SUMMARY
The regenerative capacity of the peripheral nervous system declines with age. Why this occurs, however, is unknown. We demonstrate that 24-month old mice exhibit an impairment of functional recovery after nerve injury compared to 2-month old animals. We find no difference in the intrinsic growth capacity between aged and young sensory neurons in vitro nor in their ability to activate growth-associated transcriptional programs after injury. Instead, using age-mismatched nerve transplants in vivo, we show that the extent of functional recovery depends on the age of the nerve graft, and not the age of the host. Molecular interrogation of the sciatic nerve reveals that aged Schwann cells (SCs) fail to rapidly activate a transcriptional repair program after injury. Functionally, aged SCs exhibit impaired de-differentiation, myelin clearance and macrophage recruitment. These results suggest that the age-associated decline in axonal regeneration results from diminished Schwann cell plasticity, leading to slower myelin clearance.
INTRODUCTION
Successful regeneration of the PNS requires the coordinated activity of several distinct yet interlocking systems. After axonal injury, neurons activate transcriptional "intrinsic growth" programs and begin extending new axons. Schwann cells (SCs) in the distal nerve undergo a rapid reprogramming process, de-differentiating into non-myelinating repair cells that secrete pro-regenerative factors, clear non-permissive myelin, recruit macrophages and create physical tracks onto which newly growing axons can navigate. Macrophages, in turn, enter the site of nerve injury from the circulation and help to complete clearance of myelin and axonal-derived debris, obstacles to regrowth. Remarkably, these complex intrinsic and extrinsic processes can lead to some restoration of function after peripheral nerve trauma.
As with most mammalian tissues, however, the regenerative capacity of the PNS declines with advancing age (Nagano, 1998; Pestronk et al., 1980; Tanaka and Webster, 1991; Vaughan, 1992; Verdu et al., 2000). In humans, an association between age and functional recovery has been pondered since at least World War II, and more recent clinical data quantifies this correlation, such as after brachial plexus injury or median nerve transection (Woodhall, 1956; Nagano, 1998; Lundborg and Rosen 2001). In addition, broader defects of the aging PNS have also been widely noted. For example, it is estimated that nearly 1 in 3 persons over the age of 65 suffer from some form of peripheral neuropathy and the underlying cause in the majority of these instances is considered idiopathic (Cho et al., 2006). Thus, understanding how the PNS changes with normal aging is clinically important.
To some degree, the clinical associations between age and the extent of neuronal regeneration have been recapitulated in animal models. While not addressing functional recovery directly an age-associated impairment of axonal regeneration can be detected in both mice and rats through histological assessments (Verdu et al., 2000). More recently, studies have shown similar phenomena in both zebra fish and the nematode C. elegans (Graciarena et al., 2014). Interestingly, the developmental decline in axonal regeneration found in the nematode C. elegans is the result of neuronal-intrinsic alterations (Zou et al., 2013). In the case of mammals, however, to what degree age-associated impairment of axonal regeneration represents neuronal-intrinsic alterations versus environmental perturbations remains an open and critical question, with significantly different therapeutic implications.
In this study, we used 24- and 2-month old mice along with standard models of peripheral nerve injury to assess the potential contributions of neurons, immune cells and peripheral glia to the aging phenotype. Our results demonstrate that extrinsic factors are sufficient to rejuvenate the age-associated decline in mammalian axonal regeneration, and broadly reveal how Schwann cell function and its response to axonal injury alters during normal aging, resulting in slower dedifferentiation and myelin clearance.
RESULTS
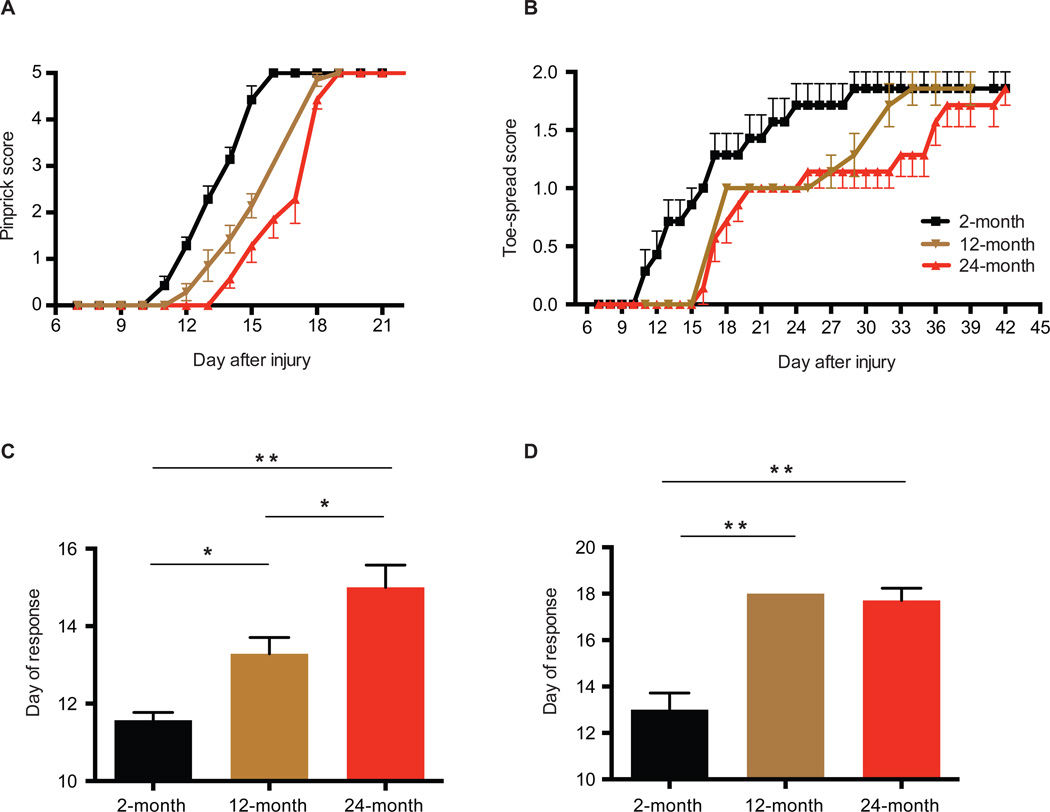
Although previous studies have established in rodents that fewer axons regenerate after injury in aged animals, it is unclear how this might translate into functional recovery, a more meaningful clinical parameter. We thus first sought to establish whether aging impaired functional recovery in mice after nerve injury. To accomplish this, we performed a sciatic nerve crush injury (a model in which full recovery is expected (Ma et al., 2011)) on cohorts of 2, 12, and 24-month old C57BL/6 mice, and assayed their sensory and motor functional recovery over time, using behavioral-based assays (see Methods). In young mice, full sensory recovery is achieved approximately 15 days after injury, while motor function is restored by day 30, reflecting the short distances and compatible with axon growth of 1–2mm per day, similar to that found in humans. Aging, however, produces a progressive defect in the onset and time to restoration of motor and sensory function in the mice, with an approximately 3–4 day (~29%) difference in maximum recovery between young and aged animals for sensory function and a 9–10 day delay (~44%%) for recovery of motor function (Figures 1A and 1B). Middle-aged animals (12-month) show an intermediate phenotype, indicating that age-dependent slowing of functional recovery is acquired gradually throughout life. Interestingly, the kinetics of these behavioral data suggest that the prominent effect of age is not a slower overall rate of regeneration, but instead a marked delay in the initiation of regeneration in the first place (Figures 1C and 1D).
