Abstract
Aim
This study was designed to determine the efficacy and tolerability of capecitabine, oxaliplatin and bevacizumab in combination with cetuximab as first-line therapy for advanced colorectal cancer.
Patients and Methods
Patients with previously untreated advanced colorectal cancer received oxaliplatin 130 mg/m2 and bevacizumab 7.5 mg/kg every three weeks, capecitabine 850 mg/m2 twice daily on days 1–14, and cetuximab at 400 mg/m2 load and 250 mg/m2 weekly. KRAS, BRAF and PI3K mutation status from paraffin-embedded tumor samples were assessed using real-time polymerase chain reaction.
Results
Thirty patients were evaluable for safety and efficacy. One patient had a complete response and 12 patients had a partial response, giving an overall response rate of 43% (95% confidence interval (CI) 25% – 63%). Fifteen patients had stable disease. The median time to progression was 10.3 months (95% CI, 6.8 – 16.3 months). The median overall survival was 18.8 months (95% CI, 14.2 – 23.7 months). Common grade ≥3 non-hematological toxicities were skin rash (37%), sensory neuropathy (27%) and diarrhea (17%). Grade ≥3 hematological toxicities were uncommon. Mutations in KRAS, BRAF and PI3K occurred in 34.5 %, 10.3% and 10.3% of patients respectively, but did not correlate with treatment outcome.
Conclusion
The addition of cetuximab to capecitabine, oxaliplatin and bevacizumab did not improve the three drug regimen activity compared to published data and was associated with significant toxicities requiring frequent dose modifications. KRAS, BRAF, and PI3K mutation status were consistent with published literature, but did not affect outcome in this small study.
Keywords: Capecitabine, oxaliplatin, bevacizumab, cetuximab, phase II, metastatic colorectal
Treatment of colorectal cancer has evolved significantly over the past decade. In the first-line setting, current therapy usually consists of 5-fluorouracil (5-FU) based combination chemotherapy plus a targeted antibody against either vascular endothelial cell growth factor (VEGF) or the epidermal growth factor receptor (EGFR) (1). Capecitabine is an oral pro-drug of 5-FU that allows greater patient convenience compared to infusional 5-FU. Randomized phase III trials have demonstrated non-inferiority of oral 5-FU in combination with oxaliplatin (XELOX) compared to infusional 5-FU and oxaliplatin (FOLFOX) in first- (2, 3) and second-line settings (4). Among biological agents added to chemotherapy, bevacizumab was the first to demonstrate clinical benefit in the first-line setting, initially with the irinotecan, 5-FU and leucovorin (IFL) regimen, and later with other regimens including 5-FU and leucovorin (5), capecitabine (6), and with FOLFOX and XELOX (7). The anti-EGFR monoclonal antibodies cetuximab and panitumumab have shown clinical benefit in patients with metastatic colorectal cancer whose tumors do not harbor a KRAS mutation. In the first-line setting, cetuximab has demonstrated a significant benefit in combination with infusional 5-FU plus irinotecan (FOLFIRI regimen) (8); however, the role for cetuximab combined with oxaliplatin-based regimens (FOLFOX or XELOX regimens) is controversial (9, 10).
At the time this study was initiated, a randomized study of eighty-three patients suggested a benefit from addition of bevacizumab to the regimen of cetuximab plus irinotecan in refractory colorectal cancer (11). Similarly, the combination of capecitabine, oxaliplatin, and bevacizumab had just been shown to be tolerable and active (12, 13). Preclinical data had demonstrated greater anti-tumor activity when anti-VEGF and anti-EGFR agents were combined with chemotherapy compared to chemotherapy alone (14, 15) and when they were combined with each other compared to each agent alone (16–18). Therefore this phase II study was conducted to evaluate the tolerability and activity of the XELOX-A regimen (capecitabine, oxaliplatin, bevacizumab) combined with cetuximab in first-line metastatic colorectal cancer. This trial was also designed to allow exploration of biomarkers of activity, including KRAS, BRAF, and PI3K, which were not yet well studied at the time of study initiation.
Patients and Methods
Patient Selection
Patients with histologically confirmed colorectal adenocarcinoma and documented metastatic or incurable recurrent disease were eligible to participate in the trial. Patients were required to be age ≥18 years, with Eastern Cooperative Group (ECOG) performance status 0–2, normal organ and marrow function defined as absolute neutrophil count (ANC) >2,000/μl, platelets >100,000/μl, total bilirubin < 2.0 X upper limit of normal (UNL), aspartate transaminase (AST) or alanine transaminase (ALT) <2.5 X UNL (<5 X UNL if known liver metastasis) and creatinine clearance > 40 ml/min.
Exclusion criteria included having received prior chemotherapy or biologic therapy for metastatic or recurrent disease (adjuvant or radiosensitizing fluoropyrimidines with or without leucovorin more than six months before study entry and adjuvant oxaliplatin more than 12 months before study entry were allowed), poorly controlled hypertension arterial thromboembolic events within six months of study entry, uncontrolled coagulopathy; major surgery within four weeks of initiation of study treatment, untreated leptomeningeal or brain metastases, grade ≥2 peripheral neuropathy, inability to tolerate oral medications, baseline urine protein: creatinine ratio >1, and pregnancy or lactation. Women of childbearing potential were required to have a negative pregnancy test within seven days prior to initiation of therapy. Effective contraception was required for sexually active female or male subjects.
