Abstract
The primary purpose of the current study was to examine the relationship between performance on executive tasks and white matter integrity, assessed by diffusion tensor imaging (DTI) in Multiple Sclerosis (MS). A second aim was to examine how processing speed affects the relationship between executive functioning and FA. This relationship was examined in two executive tasks that rely heavily on processing speed: the Color-Word Interference Test and Trail-Making Test (Delis-Kaplan Executive Function System). It was hypothesized that reduced fractional anisotropy (FA) is related to poor performance on executive tasks in MS, but that this relationship would be affected by the statistical correction of processing speed from the executive tasks. 15 healthy controls and 25 persons with MS participated. Regression analyses were used to examine the relationship between executive functioning and FA, both before and after processing speed was removed from the executive scores. Before processing speed was removed from the executive scores, reduced FA was associated with poor performance on Color-Word Interference Test and Trail-Making Test in a diffuse network including corpus callosum and superior longitudinal fasciculus. However, once processing speed was removed, the relationship between executive functions and FA was no longer significant on the Trail Making test, and significantly reduced and more localized on the Color-Word Interference Test.
Keywords: Multiple Sclerosis, Executive, Processing Speed, Diffusion Tensor Imaging, White Matter
Introduction
Cognitive impairment exists in 45-70% of individuals with Multiple Sclerosis (MS) (Chiaravalloti & DeLuca, 2008). Despite the identification of executive dysfunction in persons with MS (for review, see Genova, Sumowski, Chiaravalloti, Voelbel, & DeLuca, 2009), research examining these deficits has been limited (e.g. Benedict et al., 2002; Marie & Defer, 2001). Individuals with MS show poorer performance on tasks requiring decision-making (Kleeberg et al., 2004; Simioni et al., 2009), as well as tasks of both abstract reasoning and concept formation (e.g. Arnett et al., 1994; Beatty, Paul, Blanco, Hames, & Wilbanks, 1995; Foong et al., 1997). Impairments in response inhibition have also been shown on tasks such as the Stroop and the Go/No Go task (Pujol et al., 2001; A. M. Smith et al., 2009; Vitkovitch, Bishop, Dancey, & Richards, 2002).
Recently, neuroimaging techniques have been utilized to examine the relationship between executive dysfunction and neuronal damage in MS. For example, performance deficits on two tasks of executive function (Wisconsin Card Sorting Test and the Sorting Test from the Delis-Kaplan Executive Function System (DKEFS)) have been shown to be associated with increased pathology (i.e. lesion load and atrophy) in MS as assessed by magnetic resonance imaging (MRI) (Arnett et al., 1994; Parmenter et al., 2007) Magnetic resonance spectroscopy (MRS), which measures neurometabolites as markers of neuronal integrity, has also been used to examine the relationship between executive dysfunction and neuronal damage. Using MRS, poor performance on a task of planning and organization was found to be associated with decreased levels of N-acetyl aspartate in individuals with MS (Christodoulou et al., 2003; Pan, Krupp, Elkins, & Coyle, 2001). Diffusion tensor imaging (DTI) allows for the examination of white matter tract integrity by quantifying fractional anisotropy (FA): the extent to which the diffusion of water is constrained (e.g., by cell walls) such that it moves preferentially in certain directions (e.g., along axons). FA has been shown to be reduced in MS, indicative of white matter pathology (for review, see (Ge, Law, & Grossman, 2005). Several recent studies have employed DTI to examine the association of white matter integrity and executive functions in persons with MS, yielding mixed results. For example, (Roca et al., 2008) reported significantly reduced FA that was correlated with impaired performance on a task of planning and organization (the Hotel Task). However, (Dineen et al., 2009), using a sorting task, failed to find a significant relationship between FA and behavioral performance. While both tasks are purported to assess planning, the task employed by (Roca et al., 2008) required a timed response whereas the (Dineen et al., 2009) study did not. Therefore inconsistencies in the literature concerning the relationship between FA and planning may be due to the processing speed requirement of the task.
Indeed, a number of behavioral studies have recently shown that executive dysfunction is closely related to processing speed in MS. In two such studies, processing speed abilities were found to be associated with performance on tasks on the DKEFS, as well as the Stroop test (Drew, Starkey, & Isler, 2009; Macniven et al., 2008). (Drew et al., 2009) reported that processing speed measures were significantly correlated with both timed and untimed measures of the DKEFS, including Trail-Making Test, Verbal-Fluency, Card-Sorting and Proverbs. In addition, on many of the subtests, the processing speed measures were primary predictors of performance. Such findings support the notion that impairments in executive functioning in MS may be largely influenced by processing speed impairments (DeLuca, Sumowski, Leavitt, Chiaravalloti, & Wylie, 2010; Drew et al., 2009; Leavitt et al., Submitted).
The purpose of the current study was to examine whether poor performance on tasks of executive functioning is related to reductions in white matter integrity (FA) in MS. The current study examined two sub-tests of the DKEFS: the Color-Word Interference Test, and Trail-Making Test. A second purpose was to examine how processing speed affects the relationship between executive functioning and FA. To examine this relationship, executive tasks were statistically corrected for processing speed, to examine the extent to which processing speed affects the relationship between executive functions and FA. It was hypothesized that reduced FA would be associated with lower executive abilities in MS, but that this relationship would be significantly diminished after the contribution of processing speed was statistically removed from the data.
