Abstract
Background: This study was undertaken to determine how frequently patients receiving natalizumab for multiple sclerosis (MS) experience recrudescence of their MS symptoms at the end of the dosing cycle.
Methods: One hundred consecutive MS patients receiving natalizumab completed a survey evaluating changes in symptoms during the natalizumab dosing cycle. Ninety-one patients also completed questionnaires at two time points: the first week after natalizumab infusion and the last week of the dosing cycle. These included the Multiple Sclerosis Quality of Life–54 (MSQOL-54), Fatigue Visual Analog Scale (VAS), Fatigue Severity Scale (FSS), and Beck Depression Inventory–II (BDI-II).
Results: End of dosing interval (EDI) symptoms were reported as currently being experienced by 57% of respondents. An additional 10% reported that they previously experienced that phenomenon, but not currently, and 33% reported never experiencing this. In those with EDI symptoms, they began to occur a median of 21 days after infusion and improved again a median of 1 day after infusion. The most common symptoms reported were fatigue, weakness, walking impairment, and cognitive difficulties. No specific demographic or disease characteristics were associated with this phenomenon. In the subgroup with EDI symptoms, the MSQOL-54, Fatigue VAS, FSS, and BDI-II scores were all significantly worse in the last week of the dosing cycle when compared with the first week. No difference was seen in these scores between first and last week in the subgroup not experiencing symptom recrudescence.
Conclusions: Recrudescence of fatigue, weakness, walking impairment, or cognitive difficulties at the end of the dosing cycle occurs in about two-thirds of MS patients receiving natalizumab.
Natalizumab (Tysabri, Biogen Idec, Cambridge, MA) is a monoclonal antibody that binds to α4 integrin (also called very late antigen-4) on the cell surface of all leukocytes except neutrophils. It reduces migration of leukocytes into the central nervous system (CNS), and significantly reduces the relapse rate and risk of disability accrual in patients with relapsing forms of multiple sclerosis (MS).1 Furthermore, some natalizumab-treated patients experience subjective improvement in functional status, mood, fatigue level, and cognitive function.2–6 Natalizumab is administered as a 300-mg intravenous infusion every 4 weeks. The mean half-life of natalizumab in MS patients is 11 days (SD 4 days).7 In a typical patient at steady state, 24% of the dose remains at the time the patient is re-dosed 4 weeks later.7 However, α4 integrin saturation remains greater than 70% at 4 weeks after infusion.8
Several studies have evaluated the effect of natalizumab on quality of life, fatigue, and cognitive function. Rudick et al.2 found that natalizumab treatment resulted in significantly more improvement in Physical and Mental Component Summary scores from the 36-item Short Form Health Status Survey (SF-36) compared with placebo. Iaffaldano et al.6 reported a prospective open-label study of patients going on natalizumab in which patients had periodic cognitive testing and Fatigue Severity Scale (FSS) measurement. They found a decrease in the Cognitive Impairment Index, Beck Depression Inventory (BDI) score, and FSS score in patients while on natalizumab treatment compared with the pre-natalizumab condition. Portaccio et al.9 performed a small, nonrandomized study of patients going on natalizumab or interferon beta (IFNβ), and found a lower rate of cognitive worsening in the natalizumab group relative to the IFNβ-treated group. Yildiz et al.10 tested patients already receiving natalizumab at two time points a year apart, and found that fatigue scores actually worsened over the interval despite a very low relapse rate. However, none of these studies evaluated changes in symptoms within the natalizumab dosing cycle.
We have observed clinically that a significant number of natalizumab-treated MS patients report feeling more of their previously experienced MS symptoms at the end of their dosing cycle, and that these symptoms abate shortly after receiving their next natalizumab infusion. Though commonly experienced, this phenomenon of end of dosing interval (EDI) symptoms has not been previously studied. We performed a cross-sectional survey and cohort study of natalizumab-treated MS patients to determine the prevalence of this phenomenon, and to determine whether subjective differences in fatigue, mood, and quality of life (QOL) scores can be detected between the beginning and end of the natalizumab dosing cycle.
Patients and Methods
Patients
Consecutive patients receiving natalizumab to treat a relapsing form of MS were recruited from the Johns Hopkins Neurology infusion center from July through October 2012. Patients were excluded from participation if they were under 18 years of age, had received less than 5 consecutive cycles of natalizumab, had experienced a recent infection or MS relapse, or had cognitive impairment sufficient to interfere with the completion of the surveys.
