Abstract
Objective
We sought to develop a list of 5 tests, treatments, or services commonly used in rheumatology practice whose necessity or value should be questioned and discussed by physicians and patients.
Methods
We used a multistage process combining consensus methodology and literature reviews to arrive at the American College of Rheumatology's (ACR) Top 5 list. Rheumatologists from diverse practice settings generated items using the Delphi method. Items with high content agreement and perceived high prevalence advanced to a survey of ACR members, who comprise >90% of the US rheumatology workforce. To increase the response rate, a nested random sample of 390 rheumatologists received more intensive survey followup. The samples were combined and weighting procedures were applied to ensure generalizability. Items with high ratings underwent literature review. Final items were then selected and formulated by the task force.
Results
One hundred five unique items were proposed and narrowed down to 22 items during the Delphi rounds. A total of 1,052 rheumatologists (17% of those contacted) participated in the member-wide survey, whereas 33% of those in the nested random sample participated; respondent characteristics were similar in both samples. Based on survey results and available scientific evidence, 5 items (relating to antinuclear antibodies, Lyme disease, magnetic resonance imaging, bone absorptiometry, and biologic therapy for rheumatoid arthritis) were selected for inclusion.
Conclusion
The ACR Top 5 list is intended to promote discussions between physicians and patients about health care practices in rheumatology whose use should be questioned and to assist rheumatologists in providing high-value care.
Keywords: The American College of Rheumatology is an independent, professional, medical and scientific society which does not guarantee, warrant, or endorse any commercial product or service
INTRODUCTION
As health care costs strain federal and state budgets and threaten the long-term fiscal health of the US, increasing attention is focused on health care quality, affordability, and value. Policymakers, payers, and health systems face considerable challenges in controlling health care spending while ensuring the highest quality care. Recently, the medical profession has been called on to more actively inform these efforts by drawing attention to potentially wasteful health care spending (1). In 2011, the American Board of Internal Medicine (ABIM) Foundation embarked on the Choosing Wisely campaign, inviting medical professional societies to construct a list of 5 tests, treatments, or services that are commonly used in that specialty but whose usefulness should be reevaluated by patients and clinicians (2).
At the core of these calls to action are the principles of professionalism laid out in the Physician Charter drafted by the ABIM Foundation, American College of Physicians, and European Federation of Internal Medicine in 2002, including patient welfare, patient autonomy, and social justice (3). The latter calls on the profession to promote a fair distribution of health care resources and to engage in collective efforts to improve the health care system for the welfare of society. The American College of Rheumatology (ACR) supports these principles and has partnered with the ABIM Foundation in the Choosing Wisely campaign to generate a Top 5 list. The campaign is focused on encouraging physicians and patients to openly discuss potential waste and harms from tests and procedures that have unclear value. Rather than being a prescriptive set of rules, the Top 5 lists are meant to leave room for clinical judgment. This aspect is particularly important in rheumatology, a cognitive specialty that encompasses a wide range of complex and often poorly understood chronic diseases.
Here we present the research methods used to derive the ACR Top 5 list. To ensure the relevance of the list to current rheumatology practice, we used a multistage process that combined consensus and survey methods with reviews of the scientific evidence. Our methods reflect the fact that the overriding goal of the project was to engage US rheumatologists in a meaningful discussion about high-value care.
MATERIALS AND METHODS
The ACR convened a task force of rheumatologists and methodologists to oversee development of the rheumatology Top 5 list. All members of the Top 5 task force disclosed potential conflicts of interest at the start of the project. The task force defined the following principles for the project: 1) ideas for the rheumatology Top 5 list should be derived from practicing rheumatologists based on their clinical experiences and observations in their communities and should reflect services directly relevant to the specialty, 2) the process should attempt to engage the entire US rheumatology workforce in order to begin a national conversation about this topic and to reach professional consensus, and 3) literature reviews should be conducted to evaluate the quality of scientific evidence supporting the items appearing on the list.
Using these defining principles as a framework, the task force outlined separate methodologic steps to address each of 3 phases of the project.
Phase 1: generation of ideas
The task force assembled an ad hoc group of practicing rheumatologists to generate a broad and comprehensive list of ideas for the Top 5 list using the Delphi method (4). Twenty practicing rheumatologists comprised this Core Member Group. Members, who were nominated by the task force, were derived from diverse practice environments, including solo and small-group single-specialty practices, multispecialty groups, and academic or private health systems, and were geographically dispersed across the US. The group was also balanced for age and sex to reflect the US rheumatology workforce.
We used the Delphi method to generate ideas for the project for several reasons. The method accommodates larger groups like the one assembled for this project and provides a means to combine opinions from experts who are geographically dispersed. In addition, because respondents remain anonymous to one another, participants can voice opinions without the influence of personal status or embarrassment.
Delphi survey round 1
In the first-round Delphi survey, individuals in the Core Member Group were asked to generate items. Participants were provided examples from phase 1 of the ABIM Foundation's Choosing Wisely campaign (online at http://choosingwisely.org) and instructed to suggest items that met the following criteria: 1) commonly ordered or provided by rheumatologists, 2) among the most expensive services ordered or provided, and 3) shown by currently available evidence not to provide meaningful benefit to at least some groups for whom the test or treatment is provided. Space was provided to include a qualitative justification for the item and also to list supporting guidelines or other evidence.
Responses to the survey were then organized by the task force into themes and eventually into statements with a uniform structure (“Do not perform . . .”). These statements were then used to create a web-based survey for the second round.
Delphi survey round 2
The Core Member Group was instructed to rate their agreement with the content of each of the items derived from round 1 on a 5-point Likert scale (anchored from “strongly agree” to “strongly disagree”), and also to rate the prevalence of the particular item in their community on a 5-point Likert scale (anchored from “extremely prevalent” to “never occurs”). In addition, participants ranked the 10 items they thought would have the highest impact in terms of curbing wasteful health care spending in rheumatology. Finally, comments, suggestions for revision on each item, and ideas for additional items were sought.
Data were aggregated and items with the following characteristics were retained for the next round: 1) high content agreement (≥70% agreed) AND either 2) high prevalence rating (≥50% of members rated as at least moderately prevalent in their communities) OR 3) high impact ranking (among the top 20) items. The task force eliminated items where reasonable concerns about scientific validity were raised and incorporated revisions to improve clarity and specificity. Remaining items were then used to construct the next survey round.
Delphi survey round 3
The purpose of the third-round survey was to allow participants the opportunity to view the group results and change their ratings and ranks in light of their colleagues’ responses. Anonymous summary statistics from round 2 were, therefore, sent along with the survey. As in the previous round, participants rated items based on agreement with content and prevalence and also ranked the 10 items they thought had the highest impact in terms of curbing wasteful health care spending. Additional comments, suggestions, or revisions were sought.
Statements with 1) high content agreement (≥80% agreed) AND either 2) high prevalence rating (≥50% of members rated as at least moderately prevalent) OR 3) high impact ranking (among the top 20) were retained. A few statements with similar content were combined and additional revisions for clarity were made.
