Summary
Under mild abiotic-stress conditions, Arabidopsis atg mutants showed a functional stay-green phenotype which is probably caused by the lack of chloroplastic autophagy and the retrograde regulation of senescence-associated gene expression.
Key words: Abiotic stress, Arabidopsis thaliana, autophagy, atg5, chlorophyll degradation, leaf senescence, stay-green.
Abstract
Plant autophagy, one of the essential proteolysis systems, balances proteome and nutrient levels in cells of the whole plant. Autophagy has been studied by analysing Arabidopsis thaliana autophagy-defective atg mutants, but the relationship between autophagy and chlorophyll (Chl) breakdown during stress-induced leaf yellowing remains unclear. During natural senescence or under abiotic-stress conditions, extensive cell death and early yellowing occurs in the leaves of atg mutants. A new finding is revealed that atg5 and atg7 mutants exhibit a functional stay-green phenotype under mild abiotic-stress conditions, but leaf yellowing proceeds normally in wild-type leaves under these conditions. Under mild salt stress, atg5 leaves retained high levels of Chls and all photosystem proteins and maintained a normal chloroplast structure. Furthermore, a double mutant of atg5 and non-functional stay-green nonyellowing1-1 (atg5 nye1-1) showed a much stronger stay-green phenotype than either single mutant. Taking these results together, it is proposed that autophagy functions in the non-selective catabolism of Chls and photosynthetic proteins during stress-induced leaf yellowing, in addition to the selective degradation of Chl–apoprotein complexes in the chloroplasts through the senescence-induced STAY-GREEN1/NYE1 and Chl catabolic enzymes.
Introduction
Senescence marks the final stage of leaf development in plants. In the early phase of leaf senescence, developmental and environmental cues signal the plant cells to activate transcription factors (TFs) that modulate the expression of senescence-associated genes (SAGs) (Guo and Ecker, 2004; Balazadeh et al., 2008). The products of these SAGs conduct the highly ordered breakdown of intracellular organelles, including the degradation of proteins and macromolecules to remobilize leaf nutrients into other developing organs such as new leaves or seeds, or into storage organs (Lim et al., 2007; Robinson et al., 2012).
Autophagy, a highly conserved process in eukaryotes, functions as one of the major pathways for the massive degradation of intracellular proteins during leaf senescence (Nakatogawa et al., 2009; Reumann et al., 2010) as well as for survival under some biotic/abiotic-stress conditions (Klionsky, 2004). Autophagy occurs by two main mechanisms, microautophagy and macroautophagy. In microautophagy, an invagination of the vacuolar membrane directly engulfs the cytosolic component to be degraded (Klionsky and Ohsumi, 1999). By contrast, in macroautophagy, autophagosomes form at the periphery of damaged or overproduced proteins. Autophagosomes enclose organelles or cytosolic compounds, which are transported into the vacuole and broken down by the non-selective degradation pathway (Meijer et al., 2007). To date, more than 30 autophagy-associated (atg) genes have been identified in yeast and Arabidopsis (Arabidopsis thaliana) (Bassham et al., 2006).
In pre-senescent leaves during vegetative growth, chloroplasts contain the majority of plant nutrients. For example, chloroplastic proteins contain 75–80% of total leaf nitrogen in C3 plants (Makino and Osmond, 1991). Thus, the degradation of chloroplast proteins in old or inefficient leaves during senescence provides important nutrients for relocation to developing organs. In recent years, the degradation mechanisms of chloroplasts and chloroplast proteins during senescence have been widely studied. During leaf senescence, Rubisco, the most abundant stromal protein in the chloroplasts (Wittenback, 1978), is released from chloroplasts into the cytoplasm as small double-membrane bodies termed Rubisco-containing bodies (RCBs; Chiba et al., 2003) that are then transported to the central vacuole by autophagy for degradation (Ishida et al., 2008). RCBs were not observed in the leaves of autophagy-defective atg4 (Wada et al., 2009) and atg5 (Ishida et al., 2008) mutants in Arabidopsis, indicating the direct involvement of macroautophagy in the degradation of Rubisco during leaf senescence. For the degradation of Rubisco and stromal proteins, another extra-chloroplastic degradation system, called senescence-associated vacuoles (SAVs), was also identified. SAVs are clearly smaller than the central vacuole and contain Rubisco and other stromal proteins, including glutamine synthetase, but not the photosystem proteins (Martinez et al., 2008). This indicates that the SAV-dependent degradation system mainly functions in the degradation of stromal proteins in chloroplasts.
In contrast to the extra-chloroplastic degradation mechanisms for Rubisco and other proteins, an intra-chloroplastic degradation system mainly degrades thylakoid proteins. Plastids isolated from senescing leaves can degrade photosystem proteins under light conditions (Feller et al., 2008) indicating that senescing chloroplasts have active systems to degrade photosystem proteins. This system may include the chloroplast protease FtsH, as the Arabidopsis T-DNA insertion KO mutants of ftsH6 were unable to degrade Lhcb3 during dark-induced senescence and were also unable to degrade Lhcb1 and Lhcb3 under high light conditions (Zelisko et al., 2005).
Chlorophyll (Chl) is degraded by several Chl catabolic enzymes (CCEs; Hörtensteiner, 2013). In addition, STAY-GREEN (SGR), Mendel’s green cotyledon gene encoding a novel chloroplast protein, functions in the initiation of Chl degradation (Park et al., 2007; Ren et al., 2007). Recently, it was demonstrated that SGR and six CCEs form a complex with light-harvesting complex II (LHCII), which may allow metabolic channelling of phototoxic Chl degradation intermediates (Sakuraba et al., 2012b, 2013). Chl degradation ends with the formation of fluorescent chlorophyll catabolite (FCC), a non-toxic Chl degradation intermediate, in chloroplasts. For the final steps of Chl breakdown, FCC is transported into the vacuole and converted to non-fluorescent Chl catabolite (NCC) (Oberhuber et al., 2003; Hörtensteiner and Krautler., 2011).
Although Chl breakdown generally occurs in the chloroplast until the formation of FCC, Wada et al. (2009) detected Chl fluorescence in the central vacuole of dark-induced senescing leaves in Arabidopsis, strongly indicating that macroautophagy also functions in the transport of Chl–apoprotein complexes from chloroplasts to the vacuole during senescence. Considering this function, the autophagy-dependent degradation system and other intra-chloroplastic degradation systems seem to share target proteins or macromolecules, including Chl and Chl-binding photosystem proteins. However, the relationship among these different degradation systems remains enigmatic.
It was found here that atg5 mutants display a stay-green phenotype only under mild abiotic-stress conditions, but not under strong stress conditions; atg5 leaves showed early leaf yellowing with extensive cell death under strong abiotic-stress conditions. Under mild abiotic-stress conditions, however, atg5 acts as a functional stay-green mutant, maintaining the proper balance of Chls and photosynthetic proteins and retaining the grana thylakoid structure in the chloroplasts. Genetic analysis of atg5 and the non-functional stay-green mutant nye1-1 revealed that autophagy contributes to the non-selective breakdown of Chl-photosynthetic proteins during mild abiotic-stress-induced leaf yellowing, in addition to the selective breakdown of Chl-apoproteins through a dynamic STAY-GREEN1(SGR1)/NYE1-CCE complex in the senescing chloroplasts (Sakuraba et al., 2012b). The relationship between autophagy-induced and SGR1-dependent degradation of the Chl-apoprotein complex in chloroplasts is also discussed.
Materials and methods
Plant materials and growth conditions
The Arabidopsis thaliana plants were grown on soil at 21–23 °C under long day (LD) conditions (16/8h light/dark; 90–100 μmol m–2 s–1 cool-fluorescent white light). For dark treatment, detached or attached leaves of 3-week-old plants were placed in complete darkness. Wild type, atg5 and nye1-1 are of the Col-0 ecotype. The atg7 mutant (Ws-2 ecotype; CS39995) was obtained from the Arabidopsis Biological Resource Center (ABRC, Ohio, USA).