Assessments
Participants completed a general survey on their MS history, which included a question about EDI symptoms. If they answered that they did experience EDI symptoms, an additional survey form was given with specific questions about this phenomenon.
A subset of patients also agreed to enter a cohort study and complete additional questionnaires. Participants were asked to rate their symptoms for the prior week at two time points: 7 days after receiving natalizumab (reflecting the week of peak natalizumab level) and on the day of infusion (reflecting the week of lowest natalizumab level). The questionnaires included the Multiple Sclerosis Quality of Life–54 (MSQOL-54), a 54-question survey that includes the SF-36 and additional MS-specific questions.11 The primary outcomes of this survey are composite scores for “physical health” and “mental health,” which range from 0 to 100 with higher numbers indicating better QOL.
Fatigue was assessed with two instruments: a visual analog scale (VAS) and the FSS.12 First, patients were asked to mark their fatigue level on a 100-mm line, yielding a score from 0 (no fatigue) to 100 (worst fatigue imaginable) for the prior week. The FSS is a 9-question survey in which each question is scored from 1 to 7 with higher numbers indicating more severe fatigue. Symptoms of depression were measured using the Beck Depression Inventory–II (BDI-II).13 The BDI-II is a standard 21-question instrument in which the score for each question ranges from 0 to 3, leading to a range of possible scores from 0 to 63.
Statistical Analyses
The demographic and disease characteristics were compared between those who never experienced symptom recrudescence and those who ever experienced symptom recrudescence (currently or previously). A t test was used to compare continuous variables, and a χ2 test was used to compare binomial variables individually. In addition, a multivariate logistic regression model was generated using the following covariates: age, gender, race (white vs. nonwhite), body-mass index (BMI), number of natalizumab doses, total number of relapses, mean number of relapses per year while on natalizumab, self-reported fatigue, self-reported cognitive dysfunction, history of depression, need to use an assistive device to walk, and smoking status. The MSQOL-54 data were analyzed by calculating the Physical Health Composite Score and the Mental Health Composite Score. The mean Physical and Mental Health Composite Scores, fatigue VAS, FSS, and BDI-II were compared between the time of peak and trough natalizumab effect using a paired t test. Data were also analyzed by generating a “difference score” for each outcome by subtracting the value at the time of peak natalizumab effect from the value at the time of trough natalizumab effect. The difference scores for the outcomes were used in a regression model to evaluate whether disease or demographic factors influenced the change in score. Also, an analysis of covariance (ANCOVA) model was used to analyze whether covariates modified the effect of the presence of EDI symptoms on change in outcomes. Analyses were performed using STATA 10.0 (StataCorp, College Station, TX).
Study Protocol Approval and Patient Consent
The study protocol was approved by the Johns Hopkins Institutional Review Board. Written informed consent was obtained from all participants.
Results
Cross-Sectional Survey
One hundred five consecutive patients receiving natalizumab for a relapsing form of MS were approached to participate. One hundred out of 105 (95%) agreed to complete the survey. Demographics of the cross-sectional cohort are shown in Table 1. Patients were interviewed and their charts were reviewed at the time of enrollment to ensure that they had not experienced an infection within the last 30 days or an MS relapse within the previous 60 days. Those with a recent infection or relapse were excluded from participation. Patients were enrolled only if they had received 5 or more previous natalizumab infusions so that they had at least 5 months of experience in which to potentially identify any EDI symptoms.
