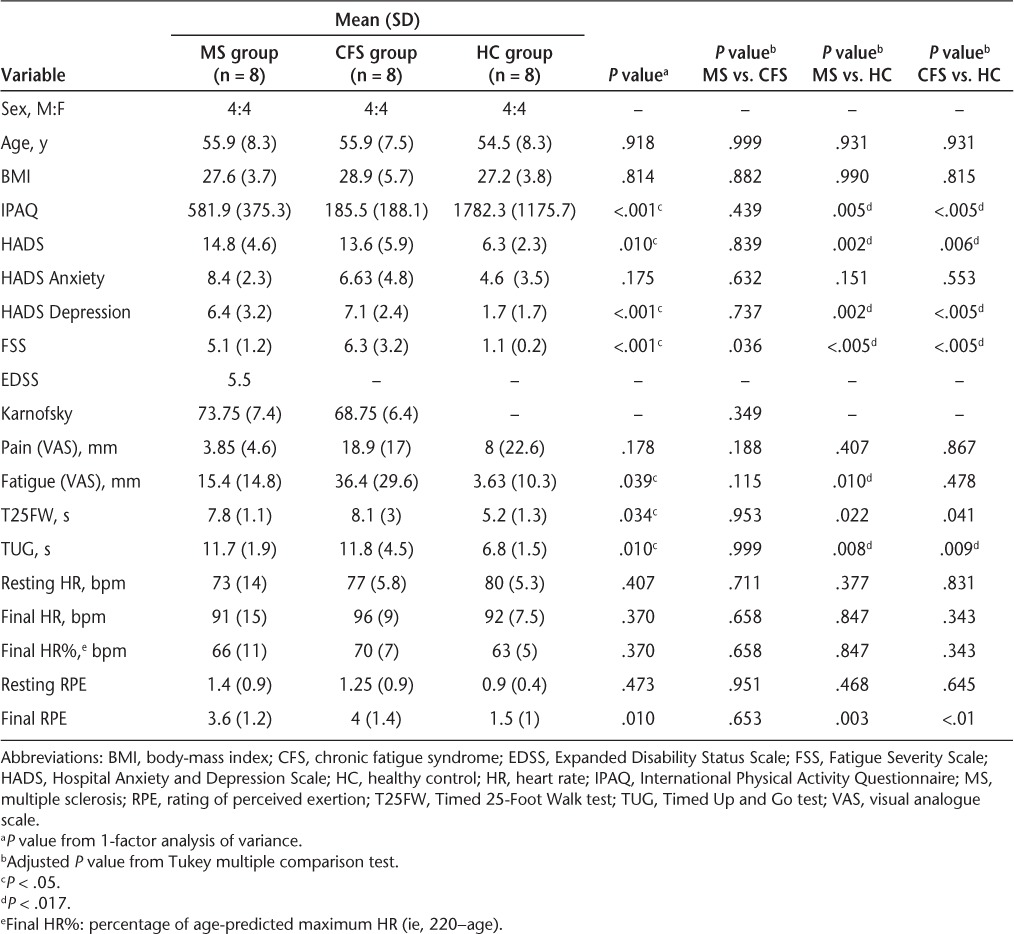
For fatigue (VAS), T25FW, and TUG, the difference between the HC group and both clinical groups was statistically significant at baseline (Table 1); no significant group differences were present for pain. There was no significant difference between the two clinical groups for any outcomes.
Effect of Exercise on Pain, Fatigue, and Function over Time
Results over time are presented in Table 2. The ANOVA for pain found a significant change over time, emerging as an increase in pain levels, but there was no significant group/time interaction.
Table 2.