Trypan blue staining
Leaves were incubated overnight in lactophenol-trypan blue solution (10ml lactic acid, 10ml glycerol, 10g phenol, and 10mg trypan blue dissolved in 10ml distilled water) (Koch and Slusarenko, 1990). Stained leaves were then boiled for 1min and then decolourized in 60% glycerol solution.
Chlorophyll quantification
Chlorophylls (Chls) were extracted from leaf tissues with 80% ice-cold acetone solution at 4 °C. Chl concentration was quantified by a spectrophotometric method (Porra et al., 1989).
Immunoblot analysis
Protein extracts were prepared from rosette leaves of Arabidopsis thaliana. A 10mg aliquot of leaf tissue was ground in liquid nitrogen and homogenized with 100 μl of sample buffer [50mM TRIS–HCl, pH 6.8, 2mM EDTA, 10% (w/v) glycerol, 2% SDS, and 6% 2-mercaptoethanol] was used to suspend the protein extracts. The protein samples were subjected to SDS-PAGE. Gels were stained with Coomassie Brilliant Blue R-250 (Sigma–Aldrich). Antibodies against photosynthetic proteins, including Lhca3, Lhcb1, Lhcb2, Lhcb4, Lhcb5, CP43, D1, and PsaA (Agrisera, Sweden), were used for immunoblot analysis. Each protein was detected using an electrochemiluminescence (ECL) system (WESTSAVE, AbFRONTIER, Seoul, Korea) according to the manufacturer’s manual.
Chl fluorescence measurement using pulse amplitude modulation
Maximal photochemical efficiency of PSII (F v/F m) was measured using the OS-30p+ instrument (OPTI-SCIENCES, USA). Detached leaves before and after salt treatment were adapted in the dark for 5min and the F v/F m ratio was measured at room temperature. This 5min dark treatment resulted in the complete oxidation of Q A.
Transmission electron microscopy
Transmission electron microscopy was conducted as previously described by Inada et al. (1998) with some modifications. Leaf tissues were fixed with modified Karnovsky’s fixative (2% paraformaldehyde, 2% glutaraldehyde, and 50mM sodium cacodylate buffer, pH 7.2). Samples were then washed with 0.05M sodium cacodylate buffer, pH 7.2, three times at 4 °C for 10min. The samples were post-fixed at 4 ºC for 2h with 1% osmium tetroxide in 0.05M sodium cacodylate buffer, pH 7.2, and washed twice with distilled water at room temperature. Samples were stained in 0.5% uranyl acetate at 4 °C overnight and dehydrated in an ethanol gradient and propylene oxide, then finally infiltrated with Spurr’s resin. Polymerization was performed at 70 °C for 24h and samples were sectioned with an ultramicrotome (MT-X). The sections were mounted on copper grids and stained with 2% uranyl acetate for 7min and with Reynolds’ lead citrate for 7min. Micrographs were made by using a LIBRA 120 transmission electron microscope (JEOL, Japan).
Ion leakage measurement
To measure ion leakage after treatment, approximately 10 rosette leaves were placed in a tube with 6ml of 0.4mM mannitol. The tubes were placed at room temperature for 3h with shaking. Conductivity of the incubated solution was measured using an electroconductivity meter (CON6 METER, LaMOTTE Co., USA), before and after boiling for 10min.
Abiotic-stress treatments
Analysis of salt stress was performed as previously described by Wu et al. (2012) with minor modifications. Detached leaves of 3-week-old plants were floated abaxial side-up, on 3mM MES buffer (pH 5.8) containing 150, 300, or 450mM NaCl. For osmotic stress, leaves were floated on buffer containing 50, 200, or 400mM mannitol. For oxidative stress, leaves were floated on buffer containing 5, 20, or 50mM H2O2.
Reverse transcription (RT) and quantitative real-time PCR (qPCR) analysis
Total RNA was extracted from the leaf tissues using the Plant RNA Extraction Kit (iNtRON Biotechnology, Seoul, Korea) including the RNase-free DNase I treatment step to remove possible genomic DNA contamination. For RT, the first-strand cDNAs were prepared with 5 μg total RNA using M-MLV reverse transcriptase and aqn oligo(dT) primer (Promega). For quantitative real-time PCR (qPCR), 20 μl reactions, including first-strand cDNAs equivalent to 50ng total RNA, 10 μl 2× Universal SYBR Green Master Mix (Roche), and gene-specific forward and reverse primers (see Supplementary Table S1 at JXB online), were analysed using a Light Cycler 480 (Roche Diagnostics). Data analysis was conducted using the Roche Optical System software (ver. 1.5). The efficiency of qPCR analysis was calculated by comparing the slope of linear regression of Ct and log10 of gene copies. Relative gene expression levels were normalized against the transcript levels of GAPDH (encoding glyceraldehyde phosphate dehydrogenase; At1g16300) as previously reported by Sakuraba et al. (2010).
Results
atg5 leaves exhibit a stay-green phenotype under mild abiotic-stress conditions
Plant autophagy affects senescence and stress tolerance; atg mutants exhibit accelerated leaf yellowing during age- and dark-induced senescence (Thompson et al., 2005), and hyper-sensitivity to abiotic stresses such as high salinity, oxidative stress, and drought (Xiong et al., 2007; Liu et al., 2009; Zhou et al., 2013). This indicates that autophagy plays an important role in maintaining the proper balance of the cellular proteome during abiotic stresses. However, stress-induced chlorophyll (Chl) degradation should be impaired when autophagy does not operate properly, because autophagy is involved in chloroplast degradation, including Chl breakdown, during senescence (Ishida et al., 2008; Wada et al., 2009; Ono et al., 2012).