The study protocol (Clinicaltrials.gov NCT00290615) was approved by the institutional review boards of each participating center and conducted in accordance with the Declaration of Helsinki and good clinical practice guidelines. Written informed consent was obtained prior to any trial related procedures.
Study design and statistical methods
This was an open-label, multicenter, non-randomized phase II trial. The primary endpoint was overall response (complete and partial response), as defined by RECIST 1.0 criteria based on an intent-to-treat analysis. Secondary endpoints included time to progression, progression-free survival, overall survival, safety and tolerability. Progression-free survival (PFS) was defined as the interval between start of treatment and the date of disease progression or death, censoring for loss to follow-up or start of new line of treatment (for patients who discontinued study treatment for reasons other than disease progression). A 2-stage modified Simon design was used to test the null hypothesis that the response rate was ≤30% versus an alternative favorable response ≥50%, with a significance level of 0.05 and power of 0.853. In the first stage, fifteen patients were to be enrolled and the trial stopped if four or fewer patients showed response. If five or more patients responded in the first stage, the trial was to enroll an additional thirty patients in the second stage. If eighteen or fewer patients out of forty-five patients showed response, the treatment was to be considered to have a response rate of ≤30% and unworthy of further investigation. The null hypothesis was to be rejected if nineteen or more patients responded. The exact method was used to calculate 95% confidence interval of proportions. Survival duration was calculated using the Kaplan-Meier method and comparison between subgroups were performed using the log-rank test. Cox proportional hazard model was used to assess the effect of KRAS, BRAF and PI3K on progression-free and overall survival.
Treatment schedule
Patients received treatment in 21-day cycles, comprising oral capecitabine 850 mg/m2 every 12 hours on days 1–14, weekly cetuximab at an initial dose of 400 mg/m2 intravenously over 120 minutes and subsequently 250 mg/m2 over 60 minutes; on day one of each cycle, oxaliplatin 130 mg/m2 was administered intravenously over two hours and bevacizumab 7.5 mg/kg was administered intravenously over 30–90 minutes. The use of growth factors was permitted. Toxicity was graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events 3.0 (NCI CTCAE), Version 3.0. Neurosensory toxicity was graded according to the Neurologic Toxicity Scale for Oxaliplatin. Treatment on day one of each cycle was delayed until recovery of ANC > 1,500/mm3 and platelet count > 75,000/mm3 and recovery from any clinically significant treatment-related non-hematologic toxicity (except alopecia, anorexia or fatigue) to grade ≤1, or bilirubin and alanine transaminase to grade ≤1. Dose reduction due to adverse events was performed for each drug as specified in the study protocol, which included algorithms to manage oxaliplatin-related neuropathy, capecitabine-related diarrhea and hand-foot syndrome, cetuximab-related acne and infusion reactions and bevacizumab-related hypertension.
Patient evaluation
Vital signs, ECOG performance status, medical history, physical examination, neurosensory assessment, complete blood count (CBC), creatinine, AST, ALT, bilirubin, magnesium, urine protein to creatinine ratio, and toxicity assessments were performed at baseline and every three weeks prior to each treatment cycle. An electrocardiogram was performed at baseline and every three cycles. Formal toxicity assessments were performed weekly for the first three cycles, as well as weekly CBC for the first two cycles.
Tumor response was assessed every two cycles (nine weeks). Study specific assessment of tumor measurements were performed by a radiologist for all patients.
The primary study outcome was ‘on treatment’ PFS, defined from the start of study treatment to date of disease progression or death, whichever occurred earlier, with censoring of patients at the time of loss to follow-up or start of new line of treatment (for patients who discontinued study treatment for reasons other than disease progression). Responses were scored according to RECIST criteria version 1.0 (19).
Correlative Studies
Formalin-fixed paraffin embedded tumor tissue blocks were obtained for each patient. The tumor content was determined by a pathologist and paraffin blocks containing greater than 70% tumor were used for genomic DNA isolation. One 10 μm cut was used to isolate the genomic DNA using the Ambion RecoverAll Total Nucleic Acid Isolation kit per manufacturer’s instructions (Foster City, CA, USA). KRAS mutation status was determined by real-time PCR using the DxS KRAS Mutation Test Kit from DxS Diagnostic Innovations (Manchester, UK), which is able to detect the seven common mutations of the KRAS gene at codons 12 and 13. PI3K mutation status was determined by real-time PCR using the DxS PI3K Mutation Test Kit from DxS Diagnostic Innovations, which is able to detect the following mutations in Exons 9 and 20 of the PIK3CA gene: H1047R, E542K, E545D, and E545K. BRAF mutational status was determined using a custom developed Taqman SNP assay from Applied Biosystems to detect the V600E mutation in the BRAF gene (Carlsbad, CA, USA).
Results
Thirty eligible patients were enrolled; fifteen patients were treated in stage one and fifteen were treated in stage two. Patient demographics are described in Table I. The median age of the patients was 57 years (range 33–77 years) and all had good baseline performance status ECOG 0–1. A minority (23%) had received prior adjuvant chemotherapy, including one patient who had received adjuvant oxaliplatin for rectal cancer. After the release of preliminary results from the CAIRO2 study (capecitabine, oxaliplatin and bevacizumab with or without cetuximab) which demonstrated a reduction in PFS with the addition of cetuximab to the combination of capecitabine, oxaliplatin and bevacizumab (20), a decision was made to close the present study before the completion of stage two.
Table I.