Summary of results over time, including comparison between groups over time
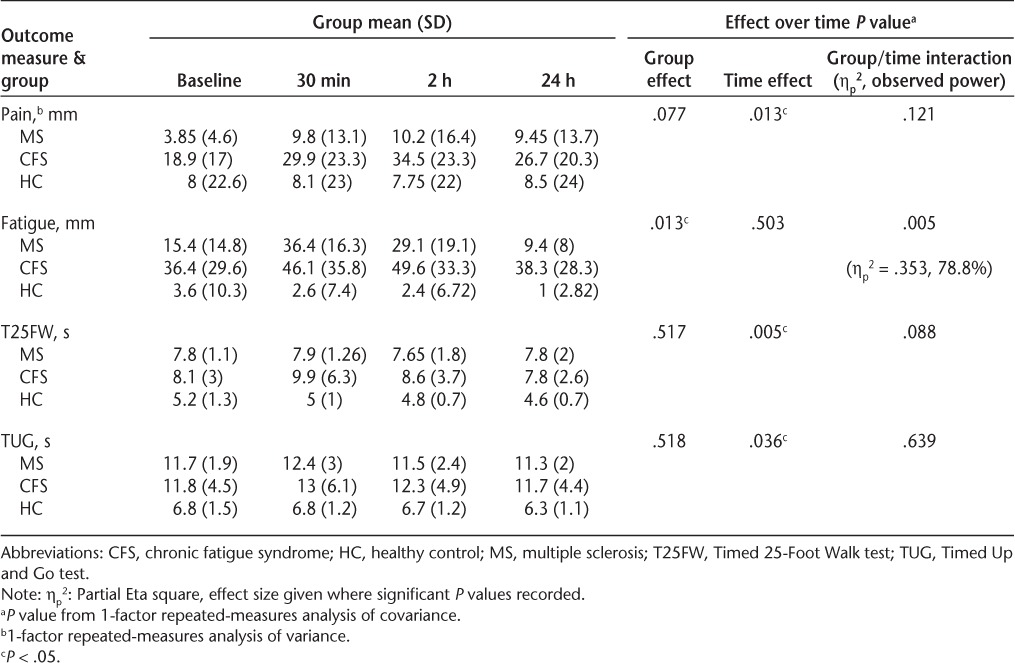
ANCOVA was performed for the other outcomes. A significant group/time interaction emerged for fatigue scores, resulting in a large effect size (P = .005, ηp2 = .353). Subsequent analysis suggested that a decrease in fatigue emerged in the MS and HC groups; however, in the CFS group there appeared to be an increase in fatigue over time. There was a significant time effect and a near-significant group/time interaction for the T25FW, with scores decreasing over time. For the TUG only a significant time effect emerged, with scores decreasing over time.
Effect of Exercise on HR and RPE
Heart rate and RPE were monitored throughout the exercise test. In addition, the final HR was converted to a percentage of age-predicted HR and is presented in Table 1. Percentage of age-predicted HR and RPE at the end of the 15-minute exercise session were, respectively, 66% (±11%) and 3.6 (±1.2) in the MS group, 63% (±5%) and 1.5 (±1) in the HC group, and 70% (±7%) and 4 (±1.4) in the CFS group. A one-way ANOVA revealed no differences in HR between groups; however, a significant difference emerged between the three groups for RPE scores (P = .01), with both clinical groups reporting higher RPE scores at the end of the test. The Tukey test revealed that these differences were only between the HC and MS groups and between the HC and CFS groups (Table 1).
Two participants with MS (EDSS = 6; Karnofsky score = 70) and three participants with CFS (Karnofsky score = 60 [n = 1], 70 [n = 2]) were unable to maintain the desired wattage to achieve a 90% work rate; however, they completed the exercise test to the best of their ability.
Discussion
This pilot study was intended to determine whether undertaking 15 minutes of moderate-intensity aerobic activity would increase pain and fatigue and decrease function (measured with the T25FW and the TUG) in people with MS and CFS. Comparison was also made with an HC group. The initial results were intended to provide data for a future study on MS and to establish the effect of exercise on HR and perceived exertion in all participants.
In contrast to our hypothesis, whereby we anticipated an immediate (within 24 hours) negative impact of exercise on symptoms and function in people with MS and CFS based on previous research,2,9–11 we found that for people with MS there was no increase in fatigue levels or decrease in function following the exercise test; fatigue levels appeared to increase for those with CFS. Additionally, there was a slight trend toward increased pain levels in both the MS and CFS groups. One previous study found that following an incremental bicycle exercise test, fatigue levels may return to baseline levels the day after exercise for people with CFS34; another CFS study found that aerobic exercise did not immediately affect CFS symptoms. Our pilot results indicate that further study is required. Despite studies6,7 showing that cycling exercise may result in improved anxiety levels and leg spasticity, we are unaware of any previous research on the immediate effect of an incremental cycle exercise test on pain, fatigue, and function in people with MS.
Taken together, these results are important because if people with MS and CFS do not experience any detrimental effects on fatigue and function following short durations of exercise, this may encourage maintenance of exercise programs and thus allow those with MS and CFS to experience the long-term benefits of exercise. However, further investigation is required of the impact of exercise on fatigue, pain, and function.