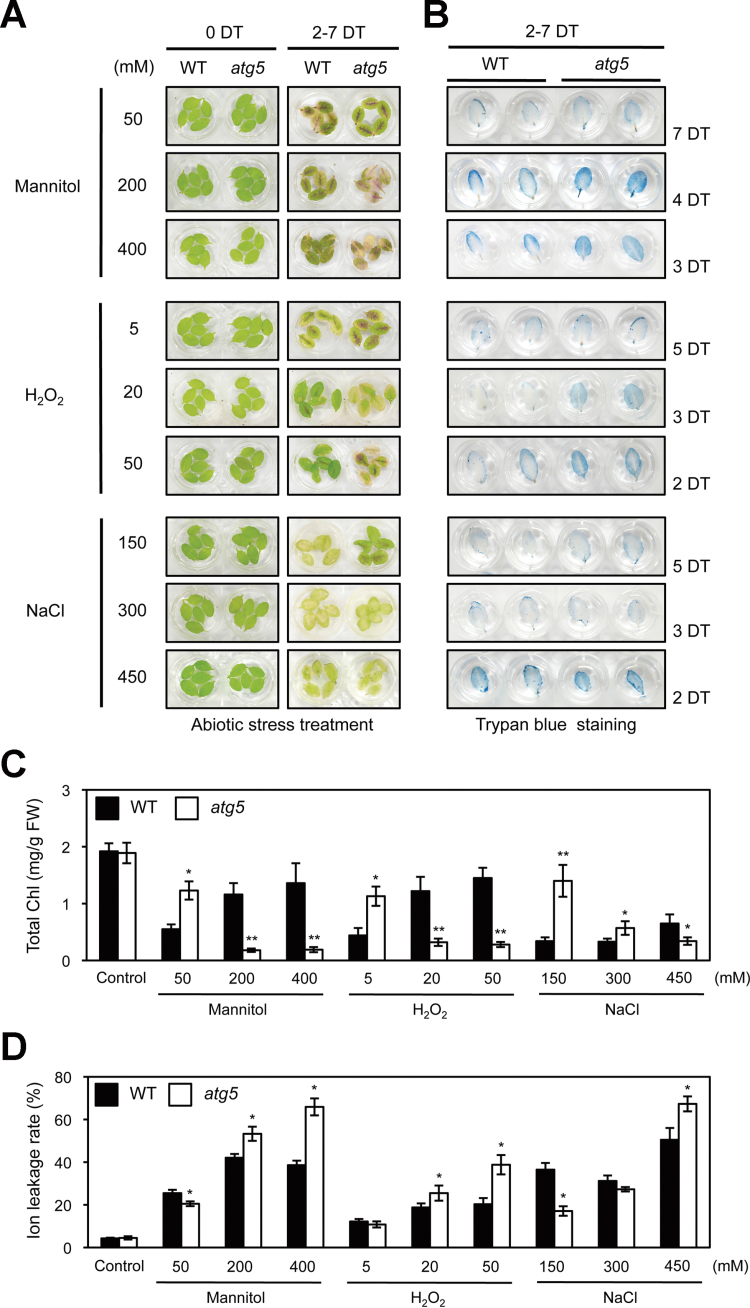
To investigate the relationship between autophagy and Chl degradation in more detail, we examined the visual phenotypes of atg5 leaves under different abiotic-stress conditions (Fig. 1), including salt (NaCl), osmotic pressure (mannitol), and oxidative reagent (H2O2) treatments. To this end, the detached rosette leaves of 3-week-old plants were used to separate the effect of autophagy defects in each plant organ (Thompson et al., 2005). As previously observed in whole plants (Thompson et al., 2005; Zhou et al., 2013), atg5 leaves exhibited an early senescence phenotype under strong abiotic-stress conditions, such as 200 and 400mM mannitol, 20 and 50mM H2O2, or 450mM NaCl (Fig. 1A). Trypan blue staining revealed that early leaf yellowing of atg5 leaves under these strong abiotic stresses resulted from cell death (Fig. 1B). By contrast, atg5 leaves exhibited a stay-green phenotype under mild abiotic-stress conditions, such as 50mM mannitol, 5mM H2O2, or 150mM NaCl treatments (Fig. 1A). Notably, under these mild abiotic stresses, atg5 leaves barely showed any cell death phenotype (Fig. 1B), suggesting that atg5 is defective in Chl degradation, although only under mild abiotic-stress conditions in which accelerated cell death hardly occurs. To understand the stay-green phenotype of atg5 leaves under mild stress conditions in more detail, total Chl levels and ion leakage rates were measured in each condition as an indicator of membrane disintegration and plant cell death. Consistent with the visible phenotypes, atg5 leaves under mild abiotic-stress conditions had significantly higher total Chl levels than wild-type leaves (Fig. 1C). Compared with wild-type leaves, atg5 leaves had significantly lower ion leakage rates, but had significantly higher rates under strong stress conditions (Fig. 1D). This confirms that the degree of cell death is closely associated with the phenotype of atg5 leaves under these abiotic-stress conditions. atg7, another autophagy mutant, was also examined. Similar to atg5 leaves, atg7 leaves also showed a stay-green phenotype under mild abiotic-stress conditions (see Supplementary Fig. S1 at JXB online).
Fig. 1.
Phenotypic characterization of atg5 leaves under different abiotic stresses. (A) Visible phenotypes of detached rosette leaves from 3-week-old wild-type (WT) and atg5 mutants under osmotic (50, 200, or 400mM mannitol), oxidative (5, 20, or 50mM H2O2), and salt (150, 300, or 450mM NaCl) stress conditions. (B) Cell death in WT and atg5 leaves under abiotic stresses, as shown by trypan blue staining. (C, D) The changes of total Chl levels (C) and ion leakage rates (D) in WT and atg5 leaves after abiotic-stress treatments in (A). Black and white bars indicate WT and atg5, respectively. DT, days of treatment. Similar results were obtained from three independent experiments. Student’s t-test (*P<0.05, **P<0.01). (This figure is available in colour at JXB online.)
Our results using detached atg5 leaves conflicted with previous results using whole plant bodies under salt-stress conditions (Zhou et al., 2013). Therefore, the whole-plant phenotype of atg5 mutants grown for 2 weeks on phytoagar plates containing a low concentration of NaCl (150mM) was examined. Consistent with the previous results (Zhou et al., 2013), older leaves (cotyledon and 1st cycle of rosettes) of atg5 plants showed a leaf necrosis phenotype (see Supplementary Fig. S2A and B at JXB online). However, younger leaves (2nd and 3rd cycle of rosettes) stayed green with higher Chl levels compared with those of wild-type leaves, suggesting that both detached and attached leaves of atg5 have a stay-green capacity under mild salt-stress conditions, although atg5 mutants exhibit a necrosis phenotype in older leaves.
atg5 exhibits a functional stay-green phenotype under mild abiotic-stress conditions
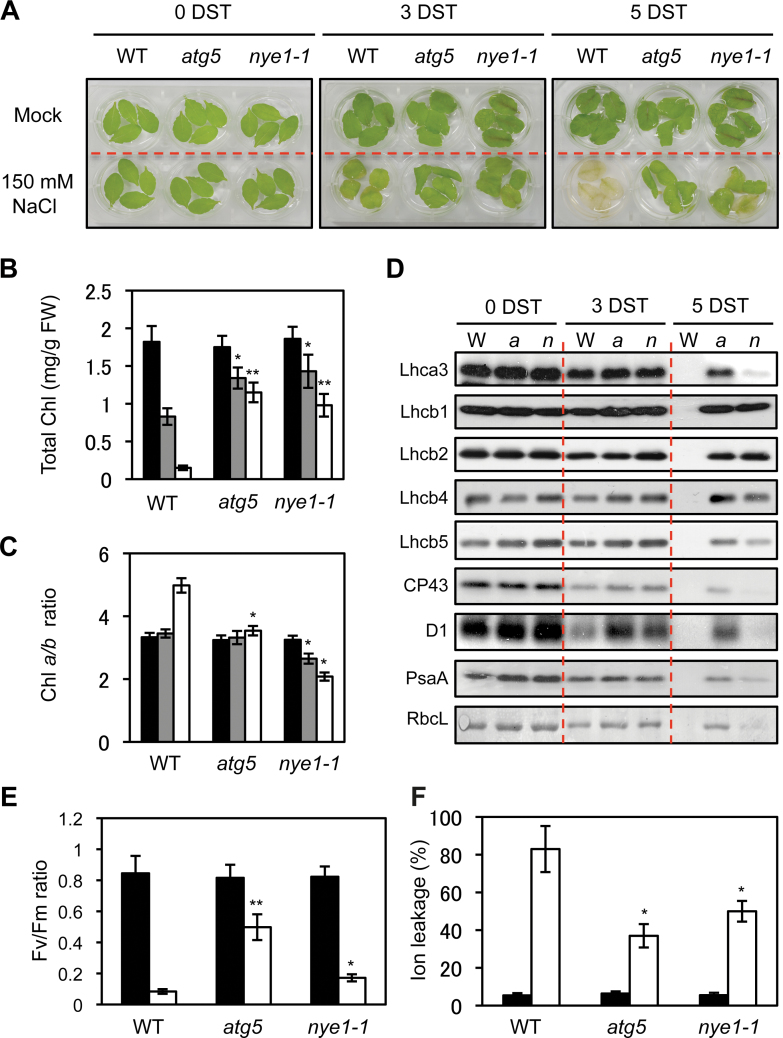
To characterize the stay-green phenotype of atg5 leaves under mild abiotic stresses in more detail, several photosynthetic parameters of atg5 were compared with a non-functional stay-green SGR1 mutant, nye1-1 (Ren et al., 2007), under mild salt-stress conditions (150mM NaCl). Similar to atg5 mutants, nye1-1 mutants also exhibited a stay-green phenotype after 3 d and 5 d of salt treatment (Fig. 2A). Consistent with the visible phenotype, atg5 and nye1-1 leaves showed significantly higher Chl retention than wild-type leaves (Fig. 2B).
Fig. 2.
Characterization of atg5 leaves under mild salt stress conditions. (A–F) Visible phenotypes (A), total Chl levels (B), Chl a/b ratios (C), photosynthetic protein levels (D), F v/F m ratios (E), and ion leakage rates (F) of wild-type (WT), atg5, and nye1-1 leaves under the mild salt-stress conditions. Detached leaves of 3-week-old WT, atg5, and nye1-1 plants were incubated in 3mM MES buffer (pH 5.8) containing 150mM NaCl for 3 d and 5 d (3 and 5 DST, days of salt stress). (B, C, E, and F) Black, grey, and white bars indicate 0, 3, and 5 DST, respectively. (D) Antibodies against PSII core (CP43 and D1), PSII antenna (Lhcb1, Lhcb2, Lhcb4, and Lhcb5), PSI antenna (Lhca1 and Lhca3), and PSI core (PsaA) were used. RbcL (Rubisco large subunit) was visualized by Coomassie Brilliant Blue (CBB) staining after immunoblot analysis. These experiments were repeated more than three times with similar results. DST: days of salt treatment. Student’s t-test (*P<0.05, **P<0.01). (This figure is available in colour at JXB online.)
