Abstract
Rationale
Mitochondrial reactive oxygen species (ROS) are implicated in aging, chronic degenerative neurological syndromes, and myopathies. Based on the free radical hypothesis, dietary, pharmacological, and genetic ROS suppression has been tested to minimize tissue damage, with remarkable therapeutic efficacy. The effects of mitochondrial-specific ROS suppression in primary mitophagic dysfunction are unknown.
Objective
An in vivo dose-ranging analysis of ROS suppression in an experimental cardiomyopathy provoked by defective mitochondrial clearance.
Methods and Results
Mice lacking mitofusin (Mfn) 2 in hearts have impaired Parkin-mediated mitophagy leading to accumulation of damaged ROS-producing organelles and progressive heart failure. As expected, cardiomyocyte-directed expression of mitochondrial targeted catalase (CAT) at modest levels normalized mitochondrial ROS production and prevented mitochondrial depolarization, respiratory impairment, and structural degeneration in Mfn2 null hearts. In contrast, CAT expression at higher levels that super-suppressed mitochondrial ROS failed to improve either mitochondrial fitness or cardiomyopathy, revealing that ROS toxicity is not the primary mechanism for cardiac degeneration. Lack of benefit from super-suppressing ROS was associated with failure to invoke secondary autophagic pathways of mitochondrial quality control, revealing a role for ROS signaling in mitochondrial clearance. Mitochondrial permeability transition pore (MPTP) function was normal, and genetic inhibition of MPTP function did not alter mitochondrial or cardiac degeneration, in Mfn2 null hearts.
Conclusions
Local mitochondrial ROS: 1. Contribute to mitochondrial degeneration and 2. Activate mitochondrial quality control mechanisms. A therapeutic window for mitochondrial ROS suppression should minimize the former while retaining the latter, which we achieved by expressing lower levels of CAT.
Keywords: Oxidant stress, mitochondria, mitophagy, mitofusin, catalase, cardiomyopathy
INTRODUCTION
Mitochondria produce ATP that fuels excitation-contraction coupling. They are also sources of damaging reactive oxygen species (ROS). Preserving cardiac mitochondrial health is therefore essential to maintaining normal cardiac function. We recently identified a central role for the mitochondrial fusion protein mitofusin (Mfn) 2 in mitochondrial quality control signaling 1. Ablation of Mfn2 in mouse hearts interrupts Parkin-mediated mitophagy of damaged organelles, impairing cardiomyocyte respiration and inducing cardiomyopathy.
Mfn2 dimers form molecular bridges linking cardiomyocyte mitochondria to sarcoplasmic reticulum (SR) 2, which is independent of mitophagy. Calcium microdomains thus created are essential to regulatory bioenergic feedback. When mitochondrial uptake of SR-derived calcium and the calcium-sensitive generation of reduced NADPH are delayed by Mfn2 ablation, mitochondrial production of superoxide (O2−) is transiently increased. However, whether increased ROS contribute to the mitophagic cardiomyopathy provoked by Mfn2 deficiency is unknown. Accordingly, we suppressed mitochondrial ROS in Mfn2 null hearts using transgenically expressed mitochondrial-targeted catalase (mCAT) 3. Remarkably, we observed an inverse dose-response: low mCAT expression improved all aspects of cardiac and mitochondrial function, whereas high mCAT expression evoked persistent organ and organelle dysfunction, despite super-suppression of ROS. These results validate the notion that local mitochondrial ROS contribute to mitochondrial degeneration, and also uncover an essential role for ROS in mitochondrial quality control signaling.
METHODS
Mfn2loxp/loxp mice crossed onto myh6-driven nuclear-directed Cre (cardiac Mfn2 KO), ppif null (cyclophilin D KO), and lowCAT and hiCAT transgenic mice have been described previously 1, 3, 4. Details of experimental and analytical protocols are in the online data supplement.
