Abstract
Every year in the United States, an estimated 500,000 babies are born preterm (before 37 completed weeks of gestation), and this number is rising, along with the recognition of brain injuries due to preterm delivery. A common underlying pathogenesis appears to be perinatal hypoxia induced by immature lung development, which causes injury to vulnerable neurons and glia. Abnormal growth and maturation of susceptible cell types, particularly neurons and oligodendrocytes, in preterm babies with very low birth weight is associated with decreased cerebral and cerebellar volumes and increases in cerebral ventricular size. Here we reconcile these observations with recent studies using models of perinatal hypoxia that show perturbations in the maturation and function of interneurons, oligodendrocytes and astroglia. Together, these findings suggest that the global mechanism by which perinatal hypoxia alters development is through a delay in maturation of affected cell types, including astroglia, oligodendroglia and neurons.
Prematurity and brain injury in the newborn
The incidence of live preterm births in the USA has increased by 14% between 1990 and 2002 and continues to rise steadily. This is believed to be due at least in part to the increased number of births to mothers over 35, increased use of fertility treatments and a myriad of environmental factors, including smoking. Nevertheless, medical, pharmacological and technological advances have enhanced the survival rate in increasingly younger infants, particularly those born at a very low birth weight (VLBW); that is, under 1,500 g and less than 32 gestational weeks of age. Because lower birth weight and earlier gestational age are predictors of pathology, this improved survival rate of VLBW children is accompanied by increased morbidity in this population: their developmental trajectory remains complicated and plagued with disabilities, even in the absence of focal brain injury. For example, VLBW infants typically show neurological sequelae including cerebral gray and white matter abnormalities, ventriculomegaly and decreased cortical and basal ganglia volumes. Long-term longitudinal follow-up studies of this population have demonstrated long term cognitive difficulties, particularly those involved with language and executive functions, and psychiatric illnesses including autistic spectrum and anxiety disorders1,2. Many aspects of cognitive and neurological sequelae improve over time when extra resources are provided, including special education services and occupational and physical therapy. Under these conditions, many VLBW children show no appreciable differences from term-born children by the time they reach adolescence3,4. Unfortunately, there are few prognostic indicators of the likelihood that a particular child will suffer long-term neurological and neuropsychological consequences. Neuroimaging studies in adolescents who were born prematurely have delineated abnormal brain asymmetries and aberrant connectivity, suggesting that cognitive improvements might be achieved by establishing unusual patterns of connectivity5, perhaps due to a prolonged period of plasticity.
Premature birth has a negative impact on structural and functional integrity of the brain, and serial magnetic resonance imaging studies clearly have demonstrated a reduction in gray and white matter volumes in premature infants as compared to term controls6,7. Diffusion tensor imaging analysis has revealed differences in fractional anisotropy between prematurely born and term infants, indicating compromised microstructural organization and integrity of white matter, which are correlated with gestational age. These alterations in structural organization last until adolescence and correlate with a broad spectrum of cognitive and neurological deficits8. Diffuse white matter injury, together with gray matter and hippocampal abnormalities9,10, are the most common types of cerebral abnormalities associated with prematurity, as focal necrotic lesions characteristic of cystic periventricular leukomalacia are now rarely observed in premature infants11.
These observations raise important questions about the cellular substrates and cellular dynamics, in both neurons and glia, associated with premature brain injury. Yet it has been difficult to reproduce in animal models alterations in the trajectory of brain development and maturation due to early brain injury and prematurity, and we still are unable to discriminate alterations that are beneficial from those that are deleterious. The ever-rising rate of premature births invokes the necessity of animal models that recapitulate the phenotype of VLBW-associated injury to further delineate causative factors and potential therapeutics for recovery from injury associated with premature birth. We propose that there is a delay in maturation following injury associated with premature birth.
Models of VLBW premature birth
While there are focal ischemic injuries associated with VLBW, the most common injuries observed clinically are a global decrease in cortical volume and ventriculomegaly, which are attributable to chronic hypoxic injury, sustained as a consequence of immature lung development. Many aspects of this global injury are recapitulated through the use of a rodent model of chronic perinatal hypoxia in which mice are reared under low oxygen between postnatal day (P) 3 and P11. In this model (see ref. 12 for a review), hypoxically reared pups initially show decreases in cortical gray and white matter volumes and increased ventricle size. Subsequently, many aspects of this early brain phenotype show substantial recovery; for example, cortical and hippocampal volumes are reestablished to levels observed in normoxic controls by early adulthood, as are the total number of excitatory neurons and oligodendrocytes (OLCs) in the cortex and hippocampus. However, there are also important aspects of this injury that do not show recovery, most notably the maturation of GABA interneurons in the cortex and myelin structure (see below). These cellular deficits correlate with long-lasting deficits in learning and memory, increased anxiety, and decreases in fine motor skills well into adulthood, which is quite similar to the observed clinical phenotype13.
Cellular, functional and behavioral abnormalities have been found in models of perinatal hypoxic-ischemic injury such as the Vannucci model (Table 1), in which carotid artery occlusion is followed by exposure to hypoxia, typically from P3 to P9 (for a review, see ref. 14). In contrast to the chronic hypoxic, the hypoxic-ischemic model induces focal and diffuse injury that leads to a more typical glial reactive response and the formation of a glial scar. Recent studies have shown that mice exposed to the hypoxic-ischemic Vannucci model also show cognitive and motor deficits that last well into adulthood. Imaging studies have suggested that the extent of behavioral deficits is correlated to that of hippocampal and white matter injury, as well as to the expression of myelin basic protein and neurofilament protein in the white matter15,16.