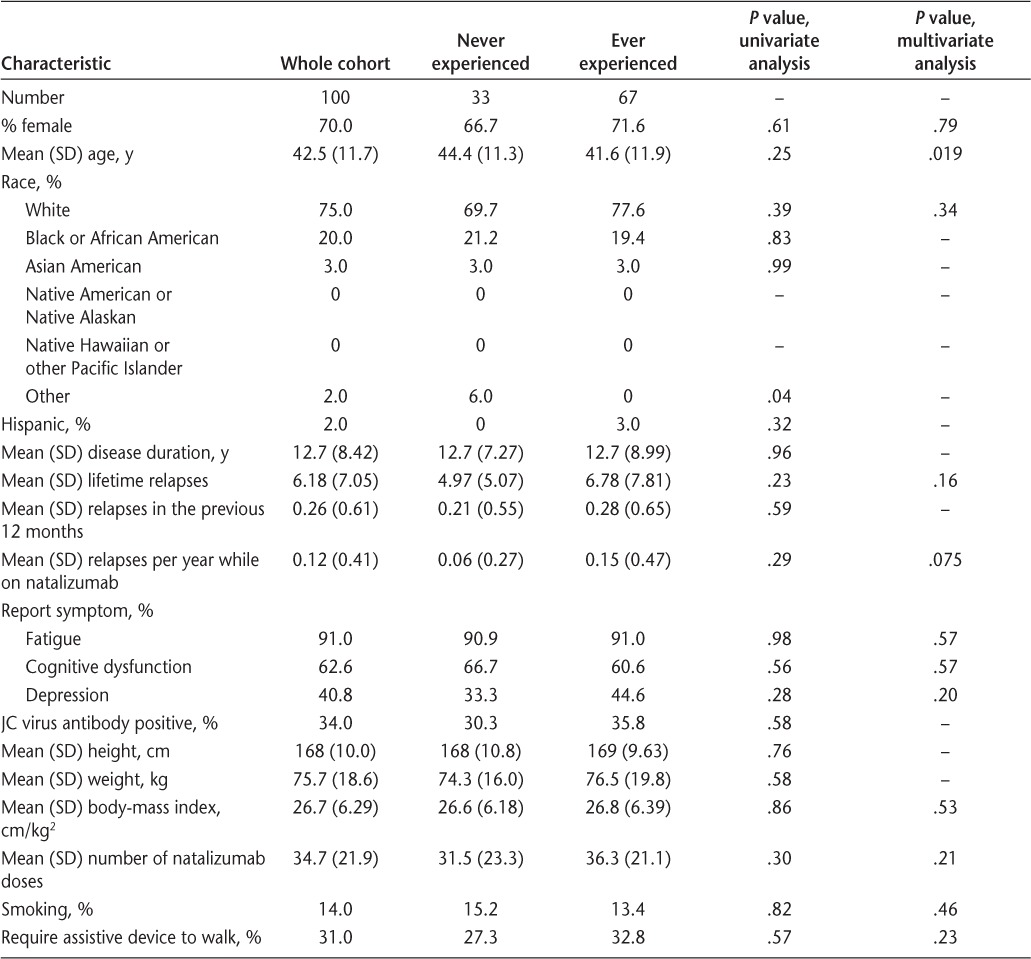
Table 1.
Demographic and disease characteristics of the whole cohort and subgroups: those who never experienced symptom recrudescence and those who ever experienced it (currently and previously)
When asked whether they felt worse at the end of the natalizumab dosing cycle than at other times during the cycle, 57% affirmed that they did currently experience that. An additional 10% reported that they previously experienced that phenomenon, but not currently, and 33% reported never experiencing that phenomenon. Among those reporting EDI symptoms, the phenomenon was present from the beginning of natalizumab use in 56% and it began later in 44%. Of those who did not experience it from the beginning, it started a median of 4 months (range, 0–18 months) after natalizumab initiation.