The baseline results suggested differences in fatigue and mobility between the three groups. Differences in fatigue have been noted between MS and CFS in the past.3 Fatigue, as measured by the FSS, was significantly higher in the clinical groups compared with the HC group; when measured with the fatigue VAS, fatigue was significantly higher in the MS group compared with the HC group, but not compared with the CFS group. The different recall period of the two fatigue measures used in this study (ie, FSS recalled over 7 days, while fatigue VAS representing current fatigue level) may also provide some explanation of these differences. This was the first study to report on functional differences between CFS and MS in comparison with an HC group; both the T25FW and TUG tests were performed more slowly at baseline in the clinical groups compared with the HC group.
The SATET test was used to provide a generic exercise stimulus; the test provided the opportunity to monitor HR and RPE. The resting HR and RPE were similar across groups before the beginning of the exercise test. The final HR response, at least for those with CFS, is similar to that when the SATET test was previously used in a CFS population (around 70% age-predicted maximum HR).12 In MS there is less comparable evidence.
In contrast to HR response, RPE response, on termination of the exercise, was different between the groups, with the clinical groups reporting more perceived exertion. Thus the results imply that those with MS and CFS perceive their effort during exercise differently from a healthy population. Similarly, Paul et al.12 found that those with CFS displayed a similar HR response as healthy participants, yet reported higher RPE levels during a 15-minute SATET exercise test compared with a healthy group. Research may be required to better understand perceived exertion response to exercise in these patient groups.
Although it is acknowledged that using age-predicted maximal HR is less accurate than using laboratory measures of exercise capacity, the former is more commonly used in clinical practice.31 Thus our research tentatively suggests that 15 minutes of aerobic cycling, at a work rate producing an HR of about 66% to 70% of age-predicted HR, may be appropriate for those with MS and CFS who have a Karnofsky performance score of 50 to 80. Further work is needed to clarify the training intensity that people with MS or CFS at different levels of disability should achieve for health benefits, while minimizing unwanted side effects, and whether this response is similar when exercise is undertaken more frequently. This knowledge would have clinical applicability in the provision of objective recommendations for exercise prescription in MS and CFS. However, five of the participants were unable to maintain the workload suggested by the SATET protocol, which is a limitation of the exercise protocol. The SATET protocol was originally standardized for use in healthy individuals, although it has subsequently been successfully used in a CFS population.12 Our results imply that it may also be a safe and appropriate exercise field test in an MS population.
These results enhance our understanding of the consequences of short-term aerobic exercise in these clinical groups. However, this study has several limitations. Participant numbers were small, although they are comparable to past similar studies in people with CFS,12 and we used an established field test to prescribe exercise intensity, rather than basing exercise prescription on results of a maximal aerobic capacity test.
An aim of this study was to establish the required number of participants in a future similar study investigating the immediate effect of a short bout of moderate-intensity aerobic cycling exercise on individuals with MS; our data indicate that an MS group of 72 subjects is required in order to detect a change of 0.6 second in the T25FW test at the 5% level of significance at a power of 80%. This level of change has recently been suggested as clinically significant for people with MS.35 Participants with MS and CFS in this study were moderately disabled, and those with MS who were on disease-modifying therapy were not included; thus the pilot sample is not representative of the wider MS and CFS populations.
This study provides important messages for those with MS and CFS and for health professionals involved in their care. Undertaking aerobic training on a stationary cycle for 15 minutes at a work rate sufficient to produce an HR of about 66% to 70% of the maximum age-predicted HR and an RPE of about 4 does not significantly affect pain and function in people with MS and CFS.
PracticePoints.
Exercise offers many benefits to people with MS or chronic fatigue syndrome (CFS).
This pilot study suggests that undertaking 15 minutes of moderate-intensity aerobic cycling exercise may not exacerbate pain or function, within a 24-hour period, in those with MS or CFS.
These initial results are promising for understanding the consequences of exercise among people with MS and CFS.
Acknowledgments
We are grateful to the staff of the Douglas Grant Rehabilitation Unit for allowing use of their facilities, in particular Alan Izat and Anne Thompson, and to the participants themselves for their patience and interest.
Footnotes
Financial Disclosures: The authors have no conflicts of interest to disclose.