Baseline patient characteristics.
| Characteristic | No | % |
|---|---|---|
| Total patients | 30 | |
| Sex | ||
| Female | 14 | 47 |
| Male | 16 | 53 |
| Ethnicity | ||
| Caucasian | 24 | 80 |
| African American | 5 | 17 |
| Others | 1 | 3 |
| Age, years | ||
| Median | 56.0 | |
| Range | 33–77 | |
| ECOG Performance Status | ||
| 0 | 22 | 73 |
| 1 | 8 | 27 |
| Primary Site | ||
| Colon | 22 | 73 |
| Rectum | 8 | 27 |
| Prior Adjuvant chemotherapy | ||
| Yes | 7 | 23 |
| No | 17 | 57 |
| Unknown | 6 | 20 |
| Number of sites of metastases | ||
| 1 | 15 | 50 |
| 2 | 4 | 13 |
| ≥3 | 5 | 17 |
| Unknown | 6 | 20 |
Dose modifications for toxicity were required in most patients. Among the thirty patients, dose reductions of at least one agent by at least one dose level occurred in twenty-one patients. Seven patients required two or more reductions; eleven required discontinuation of one or more drugs.
The treatment-related adverse events are summarized in Table II. There were no unexpected adverse events encountered with the combination therapy. The most common non-hematological adverse event of any grade were skin rash (90%), sensory neuropathy (84%), diarrhea (70%), hypomagnesemia (56%), nausea (50%), hand-foot syndrome (23%), proteinuria (20%), fatigue (17%) and hypertension (10%). The most common grade three to five non-hematological toxicities were skin rashes (37%), sensory neuropathy (27%), diarrhea (17%) and paronychia (10%). The most common hematological adverse events of any grade included thrombocytopenia (50%), neutropenia (33%) and anemia (10%). Grade three to five anemia, thrombocytopenia and neutropenia occurred in 3%, 7% and 0%, respectively.
Table II.
Selected treatment-related toxicities
| Toxicity | All Grade No. (%) |
Grade 3–5 No. (%) |
|---|---|---|
| Non-hematological | ||
| Bowel perforation | 1 (3) | 1 (3) |
| Diarrhea | 21 (70) | 5 (17) |
| Nausea | 15 (50) | 0 |
| Vomiting | 1 (3) | 1 (3) |
| Fatigue | 5 (17) | 2 (7) |
| Hand-foot syndrome | 7 (23) | 0 |
| Hypersensitivity | 2 (6) | 1 (3) |
| Hypertension | 3 (10) | 0 |
| Hypomagnesemia | 17 (56) | 1 (3) |
| Paronychia | 3 (10) | 3 (10) |
| Proteinuria | 6 (20) | 0 |
| Sensory neuropathy | 25 (84) | 8 (27) |
| Skin rash | 27 (90) | 11 (37) |
| Thromboembolism (Arterial) | 2 (7) | 2 (7) |
| Thromboembolism (Venous) | 2 (7) | 2 (7) |
| Hematological | ||
| Anemia | 3 (10) | 1 (3) |
| Thrombocytopenia | 15 (50) | 2 (7) |
| Neutropenia | 10 (33) | 0 |
Twenty serious adverse events (excluding death) were documented among ten patients, including diarrhea (n=7), dehydration (n=1), dyspnea (n=1), elevated ALT/AST (n=1), sepsis (n=1), hyperbilirubinemia (n=1), urethral obstruction from disease progression (n=1), cerebrovascular accident (n=2), bowel obstruction (n=2), bowel perforation (n=1), pulmonary embolism (n=1) and deep venous thrombosis (n=1). There were two deaths unrelated to study drug; one occurred after elective resection of liver metastasis and the other after evidence of disease progression.
Tumor tissue was available from twenty-nine of thirty patients for KRAS, BRAF and PI3K mutation status analysis. Ten patients had KRAS mutations (34.5%). BRAF mutations occurred in three of twenty-nine patients (10.3%) and were mutually exclusive of KRAS mutations. Hence, the incidence of BRAF mutations among the nineteen patients with wild type KRAS was 16%. PI3K mutations occurred in three patients (10.3%), of whom one had a mutation in KRAS but not BRAF; one patient had no mutation in either KRAS or BRAF and another patient had a mutation in BRAF but not KRAS.