Phase 2: ACR member engagement
ACR member survey. An important goal of this project was to engage rheumatologists in the discussion regarding physician stew-ardship of health care resources. Therefore, we solicited anonymous feedback on candidate items from the entire US ACR membership. An e-mail with a link to an online survey with items for the Top 5 list was sent to all ACR members (n = 6,188). To ensure that a low response rate did not threaten the generalizability of our results, we also pursued a nested random sample of 390 ACR members that reflected the demographics of the ACR membership. This group received 3 e-mail reminders rather than one.
ACR members were asked to rate their agreement with the content of each item (5-point Likert scale anchored from “strongly agree” to “strongly disagree”), to rate whether they believed the item was “high impact” (yes/no based on its prevalence, cost, or potential to reduce patient harm), and to rank the 2 items they believed were the best candidates for the Top 5 list. In addition, comments were sought on individual items and on the campaign in general.
Quantitative survey analysis
We compared member-wide survey responders to nested random sample responders on the following characteristics: age, sex, geographic location, and whether the majority of time was spent in patient care (yes/no). Because there were no significant differences in any of these key characteristics between the 2 samples and they were drawn from the same underlying population, the samples were combined.
Next, we compared all survey responders to survey nonresponders on the characteristics listed above. Because there were statistically significant differences between the 2 groups, we used inverse probability weights to make the results more representative of the entire ACR membership.
In the combined weighted and unweighted samples, we then analyzed the survey responses. Items that had the highest combined rank in each of 3 categories assessed (content agreement, impact, and rank) were selected.
Qualitative survey analysis
We used the comments submitted by ACR members in 2 ways. First, we incorporated substantive comments into revisions of the existing statements. Next, we performed a formal qualitative analysis to identify major themes raised by respondents in the survey.
All of the comments were aggregated and reviewed by 3 investigators (GS, JB, JY) to identify major categories, which resulted in the identification of common themes. After discussion, a coding frame was developed and comments were reviewed for text units (phrases) corresponding to codes (5). All 3 investigators reviewed the comments to identify codes, and differences were reconciled by discussion. Emerging themes and subthemes were added to the initial themes until thematic saturation was reached, meaning no new themes were identified.
Phase 3: scientific evidence review
A group of academic rheumatologists (JY, GS, JB, MM, JZ, LSG) working with 2 rheumatology fellows (AC, VK) compiled evidence reports for each of the 10 candidate items that remained after the Delphi rounds and member surveys were complete. Each evidence report included the following: 1) reference to major guidelines or recommendations regarding the proposed item; 2) a summary of the relevant scientific evidence gathered through searches of the Cochrane Library, PubMed, and other sources, as well as a rating of the level of evidence using a modification of the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach proposed by the American College of Chest Physicians (6), which considers the strength of the recommendation and quality of the evidence; and 3) any available cost studies or information regarding the prevalence of inappropriate use of that test or procedure.
Phase 4: selection of final items
Task force review. The evidence reports were presented to the task force, who then selected the final Top 5 items based on the strength of the scientific evidence as well as the other relevant characteristics (i.e., item ratings in the membership surveys, adequate representation of diverse aspects of rheumatology practice, measurability, and potential impact).
Patient review
The selected items were then presented to a convenience sample of 6 patients with diverse rheumatic conditions for review and qualitative feedback.
ACR Board of Directors review
Finally, the Top 5 list was presented to and approved by the ACR Board of Directors. Suggestions made by the Board were incorporated into the final wording of items.
RESULTS
Selection of items
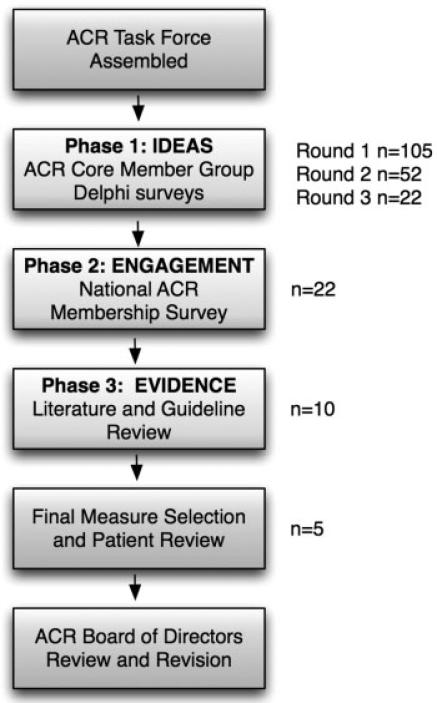
Figure 1 shows the use of a combination of consensus methods and scientific evidence review to narrow down the list of candidate items considered at each phase of the project.
Figure 1.
Flow diagram showing methods for developing the American College of Rheumatology (ACR) Top 5 list. n = candidate items for the Top 5 list considered at each phase of the project.
Core Member Group Delphi survey results
Participants in round 1 of the Delphi survey generated 105 unique candidate items for the ACR Top 5 list, covering a wide range of tests, procedures, and treatments used in rheumatology. The largest category of proposed items was regarding laboratory testing, followed by imaging and medication use. This broader list of items was narrowed down to 52 items for round 2 of the Delphi survey, and finally to 22 items in round 3. The final 22 items had high mean agreement (4.7 on the 5-point Likert scale) and were believed to be at least moderately prevalent by most respondents.
ACR member survey quantitative analysis results
Seventeen percent (n = 1,052) of ACR members who were contacted responded to the single e-mail member-wide survey. Thirty-three percent (n = 130) of those in the nested random sample (receiving 3 e-mail reminders) responded. The responders in these 2 samples were similar for all of the characteristics examined, including age, sex, census geographic region, and whether a majority of time was spent in patient care (univariate and multivariate odds of response were not statistically significant [P > 0.05] for all variables). Therefore, the samples were combined.
Responders differed from the general ACR membership. In multivariate logistic regression models, we found that the odds of response (yes/no) to the survey was higher for ACR members ages ≥50 years compared to those who were younger (odds ratio [OR] 1.4, 95% confidence interval [95% CI] 1.2–1.7), those who are men (OR 1.2, 95% CI 1.1–1.5), and those who spend a majority of their time in clinical practice (OR 1.4, 95% CI 1.2–1.7). Census geographic region was not statistically significant. We therefore built inverse probability weights based on age, sex, and time spent in clinical care. Applying these weights resulted in negligible changes to the agreement and impact ratings, suggesting that younger physicians, women, and those spending less time in patient care responded to the survey similarly to other groups. Because the weighted and unweighted results were the same, the unweighted responses are presented.
For the 22 candidate items, content agreement was very high (mean ± SD 4.5 ± 0.28 of 5, range 3.6–4.9). Impact ratings were also high for most items (a mean of 64% of items were thought to be high impact). The 10 items (Supplementary Table 1, available in the online version of this article at http://onlinelibrary.wiley.com/doi/10.1002/acr.21930/abstract) with the highest combined ranks for content agreement, rating, and top 2 rankings advanced to the literature review. Ratings for the final Top 5 items (Table 1) are shown in Table 2, as are the percentages of ACR members selecting these items as one of their top 2 picks.
