Abstract
A common treatment for anxiety disorders is chronic administration of selective serotonin reuptake inhibitors (SSRIs) such as fluoxetine. Recent data suggest that SSRIs modulate fear responses after conditioned fear extinction and that gonadal hormones influence fear extinction. In this study we investigated the influence of sex and the estrous cycle on the effects of acute (experiment 1) and chronic (experiment 2) fluoxetine treatment on fear extinction. In experiment 1, rats received tone-footshock pairings during day 1. On day 2, rats received either fluoxetine (10mg/kg in 0.5mL) or vehicle prior to extinction learning. On day 3, extinction memory was assessed during extinction recall. In experiment 2, rats were exposed to a similar behavioral protocol, except that fluoxetine and vehicle were administered for 14 consecutives days after conditioning (days 2–15). Extinction learning and extinction recall occurred on days 15 and 16, respectively. Acute administration of fluoxetine increased fear responses equally in males and females during extinction learning and extinction recall. Chronic administration of fluoxetine reduced fear responses during extinction learning and extinction recall in female but not in male rats and this effect seems to be modulated by the estrous cycle. The SSRI-induced reduction of freezing during extinction learning and recall suggest a general anxiolytic effect of the drug treatment rather than a specific effect on extinction learning per se. Our data show evidence of sex-specific anxiolytic effects of 14-day treatment of fluoxetine while the acute anxiogenic effect of SSRI seems independent of sex effects.
Keywords: fear, estrous cycle, estrogen, fluoxetine, gonadal hormones, anxiety disorders
Introduction
A common treatment for anxiety disorders such as posttraumatic stress disorder (PTSD) and other anxiety and mood disorders such as depression is the administration of selective serotonin inhibitors (SSRIs). While widely used, the mechanisms by which SSRIs facilitate treatment and remission remain unclear. In the clinic, acute SSRI treatment is known to exacerbate symptoms of anxiety [1] while chronic treatment is known to ameliorate the same symptoms [2]. Failure to extinguish conditioned fears has been reported to possibly contribute to the etiology and/or maintenance of elevated fear across psychiatric disorders such PTSD [3–5], OCD [6], and schizophrenia [7]. It has therefore been suggested that chronic treatment of patients with anxiety disorders with SSRIs may facilitate fear extinction [8, 9]. Interest and focus on testing this hypothesis is growing as evident by the emergence of recent publications examining how SSRIs may facilitate fear inhibition and extinction. These studies show that chronic administration of the SSRI fluoxetine decreases fear responses during extinction learning [10] and after extinction learning [8, 9, 11]. In contrast, a more recent study has shown that a 3-week treatment of SSRI citalopram impaired fear extinction and a 9-day treatment had no effect on extinction [12].
In fear conditioning, chronic fluoxetine treatment before extinction learning prevented the expression of fear responses after exposure to a sub-conditioning stressor [8, 9, 13] and prevented the electrophysiological changes in the hippocampus that are normally induced by fear conditioning [13]. In another study, chronic fluoxetine treatment decreased fear responses during retention testing after extinction of contextual fear conditioning in the 129SI/Svlmj (SI) inbred mouse strain [11], which are known to be deficient in extinction learning. Furthermore, a recent study showed that chronic fluoxetine treatment combined with extinction treatment attenuated spontaneous recovery and renewal suggesting erasure of conditioned memory [10]. In healthy humans, it was recently shown that a two-week treatment of Escitalopram had no effects of fear acquisition but facilitated extinction learning [14].
Although there is sufficient evidence indicating that fluoxetine decreases fear responses after extinction all of these studies have been conducted in males only, ignoring the influence of sex and gonadal hormones on SSRIs and fear extinction. Sex hormones such as estrogen have been shown to interact with SSRIs [15–17]. It is therefore possible that SSRIs may differentially affect the expression of conditioned fear in males and females, but this possibility has not been previously examined. Regarding fear extinction, we recently found that women and female rats receiving extinction training during their high gonadal hormones stages of their menstrual cycle (or estrous cycle in the rat) showed good recall of extinction memory [18], suggesting that gonadal hormones may enhance consolidation of extinction memory. Furthermore, our neuroimaging studies showed that in women high levels of estrogen are associated with increased vmPFC, hippocampal and amygdala activation during extinction recall suggesting that gonadal hormones modulate the function of brain regions involved in fear extinction [19–21]. Therefore, in this study we investigate the influence of sex and the estrous cycle phase on the effects of acute and chronic administration of fluoxetine on fear extinction. Rats were exposed to a fear conditioning and extinction protocol. Acute and chronic treatment with fluoxetine was administered prior to extinction learning. Given that acute and chronic administration has been found to be anxiogenic and anxiolytic, respectively [8, 22], we expected extinction learning to be enhanced with chronic but not acute administration of fluoxetine. These effects were expected particularly in female rats being exposed to extinction learning during the high gonadal hormone stages (proestrus/estrus) of the estrous cycle.
1. Methods
We used a total of 62 male and 98 female Sprague Dawley rats (approximately 300g and 250g for the males and females, respectively). Rats were housed individually at the Massachusetts General Hospital Center for Comparative Medicine and handled for 5 minutes/day for 2 days. Rats were maintained on a 12h light/dark cycle and were provided with free access to laboratory rat chow and water. They were transported to a holding room in our laboratory and returned to the animal facility at the end of each day. The procedures were approved by the Subcommittee on Research Animal Care (SRAC) of the Massachusetts General Hospital, in compliance with National Institutes of Health guidelines.