Efficacy
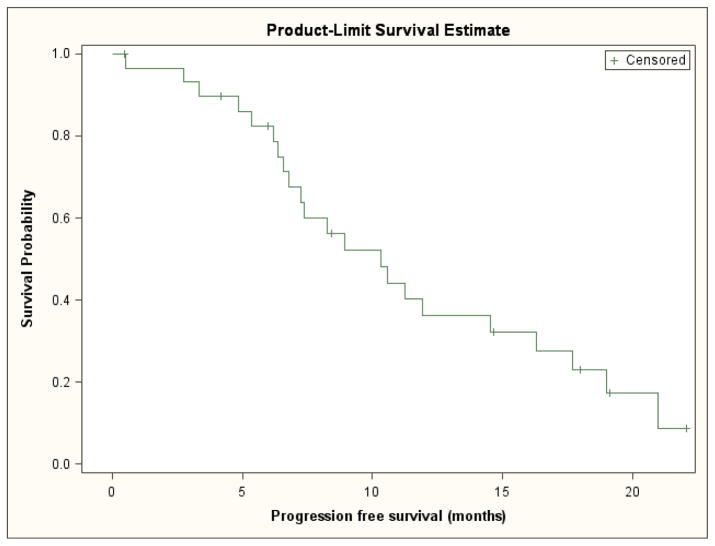
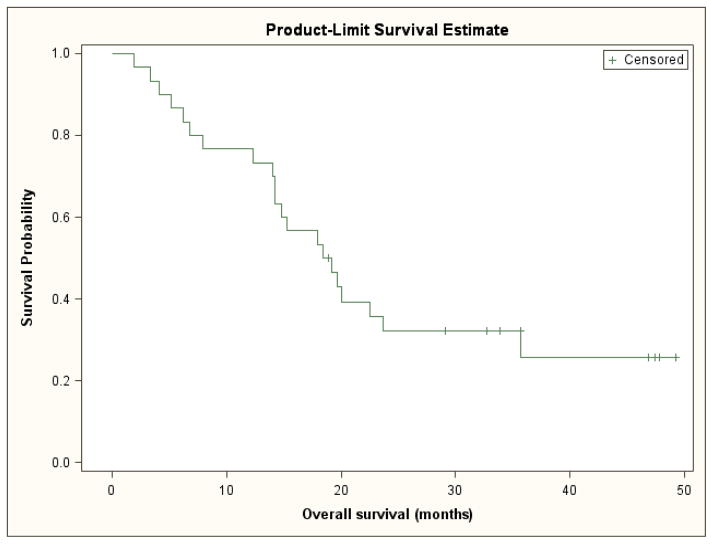
Among the entire cohort, the median PFS was 10.3 months (95% CI, 6.8 to 16.3 months) (Figure 1). Since there were no deaths prior to disease progression or censoring for progression, calculated time to progression yielded identical results to progression-free survival. The median overall survival was 18.8 months (95% CI, 14.2 to 23.7 months) (Figure 2). The overall response rate was 43% (95% CI, 25% to 63%); one patient had a complete response, twelve patients had a partial response, and fifteen patients had stable disease as their best response.
Figure 1.
Progression-free survival
Figure 2.
Overall Survival
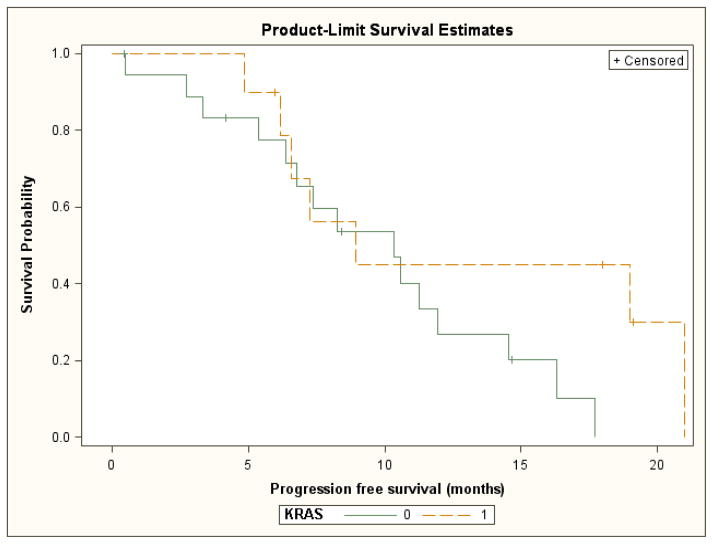
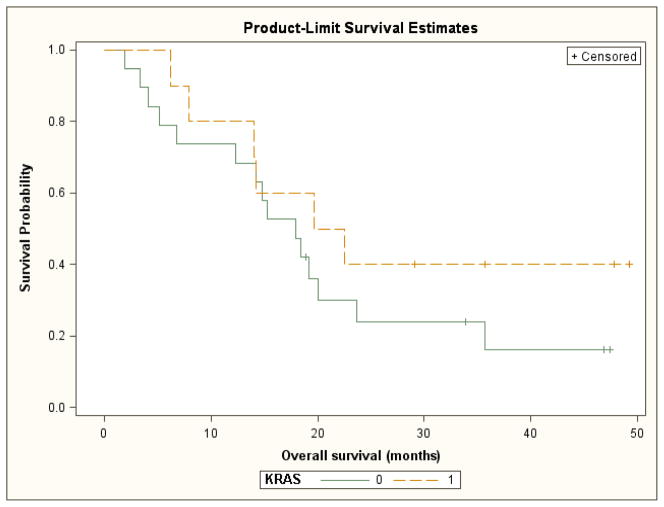
An exploratory analysis of outcomes based upon tumor mutation status was also performed. The median progression-free survival was 10.3 months for patients with wild-type KRAS tumors and 8.9 months for patients with KRAS mutant tumors. These differences were not statistically significant (log rank test p= 0.13) (Figure 3). Median overall survival was 18.0 months for patients with wild-type KRAS tumors and 21.1 months for patients with mutant KRAS tumors. These differences were also not statistically significant (log rank test p= 0.30) (Figure 4). The response rates for patients with KRAS wild-type tumors and KRAS mutant tumors were 37% and 60% respectively.
Figure 3.
Time to progression by KRAS mutation status (0=wild type, 1=mutated)
Figure 4.