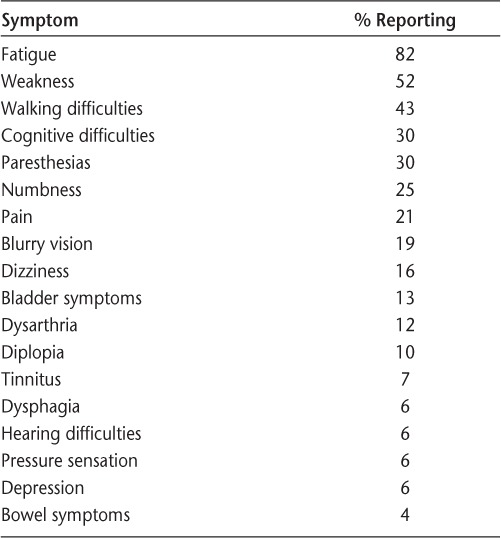
The timing of symptom change was assessed. In the group reporting EDI symptoms, they began to appear a median of 21 days (range, 12–49 days) after infusion. After receiving natalizumab, symptoms were reported to begin improving after a median of 1 day (range, 0–12 days). Among those reporting EDI symptoms, the most common symptom to recur at the end of the dosing cycle was fatigue (82%), followed by weakness (52%), walking difficulties (43%), cognitive difficulties (30%), and paresthesias (30%) (Table 2). The severity of the symptoms was categorized as mild by 53%, moderate by 42%, and severe by 5%. When asked whether this had improved over time, 37% reported that it had improved over time, 9% reported that it had worsened over time, and 54% reported no significant change. Analyses were performed to determine whether there was any difference in demographic or disease characteristics between the group of patients who ever experienced symptom recrudescence and those who never experienced it. These data are summarized in Table 1. In the univariate analysis, no specific factors were found to associate with symptom recrudescence, including age, gender, race, disease duration, number of relapses, self-reported fatigue, self-reported cognitive dysfunction, history of depression, JC virus antibody status, BMI, number of natalizumab doses, smoking status, or need to use an assistive device to walk. A multivariate logistic regression model was also used to test the association between demographic/disease characteristics and proportion experiencing EDI symptoms. In this model, keeping other covariates constant, age had a significant negative association with the presence of EDI symptoms (odds ratio, 0.94 per year; 95% confidence interval [CI], 0.897–0.990; P = .019).
Table 2.
Frequency of symptoms reported to recur at the end of the natalizumab dosing cycle by those who experience symptom recrudescence
At the direction of their treating physician, a subset of the patients (n = 21) was receiving natalizumab every 6 or 8 weeks instead of the typical every 4 weeks. The effectiveness of this extended dosing interval has not been studied, and this is an off-label use of the medication. Symptom recrudescence was not more prevalent in the group on the extended dosing schedule relative to the group on the standard dosing schedule (52.4% vs. 57.7%, P = .66). However, four patients reported that their symptoms began to recur sometime later than 28 days after infusion, suggesting that some may experience symptom recrudescence only with an extended dosing schedule.
Cohort Study
Ninety-one patients completed questionnaires in the infusion center assessing QOL, fatigue levels, and symptoms of depression for the week leading up to their infusion (ie, the time of lowest serum natalizumab levels). They were asked to complete the same series of questionnaires at home 7 days after their infusion regarding the prior week (ie, the time of peak natalizumab effect). The follow-up questionnaires were returned by 82 of 91 patients (90%). These results are summarized in Table 3. No patients experienced a relapse or an infection in the 1-week interval separating the completion of their surveys.
Table 3.
Scores for questionnaires assessing quality of life, fatigue, and depression for all patients surveyed and the subgroups of those with and without symptom recrudescence, at first and last weeks of the natalizumab dosing cycle
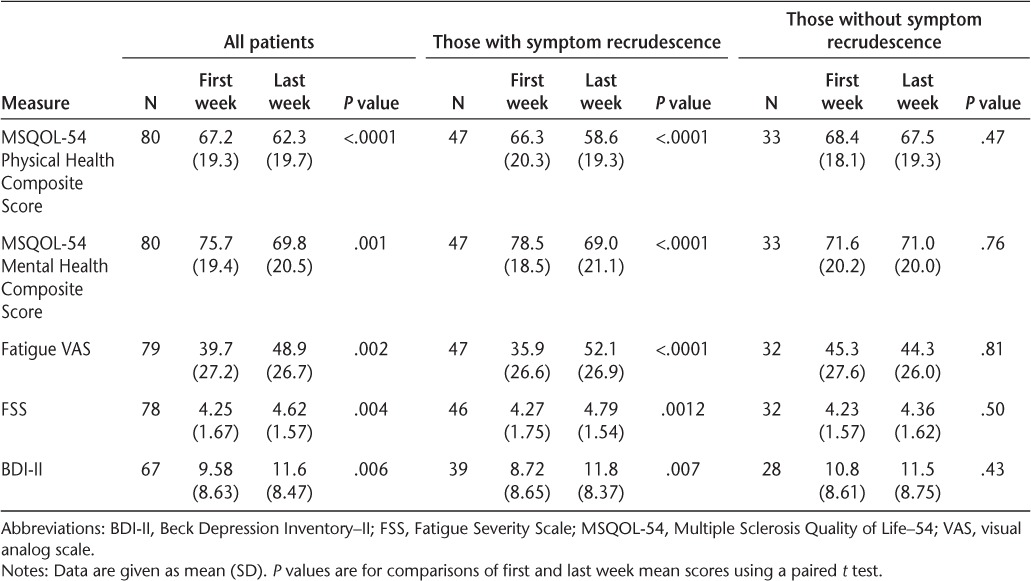
Quality of life was evaluated using the MSQOL-54, an MS-specific QOL questionnaire. For all patients surveyed, the mean Physical Health Composite Score was significantly lower (worse) at the end of the dosing cycle compared to the time of peak natalizumab level (62.3 vs. 67.2, P < .0001). The same difference was found for the mean Mental Health Composite Score (69.8 vs. 75.7, P = .001).