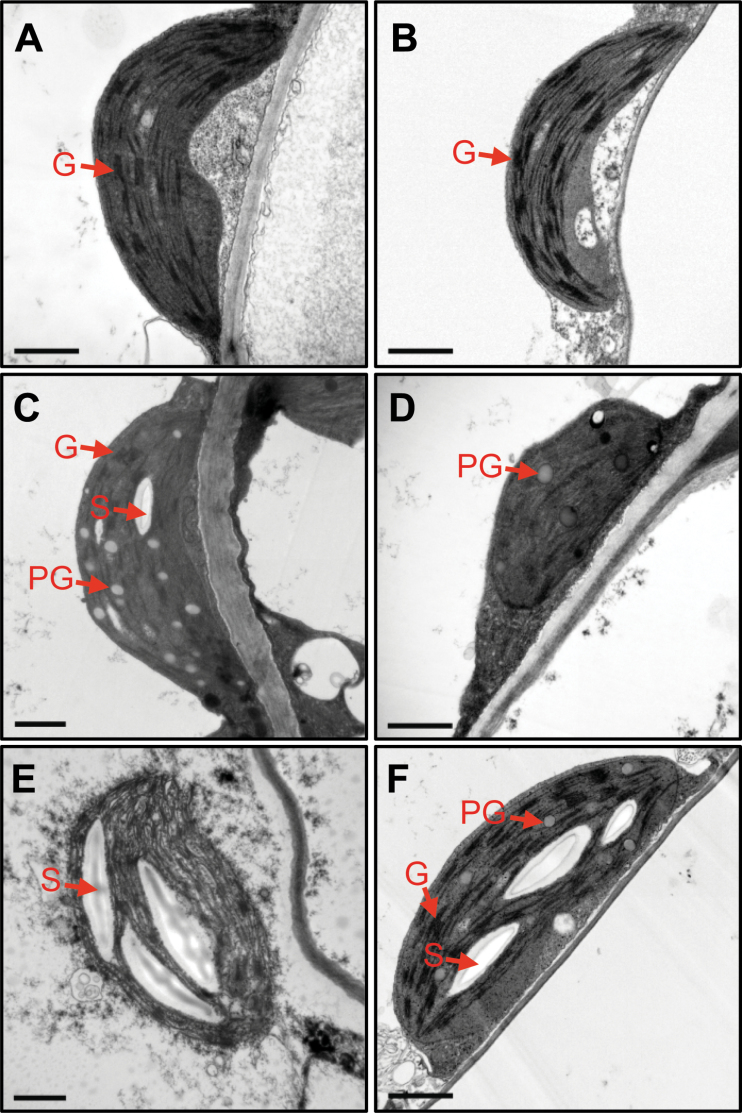
Stay-green plants can be divided into functional and non-functional types (Thomas and Howarth, 2000; Hörtensteiner, 2009). The Arabidopsis SGR1 mutant, nye1-1, and the Chl catabolism-defective mutants, nyc1-1 and pph-1, belong to the non-functional stay-green type (Kusaba et al., 2007; Sato et al., 2007; Morita et al., 2009). Several photosynthetic parameters were therefore analysed to examine whether atg5 is a functional or a non-functional stay-green type mutant under mild salt-stress conditions. First, the Chl a/b ratio of leaves was measured. Because SGR1/NYE1 contributes to Chl degradation in the light-harvesting complex of photosystem II (LHCII) with CCEs (Sakuraba et al., 2012b), the Chl a/b ratio of nye1-1 mutants gradually decreased under salt stress (Fig. 2C), and this selective stabilization of LHCII mainly contributes to a non-functional stay-green phenotype. By contrast, the Chl a/b ratio of atg5 leaves did not change under salt stress. Similar to the Chl a/b ratio in nye1-1, LHCII proteins (Lhcb1, 2, 4, and 5) were predominantly retained while other photosystem proteins (CP43, D1, Lhca1, Lhca3, and PsaA) gradually decreased in nye1-1 leaves during salt treatment (Fig. 2D). By contrast, atg5 leaves retained all photosystem proteins at high levels (Fig. 2D), indicating that ATG5 is involved in the non-selective destabilization of all photosystem proteins under salt stress-induced leaf senescence The Chl fluorescence parameter, F v /F m, representing the optimal yield of PSII, was then compared. After 4 d of salt treatment, the F v /F m ratio in atg5 leaves was higher than in wild-type and nye1-1 leaves (Fig. 2E). The ion leakage rate, an indicator of membrane disintegration and one of the important factors for determining the stay-green type, was then examined. Ion leakage rates of atg5 and nye1-1 leaves were lower than for wild-type leaves (Fig. 2F). The chloroplast structure of atg5 leaves was also examined. Before salt treatment, atg5 leaves contained normal shapes of chloroplast and grana thylakoid structures, similar to wild-type leaves (Fig. 3A, B). After 4 d of salt treatment, grana thylakoids were hardly found in the chloroplasts of wild-type leaves, and different types of degrading chloroplasts were found (Fig. 3C–E). By contrast, atg5 leaves retained the grana thylakoids (Fig. 3F).
Fig. 3.
Transmission electron microscopy of plastids in atg5 leaves under mild salt-stress conditions. (A, B) Chloroplasts in the mesophyll cells of 3-week-old wild-type (WT; A) and atg5 (B) leaves before salt (150mM) treatment. (C–F) Chloroplasts in the mesophyll cells of WT (C, D, E) and atg5 (F) leaves after 4 DST. DST, days of salt treatment; G, grana thylakoid; PG, plastoglobule; S, starch. Scale bars=1 μm. (This figure is available in colour at JXB online.)
Taken together, our results indicate that atg5 acts as a functional stay-green type mutant under mild abiotic-stress conditions, because it retains a proper balance of photosystem proteins, photosynthetic efficiency and grana thylakoid structures.