Methods
Subjects
This study was approved by the institutional review boards of both the University of Medicine and Dentistry of New Jersey and the Kessler Foundation Research Center. Subjects' consent was obtained according to the Declaration of Helsinki. Forty subjects participated in this study including 15 healthy controls (HCs) and 25 persons with MS according to the criteria of (McDonald et al., 2001). Of the MS subjects, 22 were diagnosed with relapsing-remitting MS, 1 had primary-progressive MS and 2 had secondary-progressive MS. Disease duration in the MS group ranged from 7 months to 348 months (29 years) with a mean of 115.16 ± 84.02 months (9.6 ± 7 years). In the MS group, 20/25 subjects were female and 8/15 HCs were female (χ2 (1, N = 40) = 3.175, p = .075). The mean age of the MS group (44.0 ± 7.9 years) was significantly older than the HC group (36.27 ± 10.3 years) (t (38) = -2.662, p = 0.011). There was no significant difference between the HCs (16.60 ± 2.2 years), and the MS subjects (16.28 ± 2.2 years) in years of education (t (38) = .449, p = 0.656). Due to the statistical differences in age, age was entered as a covariate in all statistical analyses. There was no difference between the groups on premorbid IQ, as assessed by Wechsler Abbreviated Scale of Intelligence (WASI) Vocabulary (F (2,36) = 2.87, p = .099). Ambulation index was obtained on 24/25 subjects with MS and 9/15 HCs. As expected, the Ambulation Index was significantly elevated in the MS group (M = 1.83, S.D. = 1.6) vs. HCs (M = .33, S.D. = 1) (t (23.7) = -3.182, p = .004).
Neuropsychological Tests
To assess executive functioning and processing speed, all subjects were administered the Color-Word Interference Test and the Trail-Making Test of the DKEFS (Delis, Kaplan, & Kramer, 2000). The first three trials from the Color-Word Interference Test were used in the current study. On the first trial “Word Reading,” subjects are asked to read the words “RED,” “GREEN,” and “BLUE,” which are printed in black ink and repeated randomly. On the second trial “Color Naming,” “XXXX” is repeated, printed in either red, green or blue ink. The subject is asked to identify the color in which the Xs are printed. On the third trial, “Inhibition”, the words “RED,” “GREEN,” and “BLUE,” are printed in red, green and blue ink, but the color of the ink does not match the written word (i.e. the word “RED” is printed in blue ink). The subject is asked to identify the color that the word is printed in (i.e. inhibiting the automatic process of word reading). The scores on each of the 3 subtests are based on time to completion. For the Trail-Making Test, trials 2,3, and 4 were of interest in the current study, which are referred to as “Number Sequencing,” “Letter Sequencing,” and “Number-Letter Switching.” On “Number Sequencing,” subjects are asked to connect consecutive targets on a page, all of which have a number (1-25). On “Letter-Sequencing,” subjects are asked to connect consecutive targets on a page that have the letters (A-Z) in alphabetical order. On “Number-Letter Switching,” subjects are asked to switch between numbers and letters in consecutive order (i.e. “1, A, 2, B”). The scores on each subtest are again time to completion. Both tasks (Trail-Making and Color-Word Interference) include other trials and scores, which were not of interest in the current study.
In addition to these tests, premorbid IQ was assessed using the Vocabulary subtest of the WASI (Wechsler, 1999).
Imaging
Patients were scanned on a 3T Siemens Allegra System. Scans included an MPRAGE and a T2-weighted image. The imaging data were acquired as part of a larger fMRI study (Chiaravalloti, Wylie, Leavitt, & Deluca, 2012; Sumowski, Wylie, Deluca, & Chiaravalloti, 2010). DTI data were acquired using a 12-direction dual spin echo sequence (TR=7300ms, TE=88ms, FOV=210mm, matrix=128x128, slice thickness 4mm, 26 slices, no gap, b=1000 s/mm2, NEX=7). After acquisition, data were transferred off-line to a Linux-based workstation and processed using FSL from the FMRIB software library (Functional Magnetic Resonance Imaging of the Brain Software Library; http://www.fmrib.ox.au.uk/fsl). For each participant, a single FA image was created from the raw diffusion data as follows: First, DICOM (Digital Imaging and Communications in Medicine) files of each DTI acquisition were converted into a single multivolume NIfTI (Neuroimaging Informatics Technology Initiative) file. FSL's Brain Extraction Tool (BET) was used to remove all non-brain tissue from each image by creating a brain mask from the B=0 (nondiffusion weighted) images. Each subject's data were then corrected for distortions due to eddy currents and head motion using affine transformations. Finally, using DTIFIT from FSL, a diffusion tensor model was fit at each voxel, resulting in a single FA image. Visual inspection of data occurred at each stage of image processing.
After the preprocessing steps had been completed, a white-matter “skeleton” was created using scripts within the Tract-Based Spatial Statistics (TBSS) package in FSL 4.1.4 (Smith et al., 2004). The TBSS method minimizes potential misalignment problems of other voxel-based whole-brain analysis methods by first determining an FA skeleton restricted only to the center of major white-matter tracts. This skeleton was derived by running a non-linear registration that aligns all FA images to the FMRIB58_FA standard image supplied in FSL. FA values for each individual were then mapped directly onto the standard skeleton (MNI152 space) for group comparison (Smith et al., 2006).
Statistical Analysis
In order to examine FA between the MS and HC group, a t-test was performed. Images were thresholded to protect against Type I error following (Baudewig, Dechent, Merboldt, & Frahm, 2003). All results were significant at a corrected alpha level of p < 0.05 (i.e., all results were corrected for multiple comparisons). The correction was done using a Monte Carlo simulation showing that with an individual probability threshold of p<0.01, clusters of at least 38 contiguous voxels (in native space) were significant at p<0.05.
In order to examine relationship between cognitive task performance and FA, multiple linear regressions were run (the 3dRegAna program in the AFNI suite of programs). Three analyses were run for each of the two tests of interest (Color-Word Interference and Trail-Making Test), resulting in 6 analyses for each group. In order to protect against type I error, we used Bonferroni correction on the corrected alpha level. That is, while we would ordinarily use a corrected alpha threshold of p<0.05, because we performed 12 tests, we used a corrected alpha threshold of p<0.004. The individual voxel probability threshold was maintained at p<0.01, and Monte Carlo simulations showed 101 contiguous voxels resulted in a corrected alpha threshold of p<0.004. All analyses included age as a predictor variable, because this variable is known to affect neuronal integrity (i.e. Nebes et al., 2006). Three questions were asked for each task: 1. What is the relationship between processing speed and FA? 2. What is the relationship between executive functioning and FA? and finally 3. What is the relationship between executive functioning and FA once processing speed is statistically removed from the executive score?
