Abstract
Background
A core vulnerability trait for substance use disorder (SUD) is behavioral disinhibition. Error processing is a central aspect of inhibitory control that determines adaptive adjustment of performance; yet it is a largely overlooked aspect of disinhibition as it relates to risk for SUD. We investigated whether differences in brain activation during both successful and failed inhibition predicts early problem substance use.
Method
Forty-five 9–12 year olds underwent a functional MRI scan during a go/no-go task. They were then followed over approximately 4 years, completing assessments of substance use. Externalizing behavior was measured at ages 3–8, 9–12 and 11–13. Participants with drug use or problem alcohol use by ages 13–16 (n=13; problem-user group) were individually matched by gender, age, and family history of alcoholism with non-substance-using children (n=13; non-user group). The remaining 19 participants provided an independent sample from which to generate unbiased regions-of-interest for hypothesis testing in the problem-user and non-user groups.
Results
No differences were observed between groups in activation during correct inhibition compared with baseline. A significant difference arose in left middle frontal gyrus (LMFG) activation during failed inhibition compared with correct inhibition, with the problem-user group demonstrating blunted activation. The problem-user group also had more externalizing problems at ages 11–13. Logistic regression found that activation of LMFG significantly predicted group membership over and above externalizing problems.
Conclusions
Blunted LMFG activation during performance errors may underlie problems adapting behavior appropriately, leading to undercontrolled behavior, early problem substance use and increased risk for SUD.
Keywords: inhibitory control, error monitoring, vulnerability, substance abuse, development
1. INTRODUCTION
Behavioral undercontrol is an established early etiologic predictor of substance use disorder (SUD; Zucker et al., 2008). Measures of behavioral undercontrol in adolescents have been associated with early age of first drink (McGue et al., 2001) and greater use of alcohol and other drugs (Miller and Plant, 2002; Wills et al., 1996). Behavioral undercontrol is also detectable in precursive form much earlier than problem onset or diagnosable SUD (Caspi et al., 1996; Kellam et al., 1983; Zucker et al., 2008).
Behavioral undercontrol is a multicomponent domain. It includes externalizing behavior (aggression and delinquency), impulsivity, and sensation-seeking, all of which are posited to share a latent trait of behavioral disinhibition, defined as a an inability, unwillingness, or failure to inhibit behavioral impulses even in the face of negative consequences (Sher and Trull, 1994). Weak response inhibition specifically is linked to liability for a range of externalizing diagnoses (Young et al., 2009). Among the most established laboratory probes of response inhibition are the stop task and the go/no-go task (Nigg, 2000). The ability to suppress a response on the stop task has been associated with family risk for alcoholism (Nigg et al., 2004) and predicted early onset of alcohol problems, independently of family history of alcoholism (Nigg et al., 2006).
The go/no-go task is more widely used in neuroimaging studies. It examines the ability to inhibit a prepotent response by engaging individuals in responding to frequent “go” signals and requiring them to inhibit the response when infrequent “no-go” signals occur. In healthy adults, response inhibition during this task has been linked to functioning of fronto-parietal and fronto-striatal-thalamic networks, primarily in the right hemisphere (e.g., Chambers et al., 2009; Garavan et al., 1999; Kelly et al., 2004; Stevens et al., 2007). The prefrontal cortex is a critical component of this circuitry. The ability to successfully inhibit a response increases markedly from childhood to young adulthood (Schachar and Logan, 1990), concomitant with ongoing maturation of the prefrontal cortex and its associated circuitry (Gogtay et al., 2004).
Functional MRI studies of the development of response inhibition show that neural activation is more global and diffuse at younger ages, but lateralizes to the right-hemisphere and becomes more localized in the prefrontal cortex by adulthood (Booth et al., 2003; Casey et al., 1997; Durston et al., 2006; Tamm et al., 2002). Therefore, significant maturational changes are occurring in response inhibition circuitry during the same developmental period that risk for engagement in problem substance use is sharply increasing.
The earliest age at which this circuitry has been investigated in the context of risk for substance abuse is 12–14 years (Norman et al., 2011; Schweinsburg et al., 2004; Whelan et al., 2012), with findings demonstrating blunted prefrontal activation during inhibitory control in youth at risk. However, early alcohol use (typically defined as use prior to the population median of age 14), initiation of problem use, and early drug use, are all known to be powerful predictors of later SUD (Grant and Dawson, 1997, 1998; Gruber et al., 1996), so understanding what mechanisms precede early onset is an important goal. The present study sought to extend the literature by capturing individuals at an earlier age, before those at heightened risk begin significant substance use. To this end, we investigate whether the functioning of impulse control circuitry at ages 9–12 predicts markers of problem substance use by ages 13–16 in a high-risk sample.
An important additional goal is to isolate components of inhibitory control that may help identify particular mechanisms in both brain and behavior related to risk. Here, we capitalized on the fact that during the go/no-go task, one can either correctly inhibit during a no-go trial or commit an error by responding to the no-go trial. It has been suggested that maturation of error-monitoring abilities may be a factor underlying age-related performance gains in inhibitory control (Stevens et al., 2009). Error processing is an important aspect of cognitive control, believed to be critical to adaptive adjustment of performance (Ridderinkhof et al., 2004). The medial prefrontal cortex, including the anterior cingulate, has been consistently associated with error detection (e.g., Carter et al., 1998; Garavan et al., 2002; Kiehl et al., 2000) and may interact with lateral prefrontal cortex to support performance adjustment (Ridderinkhof et al., 2004; Stevens et al., 2009). Thus far, the differentiation of inhibitory control to consider brain regions subserving error monitoring has not been investigated in relationship to early risk for SUD.
We investigated whether differences in brain activation during both successful inhibition and inhibitory errors predict problem substance involvement at an early age and also whether brain activation adds explanatory power beyond other potential risk factors – particularly externalizing problems, which are among the most well-established risk variables in childhood. Based on prior work investigating risk for substance abuse (Norman et al., 2011; Schweinsburg et al., 2004), we hypothesized that blunted activation in the prefrontal cortex would be related to early problem substance use. Based on work describing the network dynamics of inhibitory control (Stevens et al., 2007, 2009), we hypothesized that this effect would be observed during successful inhibition in prefrontal regions within the fronto-parietal and fronto-striatal-thalamic circuits, and during inhibitory errors in anterior cingulate and lateral prefrontal regions.
