Abstract
We previously found that a maximum innate inflammatory response induced by stimulation of Toll-like receptors (TLRs) 3, 7 and 9 requires ERα, but does not require estrogen in multiple cell types from both control and lupus-prone mice. Given the estrogen-independence, we hypothesized that ERα mediates TLR signaling by tethering to, and enhancing, the activity of downstream transcription factors such as NFκB, rather than acting classically by binding EREs on target genes. To investigate the mechanism of ERα impact on TLR signaling, we utilized mice with a knock-in ERα mutant that is unable to bind ERE. After stimulation with TLR ligands, both ex vivo spleen cells and bone marrow-derived dendritic cells (BM-DCs) isolated from mutant ERα (“KIKO”) mice produced significantly less IL-6 compared with cells from wild-type (WT) littermates. These results suggest that ERα modulation of TLR signaling does indeed require ERE binding for its effect on the innate immune response.
Keywords: ERα, TLRs, DCs
Introduction
One of the more profound features of lupus is that females have a 9:1 prevalence of disease over males. The cause of the sex bias in lupus is likely multifactorial, including differences in the sex chromosomes, sex hormones and their receptors. Although estrogen acts primarily via its receptors, estrogen receptor alpha and beta (ERα/ERβ), estrogen can also act through non-receptor mediated mechanisms. Interestingly, there is growing evidence that ERs can mediate physiologic functions independent of estrogen. We previously showed that an optimal inflammatory response by TLRs is dependent on ERα, but independent of estrogen in multiple cell types. B cells and DCs derived from both B6 ERαKO and lupus-prone ERαKO mice had a significantly blunted response to TLR 7 and 9 ligands. [1] These data suggest that ERα modulation of TLR signaling may play a role in lupus pathogenesis, and appears to be independent of estrogen effects.
Based on these findings, we hypothesized that ERα mediates TLR signaling by a genomic, but non-classical mechanism, i.e. by tethering to and enhancing the activity of downstream transcription factors such as NFκB, thereby altering the innate immune response and exacerbating inflammation. An alternative mechanism to explain this ligand independence is activation via kinase cascades including MAPKs (rapid signaling pathway). To investigate the mechanism of ERα impact on TLR signaling, we utilized mice with a knock-in ERα mutant (“KIKO”) that is unable to bind ERE, but otherwise functions normally with regard to ligand binding, activation, etc. [2] Female mice that carry a single copy of this non-classical ERα knock-in mutation are infertile due to severe ovarian and uterine defects, [3] but their immune phenotype is not known.
In this communication we show that both ex vivo spleen cells and bone-marrow-derived dendritic cells (BM-DCs) from KIKO mice (ERα DNA-binding mutant mice) behave similarly to ERαKO mice in that TLR-stimulated endpoints are blunted. We previously showed that multiple TLR-induced cytokines are impacted by ERα IL-6, MCP-1, IL-23, IL-17 among others. In this study we looked at IL-6, which has multiple pro-inflammatory effects and is a potent activator of the NFκB pathway. IL-6 is known to play a critical role in the immunopathology of SLE in both humans and mice (contributes to B cell hyperactivity and differentiation of T cells into effector cells, including Th17 cells) [4,6]. Blocking IL-6 in mouse models of SLE significantly improves disease. [7] Herein we show that DNA binding of ERα is required for TLR-induced IL-6 production in murine immune cells. These findings suggest that ERE binding is indeed required for ERα modulation of TLR-induced inflammation, despite the lack of estrogen in the system, and future studies will confirm this result in a lupus mouse model.
Materials and Methods
Mice
Female NERKI (ERα DNA-binding mutant) and Ex3a (ERanull) mice on the C57BL/6/129 background (kind gift of Ken Korach, NIEHS, NC) were crossed to obtain the “KIKO” mouse as previously described. All mice were maintained at the Ralph H. Johnson VAMC Animal Care Facility (Charleston, SC) using Institutional Animal Care and Use Committee approved protocols.