To address question 1 (What is the relationship between processing speed and FA?), we created a processing speed variable for each of the two tests, Color-Word Interference and Trail-Making (henceforth abbreviated as PSCW and PSTMT, respectively). To create PSCW, the Word-Reading and Color-Naming subtest scores of Color Word Interference Task were combined, as has been done previously (Denney & Lynch, 2009). To create PSTMT, the Number-Sequencing and Letter-Sequencing sub-test scores of the Trail-Making Task were combined. Two separate hierarchical regression analyses were performed to determine the association between FA and PS which included each of these PS variables (PSCW, PSTMT) as predictor variables. These analyses were run separately for the MS and HC groups.
To address question 2 (What is the relationship between executive functioning and FA), we used the executive score on each of the tasks. For Color-Word, we used the score on the third trial of the task (i.e. the “Inhibition” trial score), abbreviated henceforth as CW. For Trail-Making, we used the fourth trial of the task (i.e. the “Switching” trial score), abbreviated henceforth as TMT. Two separate hierarchical regression analyses were run to determine the association between FA and executive functioning which included each of these executive variables (CW, TMT) as predictor variables for each group separately.
To address question 3 (What is the relationship between executive functioning and FA once processing speed is statistically removed from the executive score? ), we created a new executive score by statistically removing processing speed from each of the existing executive scores (CW and TMT). For Color Word, we residualized the CW scores by “regressing [Color-Word Inhibition Trial Score] on [Color Naming Trial Score], obtaining the unstandardized residual score for each subject, and subtracting this score from the overall sample mean on [Color-Word Inhibition Trial Score]” (Denney & Lynch, 2009), page 453). This method of residualizing scores has been shown to be one of the most sensitive ways of isolating executive functioning from Color-Word Interference (Denney & Lynch, 2009). The new executive score (with PS removed) is abbreviated as CW-PSCW. For the Trails variable, this same approach was used by regressing Number-Letter Switching onto Letter Sequencing, and subtracting the unstandardized residual for each subject from the overall sample mean on Number-Letter Switching to create a new score, abbreviated as TMT-PSTMT. After residualization, higher scores for both CW-PSCW and TMT-PSTMT indicated better performance. Two separate hierarchical regression analyses were run to determine the association between FA and executive functioning (corrected for processing speed) which included each of these executive variables (CW-PSCW, TMT-PSTMT) as predictor variables for each group separately.
Results
Behavioral Results
Neuropsychological test results are presented in Table 1. ANCOVAs for these neuropsychological measures were performed using SPSS software to examine group differences in performance, with age entered as a covariate. The score from one HC subject for Trail-Making Number Letter Switching was excluded due to testing error. On both Trail-Making and Color Word Interference, the MS group performed significantly slower than HCs on all processing speed measures. On the executive measures, the MS group performed worse on the executive scores of both tasks. However, once the executive scores were corrected for processing speed (via residualization), there were no group differences in either task. Analyses were conducted again with gender as a covariate, and results remained the same.
Table 1.
Neuropsychological Test Performance
| Neuropsychological Test Performance | MS Mean (SD) | HC Mean (SD) | |||||
|---|---|---|---|---|---|---|---|
| Raw | Scaled | Raw | Scaled | F | p | Eta Squared | |
| WASI Vocabulary | 62.7 (8.0) | 11.6 (2.6) | 65.6 (7.4) | 12.6 (2.6) | 2.8 | .104 | .07 |
| Trail-Making Task | |||||||
| Trails-Number Sequencing (seconds to completion) | 40.3 (26.6) | 9.1 (4.0) | 22.8 (5.8) | 12.5 (1.6) | 7.3 | .010 | .165 |
| Trails-Letter Sequencing (seconds to completion) | 40.1 (26.3) | 9.2 (3.8) | 22.5 (7.8) | 12.7 (1.9) | 7.9 | .008 | .176 |
| pstmt | 80.4 (52.4) | - | 45.3 (12.1) | - | 7.8 | .008 | .174 |
| TMT | 90.8 (42.8) | 9.2 (3.2) | 50.3 (20.0) | 12.5 (1.7) | 11.0 | .001 | .249 |
| TMT-PStmt | 80.6 (20.9) | - | 90.3 (16.5) | - | 1.18 | .285 | .032 |
| Color-Word Interference | |||||||
| CWI Color Naming (seconds to completion) | 30.6 (5.4) | 9.2 (2.6) | 26 (5.5) | 11.1 (2.4) | 6.9 | .012 | .157 |
| CWI Word Reading (seconds to completion) | 23.9 (6.6) | 9.0 (3.7) | 18.1 (2.9) | 12.4 (1.8) | 15 | .000 | .289 |
| pscw | 54.6 (11.7) | - | 44.1 (7.9) | - | 11.6 | .002 | .238 |
| CW | 57 (13.0) | 9.6 (3.0) | 46.4 (10.2) | 11.8 (2.3) | 6.3 | .016 | .146 |
| CW-PScw | 51.8(9.3) | - | 55.0 (8.4) | - | 0.71 | .405 | .019 |
Note. Both raw and scaled scores are provided where applicable. ANOVAs were performed on raw scores, controlling for age. TMT = Executive (switching) score on Trail-Making, PStmt = Processing speed variable on Trail-Making, TMT-PSTMT= Executive score of Trail-Making, statistically corrected for processing speed. CW = Executive (inhibition) score on Color-Word, PScw = Processing speed variable on Color-Word, CW-PScw = Executive Score on Color-Word, statistically corrected for processing speed.