2. METHODS
2.1. Participants
2.1.1. Overview
Forty-five (35 males) participants were selected from an ongoing fMRI study. Selection was based on availability of follow-up substance use data at least through age 13. At study onset, these 45 participants were 9–12 years old and had minimal substance use (<2 lifetime uses). All participants were recruited from the Michigan Longitudinal Study (MLS), a prospective community study of families with high levels of parental alcohol use disorders (AUD) and a contrast sample of nonalcoholic families drawn from the same neighborhoods (Zucker et al., 1996). See Supplementary Material1 for further details regarding recruitment and assessment procedures for the MLS and the ongoing fMRI study.
Presence of most active primary Axis I disorders were exclusionary for entry into this study; this did not include prior history of mood disorder, or current or past history of conduct disorders or attention deficit disorder. These were allowed because exclusion would preferentially eliminate part of the phenomena of interest. Psychostimulants used for treatment of attention difficulties were discontinued for 48 hours, prior to the fMRI study. Axis I disorders were assessed by a clinical psychologist based on DSM-IV criteria with the Diagnostic Interview Schedule - Child (v4) (Shaffer et al., 2000). The parallel version (DISC-Parent) of the instrument was administered to the primary caregiver to supplement the child data. All participants were right-handed as determined by Edinburgh Handedness questionnaire (Oldfield, 1971). Further exclusionary criteria for the fMRI study were neurological, acute, uncorrected, or chronic medical illness; current (within 6 months) treatment with other centrally active medications; history of psychosis or schizophrenia in first-degree relatives; or MRI contraindications.
All participants gave written assent after explanation of the IRB-approved experimental protocol. At least one parent gave written informed consent.
2.1.2. Creation of groups
Between ages 6–10, alcohol and drug use was assessed at 3-year intervals with a Health & Daily Living Questionnaire: children were asked if they ever had more than a sip of alcohol, smoked cigarettes, or used marijuana or other drugs. If yes, then the age at which this occurred and the quantity/frequency of alcohol or cigarettes (frequency of use only if other drugs) was recorded. Beginning at age 11, alcohol and drug use was assessed annually using the self-report Drinking and Drug History Form for Children (Zucker and Fitzgerald, 1994). This form includes quantity and frequency of alcohol, nicotine and illicit drug use (marijuana and 17 other drugs, including inhalants and nonmedical use of prescription drugs) and questions on consequences/problems related to use of these substances.
Initiation of high-risk substance use was defined as a report of problem alcohol use and/or any illicit drug use. Problem alcohol use was defined as the report of one or more of following: been drunk, binged, reported a problem due to alcohol use. Thirteen of the 45 participants initiated high-risk substance use based on these criteria during follow-up (problem-user group). Participants in the problem-user group were individually matched on age, gender and family history of alcoholism to 13 participants who had not reported any substance use at all (non-user group). The remaining 19 participants were used as an independent sample for unbiased region-of interest (ROI) definition (reference group). This approach eliminated the risk of invalid statistical inference that can result from circularity inherent in non-independent ROI selection (Kriegeskorte et al., 2009).
2.2. Measures
2.2.1. Behavior problems
Early externalizing problems were assessed with the Child Behavior Checklist (CBCL; Achenbach, 1991a) filled out by each parent as part of the MLS. Earliest available data were used (mean age=5.0; range: 3.0–8.1): for 19 participants (10 non-user/9 problem-user), these data were collected at ages 3–5; for the remaining 7 (3 non-user/4 problem-user), earliest data were collected at 6–8yrs. Externalizing problems were also assessed with CBCL within the same year as the fMRI scan (ages 9–12). Later externalizing problems were assessed at ages 11–13 (mean age=12.8) with the Youth Self-Report (YSR) (Achenbach, 1991b) to capture a continuous measure of behavioral undercontrol that was more contemporaneous with onset of substance use.
2.2.2. fMRI paradigm
An event-related go/no-go task (Durston et al., 2002) was used to probe response inhibition. Participants were instructed to respond to target stimuli (letters other than X) by pressing a button (go trials) but make no response to infrequent non-target stimuli (letter X; no-go trials). Stimulus duration was 500ms, followed by 3500ms of fixation. There were 5 runs of 49 trials, each lasting 3min 2s and containing 11, 12, or 13 no-go trials for a total of 60 (25%) no-go trials out of 245 total trials. Accuracy and reaction times (RT) during the task were recorded. Before scanning, all participants had a practice session of 49 trials on a desktop computer.
2.2.3. fMRI data acquisition
Whole-brain blood oxygenated level-dependent (BOLD) images were acquired on a 3.0 Tesla GE Signa scanner (Milwaukee, WI) using a T2*-weighted single-shot combined spiral in-out sequence (Glover and Law, 2001) with the following parameters: TR =2000ms; TE =30ms; flip angle =90°; FOV =200mm; 64×64 matrix; in-plane resolution =3.12×3.12mm; and slice thickness =4mm. The entire volume of 29 axial slices was acquired every 2sec. A high-resolution anatomical T1 scan was obtained for spatial normalization (three-dimensional spoiled gradient-recalled echo, TR =25ms; min TE; FOV =25cm; 256×256 matrix, slice thickness =1.4mm). Participant motion was minimized using foam pads placed around the head along with a forehead strap and the importance of keeping as still as possible was emphasized. Motion was also controlled for statistically (see next section).
2.3. Data Analysis
2.3.1. Task performance
Proportion of inhibitory failures, proportion of correct responses to go targets (hits), and RT for hits was calculated. Two-tailed independent-samples t-tests were conducted to investigate performance differences between groups.