Figure 1. Progressive, age-dependent deficit in peripheral nerve regeneration.
Cohorts of 2, 12 and 24-month old mice were subjected to sciatic nerve crush injury and restoration of sensory (A) and motor (B) function, assayed through pinprick and toe-spreading assays, respectively. The first day in which a positive sensory (C) or motor (D) behavioral response was also recorded. *P < 0.05; **P < 0.01 in one-way ANOVA with Bonferroni's multiple comparisons test. Mean ± SEM, n = 7–8 mice per cohort.
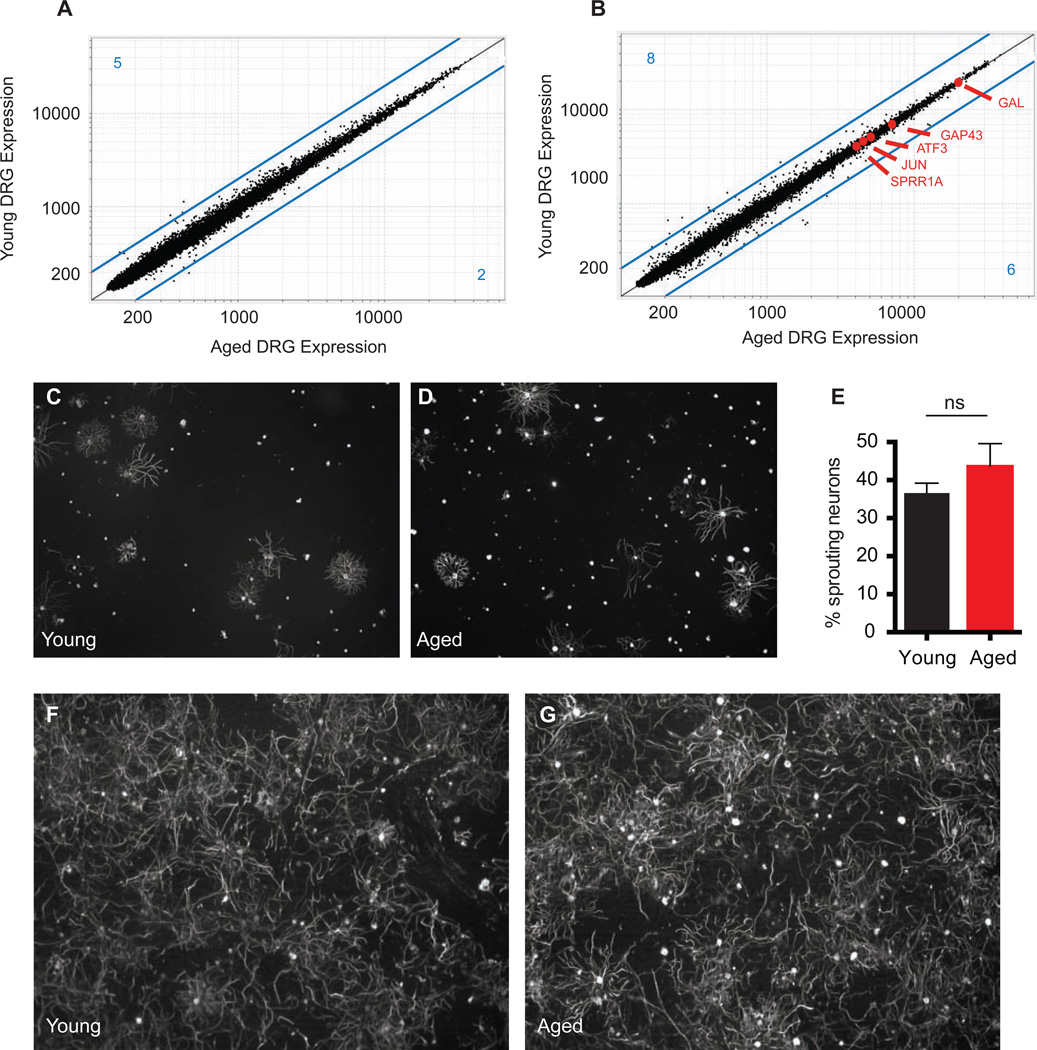
What is responsible for this defect? Because axonal regeneration in mammals is driven by the transcriptional activation of neuronal "intrinsic growth" programs after injury (Chen et al., 2007; Seijffers et al., 2007), it has been suggested that the age-related decline in regeneration may be the consequence of a diminished intrinsic growth potential of aged neurons (Pestronk et al., 1980). Unexpectedly, however, we find by transcriptional profiling of young (2-month) and aged (24-month) dorsal root ganglia (DRGs), both before and after nerve injury, extremely comparable gene-expression signatures, with similar levels of injury-induced transcriptional activation between young and old ages (Figures 2A, 2B, S1A). After nerve injury, over 250 genes in the DRG are up or down-regulated greater than 2-Fold compared to naive controls (Figure S1B). Among these, only 14 are differentially expressed (> 2-Fold) between young and aged animals. Many of these age-variant transcripts are also de-regulated in naive (uninjured) conditions, implying they are part of a "background" aging signature and not specific to nerve injury (Table S1). Important "regeneration-associated genes" such as ATF3, GAP43, and Sprr1a, were not differentially expressed between young and aged DRGs (Figure 2B) (Bonilla et al., 2002; Chong et al., 1994; Seijffers et al., 2007). As predicted from the transcriptional data, in vitro neurite outgrowth assays using dissociated neurons revealed comparable growth activity regardless of age, in both naive and pre-conditioned DRG sensory neurons, confirming no deficit with age in the degree if intrinsic neuronal growth induced by injury (Figure 2C–G). These experiments strongly argue that a mechanism extrinsic to neurons moderates the regenerative potential of aging DRG neurons in vivo.
Figure 2. Neuronal intrinsic growth responses to injury remain robust throughout life.