TBSS Analysis
Comparison of FA between groups
FA was compared between groups. There were multiple regions in which the MS group showed significantly reduced FA compared to HCs. These regions, listed in Table 2, replicate reductions in FA that have been found previously in MS (Bammer et al., 2000; Hecke et al., 2010; Roosendaal et al., 2009; Rueda et al., 2008).
Table 2.
Locations where MS FA < HC FA
| Cluster Size | X | Y | Z | Location |
|---|---|---|---|---|
| 347 | 3.0 | 19.0 | 14.0 | Body of corpus callosum |
| 64 | 38.0 | −53.0 | 18.0 | R Superior longitudinal fasciculus |
| 45 | 24.0 | −52.0 | 22.0 | R Splenium |
| 44 | 19.0 | −67.0 | 37.0 | R Inferior longitudinal fasciculus |
| 39 | −38.0 | −49.0 | 13.0 | L Superior longitudinal fasciculus |
Relationship between Trail-Making Test Performance and FA
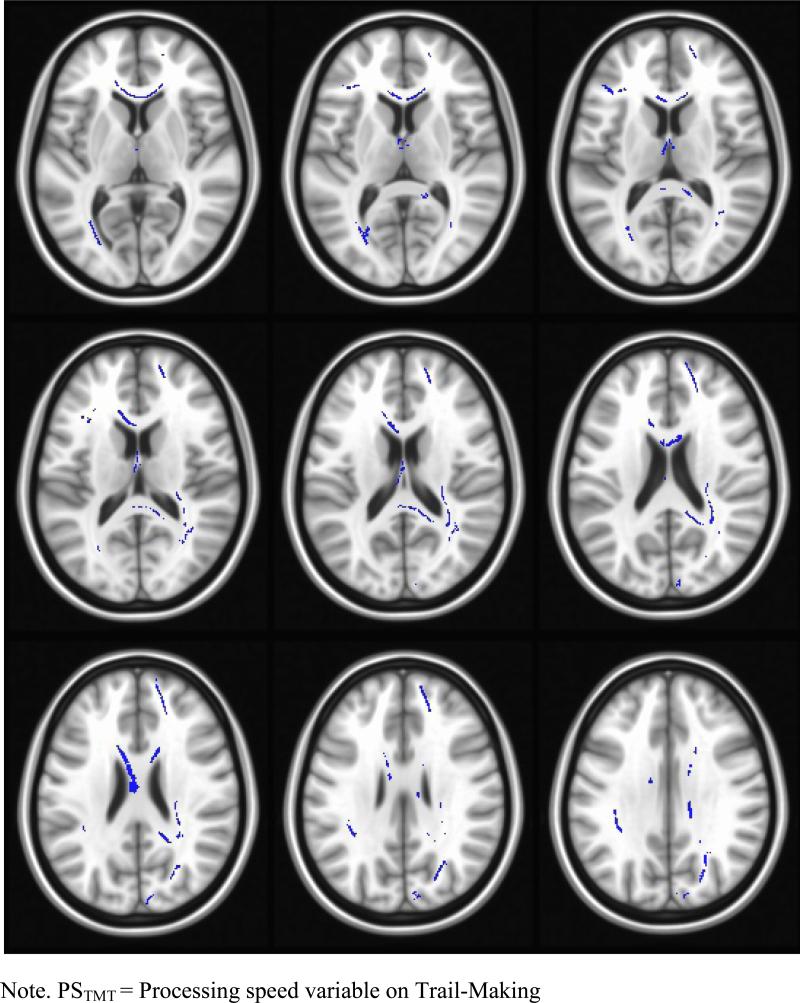
To address question 1 (What is relationship between processing speed in Trail-Making Test and FA?), hierarchical regression analyses were performed in the HC group and MS group separately with age entered first, the PSTMT serving as the predictor variable, and FA as the dependent variable. A significant association between reduced performance on PSTMT and reduced FA was found in several regions, including body and splenium of the corpus callosum, the forceps minor and major, right SLF, right inferior longitudinal fasciculus and the left external capsule, among others (see Table 3 and Figure 1).
Table 3.
Relationship between FA and PSTMT in MS
| Cluster Size | X | Y | Z | R2 | |
|---|---|---|---|---|---|
| *L Body of CC | 1433 | −7 | 6 | 24 | .5 |
| *R Forceps Minor | 416 | 19 | 46 | 21 | .48 |
| L Forceps Major | 247 | −24 | −69 | 9 | .32 |
| R Forceps Major | 101 | 11 | −86 | 28 | |
| *R Inferior Longitudinal Fasciculus | 388 | 22 | −73 | 32 | .31 |
| L Splenium of CC | 345 | −2 | −38 | 14 | .45 |
| Fornix | 233 | 0 | −8 | 16 | .33 |
| *L SLF | 212 | −36 | −39 | 29 | .57 |
| R SLF | 137 | 15 | 3 | 28 | |
| *R Posterior Corona Radiata | 209 | 30 | −46 | 21 | .51 |
| R Posterior Corona Radiata | 119 | 26 | −26 | 24 | |
| R Anterior Corona Radiata | 108 | 25 | 32 | 0 | .43 |
| *L Inferior Fronto-occipital fasciculus | 144 | −41 | 31 | 12 | .47 |
| R Posterior Thalamic Radiation | 139 | 29 | −62 | 14 | .32 |
Note. PSTMT= Processing speed variable on Trail-Making, L= Left, R= Right, SLF = Superior Longitudinal Fasciculus
Relationship between FA and TMT observed here in MS group.
Figure 1.