2.3.2. fMRI data preprocessing
Functional images were reconstructed using an iterative algorithm (Fessler et al., 2005). Subject head motion was corrected using FSL 5.0.2.2 (Analysis Group, FMRIB, Oxford, United Kingdom; Jenkinson et al., 2002), and runs exceeding 3mm translation or 3° rotation in any direction were excluded (runs excluded by group: reference=2.0%; problem-user=1.2%; non-user=2.8%). Remaining image processing was completed using statistical parametric mapping (SPM8; Wellcome Trust Centre for Neuroimaging, UCL, London, United Kingdom). Functional images were spatially normalized to a standard stereotaxic space as defined by the Montreal Neurological Institute. A 6mm full-width half-maximum Gaussian spatial smoothing kernel was applied to improve signal-to-noise ratio and account for differences in anatomy.
2.3.3. Individual subject statistical maps
Individual analyses were completed using a general linear model. Three regressors of interest (correct no-go trails, failed no-go trials, and go trials) were convolved with the canonical hemodynamic response function. Motion parameters and white matter signal intensity were modeled as nuisance regressors to remove residual motion artifacts and capture non-task-related noise, respectively. Go trials were not included in the contrasts due to their high frequency relative to other trial types; instead an implicit baseline was used as described in (Devito et al., 2013). Three contrasts of interest were modeled: correct inhibition (correct no-go trials versus baseline), failed inhibition (failed no-go trials versus baseline) and failed inhibition versus correct inhibition (failed no-go versus correct no-go). The latter contrast was included in order to investigate the specific impact of errors on brain function involved in low-probability stimulus processing (Stevens et al., 2009).
2.3.4. Task effects and ROI selection
A key concern in studies of this nature is the risk of inflated statistics and invalid inference that can result from non-independent ROI selection (Kriegeskorte et al., 2009). To circumvent this issue, we designed independent ROI selection. Task effects were determined through one-sample t-tests in the reference group (n=19). Areas of activation were deemed significant if they reached a cluster-level FDR-corrected threshold of p<0.05, where a cluster-forming threshold of uncorrected p<0.001 was used. These regions were then the focus of hypothesis testing in the problem-user and non-user groups. Effect sizes for significant clusters were extracted from individual contrasts of interest using MarsBaR Region of Interest toolbox (Brett et al., 2002) and entered into SPSS (IBM; version 21) for hypotheses testing.
2.3.5. Hypotheses testing
To investigate whether there are early differences in brain activation related to inhibitory control in youth that go on to be early problem substance users, independent-samples t-tests (two-tailed) were performed in each ROI comparing problem-user and non-user groups (Bonferonni-corrected threshold: 0.05/11 ROIs = 0.004). To investigate whether brain activation at age 9–12 significantly predicts later membership in the problem-user versus non-user group, above and beyond other risk factors, logistic regression was used.
3. RESULTS
3.1 Group characteristics
Group characteristics are given in Table 1 (see Supplementary Material2 for individual onset ages of problem use). Problem-user and non-user groups were individually matched on gender, family history of AUD, and age of baseline scan. Independent-sample t-tests (two-tailed) indicated no significant differences between problem-user and non-user groups in household income, IQ, parent-report externalizing behavior problems at ages 3–8 or 9–12, or maximum age of follow-up for substance use. Groups differed in self-report externalizing behavior problems at ages 11–13 (t24=2.7).
Table 1.
Group characteristics.
| Referencea | Non-User | Problem-User |
Non-User vs. Problem-User
|
||
|---|---|---|---|---|---|
| p | d | ||||
| N | 19 | 13 | 13 | NA | |
| Gender: Male/Female | 15/4 | 10/3 | 10/3 | NA | |
| Parent History AUD: positive/negative | 14/5 | 10/3 | 10/3 | NA | |
| Parent History: AUD only/AUD+DUD/none | 7/7/5 | 4/6/3 | 3/7/3 | 0.896 | |
| Annual Household Income ($1,000’s)b | 81(45) | 96(47) | 86(50) | 0.577 | 0.22 |
| IQb | 106(13) | 111(15) | 105(14) | 0.269 | 0.44 |
| Caucasian (n) | 14 | 10 | 11 | 0.999 | |
|
| |||||
| Baseline | |||||
| Age at baseline scanb | 10.9(1.1) | 10.9(0.9) | 11.0(1.0) | NA | |
| Baseline age range | 9.4–12.9 | 9.8–12.5 | 9.4–12.5 | ||
| Any substance use (n) | 0 | 0 | 2c | 0.480 | |
| ADHD Diagnosis | 3 | 0 | 2 | 0.480 | |
| Conduct Disorder Diagnosis | 0 | 0 | 1 | 0.999 | |
| Anxiety Diagnosis (prior history) | 0 | 1 | 0 | 0.999 | |
| Depression Diagnosis | 0 | 0 | 0 | ||
|
| |||||
| Follow-up | |||||
| Age at oldest follow-upb,d | 13.9(0.7) | 14.9(0.8) | 15.1(1.9) | 0.685 | 0.16 |
| Age range at oldest follow-up data | 13.0–16.1 | 13.9–16.5 | 13.0–16.8 | ||
| Any alcohol use [n (mean age onset)] | 3 (14.3) | 0 | 8 (12.4) | ||
| Smoked cigarettes (n) | 1 | 0 | 4 (15.0) | ||
| High risk substance use criteria [n (mean age onset)] | |||||
| Problem alcohol use | 0 | 0 | 7 (13.3) | ||
| Marijuana use | 0 | 0 | 8 (13.3) | ||
| Other illicit drug use | 0 | 0 | 5 (13.4) | ||
| ADHD Diagnosis | 3 | 0 | 2 | 0.480 | |
| Conduct Disorder Diagnosis | 0 | 0 | 1 | 0.999 | |
| Anxiety Diagnosis | 0 | 1 | 1 | 1.000 | |
| Depression Diagnosis | 0 | 0 | 2 | 0.480 | |
|
| |||||
| Externalizing Problems Age 3–8 (CBCL)b | 7.6(5.7) | 9.9(6.2) | 11.5(4.8) | 0.479 | 0.29 |
| Externalizing Problems Age 9–12 (CBCL)b | 6.9(7.1) | 6.7(5.3) | 9.9(5.4) | 0.136 | 0.61 |
| Externalizing Problems Age 11–13 (YSR)b | 8.2(4.2) | 6.5(4.2) | 11.1(4.6) | 0.013 | 1.05 |
|
| |||||
| Task performanceb | |||||
| Proportion hits | 0.94(0.10) | 0.97(0.04) | 0.94(0.06) | 0.256 | 0.46 |
| Hit reaction time | 522(133) | 503(80) | 441(64) | 0.038 | 0.86 |
| Proportion inhibitory errors | 0.47(0.19) | 0.46(0.21) | 0.52(0.16) | 0.432 | 0.31 |
AUD, Alcohol Use Disorder; DUD, Drug Use Disorder; CBCL, Child Behavior Checklist; YSR, Youth Self-Report; NA, not applicable because groups were matched on these characteristics; d, Cohen’s d effect size
See Supplementary Material6 for statistics regarding comparisons of reference group with the problem-user and non-user groups.