Fatigue for the prior week was evaluated using two scales. Patients indicated their fatigue level on a VAS, yielding a score from 0 to 100. The mean fatigue VAS score at the end of the dosing cycle was significantly higher (worse) than during the time of peak natalizumab level (48.9 vs. 39.7, P = .002). Fatigue was also assessed using the FSS, which provides a summary score from 1 to 7 with higher numbers indicating more severe fatigue. Mean FSS scores were higher at the end of the dosing cycle than at the beginning (4.62 vs. 4.25, P = .004). Depressive symptoms were assessed with the BDI-II. BDI-II scores were significantly higher (worse) at the end of the dosing cycle than at the time of peak natalizumab level (11.6 vs. 9.58, P = .006).
A prespecified subgroup analysis was performed for the group that reported currently experiencing symptom recrudescence and the group that did not. The subgroup that did report symptom recrudescence had more dramatic differences in all measured scales than the group as a whole (Table 3). By contrast, the subgroup that did not report experiencing symptom recrudescence showed no significant differences in any of the scales between the beginning and end of the dosing cycle.
The longitudinal data were also analyzed by creating a difference score for each outcome and using linear regression to evaluate whether the following variables affected amount of change in the measured outcomes: age, gender, self-reported fatigue, self-reported cognitive dysfunction, and need to use an assistive device to walk. Self-reported fatigue was associated with a greater change score in the fatigue VAS. None of the other variables were associated with the amount of change in the measured outcomes. An ANCOVA model was used to determine whether these covariates modified the effect that having EDI symptoms has on the change in the outcomes. The only effect modification that was identified was that age modifies the effect of EDI symptoms on change in FSS, such that those with EDI symptoms see less of an increase with age in FSS than those without EDI symptoms.
Discussion
This study found that a significant number of MS patients treated with natalizumab experience recrudescence of some of their MS symptoms toward the end of the natalizumab dosing cycle, and these symptoms are improved by infusion of natalizumab. Fatigue was the most common symptom that fluctuated, followed by weakness and walking difficulties. Moreover, it was found that scores for questionnaires assessing physical and mental QOL, fatigue, and depression were significantly better during the first week after receiving natalizumab than for the last week of the dosing cycle in patients who experience this. In a multivariate analysis, age was identified as negatively associated with having EDI symptoms. Since MS becomes less inflammatory as patients age, this would be consistent with a model in which EDI symptoms are due to recurrent low-grade CNS inflammation.
One other study has reported the same phenomenon of MS symptoms recurring at the end of the natalizumab dosing cycle. Gudesblatt et al.14 looked at four specific symptoms—fatigue, loss of concentration, weakness, and confusion—and found that 66% of natalizumab-treated patients had at least one of those symptoms recur at least 21 days after natalizumab infusion. Fatigue was the most common symptom to recur. They found no difference in the prevalence of these symptoms by age, BMI, body surface area, or duration of natalizumab treatment. Their results are very concordant with our findings.
Foley et al.15 evaluated the pharmacokinetics and pharmacodynamics of natalizumab infusion as a function of several variables: BMI, duration of treatment, and whether patients were on a standard 28-day infusion cycle or a longer cycle. They found that lower BMI was associated with a higher natalizumab level, and that patients on longer dosing intervals have lower mean natalizumab levels. We did not see an association between BMI or a longer dosing interval and the presence of symptom recrudescence, which would argue against its being strictly related to drug level. However, it is difficult to draw firm conclusions from this subgroup analysis because only 21 patients were on a dosing cycle longer than 28 days. Anecdotally, some patients in the study did report that they did not experience symptom recrudescence on an every-4-weeks dosing schedule, but they did experience it when they had a longer interval between doses.