RESULTS
Mitochondrial ROS production is increased in mitophagically impaired Mfn2 deficient hearts
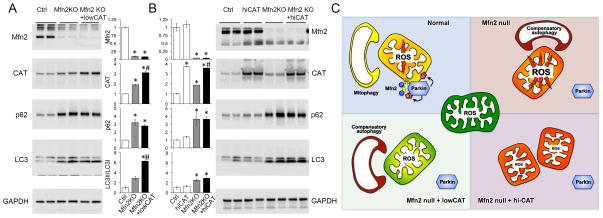
Because superoxide production is transiently increased in hemodynamically stressed young Mfn2-deficient cardiomyocytes 2, we asked if ROS are chronically increased as these mice develop their characteristic delayed cardiomyopathy 1. Basal hydrogen peroxide (H2O2) production by Mfn2-deficient cardiac mitochondria (Amplex Red 5) was increased ~3-fold over control values (Online Figure I). Immunoreactive catalase also increased with Mfn2 deficiency (see Figures 4a and 4b), consistent with an adaptive anti-oxidant response to chronically increased mitochondrial ROS 6.
Figure 4. Induction of generalized autophagy by Mfn2 ablation and different effects of low and hi-CAT.
A and B. Immunoblot analyses of autophagy markers in total myocardial proteins. Levels of Mfn2 and CAT are shown; GAPDH is loading control. Quantitative results are shown between the representative blots for comparison of lowCAT to hi-CAT. * = P<0.05 vs Ctrl; # = P<0.05 vs same treatment Mfn2 KO (ANOVA). C. Schematic depiction of how CAT-mediated modulation of mitochondrial ROS can impact secondary mitochondrial quality control pathways.
MPTP inhibition does not prevent cardiomyopathy in cardiac Mfn2 null mice
Both ROS and altered mitochondrial-SR interactions can open mitochondrial permeability transition pores (MPTP) 7–9. In contrast to a previous report 10, here (Online Figure IIa) and previously 2 we do not observe intrinsic abnormalities in calcium-stimulated MPTP opening in Mfn2 null cardiac mitochondria. Nevertheless, we tested if MPTP opening contributed to the cardiomyopathy of Mfn2 deficiency by genetically deleting the MPTP regulatory protein cyclophilin D in cardiac Mfn2 knockout mice. Compound knockout mice followed for 30 weeks exhibited no difference in cardiac hypertrophy, left ventricular contractile impairment, mitochondrial enlargement and depolarization, or mitochondrial respiratory dysfunction compared to cardiac Mfn2 null mice (Online Figures IIb–IId). Thus, inhibiting MPTP opening by genetic ablation of CypD does not improve the cardiomyopathy.
Mitochondrial ROS negatively and positively contribute to the cardiomyopathy of Mfn2 insufficiency
H2O2 is generated during oxidative phosphorylation as the normal reaction product of O2− and superoxide dismutase; its toxic effects are managed in part by catalase-mediated decomposition of H2O2 into oxygen and water. Expression of mitochondrial-targeted human catalase (mCAT) moderates the toxic effects of ROS 3, 11, 12. Accordingly, we expressed mCAT in hearts at 2 different expression levels, both of which have improved other murine cardiac disease models 3. In normal hearts the mCAT chicken β-actin driven transgene expressed at ~10-fold higher levels than the mCAT floxed-STOP bacterial artificial chromosome (here in combination with myh6-driven nuclear-targeted Cre 13), with corresponding dose-dependent effects on substrate-stimulated mitochondrial H2O2 production (Online Figures IIIa–IIIc). We therefore called these hi-CAT and lowCAT, respectively. Alone, neither hi-CAT nor lowCAT affected levels of myocardial Mfn2, electron transport chain proteins, ubiquitinated protein (Online Figures IIIb, IIId, IIIe), or any measured metric of cardiac or mitochondrial function (vide infra).