Table 1.
Animal models of VLBW premature birth
| Model | Species | Features | Advantages | Disadvantages |
|---|---|---|---|---|
| Hypoxia-ischemia (Vannucci model) | Mouse, rat, pig | Necrotic injury OLC death Axon damage Microglial activation Astrogliosis |
Focal injury Widely used |
Difficult to perform in the very young pup Variability in phenotype Unilateral injury |
| Chronic intermittent hypoxia | Mouse, rat | Neuronal and OLC death | Diffuse moderate to severe injury | Severe injury |
| Chronic hypoxia | Mouse, rat | Diffuse gray and white matter injury Ventriculomegaly Moderate neuronal cell death and altered white matter development |
Mild injury Global diffuse injury similar to that in most very preterm children Cognitive deficits and hyperactivity |
Mild injury Lack of gliosis |
| LPS-induced inflammation | Mouse, rat, rabbit, sheep | Diffuse gliosis Microglial activation Neuronal and OLC death |
Inflammatory insult to the developing brain HI can be added to increase degree of injury |
Type of induction (LPS) is not similar to human (infection) |
| Interleukin-1β | Mouse | Systemic inflammation that results in hypomyelination | Impaired OLC development Axonal damage |
No increased cell death No astrogliosis |
| Premature delivery | Baboon | Diffuse gliosis, subarachnoid hemorrhage, gray and white matter injury; microcystic PVL, moderate ventriculomegaly | Baboons are delivered prematurely and treated similarly to infants | Difficulty in performing behavioral testing Cost |
HI, hypoxia-ischemia; LPS, lipopolysaccharide; PVL, periventricular leukomalacia.
Although substantially more time-consuming and expensive than rodent models, global ischemia models in preterm fetal sheep provide opportunities for more thorough and integrated analysis of the complex pathophysiological events that contribute to injury in the premature human brain during critical periods in development. This is because large animal brains more faithfully recapitulate developmental events that occur in humans17. The fetal sheep model, in which global ischemia is induced at a gestational time point roughly corresponding to the third trimester in humans, results in global brain volume loss and necrotic and non-necrotic white matter injury characterized by reactive astrogliosis and/or reactive microglia and macrophages17.
Perturbed maturation of interneurons in models of prematurity
Unlike the focal and diffuse necrosis induced by hypoxia-ischemia, chronic postnatal hypoxia induces a general loss of cortical and hippocampal gray and white matter volume in the absence of necrosis and detectable neuronal cell death. The mechanism by which this massive volume loss occurs has been rather mysterious, and even more puzzling is how the recovery of this volume can ensue. Neurons are mostly generated in the first part of gestation in humans and during embryogenesis in rodents; hence, perturbations induced by prematurity in humans and by perinatal hypoxic injury in rodent models are unlikely to affect neurogenesis. Loss of neurons due to apoptosis may explain in part the decrease in gray matter volumes of cortex and basal ganglia in both human and mice; however, it would be necessary to invoke massive cell genesis to explain the subsequent recovery. Indeed, there is an increase in both cortical and hippocampal neurogenesis from astroglial progenitors in the period of recovery from hypoxia. Unlike astroglia residing in the dentate gyrus or subventricular zone (SVZ), which maintain stem cell–like potential throughout life, astroglial cells in the cortex and hippocampus rapidly lose pluripotency in the early postnatal period; by 2 weeks after birth, cortical astroglial cells are no longer able to give rise to self-renewing neural stem cells, indicating their progressive maturation to protoplasmic astrocytes18. In contrast, cortical astroglial cells in 2-week-old mice reared under hypoxic conditions from P3 to P11 are able to generate neurons and OLCs both in vitro and in vivo18.
Although striking, the extent of cortical neurogenesis in hypoxically reared mice is relatively small and unlikely to entirely explain the recovery of cortical gray matter volume that is observed. Other contributing mechanisms are likely to be increased growth of axons, dendrites and synaptic connections. Although dendritic growth and pruning has not been yet examined in rodent models of hypoxia, it has been recently demonstrated that the cortical volume loss in the sheep model of ischemia is associated with impaired expansion of dendritic arbors and reduced synaptic density of cortical pyramidal cells and that the abnormal growth and orientation of the apical dendrites of pyramidal cells can explain the decrease in cortical functional anisotropy observed in these animals19. This is suggestive of a neuronal maturation defect in both hypoxia and ischemia animal models of prematurity.