All apparatus and procedures were identical to those previously described (Milad 2009, 2010). Briefly, the conditioned stimulus (CS) was a 4kHz tone with an intensity of 80 dB and duration of 30s, during which an LED indicator light would turn on. The unconditioned stimulus (US) was a 0.6mA, 0.5s footshock that co-terminated with the tone presentation during conditioning. All the phases of the experiments were conducted in the same context.
We conducted 2 experiments using the same behavioral protocols. During experiment 1 we investigated the effect of acute administration of fluoxetine on fear extinction using male and female rats. On Day 1, 24 male and 51 female rats were placed in the conditioning chambers where they received 5 non-reinforced presentations of the tone alone (Habituation) followed by 7 tone-footshock pairings (Conditioning) at an average inter-trial interval (ITI) of 3 minutes. Rats were then returned to their home cages following the conditioning phase. On Day 2, fluoxetine (10mg/kg, Sigma-Aldrich, St. Louis, MO, dissolved in saline) or vehicle was administered (i.p.) to animals approximately 30 minutes before the commencement of the extinction learning phase. The dose of the SSRI used herein was determined base on previous studies [23, 24]. During extinction learning, 20 non-reinforced tone trials with an ITI of 1.5 minutes were presented. On Day 3, the animals received 15 presentations of the tone alone (extinction recall).
In experiment 2 we investigated the effects of chronic administration of fluoxetine on fear extinction on males and females. The experimental procedures were identical to those used in experiment 1 except that rats were administered daily fluoxetine (10mg/mL) (n=12) during 14 days (i.e. days 2–15) starting the day after conditioning. Fluoxetine was delivered through drinking water. The rats in the control condition were administered with saline (n=12), also in drinking water. On Days 15 and 16, rats underwent extinction learning and extinction recall phases, respectively as in experiment 1.
Vaginal swabs and cycle phase assessment were conducted for at least 10 consecutive days prior to each of the two experiments and also during the days of the experiment as previously described [19, 25]. Female rats were classified according to the phase of the estrous cycle during extinction learning. The estrous cycle consists of 4 different phases: diestrus, proestrus, estrus and metestrus. The level of gonadal hormones varies through out the different phases of the cycle (for reference see [26]). Higher level of gonadal hormones occurs during proestrus and first half of estrus; while lower levels of gonadal hormones occurs during metestrus and first half of diestrus [26]. Our previous findings showed that extinction recall was best in rats undergoing extinction learning during high level of gonadal hormones and worst in rats during low levels of gonadal hormones [19]. Therefore, when assessing the interactions between fluoxetine treatment and cycle phase, female rats were divided into 2 groups: the proestrus/estrus group and the metestrus/diestrus group.
As noted, during experiment 1, rats were intraperitoneally injected with fluoxetine. In experiment 2, however, fluoxetine was administered orally via drinking water because chronic fluoxetine injections seemed to induce pain and abdominal tissue scaring after repeated daily injections (our observations after initial pilot study). We used the absorption fraction of fluoxetine (0.85) to alter the dose to 12mg/kg in order to account for the different route of administration and the inherent difference in the drug metabolism. After fear conditioning, rats were immediately returned to their home cages and allowed free access to water for 4 hours, after which they were water deprived for 12 hours. For the next 14 days, the rats were given a new preparation of medicated water daily, which they had free access during the 24 hrs on each day. The amount of water consumed was monitored and recorded daily. Thereafter, extinction learning and extinction recall tests occurred on days 15 and 16, respectively.
Since the route of drug administration was different for both experiments we conducted an additional control experiment to confirm that the data from both modes of administration are comparable. Rats received acute administration of fluoxetine in drinking water prior to extinction learning (12 females, 12 males). We used the same absorption fraction of fluoxetine (0.85) and dose as describe above. The objective was to test whether we would be able to obtain the acute anxiogenic effect with a single oral administration of fluoxetine. On Day 1, the animals were habituated and conditioned, after which they were allowed free access to regular drinking water. The animals were immediately returned to their home cages following the conditioning phase and allowed free access to water for 4 hours, after which they were water deprived for 12 hours before being provided with medicated drinking water for the next 24 hours. Following this period, the animals were returned to the chambers, where they underwent the extinction learning protocol. Then, animals were given free access to regular drinking water. The extinction recall procedure was the same as in the other experiments.
Upon completion of the experiments, post-hoc separation of all female rats was conducted into groups that (based on vaginal swabs) underwent extinction learning while in proestrus/estrus or metestrus/diestrus. As in our previous studies [19, 27], freezing behavior was used as the measure of conditioned fear. Freezing during the tone presentation was evaluated from digital videos using motion-sensing computer software (FreezeScan, Clever System, Reston, VA, USA), and time spent freezing was recorded as a percentage of the total 30s duration of the tone. Repeated-measures analysis of variance [28] was used to analyze the freezing data with the statistical analysis software (SPSS Inc., Chicago, IL, USA). Data are shown as means of blocks of 5 trials. During extinction recall phase, we show and analyze only the first 10 trials of this phase to focus our analyses on the extinction recall and initial re-extinction parts of this training session. Error bars represent standard error of the mean (SE).