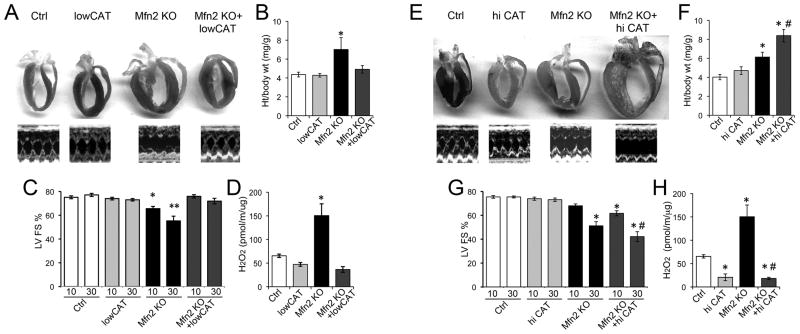
We expressed lowCAT on the cardiac Mfn2 null background to determine how mitochondrial ROS contributes to the cardiomyopathy caused by cardiac Mfn2 deficiency. As with other cardiac disease models 3, lowCAT attenuated cardiac enlargement (Figure 1a) and cardiac/cardiomyocyte hypertrophy (Figure 1b, Online Figure IV) and improved left ventricular dysfunction (Figure 1c). These benefits were associated with normalization of mitochondrial ROS production (Figure 1d).
Figure 1. Differential effects of lowCAT and hi-CAT on the cardiomyopathy induced by Mfn2 ablation. A–D: LowCAT studies.
A. Representative heart sections and m-mode echocardiograms (age 30 weeks). B. Heart weight/body weight. C. Echocardiographic left ventricular fractional shortening (LV FS) at 10 and 30 weeks. D. H2O2 production by isolated heart mitochondria. E–H: same as A–D for hi-CAT. * = P<0.05 vs Ctrl; ** = P<0.05 vs Mfn2 KO at 10 weeks; # = P<0.05 vs same treatment Mfn2 KO (ANOVA).
Ignoring Voltaire’s aphorism that “better is the enemy of good” we tested ROS super-suppression in Mfn2 deficiency by co-expressing hi-CAT. Unexpectedly, reduction of mitochondrial ROS production in Mfn2 null hearts to well below normal levels (Figure 1h) failed to improve any aspect of the hallmark cardiomyopathy. Rather, cardiac enlargement in 30 week old mice was increased further (Figures 1e, 1f) and the time-dependent decline in ventricular pump function was accelerated (Figure 1g). These results reveal an inverse dose-response relationship for catalase-mediated ROS suppression in this mitophagic cardiomyopathy.
Oxidative protein damage is minimal in Mfn2 null hearts
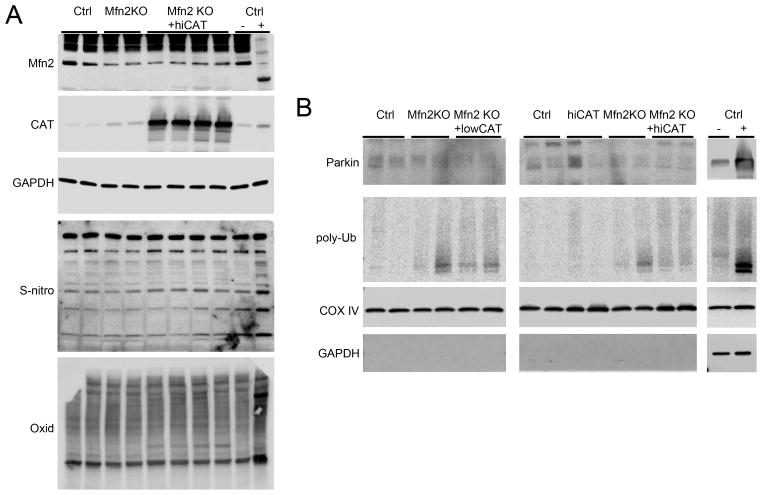
The observation that hi-CAT is more effective than lowCAT in suppressing mitochondrial ROS production in Mfn2 null hearts, but is ineffective in rescuing the cardiomyopathy, suggested that ROS cytotoxicity is not causing cardiac failure. Indeed, Mfn2 null cardiac homogenates show no evidence of increased protein S-nitrosylation or oxidation, whereas both are evident in double Mfn1/Mfn2 knockout hearts used as a positive control (Figure 2a). We confirmed that the hallmark defect in Parkin-mediated mitophagy was not affected by lowCAT or hi-CAT (Figure 2b; positive control is cardiac Parkin transgenic mouse heart).