(A and B) Microarray data from DRG neurons. Expression vs. expression of young vs. aged DRGs in (A) naive conditions and (B) 4 days after nerve crush injury. Known markers of regeneration are highlighted in red. Lines in blue represent Fold Change = 2, blue numbers in each corner represent the number of genes over- or under-expressed by more than 2-Fold. (C to G) In vitro neurite outgrowth assays of DRG sensory neurons. Sensory neurons from naive young (C) or aged (D) DRGs from aged or young animals were purified and cultured for 17 hours and neurite outgrowth measured with Neuromath (E). Mean ± SEM, n = 3 independent experiments per group, NS = not significant with unpaired t-test. DRG sensory neurons from young (F) or aged (G) animals were purified 4 days after nerve injury, and cultured for 12 hours, revealing robust pre-conditioning responses.
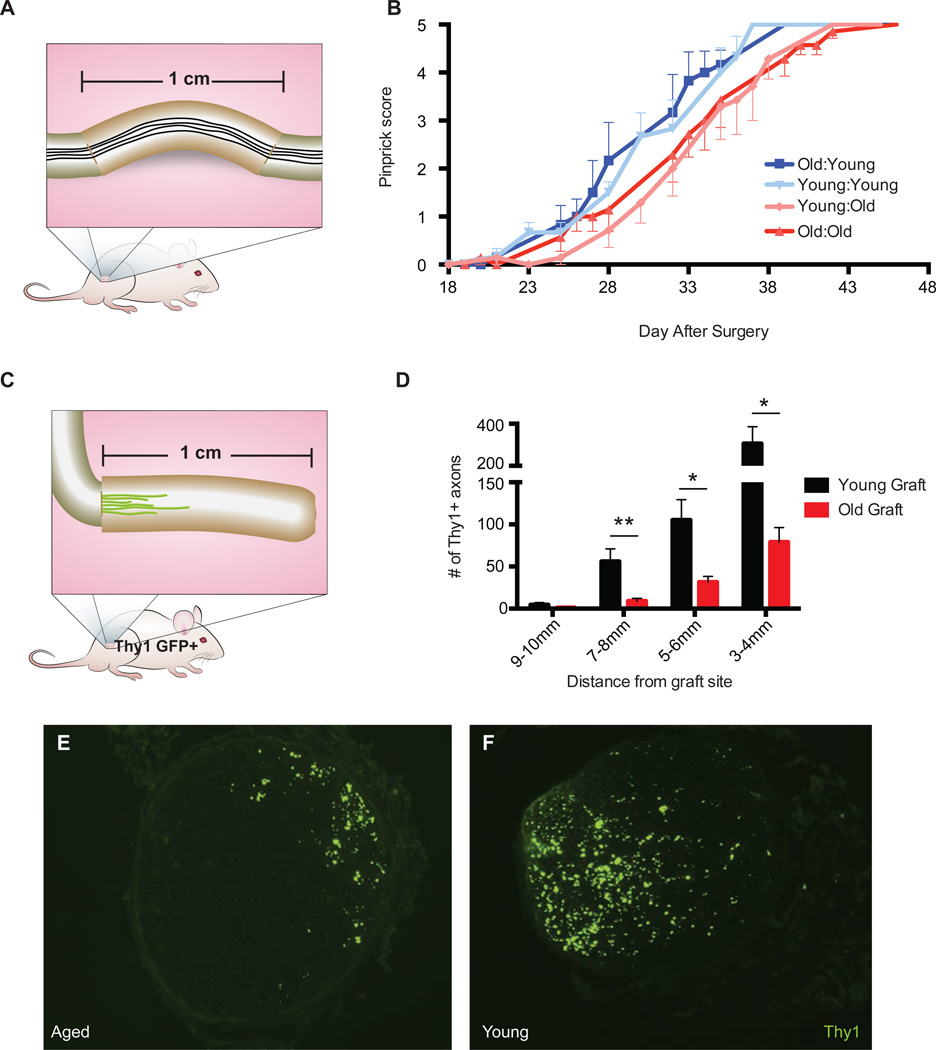
As such, we hypothesized that allowing "aged" axons to regenerate in a "youthful" environment might enhance functional recovery in aged animals. Conversely, we conjectured that forcing young axons to regenerate in an "aged" environment would result in impaired recovery. To test this, we harvested 1 cm segments of sciatic nerve from either aged or young animals and directly sutured them onto the two cut ends of transected sciatic nerves of either old or young animals. In this way, axons from the host animal must regenerate through a 1 cm segment of either age-matched or age-mismatched nerve tissue before reaching the distal end of the nerve en route to their targets in the skin (Figure 3A). We assessed sensory functional recovery to monitor regeneration. Because transection/re-suture injuries are more severe than crush injuries, motor recovery of plantar muscles in the foot is not obtained, and functional sensory recovery takes much longer (Ma et al., 2011). Strikingly, we observed that functional recovery was enhanced in aged animals receiving young nerve grafts such that it now resembled that found in young animals receiving young nerve grafts (Figure 3B). Reciprocally, young animals receiving aged nerve grafts showed slower functional recovery, similar to aged animals receiving aged nerve grafts (Figure 3B).
Figure 3. Neuronal-extrinsic factors are sufficient to recapitulate or rejuvenate the phenotype.
(A) Schematic of nerve graft strategy. 1 cm long segments from young or aged animals were sutured into the nerves of either young or aged host animals and restoration of sensory recovery assayed as in Fig. 1. (B) Time-course of sensory recovery as measured by pinprick score. (C) Schematic of Thy1 YFP+ graft strategy. 1 cm long segments of sciatic nerves from either aged or young mice were sutured onto the sciatic nerves of young Thy1 YFP+ host animals and nerves analyzed histologically after 7 days (D). Representative images of YFP+ axons in the donor aged and young nerve grafts, 5mm distal to the site of suture. (E) Quantification of the number of YFP+ fibers at each specified distance from the suture site. Axons counts were quantified using ImageJ. Mean ± SEM is plotted, n = 7 mice per group (one mouse with counts greater than 3 SD from the mean was removed from the dataset). Stats: interaction age × graft distance; P < 0.01, two-way ANOVA; F(3, 48) = 5. *P <0.05, **P <0.01 with multiple t-test, Holm-Sidak correction.
We further quantified the extent of axonal regeneration in an age-mismatched system. To accomplish this, we used a different nerve graft strategy in which either young or old 1 cm long pieces of sciatic nerve were sutured onto the proximal cut end nerves of Thy1 YFP+ young host animals (aged transgenic mice were not available for the reciprocal experiment) (Figure 3C). Thy1 YFP+ mice were chosen to enable visualization of regenerating fibers in the graft. After one week, the number of YFP+ axons growing into either young or aged donor tissue was quantified histologically. Forcing young axons to regenerate into an aged environment, as opposed to a young one, severely impaired the extent and length of growth with reduced numbers of axons at every distance from the site of the graft (Figure 3D and 3E).