2. Results
Data from experiment 1 showed that acute administration of fluoxetine induced higher freezing during extinction learning with no effect during extinction recall (Fig 1a), relative to vehicle and across both male and female rats. Repeated measures ANOVA revealed a significant main effect of group [F (1, 73)= 4.15, p= 0.045], significant main effect of trial [F (3, 219)= 15.71, p< 0.001] but no significant interaction [F (3, 219)= 1.35, p= 0.26] suggesting that acute fluoxetine administration induces an increase in fear expression during extinction learning (an acute anxiogenic effect) without facilitating the rate of extinction learning. In contrast to the acute treatment, chronic administration of fluoxetine prior to extinction learning resulted in decreased freezing during extinction learning and during extinction recall (when all male and female rats were combined) (Fig 1b). Repeated measures ANOVA during extinction learning showed a significant main effect of group [F (1, 68)= 4.19, p= 0.04], a significant effect of trial [F (3, 204)= 126.18, p< 0.001, but no significant interaction [F (2, 204)= 1.103, p= 0.35]. Repeated measures ANOVA for extinction recall showed a significant main effect of group [F (1, 68)= 3.90, p= 0.05], a significant main effect of trial [F (1, 68)= 35, p< 0.001], but no significant interaction [F (1, 68)= 97.78, p= 0.24].
Figure 1. Effects of acute and chronic fluoxetine administration during fear extinction.
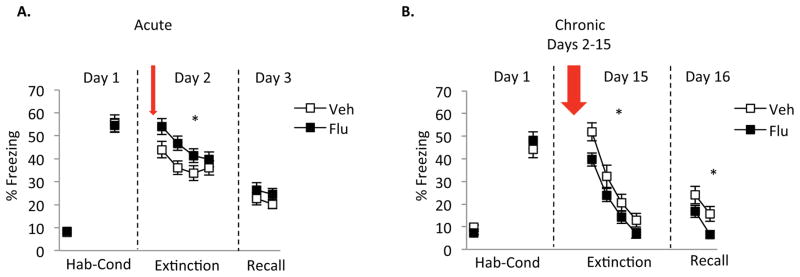
A) Acute administration of fluoxetine prior to extinction learning increased freezing during extinction learning but not during extinction recall. B) Chronic administration of fluoxetine prior to extinction learning decreased freezing during extinction learning and extinction recall. Acute vehicle rats (n=36), acute fluoxetine rats (n=39), chronic vehicle (n=32), chronic fluoxetine rats (n=38). In this and all subsequent figures, data are shown in blocks of 5 trials. For the extinction recall phase, only the first 10 of 15 trials are shown (2 blocks of trials).
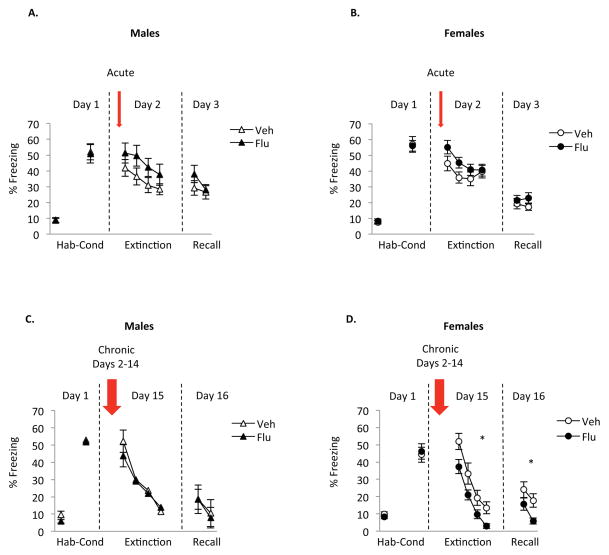
We next assessed potential sex differences in effects of both acute and chronic SSRI treatment. In both males (Fig 2a) and females (Fig 2b) analyzed separately, acute fluoxetine treatment resulted in modest elevation of freezing during extinction learning; this effect was not statistically significant. Repeated measures ANOVA showed no significant effect of group [males: F (1, 22)= 2.14, p=0.16; females: F (1,49)= 1.97, p=0.17], a significant effect of trial [males: F (3,66)= 12.53, p<0.001; females: F (3,147)= 8.95, p< 0.001] and no significant interaction [males: F (3, 66)= 0.30, p=0.83, females: F (3,147)= 1.44, p= 0.23. During extinction recall, there was no significant group difference in freezing in either male rats or female rats [males: F (1,22)= 0.88, p=0.36, females: F (1,49)= 1.06, p= 0.31].
Figure 2. Influence of sex on the effects of acute and chronic administration of fluoxetine during fear extinction.
A and C) Acute or chronic administration of fluoxetine did not affect freezing during extinction learning of extinction recall in male rats. B) Acute administration of fluoxetine did not affect freezing during extinction learning in female rats while, D) Chronic administration reduced freezing during extinction learning and extinction recall in female rats. Acute male vehicle rats (n=12), acute males fluoxetine rats (n=12), acute female vehicle rats (n=24), acute females fluoxetine rats (n=27), chronic male vehicle rats (n=9), chronic males fluoxetine rats (n=14), chronic female vehicle rats (n=23), chronic females fluoxetine rats (n=24).