Figure 2. Effects of Mfn2 deficiency without and with CAT co-expression on protein oxidation and mitophagy.
A. Immunoblot analysis of S-nitrosylated cysteines (S-nitro) and oxidatively modified protein (Oxid) in cardiac homogenates. B. Immunoblot analysis of mitphagy markers in cardiac mitochondrial proteins.
Mitochondrial ROS production contributes to mitochondrial quality control
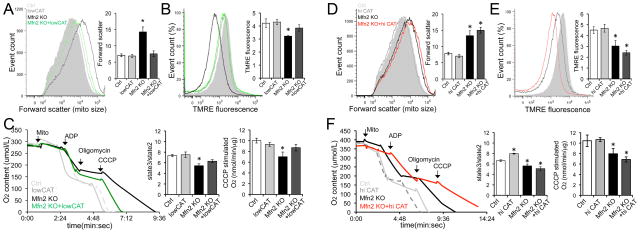
Next we tested whether mitochondrial-derived ROS were having localized effects on mitochondrial fitness in Mfn2 null hearts. ROS normalization with lowCAT (see Figure 1d) improved mitochondrial dysmorphology (Figure 3a, Online Figure V), normalized mitochondrial polarization status (a marker of mitochondrial health 14, 15, Figure 3b), and corrected respiratory dysfunction (Figure 3c). In contrast, hi-CAT expression in Mfn2 null hearts actually exaggerated the characteristic abnormalities in mitochondrial size (Figure 3d), depolarization (Figure 3e), and respiration (Figure 3f). Ultrastructural examination of Mfn2 KO + hi-CAT hearts also revealed giant mitochondria in various states of degeneration (Online Figure V). Thus, lowCAT improved overall mitochondrial quality control in Mfn2 deficient hearts, whereas hi-CAT further impeded it.
Figure 3. Differential effects of lowCAT and hi-CAT on mitochondrial dysfunction induced by Mfn2 ablation.
A–C: LowCAT studies. A. Mitochondrial size assessed by flow cytometric forward scatter. B. Mitochondrial polarization status assessed by flow cytometric TMRE fluorescence. C. Mitochondrial respiration. D–F: Same as A–C for hi-CAT. Black tracings are Mfn2 KO and colored tracings are Mfn2 KO + (low=green; hi=red) CAT; grey histograms show normal. * = P<0.05 vs Ctrl.
We posited that the general benefits of lowCAT on mitochondrial fitness, which can be explained in part by modulating mitochondrial damage, might also accrue from relieving the load on compensatory clearance mechanisms invoked by mitophagic dysfunction. Higher levels of autophagosome-associated p62/sequestosome and increased processing of the microtubule-associated protein light chain (LC3) (Figures 4a and 4b) in Mfn2 deficient hearts indicated that cell-wide autophagy, a potential secondary mechanism for culling damaged mitochondria 16, is increased. Whereas neither lowCAT nor hi-CAT alone affected autophagy, lowCAT expression further increased the ratio of LC3II/LC3I compared to Mfn2 null alone (Figure 4a). By contrast, super-suppression of mitochondrial ROS with hi-CAT did not improve LC3 processing in Mfn2 knockout hearts (Figure 4b), revealing a role for mitochondrial ROS in provoking secondary mitochondrial autophagy. In this context, combined suppression of both primary mitophagy and secondary autophagy is a likely reason why the Mfn2 null cardiomyopathy is aggravated by hi-CAT (Figure 4c).