Regions where FA was associated with PSTMT in MS
To address question 2 (What is the relationship between executive functioning and FA), hierarchical regression analyses were performed in both groups separately with age entered first, the TMT serving as the predictor variable, and FA serving as the dependent variable. The network of regions where reduced FA was associated with TMT score was virtually identical those regions where FA was associated with PSTMT (see note in Table 3). There were several unique regions in which there was a relationship between TMT and FA, not found when examining relationship between PSTMT and FA, including the genu of the corpus callosum (R2 = .48), the cingulum (R2 = .45) and superior corona radiata (R2 = .45). These unique regions may be possibly representative of areas responsible for executive abilities beyond simple processing speed.
To address question 3 (What is the relationship between executive functioning and FA once processing speed is statistically removed from the executive score?), the hierarchical regression was run with TMT-PSTMT as the predictor variable. There were no observed regions in which reduced FA was associated with TMT-PSTMT in the MS group.
The same hierarchical regressions described above were repeated for the HC group. When examining question 1 in the HC group, no regions were associated with PSTMT. When examining question 2 in HCs, there was a significant relationship between FA in HCs and TMT in the left posterior limb of internal capsule (R2 =.55). When examining question 3, no regions in which FA was associated with TMT-PSTMT were identified.
Relationship between Color-Word Interference Performance and FA
To address question 1, (What is relationship between processing speed in Color-Word Interference and FA?), hierarchical regression analyses were performed for each group separately with age (entered first) and PSCW as the predictor variable and FA as the dependent variable. A significant relationship was found between reduced PSCW scores and reduced FA in multiple regions, including the body and splenium of the corpus callosum, the right anterior and right posterior thalamic radiation, and the right forceps minor, among others, in that poor performance was associated with reduced FA in those regions (see Table 4).
Table 4.
Relationship between FA and PSCW in MS group
| Cluster Size | X | Y | Z | t-value | R2 | |
|---|---|---|---|---|---|---|
| * † L Body of Corpus Callosum | 5900 | −5 | 1 | 24 | −4.810 | .49 |
| L Splenium of Corpus Callosum | 539 | −4 | −38 | 11 | −4.163 | .45 |
| R Splenium of Corpus Callosum | 485 | 13 | −35 | 27 | −3.849 | |
| *R Forceps Minor | 446 | 15 | 54 | 20 | −7.092 | .70 |
| Fornix | 341 | 0 | −10 | 17 | −4.204 | .47 |
| L SLF | 240 | -35 | −38 | 30 | −4.715 | .51 |
| R SLF | 140 | 43 | −32 | 8 | −4.636 | |
| R SLF | 120 | 39 | −9 | 26 | −3.912 | |
| R SLF | 120 | 46 | −3 | 32 | −3.854 | |
| *R Anterior Thalamic Radiation | 284 | 44 | 20 | 16 | −4.099 | .47 |
| R Posterior Thalamic Radiation | 681 | 30 | −52 | 18 | −5.141 | .55 |
Note. PSCW = Processing speed variable on Color-Word, SLF = Superior Longitudinal Fasciculus, L = Left, R = Right
Relationship between FA and CW observed here in MS group.
Relationship between FA and PSCW was also observed in Corpus Callosum in HCs.
To address question 2 (What is the relationship between executive functioning and FA), hierarchical regression analyses were performed in both groups separately with age entered first, and the CW serving as the predictor variable, and FA serving as the dependent variable. The network of regions where reduced FA was associated with CW was similar to those regions in which there was an association between PSCW and FA and included the body of corpus callosum (R2 = .48) and forceps minor (R2 = .58) among others (See note in Table 4).
To address question 3 (What is the relationship between executive functioning and FA once processing speed is statistically removed from the executive score?) the hierarchical regression was run with CW-PSCW as the predictor variable. Reduced FA was associated with reduced performance on CW-PSCW in only one region: right anterior thalamic radiation (see Table 5). Thus, PSCW and CW scores were associated with FA in a diffuse network of white matter tracts, while CW-PSCW was associated with FA in a network localized to the anterior thalamic radiation.
Table 5.
Relationship between FA and CW-PScw in MS group
| Cluster Size | X | Y | Z | t-value | R2 | |
|---|---|---|---|---|---|---|
| R Anterior Thalamic Radiation | 128 | 18 | −2 | 8 | 3.870 | .41 |
Note. CW-PSCW = Executive Score on Color-Word, statistically corrected for processing speed, R = Right
The same hierarchical regressions described above were repeated for the HC group. The relationship between higher FA and higher PSCW was found in only the body of the corpus callosum (R2 = .69) (See note in Table 4). There was no relationship observed between FA and either CW or CW-PSCW in the HC group.
Discussion
The purpose of the current study was to examine the relationship between executive abilities and white matter integrity in MS. Because certain executive tasks are heavily weighted in PS, and processing speed impairments are prevalent in MS, we examined the relationship between executive abilities and FA before and after we controlled for processing speed to examine the influence of processing speed on this relationship. Two different executive tasks (Trail-Making and Color-Word Interference) were examined, both of which are heavily weighted in processing speed. In terms of behavioral results, the MS group performed slower than HCs on all measures of processing speed, as well as the executive tasks before they were statistically corrected for processing speed. However, once the influence of processing speed was removed from the executive test scores, there were no longer significant group differences. Thus, in MS, performance on the two executive tasks is highly dependent on processing speed. In fact, processing speed impairments in MS may be the biggest contributing factor to performance on these executive tasks.
In the current investigation we found that the white matter tracts associated with performance on the executive tasks (before controlling for processing speed) were nearly identical to those regions which were associated with processing speed. For example, in Trail-Making, both processing speed and executive scores were associated with FA in the body of the corpus callosum, the inferior longitudinal fasciculus, and the superior longitudinal fasciculus, among others. However, once processing speed was statistically removed from the executive score, there was no longer an association between FA and performance. These findings indicate that processing speed plays a critical role in the relationship between FA and executive functioning on the Trail-Making task.