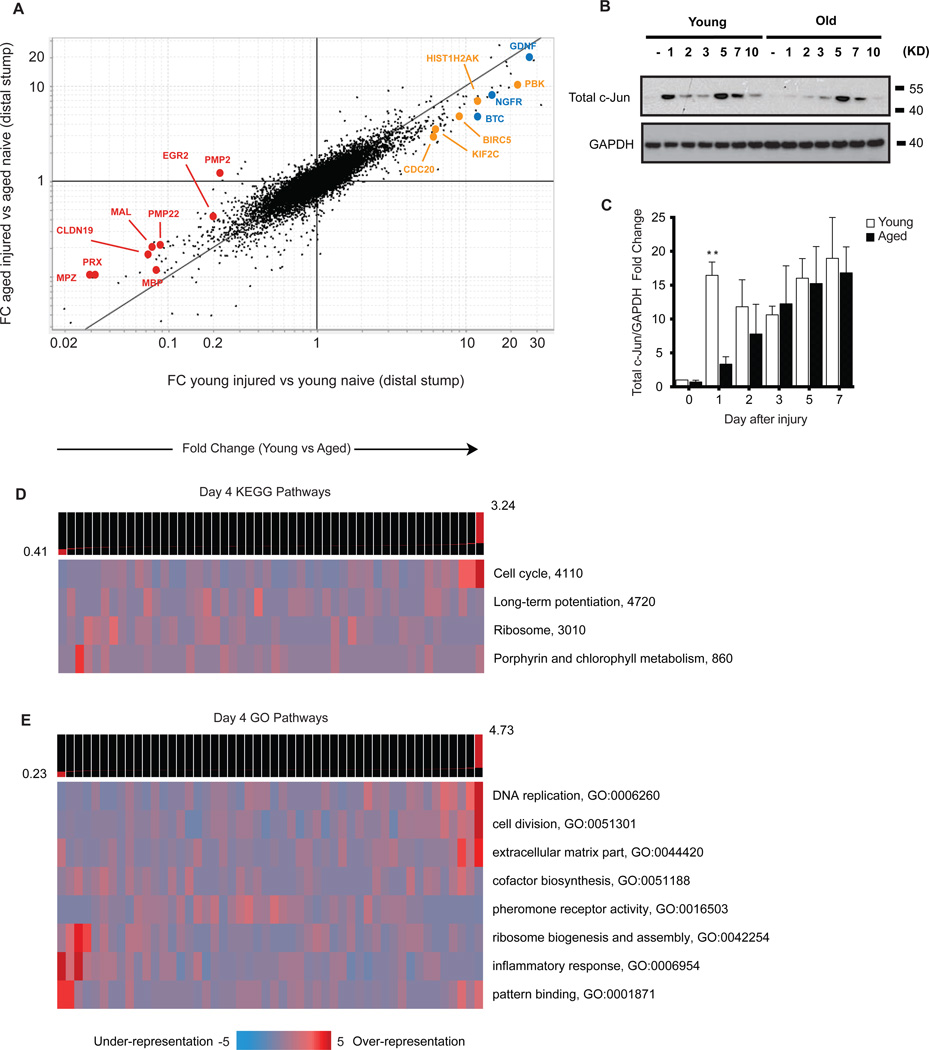
Given the age-dependent ability of factors extrinsic to the injured neurons to modulate axonal growth, we next sought to identify what age-associated changes occur within the sciatic nerve environment. To this end, transcriptional profiling of young and aged nerves distal to a sciatic nerve ligation (to prevent regenerating axons from contaminating samples) revealed widespread age-related alterations (Figure 4A–4C, S2A and S2B, Table S2, Table S3). From a global perspective, gene pathway analyses of differentially represented transcripts between young and aged nerves showed several significantly enriched pathways, using the Kyoto Encyclopedia of Genes and Genomes (KEGG) and Gene Ontology (GO) databases (Figures 4A and 4B, S3A and S3B). In young nerves after injury, this included prominent over-representation of genes associated with "cellcycle," "DNA replication" and the "extra-cellular matrix," while genes associated with the "inflammatory response" were among the pathways relatively enriched in old nerves (Figures 4A and 4B). Several pathways are also enriched in young nerves in naive conditions, such as genes associated with the "extra-cellular matrix" and "lipid biosynthesis" (Figure S3A and S3B). This analysis suggests that a greater degree of cell proliferation may occur in young than aged nerves distal to an injury.
Figure 4. Acquisition of repair cell phenotype is impaired in aged Schwann cells.
Significantly over-represented gene pathways from KEGG (A) and GO (B) databases. Columns represent groups of genes according to their fold change difference in young vs. aged sciatic nerves 4 days after crush injury. Columns to the left contain genes more over-expressed in old, whereas columns to the right contain genes more over-expressed in young. Heat-map depicts the pattern of expression of pathways across the groups of differentially expressed genes (see Goodarzi et al., 2009). (C) Expression data from the distal nerve stump of aged and young animals after nerve transection/ligation injury. X axis represents Fold Change of young injured against young naive transcripts. Y axis is Fold Change of aged injured against aged naive transcripts. Transcripts along the black y=x line represent equal up or down regulation after injury compared to naive. Red = myelin-associated genes; Blue = growth factors-associated; Orange = mitosis associated. (D) Representative Western blot analysis of total c-Jun in sciatic nerves after nerve injury. (E) Summary of Western blot analyses of c-Jun regulation in young and aged nerves (distal to the site of injury) after nerve injury. n = 3 biological replicates for all time-points except day 7, where n = 2. **P < 0.01 with multiple t-tests, Holm-Sidak correction.
The specific age-associated alterations can be seen prominently by the number of transcripts that deviate from the Y=X parity line in the fold change versus fold change plot, indicating that hundreds of genes up- or down-regulated after injury in the young are not equally regulated in aged nerves. Strikingly, we found that many of these genes are associated with a "repair program" in Schwann cells (SCs) activated after injury (Figure 4C, Table S3). After nerve injury, SCs undergo a phenotypic reprogramming process whereby they down-regulate their myelinating phenotype, rapidly divide, and acquire a non-myelinating, "repair cell" phenotype critical for the development of a growth permissive environment within the nerve that facilitates regeneration (Arthur-Farraj et al., 2012; Mirsky et al., 2008).
We observed a prominent age-related deregulation of genes encoding key repair cell functions. Genes down-regulated in aged nerves relative to young ones after injury included those associated with growth factors (BTC, NGFR, BDNF) and mitosis (e.g. KIF2C, PBK, BIRC5, CDC20) (Figure 4C, Table S3). Likewise, genes associated with myelin production (e.g. PMP2, MPZ, MAL, EGR2) were relatively over-expressed in lesioned aged animals, implying an impaired ability to down-regulate their myelinating phenotype after injury (Figure 4C).