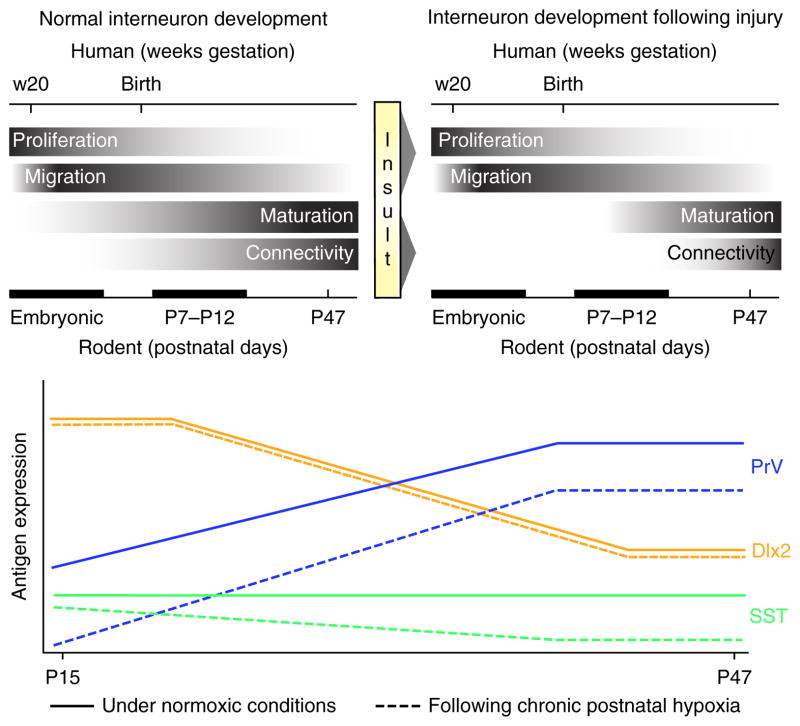
In addition to a loss of volume, a 25–30% decrease in cortical parvalbumin (PrV)- and somatostatin (SST)-positive GABAergic inhibitory neurons in the cerebral cortex of adult mice reared under hypoxia has been noted, which could be due to either a delay in maturation of these cells or to cell death (Fig. 1)20. However, no evidence was found of increased GABA interneuron cell death following hypoxia. Furthermore, experiments using a transgenic mouse line expressing GFP under the Gad1 promoter, which allows visualization of all GABAergic cells regardless of stage of maturation or subtype, found no differences between normoxically and hypoxically reared mice in the total numbers of all GFP+ interneurons, but rather a decrease in the subsets of GABAergic cells that express PrV and SST20.
Figure 1.
Chronic perinatal hypoxia causes a delay in maturation of interneurons. Top, relative time course of normal interneuron development (left) in the brains of both humans and rodents. Exposure to hypoxic injury (right) during the early postnatal period does not affect interneuron proliferation or migration but induces a delay of interneuron maturation and connectivity. Bottom, developmental pattern of interneuron antigen expression for parvalbumin (PrV), somatostatin (SST) and distal-less homeobox 2 (Dlx2) both under normal conditions (solid lines) and following hypoxic injury (dashed lines). Immature interneuron markers such as Dlx2 are unaffected by injury at this stage of development, but proteins upregulated with maturation such as PrV are vulnerable and show long-lasting perturbations in expression levels. For more details, see ref. 20.
Because there are no changes in interneuron genesis or apoptosis in hypoxic mice, at least at the time points examined, we propose that decreases in PrV and SST protein expression are due to perturbed maturation of these interneuron subtypes. Under normal conditions in mice, expression of PrV protein begins to be detectable toward the end of the second postnatal week and is contingent on excitatory input and neurotrophin signaling21. In adulthood, PrV+ and SST+ interneurons form a dense inhibitory network of synapses on adjacent pyramidal cells22,23. PrV+ neuron–mediated regulation of cortical excitability improves the efficiency of cortical information processing; firing of these neurons generates gamma oscillations, which are disrupted in several neuropsychiatric disorders24,25. In mice, a decreased number of hippocampal PrV+ interneurons correlates with the degree of deficit in spatial memory in the Morris water maze task26. Hence, the lasting decrease in PrV and SST expression and therefore dampened inhibitory neurotransmission may be responsible at least in part for the deficits in learning and spatial memory observed after hypoxic insults13.
In humans, premature birth is associated with increased incidence of seizure disorders, which has been related to decreases in cortical GABA content (for a review, see ref. 27). Interestingly, animals that are deficient in PrV neurons show increased seizures activity28. Therefore, similar differences may also occur in this model of chronic hypoxia, although this remains to be demonstrated. Nevertheless, these findings provide exciting opportunities for therapeutic interventions because we can use these mice to select pharmacotherapeutic compounds that increase GABAergic cell differentiation and maturation. Discovering compounds that enhance the maturation of GABAergic neurons in the postnatal period presents therapeutic opportunities for a variety of disorders that have shown GABAergic neuron dysregulation, including schizophrenia and perhaps autism.
Perturbed astrocyte function and aberrant myelination
In contrast to neurogenesis, gliogenesis occurs mostly during the first and second postnatal weeks in rodents and during the last part of gestation in humans. Thus, the ongoing genesis and initial maturation of these cells coincides with the timing of injury in mouse models of premature brain damage and in children born prematurely. Moreover, because this is a critical time for gliogenesis, recovery from premature brain injury also critically involves glial function. Indeed, we have found that glia, both astrocytes and OLCs, are integral to the recovery from hypoxic injury (see below). Furthermore, results from different laboratories provide converging evidence that supports the hypothesis that, as for neurons, a global delay in glial cell maturation is also observed after premature brain injury.
Astrocyte maturation is delayed by perinatal hypoxia, as demonstrated by decreased GFAP and increased nestin expression, which suggests a more immature phenotype29. Furthermore, astrocyte function is also compromised, as glial-specific glutamate transporter expression and function are reduced after hypoxia29, pointing to defective glutamate clearance and increased extracellular levels of the amino acid as likely contributors to excitotoxic death of OLCs. Conversely, another function of astrocytes that is thought to be a protective reaction to injury, reactive gliosis, was revealed in a mouse model of lipopolysaccharide-induced neonatal white matter injury and in human neonatal tissue. Activated STAT-3 signaling in astrocytes prevents TGFβ-1 production by microglial cells, which in turn inhibits OLC maturation30. Deletion of STAT-3 in astrocytes enhances TGFβ-1 in microglia and exacerbates white matter injury by further delaying OLC maturation30. In summary, astrocytes appear to play multiple roles depending on the type of injury and specific cellular interactions with other glial cell types.