Expression of SAGs in atg5 leaves under dark- or salt-stress-induced senescence conditions
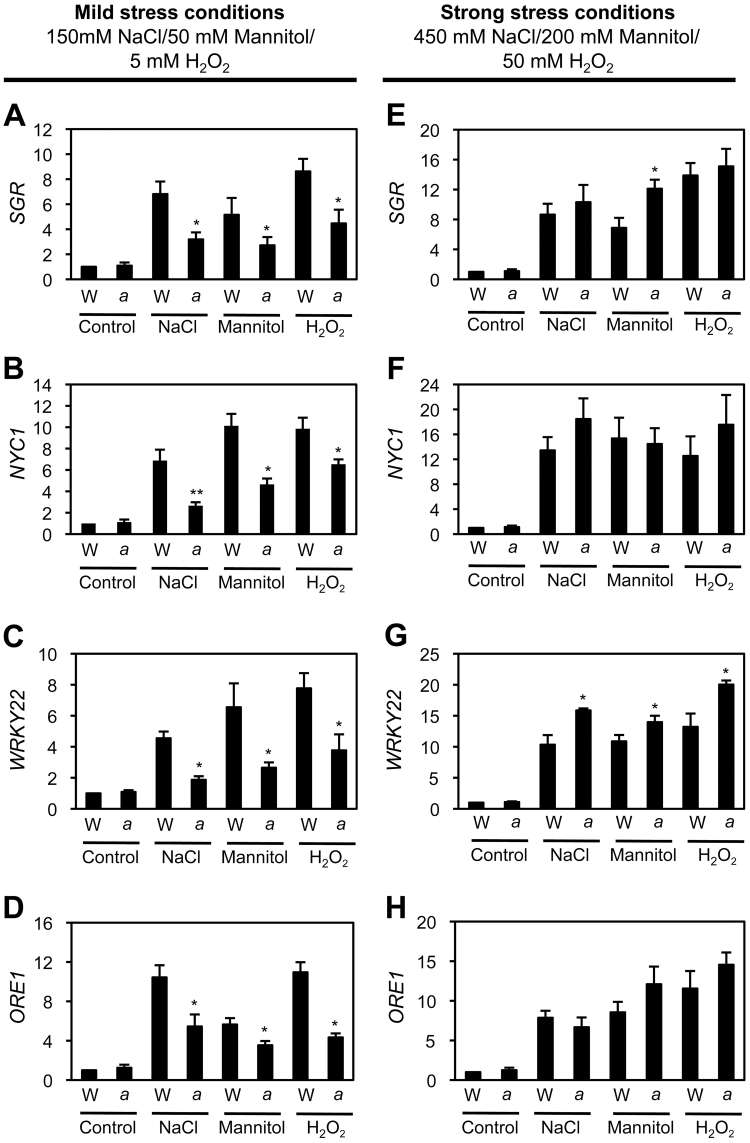
To reveal the mechanism of conditional delayed senescence in atg5 leaves under mild abiotic-stress conditions, expression levels of senescence-associated genes (SAGs), including two senescence-induced TFs, WRKY22 (Zhou et al., 2011) and ORE1 (Kim et al., 2009), and two Chl catabolism-associated proteins, SGR1/NYE1 (Park et al., 2007; Ren et al., 2007) and NYC1 (Kusaba et al., 2007), were measured under mild stress-induced senescence conditions. Expression levels of all four SAGs in wild-type leaves drastically increased after 3 d of mild stress conditions, such as 150mM NaCl, 50mM mannitol, and 5mM H2O2 treatments. However, their expression levels were significantly down-regulated in atg5 leaves (Fig. 4A–D), indicating that the down-regulation of SAGs contributes to the functional stay-green phenotype of atg5 leaves under mild abiotic-stress conditions. Although the non-functional stay-green nye1-1 mutant also retained leaf greenness (Fig. 4), it was found that, under mild abiotic stresses, expression levels of WRKY22 and ORE1 in nye1-1 leaves were almost the same as those of wild-type leaves (see Supplementary Fig. S3 at JXB online). The expression levels of four SAGs were also checked under strong abiotic-stress conditions. Except for the slight up-regulation of WRKY22, the expression levels of the other three SAGs were not significantly different in atg5 leaves and wild-type leaves (Fig. 4E–H).
Fig. 4.
Altered expression of SAGs in atg5 leaves under mild and strong abiotic-stress conditions. First-strand cDNAs were prepared from total RNA extracted from 3-week-old rosette leaves of WT and atg5 plants before (control) and after 3 d of mild abiotic-stress treatments (A–D) and strong abiotic-stress treatments (E–H). By RT-qPCR analysis, relative expression levels of SGR1/NYE1 (A, E), NYC1 (B, F), WRKY22 (C, G), and ORE1 (D, H) were obtained by normalizing to the mRNA levels of GAPDH. Mean and SD values were obtained from more than three biological replicates. These experiments were replicated at least twice with similar results. Student’s t-test (*P<0.05, **P<0.01).
The stay-green phenotype in atg5 leaves under mild stress conditions could also be caused by defects of other intra-chloroplastic catabolic systems. The expression levels of genes encoding FtsH proteases (FtsH2 and FtsH6), Clp proteases (ClpP4 and ClpC1), and Deg proteases (DegP4 and DegP8) were also examined. Expression levels of these genes significantly increase under high light, cold, and heat-stress conditions (Sinvany-Villalobo et al., 2004), indicating that these chloroplastic proteases have major roles in protein degradation under several abiotic-stress conditions. Under mild salt stress (150mM NaCl), the gene expression levels in atg5 leaves were almost the same as those in wild-type leaves (see Supplementary Fig. S4 at JXB online), indicating that these three catabolic systems are not related to the functional stay-green phenotype of atg5 leaves under mild abiotic-stress conditions.
Taking these results together, it is possible that the down-regulation of several SAGs in atg5 leaves during mild abiotic-stress conditions is controlled by retrograde signalling between chloroplasts and the nucleus, which is often observed in many functional stay-green mutants (Sakuraba et al., 2012a).
Early leaf yellowing of atg5 is independent of Chl breakdown in LHCII by the SGR1–CCEs complex in chloroplasts
Chl degradation and autophagy (macroautophagy) affect the degradation of Chls and photosynthetic proteins in the chloroplasts, a very late step in leaf senescence (Lim et al., 2007). However, atg5 and nye1-1 retain different levels of photosynthetic proteins under mild salt-stress conditions (Fig. 2). Among photosynthetic proteins, nye1-1 selectively retains LHCI and LHCII under salt stress (Fig. 2E), as the nye1-1 mutant has impaired SGR1 function. SGR1 induces Chl degradation in LHCII by recruiting Chl catabolic enzymes (CCEs) (Sakuraba et al., 2012b, 2013). However, most photosynthetic proteins are retained in atg5 leaves with a constant Chl a/b ratio (Fig. 2), indicating that the degradation of photosynthetic proteins by the SGR1–CCE complex and macroautophagy may function independently.
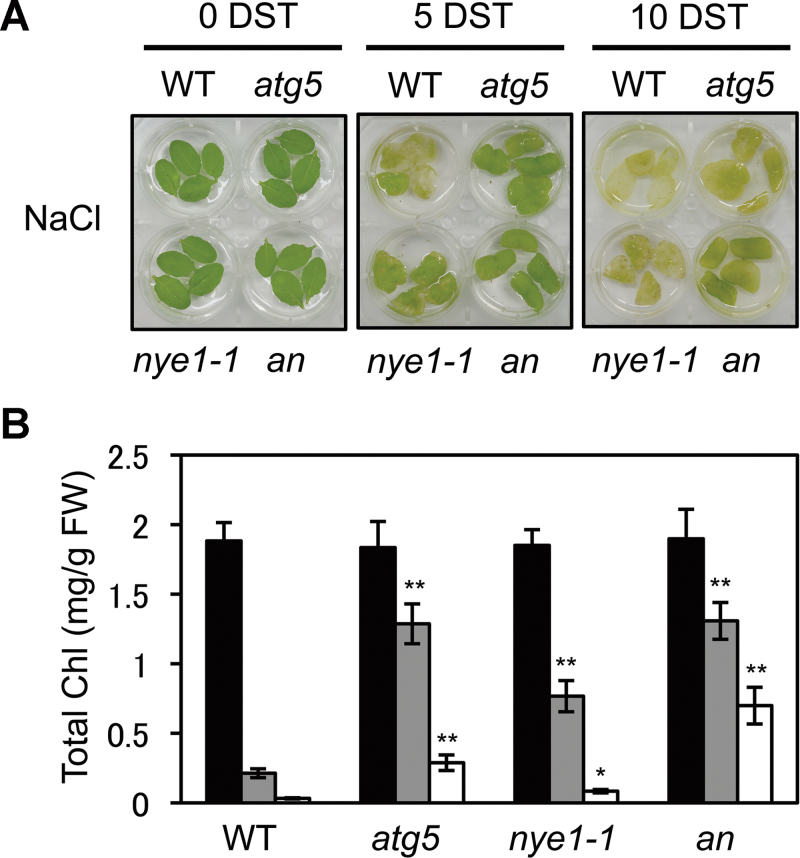
To examine this possibility, the phenotype of atg5 nye1-1 double mutants was investigated under mild salt stress (150mM NaCl). Although no differences were observed between atg5 single and atg5 nye1-1 double mutants until 5 d of salt treatment, the double mutant exhibited a stronger stay-green phenotype at 10 d (Fig. 5A) with significantly greater retention of Chls (Fig. 5B), indicating that the two mutations show an additive effect on abiotic-stress-induced leaf senescence. To examine the relationship of the two mutations in more detail, the phenotype of the double mutants was checked under dark-induced senescence conditions. During dark-induced senescence, the nye1-1 mutant shows a stay-green phenotype (Ren et al., 2007), but atg5 shows an early leaf yellowing phenotype (Thompson et al., 2005). It was found that, after 4 d of dark incubation, the double mutant exhibited an intermediate phenotype and Chl levels of the two single mutants (see Supplementary Fig. S5 at JXB online). Collectively, these results indicate that Chl degradation during senescence occurs by two independent processes, SGR1–CCE–LHCII interaction and macroautophagy.