Recently, the transcription factor c-Jun was identified as a "master regulator" of the SC repair phenotype after nerve injury, and SC-specific ablation of c-Jun results in severely impaired functional recovery (Arthur-Farraj et al., 2012). Interestingly, we noticed marked similarities between the transcriptional profiles of injured aged nerves and genes altered in mice lacking c-Jun expression in SCs after injury (e.g. MPZ, CDH1, SEMA4F) suggesting that genes under control of c-Jun were altered in aged animals (Arthur-Farraj et al., 2012). Strikingly, Western blot analysis demonstrated that the regulation of c-Jun protein was significantly different in the nerves of aged compared with young animals immediately after injury (Figures 4D and 4E). The initial burst of c-Jun expression found in the nerves of young animals 1 day after injury was approximately 5-fold higher than that in aged animals (Figure 4E). ERK and JNK, two upstream kinase pathways of c-Jun, were unaltered in aged sciatic nerves after injury (Figure S4).
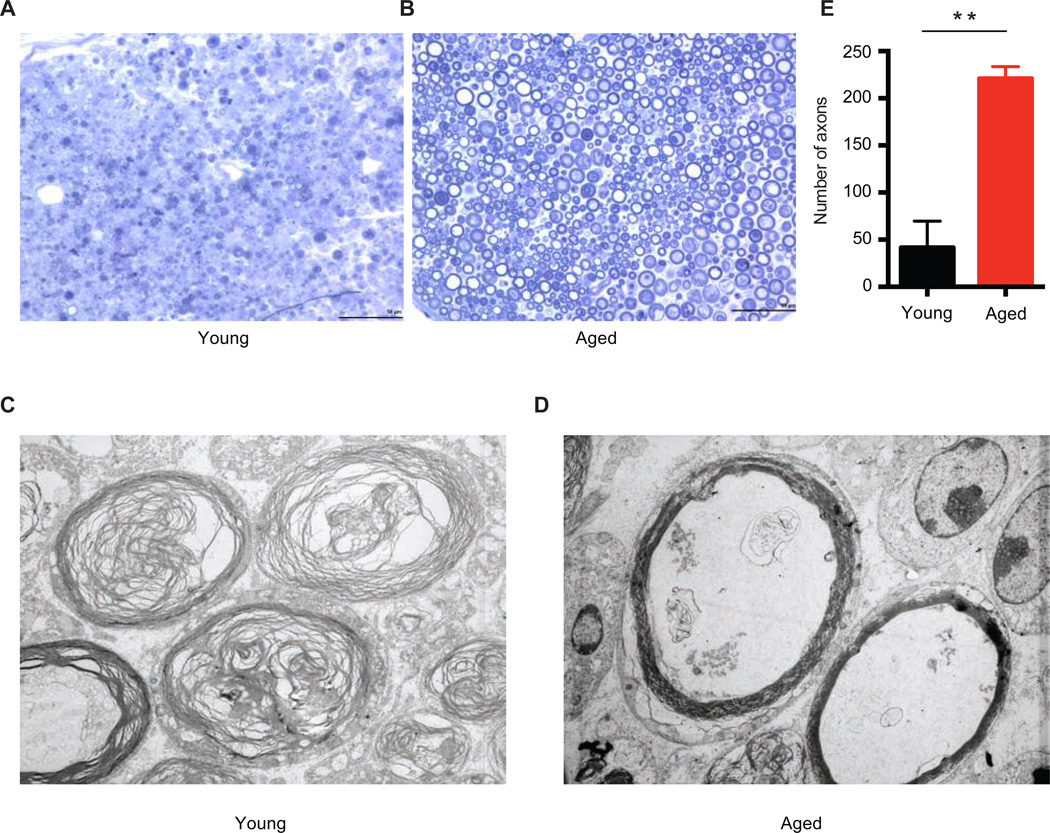
If, as suggested by these data, Schwann cell de-differentiation and repair cell functions are impaired with aging, one would predict functional consequences in the SCs, and more generally, for Wallerian degeneration in the nerve. To test this we first performed toluidine blue staining and electron microscopy examination of distal sections of young and aged sciatic nerves. These revealed stark differences in Wallerian degeneration. By 3 days after a nerve crush injury in young mice, axons in the nerve distal to the injury have degenerated and been cleared, and the myelin sheaths surrounding the axons have also degenerated, leaving the nerve landscape largely empty of any organized structure (Figures 5A and 5C). In complete contrast, however, axons in the distal nerve of aged animals are at less developed state of degeneration and clearance, and more starkly, most myelin sheaths remain intact (Figures 5B and 5D). Quantification of axons with intact myelin sheaths shows approximately 4 times the number remaining in aged, as opposed to young, animals (Figure 5E). These data are consistent with previous histological studies in rodents, and quantify the aberrant delayed state of early Wallerian degeneration in aged animals (Vaughan, 1992; Verdu et al., 2000).
Figure 5. Wallerian degeneration is disrupted in aged animals.
Toluidine blue staining of distal nerve sciatic sections 3 days after crush injury in young (A) and aged (B) animals. Electron microscopic images of same distal nerve sections from young (C) and aged (D) animals. (E) Quantification of the number of intact myelin sheaths in aged and young nerves from toluidine blue staining. n=4. ** P <0.01 with unpaired t-test. Scale bar = 50um.
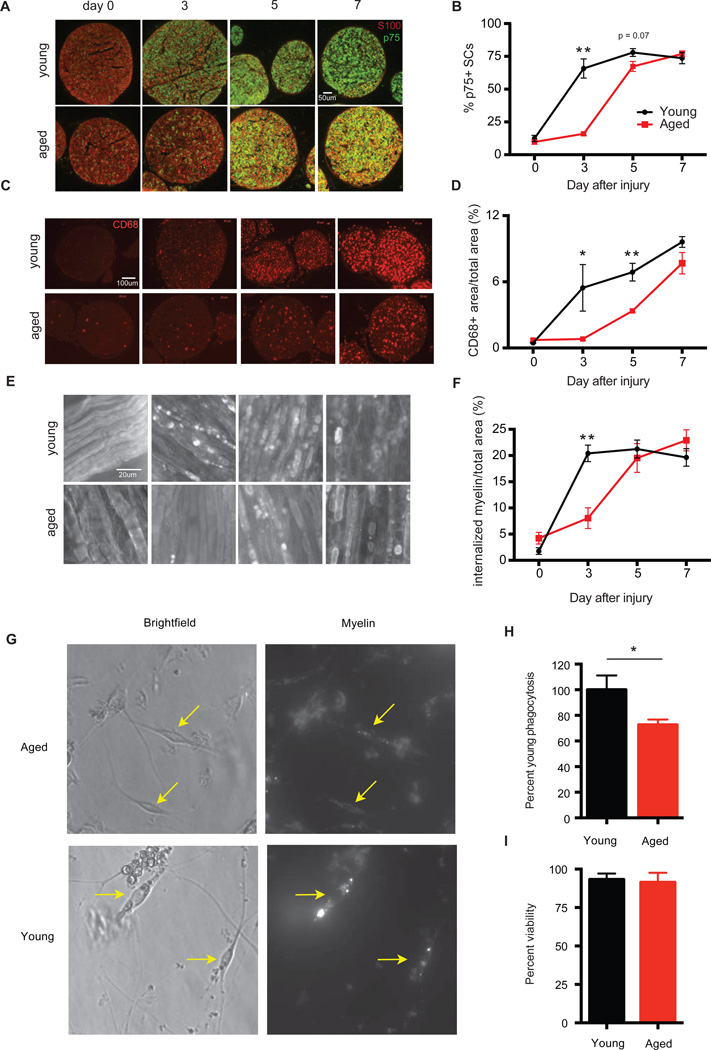
We next directly addressed if age affected Schwann cell function and repair responses after nerve injury. As expected, expression of p75, an induced marker for the repair cell phenotype was delayed in aged SCs after sciatic nerve crush injury compared to young controls. In young animals, ~65% of S100+ SCs were positive for p75 3 days after nerve injury (Figures 6A, 7A and 7B). S100 is a SC marker constitutively present in intact and dedifferentiated SCs. In aged animals, however, p75 reactivity was observed in only ~16% of S100+ SCs.