Overall survival by KRAS mutation status (0= wild type, 1= mutated)
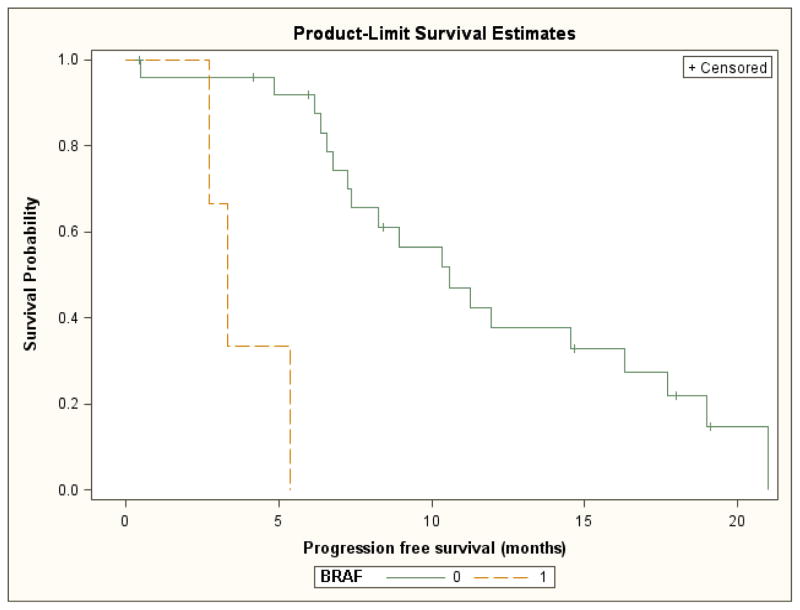
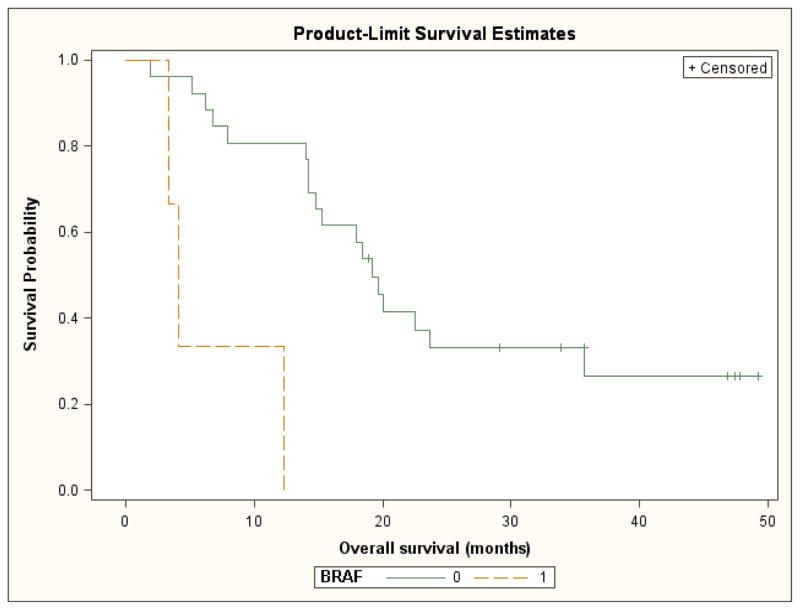
For patients with BRAF mutant versus wild-type tumors, median progression-free and overall survival were significantly shorter (3.3 vs. 10.6 month; log-rank test p=<0.001 and 4.1 months vs. 19.2 months, log rank test p=0.002, respectively). However, this analysis was limited by the small number of patients in the BRAF mutated subgroup (Figures 5 and 6). For the six patients with tumors that were wild-type for both KRAS and BRAF, the response rate was 38%.
Figure 5.
Progression free survival by BRAF mutation status (0= wild type, 1= mutated)
Figure 6.
Overall survival by BRAF mutation status (0= wild type, 1= mutated)
There were no statistically significant differences in progression-free or overall survival by PI3K mutation status (data not shown). For the seventeen patients with tumors that were wild-type for both KRAS and PI3K, the response rate was 41%. For the two patients with tumors that were KRAS wild-type but PI3K mutant, the response rate was 0%. After adjusting for KRAS and PI3K mutation status in a Cox proportional hazard regression model, BRAF mutation remained a significant adverse predictor of progression-free (hazard ratio 16.7, 95% CI, 2.6 to 109.4, p=0.003) and overall survival (hazard ratio 8.3, 95% CI, 1.9 to 37.4, p= 0.006).
Discussion
In this phase II study for first-line metastatic colorectal cancer, the regimen of capecitabine, oxaliplatin, bevacizumab, and cetuximab had a median time to progression of 10.3 months and an objective response of 43%. In patients with KRAS wild-type tumors, PFS was 10.3 months and the response rate was 37%. These results are also consistent with results from two large phase III studies, CAIRO2 and PACCE.
The CAIRO2 study compared capecitabine, oxaliplatin, and bevacizumab with and without cetuximab (20); the PACCE study compared infusional 5-FU, oxaliplatin or irinotecan, and bevacizumab with and without panitumumab (21). In the CAIRO2 study, for patients treated with cetuximab, median PFS was 9.4 months for all patients and 10.5 months for patients with wild-type tumors. In the PACCE study, for patients treated with panitumumab, median PFS was 10.0 months for all patients and 9.8 months for patients with wild-type tumors. In both studies, the addition of an anti-EGFR antibody was associated with worse clinical outcome compared to the control treatment in unselected patients and in patients with ras mutant tumors.
The frequencies of KRAS, BRAF, and PI3K mutations in the present study are comparable to those found by other groups (8, 9, 22). Mutations in KRAS and BRAF have been associated with a lack of benefit from anti-EGFR monoclonal antibodies in metastatic colorectal cancer. Mutations in PI3K have been found in 10% of colorectal cancers (23–25), and have been associated with a lack of response to anti-EGFR directed therapy in some reports (26) but not in others (27). KRAS and PI3K mutation status was not associated with treatment outcome in the current study. This may be explained in part by the small sample sizes involved. Patients whose tumors were BRAF wild-type had better clinical outcomes compared to those whose tumors carried BRAF mutations, and this effect persisted after adjusting for KRAS and PI3K mutation status. These findings are consistent with the literature supporting the prognostic role of BRAF (28–30).
