Abstract
Reactive species derived from oxygen and nitric oxide are produced during inflammation and promote oxidation and nitration of biomolecules, including unsaturated fatty acids. Among the products of these reactions are α,β-unsaturated carbonyl and nitro derivatives of fatty acids, electrophilic species whose reactivity with nucleophilic amino acids provides a means of posttranslational protein modification and signaling. These electrophilic fatty acids activate cytosolic and nuclear stress–response pathways (through Nrf2/Keap1 and PPARγ, for example). There is also growing evidence that mitochondria generate electrophilic species. This appreciation, when combined with the role of mitochondrial dysfunction in conditions where exogenously delivered electrophiles exhibit therapeutic benefit, suggests that mitochondrial electrophile targets are also important in the resolution and prevention of inflammatory injury. Cardioprotective signaling pathways in particular appear to converge on mitochondria, with nitro-fatty acids recently shown to protect against cardiac ischemia/reperfusion injury in a murine model. Although numerous mitochondrial proteins are subject to modification by electrophiles, defining the targets most relevant to cytoprotection during inflammatory stress remains a clinically relevant goal.
Keywords: electrophiles, mitochondria, redox signaling, ischemia-reperfusion
Introduction
Cardiovascular disease has been the leading cause of death in the United States every year since 1919, and myocardial ischemia in particular imposes a severe morbidity and mortality burden. It is estimated that 16.8 million Americans have coronary heart disease, and each year over one million experience a new or recurrent coronary attack.1 Timely restoration of arterial patency is required for tissue preservation following acute myocardial infarction, and thrombolysis and percutaneous angioplasty have emerged as the therapeutic gold standards. However, the benefit of these approaches is limited by reperfusion injury, in which additional tissue damage accompanies the return of oxygenated blood to the ischemic heart. No clinical strategies for abrogating reperfusion injury currently exist, though there is ample interest: agents currently under study include calcium channel blockers, free radical scavengers, and nitric oxide donors.2 The recent observation that nitrated fatty acids, endogenous electrophilic species produced during inflammation, are cardioprotective in murine ischemia-reperfusion suggests that these compounds and their cellular targets may open new clinical avenues toward the prevention of reperfusion injury.3
Reactive gases (O2, •NO), cell signaling, and inflammation
When first characterized as a signaling molecule, the gaseous free radical nitric oxide (•NO) exhibited classical high-affinity ligand-receptor binding behavior with its specific receptor, the heme iron of soluble guanylate cyclase (sGC). Observations over the ensuing 20 years have augmented this view, revealing an expansive biochemical and physiological role for •NO in mammalian biology, with an ever-growing cast of molecular, cellular, and tissue targets.
Chemically, •NO reacts with molecular oxygen (O2) and its derivatives (ROS) to generate a range of oxidation products (NOx) whose downstream reactivities greatly increase the breadth of what is termed “•NO signaling.” These reactive nitrogen species (RNS) differentially induce biomolecule oxidation, nitration (addition of NO2), and nitrosation (addition of NO), each of which has implications in cell signaling.4 One well-characterized RNS-forming reaction is that of •NO with superoxide (O2•−), which occurs at nearly diffusion-limited rates to produce peroxynitrite (ONOO−).5 ONOO−is itself strongly oxidizing, and when protonated undergoes homolytic scission to produce hydroxyl radical (•OH) and nitrogen dioxide (•NO2), the latter a nitrating agent and the former the most potent biological oxidant.5 Moreover, ONOO− reacts avidly with CO2 to produce nitrosoperoxycarbonate (ONOOCO2−), a reaction of particular importance in acidic milieus which tips the balance in successive reactions toward nitration versus oxidation, and leads to the formation of a carbonate radical (HCO3•).6 Through its own activity and that of its byproducts, ONOO− engages in lipid and protein oxidation and nitration, enabling signal transduction and changes in cellular function.7 Radical reactions of •NO with molecular oxygen lead to an additional set of RNS with distinct properties. The reaction of •NO with one of the unpaired electrons of O2 forms •NO2, which may react further with •NO to yield N2O3 or N2O4—nitrosating and nitrating compounds, respectively.8 Ultimately, this array of reactions provides the palette from which •NO draws its myriad cGMP-independent in vivo effects.
Though many RNS facilitate prooxidant reactions, •NO predominantly mediates protective effects during inflammation.9 The selective modification of biomolecules by RNS to engage signaling pathways provides an appealing explanation for the inflammation-resolving effects of •NO. In particular, RNS (and ROS) interact with unsaturated fatty acids to form electrophilic lipid derivatives, a class of molecules gaining recognition for their antiinflammatory properties.