Fig. 5.
Characterization of atg5 nye1-1 double mutant under mild salt-stress conditions. (A, B) Visible phenotypes (A) and total Chl levels (B) of detached leaves from wild-type (WT), atg5, nye1-1, and atg5 nye1-1 (an) plants during the mild salt stress. Detached leaves from 3-week-old plants were incubated abaxial side-up on 3mM MES (pH 5.8) buffer containing 150mM NaCl for 5 d and 10 d. Similar results were obtained from three independent experiments. DST, days of salt treatment. Student’s t-test (*P<0.05, **P<0.01). (This figure is available in colour at JXB online.)
Discussion
atg5 acts as a functional stay-green mutant under mild abiotic-stress conditions
Defects in chloroplast destruction or senescence-promoting mechanisms can cause leaves to retain their green colour during senescence, a phenomenon called ‘stay-green’ (Hörtensteiner, 2009). For example, the knockout mutants of CCEs exhibit a stay-green phenotype during dark-induced and natural senescence because of the impairment of Chl degradation (Schelbert et al., 2009; Horie et al., 2009). Because the degradation of chloroplast components involves autophagy (Ishida et al., 2008; Wada et al., 2009), it was expected that atg mutants would show a stay-green phenotype under senescence-inducing conditions. However, under strong stress conditions, the atg mutants exhibit a phenotype of accelerated yellowing and cell death.
In this study, this apparent inconsistency has been addressed by identifying the conditions under which atg mutants show a stay-green phenotype. Under mild abiotic-stress conditions, such as 150mM NaCl, 5mM H2O2, or 50mM mannitol, atg5 leaves exhibit a stay-green phenotype (Fig. 1A), and show little cell death (Fig. 1B). Under strong abiotic-stress conditions, however, atg5 leaves turn yellow much faster than WT leaves and show extensive cell death (Fig. 1). This rapid leaf yellowing phenotype is consistent with previous studies on the phenotype of atg plants under abiotic-stress conditions (Xiong et al., 2007; Liu et al., 2009; Zhou et al., 2013). It is speculated that the difference in atg phenotype between strong- and weak-stress conditions may reflect the importance of autophagy in adapting to severe-stress conditions. Generally, plants need to change the balance of the proteome drastically under abiotic-stress conditions, for adaptation to these extreme environments. Because atg mutants cannot properly control their proteome balance, they cannot adapt to strong abiotic-stress conditions and thus exhibit extensive cell death. However, adaptation to mild-stress conditions does not require drastic changes in the proteome balance. Thus, atg5 leaves exhibit little cell death, which leads to the stay-green phenotype (Fig. 6).
Fig. 6.
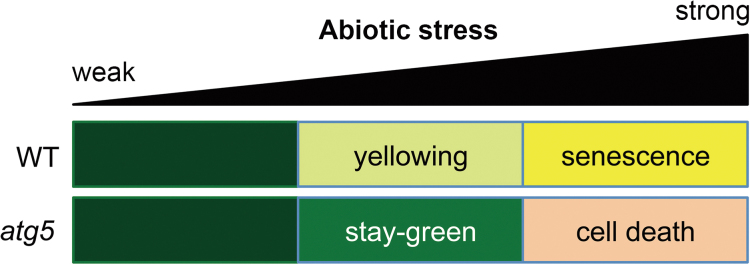
Leaf phenotypes of atg5 mutants depending on the strength of abiotic stresses. Under strong abiotic-stress conditions, atg5 leaves show an accelerated cell death phenotype; under mild abiotic-stress conditions, atg5 leaves show little or no cell death, which leads to a stay-green phenotype. (This figure is available in colour at JXB online.)
Autophagy is involved in chloroplast degradation, a downstream step in leaf senescence pathways. It was therefore expected that the stay-green phenotype of atg mutants would resemble the phenotypes of the cosmetic stay-green mutants of SGR1 and CCEs (Kusaba et al., 2007; Morita et al., 2009). However, it was found that atg5 conditionally acts as a functional, not a cosmetic stay-green type mutant. Under mild salt stress (150mM NaCl) conditions, atg5 leaves stayed green with the proper balance of photosynthetic proteins (Fig. 2D), high photosynthetic capacity (Fig. 2E), and well-retained grana thylakoids (Fig. 3). Also, atg5 leaves showed lower expression levels of several SAGs under mild abiotic-stress conditions (Fig. 4), indicating that atg5 leaves during mild abiotic-stress conditions show defects in both autophagy-dependent senescence pathways and other senescence pathways. One possibility is that the retained chloroplast proteins in atg5 leaves may induce retrograde signalling from the chloroplasts to nucleus, leading to the altered expression of the SAGs. Recently, chloroplast homeostasis has been implicated as an important factor in leaf senescence; for example, tobacco plants with reduced NADH dehydrogenase activity exhibited delayed senescence without significant alteration of their growth rate (Zapata et al., 2005). In addition, Arabidopsis plants over-expressing chlorophyllide a oxygenase (CAO) showed changes in the Chl pigment composition of the photosynthetic apparatus and also showed a functional stay-green phenotype with wide changes in SAG expression (Sakuraba et al., 2012a). These results indicate that chloroplasts have an important role in regulating nuclear gene expression during leaf senescence.
Together, these data indicate that the multiple effects of two different pathways probably cause the functional stay-green phenotype of atg5 leaves under mild abiotic-stress conditions. Because the macroautophagy pathway does not function in atg5 leaves, the degradation of chloroplasts and chloroplastic proteins itself is impaired. Simultaneously, the retention of chloroplast proteins induces chloroplast–nucleus retrograde signalling that affects the regulation of SAGs. Some enigmas remain for this hypothesis; for instance, the chloroplast component(s) that mediate the chloroplast–nucleus retrograde signalling during leaf senescence remain to be identified. Further physiological and biochemical analyses of the functional stay-green phenotype of atg mutants are essential for revealing the functions of plant autophagy.
Degradation of Chls and photosynthetic proteins requires both autophagy and intra-chloroplastic catabolic systems during leaf senescence
SGR1/NYE1 and CCEs form a dynamic protein complex for LHCII disassembly and Chl degradation (Sakuraba et al., 2012a, 2013). However, the SGR1–CCE complex does not interact with other photosynthetic proteins, such as LHCI, the PSII core complex, or the PSI core complex (Sakuraba et al., 2012a). Consistent with this LHCII-specific interaction, LHCII proteins were dominantly retained while other photosystem proteins were normally degraded in nye1-1 (Fig. 2D) and a CCE mutant nyc1-1 (Horie et al., 2009). Thus, degradation mechanisms of other photosynthetic proteins and Chls still remain enigmatic.
At least in part, our finding of the functional stay-green phenotype in atg5 leaves under mild salt-stress condition provides an important clue to solve this enigma. The levels of several photosynthetic proteins retained in atg5 and nye1-1 leaves under mild salt stress were compared. Although both atg5 and nye1-1 leaves exhibited a stay-green phenotype (Fig. 2A) with highly retained Chl levels (Fig. 2B), only LHCII proteins were predominantly retained in nye1-1 leaves, whereas all photosynthetic proteins were substantially retained in atg5 leaves (Fig. 2D). Recent reports showed that one of the autophagy pathways, the so-called chlorophagy pathway, functions in the transportation of Chls and photosystem proteins from the chloroplasts to the vacuole for their degradation (Wada et al., 2009). Considering the low selectivity of proteolysis in autophagy, the chlorophagy pathway non-selectively transported all photosystem proteins from the chloroplasts to the vacuole. Although RCBs also act in chloroplastic autophagy, RCBs do not show Chl fluorescence (Ishida et al., 2008). SAVs, another extra-chloroplastic catabolic system, contain Chl a, but not photosystem proteins. Thus, so far, chlorophagy is the only identified extra-chloroplastic degradation system for photosystem proteins. By contrast with chlorophagy, the SGR–CCE complex seems to concentrate on the destabilization of LHCII and Chls. LHCII, especially the three major, abundant subunits (Lhcb1, Lhcb2, and Lhcb3), forms aggregates because of its abundance (Ruban et al., 2012). In this sense, if specific degradation systems for LHCII exist, it is natural that the SGR1–CCE complex would be one of them.