Data presented as mean (standard deviation)
n=1 reported drinking on one occasion, n=1 reported having used marijuana on one occasion.
Drinking and drug use data were collected annually. Maximum age refers to age of participant at their last available drinking and drug use assessment; these data had to be available up to age 13 for the participant to be included in this study.
Fisher’s exact tests (two-tailed) indicated no reliable difference in family history of drug use disorder, presence of baseline substance use, or diagnoses between groups.
3.2 Performance measures
Performance measures are given in Table 1. The problem-users had significantly faster RT than non-users (t24=2.2). Proportion of hits and of inhibitory errors did not reliably differ.
3.3 Selection of ROI’s: Whole-brain task effects
Task effects for the reference group are reported in Table 2 and Supplementary Material (Figure S13). Correct inhibition activated prefrontal, striatal, and parietal regions. Failed inhibition compared with baseline activated bilateral prefrontal regions and anterior cingulate. Failed inhibition compared with correct inhibition resulted in activation localized in the left middle frontal gyrus (LMFG; Figure 1A). These 11 ROI’s became the focus of our hypothesis testing.
Table 2.
ROI’s used for hypotheses testing based on regions of activation in the whole brain analysis of the reference group.
| Region | L/R | BA | x,y,z | Size | t | pFDR |
|---|---|---|---|---|---|---|
| Correct Inhibition | ||||||
| IFG/Insula | L | 47 | −50, 8, −2 | 463 | 7.55 | <.001 |
| Middle Frontal Gyrus | R | 9/10 | 36, 46, 34 | 941 | 6.43 | <.001 |
| Middle Frontal Gyrus | L | 10 | −40, 52, 10 | 390 | 5.43 | .002 |
| Supplemental Motor Area | B | 6 | 4, 14, 46 | 652 | 5.53 | <.001 |
| Inferior Parietal Lobe | R | 40 | 54, −44, 30 | 547 | 5.59 | <.001 |
| Caudate | R | 18, 22, −4 | 245 | 6.35 | .005 | |
| Failed Inhibition | ||||||
| IFG/Insula | R | 47 | 46, 16, 0 | 333 | 5.62 | .004 |
| Middle Frontal Gyrus | R | 9 | 44, 42, 28 | 341 | 5.12 | .004 |
| Middle Frontal Gyrus | L | 9 | −26, 48, 18 | 497 | 5.87 | .001 |
| Anterior Cingulate | 24/32 | 0, 28, 34 | 531 | 5.18 | .001 | |
| Failed Inhibition > Correct Inhibition | ||||||
| Middle Frontal Gyrus | L | 9 | −20, 48, 24 | 222 | 5.62 | .024 |
L/R, left or right hemisphere, B, bilateral.; BA, Brodmann Area
Figure 1.
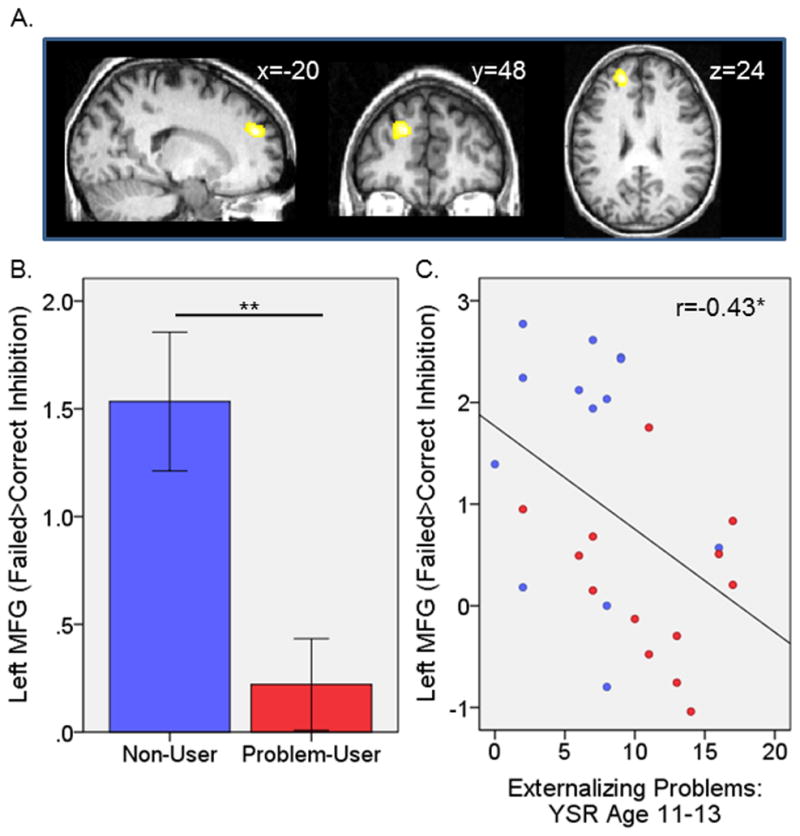
Left middle frontal gyrus activation. A) Localization of activation in the reference group to failed inhibition>correct inhibition. B) Non-user versus problem-user group differences during failed inhibition compared with correct inhibition. C) Correlation with externalizing problems at age 11–13 across problem-user and non-user groups. Blue is non-user group; red is problem-user group; MFG, middle frontal gyrus. *p<.05, **p<.01.
3.4 Problem-user versus non-user group differences in brain activation
Statistics for problem-user versus non-user group comparisons for each ROI are reported in Table 3. During correct inhibition, no reliable activation differences were observed between problem-user and non-user groups. During failed inhibition versus baseline, groups differed in activation of the LMFG but this did not survive correction for multiple comparisons. During failed inhibition versus correct inhibition groups also differed in the LMFG (Figure 1B); this effect survived correction for multiple comparisons. There were no group differences in the remaining ROIs.