The regimen of capecitabine, oxaliplatin, bevacizumab and cetuximab was associated with significant toxicities. The rate of grade 3–4 toxicities in the current report are similar to those reported in CAIRO2 and PACCE. Importantly, in the current study, more than half of all patients required either multiple dose reductions or discontinuation of one or more study drugs for toxicity. Thus, poor long-term tolerability of the regimen may limit potential efficacy, particularly for progression time points beyond six months. The reasons for lack of benefit from the addition of an anti-EGFR therapy to first-line 5-FU, oxaliplatin, and bevacizumab-based treatment are not known. Combining anti-EGFR and anti-VEGF therapies has been useful as maintenance therapy for non-small cell lung cancer (31), but has had limited success in other settings (32, 33).
In conclusion, for patients with unknown KRAS mutation status and those with KRAS wild-type tumors, the combination of cetuximab with capecitabine, oxaliplatin and bevacizumab is only moderately well tolerated and offers no apparent clinical benefit over standard three drug regimens.
Acknowledgments
We gratefully acknowledge the invaluable contributions of the patients, their families and the Duke University GI Oncology clinical trials team with special recognition to Anthony Amara and Wanda Honeycutt for data management and Anuradha Bulusu for statistical assistance.
References
- 1.Cunningham D, Atkin W, Lenz HJ, Lynch HT, Minsky B, Nordlinger B, Starling N. Colorectal cancer. Lancet. 2010;375:1030–1047. doi: 10.1016/S0140-6736(10)60353-4. [DOI] [PubMed] [Google Scholar]
- 2.Cassidy J, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Saltz L. Randomized phase III study of capecitabine plus oxaliplatin compared with fluorouracil/folinic acid plus oxaliplatin as first-line therapy for metastatic colorectal cancer. J Clin Oncol. 2008;26:2006–2012. doi: 10.1200/JCO.2007.14.9898. [DOI] [PubMed] [Google Scholar]
- 3.Ducreux M, Bennouna J, Hebbar M, Ychou M, Lledo G, Conroy T, Adenis A, Faroux R, Rebischung C, Douillard J. Efficacy and safety findings from a randomized phase III study of capecitabine (X) + oxaliplatin (O) (XELOX) vs. infusional 5- FU/LV + O (FOLFOX-6) for metastatic colorectal cancer (MCRC) J Clin Oncol. 2007;25(18S):abstr 4029. [Google Scholar]
- 4.Rothenberg ML, Cox JV, Butts C, Navarro M, Bang YJ, Goel R, Gollins S, Siu LL, Laguerre S, Cunningham D. Capecitabine plus oxaliplatin (XELOX) versus 5-fluorouracil/folinic acid plus oxaliplatin (FOLFOX-4) as second-line therapy in metastatic colorectal cancer: a randomized phase III noninferiority study. Ann Oncol. 2008;19:1720–1726. doi: 10.1093/annonc/mdn370. [DOI] [PubMed] [Google Scholar]
- 5.Kabbinavar FF, Schulz J, McCleod M, Patel T, Hamm JT, Hecht JR, Mass R, Perrou B, Nelson B, Novotny WF. Addition of bevacizumab to bolus fluorouracil and leucovorin in first-line metastatic colorectal cancer: results of a randomized phase II trial. J Clin Oncol. 2005;23:3697–3705. doi: 10.1200/JCO.2005.05.112. [DOI] [PubMed] [Google Scholar]
- 6.Tebbutt NC, Wilson K, Gebski VJ, Cummins MM, Zannino D, van Hazel GA, Robinson B, Broad A, Ganju V, Ackland SP, Forgeson G, Cunningham D, Saunders MP, Stockler MR, Chua Y, Zalcberg JR, Simes RJ, Price TJ. Capecitabine, bevacizumab, and mitomycin in first-line treatment of metastatic colorectal cancer: results of the Australasian Gastrointestinal Trials Group Randomized Phase III MAX Study. J Clin Oncol. 2010;28:3191–3198. doi: 10.1200/JCO.2009.27.7723. [DOI] [PubMed] [Google Scholar]
- 7.Saltz LB, Clarke S, Diaz-Rubio E, Scheithauer W, Figer A, Wong R, Koski S, Lichinitser M, Yang TS, Rivera F, Couture F, Sirzen F, Cassidy J. Bevacizumab in combination with oxaliplatin-based chemotherapy as first-line therapy in metastatic colorectal cancer: a randomized phase III study. J Clin Oncol. 2008;26:2013–2019. doi: 10.1200/JCO.2007.14.9930. [DOI] [PubMed] [Google Scholar]
- 8.Van Cutsem E, Kohne CH, Hitre E, Zaluski J, Chang Chien CR, Makhson A, D’Haens G, Pinter T, Lim R, Bodoky G, Roh JK, Folprecht G, Ruff P, Stroh C, Tejpar S, Schlichting M, Nippgen J, Rougier P. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N Engl J Med. 2009;360:1408–1417. doi: 10.1056/NEJMoa0805019. [DOI] [PubMed] [Google Scholar]