In this study, it was also found that atg5 nye1-1 double mutants exhibited a very strong stay-green phenotype under mild salt-stress conditions, a seemingly additive phenotype (Fig. 5). This result indicates that the autophagy- and SGR1-dependent degradation pathways function independently. Reflecting the relationship of these two pathways, the combination of intra- and extra-chloroplastic catabolic pathways acts to degrade photosystem proteins. Another catabolic system may also function in the degradation of photosystem proteins during leaf senescence. For instance, genes encoding several members of the FtsH, Clp, and Deg protease families were significantly up-regulated in senescing leaves (Gepstein et al., 2003; Andersson et al., 2004) strongly indicating that these chloroplastic proteases have important roles in the degradation of chloroplast proteins during leaf senescence as well as abiotic-stress conditions. Indeed, an FtsH protease affects the turnover of D1 protein, one of the core subunits of photosystem II, under abiotic-stress conditions (Bailey et al., 2002; Kato et al., 2009). Thus, it is possible that these proteases are involved in the degradation of photosystem proteins during senescence.
Further biochemical analyses of chloroplasts and vacuole fractions during senescence will help us to understand the complete picture of the degradation mechanisms of photosynthetic proteins and their photosynthetic pigments.
Supplementary data
Supplementary data can be found at JXB online.
Supplementary Fig. S1. Phenotype of wild-type (WT) and atg7 leaves under mild abiotic-stress conditions.
Supplementary Fig. S2. Phenotype of wild-type (WT) and atg5 plants grown on phytoagar plates containing NaCl.
Supplementary Fig. S3. Altered expression of SAGs in nye1-1 leaves under mild abiotic-stress conditions.
Supplementary Fig. S4. Expression analysis of chloroplastic protease genes in atg5 leaves under mild salt -stress conditions.
Supplementary Fig. S5. Phenotype (A) and total Chl level (B) of atg5 nye1-1 double mutants during dark-induced senescence.
Supplementary Table S1. Primers used for qPCR in this study.
Acknowledgements
We thank Dr Benke Kuai for nye1-1 seeds. This work was supported by the Strategic Korean–Swiss Cooperative Program, the National Research Foundation of Korea (NRF) grant funded by the Ministry of Education, Science, and Technology (MEST) (No. 2013K1A3A1A14055213).
Sequence data from this article can be found in the Arabidopsis Genome Initiative (AGI) or GenBank/EMBL databases under the following accession numbers: ATG5, At5g17290; ATG7, At5g45900; GAPDH, At1g16300; NYC1, At4g13250; ORE1, At5g39610; SGR1/NYE1, At4g22920; WRKY22, At4g01250; FtsH2, At2G20950: FtsH6, At5g15250; ClpP4, At5g45390; ClpC1, At5g50920; DegP4, At1g65640; DegP8, At5g39830.
YS and N-CP designed the research; YS and S-H.L performed the research; Y-SK assisted with the research; YS, OKP, SH, and N-CP analysed the data; YS and N-CP wrote the article. The authors declare that they have no conflict of interest.
References
- Andersson A, Keskitalo J, Sjodin A, et al. 2004. A transcriptional timetable of autumn senescence. Genome Biology 5, R24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey S, Thompson E, Nixon PJ, Horton P, Mullineaux CW, Robinson C, Mann NH. 2002. A critical role for the Var2 FtsH homologue of Arabidopsis thaliana in the photosystem II repair cycle in vivo . Journal of Biological Chemistry 277, 2006–2011 [DOI] [PubMed] [Google Scholar]
- Balazadeh S, Riano-Pachon DM, Mueller-Roeber B. 2008. Transcription factors regulating leaf senescence in Arabidopsis thaliana . Plant Biology 10, 63–75 [DOI] [PubMed] [Google Scholar]
- Bassham DC, Laporte M, Marty F, Moriyasu Y, Ohsumi Y, Olsen LJ, Yoshimoto K. 2006. Autophagy in development and stress responses of plants. Autophagy 2, 2–11 [DOI] [PubMed] [Google Scholar]
- Chiba A, Ishida H, Nishizawa NK, Makino A, Mae T. 2003. Exclusion of Rubisco from chloroplasts by the vesicle during natural senescence of wheat leaves. Plant and Cell Physiology 44, 914–921 [DOI] [PubMed] [Google Scholar]
- Feller U, Anders I, Mae T. 2008. Rubiscolytics: fate of Rubisco after its enzymatic function in a cell is terminated. Journal of Experimental Botany 59, 1615–1624 [DOI] [PubMed] [Google Scholar]
- Gepstein S, Sabehi G, Carp MJ, Hajouj T, Nesher MF, Yariv I, Dor C, Bassani M. 2003. Large-scale identification of leaf senescence-associated genes. The Plant Journal 36, 629–642 [DOI] [PubMed] [Google Scholar]
- Guo H, Ecker JR. 2004. The ethylene signaling pathway: new insights. Current Opinion in Plant Biology 7, 40–49 [DOI] [PubMed] [Google Scholar]
- Horie Y, Ito H, Kusaba M, Tanaka R, Tanaka A. 2009. Participation of chlorophyll b reductase in the initial step of the degradation of light-harvesting chlorophyll a/b–protein complexes in Arabidopsis. Journal of Biological Chemistry 284, 17449–17456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hörtensteiner S. 2009. Stay-green regulates chlorophyll and chlorophyll-binding protein degradation during senescence. Trends in Plant Science 14, 155–162 [DOI] [PubMed] [Google Scholar]
- Hörtensteiner S. 2013. Update on the biochemistry of chlorophyll breakdown. Plant Molecular Biology 82, 505–517 [DOI] [PubMed] [Google Scholar]
- Hörtensteiner S, Krautler B. 2011. Chlorophyll breakdown in higher plants. Biochimica et Biophysica Acta 1807, 977–988 [DOI] [PubMed] [Google Scholar]
- Inada N, Sakai A, Kuroiwa H, Kuroiwa T. 1998. Three-dimensional analysis of the senescence program in rice (Oryza sativa L.) coleoptile: investigations by fluorescence microscopy and electron microscopy. Planta 206, 585–597 [DOI] [PubMed] [Google Scholar]
- Ishida H, Yoshimoto K, Izumi M, Reisen D, Yano Y, Makino A, Ohsumi Y, Hanson MR, Mae T. 2008. Mobilization of rubisco and stroma-localized fluorescent proteins of chloroplasts to the vacuole by an ATG gene-dependent autophagic process. Plant Physiology 148, 142–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kato Y, Sakamoto W. 2009. Protein quality control in chloroplasts: a current model of D1 protein degradation in the photosystem II repair cycle. Journal of Biochemstry 146, 463–469 [DOI] [PubMed] [Google Scholar]
- Kim JH, Woo HR, Kim J, Lim PO, Lee IC, Choi SH, Hwang D, Nam HG. 2009. Trifurcate feed-forward regulation of age-dependent cell death involving miR164 in Arabidopsis. Science 323, 1053–1057 [DOI] [PubMed] [Google Scholar]
- Klionsky DJ. 2004. Cell biology: regulated self-cannibalism. Nature 431, 31–32 [DOI] [PubMed] [Google Scholar]
- Klionsky DJ, Ohsumi Y. 1999. Vacuolar import of proteins and organelles from the cytoplasm. Annual Review of Cell and Developmental Biology 15, 1–32 [DOI] [PubMed] [Google Scholar]
- Koch E, Slusarenko A. 1990. Arabidopsis is susceptible to infection by a downy mildew fungus. The Plant Cell 2, 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusaba M, Ito H, Morita R, et al. 2007. Rice NON-YELLOW COLORING1 is involved in light-harvesting complex II and grana degradation during leaf senescence. The Plant Cell 19, 1362–1375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PO, Kim HJ, Nam HG. 2007. Leaf senescence. Annual Review of Plant Biology 58, 115–136 [DOI] [PubMed] [Google Scholar]
- Liu YM, Xiong Y, Bassham DC. 2009. Autophagy is required for tolerance of drought and salt stress in plants. Autophagy 5, 954–963 [DOI] [PubMed] [Google Scholar]