Generation of BMDCs and Spleen cells
Bone marrow-derived DCs were generated using a modified version of the protocol originally described by Inaba et al. [24], without lymphocyte depletion. Briefly, equal numbers of BM cells from WT and KIKO mice were suspended in complete RPMI supplemented with 20ng/mL murine GM-CSF and 20ng/mL murine IL-4 (R&D systems, Minneapolis, MN) and cultured in T75 flasks at 1 × 106 cells/ml (~20 × 106/flask) for 7 days. BMDCs were harvested from flasks, counted, and re-plated in 6-well plates at 1 × 106 cells/ml (4 × 106/well) for 18h. For spleen cells, mice were sacrificed and spleens harvested and kept in ice-cold RPMI. Spleens were processed and subjected to red blood cell lysis. Cells were washed twice in cold RPMI before being counted and cultured in 12- or 6-well plates at 1 × 106 cells/ml (2-4 × 106/well) for 18h.
Treatment of DCs with TLR agonists
BM-DCs were harvested on day 7 from BM cultures as described above, seeded at 1 × 106 cells/ml into 6- or 12-well plates in estrogen-free phenol red-free RPMI with 10% charcoal-dextran-stripped FCS and treated with vehicle or TLR agonist: loxoribine (TLR7/8 agonist; 50-200umol, Sigma–Aldrich, St. Louis, MO), or CpG DNA (TLR9 agonist; 1μg/ml, Hycult Biotech, Canton, MA) for 18h.
Cytokine release assay
Cytokine release by DCs and spleen cells was determined by culturing 2 × 106/mL cells with either vehicle or TLR agonist. After 18h, culture supernatants were harvested and cytokine (IL-6) concentrations were measured by sandwich ELISA per the manufacturer’s protocol (eBioscience, Inc., San Diego, CA) using a micro-plate luminometer (Thermo Scientific Multiskan Ascent).
Results
Spleen counts are significantly reduced in ERα DNA binding domain mutant mice (“KIKO”) compared with wild-type mice
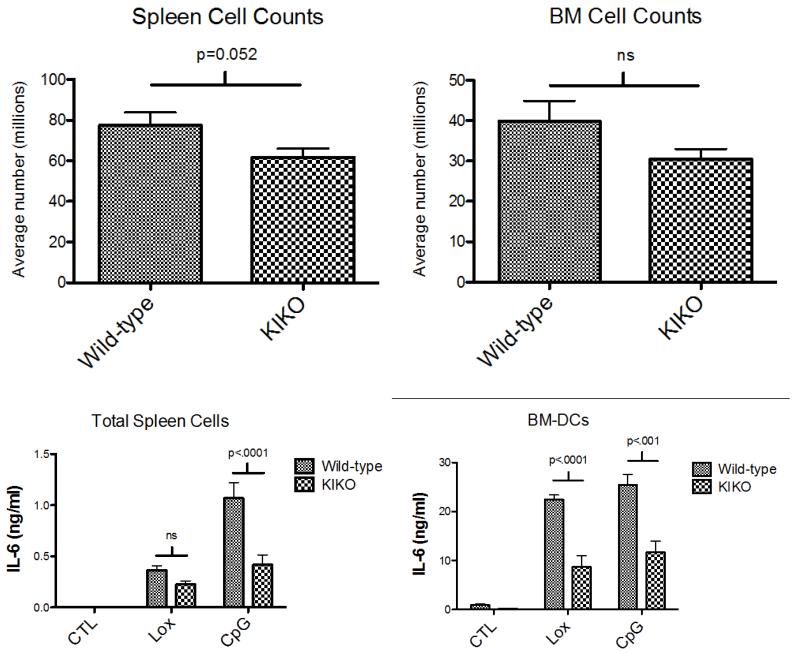
Twenty C57BL/6 mice (8 WT and 12 KIKO) were sacrificed at 18 weeks. Spleen cells were isolated and counted. Spleens from KIKO mice were smaller and there was a significant difference in spleen cell counts between WT and KIKO mice (Figure 1A). This result aligns with previously published studies demonstrating a critical role for estradiol and ERα in immune organ development (spleen, thymus) as well as murine DC development [8-12]. In a subset of animals (4 WT and 5 KIKO) femurs were harvested and bone marrow cells were counted. There was a trend toward reduced number of cells in the KIKO mice, however it did not reach significance.
Figure 1.