Characteristics and redox-dependent generation of electrophiles
An electrophile is a compound which forms a bond with a nucleophile by accepting an electron pair. This reaction occurs at an electron-poor region of the molecule whose presence can typically be attributed to a nearby electron-withdrawing substituent, most commonly a carbonyl (C=O). Electrophiles may be produced endogenously, obtained from the diet, or arise during metabolism of xenobiotics. Here we concern ourselves with endogenous electrophiles generated as byproducts of lipid oxidation.
Olefins conjugated to electron-withdrawing groups constitute a major portion of endogenously produced electrophiles. Such compounds are products of cellular redox reactions, and though important in inflammation, are also produced in a tightly regulated fashion during normal metabolism. In the latter case production is predominantly enzymatic, while free radical-based nonenzymatic pathways become significant under oxidizing conditions. Notably, polyunsaturated fatty acids (PUFA) are highly susceptible to oxidation and readily undergo peroxidation by enzymatic or free radical chain reaction mechanisms, yielding numerous electrophilic species.10
Nonenzymatic peroxidation of unsaturated fatty acids is initiated by hydrogen abstraction from a methylene carbon along the lipid backbone, leaving an unpaired electron. Under aerobic conditions, this newly formed radical (L•) reacts rapidly with oxygen to form a lipid peroxyl radical (LOO•), which can be reduced to a hydroperoxide (LOOH), propagate the chain reaction by abstracting a proton from an adjacent PUFA or form an endoperoxide via cyclization.11 L• and LOO• may also react with other radical species such as •NO or •NO2.12 Hydroperoxides and endoperoxides are unstable and decompose to form a variety of carbonyl-containing compounds, some with electrophile functionality, whereas nitro-fatty acids (NO2-FA) formed by •NO2 addition acquire electrophilic character due to the strong electron-withdrawing activity of the nitro group.10,13 PUFA oxidation and peroxidation also occur enzymatically via cyclooxygenase (COX) and lipoxygenase (LOX) activities, and when followed by dehydrogenase metabolism can yield α,β-unsaturated carbonyl-containing electrophiles.10 Arachidonic acid (a ω-6 fatty acid) and longer-chain ω-3 fatty acids can be modified to produce electrophilic species by these enzymatic pathways. In brief, fatty acid-derived electrophiles can be considered in two groups: α,β-unsaturated carbonyls and NO2-FA.
α, β-unsaturated carbonyls
α,β-unsaturated carbonyls are a class of electrophiles whose ranks include the reactive aldehydes 4-hydroxynonenal (4-HNE), 4-oxononenal (4-ONE), and acrolein (2-propenal), as well as ω-3 and ω-6 fatty acid derivatives such as the cyclopentenone prostaglandins and oxo-eicosatetraenoic acids (oxo-ETEs).14 4-HNE and 4-ONE are nonenzymatically derived, largely from decomposition of peroxidized linoleic acid. By this model, autoxidation of lineoleate forms 13-hydroperoxy-9,11-octadecaenoic acid (13-HPODE), which oxidatively decomposes to 4-hydroperoxynonenal (HPNE). HPNE can be converted to either 4-HNE or 4-ONE by reduction or dehydrogenase activity, respectively.15 Acrolein is produced during lipid peroxidation by poorly elucidated mechanisms, and is also formed during oxidation of carbohydrates and amino acids.16
ω-6-derived electrophiles are produced enzymatically via COX and LOX as products of the ω-6 fatty acid arachidonate. These include the A and J series of cyclopentenone prostaglandins, such as 15-deoxy-prostaglandin J2 (15d-PGJ2), along with the LOX-derived 5-, 12-, and 15-oxo-ETEs.14 However, nonenzymatic pathways can yield similar or identical products to the above. Endoperoxides formed as intermediates during nonenzymatic peroxidation can undergo a series of internal rearrangements and reactions to form cyclopentenone structures containing α,β-unsaturated carbonyls.17 oxo-ETEs can also be generated through nonenzymatic lipid oxidation.18 ω-3 fatty acids are subject to many of the same oxidation pathways as ω-6 fatty acids, though their corresponding electrophilic species have distinct biological properties. It has been proposed that oxidation products of ω-3 fatty acids mediate their protective cardiovascular effects.19
Nitro-fatty acids
NO2-FA are electrophilic fatty acid derivatives formed by lipid oxidation reactions involving reactive O2 and •NO-derived species. NO2-FA consist of an electron-withdrawing vinyl NO2 group present in an unsaturated fatty acid. •NO is lipophilic and tends to concentrate (roughly 20-fold) in lipid microenvironments, including the interiors of membranes and lipoproteins. Molecular O2 compartmentalizes similarly and consumes •NO, creating a molecular “lens” effect that accelerates •NO oxidation several hundred-fold and yields an array of oxidizing, nitrosating, and nitrating species.20 Multiple RNS (•NO2, ONOO−, HNO2, and NO2+) facilitate oxidation and nitration of PUFA. The nitrating compound •NO2 may form NO2-FA by hydrogen abstraction and reaction with L• radicals, whereas NO2+ mediates nitration by ionic addition mechanisms. ONOO− serves as a membrane-diffusible PUFA-nitrating agent. HNO2, which is the protonated form of nitrite (NO2−), has a pKa of 3.4, rendering it a possible player in NO2-FA formation in acidic tissues or organelles.21 Nitrite may also participate in nitration reactions indirectly, via its conversion to •NO and •NO2 by the nitrite reductase activity displayed by many heme-containing proteins. Although all of these mechanisms may have a role in NO2-FA production in vivo, it remains unknown which predominate. It is clear, however, that NO2-FA production occurs in vivo in response to pathologic stress. A recent study demonstrated that mice that have undergone cardiac ischemia via a coronary artery ligation procedure generate increased levels of NO2-FA compared to sham controls.3
Thus, during oxidative stress and inflammation, ROS, RNS, and unsaturated fatty acids combine to generate various reactive electrophiles. Due to the signaling characteristics of electrophiles, the generation of these species has implications in the propagation and resolution of inflammatory disease.