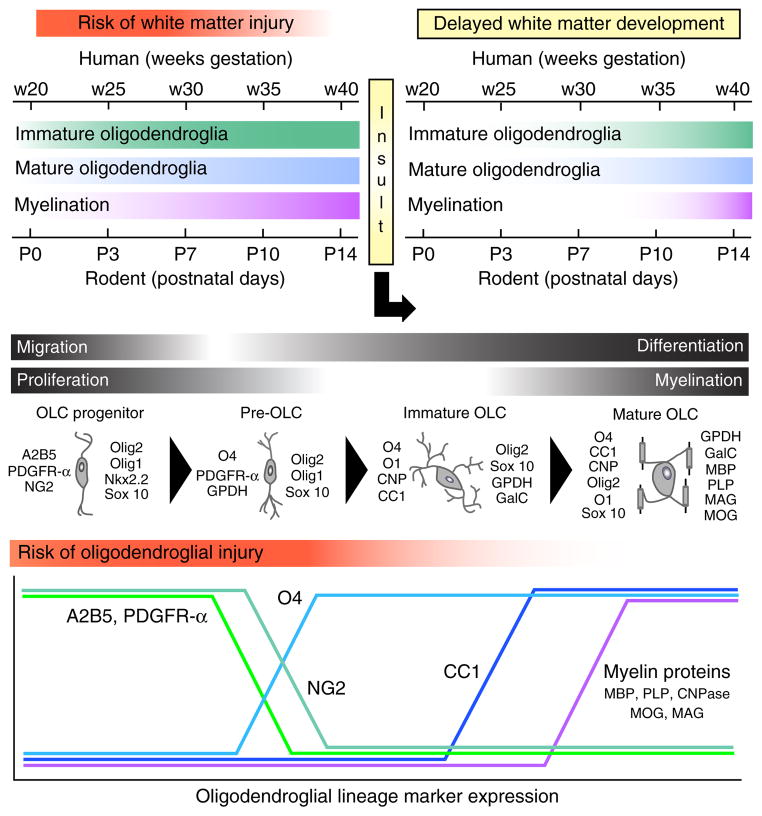
In the postnatal brain, OLCs originate from progenitor cells (OPCs) located in the parenchyma and in the SVZ. In rodents, these cells proliferate, migrate and differentiate during the first 2 postnatal weeks, whereas in humans they mostly develop between 23 and 37 weeks of gestation (Fig. 2). Previous studies in human tissue have associated OLC susceptibility with pre-OLCs or immature OLCs that are present in large numbers between 23 and 32 weeks of postconceptional age31. However, more recent analysis in animal models and in human tissue has revealed multiple cellular and molecular mechanisms underlying the effects of premature brain injury on OLCs. These include (i) induction of pre-OLC or OLC death that occurs during early phases of the injury32, (ii) a parallel robust regenerative or proliferative response of OPCs in white matter and SVZ during early phases of the injury, (iii) delayed or arrested OLC maturation31,33, and (iv) delayed myelination33 (Fig. 2).
Figure 2.
Perinatal white matter injury causes delays in OLC maturation and in developmental myelination. Top, time course of oligodendroglia development and myelination in the human and rodent brain identifies a specific time window for risk of white matter injury. A perinatal insult causes a delay in OLC maturation and myelination. Middle, OLC lineage progression under normal physiological conditions. Antigenic markers identify distinct stages of OLC maturation from the OLC progenitor to the mature, myelinating OLC stage. OLC progenitors and pre-OLCs are most abundant during the developmental time window corresponding to high risk of white matter injury. Bottom, developmental regulation of major developmental markers at different stages of OLC lineage progression. Markers: PDGFR-α, platelet-derived growth factor receptor-α; A2B5, a cell surface ganglioside epitope expressed on oligodendrocyte progenitors; NG2, neuron-glial antigen 2; Olig1, oligodendrocyte transcription factor 1; Olig2, oligodendrocyte transcription factor 2; GPDH, glycerol-3-phosphate dehydrogenase; O1, oligodendrocyte antibody 1, a mature oligodendrocyte marker; O4, oligodendrocyte antibody 4, an immature oligodendrocyte marker; CNP, cyclic nucleotide phosphodiesterase; CC1, antibody that detects the APC (adenomatous polyposis coli) protein; Sox10, SRY-related HMG-box 10 transcription factor; GalC, galactocerebroside; MBP, myelin basic protein; PLP, proteolipid protein; MAG, myelin-associated glycoprotein; MOG, myelin oligodendrocyte glycoprotein.
What causes pre-OLC and OLC death in premature-birth white matter injury? Several factors appear to be involved, including excito-toxicity and oxidative stress32. In animal models of hypoxia-ischemia, both apoptotic and excitotoxic cell death of immature OLCs have been observed34. Immature OLCs are particularly susceptible to oxidative stress and free-radical mediated injury32, and, in postmortem brain tissue from human premature infants, apoptosis was detected in the OLC lineage35. A still-open issue is whether death of mature OLCs occurs after perinatal hypoxia and prematurity or whether cell loss is limited to immature OLCs.
In mice exposed to chronic hypoxia, OLCs regenerate and myelin proteins return to normal levels within a few weeks after the injury, but myelin structure, as detected by electron microscopy, is abnormal33. Altogether, these results point to a delay in maturation of the OLC lineage during a critical developmental time window as a major cause of white matter dysfunction in premature brain injury. Future studies should test the hypothesis that delayed OLC maturation caused by hypoxia occurs because of a cellular environment that is no longer conducive to proper functional myelination. As specific cellular interactions (OLCs-axons and OLCs-astrocytes) are crucial for functional myelination, it is possible that developmentally regulated changes in electrical activity of subcortical white matter axons might contribute to the delayed and abnormal myelination after hypoxia.