Table 1.
The American College of Rheumatology’s Top 5 list*
| Do not test ANA subserologies without a positive ANA and clinical suspicion of immune-mediated disease. |
|---|
| Tests for ANA subserologies (including antibodies to double-stranded DNA, Sm, RNP, SSA, SSB, Scl-70, and centromere) are usually negative if the ANA is negative. Exceptions include anti–Jo-1, which can be positive in some forms of myositis, or occasionally, anti-SSA in the setting of lupus or Sjogren's syndrome. Broad testing of autoantibodies should be avoided; instead, the choice of autoantibodies should be guided by the specific disease under consideration. |
| Do not test for Lyme disease as a cause of musculoskeletal symptoms without an exposure history and appropriate examination findings. |
| The musculoskeletal manifestations of Lyme disease include brief attacks of arthralgia or intermittent or persistent episodes of arthritis in 1 or a few large joints at a time, especially the knee. Lyme testing in the absence of these features increases the likelihood of false-positive results and may lead to unnecessary followup and therapy. Diffuse arthralgias, myalgias, or fibromyalgia alone are not criteria for musculoskeletal Lyme disease. |
| Do not perform MRI of the peripheral joints to routinely monitor inflammatory arthritis. |
| Data evaluating MRI for the diagnosis and prognosis of RA are currently inadequate to justify widespread use of this technology for these purposes in clinical practice. Although bone edema assessed by MRI on a single occasion may be predictive of progression in certain RA populations, using MRI routinely is not cost effective compared with the current standard of care, which includes clinical disease activity assessments and plain film radiography. |
| Do not prescribe biologic agents for RA before a trial of methotrexate (or other conventional nonbiologic DMARD). |
| High-quality evidence suggests that methotrexate and other conventional nonbiologic DMARDs are effective in many patients with RA. Initial therapy for RA should be a conventional nonbiologic DMARD unless these are contraindicated. If a patient has had an inadequate response to methotrexate with or without other nonbiologic DMARDs during an initial 3-month trial, then biologic therapy can be considered. Exceptions include patients with high disease activity AND poor prognostic features (functional limitations, disease outside the joints, seropositivity, or bony damage), where biologic therapy may be appropriate first-line treatment. |
| Do not routinely repeat DXA scans more often than once every 2 years. |
| Initial screening for osteoporosis should be performed according to National Osteoporosis Foundation recommendations. The optimal interval for repeating DXA scans is uncertain, but because changes in bone density over short intervals are often smaller than the measurement error of most DXA scanners, frequent testing (e.g., <2 years) is unnecessary in most patients. Even in high-risk patients receiving drug therapy for osteoporosis, DXA changes do not always correlate with the probability of fracture. Therefore, DXA scans should only be repeated if the result will influence clinical management or if rapid changes in bone density are expected. Recent evidence also suggests that healthy women ages ≥67 years with normal bone mass may not need additional DXA testing for up to ten years provided osteoporosis risk factors do not significantly change. |
ANA = antinuclear antibody; MRI = magnetic resonance imaging; RA = rheumatoid arthritis; DMARD = disease-modifying antirheumatic drug; DXA = dual x-ray bone absorptiometry.
Table 2.
National survey results for the American College of Rheumatology's Top 5 list*
| Content agreement, raw mean ± SD (1–5 scale)† | Content disagreement, % who disagree‡ | Impact, % rating as high impact§ | Top picks, % ranking as 1st or 2nd choice | |
|---|---|---|---|---|
| ANA and subserology testing | 4.38 ± 1.00 | 9 | 66 | 15 |
| Lyme disease testing | 4.61 ± 0.77 | 4 | 71 | 11 |
| Routine MRI for monitoring arthritis | 4.39 ± 1.03 | 8 | 75 | 16 |
| Biologic agents before conventional DMARDs for rheumatoid arthritis | 4.53 ± 0.87 | 6 | 75 | 14 |
| Frequent DXA scans | 4.85 ± 0.48 | 1 | 64 | 6 |
ANA = antinuclear antibody; MRI = magnetic resonance imaging; DMARDs = disease-modifying antirheumatic drugs; DXA = dual x-ray bone absorptiometry.
Assessed on a Likert scale anchored from 1 = “strongly disagree” to 5 = “strongly agree.”
Percentage of respondents who “disagreed” or “strongly disagreed.”
Assessed by asking respondents whether they thought the item was high impact (yes/no).
ACR member survey qualitative analysis results
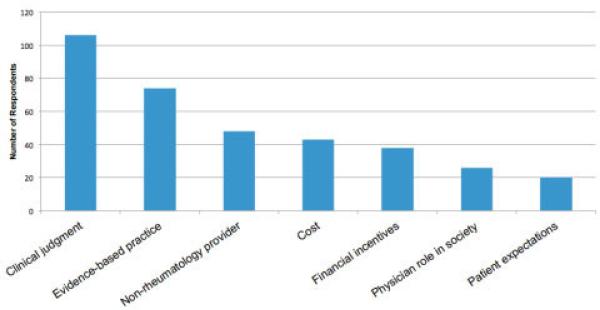
A total of 293 unique comments were received from members responding to the survey. Figure 2 shows the major themes identified. A more detailed list of themes and subthemes, as well as examples of physician responses, can be found in Supplementary Tables 2 and 3 (available in the online version of this article at http://onlinelibrary.wiley.com/doi/10.1002/acr.21930/abstract). Briefly, the single most commonly identified theme was clinical judgment. Many respondents thought that the statements were reasonable as long as they were not applied in a way that did not consider important clinical exceptions or physician judgment. For example, one respondent wrote, “All recommendations for test reduction should function well as guidelines as long as easy ‘opt-out’ is available for judicious use of clinical judgment.” The second most commonly occurring theme related to non-rheumatology providers. Many ACR members commented that a significant amount of waste occurs before patients are evaluated by rheumatologists. For example, one respondent wrote, “Many of the excesses we see in ordering of tests and imaging occur in primary care practices before referral to rheumatology.” Additional examples are provided in Supplementary Table 3 (available in the online version of this article at http://onlinelibrary.wiley.com/doi/10.1002/acr.21930/abstract).
Figure 2.
Summary of themes that emerged from qualitative analysis of the American College of Rheumatology (ACR) Top 5 List survey. ACR members were asked to provide comments on either the individual candidate items proposed for the Top 5 list or any aspect of the overall project. Free-text survey responses were analyzed using qualitative analysis to identify major themes and subthemes. For additional details, please see Supplementary Table 2 and Table 3 (available in the online version of this article at http://onlinelibrary.wiley.com/.doi/10.1002/acr.21930/abstract).
Task force item selection
After review of evidence reports corresponding to the 10 candidate items (Supplementary Table 1, available in the online version of this article at http://onlinelibrary.wiley.com/doi/10.1002/acr.21930/abstract), the task force selected the final 5 items based on the level of evidence, relevance to rheumatology practice, and member survey results, including content agreement and impact ratings. These items are shown in Table 1. Feedback from both patient reviewers and the ACR Board of Directors was considered in crafting the final statements.