Signaling actions of electrophiles
Like other reactive species later shown to be important in normal physiology, electrophiles were first recognized for their cytotoxicity. Indeed, electrophiles may have deleterious effects: alkylation of DNA by electrophilic compounds is a well-characterized phenomenon that promotes carcinogenesis, and large-scale or irreversible disruptions of protein function may lead to apoptotic or necrotic cell death. However, this view changed as various adaptive mechanisms for countering electrophilic stress were elucidated, and it is now appreciated that electrophiles at sub-toxic levels induce protective or adaptive responses to stress.22 Electrophilic lipids engage in cell signaling through Michael addition reactions with cellular nucleophiles. As these include cysteine thiols, the imidazole of histidine, and the ε-amino group of lysine, posttranslational modification is a major mode of electrophile signaling, leading to changes in protein trafficking, function and catalytic activity.23 Importantly, despite their similar chemical reactivities, different electrophiles may target distinct protein pools.24 Of particular importance are reactions with transcription factors, several of which are involved in coordinating cellular responses to electrophiles.
Electrophile-activated signaling pathways
A canonical example of genomic regulation common to most electrophiles is the Nrf2/Keap1 (Nuclear erythroid 2-related factor 2/Kelch ECH associating protein) pathway. Under basal conditions, the transcription factor Nrf2 is held in the cytoplasm and tagged for proteasomal degradation through its regulator, Keap1, which possesses several reactive and evolutionarily conserved cysteine residues critical in maintaining its interaction with Nrf2. Covalent adduction of these cysteines releases Nrf2, which translocates to the nucleus and activates the cis-acting electrophile response element (ERE), which regulates the expression of phase-II genes involved in electrophile detoxification and restoration of redox balance, including glutathione S-transferases (GSTs), heme oxygenase-1 (HO-1), thioredoxin, and components of the glutathione synthesis pathway.25 Another target of electrophiles, peroxisome proliferator-activated receptor gamma (PPARγ) is a nuclear receptor involved in regulation of lipid metabolism and cell differentiation, and the target of the thiazoladinedione (TZD) class of antihyperglycemic medications. In recent years various electrophiles have been promoted as endogenous PPARγ ligands, including the cyclopentenone prostaglandin 15d-PGJ2 and NO2-FA. Un-like TZDs, these electrophiles bind the receptor covalently, and ultimately lead to a gene expression profile distinct from that of their synthetic counterparts.26 Electrophiles, notably 15d-PGJ2, 4-HNE, and NO2-FA, also signal through the heat-shock factor (HSF) family of transcription factors, leading to upregulation of chaperones (heat-shock proteins) involved in protein folding, trafficking, and turnover.27 In addition to the activation of cytoprotective signaling by these mechanisms, electrophiles downregulate the proinflammatory gene expression induced by nuclear factor κB (NF-κB), a multisub-unit transcription factor whose nuclear translocation is inhibited by its association with inhibitor of κB (IκB) under basal conditions. The NF-κB p50 and p65 subunits and IκB possess electrophile-reactive residues of functional significance, which when modified suppress NF-κB transcription of target genes by inhibiting translocation or impairing DNA binding.14 Although these pathways illustrate the variety of cytoplasmic and nuclear signaling targets of electrophiles, mitochondrial transduction of electrophile signaling will be addressed in particular.