In Color-Word, the scenario was slightly different. Before processing speed was removed from the executive score, there was similarity between the network of regions associated with FA and performance on the processing speed variable and executive score. These regions included the body of the corpus callosum, anterior thalamic radiation and forceps minor. Once variance associated with processing speed specifically was removed from the executive score, one localized region remained associated with executive functioning: the anterior thalamic radiation. These findings indicate that like Trail-Making, performance on Color-Word appears to be highly influenced by processing speed. However, even after statistically removing the contribution of processing speed from the CW scores, the inhibition necessary for the CW task was still associated with the integrity of anterior thalamic radiation. Other recent studies have also demonstrated that damage to the anterior thalamic radiations (assessed with DTI) are linked to executive dysfunction in other clinical populations such as schizophrenia (Mamah et al., 2010; Perez-Iglesias et al., 2010), and depression (Sexton et al., 2012). Therefore, it appears that this white matter tract is critical for connecting brain regions involved in executive functioning (specifically inhibition).
Neuroimaging studies of both Trail-Making and Stroop performance are consistent with the findings of the current study. For example, in the current study, damage to the forceps minor was associated with reduced processing speed in both tasks. The forceps minor is a tract that connects the frontal lobes by passing though the genu of the corpus callosum. The association between damage in this region and reduced performance in Trail-Making has been reported in schizophrenia (Perez-Iglesias et al., 2010) and traumatic brain injury (Kraus et al., 2007). Previous studies in MS have noted a correlation between reduced FA in the forceps minor and PASAT performance (Hecke et al., 2010). The SLF was also associated with processing speed in both tasks. The SLF is one of the most prominent white matter tracts connecting the frontal, temporal, and parietal lobes. In a recent study which used fMRI guided fiber tractography, a correlation was shown between poor performance of the Paced Visual Serial Addition Test (PVSAT) (a task reliant on processing speed) and reduced FA in the SLF, and further, the SLF was the major tract connecting regions which were activated during performance of the PVSAT (Bonzano, Pardini, Mancardi, Pizzorno, & Roccatagliata, 2009). Others have also reported a significant relationship between the SLF and processing speed tasks, including the Paced Auditory Serial Addition Test (PASAT) (Dineen et al., 2009) and the Digit-Symbol test (Turken et al., 2008). Together with our findings, there is growing support for the notion that the SLF is critically involved in speeded performance.
Finally, the findings of the current paper have implications not only for the knowledge of neural networks underlying processing speed and executive functioning in MS, but also for understanding the cognitive deficits themselves as behavioral constructs. The findings of similar or almost identical networks underlying the processing speed variables and executive variables (before they were controlled for processing speed) indicate that there is significant shared variance between these two constructs. In Color-Word Interference, once processing speed is controlled for, there is still a unique region in which FA is associated with executive abilities. However, in Trail-Making, once processing speed is controlled for, no network is observed in which there is a relationship between FA and executive abilities, the anterior thalamic radiation. These findings indicate that the executive functions involved in the Trail-Making Task are more heavily dependent on processing speed than those involved in Color-Word Interference.
The current study had several limitations. First, the sample size was relatively small, and the HCs were not matched to the MS group in terms of age. Age, however, was controlled for in all analyses. A second limitation is that because the DTI data were acquired as part of a larger fMRI study, the data were acquired with lower directionality than what is available today. Increased directionality and improved methods of acquisition would increase the signal to noise ratio and be helpful in confirming the findings of the current study. A third limitation involved the tasks used to examine both executive functioning and processing speed. Executive functioning consists of multiple domains, and the current investigation focused primarily on switching and inhibition. Future research is needed to determine if these findings would generalize to other aspects of executive functioning. Similarly, our processing speed variables were highly “task-specific” and future research would be useful to determine if the current findings could be replicated for more classic tasks of processing speed. Finally, Trail-Making has a significant motor component, and given the motor deficits in MS, performance on this task might have been confounded by motor slowing. Future examinations will benefit by using a variety of processing speed tasks.
The current study examined the relationship of three factors known to be impacted by MS: white matter integrity, executive abilities and processing speed. The findings indicate a relationship between white matter integrity and executive ability in the frontal subcortical white matter tracts involving the anterior thalamic radiation. These data also indicate that damage to a diffuse network of white matter tracts contribute to reduced processing speed in task-specific abilities.
Acknowledgements
We would like to acknowledge Dr. Matthew Hoptman for his help in the analysis of the diffusion tensor imaging data.
Funding: This work was supported in part by the National Multiple Sclerosis Society [PP1364 to G.W., RG3330 to N.C.]; and National Institutes of Health [1R01HD045798 to N.C., 1R01HD045798S to N.C.].