In the chronic administration experiment, we observed a sex-specific effect (Fig 2c and 2d). Chronic administration of fluoxetine induced a significant decrease in freezing during extinction learning and extinction recall only in females (Fig 2d). Repeated measures ANOVA during extinction learning and extinction recall showed a main effect of group [extinction learning: F (1,45)= 6.2, p=0.02; recall: F (1,45)= 4.5, p=0.04], a significant effect of trial [extinction learning: F (1,45)= 94.82, p<0.001; recall: F (1,45)= 18.72, p<0.001] and no significant interaction [extinction learning: F (3,135)= 0.43, p= 0.73; recall: F (1,45)= 0.90, p= 0.35]. Thus, the anxiolytic effect of chronic fluoxetine treatment appears to be specific to females.
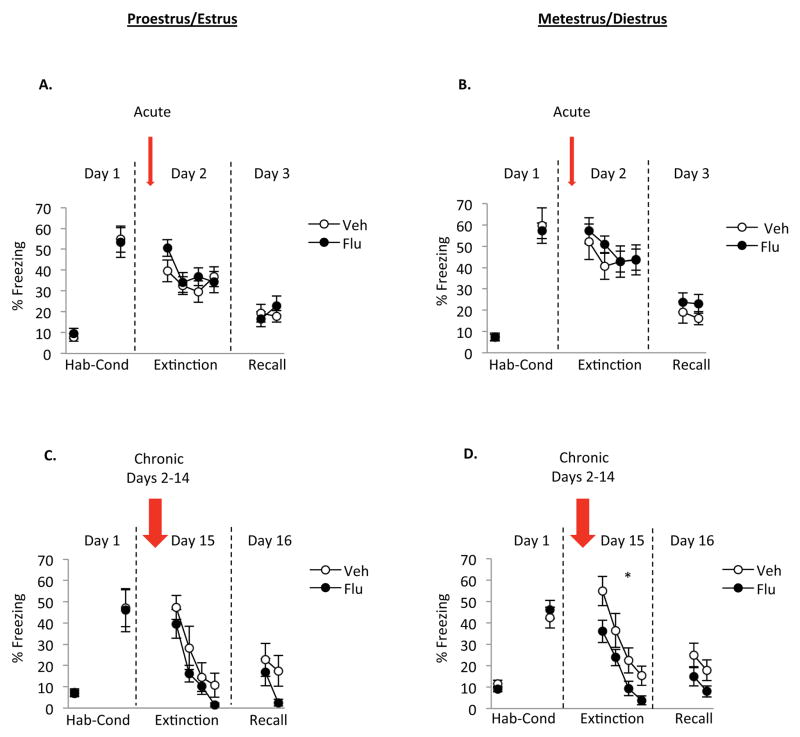
Previous findings from our lab showed that naturally cycling rats in the proestrus phase of the estrous cycle during extinction learning showed enhanced memory of extinction during extinction recall relative to rats undergoing extinction learning while in the metestrus phase [19, 20]. To examine the potential interaction between estrous cycle phase and the effects of fluoxetine administration on fear extinction, data from female rats in experiment 1 and 2 were further divided and analyzed based on cycle phase. Since gonadal hormones are higher during proestrus and first half of estrus and lower during metestrus and first half of diestrus, female rats were divided in 2 groups: proestrus/estrus group and metestrus/diestrus group. For the acute treatment, there was no significant difference during extinction learning and recall when comparing fluoxetine proestrus/estrus rats with vehicle proestrus/estrus rats (Fig 3a) and when comparing fluoxetine metestrus/diestrus with vehicle metestrus/diestrus rats (Fig 3b). For the chronic treatment, freezing was significantly decreased during extinction learning in metestrus/diestrus fluoxetine rats relative to metestrus/diestrus vehicle rats (Fig 3d). Repeated measures ANOVA showed a significant effect of group during extinction learning [F (1,27)= 4.96, p=0.03] but not during extinction recall [F (1,27)= 2.79, p=0.11], a significant effect of trial [extinction learning: F (3,81)= 54.38, p<0.001; recall: F (1,27)= 9.16, p=0.005] and no significant interaction [extinction learning: F (3,81)= 0.58, p=0.63; recall: F (1,27)< 0.001, p=0.988]. No significant differences in freezing were observed during extinction learning and recall in the proestrus/estrus fluoxetine rats relative to the proestrus/estrus vehicle rats (Fig 3c). All together, these data suggest that chronic administration of fluoxetine has sex-specific effects and that such effects appear to be specific to low gonadal hormone phases of the estrous cycle.
Figure 3. Influence of the estrous cycle on the effects of acute SSRI and chronic SSRI during fear extinction.
A and B) Acute administration of fluoxetine did not affect freezing during extinction learning or extinction recall in the proestrus/estrus rats or in the metestrus/diestrus rats, respectively. C) Chronic administration of fluoxetine did not affect freezing during extinction learning or extinction recall in the proestrus/estrus rats, but D) reduced freezing during extinction learning in the metestrus/diestrus rats. Acute proestrus/estrus vehicle rats (n=14), acute proestrus/estrus fluoxetine rats (n=9), acute metestrus/diestrus vehicle rats (n=10), acute metestrus/diestrus fluoxetine rats (n=18), chronic proestrus/estrus vehicle rats (n=9), chronic proestrus/estrus fluoxetine rats (n=9), chronic metestrus/diestrus vehicle rats (n=14), chronic metestrus/diestrus fluoxetine rats (n=15).