The mitochondrion as a source and target of electrophiles
Electrophile generation by mitochondria
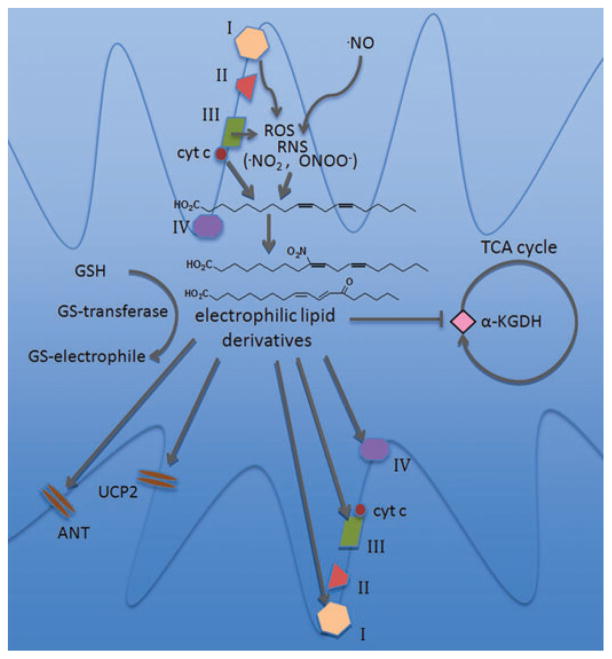
As noted above, the generation of electrophiles frequently involves the interaction of oxidizing species and PUFA. Mitochondria are a prominent source of reactive oxygen and nitrogen species and possess an abundance of unsaturated fatty acids on the vast surface area of their inner membranes, thus representing a fertile environment for lipid peroxidation and electrophile production.28 In addition, cytochrome c, along with its involvement in electron transport, functions as a peroxidase when structurally destabilized by oxidation or upon interactions with anionic phospholipids. The result of this activity is primarily formation of hydroxy and hydroperoxy derivatives of cardiolipin, a phospholipid found exclusively in mitochondrial membranes. These oxidized species are critical factors in the induction of apoptosis through cytochrome c release.29 Oxidized fatty acids are preferentially hydrolyzed by mitochondrial phospholipase A2 (PLA2) activity, and may be modulated by ROS production—one mitochondrial PLA2 isoform is activated by superoxide.30 Combined, these activities may provide a mechanism for the generation of free electrophilic fatty acid derivatives by mitochondria (Fig. 1).
Figure 1.
Mitochondrial electrophile generation and signaling. Reactive oxygen species (ROS) generated by the electron transport chain interact with nitric oxide to form reactive nitrogen species (RNS). ROS, RNS, and the peroxidase activity of cytochrome c lead to the formation of electrophilic lipid species from polyunsaturated fatty acids. Proximal targets of mitochondrial electrophiles include electron transport chain components (complexes I, III, and IV), membrane transport proteins and ion channels (the adenine nucleotide transporter [ANT] and uncoupling protein 2 [UCP2]), and matrix metabolic enzymes such as α-ketoglutarate dehydrogenase (α-KGDH). Alternatively, mitochondrial glutathione S-transferases (GSTs) may inactivate electrophiles by catalyzing their conjugation to glutathione (GSH).
Mitochondria are also involved in the production of NO2-FA. For example, mitochondrial NO2-FA levels increase following ischemic preconditioning of ex vivo Langendorff-perfused mouse hearts, to an estimated concentration of several hundred nanomolar within the organelle.31 Additional evidence of mitochondrial NO2-FA production comes from recent studies using isolated rat liver mitochondria, which produce [15N] nitro-oleic and nitro-linoleic acid when supplied with only [15N] NO2−, as demonstrated by LC/MS analysis. NO2-FA production increases only slightly in the presence of substrate, but is abolished by addition of sodium cyanide. However, inhibition of complex I with rotenone has no effect on NO2-FA levels, suggesting that function of only downstream respiratory chain components is required.32 The mechanism of NO2-FA synthesis under these conditions remains undefined, and work to determine factors that modulate NO2-FA production from NO2− is ongoing.