Table 3.
Comparison of non-user versus problem-user groups in ROI activation
| Region | Non-Users | Problem-users | t (df=24) | p | d | ||
|---|---|---|---|---|---|---|---|
| Mean | SEM | Mean | SEM | ||||
| Correct Inhibition | |||||||
| L IFG/Insula | 1.25 | 0.54 | 1.36 | 0.54 | −0.15 | 0.885 | 0.06 |
| R Middle Frontal Gyrus | 1.06 | 0.63 | 0.52 | 0.40 | 0.72 | 0.482 | 0.28 |
| L Middle Frontal Gyrus | 1.50 | 0.65 | 0.65 | 0.55 | 1.00 | 0.329 | 0.39 |
| Supplemental Motor Area | 0.76 | 0.22 | 0.95 | 0.18 | −0.66 | 0.513 | 0.26 |
| R Inferior Parietal Lobe | 1.04 | 0.65 | 0.25 | 0.24 | 1.13 | 0.269 | 0.44 |
| R Caudate | 2.05 | 0.86 | 1.09 | 0.29 | 1.06 | 0.301 | 0.41 |
| Failed Inhibition | |||||||
| R IFG/Insula | 1.16 | 0.80 | 1.39 | 0.62 | −0.22 | 0.828 | 0.09 |
| R Middle Frontal Gyrus | 0.94 | 0.54 | 0.40 | 0.34 | 0.83 | 0.414 | 0.32 |
| L Middle Frontal Gyrus | 1.32 | 0.29 | 0.50 | 0.19 | 2.31 | 0.030 | 0.91 |
| Anterior Cingulate | 2.20 | 0.61 | 1.32 | 0.50 | 1.10 | 0.283 | 0.43 |
| Failed Inhibition > Correct Inhibition | |||||||
| L Middle Frontal Gyrus | 1.53 | 0.32 | 0.22 | 0.21 | 3.40 | 0.002 | 1.36 |
L, Left; R, right; d, Cohen’s d effect size
3.5 Logistic Regression to predict outcome
The prior analyses revealed three variables related to the outcome of interest (i.e., group membership): RT, externalizing at ages 11–13, and LMFG activation during failed vs. correct inhibition. These variables became the focus of the logistic regression to determine whether RT or LMFG activation informed risk prediction over and above the well-established predictor of externalizing behavior. The relationship between the three variables was first tested. Externalizing at age 11–13 was negatively correlated with LMFG activation across all subjects in the problem-user and non-user groups (Figure 1C; n=26, r=−.43, p=0.031). There were no other reliable correlations between the three variables (RT and externalizing: r=.04; p=0.865; RT and LMFG: r=.01, p=0.948).
Results of the logistic regression are given in Table 4. The first block included RT and externalizing at 11–13 simultaneously. These variables significantly predicted problem-user versus non-user status (prediction accuracy: overall 80.8%; non-user group 84.6%; problem-user group 76.9%). In the second block, LMFG activation during failed vs. correct inhibition was added to determine whether brain activation at age 9–12 improved prediction of group membership. Inclusion of LMFG significantly improved the model (prediction accuracy: overall 88.5%; non-user group: 84.6%; problem-user group: 92.3%). LMFG activation was the only significant predictor in the final model, although RT approached significance. Results remained significant when removing: 1) those with externalizing diagnoses (ADHD or CD; n=3); 2) those with a lifetime history of depression (n=2); and 3) those with (minimal) baseline substance use (n=2) (see Supplementary Material4).
Table 4.
Results of logistic regression analysis predicting non-user versus problem-user group membership
| R-sq or βa | Chi-sq or Waldb | p | Change | ||
|---|---|---|---|---|---|
| Chi-sq | p | ||||
| Block 1: Method-Enter | |||||
| Model | 0.39 | 12.7 | .002 | ||
| Reaction Time | −0.02 | 4.1 | .044 | ||
| Externalizing ages 11–13 | 0.29 | 5.4 | .021 | ||
|
| |||||
| Block 2: Method-Enter Left MFG (FI>CI) | |||||
| Model | 0.54 | 20.2 | <.001 | 7.5 | .006 |
| Reaction Time | −0.03 | 3.7 | .053 | ||
| Externalizing ages 11–13 | 0.20 | 2.1 | .149 | ||
| Left MFG (FI>CI) | −1.72 | 4.6 | .032 | ||
MFG, middle frontal gyrus; FI, failed inhibition; CI, correct inhibition.
Cox & Snell R2 reported for model fits; beta’s reported for individual variables.
Chi-squared reported for model fits; Wald statistic reported for individual variables
4. DISCUSSION
The present study investigated whether differences in the functioning of neural mechanisms underlying behavioral disinhibition predicts early problem substance use. Participants were individually matched on family history of alcoholism, gender, and age. We found that less activation in the left middle frontal gyrus during inhibitory errors compared with correct inhibition at ages 9–12 was a significant predictor of problem substance use by ages 13–16, above and beyond externalizing problems and RT (an indicator of impulsive responding). These results should be considered somewhat preliminary due to the small sample size and replication is necessary. However, this is the earliest age at which baseline neuroimaging has been done to prospectively investigate neural risk factors for substance abuse. This allowed for the characterization of very early risk markers for those at the greatest risk of poor outcome – i.e., those initiating early problem use. This is also the first study of SUD prospective risk to specifically differentiate between aspects of inhibitory control during the go/no-go task by investigating both successful inhibition and inhibitory errors.
The problem-user group had blunted activation within the left middle frontal gyrus during unsuccessful versus successful inhibition compared to the non-user group. This region is part of a network believed to processes low-probability stimuli (Stevens et al., 2009). These authors show that, during a go/no-go task, this network is engaged to all no-go stimuli, but activation is augmented during errors compared to correctly inhibited trials, suggesting involvement in the signaling of suboptimal performance. Furthermore, Garavan and colleagues (2002) demonstrated that the left lateral prefrontal cortex activated during successful behavior adjustment to error during a go/no-go task. Therefore this network is believed to be involved in subsequent performance adjustment (Ridderinkhof et al., 2004; Stevens et al., 2009). A behavioral consequence of blunted activity in this region may be difficulty adjusting one’s own behavior appropriately. Indeed, we observed that activation of the left middle frontal gyrus to inhibitory errors was negatively correlated with externalizing behavior – those with less activation showed more behavioral undercontrol.