These findings raise important questions about the molecular mechanisms underlying the effects of hypoxia on OLC development. On the basis of the hypothesis that at least some of the effects of hypoxia on delayed OLC differentiation and increased OPC proliferation could be related, cell cycle regulatory mechanisms were examined, in particular regulators of G1–S phase transition. Major changes in the Cdk2-p27Kip1 pathway were found in white matter OLCs after hypoxia: in particular, an activation of Cdk2 in OPCs, which promotes their proliferation, as well as a transient reduction in p27Kip1 during phases of delayed differentiation33. Consistent with these findings, reduced p27Kip1 was also observed in subcortical white matter of preterm neonates with hypoxic-ischemic encephalopathy33. Finally, loss- and gain-of-function analysis also demonstrated direct involvement of Cdk2 and p27Kip1 in the delay of OLC maturation observed after hypoxia33. Both Cdk2 and p27Kip1 are crucial in timing OLC development under normal physiological conditions (for a review, see ref. 36). These results, together with studies from other laboratories37,38, indicate that inappropriate reactivation of developmental mechanisms is likely involved in the delayed OLC maturation observed in neonatal brain injury.
Another mechanism likely to be crucial in preventing OLC differentiation after premature-birth brain injury involves OPC-astrocyte interactions, particularly reactive astrocyte–derived high-molecular-weight hyaluronic acid39. Both this proteoglycan and its putative receptor CD44 are highly expressed in diffuse white matter injury31, and a specific hyaluronidase, PH20, has been identified that digest hyaluronic acid to generate bioactive fragments that actively inhibit OLC differentiation and myelination40.
Further analysis in animal models having white matter structures that more closely resemble their human counterparts and more extensive analysis of human tissue will provide crucial information about selective sensitivities of different stages of the OLC lineage to premature white matter injury. Finally, improved functional imaging techniques will allow more detailed analysis of developmental myelination under pathological conditions in the human brain and will define the relevance of animal models to premature injury in infants.
Potential interventions
The cellular findings described above demonstrate the considerable complexity of gray and white matter injury observed in the premature brain and identify a need for global interventions that involve not only preventing cell death but also resynchronizing neuronal and glial developmental programs to promote timely establishment of neuronal maturation and connectivity (particularly for inhibitory neurons), as well as functional myelination. Although it is most likely that no single approach will prevent all neurocognitive difficulties and promote repair at multiple levels, findings in premature infants and in animal models point to some avenues that deserve further exploration.
Results obtained in premature infants indicate that the environment affects plasticity and recovery after neonatal brain injury. Two of the strongest predictors of long-term neurological outcome of VLBW infants are level of maternal education and the presence of a two-parent household, even when controlling for variables such as income. The fact that these factors mediate long-term outcome suggests that there are environmental moderators of recovery; hence, knowledge of the underlying mechanisms may lead to new therapeutics. Studies have tried to mimic these environmental factors by providing a stimulating environment to mice following hypoxic injury. Results showed that even only 2 weeks of enrichment reverses deficits in learning and memory observed in hypoxically reared mice41. Moreover, hypoxic mice reared under enriched conditions show increased levels of PrV and SST protein expression that are similar to those of normoxic controls by the time they reach early adulthood20. Hence, the behavioral improvement observed after environmental enrichment41 might in part be mediated by normalization of interneuron neurochemical properties.
We hypothesize that enrichment may support or even accelerate maturation processes and therefore act to reverse delayed maturation observed in central cell types. Certainly, in addition to increased cell proliferation and survival, environmental enrichment has been demonstrated to increase angiogenesis, dendritic complexity and synaptic connectivity and to increase maturation in other neural systems, including the visual system42. Furthermore, enrichment has been demonstrated to upregulate several trophic factors, among them BDNF and its receptor TrkB, which are crucial in the postnatal maturation of PV+ interneurons21. Research focused on elucidating the molecular mechanisms that induce these factors under enriched conditions and experimental manipulations to understand their specific and causal role in the beneficial effects of enrichment during functional recovery are necessary and will undoubtedly reveal potential therapeutic avenues for a myriad of injuries. Unveiling these mechanisms is particularly crucial because, in spite of the important beneficial effects of environmental enrichment, the cost, feasibility and labor intensiveness of enrichment interventions in premature infants is challenging. Therefore, the discovery of key molecules that could potentially be targeted for pharmacotherapy of premature brain injury is imperative.
Exploratory studies have already led to the identification of other promising therapeutic avenues, whose interaction and potential synergism with environmental enrichment is not yet known. Wnt–β-catenin signaling is important in OLC development and regeneration37. Axin 2 is an essential element of this pathway, is highly expressed in OPCs and negatively regulates the Wnt signaling pathway by promoting β-catenin degradation. Fancy et al. (2011) demonstrated that Axin2 functional stability is essential for timely myelination after hypoxic injury. Treatment with a small-molecule inhibitor (XAV939) that targets the enzyme tankyrase to stabilize Axin2 levels promotes oligodendrogenesis and myelination, indicating that small compounds that easily cross the blood-brain barrier could be incorporated into future therapeutic approaches aimed at promoting premature brain injury. Small compounds could also be designed to target PH20 hyaluronidase and prevent hyaluronic acid breakdown, and/or activate the p27Kip1 pathway to promote OLC maturation.