References
- Arnett PA, Rao SM, Bernardin L, Grafman J, Yetkin FZ, Lobeck L. Relationship between frontal lobe lesions and Wisconsin Card Sorting Test performance in patients with multiple sclerosis. Neurology. 1994;44(3 Pt 1):420–425. doi: 10.1212/wnl.44.3_part_1.420. [DOI] [PubMed] [Google Scholar]
- Bammer R, Augustin M, Strasser-Fuchs S, Seifert T, Kapeller P, Stollberger R. Magnetic resonance diffusion tensor imaging for characterizing diffuse and focal white matter abnormalities in multiple sclerosis. Magnetic Resonance in Medicine. 2000;44(4):583–591. doi: 10.1002/1522-2594(200010)44:4<583::aid-mrm12>3.0.co;2-o. doi: 10.1002/1522-2594(200010)44:4<583. [DOI] [PubMed] [Google Scholar]
- Baudewig J, Dechent P, Merboldt KD, Frahm J. Thresholding in correlation analyses of magnetic resonance functional neuroimaging. Magnetic Resonance Imaging. 2003;21(10):1121–1130. doi: 10.1016/j.mri.2003.08.013. doi: S0730725X03003230. [DOI] [PubMed] [Google Scholar]
- Beatty WW, Paul RH, Blanco CR, Hames KA, Wilbanks SL. Attention in multiple sclerosis: correlates of impairment on the WAIS-R Digit Span Test. Applied Neuropsychology. 1995;2(3-4):139–144. doi: 10.1080/09084282.1995.9645351. doi: 10.1207/s15324826an0203&4_6. [DOI] [PubMed] [Google Scholar]
- Bonzano L, Pardini M, Mancardi GL, Pizzorno M, Roccatagliata L. Structural connectivity influences brain activation during PVSAT in Multiple Sclerosis. Neuroimage. 2009;44(1):9–15. doi: 10.1016/j.neuroimage.2008.08.015. doi: S1053-8119(08)00940-3. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, DeLuca J. Cognitive impairment in multiple sclerosis. Lancet Neurology. 2008;7(12):1139–1151. doi: 10.1016/S1474-4422(08)70259-X. doi: S1474-4422(08)70259-X. [DOI] [PubMed] [Google Scholar]
- Chiaravalloti ND, Wylie G, Leavitt V, Deluca J. Increased cerebral activation after behavioral treatment for memory deficits in MS. Journal of Neurology. 2012;259(7):1337–1346. doi: 10.1007/s00415-011-6353-x. doi: 10.1007/s00415-011-6353-x. [DOI] [PubMed] [Google Scholar]
- Christodoulou C, Krupp LB, Liang Z, Huang W, Melville P, Roque C. Cognitive performance and MR markers of cerebral injury in cognitively impaired MS patients. Neurology. 2003;60(11):1793–1798. doi: 10.1212/01.wnl.0000072264.75989.b8. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH, editors. Delis-Kaplan Executive Function System. The Psychological Corporation; San Antonio, Texas: 2000. [Google Scholar]
- DeLuca J, Sumowski JF, Leavitt VM, Chiaravalloti N, Wylie GR. Is executive dysfunction an independent deficit in multiple sclerosis, or merely a product of slowed processing speed? Journal of the International Neuropsychology Society. 2010;16(S1):2. [Google Scholar]
- Denney DR, Lynch SG. The impact of multiple sclerosis on patients’ performance on the Stroop Test: processing speed versus interference. Journal of the International Neuropsychology Society. 2009;15(3):451–458. doi: 10.1017/S1355617709090730. doi: S1355617709090730. [DOI] [PubMed] [Google Scholar]
- Dineen RA, Vilisaar J, Hlinka J, Bradshaw CM, Morgan PS, Constantinescu CS. Disconnection as a mechanism for cognitive dysfunction in multiple sclerosis. Brain. 2009;132(Pt 1):239–249. doi: 10.1093/brain/awn275. doi: awn275. [DOI] [PubMed] [Google Scholar]
- Drew MA, Starkey NJ, Isler RB. Examining the link between information processing speed and executive functioning in multiple sclerosis. Archives of Clinical Neuropsychology. 2009;24(1):47–58. doi: 10.1093/arclin/acp007. doi: acp007. [DOI] [PubMed] [Google Scholar]
- Foong J, Rozewicz L, Quaghebeur G, Davie CA, Kartsounis LD, Thompson AJ. Executive function in multiple sclerosis. The role of frontal lobe pathology. Brain. 1997;120(Pt 1):15–26. doi: 10.1093/brain/120.1.15. [DOI] [PubMed] [Google Scholar]
- Ge Y, Law M, Grossman RI. Applications of diffusion tensor MR imaging in multiple sclerosis. Annals of the New York Academy of Sciences. 2005;1064:202–219. doi: 10.1196/annals.1340.039. doi: 1064/1/202. [DOI] [PubMed] [Google Scholar]
- Genova HM, Sumowski JF, Chiaravalloti N, Voelbel GT, DeLuca J. Cognition in multiple sclerosis: a review of neuropsychological and fMRI research. Frontiers in Bioscience. 2009;14:1730–1744. doi: 10.2741/3336. doi: 3336. [DOI] [PubMed] [Google Scholar]
- Hecke WV, Nagels G, Leemans A, Vandervliet E, Sijbers J, Parizel PM. Correlation of cognitive dysfunction and diffusion tensor MRI measures in patients with mild and moderate multiple sclerosis. Journal of Magnetic Resonance Imaging. 2010;31(6):1492–1498. doi: 10.1002/jmri.22198. doi: 10.1002/jmri.22198. [DOI] [PubMed] [Google Scholar]
- Kleeberg J, Bruggimann L, Annoni JM, van Melle G, Bogousslavsky J, Schluep M. Altered decision-making in multiple sclerosis: a sign of impaired emotional reactivity? Annals of Neurology. 2004;56(6):787–795. doi: 10.1002/ana.20277. doi: 10.1002/ana.20277. [DOI] [PubMed] [Google Scholar]
- Kraus MF, Susmaras T, Caughlin BP, Walker CJ, Sweeney JA, Little DM. White matter integrity and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130(Pt 10):2508–2519. doi: 10.1093/brain/awm216. doi: awm216. [DOI] [PubMed] [Google Scholar]
- Leavitt V, Sumowski JF, Wylie G, Krch D, Chiaravalloti N, DeLuca J. Does slowed processing speed account for executive deficits in MS? Evidence from neuropsychological performance and structure neuroimaging. doi: 10.1037/a0037517. [DOI] [PubMed] [Google Scholar]