This work supports and extends other studies investigating inhibitory control circuitry during the go/no-go task in the context of risk for substance abuse. In a similarly-sized (n=26) sample of 12–14 year olds, youth with parental AUD (n=12) showed blunted activation in the left middle frontal gyrus during no-go trials (Schweinsburg et al., 2004). Furthermore, blunted activation in widespread regions, including the left middle frontal gyrus, during no-go trials was reported in a sample of 21 12–14 year olds who transitioned to heavy drinking approximately four years later, compared with 17 controls (Norman et al., 2011). Because these studies did not distinguish between correct and incorrect inhibitory trials, however, it is unclear whether the findings reflect weakness in error monitoring as opposed to the related but dissociable process of response suppression. The present study not only extends the evidence to an earlier age, but adds a critical distinction between successful inhibition and inhibitory errors. This distinction allows for a more thorough understanding of the specific mechanism underlying the behavioral undercontrol that leads to risky substance use.
An emerging literature on the maturation of response suppression versus error detection circuitry lends context within which to consider these findings. The fronto-striatal-thalamic network has been found to be less engaged during response suppression in adolescents (aged 11–17) compared with adults, and showed less coupling with the fronto-parietal network involved in higher-order response contingencies to no-go stimuli (Stevens et al., 2007). Further, less coupling between these networks in adolescents was associated with more inhibitory errors. This suggests these networks are continuing to undergo maturational changes in support of inhibitory performance throughout adolescence. In contrast, no differences were found between 11–17 year olds and adults in the network supporting performance adjustment, suggesting this neural circuitry matures at an earlier age (Stevens et al., 2009). Within this context, we propose that dysfunction in early-maturing circuitry may be an initial indicator of risk – identifiable in middle childhood (i.e., ages 9–12). In adolescence, however, risk markers may become more evident in later-maturing circuitry supporting inhibitory control. For example, in addition to the left middle frontal gyrus region already discussed, Norman et al (Norman et al., 2011) report blunted activation in the fronto-striatal and fronto-parietal networks in 12–14 year olds who went on to become heavy alcohol users. These networks have been shown to be specifically involved in successful response suppression and are dissociable from error detection and performance adjustment networks (Garavan et al., 2002; Stevens et al., 2007, 2009). Furthermore, Whelan et al (2012) found hypoactivation potentially related to substance use vulnerability in a large sample (n=1593) of 14-year olds in an orbitofrontal network supporting successful response suppression. Taken together, there is some evidence across studies to indicate that the underlying neural mechanisms related to risky substance use may change with maturation; however, this cannot be determined conclusively from comparisons of cross-sectional studies.
An additional consideration is that the work reported here, identifying error detection/performance adjustment circuitry as a prospective indicator of early problem substance use, may be specific to those at greatest risk of developing SUD. The problem-user group is unquestionably at heightened risk; on average, the onset age of drinking was 12 and for problem drinking or drug use it was 13. Based on the literature indicating that substance use by age 13 significantly increases risk for later dependence (Grant and Dawson, 1997, 1998), this group is demonstrating non-normative, high risk use. In addition, initiation of multiple substances by age 16 has recently been reported to be associated with high rates of adult SUD (Moss et al., 2014), further highlighting the high-risk nature of use in this group. It is difficult to disentangle the neuro-developmental effects of an earlier baseline age, as compared to other studies, from the high-risk nature of the substance use captured here. Continued longitudinal work starting at early ages, before onset of significant substance use, is necessary to fully understand the relationship between brain maturation and the unfolding of risk across development.
A limitation of this study is the relatively small sample size, suggesting that results should be interpreted as somewhat preliminary and, in particular, that additional differences between groups in this age range may have been missed. However, other methodological strengths add confidence to the novel finding here: groups were carefully matched, and we took an innovative and statistically conservative approach by using an independent reference group to generate ROIs for hypotheses testing, thereby eliminating the risk of bias in statistical inference, a particular concern with a small sample size. Further, the primary finding involving left middle frontal gyrus not only replicates prior reports (Norman et al., 2011; Schweinsburg et al., 2004), but converges with theory and literature on error detection effects.
A potential caveat about this work is that the sample was primarily male, which may limit the generalizability of the findings. We explored whether the main findings showed trends in the same direction for girls as for boys and found that they did (reported in Supplementary Material5). Furthermore, prior studies report similar findings in groups with a balanced gender representation (Norman et al., 2011; Schweinsburg et al., 2004), although these studies did not specifically investigate gender differences. Although there is no evidence thus far to suggest that the relationship between inhibitory control and risk for problem substance use differs by gender in childhood and early adolescence, this is an important issue and should be investigated in future work.
Although disinhibition is accepted as a core vulnerability trait, the mechanisms underlying this trait have not previously been parsed in the context of substance abuse risk. While clinical implications should be drawn with considerable caution from this initial exploration, developmental timing is central to early risk identification and prevention not only in terms of choosing when to start interventions, but also in determining the specific mechanisms to target. The present work suggests that strengthening the ability to adjust one’s behavior in response to an error may be a promising target for preventative interventions at very early ages. These clinical possibilities warrant further exploration in follow-up work.
Supplementary Material
Acknowledgments
Role of funding source
This work was supported by NIH grants R01 DA027261 to MMH, JKZ and RAZ, R01 AA12217 to RAZ and MMH, R37 AA07065 to RAZ, and K01 DA020088 to MMH, and by the Phil F. Jenkins Research Fund (JKZ). The NIH and the Jenkins Foundation had no further role in study design; in the collection, analysis and interpretation of data; in the writing of the report; or in the decision to submit the paper for publication.
Footnotes
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper at http://dx.doi.org and by entering doi:...
Supplementary material can be found by accessing the online version of this paper. Please see Appendix A for more information.