DISCUSSION
Hydrogen peroxide can function as a signaling molecule during metabolic stress 17. Here, we show that the mitophagic cardiomyopathy evoked by cardiomyocyte-specific Mfn2 deletion can benefit from suppression, but not virtual elimination, of mitochondrial H2O2. There are three major findings: 1. ROS-mediated cytotoxicity is not the primary driver of mitophagic cardiomyopathy, which is likely caused by many different forms of mitochondrial dysfunction (including loss of polarization and respiratory impairment that we dissociated from ROS levels using hi-CAT) evoked by interrupted culling; 2. High levels of ROS found in Mfn2 null cardiac mitochondria contribute to ongoing mitochondrial damage when clearance is suppressed (because lowCAT normalized these and enhanced mitochondrial fitness); and 3. Some threshold level of mitochondrial ROS is required, likely in combination with other signals, to initiate autophagy as a secondary mechanism for mitochondrial clearance when mitophagy fails. Our discovery of a therapeutic window for mitochondrial ROS suppression validates the idea that low levels of mitochondrial-derived ROS can be therapeutic by promoting mitophagy/autophagy signaling, whereas high levels are deleterious because they produce mitochondrial and cellular toxicity 18.
To our knowledge this is the first evaluation of the dose-dependent effectiveness of ROS suppression in a condition caused by a primary defect in mitochondrial quality control. The bimodal dose-response reveals multiple levels of interconnectedness between mitochondrial dysfunction and culling. To maintain proper cellular homeostasis mitophagy must selectively target only dysfunctional organelles; polarization status is a central determinant of whether a given mitochondrion will be retained or sequestered and mitophagically eliminated 14, 15. Mitochondrial inner membrane potential is normally reciprocally related to mitochondrial ROS production; depolarized mitochondria produce toxic ROS that may mark the organelle for Parkin-mediated mitophagy. When lack of Mfn2 interrupts normal Parkin signaling, chronically increased mitochondrial ROS contributes to mitochondrial damage, establishing a positive feedback loop. Accumulation of depolarized, ROS-producing organelles invokes a back-up mechanism for disposing of damaged cellular components, cell-wide autophagy. Suppressing mitochondrial ROS production to levels that are near-normal (as with lowCAT expression) interrupts this vicious cycle (Figure 4c).
In contrast, hi-CAT expression in Mfn2 KO hearts dissociates mitochondrial polarization status and ROS production; ROS levels drop to a fraction of normal, despite marked organelle depolarization. Thus, any ROS contribution to the signal for mitochondrial elimination via secondary autophagy is lost and damaged mitochondria continue to accumulate. These findings provide in vivo evidence for an essential role of mitochondrial-derived ROS in mitochondrial quality control via mitophagy and/or autophagy. The deleterious effects conferred by mitochondrial ROS super-suppression warrant consideration of anti-oxidant dose-effect in the heart and other organs when evaluating therapeutics for disorders, such as Parkinson’s Disease or aging, wherein mitophagy defects are central to observed pathology.
Supplementary Material
Novelty and Significance.
What Is Known?
Damaged or senescent mitochondria produce toxic ROS and have to be culled through mitophagy.
When mitophagy is impaired as in aging or some chronic diseases, ROS levels increase.
Suppressing mitochondrial ROS has been beneficial in experimental aging and disease models.
What New Information Does This Article Contribute?
Mitochondrial ROS toxicity is not a major direct cause of the cardiomyopathy that follows suppression of mitophagy.
Mitochondrial ROS contribute to mitochondrial dysfunction in mitophagically impaired hearts, which is relieved by ROS normalization with mitochondrial catalase.
ROS super-suppression with highly expressed catalase interrupts compensatory mitochondrial autophagy, thereby aggravating mitophagic cardiomyopathy.