- 9.Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, Donea S, Ludwig H, Schuch G, Stroh C, Loos AH, Zubel A, Koralewski P. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. 2009;27:663–671. doi: 10.1200/JCO.2008.20.8397. [DOI] [PubMed] [Google Scholar]
- 10.Maughan TS, Adams R, Smith CG, Seymour MT, Wilson RH, Meade AM, Fisher D, Madi A, Cheadle J, Kaplan RS MCT COIN Investigators. Identification of potentially responsive subsets when cetuximab is added to oxaliplatin-fluoropyrimidine chemotherapy (CT) in first-line advanced colorectal cancer (aCRC): Mature results of the MRC COIN trial. J Clin Oncol. 2010;28(15s):abstr 3502. [Google Scholar]
- 11.Saltz LB, Lenz HJ, Kindler HL, Hochster HS, Wadler S, Hoff PM, Kemeny NE, Hollywood EM, Gonen M, Quinones M, Morse M, Chen HX. Randomized phase II trial of cetuximab, bevacizumab, and irinotecan compared with cetuximab and bevacizumab alone in irinotecan-refractory colorectal cancer: the BOND-2 study. J Clin Oncol. 2007;25:4557–4561. doi: 10.1200/JCO.2007.12.0949. [DOI] [PubMed] [Google Scholar]
- 12.Fernando N, Yu D, Morse MA, Blobe GC, Odogwu L, Crews J, Polito A, Honeycutt W, Franklin A, Hurwitz HI. A phase II study of oxaliplatin, capecitabine and bevacizumab in the treatment of metastatic colorectal cancer. J Clin Oncol. 2005;23(16S):abstr 3556. [Google Scholar]
- 13.Hochster HS, Hart LL, Ramanathan RK, Childs BH, Hainsworth JD, Cohn AL, Wong L, Fehrenbacher L, Abubakr Y, Saif MW, Schwartzberg L, Hedrick E. Safety and efficacy of oxaliplatin and fluoropyrimidine regimens with or without bevacizumab as first-line treatment of metastatic colorectal cancer: results of the TREE Study. J Clin Oncol. 2008;26:3523–3529. doi: 10.1200/JCO.2007.15.4138. [DOI] [PubMed] [Google Scholar]
- 14.Baselga J, Pfister D, Cooper MR, Cohen R, Burtness B, Bos M, D’Andrea G, Seidman A, Norton L, Gunnett K, Falcey J, Anderson V, Waksal H, Mendelsohn J. Phase I studies of anti-epidermal growth factor receptor chimeric antibody C225 alone and in combination with cisplatin. J Clin Oncol. 2000;18:904–914. doi: 10.1200/JCO.2000.18.4.904. [DOI] [PubMed] [Google Scholar]
- 15.Fan Z, Baselga J, Masui H, Mendelsohn J. Antitumor effect of anti-epidermal growth factor receptor monoclonal antibodies plus cis-diamminedichloroplatinum on well established A431 cell xenografts. Cancer Res. 1993;53:4637–4642. [PubMed] [Google Scholar]
- 16.Clarke K, Smith K, Gullick WJ, Harris AL. Mutant epidermal growth factor receptor enhances induction of vascular endothelial growth factor by hypoxia and insulin-like growth factor-1 via a PI3 kinase dependent pathway. Br J Cancer. 2001;84:1322–1329. doi: 10.1054/bjoc.2001.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung YD, Mansfield PF, Akagi M, Takeda A, Liu W, Bucana CD, Hicklin DJ, Ellis LM. Effects of combination anti-vascular endothelial growth factor receptor and anti-epidermal growth factor receptor therapies on the growth of gastric cancer in a nude mouse model. Eur J Cancer. 2002;38:1133–1140. doi: 10.1016/s0959-8049(02)00013-8. [DOI] [PubMed] [Google Scholar]
- 18.Petit AM, Rak J, Hung MC, Rockwell P, Goldstein N, Fendly B, Kerbel RS. Neutralizing antibodies against epidermal growth factor and ErbB-2/neu receptor tyrosine kinases down-regulate vascular endothelial growth factor production by tumor cells in vitro and in vivo: angiogenic implications for signal transduction therapy of solid tumors. Am J Pathol. 1997;151:1523–1530. [PMC free article] [PubMed] [Google Scholar]
- 19.Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 20.Tol J, Koopman M, Cats A, Rodenburg CJ, Creemers GJ, Schrama JG, Erdkamp FL, Vos AH, van Groeningen CJ, Sinnige HA, Richel DJ, Voest EE, Dijkstra JR, Vink-Borger ME, Antonini NF, Mol L, van Krieken JH, Dalesio O, Punt CJ. Chemotherapy, bevacizumab, and cetuximab in metastatic colorectal cancer. N Engl J Med. 2009;360:563–572. doi: 10.1056/NEJMoa0808268. [DOI] [PubMed] [Google Scholar]
- 21.Hecht JR, Mitchell E, Chidiac T, Scroggin C, Hagenstad C, Spigel D, Marshall J, Cohn A, McCollum D, Stella P, Deeter R, Shahin S, Amado RG. A randomized phase IIIB trial of chemotherapy, bevacizumab, and panitumumab compared with chemotherapy and bevacizumab alone for metastatic colorectal cancer. J Clin Oncol. 2009;27:672–680. doi: 10.1200/JCO.2008.19.8135. [DOI] [PubMed] [Google Scholar]