- Macniven JAB, Davis C, Ho MY, Bradshaw CM, Szabadi E, Constantinescu CS. Stroop performance in mulitple sclerosis: Information processing, selective attention, or executive functioning. Journal of the International Neuropsychology Society. 2008;14:805–814. doi: 10.1017/S1355617708080946. [DOI] [PubMed] [Google Scholar]
- Mamah D, Conturo TE, Harms MP, Akbudak E, Wang L, McMichael AR. Anterior thalamic radiation integrity in schizophrenia: a diffusion-tensor imaging study. Psychiatry Research. 2010;183(2):144–150. doi: 10.1016/j.pscychresns.2010.04.013. doi: S0925-4927(10)00149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald WI, Compston A, Edan G, Goodkin D, Hartung HP, Lublin FD. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Annals of Neurology. 2001;50(1):121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- Nebes RD, Meltzer CC, Whyte EM, Scanlon JM, Halligan EM, Saxton JA. The relation of white matter hyperintensities to cognitive performance in the normal old: education matters. Neuropsychology, Development, Cognition, Section B Aging, Neuropsychology Cognition. 2006;13(3-4):326–340. doi: 10.1080/138255890969294. doi: VRJX75722034U067. [DOI] [PubMed] [Google Scholar]
- Pan JW, Krupp LB, Elkins LE, Coyle PK. Cognitive dysfunction lateralizes with NAA in multiple sclerosis. Applied Neuropsychology. 2001;8(3):155–160. doi: 10.1207/S15324826AN0803_4. [DOI] [PubMed] [Google Scholar]
- Parmenter BA, Zivadinov R, Kerenyi L, Gavett R, Weinstock-Guttman B, Dwyer MG. Validity of the Wisconsin Card Sorting and Delis-Kaplan Executive Function System (DKEFS) Sorting Tests in multiple sclerosis. Journal of Clinical and Experimental Neuropsychology. 2007;29(2):215–223. doi: 10.1080/13803390600672163. doi: 770386674. [DOI] [PubMed] [Google Scholar]
- Perez-Iglesias R, Tordesillas-Gutierrez D, McGuire PK, Barker GJ, Roiz-Santianez R, Mata I. White matter integrity and cognitive impairment in first-episode psychosis. The American Journal of Psychiatry. 2010;167(4):451–458. doi: 10.1176/appi.ajp.2009.09050716. doi: appi.ajp.2009.09050716. [DOI] [PubMed] [Google Scholar]
- Pujol J, Vendrell P, Deus J, Junque C, Bello J, Marti-Vilalta JL. The effect of medial frontal and posterior parietal demyelinating lesions on stroop interference. Neuroimage. 2001;13(1):68–75. doi: 10.1006/nimg.2000.0662. doi: 10.1006/nimg.2000.0662S1053811900906621. [DOI] [PubMed] [Google Scholar]
- Roca M, Torralva T, Meli F, Fiol M, Calcagno M, Carpintiero S. Cognitive deficits in multiple sclerosis correlate with changes in fronto-subcortical tracts. Multiple Sclerosis. 2008;14(3):364–369. doi: 10.1177/1352458507084270. doi: 1352458507084270. [DOI] [PubMed] [Google Scholar]
- Roosendaal SD, Geurts JJ, Vrenken H, Hulst HE, Cover KS, Castelijns JA. Regional DTI differences in multiple sclerosis patients. Neuroimage. 2009;44(4):1397–1403. doi: 10.1016/j.neuroimage.2008.10.026. doi: S1053-8119(08)01155-5. [DOI] [PubMed] [Google Scholar]
- Rueda F, Hygino LC, Jr., Domingues RC, Vasconcelos CC, Papais-Alvarenga RM, Gasparetto EL. Diffusion tensor MR imaging evaluation of the corpus callosum of patients with multiple sclerosis. Arquivos de Neuropsiquiatria. 2008;66(3A):449–453. doi: 10.1590/s0004-282x2008000400001. doi: S0004-282X2008000400001. [DOI] [PubMed] [Google Scholar]
- Sexton CE, McDermott L, Kalu UG, Herrmann LL, Bradley KM, Allan CL. Exploring the pattern and neural correlates of neuropsychological impairment in late-life depression. Psychol Med. 2012;42(6):1195–1202. doi: 10.1017/S0033291711002352. doi: S0033291711002352. [DOI] [PubMed] [Google Scholar]
- Simioni S, Ruffieux C, Kleeberg J, Bruggimann L, du Pasquier RA, Annoni JM. Progressive decline of decision-making performances during multiple sclerosis. Journal of the International Neuropsychology Society. 2009;15(2):291–295. doi: 10.1017/S1355617709090262. doi: S1355617709090262. [DOI] [PubMed] [Google Scholar]
- Smith AM, Walker LA, Freedman MS, DeMeulemeester C, Hogan MJ, Cameron I. fMRI investigation of disinhibition in cognitively impaired patients with multiple sclerosis. Journal of the Neurological Sciences. 2009;281(1-2):58–63. doi: 10.1016/j.jns.2009.02.366. doi: S0022-510X(09)00448-1. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. doi: S1053-8119(06)00138-8. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Sumowski JF, Wylie GR, Deluca J, Chiaravalloti N. Intellectual enrichment is linked to cerebral efficiency in multiple sclerosis: functional magnetic resonance imaging evidence for cognitive reserve. Brain. 2010;133(Pt 2):362–374. doi: 10.1093/brain/awp307. doi: awp307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken A, Whitfield-Gabrieli S, Bammer R, Baldo JV, Dronkers NF, Gabrieli JD. Cognitive processing speed and the structure of white matter pathways: convergent evidence from normal variation and lesion studies. Neuroimage. 2008;42(2):1032–1044. doi: 10.1016/j.neuroimage.2008.03.057. doi: S1053-8119(08)00286-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitkovitch M, Bishop S, Dancey C, Richards A. Stroop interference and negative priming in patients with multiple sclerosis. Neuropsychologia. 2002;40(9):1570–1576. doi: 10.1016/s0028-3932(02)00022-2. doi: S0028393202000222. [DOI] [PubMed] [Google Scholar]
- Wechsler D. Wechsler Abbreviated Scale of Intelligence Manual (WASI) Harcourt Assessment; San Antonio, TX.: 1999. [Google Scholar]