- Makino A, Osmond B. 1991. Effects of nitrogen nutrition on nitrogen partitioning between chloroplasts and mitochondria in pea and wheat. Plant Physiology 96, 355–362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez DE, Costa ML, Guiamet JJ. 2008. Senescence-associated degradation of chloroplast proteins inside and outside the organelle. Plant Biology (Stuttgart) 10, Supplement 1, 15–22 [DOI] [PubMed] [Google Scholar]
- Meijer WH, van der Klei IJ, Veenhuis M, Kiel JA. 2007. ATG genes involved in non-selective autophagy are conserved from yeast to man, but the selective Cvt and pexophagy pathways also require organism-specific genes. Autophagy 3, 106–116 [DOI] [PubMed] [Google Scholar]
- Morita R, Sato Y, Masuda Y, Nishimura M, Kusaba M. 2009. Defect in non-yellow coloring 3, an alpha/beta hydrolase-fold family protein, causes a stay-green phenotype during leaf senescence in rice. The Plant Journal 59, 940–952 [DOI] [PubMed] [Google Scholar]
- Nakatogawa H, Suzuki K, Kamada Y, Ohsumi Y. 2009. Dynamics and diversity in autophagy mechanisms: lessons from yeast. Nature Reviews Molecular Cell Biology 10, 458–467 [DOI] [PubMed] [Google Scholar]
- Oberhuber M, Berghold J, Breuker K, Hörtensteiner S, Krautler B. 2003. Breakdown of chlorophyll: a non-enzymatic reaction accounts for the formation of the colorless “non-fluorescent” chlorophyll catabolites. Proceedings of the National Academy of Sciences, USA 100, 6910–6915 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono Y, Wada S, Izumi M, Makino A, Ishida H. 2012. Evidence for contribution of autophagy to Rubisco degradation during leaf senescence in Arabidopsis thaliana . Plant Cell and Environment 36, 1147–1159 [DOI] [PubMed] [Google Scholar]
- Park SY, Yu JW, Park JS, et al. 2007. The senescence-induced staygreen protein regulates chlorophyll degradation. The Plant Cell 19, 1649–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE. 1989. Determination of accurate extinction coefficients and simultaneous-equations for assaying chlorophyll a and chlorophyll b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic-absorption spectroscopy. Biochimica et Biophysica Acta 975, 384–394 [Google Scholar]
- Ren GD, An K, Liao Y, Zhou X, Cao YJ, Zhao HF, Ge XC, Kuai BK. 2007. Identification of a novel chloroplast protein AtNYE1 regulating chlorophyll degradation during leaf senescence in arabidopsis. Plant Physiology 144, 1429–1441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reumann S, Voitsekhovskaja O, Lillo C. 2010. From signal transduction to autophagy of plant cell organelles: lessons from yeast and mammals and plant-specific features. Protoplasma 247, 233–256 [DOI] [PubMed] [Google Scholar]
- Robinson WD, Carson I, Ying S, Ellis K, Plaxton WC. 2012. Eliminating the purple acid phosphatase AtPAP26 in Arabidopsis thaliana delays leaf senescence and impairs phosphorus remobilization. New Phytologist 196, 1024–1029 [DOI] [PubMed] [Google Scholar]
- Ruban AV, Johnson MP, Duffy CDP. 2012. The photoprotective molecular switch in the photosystem II antenna. Biochimica et Biophysica Acta—Bioenergetics 1817, 167–181 [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Balazadeh S, Tanaka R, Mueller-Roeber B, Tanaka A. 2012a. Overproduction of Chl b retards senescence through transcriptional reprogramming in Arabidopsis. Plant and Cell Physiology 53, 505–517 [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Kim YS, Yoo SC, Hörtensteiner S, Paek NC. 2013. 7-Hydroxymethyl chlorophyll a reductase functions in metabolic channeling of chlorophyll breakdown intermediates during leaf senescence. Biochemical and Biophysical Research Communications 430, 32–37 [DOI] [PubMed] [Google Scholar]
- Sakuraba Y, Schelbert S, Park SY, Han SH, Lee BD, Andres CB, Kessler F, Hörtensteiner S, Paek NC. 2012b. STAY-GREEN and chlorophyll catabolic enzymes interact at light-harvesting complex II for chlorophyll detoxification during leaf senescence in Arabidopsis. The Plant Cell 24, 507–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakuraba Y, Yokono M, Akimoto S, Tanaka R, Tanaka A. 2010. Deregulated chlorophyll b synthesis reduces the energy transfer rate between photosynthetic pigments and induces photodamage in Arabidopsis thaliana . Plant and Cell Physiology 51, 1055–1065 [DOI] [PubMed] [Google Scholar]
- Sato Y, Morita R, Nishimura M, Yamaguchi H, Kusaba M. 2007. Mendel’s green cotyledon gene encodes a positive regulator of the chlorophyll-degrading pathway. Proceedings of the National Academy of Sciences, USA 104, 14169–14174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schelbert S, Aubry S, Burla B, Agne B, Kessler F, Krupinska K, Hörtensteiner S. 2009. Pheophytin pheophorbide hydrolase (pheophytinase) is involved in chlorophyll breakdown during leaf senescence in Arabidopsis. The Plant Cell 21, 767–785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinvany-Villalobo G, Davydov O, Ben-Ari G, Zaltsman A, Raskind A, Adam Z. 2004. Expression in multigene families. Analysis of chloroplast and mitochondrial proteases. Plant Physiology 135, 1336–1345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas H, Howarth CJ. 2000. Five ways to stay green. Journal of Experimental Botany 51, 329–337 [DOI] [PubMed] [Google Scholar]
- Thompson AR, Doelling JH, Suttangkakul A, Vierstra RD. 2005. Autophagic nutrient recycling in Arabidopsis directed by the ATG8 and ATG12 conjugation pathways. Plant Physiology 138, 2097–2110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada S, Ishida H, Izumi M, Yoshimoto K, Ohsumi Y, Mae T, Makino A. 2009. Autophagy plays a role in chloroplast degradation during senescence in individually darkened leaves. Plant Physiology 149, 885–893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittenbach VA. 1978. Breakdown of ribulose bisphosphate carboxylase and change in proteolytic activity during dark-induced senescence of wheat seedlings. Plant Physiology 62, 604–608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu NN, Huang X, Yang XQ, Guo J, Zheng EL, Yin SW, Zhu JH, Qi JR, He XT, Zhang JB. 2012. Stabilization of soybean oil body emulsions using i-carrageenan: effects of salt, thermal treatment and freeze–thaw cycling. Food Hydrocolloids 28, 110–120 [Google Scholar]
- Xiong Y, Contento AL, Nguyen PQ, Bassham DC. 2007. Degradation of oxidized proteins by autophagy during oxidative stress in Arabidopsis. Plant Physiology 143, 291–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zapata JM, Guera A, Esteban-Carrasco A, Martin M, Sabater B. 2005. Chloroplasts regulate leaf senescence: delayed senescence in transgenic ndhF-defective tobacco. Cell Death and Differentiation 12, 1277–1284 [DOI] [PubMed] [Google Scholar]
- Zelisko A, Garcia-Lorenzo M, Jackowski G, Jansson S, Funk C. 2005. AtFtsH6 is involved in the degradation of the light-harvesting complex II during high-light acclimation and senescence. Proceedings of the National Academy of Sciences, USA 102, 13699–13704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou J, Wang J, Cheng Y, Chi YJ, Fan BF, Yu JQ, Chen ZX. 2013. NBR1-mediated selective autophagy targets insoluble ubiquitinated protein aggregates in plant stress responses. Plos Genetics 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou X, Jiang YJ, Yu DQ. 2011. WRKY22 transcription factor mediates dark-induced leaf senescence in Arabidopsis. Molecules and Cells 31, 303–313 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.