Although a comprehensive review of the literature summarizing the supporting evidence for each item on the ACR Top 5 list is beyond the scope of this article, we briefly summarize key studies below. We also frame the scientific evidence in 2 ways. First, we apply a framework deemed applicable to the Top 5 list that has been recently proposed by Hoffman and Pearson, modified from a previous classification scheme (7). Next, we apply a modified version of the GRADE system that classifies recommendations as strong (grade 1) or weak (grade 2) ac cording to the balance among benefits, risks, burdens, and cost, and the quality of evidence as high (grade A), moderate (grade B), or low (grade C) according to study design, consistency of results, and directness of the evidence (6).
FINAL TOP 5 ITEMS
1. Do not test antinuclear antibody (ANA) subserologies without a positive ANA and clinical suspicion of immune-mediated disease
Framework. Adequate evidence demonstrating no additional benefits and higher risks and/or costs.
Level of evidence. Grade 1C.
Guidelines. American College of Pathologists (8), ACR (9), Italian Society of Laboratory Medicine Guideline (10). Issues surrounding laboratory testing for ANAs were the most commonly identified ideas for the ACR Top 5 list, reflecting their frequent use and perceived misuse in clinical practice. ANA testing was recently cited in an article regarding the appropriate use of screening and diagnostic tests to foster high-value, cost-conscious care by the American College of Physicians, in which they named clinical situations in which a test does not reflect high-value care. Regarding use of ANAs in the primary care setting, the authors wrote: “Do not screen for ANAs in patients with non-specific symptoms, such as fatigue or myalgia, or in patients with fibromyalgia” (11). This suggestion follows from several principles regarding use of diagnostic tests, including that tests should not be performed if results will not change management or if the pretest probability of disease is low enough to raise the likelihood of a false-positive test higher than the likelihood of a true-positive result. In our surveys, rheumatologists raised additional concerns related to ANA testing, including serial testing of ANAs to follow immunologic disease activity as a bio-marker (Supplementary Table 1, available in the online version of this article at http://onlinelibrary.wiley.com/doi/10.1002/acr.21930/abstract). Current evidence does not support the use of serial testing for this purpose (9). The final item regarding testing for ANA subserologies was selected because of its direct relevance to rheumatology practice and its high impact ratings and rank in the ACR member survey.
The selected item has 2 separate components. The first relates to ordering ANA subserologies before it is known that the ANA is positive. While potentially efficient for the clinician, such additional testing can be costly because tests for ANA subserologies (including antibodies to double-stranded DNA [dsDNA], Sm, RNP, SSA, SSB, Scl-70, and centromere) are usually negative if the ANA is negative (8,12–14). There are important exceptions where subserology testing may be useful in the context of a negative ANA test. These include anti–Jo-1, which can be positive in a unique clinical subset of myositis (8,15), or sometimes, anti-SSA in the setting of lupus or Sjögren's syndrome (16–18). Despite these exceptions, in most clinical situations, a stepwise approach in which ANA subserologies are ordered only after an ANA is known to be positive is most appropriate. It should be noted that the method of ANA testing is important and current recommendations refer primarily to the immunofluorescence ANA test, which is considered the “gold standard” by the ACR (19).
The second portion of the statement refers to ordering subserologies only when there is clinical suspicion of a specific immune-mediated disease. The recent proliferation of broad “rheumatology panels” is perhaps the most dramatic example of nondirected testing. A panel may be triggered when an ANA is detected, and automatic testing for some combination of the following autoantibodies occurs: dsDNA, histone, Sm, RNP, SSA, SSB, Scl-70, Jo-1, chromatin, striated muscle, parietal cell, myocardial, mitochondrial, actin, and ribosomal P. While individual tests have specific disease or prognostic associations, the utility of such broad screens is unproven, especially because the pretest probability for the diseases related to these different serologies usually varies within an individual patient.
Immunologic laboratory tests are continuing to rapidly evolve with new high throughput technologies that will enable more rapid tests to be done at lower costs. However, newer testing may complicate patient care if it results in undirected or very broad testing that increases false-positive results, leading to unnecessary followup tests and patient concern. As experts in the evaluation of immune-mediated diseases, it is important for rheumatologists to take a proactive role within their practices, local communities, and health systems to ensure that rational approaches to ANA and ANA subserology testing are adopted.
2. Do not test for Lyme disease as a cause of musculoskeletal symptoms without an exposure history and appropriate examination findings
Framework
Adequate evidence demonstrating no additional benefits and higher risks and/or costs.
Level of evidence
Grade 1A.
Guidelines
Centers for Disease Control and Prevention (20), American College of Physicians (21,22), Infectious Diseases Society of America (23).
The inappropriate use of testing for Lyme disease is prevalent and often leads to both wasteful health care spending and significant patient harm. Studies demonstrate that Lyme testing is ordered appropriately in only 20–40% of cases in endemic areas (24–27), and in <10% of cases in nonendemic areas (28). Clinical scenarios associated with overuse of testing include patients presenting with a characteristic erythema migrans rash, patients reporting tick bites who do not have appropriate symptoms or clinical findings, and patients requesting testing in the setting of nonspecific symptoms. Unfortunately, between 10% and 79% of patients who are inappropriately tested receive antibiotics irrespective of serologic results (24,27), sometimes resulting in preventable harm because of the adverse outcomes associated with therapy, and more generally, contributing to antibiotic overuse and resistance (26,28,29).
Testing in patients with musculoskeletal symptoms should be considered only in those who live in or have traveled to endemic areas, have risk factors for tick exposure, and have symptoms consistent with Lyme disease. Lyme disease can manifest in 3 phases: acute localized disease, early disseminated disease, and late disseminated disease. Patients with recent tick exposure (3–30 days) may develop acute localized disease manifesting as a typical erythema migrans rash, and may also experience transient arthralgias, myalgias, or fever (22). Patients with a characteristic erythema migrans rash should be treated without testing because their pretest probability for Lyme disease is extremely high (21,23).
Early disseminated disease, which also usually occurs within 30 days of tick exposure, represents hematogenous spread of the culprit spirochete to various sites, including the joints, nervous system, and heart. At this stage of disease, patients may experience recurrent brief attacks of monarticular or oligoarticular arthritis. Late disseminated disease, which occurs months to years after the original tick exposure, can result in intermittent or chronic monarticular or oligoarticular arthritis, most commonly in the knees (22,30). The presence of objective findings on clinical examination coupled with an appropriate exposure history and duration of symptoms determine the pretest probability of Lyme disease and define the subgroups of patients in whom testing or therapy is appropriate. It should be noted that patients presenting solely with nonspecific symptoms such as chronic fatigue or chronic diffuse arthralgias and myalgias should not be tested for Lyme disease even if they reside in endemic areas (22).
3. Do not perform magnetic resonance imaging (MRI) of the peripheral joints to routinely monitor inflammatory arthritis
Framework
Insufficient evidence to evaluate comparative benefits when used beyond the boundaries of established indication(s), frequency, intensity, or dosage.