- 22.Di Nicolantonio F, Martini M, Molinari F, Sartore-Bianchi A, Arena S, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A. Wild-type BRAF is required for response to panitumumab or cetuximab in metastatic colorectal cancer. J Clin Oncol. 2008;26:5705–5712. doi: 10.1200/JCO.2008.18.0786. [DOI] [PubMed] [Google Scholar]
- 23.Lievre A, Bachet JB, Le Corre D, Boige V, Landi B, Emile JF, Cote JF, Tomasic G, Penna C, Ducreux M, Rougier P, Penault-Llorca F, Laurent-Puig P. KRAS mutation status is predictive of response to cetuximab therapy in colorectal cancer. Cancer Res. 2006;66:3992–3995. doi: 10.1158/0008-5472.CAN-06-0191. [DOI] [PubMed] [Google Scholar]
- 24.Moroni M, Veronese S, Benvenuti S, Marrapese G, Sartore-Bianchi A, Di Nicolantonio F, Gambacorta M, Siena S, Bardelli A. Gene copy number for epidermal growth factor receptor (EGFR) and clinical response to antiEGFR treatment in colorectal cancer: a cohort study. Lancet Oncol. 2005;6:279–286. doi: 10.1016/S1470-2045(05)70102-9. [DOI] [PubMed] [Google Scholar]
- 25.Perrone F, Lampis A, Orsenigo M, Di Bartolomeo M, Gevorgyan A, Losa M, Frattini M, Riva C, Andreola S, Bajetta E, Bertario L, Leo E, Pierotti MA, Pilotti S. PI3KCA/PTEN deregulation contributes to impaired responses to cetuximab in metastatic colorectal cancer patients. Ann Oncol. 2009;20:84–90. doi: 10.1093/annonc/mdn541. [DOI] [PubMed] [Google Scholar]
- 26.Sartore-Bianchi A, Martini M, Molinari F, Veronese S, Nichelatti M, Artale S, Di Nicolantonio F, Saletti P, De Dosso S, Mazzucchelli L, Frattini M, Siena S, Bardelli A. PIK3CA mutations in colorectal cancer are associated with clinical resistance to EGFR-targeted monoclonal antibodies. Cancer Res. 2009;69:1851–1857. doi: 10.1158/0008-5472.CAN-08-2466. [DOI] [PubMed] [Google Scholar]
- 27.Prenen H, De Schutter J, Jacobs B, De Roock W, Biesmans B, Claes B, Lambrechts D, Van Cutsem E, Tejpar S. PIK3CA mutations are not a major determinant of resistance to the epidermal growth factor receptor inhibitor cetuximab in metastatic colorectal cancer. Clin Cancer Res. 2009;15:3184–3188. doi: 10.1158/1078-0432.CCR-08-2961. [DOI] [PubMed] [Google Scholar]
- 28.Peeters M, Oliner KS, Parker A, Huang J, Siena S, Yves Humblet Y, Van Laethem JL, André T, Wiezorek J, Reese D, Patterson SD, Van Cutsem E. Use of massively parallel, next-generation sequencing to identify gene mutations beyond KRAS that predict response to panitumumab in a randomized, phase 3, monotherapy study of metastatic colorectal cancer (mCRC). Proceedings of the 101st Annual Meeting of the American Association for Cancer Research; Washington, DC. 2010 Apr 17–21; 2010. p. abstr LB-174. [Google Scholar]
- 29.Tol J, Nagtegaal ID, Punt CJ. BRAF mutation in metastatic colorectal cancer. N Engl J Med. 2009;361:98–99. doi: 10.1056/NEJMc0904160. [DOI] [PubMed] [Google Scholar]
- 30.Van Cutsem E, Lang I, Folprecht G, Nowacki M, Cascinu S, Shchepotin I, Maurel J, Cunningham D, Celik I, Kohne C. Cetuximab plus FOLFIRI in the treatment of metastatic colorectal cancer (mCRC): The influence of KRAS and BRAF biomarkers on outcome: Updated data from the CRYSTAL trial. 2010 ASCO Gastrointestinal Cancers Symposium; 2010. p. abstr 281. [Google Scholar]
- 31.Miller VA, O’Connor P, Soh C, Kabbinavar F ATLAS Investigators. A randomized, double-blind, placebo-controlled, phase IIIb trial (ATLAS) comparing bevacizumab (B) therapy with or without erlotinib (E) after completion of chemotherapy with B for first-line treatment of locally advanced, recurrent, or metastatic non-small cell lung cancer (NSCLC) J Clin Oncol. 2009;27(18s):abstr LBA8002. doi: 10.1200/JCO.2012.47.3983. [DOI] [PubMed] [Google Scholar]
- 32.De Boer R, Arrieta Ó, Gottfried M, Blackhall FH, Raats J, Yang CH, Langmuir P, Milenkova T, Read J, JV Vandetanib plus pemetrexed versus pemetrexed as second-line therapy in patients with advanced non-small cell lung cancer (NSCLC): A randomized, double-blind phase III trial (ZEAL) J Clin Oncol. 2009;27:15s. doi: 10.1200/JCO.2010.29.5717. [DOI] [PubMed] [Google Scholar]
- 33.Natale RB, Thongprasert S, Greco FA, Thomas M, Tsai CM, Sunpaweravong P, Ferry D, Langmuir P, Rowbottom JA, Goss GD. Vandetanib versus erlotinib in patients with advanced non-small cell lung cancer (NSCLC) after failure of at least one prior cytotoxic chemotherapy: A randomized, double-blind phase III trial (ZEST) J Clin Oncol. 2009;27:5s. [Google Scholar]