Funding/Support: This study was funded by the National Health Service Ayrshire and Arran, Bevan Endowment Fund, Ayrshire and Arran Branch of the Multiple Sclerosis Society, and by a grant from the Medical Development Fund, University of Glasgow.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Nijs J, Aelbrecht S, Meeus M, Van Oosterwijck J, Zinzen E, Clarys P. Tired of being inactive: a systematic literature review of physical activity, physiological exercise capacity and muscle strength in patients with chronic fatigue syndrome. Disabil Rehabil. 2011;33:1493–1500. doi: 10.3109/09638288.2010.541543. [DOI] [PubMed] [Google Scholar]
- 3.Vercoulen J, Bazelmans E, Swanink CMA. Physical activity in chronic fatigue syndrome: assessment and its role in fatigue. J Psychiatr Res. 1997;31:661–673. doi: 10.1016/s0022-3956(97)00039-3. et al. [DOI] [PubMed] [Google Scholar]
- 4.Edmonds M, McGuire H, Price J. Exercise therapy for chronic fatigue syndrome. Cochrane Database Syst Rev. 2004;(3):CD003200. doi: 10.1002/14651858.CD003200.pub2. [DOI] [PubMed] [Google Scholar]
- 5.Dalgas U, Stenager E, Ingemann-Hansen T. Multiple sclerosis and physical exercise: recommendations for the application of resistance-, endurance- and combined training. Mult Scler. 2008;14:35–53. doi: 10.1177/1352458507079445. [DOI] [PubMed] [Google Scholar]
- 6.Petruzzello SJ, Snook EM, Gliottoni RC, Motl RW. Anxiety and mood changes associated with acute cycling in persons with multiple sclerosis. Anxiety Stress Coping. 2009;22:297–307. doi: 10.1080/10615800802441245. [DOI] [PubMed] [Google Scholar]
- 7.Motl RW, Snook EM, Hinkle ML. Effect of acute unloaded leg cycling on spasticity in individuals with multiple sclerosis using anti-spastic medications. Int J Neurosci. 2007;117:895–901. doi: 10.1080/00207450600910671. [DOI] [PubMed] [Google Scholar]
- 8.Mostert S, Kesselring J. Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult Scler. 2002;8:161–168. doi: 10.1191/1352458502ms779oa. [DOI] [PubMed] [Google Scholar]
- 9.Dodd KJ, Taylor NF, Denisenko S, Prasad D. A qualitative analysis of a progressive resistance exercise programme for people with multiple sclerosis. Disabil Rehabil. 2006;28:1127–1134. doi: 10.1080/09638280500531842. [DOI] [PubMed] [Google Scholar]
- 10.Smith C, Hale L, Olson K, Schneiders AG. How does exercise influence fatigue in people with multiple sclerosis? Disabil Rehabil. 2009;31:685–692. doi: 10.1080/09638280802273473. [DOI] [PubMed] [Google Scholar]
- 11.Learmonth YC, Marshall-McKenna R, Paul L, Mattison P, Miller L. A qualitative exploration of the impact of a 12-week group exercise class for those moderately affected with multiple sclerosis. Disabil Rehabil. 2012;35:81–88. doi: 10.3109/09638288.2012.688922. [DOI] [PubMed] [Google Scholar]
- 12.Paul L, Wood L, Maclaren W. The effect of exercise on gait and balance in patients with chronic fatigue syndrome. Gait Posture. 2001;14:19–27. doi: 10.1016/s0966-6362(00)00105-3. [DOI] [PubMed] [Google Scholar]
- 13.Wen CP, Wu X. Stressing harms of physical inactivity to promote exercise. Lancet. 2012;380:192–193. doi: 10.1016/S0140-6736(12)60954-4. [DOI] [PubMed] [Google Scholar]
- 14.Borg GA. Psychophysical bases of perceived exertion. Med Sci Sports Exerc. 1982;14:377–381. [PubMed] [Google Scholar]
- 15.Fukuda K, Strauss SE, Hickie I, Sharpe MC, Dobbins JG, Komaroff A. The chronic fatigue syndrome. Ann Intern Med. 1994;1:67–84. doi: 10.7326/0003-4819-121-12-199412150-00009. [DOI] [PubMed] [Google Scholar]
- 16.Carruthers BM, Jain AK, De Meirleir KL. Myalgic encephalomyelitis/chronic fatigue syndrome. J Chron Fatigue Syndrome. 2003;11:7–115. et al. [Google Scholar]
- 17.Crooks V, Waller S, Smith T, Hahn TJ. The use of the Karnofsky Performance Scale in determining outcomes and risk in geriatric outpatients. J Gerontol. 1991;46:M139–M144. doi: 10.1093/geronj/46.4.m139. [DOI] [PubMed] [Google Scholar]