Level of evidence
Grade 1B.
Guidelines
2012 update of the 2008 ACR recommendations for the use of disease-modifying antirheumatic drugs (DMARDs) and biologic agents in the treatment of rheumatoid arthritis (RA) (31), European League Against Rheumatism (EULAR) 2006 recommendations for the management of early arthritis (32).
MRI is currently a topic of active investigation in rheumatology, and researchers are assessing its usefulness in improving the capacity to diagnose early inflammatory arthritis, predict disease progression, gauge prognosis, and perhaps define clinical remission (33–36). In the clinical setting, the availability of physician office–based MRI machines has likely contributed to self-referral and higher utilization of this technology for the evaluation of inflammatory arthritis. While it is not known exactly how often MRI is utilized in daily clinical practice by rheumatologists, one unpublished survey reportedly found that >30% of rheumatologists had used MRI for the management of RA patients within the prior 12 months (37).
Although we anticipate that research will help define the future role of MRI in the management of rheumatic disease, data are currently inadequate to justify use of this technology for these purposes in clinical practice. In a systematic review of 17 studies addressing MRI use in early inflammatory arthritis, the ability of MRI to predict progressive radiographic damage varied dramatically (18–100% sensitivity and 6–97% specificity) (37). Importantly, there is no consensus on definitive MRI criteria for serial monitoring of disease activity. Although bone edema assessed by MRI on a single occasion may be predictive of progression in certain RA populations, using MRI routinely is not cost effective compared with the current standard of care (38).
Currently, the ACR and EULAR recommend the use of plain film radiography and clinical disease activity assessments, which include some combination of physician global assessments, tender and swollen joint counts, inflammatory markers, and patient-reported outcomes such as global assessments of pain or function to follow RA disease activity (31,32,39).
4. Do not prescribe biologic agents for RA before a trial of methotrexate (or other conventional nonbiologic DMARD)
Framework
Adequate evidence demonstrating no additional benefits and higher risks and/or costs.
Level of evidence
Grade 1A.
Guidelines
2012 update of the 2008 ACR recommendations for the use of DMARDs and biologic agents in the treatment of RA (31), EULAR recommendations for the management of RA with synthetic and biologic DMARDs (39).
Innovations in RA therapy using combinations of conventional nonbiologic DMARDs in the early 1990s followed by the introduction of tumor necrosis factor α inhibitors and other biologic agents helped make the current paradigms of early therapy and tight control in RA a reality. Many patients with RA can now expect excellent disease control and less disease-related disability. However, given that the typical cost of biologic DMARDs is between $20,000 and $50,000/year, an amount that dwarfs spending for most other categories of medications in the US, the judicious use of biologic agents is mandatory and critical to upholding the Physician Charter, which calls on the profession to help ensure the fair distribution of health care resources in society (3).
There is little debate that biologic DMARDs are cost effective for individuals with RA who have failed adequate trials of conventional nonbiologic DMARDs. In patients with newly diagnosed RA, however, conventional nonbiologic DMARDs such as methotrexate have been shown to be effective and at a significantly less cost. The recent 2012 ACR recommendations for RA state that patients should receive initial therapy with conventional nonbio-logic DMARDs unless these are contraindicated (31). Rheumatologists participating in our surveys widely agreed that at minimum, patients should receive an adequate trial of methotrexate, defined as receiving a stable dose for ≥3 months, before progressing to biologic DMARDs unless there is a clear contraindication to this drug. The one exception to this general strategy relates to a subgroup of patients who have especially severe presentations, such as those with high disease activity on standard disease activity measures (such as the Disease Activity Score in 28 joints) along with poor prognostic features (positive rheumatoid factor or positive anti–cyclic citrullinated peptide antibodies, erosions on radiographs, extraarticular manifestations of RA, and impaired function) (31). These select patients may be candidates for early biologic therapies given studies demonstrating that at least some outcomes are improved over methotrexate alone with this approach (40).
The current discussion regarding biologic DMARDs will likely have relevance to all novel therapies for RA, including new nonbiologic agents such as oral JAK inhibitors. Research is ongoing to evaluate the optimal timing and combination of these biologic, novel nonbiologic, and conventional nonbiologic DMARDS in RA. However, available scientific evidence coupled with the current cost of biologic DMARDs suggests that an initial trial of conventional nonbiologic DMARDs is warranted in most patients (41,42).
5. Do not routinely repeat dual x-ray absorptiometry (DXA) scans more often than once every 2 years
Framework
Insufficient evidence to evaluate comparative benefits when used beyond the boundaries of established indication(s), frequency, intensity, or dosage.
Level of evidence
Grade 1C.
Guidelines
ACR glucocorticoid-induced osteoporosis recommendations (43), National Osteoporosis Foundation guidelines (44), US Preventative Services Task Force recommendations (45).
DXA scans remain the gold standard for detecting low bone mineral density (BMD) and are generally underutilized in screening for osteoporosis in many populations (46–48). Initial screening for osteoporosis should be performed according to National Osteoporosis Foundation recommendations (44). The optimal interval for repeat DXA scans, expecially in high-risk patients such as those initiating glucocorticoids, is uncertain (49,50). Despite this uncertainty, availability of physician office–based DXA scanners and a Medicare policy that reimburses for repeat DXA scans every 2 years have likely contributed to higher utilization of this technology in some groups of patients (51).
For healthy older women, repeat BMD measurement even 8–15 years after the initial screen may not improve the predictive value for fractures beyond the initial measurement (50,52). However, for women with DXA results showing low bone mass, repeat testing after 1–5 years may reveal that a significant fraction has progressed to osteoporosis (52,53). In practice, many high-risk patients with low bone mass would have therapy initiated, so an additional DXA scan is unlikely to affect clinical treatment decisions. Importantly, changes in bone density over short intervals (i.e., <2 years) are often smaller than the significant change detectable by most DXA scanners, which also reduces the utility of frequent testing (54). Moreover, even in high-risk patients receiving drug therapy for osteoporosis, DXA changes do not always correlate with the probability of fracture (49,53). Data from clinical trials indicate that treatment may decrease fracture risk even when there is no apparent increase in BMD (53,55,56).
Further research is needed on the timing and frequency of DXA testing in patients at risk for osteoporosis and those with known osteoporosis who are receiving therapy. In addition, it remains unclear how often DXA scans should be repeated in patients who have discontinued bisphosphonates (taken a “drug holiday”). However, at minimum, current data suggest that for most patients, repeating DXA scans within a 2-year interval is unlikely to add value or alter clinical management. DXA scans should only be repeated if the result will influence clinical management or if very rapid changes in bone density are expected (57).
DISCUSSION
In this study, we used a systematic, consensus, and evidence-based approach to develop a list of tests and treatments in rheumatology whose use in certain clinical situations should be questioned and discussed by physicians and patients. The ACR's Top 5 list covers a range of services and tests that are commonly provided by or ordered by rheumatologists. Items are either based on high-quality evidence or describe practices that currently have insufficient scientific evidence to support their use. Our methods demonstrate that it is possible to engage the physician community in a meaningful conversation about the appropriate use of resources and to achieve consensus at a national level regarding what constitutes cost-conscious, high-value care.