(A) Twenty mice (n = 8 WT, 12 KIKO) were sacrificed at 18 weeks. Spleens from KIKO mice were smaller and there was a significant difference in spleen cell counts between WT and KIKO mice. In a subset of animals (n = 4 WT, 5 KIKO), femurs were harvested and bone marrow cells were counted. There was a trend toward reduced numbers in the KIKO mice that did not reach significance. (B) Ex vivo spleen cells (n = 4 WT, 7 KIKO) and cultured BM-DCs (n = 4 WT, 5 KIKO) were stimulated overnight (18h) with 200umol loxoribine (TLR 7 ligand) or 1ug/mL CpG DNA (TLR9 ligand) under estrogen-free media conditions. TLR stimulation resulted in increased IL-6 production that was significantly decreased in media from KIKO mice compared to wild-type mice.
TLR7- and 9-induced IL-6 production by ex vivo spleen cells and BM-DCs is significantly reduced in KIKO mice
We examined IL-6 production by spleen cells following TLR stimulation. Spleens were harvested and spleen cells were stimulated overnight (18h) with loxoribine (TLR 7 ligand) or CpG DNA (TLR9 ligand) under estrogen-free media conditions. Both TLR7 and TLR9 stimulation resulted in significantly increased levels of IL-6 production by spleen cells (Figure 1), however IL-6 produced by cells from KIKO animals was significantly reduced compared to wild-type mice, as measured by ELISA. This result suggests that TLR-induced inflammatory cytokine production is modulated by ERα and requires direct ERE binding, despite the absence of estrogen.
DCs express high levels of TLRs and are key mediators of the innate immune response. We isolated bone marrow hematopoietic cells from WT or KIKO mice and derived DCs with selective/supplemented media. Following harvest on d7, DCs were stimulated under estrogen-free conditions with loxoribine or CpG DNA for 18h. In DC cultures derived from WT mice, both the TLR7 and TLR9 ligands stimulated robust IL-6 production, however, KIKO DC IL-6 production was significantly decreased. TLR stimulation increased IL-6 levels more than 20-fold in media from B6 WT DCs, with the stimulation index reduced by ~50% in KIKO animals. These data indicate that ERα significantly modulates TLR7 and 9 responses by DCs via direct ERE binding on target genes.
Discussion and Conclusion
We previously reported that lupus prone ERαKO mice had significantly reduced renal disease and significantly prolonged survival. [13] We subsequently demonstrated that ERα modulates TLR signaling in both C57BL/6 and lupus prone mice (NZM2410 and MRL/lpr). [1] The mechanism of ERα effect on TLR-induced inflammatory endpoints is currently unknown and is the focus of the current report. The major finding in this study is the requirement for ligand-independent ERE binding by ERα for robust stimulation of TLR-induced inflammatory endpoints.
It is well known that estrogen can modulate IL-6 gene expression. [14,15] Classically, this would occur via ERα binding to an estrogen response element (ERE). There is growing evidence, however, that the molecular mechanisms, by which ERα exerts its effects, on IL-6 and other target genes, are more complicated than the classic pathway of ligand-activated transcriptional activation. ERα also acts via multiple non-classical signaling pathways to regulate cellular responses. For example, ERα may bind to other transcription factors such as AP-1, C/EBPβ, and NFκB to regulate transcription of IL-6 and others. [16-21] It is also possible that ERα exerts some of its effects by differentially recruiting co-activators or co-repressors, such as p300 to the transcriptional complex to impact gene expression depending on the cell type and environment. [22,23] This study provides additional evidence for ligand-independent actions of ERα, since the experiments reported herein were done under estrogenfree conditions. Our results were unexpected however, in that we hypothesized the mechanism of ERα impact on TLR signaling would be both estrogen-independent and ERE-independent. We speculated that the effect would be genomic, by ERα tethering to other transcription factors, but would not require direct DNA binding.