Mitochondrial targets of electrophiles
In light of these observations, mitochondria likely experience high local concentrations of electrophilic fatty acid derivatives, and electrophile effects within mitochondria should be relevant in normal and oxidatively perturbed physiology. Importantly, mitochondria are enriched in thiol-containing proteins that are subject to Michael addition reactions, and the relatively high matrix pH increases the amount of thiolate anion available for these reactions. One broad proteomic analysis identified 809 independent protein targets of electrophiles in mitochondria, with diverse metabolic and signaling roles.24
Effects on oxidative phosphorylation are not consistent among electrophiles, or even with identical chemical species in different experimental contexts. For example, 4-HNE inhibits respiration at cytochrome c oxidase in mitochondria isolated from rat hearts, but inhibits complex III more potently in rat brain mitochondria and may also inhibit NADH-linked respiration via α-ketoglutarate dehydrogenase.33,34 However, intact cardiac myocytes increase their respiration rates upon exposure to low micromolar concentrations of 4-HNE.35 Similarly, 15d-PGJ2, which inhibits complex I in isolated mitochondria, leads to increased complex I activity in endothelial cells, possibly by upregulating ROS production.36 A similar pattern may emerge with NO2-FA: in a study on cardiac myocytes, exposure to nitro-linoleic acid precipitated mild uncoupling via activation of uncoupling protein 2 (UCP2) and the adenine nucleotide transporter (ANT), seen as an increase in oligomycin-clamped state four respiration, whereas preliminary data from isolated mitochondria indicate that NO2-FA inhibit respiration.31,32 These studies highlight the importance of assessing electrophile effects in cell and organ systems along with subcellular fractions. Mitochondrial ROS production is also affected by many electrophiles. In addition to its respiratory effects via complex I, 15d-PGJ2 induces ROS formation in intact cells, even at nontoxic concentrations. The mitochondrial origin of ROS was confirmed by dichlorofluorescein (DCF) imaging studies in endothelial and Rho0 cells and reversal of ROS production with rotenone.37 4-HNE, despite having distinct effects on the respiratory chain from 15d-PGJ2, also initiates ROS generation by mitochondria in various cell lines.38,39 Importantly, pathological levels of electrophiles may also deplete mitochondrial GSH stores through the activity of GSTs, impairing the organelle’s antioxidant capacity (Fig. 1).40
Current data suggest that the influence of electrophilic species on mitochondria can limit the initiation and progression of diseases whose pathogenesis involves mitochondrial dysfunction.
Mitochondria as mediators and mitigators of tissue responses
In addition to their critical role in ATP synthesis, mitochondria are central coordinators of stress responses, integrating diverse cellular signals regarding energy balance, apoptosis, oxygen tension, and redox balance while driving downstream signaling events.41 The dysregulation of mitochondrial function observed in human diseases such as diabetes and cardiac ischemia/reperfusion exemplifies the importance of this regulatory ability.
Metabolic stress—diabetes
Mitochondrial dysfunction is involved in the pathogenesis of diabetes. Type 2 diabetics have impaired aerobic capacity at the whole-organism level, and examination of specific insulin-resistant tissues has revealed impaired oxidative phosphorylation with decreased expression of respiratory chain proteins and numerous other aberrant metabolic behaviors.42 Adipose tissue and skeletal muscle display dramatic changes in mitochondrial morphology in diabetics, including smaller size and lower numbers.43 The molecular explanations for these changes are still under investigation, but include downregulation of PPAR-γ coactivator 1α (PGC-1α), which is a key activator of respiratory complex expression and mitochondrial biogenesis.42 In addition, muscle tissues in T2DM exhibit “metabolic inflexibility,” or an inability to switch between glucose and lipid oxidation, resulting in preferential uptake and oxidation of lipids and decreased glucose metabolism. In cardiac muscle this effect is partially mediated by activation of PPAR-α, likely through interactions with long-chain fatty acid ligands.44 Enhanced fatty acid oxidation leads to redox imbalance via an increased NADH:NAD+ ratio, which further inhibits glucose oxidation by impeding pyruvate dehydrogenase activity and promotes mitochondrial ROS formation. Mitochondrial ROS are implicated in many disease-related complications, including insulin resistance, microvascular dysfunction, and diabetic cardiomyopathy. In the latter case, one model proposes that ROS and lipid peroxidation products lead to uncoupling protein (UCP) activation, lowering the inner mitochondrial membrane (IMM) potential, increasing O2 consumption, and decreasing contractile efficiency.45
Cardiac ischemia/reperfusion
Aberrant mitochondrial function is central to the pathology of cardiac ischemia/reperfusion injury. Severe ischemia depletes myocardial ATP, leading to ion pump failure and ionic imbalance, including an excess of cytosolic Ca2+ following loss of SERCA activity.46 The permeability transition pore (PTP) remains closed due to the low pH and low intramitochondrial Ca2+ concentrations during ischemia. These conditions are reversed upon reperfusion: pH increases and the IMM potential is restored, leading to Ca2+ influx to mitochondria, favoring PTP opening and ultimately apoptotic cell death.47 An additional injurious factor during reperfusion is ROS generation, which occurs at high levels. ROS and their reactive byproducts contribute to apoptosis and necrosis, as evidenced by effective antioxidant therapeutic interventions in animal models.48
Though energetic failure is a precipitating event in I/R injury, pharmacologic, or physiologic disruption of mitochondrial metabolism is associated with cardioprotection. Electron transport chain inhibitors acting at each of the respiratory complexes have been shown to improve outcomes in I/R, and ischemic preconditioning leads to ETC inhibition at multiple levels. The mechanisms underlying this seemingly paradoxical effect are not well understood, but it is proposed that respiratory chain inhibition at the time of reperfusion weakens the accompanying ROS burst and Ca2+ influx to mitochondria.49 Another metabolic pathway relevant to cardioprotection is fatty acid oxidation (FAO). Glucose oxidation is more efficient than fatty acid oxidation per mole O2 consumed, a fact which becomes especially salient in situations of low O2 supply, and which has been pharmacologically exploited: the 3-ketoacyl-coenzyme A thiolase inhibitor class of heart failure medications, which includes trimetazidine and ranolazine, inhibit FAO and shift myocardial substrate usage from fatty acids to glucose.50
Conclusion
Mitochondria are a critical nexus of cell signaling processes in inflammation. The increased redox activity of mitochondria during metabolic stress and cardiac ischemia/reperfusion favors formation of electrophilic lipid derivatives through PUFA interactions with ROS and RNS. These species adduct proteins at nucleophilic cysteine, histidine, and lysine residues, and activate adaptive signaling pathways by regulating transcription factor activity. In addition, mitochondrial targets of electrophiles are numerous, and electrophiles can be cytoprotective in settings of mitochondrial dysfunction (NO2-FA in models of type 2 diabetes and cardiac I/R, for instance). The electrophile targets in mitochondria which promote protection are incompletely characterized, but may include respiratory chain components and UCPs (modulating ATP and ROS production and O2 consumption), matrix metabolic enzymes (altering the contribution of fatty acid oxidation to cellular bioenergetics), and apoptotic machinery. Improving our understanding of electrophile signaling in mitochondria will aid in the development of effective interventions for metabolic and inflammatory disease.