Over the coming months, the ACR will work with the ABIM Foundation and Consumer Reports, partners in the Choosing Wisely campaign, to disseminate the Top 5 list to both medical professionals and the public. The intent of the dissemination activities will be not only to ensure that rheumatologists are up to date on the appropriate use of the tests and services outlined in the Top 5 list, but also to prompt discussions between rheumatologists, their patients, and referring providers. Increasingly, with ubiquitous access to web-based sources of information and direct-to-consumer advertising, patients may request or even demand specific health care tests, procedures, or treatments. On occasion, these services are not in their best interest, either because they may lead to harm, wasted personal resources, or unnecessary followup testing or treatment. Saying “no” to patients in such situations, while difficult, upholds 2 of the key principles of professionalism laid out in the Physician Charter: the primacy of patient welfare and social justice, which calls on physicians to do their part to ensure the equitable distribution of health care resources in society (3). Conversely, as patients become more educated consumers of health care, they may reasonably decline health care services that are offered to them for a variety of reasons, and respecting these wishes upholds another principle of professionalism: patient autonomy.
Rheumatologists care for patients with some of the most complex and poorly understood diseases afflicting humans, and are specifically trained to act as medical detectives. The practice of rheumatology is still very much an art, one in which a careful history and physical examination and a solid breadth of knowledge across multiple medical specialties are important complements to the latest scientific evidence. As such, it is not surprising that rheumatologists responding to our national survey raised the issue of clinical judgment more frequently than any other in our qualitative analysis. We would like to highlight that the ACR Top 5 list is not meant to supplant the judicious use of clinical judgment, and it would be inappropriate to apply these items as a rigid set of rules in clinical care. In addition, it is important to note that the list was developed by and for rheumatologists caring for adult patients, and application of these items to pediatric rheumatology may be inappropriate.
Although the ACR Top 5 list was derived from practicing rheumatologists based on their observations in their communities, a limitation of this work is a lack of precise estimates of the prevalence of these practices. Therefore, the potential cost savings or value improvement associated with the items is unclear and should be the subject of future systematic investigations. In addition, we acknowledge that the field of rheumatology is changing rapidly, and emerging knowledge has the potential to change our thinking about each of these items in the coming years. Moreover, clinical research applications are excluded from the intent of the recommendations. The list should therefore be viewed as dynamic, and the ACR will need to update its content as new evidence emerges.
As health care reform efforts move forward in the coming years, it is critical for all physicians to actively embrace their role as stewards of finite health care resources and meaningfully engage in efforts to improve the quality and safety of health care while working toward curbing wasteful health care spending. We are encouraged by the willingness and enthusiasm of clinicians in our specialty to participate in this initial exercise, and hope that the ACR Top 5 list informs future discussions about when less is more in rheumatology.
Supplementary Material
ACKNOWLEDGMENTS
The authors would like to thank Amy Miller, Rachel Myslinski, Regina Parker, Tammy Tilley, and Kevin Whorton for assistance with administration of the project. We also thank Mary Nakamura, MD, Allen C. Steere, MD, Robert Kalish, MD, Lisa Suter, MD, Alice H. Chen, MD, MPH, and Sophia Chang, MD, MPH, for their assistance.
Supported by the NIH (grant K23-AR060259 and the American College of Rheumatology).
APPENDIX A: MEMBERS OF THE AMERICAN COLLEGE OF RHEUMATOLOGY CORE MEMBERSHIP GROUP
Members of the American College of Rheumatology Core Membership Group included: Ryan Antolini, Neal Birnbaum, Kori Dewing, Sean Fahey, Madelaine Feldman, Joseph Flood, Jody Hargrove, Ray Hong, Herb Kaplan, Alex Limanni, Taraneh Mehrani, Gwenesta Melton, Rudy Molina, Eric Schned, Robert Shmerling, Christine Stamatos, Christine Thorburn, Cindy Weaver, Kelly Weselman, and Angus Worthing.
Footnotes
Members of the American College of Rheumatology Core Membership Group are listed in Appendix A.
Dr. Beall has received speaking fees from Abbott (less than $10,000). Dr. Gensler has received consulting fees from Abbott and UCB (less than $10,000 each). Dr. Saag has received consulting fees, speaking fees, and/or honoraria from Eli Lilly, Merck, Amgen, Savient, Ardea, Regeneron, URL, and Abbott (less than $10,000 each).
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Yazdany had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Yazdany, Schmajuk, Robbins, Daikh, Beall, Yelin, Barton, Zell, Saag, King.
Acquisition of data. Yazdany, Schmajuk, Beall, Barton, Carlson, Margaretten, Gensler, Kelly, Saag, King.
Analysis and interpretation of data. Yazdany, Schmajuk, Robbins, Daikh, Beall, Yelin, Barton, Carlson, Margaretten, Zell, Kelly, Saag, King
REFERENCES
- 1.Brody H. Medicine's ethical responsibility for health care reform: the Top Five list. N Engl J Med. 2010;362:283–5. doi: 10.1056/NEJMp0911423. [DOI] [PubMed] [Google Scholar]
- 2.Cassel CK, Guest JA. Choosing wisely: helping physicians and patients make smart decisions about their care. JAMA. 2012;307:1801–2. doi: 10.1001/jama.2012.476. [DOI] [PubMed] [Google Scholar]
- 3.ABIM Foundation, American Board of Internal Medicine, ACP-ASIM Foundation, American College of Physicians-American Society of Internal Medicine, European Federation of Internal Medicine Medical professionalism in the new millennium: a physician charter. Ann Intern Med. 2002;136:243–6. doi: 10.7326/0003-4819-136-3-200202050-00012. [DOI] [PubMed] [Google Scholar]
- 4.Linstone A, Turoff M. The Delphi method: techniques and applications. Addison-Wesley; Reading (MA): 1975. [Google Scholar]
- 5.Charmaz K. Constructing grounded theory: a practical guide through qualitative analysis. Sage Publications; Thousand Oaks (CA): 2006. [Google Scholar]