Similar to ERαKO animals, however, IL-6 expression levels in response to TLR stimulation were significantly decreased in the setting of an ERα DNA binding mutant. This DNA binding domain mutant (NERKI) retains all other functions of ERα, including tethering and ligand binding. [2] The mutant also retains the rapid action effects of cytoplasmic ERα (ex. MAPK signaling). If either of these ERα mechanisms were involved, we would have expected to see no change in TLR-stimulated IL-6 production compared with WT levels. Again, all stimulation experiments were carried out under estrogen-free conditions, thus, despite the mutant having an intact ligand-binding domain, the effect was estrogen-independent. One caveat to this, however, is that estrogen is required for the normal development of immune cells such as DCs. As shown by our lab and others, this development also requires ERα. [1,12] Thus, cells are exposed to estrogen in vivo and during initial culture. There may be some threshold or triggering event dependent on estrogen that impacts (imprints on) future signaling (i.e. a developmental effect). Our results suggest that if imprinting on immune cell development is the underlying mechanism, then ERE binding by ERα is also required for immune cell development. Further work is needed with conditional knockout animals and/or in vitro knockdown experiments to determine whether the estrogen-independent effect of ERα on TLR signaling is absolute.
This study provides further evidence for a ligand-independent effect of ERα on TLR-induced gene expression in spleen cells and bone marrow-derived dendritic cells, which we have shown is ERE-dependent. Additional work is needed to elucidate the specific mediators of TLR signaling upstream of ERE binding by ERα. Defining the molecular mechanism(s) of ERα effects on TLR signaling is critical to our understanding of female-predominant autoimmune diseases such as SLE, and may lead to future the rapiesthat target particular ERα actions and modulate innate immunity.
Abbreviations
- ERα
Estrogen receptor alpha
- TLRs
Toll-like receptors
- DCs
Dendritic cells
References
- 1.Cunningham MA, Naga OS, Eudaly JG, Scott JL, Gilkeson GS. Estrogen receptor alpha modulates Toll-like receptor signaling in murine lupus. Clin Immunol. 2012;144(1):1–12. doi: 10.1016/j.clim.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sinkevicius KW, Burdette JE, Woloszyn K, Hewitt SC, Hamilton K, et al. An estrogen receptor-alpha knock-in mutation provides evidence of ligand-independent signaling and allows modulation of ligand-induced pathways in vivo. Endocrinology. 2008;149(6):2970–2979. doi: 10.1210/en.2007-1526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jakacka M, Ito M, Martinson F, Ishikawa T, Lee EJ, et al. An estrogen receptor (ER) alpha deoxyribonucleic acid-binding domain knock-in mutation provides evidence for nonclassical ER pathway signaling in vivo. Mol Endocrinol. 2002;16(10):2188–2201. doi: 10.1210/me.2001-0174. [DOI] [PubMed] [Google Scholar]
- 4.Richards HB, Satoh M, Shaw M, Libert C, Poli V, Reeves WH. Interleukin 6 dependence of anti-DNA antibody production: evidence for two pathways of autoantibody formation in pristane-induced lupus. J Exp Med. 1998;188(5):985–90. doi: 10.1084/jem.188.5.985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ryffel B, Car BD, Gunn H, Roman D, Hiestand P, et al. Interleukin-6 exacerbates glomerulonephritis in (NZB × NZW)F1 mice. Am J Pathol. 1994;144(5):927–937. [PMC free article] [PubMed] [Google Scholar]
- 6.Finck BK, Chan B, Wofsy D. Interleukin 6 promotes murine lupus in NZB/NZW F1 mice. J Clin Invest. 1994;94(2):585–591. doi: 10.1172/JCI117373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mihara M, Takagi N, Takeda Y, Ohsugi Y. IL-6 receptor blockage inhibits the onset of autoimmune kidney disease in NZB/W F1 mice. Clin Exp Immunol. 1998;112(3):397–402. doi: 10.1046/j.1365-2249.1998.00612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carreras E, Turner S, Frank MB, Knowlton N, Osban J, et al. Estrogen receptor signaling promotes dendritic cell differentiation by increasing expression of the transcription factor IRF4. Blood. 2010;115(2):238–246. doi: 10.1182/blood-2009-08-236935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carreras E, Turner S, Paharkova-Vatchkova V, Mao A, Dascher C, et al. acts directly on bone marrow myeloid progenitors to differentially regulate GM-CSF or Flt3 ligand-mediated dendritic cell differentiation. J Immunol. 2008;180(2):727–738. doi: 10.4049/jimmunol.180.2.727. [DOI] [PubMed] [Google Scholar]