The free radical hypothesis implicating reactive oxygen species (ROS) in aging and numerous chronic diseases is widely accepted and has spawned attempts to prevent senescence or organ degeneration by ROS scavenging. However, ROS may also act as indicators of cell/organelle damage, thereby providing a signal for necessary repair or removal. Under this circumstance, overly aggressive ROS suppression might conceivably interfere with normal adaptive responses. In our study we have, for the first time, validated this scenario. First, we show that mitochondrial ROS production is chronically increased in the mitophagically impaired mitofusin 2-deficient heart model. We then demonstrate that expression of mitochondrial-directed catalase (mtCAT) at levels that normalize ROS corrects all mitochondrial abnormalities and prevents the cardiomyopathy evoked by Mfn2 deletion. Strikingly however, expression of mtCAT at higher levels that super-suppress mitochondrial ROS actually exacerbates mitochondrial and cardiac dysfunction. Mechanistically, this is explained by failure to invoke compensatory autophagy when ROS levels are too low. These findings uncover the essential role for mitochondrial-derived ROS in mitochondrial quality control signaling and provide an example of how excessive suppression of mitochondrial ROS can actually cause harm, sounding a cautionary note for any chronic disease being considered for anti-oxidant therapy.
Acknowledgments
SOURCES OF FUNDING
Supported by R01 HL 59888 from the National Institutes of Health (GWD) and an American Heart Association predoctoral fellowship award (MS).
Nonstandard Abbreviations and Acronyms
- mCAT
mitochondrial-targeted catalase
- LC3
microtubule-associated protein light chain
- Mfn
mitofusin
- MPTP
mitochondrial permeability transition pore
- ROS
reactive oxygen species
- SR
sarcoplasmic reticulum
- TMRE
tetramethylrhodamine ethyl ester
Footnotes
DISCLOSURES
The authors declare that they have no conflicts of interest.
References
- 1.Chen Y, Dorn GW., 2nd PINK1-phosphorylated mitofusin 2 is a Parkin receptor for culling damaged mitochondria. Science. 2013;340:471–475. doi: 10.1126/science.1231031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chen Y, Csordas G, Jowdy C, Schneider TG, Csordas N, Wang W, Liu Y, Kohlhaas M, Meiser M, Bergem S, Nerbonne JM, Dorn GW, 2nd, Maack C. Mitofusin 2-containing mitochondrial-reticular microdomains direct rapid cardiomyocyte bioenergetic responses via interorganelle Ca2+ crosstalk. Circ Res. 2012;111:863–875. doi: 10.1161/CIRCRESAHA.112.266585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dai DF, Johnson SC, Villarin JJ, Chin MT, Nieves-Cintron M, Chen T, Marcinek DJ, Dorn GW, 2nd, Kang YJ, Prolla TA, Santana LF, Rabinovitch PS. Mitochondrial oxidative stress mediates angiotensin II-induced cardiac hypertrophy and Galphaq overexpression-induced heart failure. Circ Res. 2011;108:837–846. doi: 10.1161/CIRCRESAHA.110.232306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baines CP, Kaiser RA, Purcell NH, Blair NS, Osinska H, Hambleton MA, Brunskill EW, Sayen MR, Gottlieb RA, Dorn GW, 2nd, Robbins J, Molkentin JD. Loss of cyclophilin D reveals a critical role for mitochondrial permeability transition in cell death. Nature. 2005;434:658–662. doi: 10.1038/nature03434. [DOI] [PubMed] [Google Scholar]