There is also evidence for the importance of fibroblast growth factors (FGF) for stimulating the recovery of neuron number and gray matter volume after hypoxic injury. FGF2 is a growth factor involved in neurogenesis and gliogenesis both during embryonic and postnatal development43. FGF2 is normally upregulated in parenchymal astrocytes in the first 3 weeks after birth in rodents, and these GFAP+ cells express the FGF receptors Fgfr1, Fgfr2 and Fgfr3. The conditional knockout of Fgfr1 or Fgfr2 in GFAP+ cells per se produces a 30% loss of cortical PrV GABAergic interneurons44, possibly owing to abnormal astrocyte-to-interneuron signaling. Furthermore, the expression of FGF2 and Fgfr1 is increased in mice recovering from chronic hypoxia45, and the deletion of Fgfr1 in GFAP+ cells precludes the recovery of cortical volume and cortical excitatory neurons and worsens the decrease in PV+ interneurons in hypoxic mice46. Given this evidence, we hypothesize that FGF signaling in astroglial cells is sufficient to stimulate the recovery of excitatory neurons but insufficient to boost the deficient maturation of inhibitory neurons.
Future directions
Analysis in animal models of premature brain injury has been critical in beginning to identify specific cellular and functional abnormalities in gray and white matter regions of the brain. Using a model of chronic neonatal hypoxia has allowed the identification of different phases of the injury and the characterization of the progenitor and stem cells involved in neuronal and glial regeneration after injury. Overall, these findings are consistent with those obtained from other animal models and support the notion that premature brain injury dysregulates neuronal and glial developmental programs, resulting in delays and abnormalities in functional maturation of different brain regions.
It is important to emphasize that this analysis has not only identified the cellular basis for delayed maturation, but has also defined critical periods of intervention—based on temporal windows of prolonged neural plasticity—to promote cellular, structural and functional recovery. Characterization of specific signaling pathways activated during these critical periods will also help in identifying cellular and molecular targets that can be manipulated for therapeutic purposes. Furthermore, manipulation of the environment during brain development to provide an enriched experience appears to be a viable approach, either by itself or in combination with cellular and pharmacological manipulations to maximize functional recovery after neonatal brain injury.
In light of the complexity of neonatal brain injuries, future approaches to promote regeneration and functional recovery will have to be tested in different animal models that more closely resemble the human brain. For example, producing white matter injury in rodents has been challenging because they have less white matter than species with larger brains, and the distribution of the white matter differs from that of larger mammals because of the lissencephalic nature of the rodent brain. Both sheep and baboons have been used to model premature brain injury, and furthermore, we have recently extended analyses of developing white matter injury to early postnatal piglets, in which white matter development closely resembles that of its human counterpart47. The sheep and baboon models show many similarities to the clinical situation observed in premature infants, including enlarged ventricles, neuronal and glial death, and microglia activation in white matter. Although these larger animal models have several drawbacks, including high cost and very limited potential for the genetic manipulations that are routinely performed in rodents, they allow cell dynamics studies that are not feasible in the developing human brain and better physiologic control and monitoring of several variables, including cerebral blood flow. Therefore, they might represent better testing grounds for human drugs. In the future, information from different experimental designs and tissue analysis from distinct animal models will need to become more integrated to define feasible and successful therapeutic approaches that will combine molecular and cellular results with the more complex physiology and pathology of higher mammals. Finally, it will be essential to integrate findings from animal models with analysis in postmortem human tissue. For this purpose, banking brain tissue that is representative of the broad spectrum of injuries in premature neonates is a high priority.
The issues associated with the use of human tissue could be at least in part overcome by exploiting cellular models that utilize human neural progenitor/stem cells or inducible pluripotent stem cell (iPSC) technology. Although neuronal and glial progenitors are attractive cellular elements for developing cell replacement strategies after neonatal brain injury, it has been very difficult to obtain homogeneous cell populations capable of generating neurons or myelinating glia suitable for clinical use. Nevertheless, preclinical studies have shown that human progenitors can be (i) widely dispersed after grafting in the host brain, (ii) able survive at a high density after transplantation and (iii) able to properly myelinate host axons to promote functional recovery48. The use of human cord blood cells and their potential to repair neonatal hypoxic-ischemic brain injury has recently been investigated in both preclinical studies and clinical trials49. The main advantages of iPSCs with respect to other sources of human precursor cells is that they are an unlimited source of isogenic cells that would not be subjected to rejection. Very recent studies are encouraging in that they have suggested that neuronal precursors derived from human iPSCs can populate the cerebral cortex and the basal ganglia and can ameliorate symptoms in a rodent model of Huntington disease50. Hence, there is hope that iPSCs can be used therapeutically in neonatal hypoxic injury.
Acknowledgments
This research has been supported by the US National Institutes of Health NINDS (P01 NS062686 and R01 NS060750 to F.M.V. and R01NS045702 to V.G.), NICHD (P30HD040677 to V.G.) and NIA (R21 AG034495 to FMV), and by grants from the Brain and Behavior Research Foundation (F.M.V.), the state of Connecticut (F.M.V.), Women’s Health Research at Yale (F.M.V.) and the Cerebral Palsy International Research Foundation (V.G.). N.S. is recipient of a Canadian Institute of Health Research fellowship. J.S. is the recipient of K08NS073793 (NINDS). We are particularly grateful to L. Ment for support and discussion. We thank J. Ritter for help with figures.
Footnotes
Reprints and permissions information is available online at http://www.nature.com/reprints/index.html.
COMPETING FINANCIAL INTERESTS
The authors declare no competing financial interests.