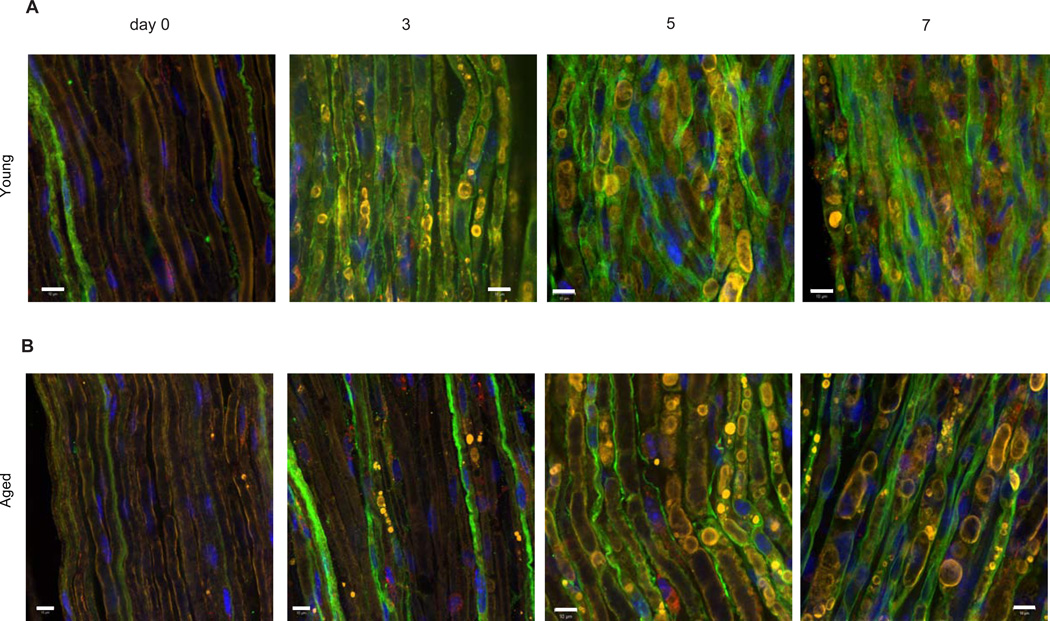
Figure 6. Aged Schwann cell injury responses are delayed.
Representative confocal images of sciatic nerves from young (A) and aged (B) animals at day 0, 3, 5 and 7 after nerve crush injury. Sections were stained with p75 (green), MBP (yellow) and Lamp-1 (red). Note strong co-localization between myelin and SCs at all time-points observed. Scale bar = 10um.
Figure 7. Functional defects in aged Schwann cells.
Cross-sections and whole mounts of sciatic nerves from young and aged animals at day 0, 3, 5 and 7 after nerve crush injury stained with (A) S100 and p75, (B) CD68, and (C) MBP. (D, E and F) Quantification of staining from (A, B and C). Mean ± SEM is plotted, n = 3–4 mice per group. P < 0.05 by two-way ANOVA for (D–F). *P < 0.05, **P < 0.01 with mutliple t-tests, Holm-Sidak correction. SCs were purified from the sciatic nerves of young and aged animals 3 days after sciatic nerve crush injury and incubated with myelin for 2 hours. (G) Representative images of purified young and aged SCs with myelin (yellow arrows). (H) Quantification of myelin contained within SCs by FACS analysis. (I) Cell viability as determined by DAPI staining. n=3–4 per group. **P <0.01 with unpaired t-test.
Repair SCs play several key roles to promote axonal regeneration and Wallerian degeneration. For example, macrophage recruitment into the degenerating nerve is depends upon factors secreted by de-differentiated SCs (Napoli et al., 2012). We thus measured macrophage recruitment into the degenerating nerve by tracking the expression of CD68, a standard marker for activated macrophages, over time. This revealed a markedly delayed recruitment of CD68+ macrophages after injury in aged animals of up to 7 days (Figures 7C and 7D). This delay in immune cell recruitment is unlikely to be the result of a defect intrinsic to aged immune cells, because heterochronic parabiosis, which provides aged animals with access to young hematopoietic cells, failed to rescue the aging phenotype (Figures S5A–S5F). This model successfully enhances re-myelination in the central nervous system in a macrophage dependent manner (Ruckh et al., 2012) and immune cell chimerism was detected in both the spleens and sciatic nerves of conjoined animals after nerve injury (Figure S5B–S5D).
Another critical downstream function of SCs after injury is the physical fragmentation of their myelin sheaths followed by intracellular degradation of myelin proteins and lipids. Myelin is an inhibitory substrate for axon growth, and it is thus essential that myelin debris is cleared from the distal injury site before regeneration can occur (McKerracher et al., 1994). SCs from aged mice showed a deficit in their ability to initiate myelin clearance during early time points after injury, in parallel with the delay in expression of p75 (Figures 7E and 7F). Dense MBP positive myelin accumulations were co-localized within p75 positive (dedifferentiated) SCs soon after injury in young nerves but not in p75 positive aged SCs (Figures 6A and 6B). The defect can be clearly seen from myelin staining in distal segments of the sciatic nerve after crush injury, where 3 days after injury to young nerves, SCs show large, bright, MBP-positive accumulation of fragmented myelin, indicating that myelin breakdown is underway, while in aged animals most MBP staining is evenly distributed in continuous sheaths, similar to intact nerves (Fig. 7E and 7F).