- 10.Kovats S, Carreras E. Regulation of dendritic cell differentiation and function by estrogen receptor ligands. Cell Immunol. 2008;252(1-2):81–90. doi: 10.1016/j.cellimm.2007.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mao A, Paharkova-Vatchkova V, Hardy J, Miller MM, Kovats S. Estrogen selectively promotes the differentiation of dendritic cells with characteristics of Langerhans cells. J Immunol. 2005;175(8):5146–5151. doi: 10.4049/jimmunol.175.8.5146. [DOI] [PubMed] [Google Scholar]
- 12.Paharkova-Vatchkova V, Maldonado R, Kovats S. Estrogen preferentially promotes the differentiation of CD11c+ CD11b(intermediate) dendritic cells from bone marrow precursors. J Immunol. 2004;172(3):1426–1436. doi: 10.4049/jimmunol.172.3.1426. [DOI] [PubMed] [Google Scholar]
- 13.Svenson JL, EuDaly J, Ruiz P, Korach KS, Gilkeson GS. Impact of estrogen receptor deficiency on disease expression in the NZM2410 lupus prone mouse. Clin Immunol. 2008;128(2):259–268. doi: 10.1016/j.clim.2008.03.508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jilka RL, Hangoc G, Girasole G, Passeri G, Williams DC, et al. Increased osteoclast development after estrogen loss: mediation by interleukin-6. Science. 1992;257(5066):88–91. doi: 10.1126/science.1621100. [DOI] [PubMed] [Google Scholar]
- 15.Ray A, Prefontaine KE, Ray P. Down-modulation of interleukin-6 gene expression by 17 beta-estradiol in the absence of high affinity DNA binding by the estrogen receptor. J Biol Chem. 1994;269(17):12940–12946. [PubMed] [Google Scholar]
- 16.Biswas DK, Singh S, Shi Q, Pardee AB, Iglehart JD. Crossroads of estrogen receptor and NF-kappaB signaling. Sci STKE. 2005;2005(288):pe27. doi: 10.1126/stke.2882005pe27. [DOI] [PubMed] [Google Scholar]
- 17.Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276(17):13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- 18.Safe S. Transcriptional activation of genes by 17 beta-estradiol through estrogen receptor-Sp1 interactions. Vitam Horm. 2001;62:231–252. doi: 10.1016/s0083-6729(01)62006-5. [DOI] [PubMed] [Google Scholar]
- 19.Ray P, Ghosh SK, Zhang DH, Ray A. Repression of interleukin-6 gene expression by 17 beta-estradiol: inhibition of the DNA-binding activity of the transcription factors NF-IL6 and NF-kappa B by the estrogen receptor. FEBS Lett. 1997;409(1):79–85. doi: 10.1016/s0014-5793(97)00487-0. [DOI] [PubMed] [Google Scholar]
- 20.Kurebayashi S, Miyashita Y, Hirose T, Kasayama S, Akira S, et al. Characterization of mechanisms of interleukin-6 gene repression by estrogen receptor. J Steroid Biochem Mol Biol. 1997;60(1-2):11–7. doi: 10.1016/s0960-0760(96)00175-6. [DOI] [PubMed] [Google Scholar]
- 21.Galien R, Garcia T. Estrogen receptor impairs interleukin-6 expression by preventing protein binding on the NF-kappaB site. Nucleic Acids Res. 1997;25(12):2424–2429. doi: 10.1093/nar/25.12.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Louthrenoo W, Kasitanon N, Sukitawut W, Wichainun R. A clinical study of crystal-proven gouty arthritis in a university hospital. J Med Assoc Thai. 2003;86(9):868–875. [PubMed] [Google Scholar]
- 23.Louthrenoo W, Kasitanon N, Mahanuphab P, Bhoopat L, Thongprasert S. Kaposi’s sarcoma in rheumatic diseases. Semin Arthritis Rheum. 2003;32(5):326–333. doi: 10.1053/sarh.2002.50000. [DOI] [PubMed] [Google Scholar]
- 24.Inaba K, Inaba M, Romani N, Aya H, Deguchi M, et al. Generation of large numbers of dendritic cells from mouse bone marrow cultures supplemented with granulocyte/macrophage colony-stimulating factor. J Exp Med. 1992;176(6):1693–1702. doi: 10.1084/jem.176.6.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]