Given that the route of fluoxetine administration differed between the acute (i.p.) and chronic (oral), we conducted an additional control experiment in which we aimed to examine if the freezing levels in i.p. injected animals during extinction learning would be comparable to animals receiving a single oral dose of fluoxetine. Our results show that indeed this is the case. There were no significant differences between the two groups [F (1,49)= 0.28, p=0.60].
3. Discussion
We investigated sex differences and the influence of the estrous cycle on the effects of acute and chronic administrations of fluoxetine, an SSRI, during extinction of conditioned fear. Our findings suggest that acute administration of fluoxetine enhances the expression of fear responses during extinction learning. Albeit significant, this acute anxiogenic effect of SSRIs on fear extinction appears small; this effect was no longer significant when the number of animals was reduced in the male and female analysis (figure 2a,b). Chronic administration of fluoxetine, on the other hand, decreased the expression of fear responses during extinction learning and extinction recall. These anxiolytic effects were observed only in female rats. Furthermore, the reduction in fear responses after chronic administration was more pronounced when female rats underwent extinction learning during a low estrogen phase (metestrus/diestrus). We therefore propose that gonadal hormones may modulate the effect of SSRIs on fear extinction after chronic administration. It is important to note that the effect of chronic administration of fluoxetine seems to reduce conditioned freezing in general rather than a specific influence on extinction learning and its associated neural plasticity.
In rodents, anxiogenic-like reactions has been shown with acute administration of fluoxetine in different experimental models of anxiety [22, 29, 30]. In auditory fear conditioning, acute administration of the SSRI citalopram prior to and after fear conditioning increases the expression of fear responses and the formation of fear memory [23, 31] possibly via enhance activation of 5-HT2c receptors [23, 32]. Ravinder et al. (2011) showed that acute fluoxetine treatment enhanced anxiety-like behavior in elevated plus maze possibly via enhancing excitability of BLA neurons [33]. Regarding anxiolytic effects of SSRIs, recent rodents studies showed that chronic administration of SSRIs decreases fear responses during different experimental models of anxiety. For example, in fear conditioning, chronic fluoxetine treatment before extinction learning prevented the reactivation of fear responses after exposure to a sub-conditioning stressor [9, 13] and prevented the electrophysiological changes in the hippocampus that are normally induced by fear conditioning [13]. In another study, chronic fluoxetine treatment reduced fear responses after extinction of contextual fear conditioning in an inbred mouse strain known to be deficient in extinction learning [11]. Furthermore, chronic fluoxetine treatment combined with extinction treatment attenuated spontaneous recovery and renewal [10].
The significant SSRI-induced reduction in freezing was observed in female rats undergoing extinction learning in the metestrus/diestrus phase of the cycle, when estrogen and progesterone levels are low. We have previously shown that female rats that undergo extinction learning during the metestrus phase exhibit impaired fear extinction learning relative to female rats that undergo extinction learning during the proestrus phase of the estrus cycle (when there are high levels of estradiol). Thus, chronic SSRI treatment reduced fear expression during fear extinction only in female rats that exhibit exaggerated fear during extinction learning, i.e. the metestrus/diestrus females. Along those lines, female rats undergoing extinction during proestrus/estrus would not require extinction facilitation, and therefore there may be a “floor effect” of SSRI treatment on extinction. In support of this hypothesis, recent studies conducted in male rats reported SSRI facilitated extinction only in strains of animals that exhibited deficient extinction learning [11]. It is important to note that in contrast to the studies discussed above showing that chronic SSRIs treatment in males decreases freezing responses, a recent study has in fact reported that extinction was impaired with a longer treatment of SSRIs (3 weeks) [12]. Perhaps this conflicting evidence between studies may be due to differences in the longevity of treatment, dose of the SSRI administered, or the type of SSRI used. Another possibility for these differences may be due the route of administration. However, this is unlikely given that our control experiment indicate no differences in the findings when comparing the effects of acute injection of fluoxetine with acute fluoxetine in drinking water. Regardless, however, the primary effects of SSRIs we observed in our current study were noted in females and may be due to a role of gonadal hormones in modulating the effects of fluoxetine. It would be interesting to compare, in future studies, the effects of different SSRIs, and the duration of treatment (i.e. 2 weeks vs. 3 weeks) on fear extinction, in both males and females.
How could SSRIs and gonadal hormones interact to modulate fear extinction in females? The answer to this question remains unclear. Recent studies suggest that gonadal hormones influence the expression of fear responses in different paradigms suggesting that gonadal hormones may modulate the formation and/or the expression of fear memories [21, 34]. For example, during extinction of cued fear conditioning, female rats and women with high levels of gonadal hormones showed low fear responses 24 hours after extinction learning [25]. Donner and Honda [17] recently showed that systemic and local administration of the estrogen receptor beta agonist enhances the expression of the rate-limiting enzyme for serotonin, tryptophan-hydroxylase 2 (TPH2), in the dorsal raphe nuclei while inducing anxiolytic effects in open field and elevated plus mazes. Estrogen receptors are expressed in the vmPFC [35], which is known to be critical for inhibiting fear responses during fear extinction [36, 37]. Moreover, estrogen normally regulates the expression of serotonin receptors 5-HT (1A) and serotonin turnover in the fear circuitry, including amygdala, hippocampus and PFC [38, 39]. Recently it has been shown that the serotonin transporter (5-HTT) is associated with deficits during extinction recall of conditioned fear [40]. All together, these studies support the idea that estrogen and serotonin interactions may be critical during fear extinction.