Contributors
MMH, JKZ and RAZ designed the study and wrote the protocol. DS collected the data. JEH and MS performed statistical analyses. MMH, JEH and DS managed the literature searches and summaries of previous related work. MH wrote the first draft of the manuscript. JKZ, RAZ and JTN edited the manuscript. All authors contributed to and have approved the final manuscript.
Conflict of Interest
No conflict declared.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 Profile. University of Vermont, Department of Psychiatry; Burlington: 1991a. [Google Scholar]
- Achenbach TM. Manual for the Youth Self-report Form and 1991 Profile. University Associates in Psychiatry; Burlington, VT: 1991b. [Google Scholar]
- Booth JR, Burman DD, Meyer JR, Lei Z, Trommer BL, Davenport ND, Li W, Parrish TB, Gitelman DR, Mesulam MM. Neural development of selective attention and response inhibition. Neuroimage. 2003;20:737–751. doi: 10.1016/S1053-8119(03)00404-X. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J-L, Valabregue R, Poline JB. Region of Interest Analysis Using an SPM Toolbox. 8th International Conference on Functional Mapping of the Human Brain; Sendai, Japan. 2002. [Google Scholar]
- Carter CS, Braver TS, Barch DM, Botvinick MM, Noll D, Cohen JD. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280:747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Casey B, Trainor R, Orendi J, Schubert A, Nystrom L, Giedd J, Castellanos X, Haxby J, Noll D, Cohen J, Forman S, Dahl R, Rapoport J. A developmental functional MRI study of prefrontal activation during performance of a go-no-go task. J Cogn Neurosci. 1997;9:835–847. doi: 10.1162/jocn.1997.9.6.835. [DOI] [PubMed] [Google Scholar]
- Caspi A, Moffitt TE, Newman DL, Silva PA. Behavioral observations at age 3 years predict adult psychiatric disorders. Longitudinal evidence from a birth cohort Arch Gen Psychiatry. 1996;53:1033–1039. doi: 10.1001/archpsyc.1996.01830110071009. [DOI] [PubMed] [Google Scholar]
- Chambers CD, Garavan H, Bellgrove MA. Insights into the neural basis of response inhibition from cognitive and clinical neuroscience. Neurosci Biobehav Rev. 2009;33:631–646. doi: 10.1016/j.neubiorev.2008.08.016. [DOI] [PubMed] [Google Scholar]
- Devito EE, Meda SA, Jiantonio R, Potenza MN, Krystal JH, Pearlson GD. Neural correlates of impulsivity in healthy males and females with family histories of alcoholism. Neuropsychopharmacology. 2013;38:1854–1863. doi: 10.1038/npp.2013.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Durston S, Davidson MC, Tottenham N, Galvan A, Spicer J, Fossella JA, Casey BJ. A shift from diffuse to focal cortical activity with development. Dev Sci. 2006;9:1–8. doi: 10.1111/j.1467-7687.2005.00454.x. [DOI] [PubMed] [Google Scholar]
- Durston S, Thomas KM, Worden MS, Yang Y, Casey BJ. The effect of preceding context on inhibition: an event-related fMRI study. Neuroimage. 2002;16:449–453. doi: 10.1006/nimg.2002.1074. [DOI] [PubMed] [Google Scholar]
- Fessler J, Lee S, Olafsson V, Shi H, Noll D. Toeplitz-based iterative image reconstruction for MRI with correction for magnetic field inhomogeneity. IEEE Trans Signal Processi. 2005;53:3393–3402. [Google Scholar]
- Garavan H, Ross TJ, Murphy K, Roche RA, Stein EA. Dissociable executive functions in the dynamic control of behavior: inhibition, error detection, and correction. Neuroimage. 2002;17:1820–1829. doi: 10.1006/nimg.2002.1326. [DOI] [PubMed] [Google Scholar]
- Garavan H, Ross TJ, Stein EA. Right hemispheric dominance of inhibitory control: an event-related functional MRI study. Proc Natl Acad Sci U S A. 1999;96:8301–8306. doi: 10.1073/pnas.96.14.8301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover GH, Law CS. Spiral-in/out BOLD fMRI for increased SNR and reduced susceptibility artifacts. Magn Reson Med. 2001;46:515–522. doi: 10.1002/mrm.1222. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen LS, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age at onset of alcohol use and its association with DSM-IV alcohol abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1997;9:103–110. doi: 10.1016/s0899-3289(97)90009-2. [DOI] [PubMed] [Google Scholar]
- Grant BF, Dawson DA. Age of onset of drug use and its association with DSM-IV drug abuse and dependence: results from the National Longitudinal Alcohol Epidemiologic Survey. J Subst Abuse. 1998;10:163–173. doi: 10.1016/s0899-3289(99)80131-x. [DOI] [PubMed] [Google Scholar]
- Gruber E, DiClemente RJ, Anderson MM, Lodico M. Early drinking onset and its association with alcohol use and problem behavior in late adolescence. Prev Med. 1996;25:293–300. doi: 10.1006/pmed.1996.0059. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Kellam SG, Stevenson DL, Rubin BR. How specific are the early predictors of teenage drug use? NIDA Res Monogr. 1983;43:329–334. [PubMed] [Google Scholar]
- Kelly AM, Hester R, Murphy K, Javitt DC, Foxe JJ, Garavan H. Prefrontal-subcortical dissociations underlying inhibitory control revealed by event-related fMRI. Eur J Neurosci. 2004;19:3105–3112. doi: 10.1111/j.0953-816X.2004.03429.x. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Liddle PF, Hopfinger JB. Error processing and the rostral anterior cingulate: an event-related fMRI study. Psychophysiology. 2000;37:216–223. [PubMed] [Google Scholar]
- Kriegeskorte N, Simmons WK, Bellgowan PS, Baker CI. Circular analysis in systems neuroscience: the dangers of double dipping. Nat Neurosci. 2009;12:535–540. doi: 10.1038/nn.2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGue M, Iacono WG, Legrand LN, Malone S, Elkins I. Origins and consequences of age at first drink. I Associations with substance-use disorders, disinhibitory behavior and psychopathology, and P3 amplitude. Alcohol Clin Exp Res. 2001;25:1156–1165. [PubMed] [Google Scholar]