Footnotes
Conflict of interest
B.A.F. acknowledges financial interest in Complexa, Inc.
References
- 1.Lloyd-Jones D, et al. Heart disease and stroke statistics–2009 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2009;119:480–486. doi: 10.1161/CIRCULATIONAHA.108.191259. [DOI] [PubMed] [Google Scholar]
- 2.Wang QD, et al. Pharmacological possibilities for protection against myocardial reperfusion injury. Cardiovasc Res. 2002;55:25–37. doi: 10.1016/s0008-6363(02)00261-4. [DOI] [PubMed] [Google Scholar]
- 3.Rudolph V, et al. Endogenous generation and protective effects of nitro-fatty acids in a murine model of focal cardiac ischaemia and reperfusion. Cardiovasc Res. 2009;85:155–166. doi: 10.1093/cvr/cvp275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Forman HJ, et al. The chemistry of cell signaling by reactive oxygen and nitrogen species and 4-hydroxynonenal. Arch Biochem Biophys. 2008;477:183–195. doi: 10.1016/j.abb.2008.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beckman JS, et al. Apparent hydroxyl radical production by peroxynitrite: implications for endothelial injury from nitric oxide and superoxide. Proc Natl Acad Sci USA. 1990;87:1620–1624. doi: 10.1073/pnas.87.4.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Denicola A, et al. Peroxynitrite reaction with carbon dioxide/bicarbonate: kinetics and influence on peroxynitrite-mediated oxidations. Arch Biochem Biophys. 1996;333:49–58. doi: 10.1006/abbi.1996.0363. [DOI] [PubMed] [Google Scholar]
- 7.Greenacre SA, Ischiropoulos H. Tyrosine nitration: localisation, quantification, consequences for protein function and signal transduction. Free Radic Res. 2001;34:541–581. doi: 10.1080/10715760100300471. [DOI] [PubMed] [Google Scholar]
- 8.Wink DA, et al. Reactions of the bioregulatory agent nitric oxide in oxygenated aqueous media: determination of the kinetics for oxidation and nitrosation by intermediates generated in the NO/O2 reaction. Chem Res Toxicol. 1993;6:23–27. doi: 10.1021/tx00031a003. [DOI] [PubMed] [Google Scholar]
- 9.Gutierrez HH, et al. Nitric oxide regulation of superoxide-dependent lung injury: oxidant-protective actions of endogenously produced and exogenously administered nitric oxide. Free Radic Biol Med. 1996;21:43–52. doi: 10.1016/0891-5849(95)02226-0. [DOI] [PubMed] [Google Scholar]
- 10.Ceaser EK, et al. Mechanisms of signal transduction mediated by oxidized lipids: the role of the electrophile-responsive proteome. Biochem Soc Trans. 2004;32:151–155. doi: 10.1042/bst0320151. [DOI] [PubMed] [Google Scholar]
- 11.Halliwell B, Chirico S. Lipid peroxidation: its mechanism, measurement, and significance. Am J Clin Nutr. 1993;57:715S–724S. doi: 10.1093/ajcn/57.5.715S. discussion 724S–725S. [DOI] [PubMed] [Google Scholar]
- 12.O’Donnell VB, Freeman BA. Interactions between nitric oxide and lipid oxidation pathways: implications for vascular disease. Circ Res. 2001;88:12–21. doi: 10.1161/01.res.88.1.12. [DOI] [PubMed] [Google Scholar]
- 13.Freeman BA, et al. Nitro-fatty acid formation and signaling. J Biol Chem. 2008;283:15515–15519. doi: 10.1074/jbc.R800004200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rudolph TK, Freeman BA. Transduction of redox signaling by electrophile-protein reactions. Sci Signal. 2009;2:re7. doi: 10.1126/scisignal.290re7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schneider C, et al. Two distinct pathways of formation of 4-hydroxynonenal. Mechanisms of nonenzymatic transformation of the 9- and 13-hydroperoxides of linoleic acid to 4-hydroxyalkenals. J Biol Chem. 2001;276:20831–20838. doi: 10.1074/jbc.M101821200. [DOI] [PubMed] [Google Scholar]