There are some important caveats to consider in the present study. First, the absence of an effect of chronic SSRI treatment on the fear expression in rats tested during the proestrus/estrus phase may be related to insufficient statistical power. Second, our study was conducted using an AAA experimental design (no contextual manipulations) so that we can compare our results from the current study to those previously collected by our group. It is unclear if the same sex-specific effects of chronic SSRIs treatment on fear expression would be observed with contextual manipulations of extinction learning (i.e. using an ABA design). Future studies are needed to consider many factors related to the present findings. Those factors include larger numbers of animals per group (for cycle phase), contextual manipulations of conditioning and extinction, and manipulations of the duration of the chronic treatment, route of administration and type of SSRI.
Our findings of acute and chronic fluoxetine treatment during fear extinction are consistent with the anxiogenic and anxiolytic effects of SSRIs seen in patients with anxiety disorders and depression [41–43]. In healthy subjects, acute SSRI treatment increases recognition of fearful faces indicating an increase in the processing of anxiety-related stimuli [44]. In another study, a single dose of SSRI citalopram increased fear potentiated startle in healthy humans [45]. Epidemiological studies indicate that women have a higher prevalence [46, 47] and higher symptom duration [48–50] for anxiety disorders and depression. However, all the studies discussed above focus on males and ignore the possibility of sex differences in the effects of SSRIs treatment on fear and anxiety. Such an understanding may help develop sex-specific therapies, which will be more beneficial for women. For example, understanding how SSRIs may interact with estrogens could help improve the efficacy of current pharmacotherapy used to treat anxiety disorders in women.
Acknowledgments
This work was supported by the National Institute of Mental Health Grant 1R01MH097880-001 and institutional funds from the Department of Psychiatry at Massachusetts General Hospital to MRM. The authors also appreciate Kara Cover, Edward Pace-Schott, Marie-France Marin and Aaron Landau for reviewing the manuscript.
References
- 1.Spigset O. Adverse reactions of selective serotonin reuptake inhibitors: reports from a spontaneous reporting system. Drug Saf. 1999;20:277–87. doi: 10.2165/00002018-199920030-00007. [DOI] [PubMed] [Google Scholar]
- 2.Boyer WF, Feighner JP. An overview of fluoxetine, a new serotonin-specific antidepressant. Mt Sinai J Med. 1989;56:136–40. [PubMed] [Google Scholar]
- 3.Milad MR, Pitman RK, Ellis CB, Gold AL, Shin LM, Lasko NB, et al. Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. BiolPsychiatry. 2009;66:1075–82. doi: 10.1016/j.biopsych.2009.06.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depress Anxiety. 2010;27:244–51. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milad MR, Orr SP, Lasko NB, Chang Y, Rauch SL, Pitman RK. Presence and acquired origin of reduced recall for fear extinction in PTSD: results of a twin study. J Psychiatr Res. 2008;42:515–20. doi: 10.1016/j.jpsychires.2008.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Milad MR, Rauch SL. Obsessive-compulsive disorder: beyond segregated cortico-striatal pathways. Trends Cogn Sci. 2012;16:43–51. doi: 10.1016/j.tics.2011.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Holt DJ, Lebron-Milad K, Milad MR, Rauch SL, Pitman RK, Orr SP, et al. Extinction memory is impaired in schizophrenia. BiolPsychiatry. 2009;65:455–63. doi: 10.1016/j.biopsych.2008.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deschaux O, Spennato G, Moreau JL, Garcia R. Chronic treatment with fluoxetine prevents the return of extinguished auditory-cued conditioned fear. Psychopharmacology (Berl) 2011;215:231–7. doi: 10.1007/s00213-010-2134-y. [DOI] [PubMed] [Google Scholar]
- 9.Deschaux O, Zheng X, Lavigne J, Nachon O, Cleren C, Moreau JL, et al. Post-extinction fluoxetine treatment prevents stress-induced reemergence of extinguished fear. Psychopharmacology (Berl) 2013;225:209–16. doi: 10.1007/s00213-012-2806-x. [DOI] [PubMed] [Google Scholar]