The cause of this symptom fluctuation during the natalizumab dosing cycle is unknown, but several possibilities could be hypothesized. One possibility is that it is due to a low level of CNS inflammation occurring when natalizumab levels are lower. As serum natalizumab levels drop and α4 integrin saturation decreases, it is possible that small numbers of leukocytes return to the CNS. Natalizumab treatment has been associated with a higher percentage of activated leukocytes in the blood,16 so MS symptoms could be temporarily worsened when small numbers of these cells migrate into the CNS and release proinflammatory cytokines. In this scenario, it is possible that a reason this phenomenon is not experienced by some people is that they may maintain relatively higher drug levels or a higher level of α4 integrin saturation. This could be tested by measuring serum natalizumab levels or receptor saturation at the end of a cycle and seeing if it differs for patients with EDI symptoms versus those without. A challenge to this hypothesis is that many people feel improvement within a day of receiving natalizumab, which is faster than we may expect to see a reduction in the leukocyte levels in the CNS. Also, the fact that EDI symptoms were not more common in patients with higher BMI or longer dosing interval argues somewhat against its being strictly related to serum levels. A second possible mechanism of this phenomenon would be that natalizumab may affect the release of soluble factors. For example, release of anti-inflammatory cytokines or corticosteroids could also create a temporary alteration in symptoms. A third possible contributor to this phenomenon is the placebo effect. The expectation of feeling different soon after receiving natalizumab may contribute to a subjective improvement and later decline. Another possible factor is that some patients were primed to expect to experience symptom recrudescence by hearing other patients in the infusion center discuss their experiences. However, the very similar findings seen in another study at a different infusion center would argue against its being specific to our center.14
A strength of this study is that a very high proportion of the patients receiving natalizumab at our center responded to the questionnaire about EDI symptoms (95%), allowing us to estimate the prevalence of this phenomenon without significant selection bias. Data from multiple validated questionnaires confirmed the finding that symptoms vary for many patients during the natalizumab dosing cycle. A limitation of this study is that the process of explaining the study to participants may have biased them to be more likely to report EDI symptoms. We attempted to mitigate this effect by having only a single question on symptom recrudescence within a larger initial questionnaire, so as not to draw particular focus to that question. The patients were recruited from an academic MS center, so they may differ somewhat from those receiving natalizumab in a different setting. However, we expect that these results are generalizable to the larger population of MS patients receiving natalizumab.
A significant proportion of patients receiving natalizumab will notice recurrence of symptoms such as fatigue, weakness, gait difficulties, and cognitive dysfunction at the end of their dosing cycle. It is important for natalizumab prescribers to be aware of this phenomenon so that these symptoms are not mistaken for an MS relapse. Understanding why serum natalizumab levels affect MS symptoms may help us better understand the fundamental cause of fluctuating chronic MS symptoms. Additional studies of this phenomenon should focus on the relation between EDI symptoms and serum or cerebrospinal fluid levels of natalizumab and cytokines.
PracticePoints.
Some MS patients receiving natalizumab will experience a recrudescence of MS symptoms toward the end of their dosing cycle, which is reversed by natalizumab infusion.
The most common symptoms reported to recur are fatigue, weakness, walking impairment, and cognitive dysfunction.
When a natalizumab-treated patient reports neurologic symptoms, one should consider this end of dosing cycle effect as a possible cause once an inflammatory relapse and infection are ruled out.
Acknowledgments
The authors thank Peter Calabresi for providing comments on the manuscript and Hayley Baker for assistance with data entry.
Footnotes
Financial Disclosures: Dr. Ratchford consults for Biogen Idec and Genzyme. He receives research funding for clinical trials from Biogen Idec, Novartis, and Sun Pharmaceuticals. Ms. Costello is a consultant and scientific advisory board participant for Teva Neuroscience, Biogen Idec, EMD Serono, Genzyme, Questcor, and Novartis. Ms. Brock-Simmons, Ms. Augsburger, Ms. Steele, Ms. Mohn, Ms. Rhone, and Ms. Bo have no conflicts of interest to disclose.
Funding/Support: This work was supported by philanthropic grants from John and Patricia Malin and the John J. Horan Memorial Fund.