The Schwann cell-mediated mechanism of intracellular myelin degradation is believed to depend on SC phagocytosis of extracellular myelin debris (Brosius Lutz and Barres, 2014). To directly address cell-intrinsic SC phagocytosis, we purified populations of SCs from young and aged sciatic nerves and cultured them in the presence of labeled myelin (Figures 7G). Quantification of the amount of myelin contained within SCs using Florescent-activated cell sorting revealed 35% less phagocytosis of myelin in aged versus young SCs (Figure 7H). This defect was not the result of reduced viability of aged SCs (Figure 7I). Thus, aged SCs exhibit a reduced capacity for myelin breakdown and clearance in vitro and in vivo.
DISCUSSION
We have found that aging introduces a progressive decline in the initiation of axonal regeneration after injury in mammals. This age-dependent slowing in regenerative potential may contribute to the suboptimal clinical outcomes reported after nerve trauma in elderly individuals (Nagano, 1998; Lunborg and Rosen, 2001).
Our data also demonstrate that the regenerative capacity of the aging mammalian PNS is not limited by diminished neuronal growth responses, but is instead profoundly influenced by extrinsic factors in the surrounding tissue. We found that neuronal intrinsic growth responses are unaltered with normal aging and that aged neurons can be fully "primed" to regenerate after an axonal injury supported by an undiminished injury-induced transcriptional change. This notion is consistent with recently published data demonstrating that the rate of axonal regeneration does not differ between young and aged mice in vivo (Kang and Lichtman, 2013). We further demonstrated that the ectopic introduction of aged tissue impeded regeneration in young, healthy mice, whereas grafting of young tissue enabled aged neurons to express a robust repair potential. These observations indicate that deficient axonal regeneration in aged animals arises from a dominant extrinsic inhibition of neuronal outgrowth by the aged microenvironment, and suggest that functional recovery after axotomy may be enhanced throughout life via neuron-extrinsic mechanisms.
Our analysis demonstrates that SC injury responses are substantially altered in aged animals, a defect due we suggest, to a failure of aged SCs to effectively activate an appropriate transcriptional repair response after axonal denervation. Although our studies were limited to C57/BL mice their congruence with rat and human observations indicates that the phenomena described here are not likely to be restricted to this strain, but the strain-dependence of this phenotype will need to be formally tested. Our data implicate aged SCs as key mediators of age-dependent inhibition of axon regeneration in the PNS. Although it remains to be determined whether the altered SCs responses of aged animals result entirely from intrinsic defects (such as age-acquired DNA damage) or whether this represents a failure in an upstream signaling cascade initiated by a change in the distal cut axons leading to delayed c-Jun expression, it is clear that the failure of aged SCs to efficiently acquire a repair phenotype after nerve injury impinges on axonal regeneration. In the future, it will be of great interest to determine both the exact cause for these alterations in the SC compartment and whether they also contribute to other age-associated disorder of the PNS, such as idiopathic age related peripheral neuropathy.
In terms of what specific SC defects impair the initiation of axonal regeneration, it is probable that inefficient SC de-differentiation in aged animals may result in a variety of downstream consequences that could impair axonal growth. For example, as macrophages are required for proper axonal regeneration, the delay in macrophage recruitment we observed could contribute to the aging phenotype (Barrette et al., 2008). In addition, as seen from our profiling studies, in the aging nerve environment there is less growth factor production after injury. A distinct SC related defect that likely impedes optimal axonal growth was that aged SCs have a marked reduced capacity for myelin clearance, a critical component for establishing the regeneration-enabling environment of the injured PNS. Moreover, our data highlight the myelin clearance function of SCs early during the course of Wallerian degeneration, a role that has perhaps been underappreciated compared to their macrophage counterparts, which operate later, indeed at a time when most myelin is already cleared in young nerves. The mechanism of myelin breakdown by SCs is then an area requiring urgent future effort (Brosius Lutz and Barres, 2014).
SCs are potentially unique among adult, differentiated cells in that, after injury, they undergo a phenotypic reprogramming process that resembles trans-differentiation (Arthur-Farraj et al., 2012) and in this way acquire a de-differentiated highly proliferative almost stem cell-like state. Given the well-known alterations that occur in stem-cell compartments during normal aging, it is perhaps not surprising that SCs, which undergo major transcriptional and phenotypic changes after injury, may be relatively vulnerable to age-acquired alterations. Glial cells in the central nervous system also exhibit an ageassociated decline in function the differentiation potential of oligodendrocyte progenitor cells declines with age, resulting in decreased myelination (Shen et al., 2008). Unlike what we have observed with SCs, however, this diminished function in CNS glia results in part from extrinsic signaling via hematopoietic cells (Ruckh et al., 2012).
Finally, our data may have bearing on therapeutic strategies for promoting neural repair in aged patients, where replication of a youthful extrinsic environment may be conducive to greater recovery. Although rare, limb transplants are now successfully performed and our work would suggest that the age of the donor may influence outcomes more than that of the host.
Supplementary Material
ACKNOWLEDGEMENTS