- 6.Guyatt G, Gutterman D, Baumann MH, Addrizzo-Harris D, Hylek EM, Phillips B, et al. Grading strength of recommendations and quality of evidence in clinical guidelines: report from an American College of Chest Physicians task force. Chest. 2006;129:174–81. doi: 10.1378/chest.129.1.174. [DOI] [PubMed] [Google Scholar]
- 7.Hoffman A, Pearson SD. ‘Marginal medicine’: targeting comparative effectiveness research to reduce waste. Health Aff (Millwood) 2009;28:w710–8. doi: 10.1377/hlthaff.28.4.w710. [DOI] [PubMed] [Google Scholar]
- 8.Kavanaugh A, Tomar R, Reveille J, Solomon DH, Homburger HA, American College of Pathologists Guidelines for clinical use of the antinuclear antibody test and tests for specific autoantibodies to nuclear antigens. Arch Pathol Lab Med. 2000;124:71–81. doi: 10.5858/2000-124-0071-GFCUOT. [DOI] [PubMed] [Google Scholar]
- 9.Solomon DH, Kavanaugh AJ, Schur PH, American College of Rheumatology Ad Hoc Committee on Immunologic Testing Guidelines Evidence-based guidelines for the use of immunologic tests: antinuclear antibody testing. Arthritis Rheum. 2002;47:434–44. doi: 10.1002/art.10561. [DOI] [PubMed] [Google Scholar]
- 10.Tozzoli R, Bizzaro N, Tonutti E, Villalta D, Bassetti D, Manoni F, et al. Guidelines for the laboratory use of autoantibody tests in the diagnosis and monitoring of autoimmune rheumatic diseases. Am J Clin Pathol. 2002;117:316–24. doi: 10.1309/Y5VF-C3DM-L8XV-U053. [DOI] [PubMed] [Google Scholar]
- 11.Qaseem A, Alguire P, Dallas P, Feinberg LE, Fitzgerald FT, Horwitch C, et al. Appropriate use of screening and diagnostic tests to foster high-value, cost-conscious care. Ann Intern Med. 2012;156:147–9. doi: 10.7326/0003-4819-156-2-201201170-00011. [DOI] [PubMed] [Google Scholar]
- 12.Thomson KF, Murphy A, Goodfield MJ, Misbah SA. Is it useful to test for antibodies to extractable nuclear antigens in the presence of a negative antinuclear antibody on Hep-2 cells? [letter]. J Clin Pathol. 2001;54:413. doi: 10.1136/jcp.54.5.413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cross LS, Aslam A, Misbah SA. Antinuclear antibody-negative lupus as a distinct diagnostic entity: does it no longer exist? QJM. 2004;97:303–8. doi: 10.1093/qjmed/hch048. [DOI] [PubMed] [Google Scholar]
- 14.Slater CA, Davis RB, Shmerling RH. Antinuclear antibody testing: a study of clinical utility. Arch Intern Med. 1996;156:1421–5. [PubMed] [Google Scholar]
- 15.Bahrt KM, McCarty GA. A negative fluorescent antinuclear antibody test in a patient with Jo-1 antibody. J Rheumatol. 1985;12:624–5. [PubMed] [Google Scholar]
- 16.Hoffman IE, Peene I, Veys EM, De Keyser F. Detection of specific antinuclear reactivities in patients with negative anti-nuclear antibody immunofluorescence screening tests. Clin Chem. 2002;48:2171–6. [PubMed] [Google Scholar]
- 17.Dahle C, Skogh T, Aberg AK, Jalal A, Olcen P. Methods of choice for diagnostic antinuclear antibody (ANA) screening: benefit of adding antigen-specific assays to immunofluorescence microscopy. J Autoimmun. 2004;22:241–8. doi: 10.1016/j.jaut.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 18.Bossuyt X, Luyckx A. Antibodies to extractable nuclear antigens in antinuclear antibody-negative samples [letter]. Clin Chem. 2005;51:2426–7. doi: 10.1373/clinchem.2005.058552. [DOI] [PubMed] [Google Scholar]
- 19.American College of Rheumatology Position Statement, methodology of testing for antinuclear antibodies. 2011 URL: http://www.rheumatology.org/practice/clinical/position/ana_ position_stmt.pdf.
- 20.Centers for Disease Control and Prevention Lyme disease diagnosis and treatment. 2011 URL: www.cdc.gov/lyme/diagnosistreatment/index.html.
- 21.American College of Physicians Guidelines for laboratory evaluation in the diagnosis of Lyme disease. Ann Intern Med. 1997;127:1106–8. doi: 10.7326/0003-4819-127-12-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 22.Hu LT. Lyme disease. Ann Intern Med. 2012;157:ITC2–1. doi: 10.7326/0003-4819-157-3-201208070-01002. [DOI] [PubMed] [Google Scholar]
- 23.Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS, et al. The clinical assessment, treatment, and prevention of Lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America. Clin Infect Dis. 2006;43:1089–134. doi: 10.1086/508667. [DOI] [PubMed] [Google Scholar]
- 24.Fix AD, Strickland GT, Grant J. Tick bites and Lyme disease in an endemic setting: problematic use of serologic testing and prophylactic antibiotic therapy. JAMA. 1998;279:206–10. doi: 10.1001/jama.279.3.206. [DOI] [PubMed] [Google Scholar]
- 25.Magri JM, Johnson MT, Herring TA, Greenblatt JF. Lyme disease knowledge, beliefs, and practices of New Hampshire primary care physicians. J Am Board Fam Pract. 2002;15:277–84. [PubMed] [Google Scholar]
- 26.Qureshi MZ, New D, Zulqarni NJ, Nachman S. Overdiagnosis and overtreatment of Lyme disease in children. Pediatr Infect Dis J. 2002;21:12–4. doi: 10.1097/00006454-200201000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Ramsey AH, Belongia EA, Chyou PH, Davis JP. Appropriateness of Lyme disease serologic testing. Ann Fam Med. 2004;2:341–4. doi: 10.1370/afm.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ley C, Le C, Olshen EM, Reingold AL. The use of serologic tests for Lyme disease in a prepaid health plan in California. JAMA. 1994;271:460–3. [PubMed] [Google Scholar]
- 29.Reid MC, Schoen RT, Evans J, Rosenberg JC, Horwitz RI. The consequences of overdiagnosis and overtreatment of Lyme disease: an observational study. Ann Intern Med. 1998;128:354–62. doi: 10.7326/0003-4819-128-5-199803010-00003. [DOI] [PubMed] [Google Scholar]
- 30.Steere AC. Diagnosis and treatment of Lyme arthritis. Med Clin North Am. 1997;81:179–94. doi: 10.1016/s0025-7125(05)70510-1. [DOI] [PubMed] [Google Scholar]
- 31.Singh JA, Furst DE, Bharat A, Curtis JR, Kavanaugh AF, Kremer JM, et al. 2012 update of the 2008 American College of Rheumatology recommendations for the use of disease-modifying antirheumatic drugs and biologic agents in the treatment of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2012;64:625–39. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Combe B, Landewe R, Lukas C, Bolosiu HD, Breedveld F, Dougados M, et al. EULAR recommendations for the management of early arthritis: report of a Task Force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis. 2007;66:34–45. doi: 10.1136/ard.2005.044354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.American College of Rheumatology Extremity Magnetic Resonance Imaging Task Force Extremity magnetic resonance imaging in rheumatoid arthritis: report of the American College of Rheumatology Extremity Magnetic Resonance Imaging Task Force. Arthritis Rheum. 2006;54:1034–47. doi: 10.1002/art.21763. [DOI] [PubMed] [Google Scholar]