References
- 1.Polman CH, O'Connor PW, Havrdova E. A randomized, placebo-controlled trial of natalizumab for relapsing multiple sclerosis. N Engl J Med. 2006;354:899–910. doi: 10.1056/NEJMoa044397. et al. [DOI] [PubMed] [Google Scholar]
- 2.Rudick RA, Miller D, Hass S. Health-related quality of life in multiple sclerosis: effects of natalizumab. Ann Neurol. 2007;62:335–346. doi: 10.1002/ana.21163. et al. [DOI] [PubMed] [Google Scholar]
- 3.Putzki N, Yaldizli O, Tettenborn B. Multiple sclerosis associated fatigue during natalizumab treatment. J Neurol Sci. 2009;285:109–113. doi: 10.1016/j.jns.2009.06.004. et al. [DOI] [PubMed] [Google Scholar]
- 4.Morrow SA, O'Connor PW, Polman CH. Evaluation of the symbol digit modalities test (SDMT) and MS neuropsychological screening questionnaire (MSNQ) in natalizumab-treated MS patients over 48 weeks. Mult Scler. 2010;16:1385–1392. doi: 10.1177/1352458510378021. et al. [DOI] [PubMed] [Google Scholar]
- 5.Yildiz M, Tettenborn B, Putzki N. Multiple sclerosis-associated fatigue during disease-modifying treatment with natalizumab, interferon-beta and glatiramer acetate. Eur Neurol. 2011;65:231–232. doi: 10.1159/000324028. [DOI] [PubMed] [Google Scholar]
- 6.Iaffaldano P, Viterbo RG, Paolicelli D. Impact of natalizumab on cognitive performances and fatigue in relapsing multiple sclerosis: a prospective, open-label, two years observational study. PLoS One. 2012;7:e35843. doi: 10.1371/journal.pone.0035843. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.TYSABRI [package insert] Cambridge, MA: Biogen Idec, Inc.; 2012. [Google Scholar]
- 8.Khatri BO, Man S, Giovannoni G. Effect of plasma exchange in accelerating natalizumab clearance and restoring leukocyte function. Neurology. 2009;72:402–409. doi: 10.1212/01.wnl.0000341766.59028.9d. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Portaccio E, Stromillo ML, Goretti B. Natalizumab may reduce cognitive changes and brain atrophy rate in relapsing-remitting multiple sclerosis: a prospective, non-randomized pilot study. Eur J Neurol. 2013;20:986–990. doi: 10.1111/j.1468-1331.2012.03882.x. et al. [DOI] [PubMed] [Google Scholar]
- 10.Yildiz M, Tettenborn B, Borgwardt S. Trajectory of fatigue severity in natalizumab treated multiple sclerosis patients. Clin Neurol Neurosurg. 2013;115:902–903. doi: 10.1016/j.clineuro.2012.08.039. [DOI] [PubMed] [Google Scholar]
- 11.Vickrey BG, Hays RD, Harooni R. A health-related quality of life measure for multiple sclerosis. Qual Life Res. 1995;4:187–206. doi: 10.1007/BF02260859. et al. [DOI] [PubMed] [Google Scholar]
- 12.Krupp LB, LaRocca NG, Muir-Nash J. The fatigue severity scale: application to patients with multiple sclerosis and systemic lupus erythematosus. Arch Neurol. 1989;46:1121–1123. doi: 10.1001/archneur.1989.00520460115022. et al. [DOI] [PubMed] [Google Scholar]
- 13.Beck AT, Steer RA, Brown GK. Manual for the Beck Depression Inventory–II. San Antonio, TX: Psychological Corp; 1996. [Google Scholar]
- 14.Gudesblatt M, Zarif M, Bumstead B. Multiple sclerosis and natalizumab: “between the dose symptoms” [abstract P982] Mult Scler. 2012;18(suppl 4):P982. et al. [Google Scholar]
- 15.Foley JF, Christensen A, Hoyt TA. Natalizumab kinetics and dose extension: defining the therapeutic envelope. Neurology. 2012;78(suppl 1):P06.174. et al. [Google Scholar]
- 16.Kivisakk P, Healy BC, Viglietta V. Natalizumab treatment is associated with peripheral sequestration of proinflammatory T cells. Neurology. 2009;72:1922–1930. doi: 10.1212/WNL.0b013e3181a8266f. et al. [DOI] [PMC free article] [PubMed] [Google Scholar]