- 5.Lagranha CJ, Deschamps A, Aponte A, Steenbergen C, Murphy E. Sex differences in the phosphorylation of mitochondrial proteins result in reduced production of reactive oxygen species and cardioprotection in females. Circ Res. 2010;106:1681–1691. doi: 10.1161/CIRCRESAHA.109.213645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rindler PM, Plafker SM, Szweda LI, Kinter M. High dietary fat selectively increases catalase expression within cardiac mitochondria. J Biol Chem. 2013;288:1979–1990. doi: 10.1074/jbc.M112.412890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rodriguez-Enriquez S, He L, Lemasters JJ. Role of mitochondrial permeability transition pores in mitochondrial autophagy. Int J Biochem Cell Biol. 2004;36:2463–2472. doi: 10.1016/j.biocel.2004.04.009. [DOI] [PubMed] [Google Scholar]
- 8.Diwan A, Matkovich SJ, Yuan Q, Zhao W, Yatani A, Brown JH, Molkentin JD, Kranias EG, Dorn GW., 2nd Endoplasmic reticulum-mitochondria crosstalk in NIX-mediated murine cell death. J Clin Invest. 2009;119:203–212. doi: 10.1172/JCI36445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen Y, Lewis W, Diwan A, Cheng EH, Matkovich SJ, Dorn GW., 2nd Dual autonomous mitochondrial cell death pathways are activated by Nix/BNip3L and induce cardiomyopathy. Proc Natl Acad Sci U S A. 2010;107:9035–9042. doi: 10.1073/pnas.0914013107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Papanicolaou KN, Khairallah RJ, Ngoh GA, Chikando A, Luptak I, O’Shea KM, Riley DD, Lugus JJ, Colucci WS, Lederer WJ, Stanley WC, Walsh K. Mitofusin-2 maintains mitochondrial structure and contributes to stress-induced permeability transition in cardiac myocytes. Mol Cell Biol. 2011;31:1309–1328. doi: 10.1128/MCB.00911-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dai DF, Santana LF, Vermulst M, Tomazela DM, Emond MJ, MacCoss MJ, Gollahon K, Martin GM, Loeb LA, Ladiges WC, Rabinovitch PS. Overexpression of catalase targeted to mitochondria attenuates murine cardiac aging. Circulation. 2009;119:2789–2797. doi: 10.1161/CIRCULATIONAHA.108.822403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schriner SE, Linford NJ, Martin GM, Treuting P, Ogburn CE, Emond M, Coskun PE, Ladiges W, Wolf N, Van Remmen H, Wallace DC, Rabinovitch PS. Extension of murine life span by overexpression of catalase targeted to mitochondria. Science. 2005;308:1909–1911. doi: 10.1126/science.1106653. [DOI] [PubMed] [Google Scholar]
- 13.Oka T, Maillet M, Watt AJ, Schwartz RJ, Aronow BJ, Duncan SA, Molkentin JD. Cardiac-specific deletion of Gata4 reveals its requirement for hypertrophy, compensation, and myocyte viability. Circ Res. 2006;98:837–845. doi: 10.1161/01.RES.0000215985.18538.c4. [DOI] [PubMed] [Google Scholar]
- 14.Narendra DP, Jin SM, Tanaka A, Suen DF, Gautier CA, Shen J, Cookson MR, Youle RJ. PINK1 is selectively stabilized on impaired mitochondria to activate Parkin. PLoS Biol. 2010;8:e1000298. doi: 10.1371/journal.pbio.1000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsuda N, Sato S, Shiba K, Okatsu K, Saisho K, Gautier CA, Sou YS, Saiki S, Kawajiri S, Sato F, Kimura M, Komatsu M, Hattori N, Tanaka K. PINK1 stabilized by mitochondrial depolarization recruits Parkin to damaged mitochondria and activates latent Parkin for mitophagy. J Cell Biol. 2010;189:211–221. doi: 10.1083/jcb.200910140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirkin V, McEwan DG, Novak I, Dikic I. A role for ubiquitin in selective autophagy. Mol Cell. 2009;34:259–269. doi: 10.1016/j.molcel.2009.04.026. [DOI] [PubMed] [Google Scholar]
- 17.Salmeen A, Andersen JN, Myers MP, Meng TC, Hinks JA, Tonks NK, Barford D. Redox regulation of protein tyrosine phosphatase 1B involves a sulphenyl-amide intermediate. Nature. 2003;423:769–773. doi: 10.1038/nature01680. [DOI] [PubMed] [Google Scholar]
- 18.Scherz-Shouval R, Elazar Z. Regulation of autophagy by ROS: physiology and pathology. Trends Biochem Sci. 2011;36:30–38. doi: 10.1016/j.tibs.2010.07.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.