- 16.Stevens JF, Maier CS. Acrolein: sources, metabolism, and biomolecular interactions relevant to human health and disease. Mol Nutr Food Res. 2008;52:7–25. doi: 10.1002/mnfr.200700412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fam SS, et al. Formation of highly reactive A-ring and J-ring isoprostane-like compounds (A4/J4-neuroprostanes) in vivo from docosahexaenoic acid. J Biol Chem. 2002;277:36076–36084. doi: 10.1074/jbc.M205638200. [DOI] [PubMed] [Google Scholar]
- 18.Hall LM, Murphy RC. Electrospray mass spectrometric analysis of 5-hydroperoxy and 5-hydroxyeicosatetraenoic acids generated by lipid peroxidation of red blood cell ghost phospholipids. J Am Soc Mass Spectrom. 1998;9:527–532. doi: 10.1016/S1044-0305(98)00013-0. [DOI] [PubMed] [Google Scholar]
- 19.Milne GL, Morrow JD. Isoprostanes and related compounds: update 2006. Antioxid Redox Signal. 2006;8:1379–1384. doi: 10.1089/ars.2006.8.1379. [DOI] [PubMed] [Google Scholar]
- 20.Liu X, et al. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc Natl Acad Sci USA. 1998;95:2175–2179. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.O’Donnell VB, et al. Nitration of unsaturated fatty acids by nitric oxide-derived reactive nitrogen species peroxynitrite, nitrous acid, nitrogen dioxide, and nitronium ion. Chem Res Toxicol. 1999;12:83–92. doi: 10.1021/tx980207u. [DOI] [PubMed] [Google Scholar]
- 22.Parola M, et al. 4-Hydroxynonenal as a biological signal: molecular basis and pathophysiological implications. Antioxid Redox Signal. 1999;1:255–284. doi: 10.1089/ars.1999.1.3-255. [DOI] [PubMed] [Google Scholar]
- 23.Isom AL, et al. Modification of Cytochrome c by 4-hydroxy- 2-nonenal: evidence for histidine, lysine, and arginine-aldehyde adducts. J Am Soc Mass Spectrom. 2004;15:1136–1147. doi: 10.1016/j.jasms.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 24.Wong HL, Liebler DC. Mitochondrial protein targets of thiol-reactive electrophiles. Chem Res Toxicol. 2008;21:796–804. doi: 10.1021/tx700433m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Osburn WO, Kensler TW. Nrf2 signaling: an adaptive response pathway for protection against environmental toxic insults. Mutat Res. 2008;659:31–39. doi: 10.1016/j.mrrev.2007.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Itoh T, et al. Structural basis for the activation of PPARgamma by oxidized fatty acids. Nat Struct Mol Biol. 2008;15:924–931. doi: 10.1038/nsmb.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jacobs AT, Marnett LJ. Heat shock factor 1 attenuates 4-Hydroxynonenal-mediated apoptosis: critical role for heat shock protein 70 induction and stabilization of Bcl-XL. J Biol Chem. 2007;282:33412–33420. doi: 10.1074/jbc.M706799200. [DOI] [PubMed] [Google Scholar]
- 28.Gutierrez J, et al. Free radicals, mitochondria, and oxidized lipids: the emerging role in signal transduction in vascular cells. Circ Res. 2006;99:924–932. doi: 10.1161/01.RES.0000248212.86638.e9. [DOI] [PubMed] [Google Scholar]
- 29.Kagan VE, et al. Cytochrome c/cardiolipin relations in mitochondria: a kiss of death. Free Radic Biol Med. 2009;46:1439–1453. doi: 10.1016/j.freeradbiomed.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Madesh M, Balasubramanian KA. Activation of liver mitochondrial phospholipase A2 by superoxide. Arch Biochem Biophys. 1997;346:187–192. doi: 10.1006/abbi.1997.0288. [DOI] [PubMed] [Google Scholar]
- 31.Nadtochiy SM, et al. Mitochondrial nitroalkene formation and mild uncoupling in ischaemic preconditioning: implications for cardioprotection. Cardiovasc Res. 2009;82:333–340. doi: 10.1093/cvr/cvn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bonacci G, Schopfer FJ, Freeman BA. Unpublished Observations 2009 [Google Scholar]
- 33.Kaplan P, et al. Oxidative modifications of cardiac mitochondria and inhibition of cytochrome c oxidase activity by 4-hydroxynonenal. Redox Rep. 2007;12:211–218. doi: 10.1179/135100007X200308. [DOI] [PubMed] [Google Scholar]