We thank David Roberson for help with figures, David Segal for assistance with behavioral experiments and set-up, Olusegun Babaniyi for animal care, Giovanni Coppola for microarray data processing, David Parkinson and Xin-Peng Dun for advice on the whole mount staining protocol, and Michael Costigan and Nadia Cohen for critical comments on the manuscript. We also thank Nick Andrews for advice on statistical analyses. This work was supported by the Sheldon and Miriam G. Adelson Medical Research Foundation (C.W., L.A.H., and B.B.), the Bertarelli Foundation (C.W.), NIH R01 NS038253 and P30 HD018655 (CJW), NIH RO1 AG033053 and UO1 HL100402 (A.J.W.), NIH NRSA F31 AG042227-02 (M.P.), S10RR027431-01 (B.B), NIH NS069375 (B.B.), NIH T32 HD007249 Developmental and Neonatal Biology Training Program (A.B-L.) and the Stanford MSTP and School of Medicine (A.B-L.). A.J.W. is an Early Career Scientist of the Howard Hughes Medical Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arthur-Farraj PJ, Latouche M, Wilton DK, Quintes S, Chabrol E, Banerjee A, Woodhoo A, Jenkins B, Rahman M, Turmaine M, et al. c-Jun reprograms Schwann cells of injured nerves to generate a repair cell essential for regeneration. Neuron. 2012;75:633–647. doi: 10.1016/j.neuron.2012.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrette B, Hebert MA, Filali M, Lafortune K, Vallieres N, Gowing G, Julien JP, Lacroix S. Requirement of myeloid cells for axon regeneration. J Neurosci. 2008;28:9363–9376. doi: 10.1523/JNEUROSCI.1447-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilla IE, Tanabe K, Strittmatter SM. Small proline-rich repeat protein 1A is expressed by axotomized neurons and promotes axonal outgrowth. J Neurosci. 2002;22:1303–1315. doi: 10.1523/JNEUROSCI.22-04-01303.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brosius Lutz A, Barres BA. Contrasting the glial response to axon injury in the central and peripheral nervous systems. Dev Cell. 2014;1:7–17. doi: 10.1016/j.devcel.2013.12.002. [DOI] [PubMed] [Google Scholar]
- Brosius Lutz A. Purification of Schwann cells from the Neonatal and injured adult mouse peripheral nerve. In: Barres Ben A, Stevens Beth., editors. Purifying and culturing neural cells: a laboratory manual. Cold Spring Harbor Laboratory Press; 2014. pp. 177–188. [DOI] [PubMed] [Google Scholar]
- Chen ZL, Yu WM, Strickland S. Peripheral regeneration. Annu Rev Neurosci. 2007;30:209–233. doi: 10.1146/annurev.neuro.30.051606.094337. [DOI] [PubMed] [Google Scholar]
- Cho DY, Mold JW, Roberts M. Further investigation of the negative association between hypertension and peripheral neuropathy in the elderly: an Oklahoma Physicians Resource/Research Network (OKPRN) Study. Journal of the American Board of Family Medicine : JABFM. 2006;19:240–250. doi: 10.3122/jabfm.19.3.240. [DOI] [PubMed] [Google Scholar]
- Chong MS, Reynolds ML, Irwin N, Coggeshall RE, Emson PC, Benowitz LI, Woolf CJ. GAP-43 expression in primary sensory neurons following central axotomy. J Neurosci. 1994;14:4375–4384. doi: 10.1523/JNEUROSCI.14-07-04375.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costigan M, Mannion RJ, Kendall G, Lewis SE, Campagna JA, Coggeshall RE, Meridith-Middleton J, Tate S, Woolf CJ. Heat shock protein 27: developmental regulation and expression after peripheral nerve injury. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1998;18:5891–5900. doi: 10.1523/JNEUROSCI.18-15-05891.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodarzi H, Elemento O, Tavazoie S. Revealing global regulatory perturbations across human cancers. Mol Cell. 2009;21:7014–7023. doi: 10.1016/j.molcel.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graciarena M, Dambly-Chaudiere C, Ghysen A. Dynamics of axonal regeneration in adult and aging zebrafish reveal the promoting effect of a first lesion. Proc. Natl. Acad. Sci. U S A. 2014;4:1610–1615. doi: 10.1073/pnas.1319405111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larocca JN, Norton WT. Current Protocols in Cell Biology. John Wiley & Sons, Inc; 2006. Isolation of myelin; pp. 3.25.1–3.25.19. [DOI] [PubMed] [Google Scholar]
- Lundborg G, Rosen B. Sensory relearning after nerve repair. Lancet. 2001;358:809–810. doi: 10.1016/S0140-6736(01)06001-9. [DOI] [PubMed] [Google Scholar]
- Kang H, Lichtman JW. Motor axon regeneration and muscle reinnervation in young adult and aged animals. J Neurosci. 2013;50:19480–19491. doi: 10.1523/JNEUROSCI.4067-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma CH, Omura T, Cobos EJ, Latremoliere A, Ghasemlou N, Brenner GJ, van Veen E, Barrett L, Sawada T, Gao F, et al. Accelerating axonal growth promotes motor recovery after peripheral nerve injury in mice. The Journal of clinical investigation. 2011;121:4332–4347. doi: 10.1172/JCI58675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKerracher L, David S, Jackson DL, Kottis V, Dunn RJ, Braun PE. Identification of myelin-associated glycoprotein as a major myelin-derived inhibitor of neurite growth. Neuron. 1994;13:805–811. doi: 10.1016/0896-6273(94)90247-x. [DOI] [PubMed] [Google Scholar]
- Mirsky R, Woodhoo A, Parkinson DB, Arthur-Farraj P, Bhaskaran A, Jessen KR. Novel signals controlling embryonic Schwann cell development, myelination and dedifferentiation. J Peripher Nerv Syst. 2008;13:122–135. doi: 10.1111/j.1529-8027.2008.00168.x. [DOI] [PubMed] [Google Scholar]
- Nagano A. Treatment of brachial plexus injury. J Orthop Sci. 1998;3:71–80. doi: 10.1007/s007760050024. [DOI] [PubMed] [Google Scholar]
- Napoli I, Noon LA, Ribeiro S, Kerai AP, Parrinello S, Rosenberg LH, Collins MJ, Harrisingh MC, White IJ, Woodhoo A, et al. A Central Role for the ERK-Signaling Pathway in Controlling Schwann Cell Plasticity and Peripheral Nerve Regeneration In Vivo. Neuron. 2012;73:729–742. doi: 10.1016/j.neuron.2011.11.031. [DOI] [PubMed] [Google Scholar]
- Pestronk A, Drachman DB, Griffin JW. Effects of aging on nerve sprouting and regeneration. Exp Neurol. 1980;70:65–82. doi: 10.1016/0014-4886(80)90006-0. [DOI] [PubMed] [Google Scholar]
- Ruckh JM, Zhao JW, Shadrach JL, van Wijngaarden P, Rao TN, Wagers AJ, Franklin RJ. Rejuvenation of regeneration in the aging central nervous system. Cell stem cell. 2012;10:96–103. doi: 10.1016/j.stem.2011.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seijffers R, Mills CD, Woolf CJ. ATF3 increases the intrinsic growth state of DRG neurons to enhance peripheral nerve regeneration. J Neurosci. 2007;27:7911–7920. doi: 10.1523/JNEUROSCI.5313-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen S, Sandoval J, Swiss VA, Li J, Dupree J, Franklin RJ, Casaccia-Bonnefil P. Age-dependent epigenetic control of differentiation inhibitors is critical for remyelination efficiency. Nat Neurosci. 2008;11:1024–1034. doi: 10.1038/nn.2172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka K, Webster HD. Myelinated fiber regeneration after crush injury is retarded in sciatic nerves of aging mice. J Comp Neurol. 1991;308:180–187. doi: 10.1002/cne.903080205. [DOI] [PubMed] [Google Scholar]
- Vaughan DW. Effects of advancing age on peripheral nerve regeneration. J Comp Neurol. 1992;323:219–237. doi: 10.1002/cne.903230207. [DOI] [PubMed] [Google Scholar]
- Verdu E, Ceballos D, Vilches JJ, Navarro X. Influence of aging on peripheral nerve function and regeneration. J Peripher Nerv Syst. 2000;5:191–208. doi: 10.1046/j.1529-8027.2000.00026.x. [DOI] [PubMed] [Google Scholar]
- Woodhall BBG. US Government Printing Office; 1956. Peripheral Nerve Regeneration: A Follow-up Study oOf 3,656 World War II Injuries. [Google Scholar]
- Zou Y, Chiu H, Zinovyeva A, Ambros V, Chuang CF, Chang C. Developmental decline in neuronal regeneration by the progressive change of two intrinsic timers. Science. 2013;340:372–376. doi: 10.1126/science.1231321. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.