- 10.Karpova NN, Pickenhagen A, Lindholm J, Tiraboschi E, Kulesskaya N, Agustsdottir A, et al. Fear erasure in mice requires synergy between antidepressant drugs and extinction training. Science. 2011;334:1731–4. doi: 10.1126/science.1214592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Camp MC, Macpherson KP, Lederle L, Graybeal C, Gaburro S, Debrouse LM, et al. Genetic strain differences in learned fear inhibition associated with variation in neuroendocrine, autonomic, and amygdala dendritic phenotypes. Neuropsychopharmacology. 2012;37:1534–47. doi: 10.1038/npp.2011.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burghardt NS, Sigurdsson T, Gorman JM, McEwen BS, Ledoux JE. Chronic Antidepressant Treatment Impairs the Acquisition of Fear Extinction. Biol Psychiatry. 2013;73:1078–86. doi: 10.1016/j.biopsych.2012.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spennato G, Zerbib C, Mondadori C, Garcia R. Fluoxetine protects hippocampal plasticity during conditioned fear stress and prevents fear learning potentiation. Psychopharmacology (Berl) 2008;196:583–9. doi: 10.1007/s00213-007-0993-7. [DOI] [PubMed] [Google Scholar]
- 14.Bui EOS, Jacoby RJ, Keshaviah A, LeBlanc NJ, Milad MR, Pollack MH, Simon NM. Two weeks of Pretreatment with Escitalopram Facilitates Extinction Learning in Healthy Individuals. Human Psychopharmacology: Clinical and Experimental Psyc. doi: 10.1002/hup.2330. in press. [DOI] [PubMed] [Google Scholar]
- 15.Benmansour S, Weaver RS, Barton AK, Adeniji OS, Frazer A. Comparison of the effects of estradiol and progesterone on serotonergic function. Biol Psychiatry. 2012;71:633–41. doi: 10.1016/j.biopsych.2011.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suzuki H, Barros RP, Sugiyama N, Krishnan V, Yaden BC, Kim HJ, et al. Involvement of estrogen receptor beta in maintenance of serotonergic neurons of the dorsal raphe. Mol Psychiatry. 2013;18:674–80. doi: 10.1038/mp.2012.62. [DOI] [PubMed] [Google Scholar]
- 17.Donner N, Handa RJ. Estrogen receptor beta regulates the expression of tryptophan-hydroxylase 2 mRNA within serotonergic neurons of the rat dorsal raphe nuclei. Neuroscience. 2009;163:705–18. doi: 10.1016/j.neuroscience.2009.06.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grothe DR, Piscitelli SC, Dukoff R, Fullerton T, Sunderland T, Molchan SE. Penetration of tacrine into cerebrospinal fluid in patients with Alzheimer’s disease. J Clin Psychopharmacol. 1998;18:78–81. doi: 10.1097/00004714-199802000-00013. [DOI] [PubMed] [Google Scholar]
- 19.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–95. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–8. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biology of Mood & Anxiety Disorders. 2012;2:3–15. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Javelot H, Weiner L, Terramorsi R, Rougeot C, Lalonde R, Messaoudi M. Efficacy of chronic antidepressant treatments in a new model of extreme anxiety in rats. Depress Res Treat. 2011;2011:531435. doi: 10.1155/2011/531435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Burghardt NS, Bush DE, McEwen BS, LeDoux JE. Acute selective serotonin reuptake inhibitors increase conditioned fear expression: blockade with a 5-HT(2C) receptor antagonist. Biol Psychiatry. 2007;62:1111–8. doi: 10.1016/j.biopsych.2006.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Silva RC, Brandao ML. Acute and chronic effects of gepirone and fluoxetine in rats tested in the elevated plus-maze: an ethological analysis. Pharmacol Biochem Behav. 2000;65:209–16. doi: 10.1016/s0091-3057(99)00193-8. [DOI] [PubMed] [Google Scholar]
- 25.Zeidan MA, Igoe SA, Linnman C, Vitalo A, Levine JB, Klibanski A, et al. Estradiol Modulates Medial Prefrontal Cortex and Amygdala Activity During Fear Extinction in Women and Female Rats. Biol Psychiatry. 2011;70:920–7. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Goldman JM, Murr AS, Cooper RL. The rodent estrous cycle: characterization of vaginal cytology and its utility in toxicological studies. Birth Defects Res B Dev Reprod Toxicol. 2007;80:84–97. doi: 10.1002/bdrb.20106. [DOI] [PubMed] [Google Scholar]
- 27.Graham BM, Milad MR. Blockade of Estrogen by Hormonal Contraceptives Impairs Fear Extinction in Female Rats and Women. Biol Psychiatry. 2012;73:371–8. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stefanova N, Ovtscharoff W. Sexual dimorphism of the bed nucleus of the stria terminalis and the amygdala. Adv Anat Embryol Cell Biol. 2000;158:III–X. 1–78. doi: 10.1007/978-3-642-57269-2. [DOI] [PubMed] [Google Scholar]
- 29.Salchner P, Singewald N. Neuroanatomical substrates involved in the anxiogenic-like effect of acute fluoxetine treatment. Neuropharmacology. 2002;43:1238–48. doi: 10.1016/s0028-3908(02)00329-5. [DOI] [PubMed] [Google Scholar]
- 30.Ando RD, Adori C, Kirilly E, Molnar E, Kovacs GG, Ferrington L, et al. Acute SSRI-induced anxiogenic and brain metabolic effects are attenuated 6 months after initial MDMA-induced depletion. Behav Brain Res. 2010;207:280–9. doi: 10.1016/j.bbr.2009.10.011. [DOI] [PubMed] [Google Scholar]