- Miller P, Plant M. Heavy cannabis use among UK teenagers: an exploration. Drug Alcohol Depend. 2002;65:235–242. doi: 10.1016/s0376-8716(01)00165-x. [DOI] [PubMed] [Google Scholar]
- Moss HB, Chen CM, Yi HY. Early adolescent patterns of alcohol, cigarettes, and marijuana polysubstance use and young adult substance use outcomes in a nationally representative sample. Drug Alcohol Depend. 2014;136:51–62. doi: 10.1016/j.drugalcdep.2013.12.011. [DOI] [PubMed] [Google Scholar]
- Nigg JT. On inhibition/disinhibition in developmental psychopathology: views from cognitive and personality psychology and a working inhibition taxonomy. Psychol Bull. 2000;126:220–246. doi: 10.1037/0033-2909.126.2.220. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Glass JM, Wong MM, Poon E, Jester JM, Fitzgerald HE, Puttler LI, Adams KM, Zucker RA. Neuropsychological executive functioning in children at elevated risk for alcoholism: findings in early adolescence. J Abnorm Psychol. 2004;113:302–314. doi: 10.1037/0021-843X.113.2.302. [DOI] [PubMed] [Google Scholar]
- Nigg JT, Wong MM, Martel MM, Jester JM, Puttler LI, Glass JM, Adams KM, Fitzgerald HE, Zucker RA. Poor response inhibition as a predictor of problem drinking and illicit drug use in adolescents at risk for alcoholism and other substance use disorders. J Am Acad Child Adolesc Psychiatry. 2006;45:468–475. doi: 10.1097/01.chi.0000199028.76452.a9. [DOI] [PubMed] [Google Scholar]
- Norman AL, Pulido C, Squeglia LM, Spadoni AD, Paulus MP, Tapert SF. Neural activation during inhibition predicts initiation of substance use in adolescence. Drug Alcohol Depend. 2011;119:216–223. doi: 10.1016/j.drugalcdep.2011.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Ridderinkhof KR, Ullsperger M, Crone EA, Nieuwenhuis S. The role of the medial frontal cortex in cognitive control. Science. 2004;306:443–447. doi: 10.1126/science.1100301. [DOI] [PubMed] [Google Scholar]
- Schachar R, Logan GD. Impulsivity and inhibitory control in normal development and childhood psychopathology. Dev Psychol. 1990;26:710–720. [Google Scholar]
- Schweinsburg AD, Paulus MP, Barlett VC, Killeen LA, Caldwell LC, Pulido C, Brown SA, Tapert SF. An FMRI study of response inhibition in youths with a family history of alcoholism. Ann N Y Acad Sci. 2004;1021:391–394. doi: 10.1196/annals.1308.050. [DOI] [PubMed] [Google Scholar]
- Shaffer D, Fisher P, Lucas CP, Dulcan MK, Schwab-Stone ME. NIMH Diagnostic Interview Schedule for Children Version IV (NIMH DISC-IV): description, differences from previous versions, and reliability of some common diagnoses. J Am Acad Child Adolesc Psychiatry. 2000;39:28–38. doi: 10.1097/00004583-200001000-00014. [DOI] [PubMed] [Google Scholar]
- Sher KJ, Trull TJ. Personality and disinhibitory psychopathology: alcoholism and antisocial personality disorder. J Abnorm Psychol. 1994;103:92–102. doi: 10.1037//0021-843x.103.1.92. [DOI] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Functional neural networks underlying response inhibition in adolescents and adults. Behav Brain Res. 2007;181:12–22. doi: 10.1016/j.bbr.2007.03.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevens MC, Kiehl KA, Pearlson GD, Calhoun VD. Brain network dynamics during error commission. Hum Brain Mapp. 2009;30:24–37. doi: 10.1002/hbm.20478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamm L, Menon V, Johnston CK, Hessl DR, Reiss AL. fMRI study of cognitive interference processing in females with fragile X syndrome. J Cogn Neurosci. 2002;14:160–171. doi: 10.1162/089892902317236812. [DOI] [PubMed] [Google Scholar]
- Whelan R, Conrod PJ, Poline JB, Lourdusamy A, Banaschewski T, Barker GJ, Bellgrove MA, Buchel C, Byrne M, Cummins TD, Fauth-Buhler M, Flor H, Gallinat J, Heinz A, Ittermann B, Mann K, Martinot JL, Lalor EC, Lathrop M, Loth E, Nees F, Paus T, Rietschel M, Smolka MN, Spanagel R, Stephens DN, Struve M, Thyreau B, Vollstaedt-Klein S, Robbins TW, Schumann G, Garavan H. Adolescent impulsivity phenotypes characterized by distinct brain networks. Nat Neurosci. 2012;15:920–925. doi: 10.1038/nn.3092. [DOI] [PubMed] [Google Scholar]
- Wills TA, Vaccaro D, McNamara G, Hirky AE. Escalated substance use: a longitudinal grouping analysis from early to middle adolescence. J Abnorm Psychol. 1996;105:166–180. doi: 10.1037//0021-843x.105.2.166. [DOI] [PubMed] [Google Scholar]
- Young SE, Friedman NP, Miyake A, Willcutt EG, Corley RP, Haberstick BC, Hewitt JK. Behavioral disinhibition: liability for externalizing spectrum disorders and its genetic and environmental relation to response inhibition across adolescence. J Abnorm Psychol. 2009;118:117–130. doi: 10.1037/a0014657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Donovan JE, Masten AS, Mattson ME, Moss HB. Early developmental processes and the continuity of risk for underage drinking and problem drinking. Pediatrics. 2008;121(Suppl 4):S252–272. doi: 10.1542/peds.2007-2243B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zucker RA, Ellis DA, Bingham CR, Fitzgerald HE, Sanford KP. Other evidence for at least two alcoholisms, II: Life course variation in antisociality and heterogeneity of alcoholic outcome. Dev Psychopathol. 1996;8:831–848. [Google Scholar]
- Zucker RA, Fitzgerald HE. Drinking and Drug History Form for Children. University of Michigan Department of Psychiatry, Addiction Research Center; Ann Arbor: 1994. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