References
- 1.Anderson P, Doyle LW Victorian Infant Collaborative Study Group. Neurobehavioral outcomes of school-age children born extremely low birth weight or very preterm in the 1990s. J Am Med Assoc. 2003;289:3264–3272. doi: 10.1001/jama.289.24.3264. [DOI] [PubMed] [Google Scholar]
- 2.Johnson S, et al. Psychiatric disorders in extremely preterm children: longitudinal finding at age 11 years in the EPICure study. J Am Acad Child Adolesc Psychiatry. 2010;49:453–463. [PubMed] [Google Scholar]
- 3.Luu TM, et al. Evidence for catch-up in cognition and receptive vocabulary among adolescents born very preterm. Pediatrics. 2011;128:313–322. doi: 10.1542/peds.2010-2655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ment LR, et al. Change in cognitive function over time in very low-birth-weight infants. J Am Med Assoc. 2003;289:705–711. doi: 10.1001/jama.289.6.705. [DOI] [PubMed] [Google Scholar]
- 5.Myers EH, et al. Functional connectivity to a right hemisphere language center in prematurely born adolescents. Neuroimage. 2010;51:1445–1452. doi: 10.1016/j.neuroimage.2010.03.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ball G, et al. The effect of preterm birth on thalamic and cortical development. Cereb Cortex. 2012;22:1016–1024. doi: 10.1093/cercor/bhr176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Inder TE, et al. Periventricular white matter injury in the premature infant is followed by reduced cerebral cortical gray matter volume at term. Ann Neurol. 1999;46:755–760. doi: 10.1002/1531-8249(199911)46:5<755::aid-ana11>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 8.Eikenes L, et al. Young adults born preterm with very low birth weight demonstrate widespread white matter alterations on brain DTI. Neuroimage. 2011;54:1774–1785. doi: 10.1016/j.neuroimage.2010.10.037. [DOI] [PubMed] [Google Scholar]
- 9.Beauchamp MH, et al. Preterm infant hippocampal volumes correlate with later working memory deficits. Brain. 2008;131:2986–2994. doi: 10.1093/brain/awn227. [DOI] [PubMed] [Google Scholar]
- 10.Brown NC, et al. Neurobehavior at term and white and gray matter abnormalities in very preterm infants. J Pediatr. 2009;155:32–38. doi: 10.1016/j.jpeds.2009.01.038. [DOI] [PubMed] [Google Scholar]
- 11.Volpe JJ. Cerebral white matter injury of the premature infant-more common than you think. Pediatrics. 2003;112:176–180. doi: 10.1542/peds.112.1.176. [DOI] [PubMed] [Google Scholar]
- 12.Scafidi J, et al. Modeling premature brain injury and recovery. Int J Dev Neurosci. 2009;27:863–871. doi: 10.1016/j.ijdevneu.2009.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Salmaso N, et al. Environmental enrichment increases the GFAP+ stem cell pool and reverses hypoxia-induced cognitive deficits in juvenile mice. J Neurosci. 2012;32:8930–8939. doi: 10.1523/JNEUROSCI.1398-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Vannucci RC, Vannucci SJ. Perinatal hypoxic-ischemic brain damage: evolution of an animal model. Dev Neurosci. 2005;27:81–86. doi: 10.1159/000085978. [DOI] [PubMed] [Google Scholar]
- 15.Cengiz P, et al. Chronic neurological deficits in mice after perinatal hypoxia and ischemia correlate with hemispheric tissue loss and white matter injury detected by MRI. Dev Neurosci. 2011;33:270–279. doi: 10.1159/000328430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fan LW, et al. Hypoxia-ischemia induced neurological dysfunction and brain injury in the neonatal rat. Behav Brain Res. 2005;165:80–90. doi: 10.1016/j.bbr.2005.06.033. [DOI] [PubMed] [Google Scholar]
- 17.Back SA, et al. The instrumented fetal sheep as a model of cerebral white matter injury in the premature infant. Neurotherapeutics. 2012;9:359–370. doi: 10.1007/s13311-012-0108-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bi B, et al. Cortical glial fibrillary acidic protein-positive cells generate neurons after perinatal hypoxic injury. J Neurosci. 2011;31:9205–9221. doi: 10.1523/JNEUROSCI.0518-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean JM, et al. Prenatal cerebral ischemia disrupts MRI-defined cortical microstructure through disturbances in neuronal arborization. Sci Transl Med. 2013;5:168ra7. doi: 10.1126/scitranslmed.3004669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Komitova M, et al. Hypoxia-induced developmental delays of inhibitory interneurons are reversed by environmental enrichment in the postnatal mouse forebrain. J Neurosci. 2013;33:13375–13387. doi: 10.1523/JNEUROSCI.5286-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Patz S, et al. Parvalbumin expression in visual cortical interneurons depends on neuronal activity and TrkB ligands during an early period of postnatal development. Cereb Cortex. 2004;14:342–351. doi: 10.1093/cercor/bhg132. [DOI] [PubMed] [Google Scholar]
- 22.Packer AM, Yuste R. Dense, unspecific connectivity of neocortical parvalbumin-positive interneurons: a canonical microcircuit for inhibition? J Neurosci. 2011;31:13260–13271. doi: 10.1523/JNEUROSCI.3131-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fino E, Yuste R. Dense inhibitory connectivity in neocortex. Neuron. 2011;69:1188–1203. doi: 10.1016/j.neuron.2011.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartos M, Vida I, Jonas P. Synaptic mechanisms of synchronized gamma oscillations in inhibitory interneuron networks. Nat Rev Neurosci. 2007;8:45–56. doi: 10.1038/nrn2044. [DOI] [PubMed] [Google Scholar]