- 34.Humphries KM, Yoo Y, Szweda LI. Inhibition of NADH-linked mitochondrial respiration by 4-hydroxy-2-nonenal. Biochemistry. 1998;37:552–557. doi: 10.1021/bi971958i. [DOI] [PubMed] [Google Scholar]
- 35.Hill BG, et al. Importance of the bioenergetic reserve capacity in response to cardiomyocyte stress induced by 4-hydroxynonenal. Biochem J. 2009;424:99–107. doi: 10.1042/BJ20090934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ceaser EK, et al. Oxidized low-density lipoprotein and 15-deoxy-delta 12,14-PGJ2 increase mitochondrial complex I activity in endothelial cells. Am J Physiol Heart Circ Physiol. 2003;285:H2298–H2308. doi: 10.1152/ajpheart.00508.2003. [DOI] [PubMed] [Google Scholar]
- 37.Landar A, et al. Interaction of electrophilic lipid oxidation products with mitochondria in endothelial cells and formation of reactive oxygen species. Am J Physiol Heart Circ Physiol. 2006;290:H1777–H1787. doi: 10.1152/ajpheart.01087.2005. [DOI] [PubMed] [Google Scholar]
- 38.Nakamura K, et al. 4-Hydroxy-2-nonenal induces calcium overload via the generation of reactive oxygen species in isolated rat cardiac myocytes. J Card Fail. 2009;15:709–716. doi: 10.1016/j.cardfail.2009.04.008. [DOI] [PubMed] [Google Scholar]
- 39.Lee JY, et al. 4-Hydroxynonenal induces vascular smooth muscle cell apoptosis through mitochondrial generation of reactive oxygen species. Toxicol Lett. 2006;166:212–221. doi: 10.1016/j.toxlet.2006.07.305. [DOI] [PubMed] [Google Scholar]
- 40.Uchida K. 4-Hydroxy-2-nonenal: a product and mediator of oxidative stress. Prog Lipid Res. 2003;42:318–343. doi: 10.1016/s0163-7827(03)00014-6. [DOI] [PubMed] [Google Scholar]
- 41.Manoli I, et al. Mitochondria as key components of the stress response. Trends Endocrinol Metab. 2007;18:190–198. doi: 10.1016/j.tem.2007.04.004. [DOI] [PubMed] [Google Scholar]
- 42.Bugger H, Abel ED. Molecular mechanisms for myocardial mitochondrial dysfunction in the metabolic syndrome. Clin Sci (Lond) 2008;114:195–210. doi: 10.1042/CS20070166. [DOI] [PubMed] [Google Scholar]
- 43.Kelley DE, et al. Dysfunction of mitochondria in human skeletal muscle in type 2 diabetes. Diabetes. 2002;51:2944–2950. doi: 10.2337/diabetes.51.10.2944. [DOI] [PubMed] [Google Scholar]
- 44.Finck BN, et al. The cardiac phenotype induced by PPARalpha overexpression mimics that caused by diabetes mellitus. J Clin Invest. 2002;109:121–130. doi: 10.1172/JCI14080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Boudina S, Abel ED. Mitochondrial uncoupling: a key contributor to reduced cardiac efficiency in diabetes. Physiology (Bethesda) 2006;21:250–258. doi: 10.1152/physiol.00008.2006. [DOI] [PubMed] [Google Scholar]
- 46.Karmazyn M. The myocardial sodium-hydrogen exchanger (NHE) and its role in mediating ischemic and reperfusion injury. Keio J Med. 1998;47:65–72. doi: 10.2302/kjm.47.65. [DOI] [PubMed] [Google Scholar]
- 47.Griffiths EJ, Halestrap AP. Mitochondrial non-specific pores remain closed during cardiac ischaemia, but open upon reperfusion. Biochem J. 1995;307(Pt 1):93–98. doi: 10.1042/bj3070093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zweier JL, Talukder MA. The role of oxidants and free radicals in reperfusion injury. Cardiovasc Res. 2006;70:181–190. doi: 10.1016/j.cardiores.2006.02.025. [DOI] [PubMed] [Google Scholar]
- 49.Burwell LS, Nadtochiy SM, Brookes PS. Cardioprotection by metabolic shut-down and gradual wake-up. J Mol Cell Cardiol. 2009;46:804–810. doi: 10.1016/j.yjmcc.2009.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Fragasso G, et al. Modulation of fatty acids oxidation in heart failure by selective pharmacological inhibition of 3-ketoacyl coenzyme-A thiolase. Curr Clin Pharmacol. 2007;2:190–196. doi: 10.2174/157488407781668776. [DOI] [PubMed] [Google Scholar]