- 18.Craig CL, Marshall AL, Sjostrom M. International Physical Activity Questionnaire: 12-country reliability and validity. Med Sci Sports Exerc. 2003;35:1381–1395. doi: 10.1249/01.MSS.0000078924.61453.FB. et al. [DOI] [PubMed] [Google Scholar]
- 19.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–370. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 20.Krupp LB, LaRocca NG, Muir-Nash J, Steinberg AD. The Fatigue Severity Scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. [DOI] [PubMed] [Google Scholar]
- 21.McCue P, Martin C, Buchanan T, Rodgers J, Scholey A. An investigation into the psychometric properties of the Hospital Anxiety and Depression Scale in individuals with chronic fatigue syndrome. Psychol Health Med. 2003;8:425–439. doi: 10.1080/1354850310001604568. [DOI] [PubMed] [Google Scholar]
- 22.Honarmand K, Feinstein A. Validation of the Hospital Anxiety and Depression Scale for use with multiple sclerosis patients. Mult Scler. 2009;15:1518–1524. doi: 10.1177/1352458509347150. [DOI] [PubMed] [Google Scholar]
- 23.Flachenecker P, Kümpfel T, Kallmann B. Fatigue in multiple sclerosis: a comparison of different rating scales and correlation to clinical parameters. Mult Scler. 2002;8:523–526. doi: 10.1191/1352458502ms839oa. et al. [DOI] [PubMed] [Google Scholar]
- 24.Jason L, Evans JM, Brown M. Fatigue scales and chronic fatigue syndrome: issues of sensitivity and specificity. Disabil Stud Q. 2011;31:1375. et al. [PMC free article] [PubMed] [Google Scholar]
- 25.Castro-Sánchez AM, Matarán-Peñarrocha GA, Lara-Palomo I, Saavedra-Hernández M, Arroyo-Morales M, Moreno-Lorenzo C. Hydrotherapy for the treatment of pain in people with multiple sclerosis: a randomized controlled trial. Evid Based Complement Alternat Med. 2012;2012:1–8. doi: 10.1155/2012/473963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kersten P, Küçükdeveci A, Tennant A. The use of the Visual Analogue Scale (VAS) in rehabilitation outcomes. J Rehabil Med. 2012;44:609–610. doi: 10.2340/16501977-0999. [DOI] [PubMed] [Google Scholar]
- 27.Cutter GR, Baier ML, Rudick RA. Development of a multiple sclerosis functional composite as a clinical trial outcome measure. Brain. 1999;122(pt 5):871–882. doi: 10.1093/brain/122.5.871. et al. [DOI] [PubMed] [Google Scholar]
- 28.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc. 1991;39:142–148. doi: 10.1111/j.1532-5415.1991.tb01616.x. [DOI] [PubMed] [Google Scholar]
- 29.Learmonth YC, Paul L, Miller L, Mattison P, McFadyen AK. The effects of a 12-week leisure centre-based, group exercise intervention for people moderately affected with multiple sclerosis: a randomized controlled pilot study. Clin Rehabil. 2012;26:579–593. doi: 10.1177/0269215511423946. [DOI] [PubMed] [Google Scholar]
- 30.Nashef L, Lane RJ. Screening for mitochondrial cytopathies: the sub-anaerobic threshold exercise test (SATET) J Neurol Neurosurg Psychiatry. 1989;52:1090–1094. doi: 10.1136/jnnp.52.9.1090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Robergs RA, Landwehr R. The surprising history of the “HRmax = 220-age” equation. J Exerc Physiol. 2002;5:1–10. [Google Scholar]
- 32.Senn S. Change from baseline and analysis of covariance revisited. Stat Med. 2006;25:4334–4344. doi: 10.1002/sim.2682. [DOI] [PubMed] [Google Scholar]
- 33.Van Breukelen GJP. ANCOVA versus change from baseline had more power in randomized studies and more bias in nonrandomized studies. J Clin Epidemiol. 2006;59:920–925. doi: 10.1016/j.jclinepi.2006.02.007. [DOI] [PubMed] [Google Scholar]
- 34.Bazelmans E, Bleijenberg G, Voeten MJM, Van der Meer JWM, Folgering H. Impact of a maximal exercise test on symptoms and activity in chronic fatigue syndrome. J Psychiatr Res. 2005;59:201–208. doi: 10.1016/j.jpsychores.2005.04.003. [DOI] [PubMed] [Google Scholar]
- 35.Learmonth YC, Dlugonski D, Pilutti LA, Sandroff BM, Motl RW. The reliability, precision and clinically meaningful change of walking assessments in multiple sclerosis. Mult Scler. 2013;19:1784–1791. doi: 10.1177/1352458513483890. [DOI] [PubMed] [Google Scholar]