- 25.Sohal VS, et al. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature. 2009;459:698–702. doi: 10.1038/nature07991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stevens HE, et al. Learning and memory depend on fibroblast growth factor receptor 2 functioning in hippocampus. Biol Psychiatry. 2012;71:1090–1098. doi: 10.1016/j.biopsych.2012.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jensen FE. Neonatal seizures: an update on mechanisms and management. Clin Perinatol vii. 2009;36:881–900. doi: 10.1016/j.clp.2009.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schwaller B, et al. Parvalbumin deficiency affects network properties resulting in increased susceptibility to epileptic seizures. Mol Cell Neurosci. 2004;25:650–663. doi: 10.1016/j.mcn.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 29.Raymond M, et al. Chronic perinatal hypoxia reduces glutamate-aspartate transporter function in astrocytes through the Janus kinase/signal transducer and activator of transcription pathway. J Neurosci. 2011;31:17864–17871. doi: 10.1523/JNEUROSCI.3179-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nobuta H, et al. STAT3-mediated astrogliosis protects myelin development in neonatal brain injury. Ann Neurol. 2012;72:750–765. doi: 10.1002/ana.23670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buser JR, et al. Arrested preoligodendrocyte maturation contributes to myelination failure in premature infants. Ann Neurol. 2012;71:93–109. doi: 10.1002/ana.22627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Back SA, et al. Maturation-dependent vulnerability of oligodendrocytes to oxidative stress-induced death caused by glutathione depletion. J Neurosci. 1998;18:6241–6253. doi: 10.1523/JNEUROSCI.18-16-06241.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jablonska B, et al. Oligodendrocyte regeneration after neonatal hypoxia requires FoxO1-mediated p27Kip1 expression. J Neurosci. 2012;32:14775–14793. doi: 10.1523/JNEUROSCI.2060-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ness JK, et al. Perinatal hypoxia-ischemia induces apoptotic and excitotoxic death of periventricular white matter oligodendrocyte progenitors. Dev Neurosci. 2001;23:203–208. doi: 10.1159/000046144. [DOI] [PubMed] [Google Scholar]
- 35.Back SA, et al. Selective vulnerability of late oligodendrocyte progenitors to hypoxia-ischemia. J Neurosci. 2002;22:455–463. doi: 10.1523/JNEUROSCI.22-02-00455.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Nguyen L, et al. The Yin and Yang of cell cycle progression and differentiation in the oligodendroglial lineage. Ment Retard Dev Disabil Res Rev. 2006;12:85–96. doi: 10.1002/mrdd.20103. [DOI] [PubMed] [Google Scholar]
- 37.Fancy SP, et al. Axin2 as a regulatory and therapeutic target in newborn brain injury and remyelination. Nat Neurosci. 2011;14:1009–1016. doi: 10.1038/nn.2855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reid MV, et al. Delayed myelination in an intrauterine growth retardation model is mediated by oxidative stress upregulating bone morphogenetic protein 4. J Neuropathol Exp Neurol. 2012;71:640–653. doi: 10.1097/NEN.0b013e31825cfa81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Back SA, et al. Hyaluronan accumulates in demyelinated lesions and inhibits oligodendrocyte progenitor maturation. Nat Med. 2005;11:966–972. doi: 10.1038/nm1279. [DOI] [PubMed] [Google Scholar]
- 40.Preston M, et al. Digestion products of the PH20 hyaluronidase inhibit remyelination. Ann Neurol. 2013;73:266–280. doi: 10.1002/ana.23788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Salmaso N, et al. Environmental enrichment increases the GFAP+ stem cell pool and reverses hypoxia-induced cognitive deficits in juvenile mice. J Neurosci. 2012;32:8930–8939. doi: 10.1523/JNEUROSCI.1398-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Baroncelli L, et al. Nurturing brain plasticity: impact of environmental enrichment. Cell Death Differ. 2010;17:1092–1103. doi: 10.1038/cdd.2009.193. [DOI] [PubMed] [Google Scholar]
- 43.Vaccarino FM, et al. Fibroblast growth factor signaling regulates growth and morphogenesis at multiple steps during brain development. In: Pedersen RA, Shatten G, editors. Current Topics in Developmental Biology. Academic; San Diego: 1999. pp. 179–200. [DOI] [PubMed] [Google Scholar]
- 44.Stevens HE, et al. Fgfr2 is required for the development of the medial prefrontal cortex and its connections with limbic circuits. J Neurosci. 2010;30:5590–5602. doi: 10.1523/JNEUROSCI.5837-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ganat Y, et al. Chronic hypoxia up-regulates fibroblast growth factor ligands in the perinatal brain and induces fibroblast growth factor-responsive radial glial cells in the sub-ependymal zone. Neuroscience. 2002;112:977–991. doi: 10.1016/s0306-4522(02)00060-x. [DOI] [PubMed] [Google Scholar]
- 46.Fagel DM, et al. Fgfr1 is required for cortical regeneration and repair after perinatal hypoxia. J Neurosci. 2009;29:1202–1211. doi: 10.1523/JNEUROSCI.4516-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ishibashi N, et al. White matter protection in congenital heart surgery. Circulation. 2012;125:859–871. doi: 10.1161/CIRCULATIONAHA.111.048215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Goldman SA, Nedergaard M, Windrem MS. Glial progenitor cell-based treatment and modeling of neurological disease. Science. 2012;338:491–495. doi: 10.1126/science.1218071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Carroll J. Human cord blood for the hypoxic-ischemic neonate. Pediatr Res. 2012;71:459–463. doi: 10.1038/pr.2011.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Jeon I, et al. Neuronal properties, in vivo effects, and pathology of a Huntington’s disease patient-derived induced pluripotent stem cells. Stem Cells. 2012;30:2054–2062. doi: 10.1002/stem.1135. [DOI] [PubMed] [Google Scholar]