- 31.Burghardt NS, Sullivan GM, McEwen BS, Gorman JM, LeDoux JE. The selective serotonin reuptake inhibitor citalopram increases fear after acute treatment but reduces fear with chronic treatment: a comparison with tianeptine. Biol Psychiatry. 2004;55:1171–8. doi: 10.1016/j.biopsych.2004.02.029. [DOI] [PubMed] [Google Scholar]
- 32.Bagdy G, Graf M, Anheuer ZE, Modos EA, Kantor S. Anxiety-like effects induced by acute fluoxetine, sertraline or m-CPP treatment are reversed by pretreatment with the 5-HT2C receptor antagonist SB-242084 but not the 5-HT1A receptor antagonist WAY-100635. Int J Neuropsychopharmacol. 2001;4:399–408. doi: 10.1017/S1461145701002632. [DOI] [PubMed] [Google Scholar]
- 33.Ravinder S, Pillai AG, Chattarji S. Cellular correlates of enhanced anxiety caused by acute treatment with the selective serotonin reuptake inhibitor fluoxetine in rats. Front Behav Neurosci. 2011;5:88. doi: 10.3389/fnbeh.2011.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Luine V, Frankfurt M. Interactions between estradiol, BDNF and dendritic spines in promoting memory. Neuroscience. 2012;239:34–5. doi: 10.1016/j.neuroscience.2012.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montague D, Weickert CS, Tomaskovic-Crook E, Rothmond DA, Kleinman JE, Rubinow DR. Oestrogen receptor alpha localisation in the prefrontal cortex of three mammalian species. J Neuroendocrinol. 2008;20:893–903. doi: 10.1111/j.1365-2826.2008.01743.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sierra-Mercado D, Padilla-Coreano N, Quirk GJ. Dissociable roles of prelimbic and infralimbic cortices, ventral hippocampus, and basolateral amygdala in the expression and extinction of conditioned fear. Neuropsychopharmacology. 2011;36:529–38. doi: 10.1038/npp.2010.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–4. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 38.Amin Z, Canli T, Epperson CN. Effect of estrogen-serotonin interactions on mood and cognition. BehavCogn NeurosciRev. 2005;4:43–58. doi: 10.1177/1534582305277152. [DOI] [PubMed] [Google Scholar]
- 39.Osterlund MK, Halldin C, Hurd YL. Effects of chronic 17beta-estradiol treatment on the serotonin 5-HT(1A) receptor mRNA and binding levels in the rat brain. Synapse. 2000;35:39–44. doi: 10.1002/(SICI)1098-2396(200001)35:1<39::AID-SYN5>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 40.Narayanan V, Heiming RS, Jansen F, Lesting J, Sachser N, Pape HC, et al. Social Defeat: Impact on Fear Extinction and Amygdala-Prefrontal Cortical Theta Synchrony in 5-HTT Deficient Mice. PLoS One. 2011;6:e22600. doi: 10.1371/journal.pone.0022600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gorman JM, Liebowitz MR, Fyer AJ, Goetz D, Campeas RB, Fyer MR, et al. An open trial of fluoxetine in the treatment of panic attacks. J Clin Psychopharmacol. 1987;7:329–32. [PubMed] [Google Scholar]
- 42.Goldstein BJ, Goodnick PJ. Selective serotonin reuptake inhibitors in the treatment of affective disorders--III. Tolerability, safety and pharmacoeconomics. J Psychopharmacol. 1998;12:S55–87. doi: 10.1177/0269881198012003041. [DOI] [PubMed] [Google Scholar]
- 43.Goodnick PJ, Goldstein BJ. Selective serotonin reuptake inhibitors in affective disorders--II. Efficacy and quality of life. J Psychopharmacol. 1998;12:S21–54. doi: 10.1177/0269881198012003031. [DOI] [PubMed] [Google Scholar]
- 44.Browning M, Reid C, Cowen PJ, Goodwin GM, Harmer CJ. A single dose of citalopram increases fear recognition in healthy subjects. J Psychopharmacol. 2007;21:684–90. doi: 10.1177/0269881106074062. [DOI] [PubMed] [Google Scholar]
- 45.Grillon C, Levenson J, Pine DS. A single dose of the selective serotonin reuptake inhibitor citalopram exacerbates anxiety in humans: a fear-potentiated startle study. Neuropsychopharmacology. 2007;32:225–31. doi: 10.1038/sj.npp.1301204. [DOI] [PubMed] [Google Scholar]
- 46.Pigott TA. Anxiety disorders in women. Psychiatr Clin North Am. 2003;26:621—vii. doi: 10.1016/s0193-953x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 47.Kinrys GWLE. Anxiety disorders in women: does gender matter to treatment? Rev Bras Psiquiatr. 2005;27:S43–S50. doi: 10.1590/s1516-44462005000600003. [DOI] [PubMed] [Google Scholar]
- 48.Breslau N, Davis GC, Andreski P, Peterson EL, Schultz LR. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry. 1997;54:1044–8. doi: 10.1001/archpsyc.1997.01830230082012. [DOI] [PubMed] [Google Scholar]
- 49.Breslau N, Anthony JC. Gender differences in the sensitivity to posttraumatic stress disorder: An epidemiological study of urban young adults. J Abnorm Psychol. 2007;116:607–11. doi: 10.1037/0021-843X.116.3.607. [DOI] [PubMed] [Google Scholar]
- 50.Breslau N, Davis GC, Peterson EL, Schultz L. Psychiatric sequelae of posttraumatic stress disorder in women. Arch Gen Psychiatry. 1997;54:81–7. doi: 10.1001/archpsyc.1997.01830130087016. [DOI] [PubMed] [Google Scholar]