- 34.Machado PM, Koevoets R, Bombardier C, van der Heijde DM. The value of magnetic resonance imaging and ultrasound in undifferentiated arthritis: a systematic review. J Rheumatol Suppl. 2011;87:31–7. doi: 10.3899/jrheum.101072. [DOI] [PubMed] [Google Scholar]
- 35.Ostergaard M, Poggenborg RP. Magnetic resonance imaging in psoriatic arthritis: update on current status and future perspectives: a report from the GRAPPA 2010 annual meeting. J Rheumatol. 2012;39:408–12. doi: 10.3899/jrheum.111235. [DOI] [PubMed] [Google Scholar]
- 36.Ostergaard M, Peterfy C, Conaghan P, McQueen F, Bird P, Ejbjerg B, et al. OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging Studies: core set of MRI acquisitions, joint pathology definitions, and the OMERACT RA-MRI scoring system. J Rheumatol. 2003;30:1385–6. [PubMed] [Google Scholar]
- 37.Suter LG, Fraenkel L, Braithwaite RS. Role of magnetic resonance imaging in the diagnosis and prognosis of rheumatoid arthritis. Arthritis Care Res (Hoboken) 2011;63:675–88. doi: 10.1002/acr.20409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Suter LG, Fraenkel L, Braithwaite RS. Cost-effectiveness of adding magnetic resonance imaging to rheumatoid arthritis management. Arch Intern Med. 2011;171:657–67. doi: 10.1001/archinternmed.2011.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Smolen JS, Landewe R, Breedveld FC, Dougados M, Emery P, Gaujoux-Viala C, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs. Ann Rheum Dis. 2010;69:964–75. doi: 10.1136/ard.2009.126532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Haraoui B, Pope J. Treatment of early rheumatoid arthritis: concepts in management. Semin Arthritis Rheum. 2011;40:371–88. doi: 10.1016/j.semarthrit.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 41.Van der Velde G, Pham B, Machado M, Ieraci L, Witteman W, Bombardier C, et al. Cost-effectiveness of biologic response modifiers compared to disease-modifying antirheumatic drugs for rheumatoid arthritis: a systematic review [review]. Arthritis Care Res (Hoboken) 2011;63:65–78. doi: 10.1002/acr.20338. [DOI] [PubMed] [Google Scholar]
- 42.Finckh A, Bansback N, Liang MH. Cost-effectiveness of biologics in early rheumatoid arthritis [letter]. Ann Intern Med. 2010;152:333–4. doi: 10.7326/0003-4819-152-5-201003020-00018. [DOI] [PubMed] [Google Scholar]
- 43.Grossman JM, Gordon R, Ranganath VK, Deal C, Caplan L, Chen W, et al. American College of Rheumatology 2010 recommendations for the prevention and treatment of glucocorticoid-induced osteoporosis. Arthritis Care Res (Hoboken) 2010;62:1515–26. doi: 10.1002/acr.20295. [DOI] [PubMed] [Google Scholar]
- 44.National Osteoporosis Foundation . Clinician's guide to prevention and treatment of osteoporosis. National Osteoporosis Foundation; Washington, DC: 2010. pp. 1–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.US Preventive Services Task Force Screening for osteoporosis: US Preventive Services Task Force recommendation statement. Ann Intern Med. 2011;154:356–64. doi: 10.7326/0003-4819-154-5-201103010-00307. [DOI] [PubMed] [Google Scholar]
- 46.Curtis JR, Carbone L, Cheng H, Hayes B, Laster A, Matthews R, et al. Longitudinal trends in use of bone mass measurement among older Americans, 1999-2005. J Bone Miner Res. 2008;23:1061–7. doi: 10.1359/JBMR.080232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.King AB, Fiorentino DM. Medicare payment cuts for osteoporosis testing reduced use despite tests’ benefit in reducing fractures. Health Aff (Millwood) 2011;30:2362–70. doi: 10.1377/hlthaff.2011.0233. [DOI] [PubMed] [Google Scholar]
- 48.Schmajuk G, Yelin E, Chakravarty E, Nelson LM, Panopolis P, Yazdany J. Osteoporosis screening, prevention, and treatment in systemic lupus erythematosus: application of the systemic lupus erythematosus quality indicators. Arthritis Care Res (Hoboken) 2010;62:993–1001. doi: 10.1002/acr.20150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kanis JA, Johnell O, Oden A, Jonsson B, De Laet C, Dawson A. Prediction of fracture from low bone mineral density measurements overestimates risk. Bone. 2000;26:387–91. doi: 10.1016/S8756-3282(00)00238-6. [DOI] [PubMed] [Google Scholar]
- 50.Hillier TA, Stone KL, Bauer DC, Rizzo JH, Pedula KL, Cauley JA, et al. Evaluating the value of repeat bone mineral density measurement and prediction of fractures in older women: the study of osteoporotic fractures. Arch Intern Med. 2007;167:155–60. doi: 10.1001/archinte.167.2.155. [DOI] [PubMed] [Google Scholar]
- 51.Zhang J, Delzell E, Zhao H, Laster AJ, Saag KG, Kilgore ML, et al. Central DXA utilization shifts from office-based to hospital-based settings among Medicare beneficiaries in the wake of reimbursement changes. J Bone Miner Res. 2012;27:858–64. doi: 10.1002/jbmr.1534. [DOI] [PubMed] [Google Scholar]
- 52.Gourlay ML, Fine JP, Preisser JS, May RC, Li C, Lui LY, et al. Bone-density testing interval and transition to osteoporosis in older women. N Engl J Med. 2012;366:225–33. doi: 10.1056/NEJMoa1107142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Watts NB, Geusens P, Barton IP, Felsenberg D. Relationship between changes in BMD and nonvertebral fracture incidence associated with risedronate: reduction in risk of nonvertebral fracture is not related to change in BMD. J Bone Miner Res. 2005;20:2097–104. doi: 10.1359/JBMR.050814. [DOI] [PubMed] [Google Scholar]
- 54.Nelson HD, Helfand M, Woolf SH, Allan JD. Screening for postmenopausal osteoporosis: a review of the evidence for the US Preventive Services Task Force. Ann Intern Med. 2002;137:529–41. doi: 10.7326/0003-4819-137-6-200209170-00015. [DOI] [PubMed] [Google Scholar]
- 55.Sarkar S, Mitlak BH, Wong M, Stock JL, Black DM, Harper KD. Relationships between bone mineral density and incident vertebral fracture risk with raloxifene therapy. J Bone Miner Res. 2002;17:1–10. doi: 10.1359/jbmr.2002.17.1.1. [DOI] [PubMed] [Google Scholar]
- 56.Bauer DC, Black DM, Garnero P, Hochberg M, Ott S, Orloff J, et al. Change in bone turnover and hip, non-spine, and vertebral fracture in alendronate-treated women: the fracture intervention trial. J Bone Miner Res. 2004;19:1250–8. doi: 10.1359/JBMR.040512. [DOI] [PubMed] [Google Scholar]
- 57.Bates DW, Black DM, Cummings SR. Clinical use of bone densitometry: clinical applications. JAMA. 2002;288:1898–900. doi: 10.1001/jama.288.15.1898. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.